Abstract
Background
COVID-19 has changed the world as we know it, and the United States continues to accumulate the largest number of COVID-related deaths worldwide. There exists a paucity of data regarding the effect of COVID-19 on adult cardiac surgery trends and outcomes on regional and national levels.
Methods
The Society of Thoracic Surgeons Adult Cardiac Surgery Database was queried from January 1, 2018, to June 30, 2020. The Johns Hopkins COVID-19 database was queried from February 1, 2020, to January 1, 2021. Surgical and COVID-19 volumes, trends, and outcomes were analyzed on a national and regional level. Observed-to-expected ratios were used to analyze risk-adjustable mortality.
Results
The study analyzed 717 103 adult cardiac surgery patients and more than 20 million COVID-19 patients. Nationally, there was a 52.7% reduction in adult cardiac surgery volume and a 65.5% reduction in elective cases. The Mid-Atlantic region was most affected by the first COVID-19 surge, with 69.7% reduction in overall case volume and 80.0% reduction in elective cases. In the Mid-Atlantic and New England regions, the observed-to-expected mortality for isolated coronary bypass increased as much as 1.48 times (148% increase) pre-COVID rates. After the first COVID-19 surge, nationwide cardiac surgical case volumes did not return to baseline, indicating a COVID-19–associated deficit of cardiac surgery patients.
Conclusions
This large analysis of COVID-19–related impact on adult cardiac surgery volume, trends, and outcomes found that during the pandemic, cardiac surgery volume suffered dramatically, particularly in the Mid-Atlantic and New England regions during the first COVID-19 surge, with a concurrent increase in observed-to-expected 30-day mortality.
Adult Cardiac Surgery:
The Annals of Thoracic Surgery CME Program is located online at http://www.annalsthoracicsurgery.org/cme/home. To take the CME activity related to this article, you must have either an STS member or an individual non-member subscription to the journal.
Dr Nguyen discloses a financial relationship with Edwards Lifesciences, Abbott, LivaNova, and CryoLife; Dr Thourani with Abbott, Boston Scientific, Edwards Lifesciences, W. L. Gore & Associates, and JenaValve; and Dr Badhwar with Abbott.
Coronavirus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a highly infectious disease thought to have emerged from Wuhan, China in 2019.1 On March 11, 2020, COVID-19 was recognized by the World Health Organization as a global pandemic, and as of January 2021, the United States (US) has had more reported cases than any other nation.2 Despite shelter-in-place and physical distancing mandates, hospitals were initially overwhelmed with a massive influx of patients with COVID-19, requiring dramatic changes to resource allocation, redeployment of staff, operative volumes, and infection mitigation strategies.3 , 4 Despite improvements in our understanding of COVID-19 pathophysiology and the utility of early steroids, remdesivir, and/or mechanical circulatory support, many questions remain unanswered.5, 6, 7, 8, 9 Previous reports have demonstrated significant morbidity and mortality risk for patients requiring elective or emergent operations who are diagnosed with COVID-19, regardless of preoperative or postoperative COVID-19 contraction.10 Thoracic surgery patients represented a minority of this previously reported cohort, and data specific to adult cardiac surgery patients with COVID-19 are limited to case series, with a relative paucity of larger scale reports.11, 12, 13
Using the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (ACSD), we conducted the study with the goal to better understand the impact of COVID-19 on adult cardiac surgery volumes, trends, and the influence of the COVID-19 surge on risk-adjusted mortality on a national and regional scale.
Patients and Methods
Patient Population
We used data from the STS ACSD to examined patients undergoing cardiac operations from January 1, 2018, to June 30, 2020. Cardiac surgery patients were grouped into pre-COVID, early COVID, and COVID-storm groups to examine demographic differences, while highlighting potentially unique factors among those operated on during the first COVID-19 case surge. When examining overall surgical volume trends, we considered all operations, with additional stratified reporting based on case status as elective or nonelective (urgent, emergent, or emergent salvage cases). As defined by the STS, urgent procedures represent those required during the same hospitalization to prevent further clinical deterioration, emergent procedures involve patients with refractory or ongoing cardiac compromise not responsive to any therapy other than cardiac surgery, and emergent salvage procedures include patients undergoing cardiopulmonary resuscitation or extracorporeal membrane oxygenation (ECMO) to sustain life.14 Patients undergoing procedures at STS reporting sites outside the US were excluded. Preoperative variables and postoperative outcome definitions included those provided by the STS, as previously defined.14 Our primary research goals were to examine adult cardiac surgery temporal volume trends before, during, and after the first COVID-19 peak on national and regional levels as well as to examine any association of COVID-19 surges on short-term mortality, both nationally and in regions that were disproportionately affected by COVID-19.
COVID-19 data were downloaded from the data repository for the 2019 Novel Coronavirus Visual Dashboard operated by the Johns Hopkins University Center for Systems Science and Engineering (JHU CSSE)2 at https://github.com/CSSEGISandData/COVID-19. All US confirmed COVID-19 cases from January 22, 2020, to January 30, 2021, were included in the study. These data were used to plot COVID-19 cases against cardiac surgical case volume over time.
Statistical Analysis
Univariate tests for categorical variables were conducted using the χ2 or Fisher exact test, and continuous variables were compared using the Student t test or Mann-Whitney U test based on distributional assumptions. All calculations analyses were performed using R software (R Foundation for Statistical Computing).15
Cardiac surgical volume trends were analyzed based on monthly volume, and COVID-19 cases were ascertained in weekly volumes to allow sufficient granularity for analysis and reporting. When calculating observed-to-expected (O/E) mortality ratios, only operations with STS risk models were considered: (1) isolated coronary artery bypass grafting (CABG), (2) isolated aortic valve replacement (AVR), (3) isolated mitral valve replacement (MVR), (4) AVR + CABG, (5) MVR + CABG, (6) isolated mitral valve repair (MV repair), and (7) MV repair + CABG. Risk-adjusted mortality is reported for these procedures, as previously described.16 , 17 As a form of risk-adjustment of the 30-day mortality primary outcome trends (pre- and post-COVID), we calculated the monthly O/E mortality ratios considering (1) all 7 procedures with available STS risk models combined and (2) isolated CABG cases only. Given the time frame of this analysis, the volume and outcome comparisons in the US were stratified to early affected regions (New England and Mid-Atlantic) vs all other US regions that were most affected at a later time.
Results
Patient Population
From January 1, 2018, to June 30, 2020, 717 103 adult cardiac surgery patients were isolated in the STS ACSD (299 645 in 2018, 299 310 in 2019, and 118 148 in the first half of 2020). This included 547 605 adult cardiac surgery patients undergoing one of the previously listed procedures for which STS risk models exist (230 351 in 2018, 228 544 in 2019, and 88 710 in the first half of 2020). Among all cases, 356 714 (49.7%) were classified as elective (150 725 in 2018, 150 685 in 2019, and 55 304 in the first half of 2020), and the remainder were collectively classified as nonelective (urgent, emergent, and emergent salvage).
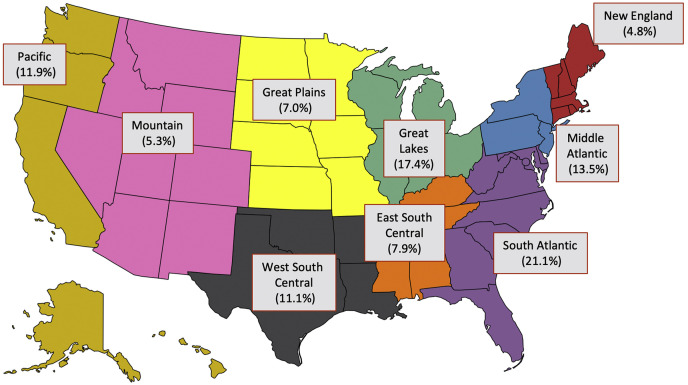
The Table lists demographic and preoperative characteristics of our study cohort. Regional contributions from the 9 STS regions to the overall study cohort are shown in Figure 1 , with the South Atlantic (21.1%) and Great Lakes (17.4%) regions contributing the most patients during the study period. All confirmed US COVID-19 cases in the Johns Hopkins CSSE from 22 January 22, 2020, to January 30, 2021, were included, which totaled more than 20 million patients.
Figure 1.
United States map demonstrates the 9 The Society of Thoracic Surgeons regions and their respective contributions to the overall study cohort.
National Cardiac Surgery Volume Trends
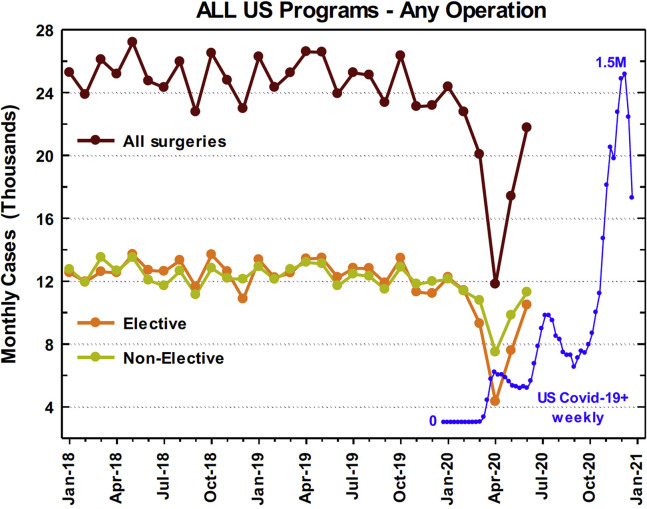
National cardiac surgery volume trends for all cases during the study period are plotted in Figure 2 , together with COVID-19 case volumes. April represented a nadir in cardiac surgical case volumes, which coincided with the first US COVID-19 surge. The nationwide monthly overall cardiac case average for January 2018 to December 2019 was 24 956, compared with 11 803 cases nationwide in April 2020, representing a 52.7% overall case reduction. This included a 65.5% reduction in elective cases (12 559 average for January 2018 through December 2019 vs 4327 in April 2020), and a 39.7% reduction in nonelective cases (12 397 average for January 2018-December 2019 vs 7476 in April 2020). When considering only the previously listed 7 operations for which STS risk-adjustment models exist, these procedures sustained a 53.8% reduction in volume (19 120 average for January 2018-December 2019 vs 8836 in April 2020). Isolated CABG, which represents the most commonly performed operation in the STS ACSD, experienced a 51.2% reduction (13 360 average for January 2018-December 2019 vs 6517 in April 2020) overall. Isolated valve cases (including AVR, MVR, or MV repair) sustained a 62.2% reduction (3871 average for January 2018-December 2019 vs 1464 in April 2020) in volume nationwide. Nationwide case volumes returned to 87.2% of baseline (24 956 average for January 2018-December 2019 vs 21 759 in June 2020) after the first nadir.
Figure 2.
Case decline in the United States (US) with overlayed number of new regional COVID-19 cases during the same time frame.
National and Regional COVID-19 Trends
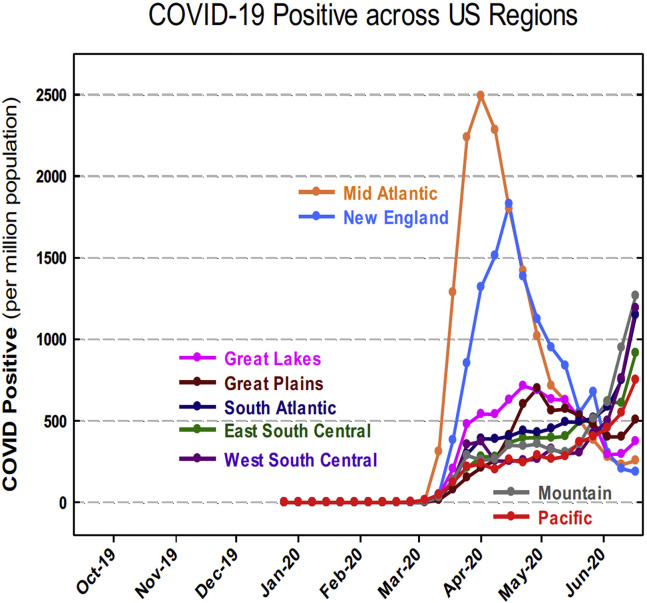
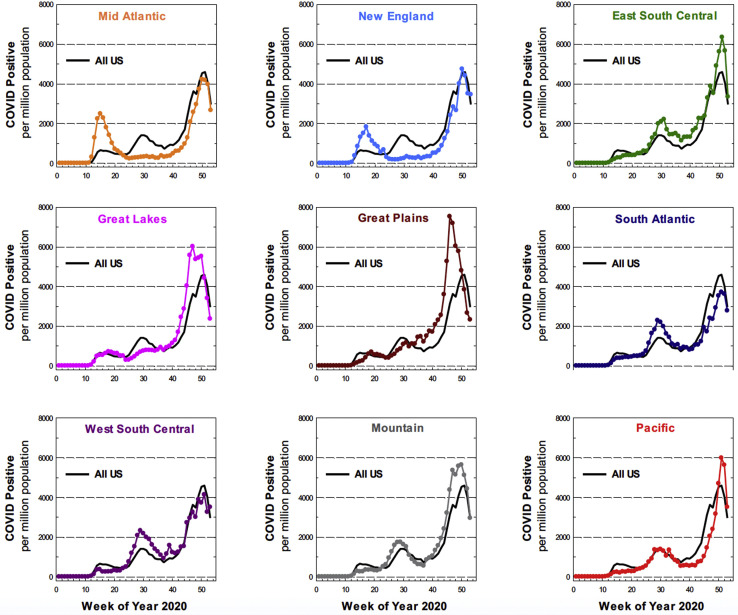
An examination of the geographic variability of the first COVID-19 surge in the US found highly variable rates of new COVID-19 cases across the 9 STS regions, as demonstrated in Figure 3 , with the Mid-Atlantic and New England regions accruing the first and second most COVID-19 cases, respectively, during the first COVID-19 surge. While the Mid-Atlantic and New England regions were most heavily affected by early COVID-19 cases, each of the 9 STS regions experienced its own unique COVID-19 case trends, as demonstrated in Figure 4 . Any regional deviation above the national COVID-19 case rate represents a regional surge compared with the national COVID-19 case rate at a given time.
Figure 3.
United States (US) regional COVID-19 rates per million population.
Figure 4.
Regional (colors) and United States (US) (solid black) COVID-19 trends, normalized per million population. Any deviation of the colored line from the black line indicates regional rates of COVID-19 that are increasing greater than the national rate.
Regional Cardiac Surgery Volume Trends
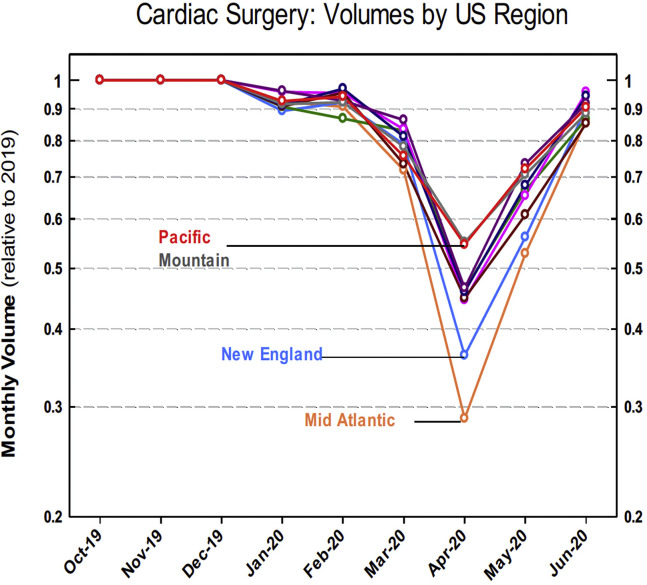
Regional cardiac surgery case volumes during the first COVID-19 surge were compared with mean regional case volumes of the respective months in previous years, as plotted in Figure 5 , demonstrating the greatest reduction in regional surgical case volumes in the Mid-Atlantic and New England regions. Given that the Mid-Atlantic region was impacted with both the highest number of COVID-19 cases during the first surge in the US as well as the greatest relative reduction in cardiac surgical case volumes, this region was chosen for a regional subgroup analysis of cardiac volume trends during the early COVID-19 pandemic.
Figure 5.
United States (US) regional monthly cardiac surgery volumes as a percentage of mean volumes for the previous monthly value.
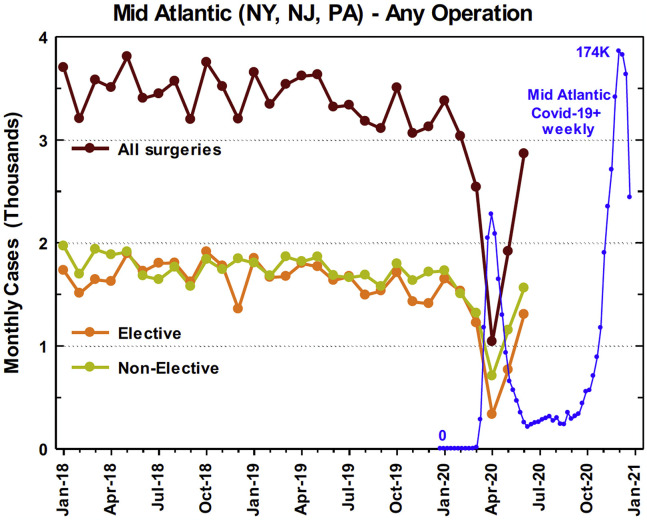
The Mid-Atlantic monthly cardiac surgical case average for January 2018 through December 2019 was 3430 cases compared with 1040 cases in April 2020, representing a 69.7% overall case reduction. This included a reduction in elective cases by 80.0% (1668 average for January 2018-December 2019 vs 333 in April 2020) and a reduction in nonelective cases by 59.8% (1762 average for January 2018-December 2019 vs 707 in April 2020) (Figure 6 ). The 7 operations for which STS risk-adjustment models exist underwent a 69.7% reduction in volume (2505 average for January 2018-December 2019 vs 759 in April 2020), including a 68.5% reduction (1625 average for January 2018-December 2019 vs 512 in April 2020) in isolated CABG and a 73.6% reduction (602 average for January 2018-December 2019 vs 159 in April 2020) in isolated valve case volumes in the region. Regional cardiac surgery case volumes returned to 83.5% of baseline (3430 average for January 2018-December 2019 vs 2865 in June 2020) after the first nadir.
Figure 6.
Mid-Atlantic regional case decline with overlayed number of new regional COVID-19 cases during the same time frame.
Cardiac Surgical Outcomes
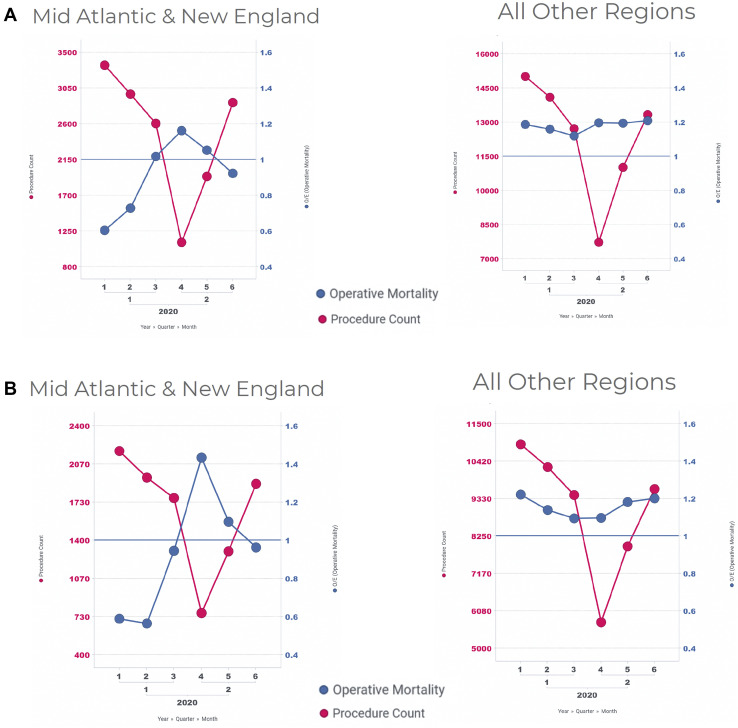
Figure 7A shows the 30-day mortality O/E ratio for all 7 STS risk-adjustable procedures in the Mid-Atlantic and New England regions vs all other US regions. The average mortality O/E ratio increased from a pre–COVID-19 (January and February 2020) of 0.66 to a high of 1.16 during April 2020 (peak of COVID-19 in those regions). This represented a 75% increase in the mortality O/E ratio in the Mid-Atlantic and New England, the 2 regions most affected by the first COVID-19 surge. When the isolated CABG 30-day mortality O/E ratio was examined, a similar comparison of the Mid-Atlantic and New England regions vs all other regions was found (Figure 7B). The O/E ratio increased from a pre–COVID-19 (January and February 2020) average of 0.58, to a high of 1.43 in April 2020, representing a 148% increase in 30-day mortality O/E ratio for isolated CABG in the same 2 regions. In comparison, the 30-day mortality O/E ratio for all other regions did not dramatically change when risk-adjusted outcomes for all procedures and for isolated CABG were examined.
Figure 7.
Mortality observed/expected (O/E) ratios for (A) all risk-adjustable operations and for (B) isolated coronary artery bypass grafting in the Mid-Atlantic and New England regions vs all other regions.
Comment
The COVID-19 pandemic continues to represent an unprecedented global health challenge as we continue to confront issues related to health care resources, personnel, and methods for safe delivery of care under new circumstances. We report the largest and most comprehensive description of the impact of the COVID-19 pandemic on cardiac surgical volumes, trends, and outcomes on a regional and national scale. This effort is unique, because unlike many other surgical databases, the STS ACSD captures more than 98% of all adult cardiac surgery cases in the US based on the most recent analyses.
After examination of nearly three-quarters of a million adult cardiac surgical cases and more than 20 million COVID-19 cases, a clear nadir of cardiac surgical volumes was seen in April 2020, with reductions to less than half of the previous level, notably including a nearly 40% reduction in all nonelective cases. When the temporal focus was on the first nationwide COVID-19 surge in Spring 2020, cardiac case volumes in the Mid-Atlantic and New England regions were affected to the greatest degree, with an even greater reduction in nonelective cases by nearly 60% in the Mid-Atlantic.
However, when we examined cardiac surgical volumes after April 2020, although some volume recovery was seen, there was not a rebound of case volumes to above-baseline levels that would adequately account for the previous nonelective cardiac surgical case deficit, neither regionally nor nationally. This finding suggests a COVID-19–related deficit of untreated adult cardiac surgery patients.
The consequence of these untreated patients remains unclear. As described previously, the STS defines even the healthiest of this subset of patients as “urgent,” requiring cardiac surgery during the same hospitalization to prevent further deterioration. There are several possible explanations for the absence of a sufficient rebound in nonelective cases. First, some patients who were potential candidates for cardiac surgery or less invasive procedures may have been directed toward less invasive alternatives, favoring shorter hospitalization and less resource use (eg, percutaneous coronary intervention rather than CABG, or transcatheter aortic valve implantation rather than AVR). However, data from other hotspot regions actually suggest a concomitant reduction in overall and relative percutaneous coronary intervention rates during the early COVID-19 surge, which does not support this explanation.18 While low-risk transcatheter aortic valve implantation approval may have contributed slightly to a decline in patient referral for surgical AVR during the pandemic, it is again unlikely that this factor alone would account for such a dramatic nadir in operative volume.
Second, patients for whom elective vs urgent status is unclear or borderline in the estimation of the heart team may be recategorized as elective and monitored closely as outpatients until an appropriate surgical date can be arranged based on local COVID-19 rates. Previous examinations of attrition rates while awaiting various cardiac procedures have demonstrated approximately 2% to 11% for CABG at 1 month and approximately 4% for AVR at 1 month.19 , 20 These rates remain concerning and may account for some loss of patients whose previously nonelective operation was deferred, although the exact proportion of such cases remains unclear.
Third, patients may be reluctant to seek appropriate in-hospital care at all in the midst of a pandemic because they fear contracting COVID-19 or further overwhelming their local health care system. Although this mechanism is difficult to quantify nationally, it is supported by the growing number of late presenting complications of myocardial infarction and other acute cardiac diseases that timely, invasive therapies might have mitigated.21, 22, 23 This final consideration is strengthened and is especially concerning because of reportedly increased rates of late presenting ST-elevation myocardial infarctions and associated mechanical complications during the pandemic that are independent of local COVID-19 case volumes.24
Collectively, these various mechanisms may account for some lack of a rebound in surgical case volumes after April 2020. However, the inability to clearly account for these patients, particularly the large number of nonelective cases, remains highly concerning for a COVID-19–related patient deficit. Namely, this case deficit likely includes significant patient attrition while awaiting operation and additional mortality risk among patients requiring cardiac surgery related to the ongoing COVID-19 pandemic, regardless of individual patient COVID-19 infection status. This effect may have been compounded by the association for higher COVID-19–related mortality among older patients with underlying cardiopulmonary comorbidities. The continued importance of seeking health care during the pandemic deserves emphasis, highlighting areas for improvement regarding patient education, follow-up, resource allocation, and empowerment.
Although nationwide reductions in cardiac surgical case volumes occurred in a seemingly synchronous fashion centered around April 2020 (Figure 5), the reasons for the increased O/E mortality ratio in the Mid-Atlantic and New England regions compared with relatively unchanged O/E mortality ratio in other regions warrants further examination, and they may be related to the cardiac case volume reductions at regional and local levels. Although “statistically significant” differences are shown in cardiac surgery patient baseline characteristics before and during the COVID-19 pandemic, this is primarily related to the large overall population studied, while the absolute numerical differences between groups remain relatively minimal (Table ).
Table.
Preoperative Patient Characteristics
| Variable | Pre-COVID (2018-Dec 2020) | Early COVID (Jan-Mar 2020) | COVID Storm (Apr-Jun 2020) | P value |
|---|---|---|---|---|
| Patients (N = 717 103) | 599 304 | 67 169 | 50 630 | |
| Age, y | 65.11 ± 11.8 | 65.02 ± 11.8 | 64.61 ± 11.9 | NS |
| Sex | 8.73E-12 | |||
| Male | 427 477 (70.3) | 48 506 (71.1) | 36 739 (71.5) | |
| Female | 180 732 (29.7) | 19 668 (28.8) | 14 649 (28.5) | |
| Diabetes | 1.27E-05 | |||
| Yes | 220 524 (36.2) | 25 322 (37.1) | 19 690 (38.3) | |
| No | 378 261 (62.2) | 42 268 (62.0) | 31 265 (60.8) | |
| Dialysis | 0.07176 | |||
| Yes | 18 786 (3.1) | 2238 (3.3) | 1766 (3.4) | |
| No | 587 716 (96.6) | 65 685 (96.3) | 49 422 (96.2) | |
| Heart failure | 2.20E-16 | |||
| Yes | 220 524 (36.2) | 25 322 (37.1) | 19 690 (38.3) | |
| No | 378 261 (62.2) | 42 268 (62.0) | 31 265 (60.8) | |
| Left ventricular ejection fraction | 2.20E-16 | |||
| Hyperdynamic (>0.70) | 28 734 (4.7) | 2973 (4.4) | 2110 (4.1) | |
| Normal (0.50-0.70) | 402 480 (66.2) | 45 147 (66.2) | 33 541 (65.3) | |
| Mild (<0.50) | 735 38 (12.1) | 8324 (12.2) | 6447 (12.5) | |
| Moderate (<0.40) | 45 024 (7.4) | 5203 (7.6) | 4101 (8.0) | |
| Severe (<0.30) | 38 932 (6.4) | 4542 (6.7) | 3663 (7.1) | |
| Smoking | 9.32E-08 | |||
| Nonsmoker | 254 120 (41.8) | 28 746 (42.2) | 21 304 (41.4) | |
| Current | 113 252 (18.6) | 12 548 (18.4) | 9895 (19.3) | |
| Former | 237 000 (39.0) | 26 391 (38.7) | 19 773 (38.5) | |
| Lung Disease | 1.85E-13 | |||
| None | 433 613 (71.3) | 48 143 (70.6) | 36 641 (71.3) | |
| Mild | 65 222 (10.7) | 7871 (11.5) | 5572 (10.8) | |
| Moderate/Severe | 58 579 (9.6) | 6579 (9.6) | 4660 (9.1) |
Continuous variables are presented as the mean ± SD, and categorical variables are presented as frequency (%).
Potential mechanisms include hospital resources and manpower issues, including urgent reallocation of hospital bed space, resources, and cross-deployment of physician and physician personnel, as well as separate state and local governmental mandates to cease all elective surgical cases to prevent overwhelming local health care systems. In the Mid-Atlantic and New England regions, where peak case reductions and increased COVID-19 case volumes occurred simultaneously, the O/E mortality ratio increased sharply, unlike other regions, where cardiac case volume reductions preceded regional peaks in COVID-19 cases.
This suggests that the reason for cardiac case decreases in the Mid-Atlantic and New-England regions may have been more influenced by hospital resources, manpower, and reallocation, without sufficient lead time to necessarily reduce case volumes in a planned manner to preserve these resources. Conversely, after a reduction in operative volumes in April 2020, other regions showed subsequent increased cardiac surgical cases towards baseline levels, even during months when regional COVID-19 cases were rising, suggesting that the first nadir in cases may have been driven by implementation of forced prophylactic surgical case reductions rather than being faced with unanticipated resource scarcity. We may speculate that this contributed, at least partially, to the increased O/E mortality ratio uniquely in the Mid-Atlantic and New England regions during the first COVID-19 surge, compared with other regions.
In addition to resource and manpower scarcity, other factors may account for the COVID-19 effect on short-term mortality in the Mid-Atlantic and New England regions. These dramatic O/E shifts are completely idiosyncratic, especially because these regions were collectively performing better than expected (O/E mortality ratios well below 1) in the pre-COVID-19 era. Additional, potential mechanisms include the risk of perioperative COVID-19 infection in these hotspot regions, physician and nonphysician provider fatigue, and selection of higher-risk patients in a manner that is not completely characterized by the current STS risk models for the risk-adjustable procedures being reported.
Based on the above findings, as well as the breadth and depth of data captured by the STS ACSD, we plan future research to specifically focus on many of the unresolved issues described in this report. Additional data regarding individual patient COVID-19 status, unique perioperative risk factors for morbidity and mortality, and specific rates of complications (eg, tracheostomy, bleeding, need for postoperative ECMO, early valve-related thrombotic complications) will contribute substantially to these ongoing analyses. As we continue to face this pandemic, continued collaboration, use of data sets such as the STS ACSD, and database linkages will be critical to answer these important questions.
Study Limitations
Our study has several limitations, including the lack of granularity regarding the exact reason(s) for the effect of COVID-19 on O/E mortality ratios in regions most affected by the first COVID-19 peak. We also lack individual patient COVID-19 infection status, which through database linkages will enhance future analyses. Finally, our analysis is primarily focused on the first COVID-19 surge in the US, although it is our hope that other countries will learn from these early experiences.
Conclusion
We present the largest description of the impact of COVID-19 on national and regional cardiac surgical case volumes, trends, and outcomes. We have quantified dramatic cardiac case volume reductions nationwide, further magnified in hotspot regions during the first COVID-19 case surge in the US. These findings were accompanied by dramatic increases in mortality O/E ratios even in regions that previously performed at better-than-expected levels. The reasons for case volume reductions are multiple, vary by region, and may partially explain the source of regional increases in the 30-day mortality O/E ratio.
Notably, a rebound of case volumes to account for previous reductions in nonelective cases has yet to be seen. This COVID-19–related cardiac surgical case deficit, particularly among nonelective cases, remains concerning, because delaying or avoiding appropriate cardiac surgical care may lead to unnecessary morbidity and mortality, regardless of infection status. We plan further research to address these issues.
Acknowledgments
The data for this research were provided by The Society of Thoracic Surgeons’ National Database Participant User File Research Program. Data analysis was performed at the investigators’ institution(s).
Footnotes
Presented at the Fifty-seventh Annual Meeting of The Society of Thoracic Surgeons, Virtual Meeting, Jan 29-31, 2021.
References
- 1.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–554. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George I., Salna M., Kobsa S., et al. The rapid transformation of cardiac surgery practice in the coronavirus disease 2019 (COVID-19) pandemic: Insights and clinical strategies from a center at the epicenter. J Thorac Cardiovasc Surg. 2020;160:937–947.e2. doi: 10.1016/j.jtcvs.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelman D.T., Lother S., George I., et al. Adult cardiac surgery and the COVID-19 pandemic: aggressive infection mitigation strategies are necessary in the operating room and surgical recovery. Ann Thorac Surg. 2020;110:707–711. doi: 10.1016/j.athoracsur.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19–final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman J.D., Lye D.C.B., Hui D.S., et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horby P., Lim W.S., Emberson J.R., et al. RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rassaf T., Totzeck M., Mahabadi A.A., et al. Ventricular assist device for a coronavirus disease 2019-affected heart. ESC Heart Fail. 2021;8:162–166. doi: 10.1002/ehf2.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zayat R., Kalverkamp S., Grottke O., et al. Role of extracorporeal membrane oxygenation in critically ill COVID-19 patients and predictors of mortality. Artif Organs. 2021;45:E158–E170. doi: 10.1111/aor.13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.COVIDSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fattouch K, Corrao S, Augugliaro E, et al. Cardiac surgery outcomes in patients with coronavirus disease 2019 (COVID-19): a case-series report. J Thorac Cardiovasc Surg. Published online October 22, 2020. https://doi.org/10.1016/j.jtcvs.2020.09.138 [DOI] [PMC free article] [PubMed]
- 12.Salna M., Polanco A., Bapat V., George I., Argenziano M., Takeda K. A case of coronavirus disease 2019 (COVID-19) presenting after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2020;160:e193–e195. doi: 10.1016/j.jtcvs.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ad N., Luc J.G.Y., Nguyen T.C., COVID-19 North American Cardiac Surgery Survey Working Group Cardiac surgery in North America and coronavirus disease 2019 (COVID-19): regional variability in burden and impact. J Thorac Cardiovasc Surg. 2021;162:893–903.e4. doi: 10.1016/j.jtcvs.2020.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Society of Thoracic Surgeons STS SCA Data Specifications v2.9. Data Collection Form Fields: Updated: October 2017. https://www.sts.org/sites/default/files/documents/ACSDTrainingManual_V2-9_October2017.pdf Accessed December 20, 2020.
- 15.R: A Language and Environment for Statistical Computing. https://www.r-project.org/ Accessed December 20, 2020.
- 16.O’Brien S.M., Feng L., He X., et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-statistical methods and results. Ann Thorac Surg. 2018;105:1419–1428. doi: 10.1016/j.athoracsur.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Shahian D.M., Jacobs J.P., Badhwar V., et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: part 1-background, design considerations, and model development. Ann Thorac Surg. 2018;105:1411–1418. doi: 10.1016/j.athoracsur.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Piccolo R., Bruzzese D., Mauro C., et al. Population trends in rates of percutaneous coronary revascularization for acute coronary syndromes associated with the COVID-19 outbreak. Circulation. 2020;141:2035–2037. doi: 10.1161/CIRCULATIONAHA.120.047457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malaisrie S.C., McDonald E., Kruse J., et al. Mortality while waiting for aortic valve replacement. Ann Thorac Surg. 2014;98:1564–1570. doi: 10.1016/j.athoracsur.2014.06.040. [discussion: 1570-1571] [DOI] [PubMed] [Google Scholar]
- 20.Rexius H., Brandrup-Wognsen G., Oden A., Jeppsson A. Mortality on the waiting list for coronary artery bypass grafting: incidence and risk factors. Ann Thorac Surg. 2004;77:769–774. doi: 10.1016/j.athoracsur.2003.05.007. [discussion: 774-775] [DOI] [PubMed] [Google Scholar]
- 21.Allende N.G., Santos R., Sokn F.J., et al. Unusual presentations of cardiac rupture during COVID-19 pandemic. Echocardiography. 2021;38:469–472. doi: 10.1111/echo.15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakhshi H., Gattani R., Ekanem E., et al. Ventricular septal rupture and cardiogenic shock complicating STEMI during COVID-19 pandemic: an old foe re-emerges. Heart Lung. 2020;50:292–295. doi: 10.1016/j.hrtlng.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitahara S., Fujino M., Honda S., et al. COVID-19 pandemic is associated with mechanical complications in patients with ST-elevation myocardial infarction. Open Heart. 2021;8 doi: 10.1136/openhrt-2020-001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yong C.M., Ang L., Welt F.G.P., et al. Cardiac procedural deferral during the coronavirus (COVID-19) pandemic. Catheter Cardiovasc Interv. 2020;96:1080–1086. doi: 10.1002/ccd.29262. [DOI] [PubMed] [Google Scholar]