Abstract
Nowadays, the coronavirus disease (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) represents a major global health problem. Intensive efforts are being employed to better understand this pathology and develop strategies enabling its early diagnosis and efficient treatment. In this study, we compared the signature of circulating miRNAs in plasma of COVID-19 patients versus healthy donors. MiRCURY LNA miRNA miRNome qPCR Panels were performed for miRNA signature characterization. Individual quantitative real-time PCR (qRT-PCR) was carried out to validate miRNome qPCR results. Receiver-operator characteristic (ROC) curve analysis was applied to assess the diagnostic accuracy of the most significantly deregulated miRNA(s) as potential diagnostic biomarker(s). Eight miRNAs were identified to be differentially expressed with miR-17-5p and miR-142-5p being down-regulated whilst miR-15a-5p, miR-19a-3p, miR-19b-3p, miR-23a-3p, miR-92a-3p and miR-320a being up-regulated in SARS-CoV-2-infected patients. ROC curve analyses revealed an AUC (Areas Under the ROC Curve) of 0.815 (P = 0.031), 0.875 (P = 0.012), and 0.850 (P = 0.025) for miR-19a-3p, miR-19b-3p, and miR-92a-3p, respectively. Combined ROC analyses using these 3 miRNAs showed a greater AUC of 0.917 (P = 0.0001) indicating a robust diagnostic value of these 3 miRNAs. These results suggest that plasma miR-19a-3p, miR-19b-3p, and miR-92a-3p expression levels could serve as potential diagnostic biomarker and/or a putative therapeutic target during SARS-CoV-2-infection.
Keywords: Coronavirus, Severe acute respiratory syndrome, miRNAs, Biomarkers, Plasma
1. Introduction
In December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection started in Wuhan, China and rapidly spread worldwide, leading to the coronavirus disease 2019 (COVID-19) pandemic. SARS-CoV-2 is a novel enveloped, non-segmented, single-stranded, positive-sense RNA coronavirus belonging to the sarbecovirus family which can infect humans and other mammals (Tahir ul Qamar et al., 2020). The COVID-19 infected patients could present mild clinical manifestation such as fever, dry cough, and fatigue whilst others could have some more serious complications including shortness of breath, acute respiratory distress syndrome (ARDS) and cytokine storm, all of which are indicators of severe pneumonia which may lead to death (Pan et al., 2020). Currently, COVID-19 pandemic represents a serious global health problem as most countries are reporting increased numbers of new COVID-19 cases and deaths every day. Early and accurate diagnosis of infected individuals as well as identifying therapeutic targets are therefore essential to assist in controlling the spread of the disease. MicroRNAs (miRNAs) are highly conserved short (19–22-nt) single-stranded non-coding RNA molecules that are widely present in plants and animals. MicroRNAs could regulate gene expression at a posttranscriptional level upon interacting with the 3′ untranslated region (3’UTRs) of target mRNAs, to induce either mRNA degradation or translation inhibition (Bartel, 2004). Currently, miRNAs are well known to be involved in regulating a broad range of biological activities where abberant miRNA expression could be associated with the development of different pathological conditions such as cancer (Tüfekci et al., 2014). In addition, miRNAs have recently emerged as important clinical biomarkers and therapeutic tools (Kreth et al., 2018). For instance, circulating miRNAs have been used as diagnostic/prognostic biomarkers for various infectious diseases including human tuberculosis (triggered by Mycobacterium tuberculosis), sepsis (caused by various microbial infections), viral hepatitis (caused by hepatitic B and C) and Human T-cell leukemia virus type 1 (HTLV-1) infection (Correia et al., 2017; Fayyad-Kazan et al., 2019). On the other hand, during viral infection, miRNAs could be implicated throughout the various stages of infection, from viral entry to disease development where miRNA-mediated antiviral activities have been reported for distinct viruses such as Hepatitis C virus (HCV) and Dengue virus (DENV) (Jopling et al., 2005; Wong et al., 2020).
In this study, we aimed at characterizing the signature of circulating miRNAs in the plasma of COVID-19 patients versus healthy individuals. We identified, several dysregulated miRNAs among which are miR-19a-3p, miR-19b-3p, and miR-92a-3p that were upregulated in infected individuals and could serve as potential biomarkers for diagnosis of SARS-CoV-2 infection based on ROC curve analysis. Moreover, targeting the identified deregulated miRNAs could have a therapeutic potential against COVID-19.
2. Materials and methods
2.1. Patients and healthy controls
A total of 33 COVID-19 patients (20 Males and 13 females; mean age 45 years, range 30–60) (Table 1 ) and 10 healthy individuals were included in this study. The COVID-19 patients were diagnosed using RT-qPCR Detection Kit (Taq Path™ COVID-19 CE-IVD RT-PCR; Thermo Fisher). Peripheral blood (10 mL) was drawn into EDTA anticoagulated tubes (BD Vacutainer) from COVID-19 patients at the time of diagnosis and from healthy volunteers and kept at 4 °C until further processing (within two hours of collection). Plasma samples were subjected to a two-step centrifugation protocol (2500 ×g and 16,000 ×g; 10–10 min, 4 °C) to obtain plasma. After separation, the cell-free plasma samples were homogenized, aliquoted, and stored at −80 °C until further processing. This study was carried out in accordance with the Declaration of Helsinki (1964) and approved by the local ethics committee of the “Lebanese University”. Written informed consent was obtained from all donors.
Table 1.
List of patients included in this study.
| Patient number | Gender | Age | Disease severity |
|---|---|---|---|
| 1 | M | 31 | Moderate |
| 2 | M | 45 | Moderate |
| 3 | M | 50 | Moderate |
| 4 | M | 44 | Mild |
| 5 | M | 36 | Mild |
| 6 | M | 32 | Mild |
| 7 | M | 47 | Mild |
| 8 | M | 55 | Severe |
| 9 | M | 60 | Moderate |
| 10 | M | 59 | Severe |
| 11 | M | 48 | Mild |
| 12 | M | 31 | Mild |
| 13 | M | 37 | Mild |
| 14 | M | 55 | Severe |
| 15 | M | 52 | Moderate |
| 16 | M | 44 | Moderate |
| 17 | M | 30 | Mild |
| 18 | M | 60 | Severe |
| 19 | M | 48 | Moderate |
| 20 | M | 51 | Mild |
| 21 | F | 30 | Mild |
| 22 | F | 45 | Mild |
| 23 | F | 47 | Mild |
| 24 | F | 52 | Moderate |
| 25 | F | 59 | Severe |
| 26 | F | 51 | Moderate |
| 27 | F | 38 | Mild |
| 28 | F | 34 | Mild |
| 29 | F | 55 | Moderate |
| 30 | F | 58 | Severe |
| 31 | F | 44 | Moderate |
| 32 | F | 37 | Moderate |
| 33 | F | 33 | Moderate |
2.2. RNA extraction and cDNA synthesis
Prior to RNA isolation, plasma samples were thawed on ice, then circulating RNA was isolated from 200 μL plasma samples using the miRNeasy Serum/Plasma RNA isolation kit (Qiagen, Antwerp, Belgium) according to the manufacturer's protocol. The first strand cDNA synthesis was performed using the miRCURY LNA RT Kit (cat. no. 339340, Qiagen, Antwerp, Belgium) according to the manufacturer's protocol.
2.3. MiRNA profiling using miRCURY LNA miRNome qPCR panels and individual confirmation using individual miRCURY LNA miRNA PCR assays
MiRCURY LNA miRNA miRNome qPCR panels enable exceptionally sensitive and specific miRNA expression profiling using LNA technology. The predesigned PCR primer sets are pre-aliquoted in 384-well PCR plates and are ready for use. The panels are provided in 384-well plates that contain predesigned dried-down PCR primers for one reaction per well and simply require addition of cDNA and miRCURY LNA SYBR Green Master Mix prior to real-time PCR amplification. Thus, following cDNA synthesis, miRCURY LNA SYBR Green master mix and RNase free water were added and then aliquoted into the 384-well PCR plate containing the pre-aliquoted miRCURY LNA miRNA miRNome qPCR Panel I (YAHS-301, Qiagen, Antwerp, Belgium). The cycling conditions were 95 °C for 2 min for heat inactivation, followed by 40 cycles at 95 °C for 60 s and 56 °C for 1 min. For individual assays, a PCR primer mix was used for each of the differentially expressed miRs. MiR-502-5p transcript was used for normalization of expression. The relative expression levels of miRNAs were calculated using the comparative ΔΔCt method as described previously (Schmittgen and Livak, 2008). The fold changes in miRNAs were calculated by the equation 2−ΔΔCt.
2.4. Functional enrichment analysis
GO functional enrichment analysis and KEGG pathway enrichment analysis were performed using DIANA TOOLS MirPath version 3. P < 0.05 was considered as a statistically significant difference.
2.5. Statistical analysis
Using Kolmogorov-Smirnov and Shapiro-Wilk normality tests, our data appeared to be characterized by a normal distribution and was expressed as the mean and standard deviation. Widely presented using the 2-ΔΔCt method, the relative gene expression involves the gene of interest data (Ct gene of interest) relative to an internal control gene (Ct internal control gene), named delta Ct. The calculated delta Ct ± SD for the patients was compared with the delta Ct ± SD (SD stands for the standard deviation of the average delta Ct of the group) for the healthy control group and tested for statistical significance. Receiver Operator Characteristic (ROC) analysis was applied to determine sensitivity, specificity, and the area under the curve (AUC) for plasma miRNAs. Data was analyzed by unpaired Student's t-test. P-values <0.05 (*), <0.01 (**), and < 0.001(***) were considered statistically significant.
3. Results
3.1. Comparison of circulating miRNA signature in the plasma of COVID-19 patients versus healthy donors
In order to identify miRNAs that are differentially expressed in the plasma of infected patients versus healthy donors, SYBR Green qRT-PCR was performed using MiRCURY LNA miRNA miRNome PCR Panels. We identified 8 miRNAs that were upregulated and 4 miRNAs that were downregulated in the plasma of the COVID-19 patients. Table 2 summarizes the identified deregulated miRNAs (Table 2).
Table 2.
Differentially expressed miRNAs in the plasma of six COVID-19 patients versus six Healthy individuals as revealed by qPCR panels.
| MicroRNA | Fold change | P-value |
|---|---|---|
| Upregulated | ||
| hsa-miR-15a-5p | 9.1 | 0.02 |
| hsa-miR-19a-3p | 10.2 | 0.002 |
| hsa-miR-19b-3p | 7.2 | 0.0019 |
| hsa-miR-23a-3p | 8.2 | 0.018 |
| hsa-miR-92a-3p | 20 | 0.0011 |
| hsa-miR-140-3p | 4.2 | 0.04 |
| hsa-miR-194-5p | 6.5 | 0.03 |
| hsa-miR-320a | 11.7 | 0.01 |
| Downregulated | ||
| hsa-miR-17-5p | 0.19 | 0.018 |
| hsa-miR-142-5p | 0.32 | 0.01 |
| hsa-miR-191-5p | 0.22 | 0.039 |
| hsa-miR-374a-5p | 0.18 | 0.042 |
3.2. Validation of differential miRNA expression in infected patients
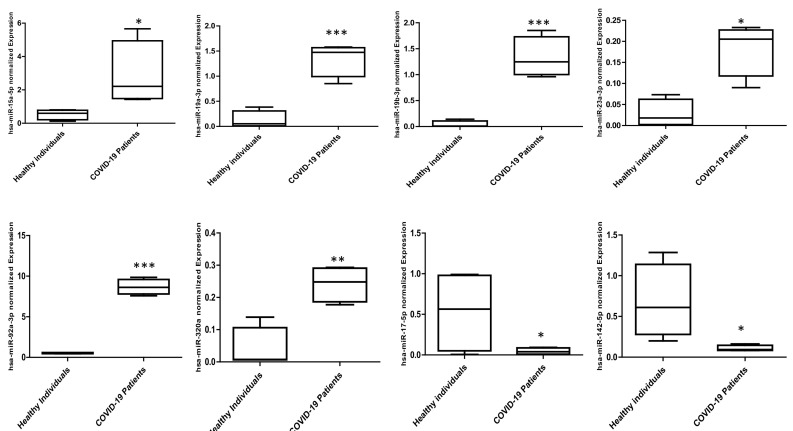
In a second step, individual SYBR Green Real Time PCR (qRT-PCR) was applied to validate the differential expression of the identified deregulated miRNAs. The change in expression profiles for the candidate miRNAs in infected patients versus the healthy donors is shown in Fig. 1 . In fact, plasma derived from infected patients were characterized by downregulated expression of miR-17-5p and miR-142-5p but upregulation of miR-15a-5p, miR-19a-3p, miR-19b-3p, miR-23a-3p, miR-92a-3p and miR-320a in comparison to healthy donors-derived plasma (Fig. 1).
Fig. 1.
Differentially expressed miRNAs in plasma of SARS-CoV-2-infected patients versus healthy individuals. Plasma was obtained from 12 individuals (6 SARS-CoV-2-infected patients and 6 healthy individuals). The relative expression of miRNAs was quantified by quantitative RT-PCR. *p < 0.05, **p < 0.01, ***p < 0.001 infected patients versus Healthy individuals (Unpaired Student's t-test).
3.3. GO function and KEGG pathway enrichment analysis
To investigate the biological functions of the genes targeted by the identified deregulated miRNAs, GO function and KEGG pathway enrichment analysis was performed. Our analysis revealed that those deregulated miRNAs are involved in biological process including viral carcinogenesis, bacterial invasion of epithelial cells and different cancers (Table 3, Table 4 ).
Table 3.
Common KEGG pathways for the upregulated miRNAs.
| KEGG pathway | P-value |
|---|---|
| Proteoglycans in cancer (hsa05205) | <0.0001 |
| Renal cell carcinoma (hsa05211) | <0.0001 |
| Chronic myeloid leukemia (hsa05220) | <0.0001 |
| Prostate cancer (hsa05215) | <0.0001 |
| Hepatitis B (hsa05161) | <0.0001 |
| Glioma (hsa05214) | <0.0001 |
| Endometrial cancer (hsa05213) | 0.00012 |
| Bacterial invasion of epithelial cells (hsa05100) | 0.00013 |
| Non-small cell lung cancer (hsa05223) | 0.00015 |
| Colorectal cancer (hsa05210) | 0.0002 |
| Pancreatic cancer (hsa05212) | 0.0006 |
| Small cell lung cancer (hsa05222) | 0.004 |
| Central carbon metabolism in cancer (hsa05230) | 0.006 |
| Epstein-Barr virus infection (hsa05169) | 0.007 |
| Melanoma (hsa05218) | 0.007 |
| Bladder cancer (hsa05219) | 0.01 |
| HTLV-I infection (hsa05166) | 0.02 |
| Acute myeloid leukemia (hsa05221) | 0.03 |
| Thyroid cancer (hsa05216) | 0.04 |
Table 4.
Common KEGG pathways for the down-regulated miRNAs.
| KEGG pathway | P-value |
|---|---|
| Hepatitis B (hsa05161) | <0.0001 |
| Proteoglycans in cancer (hsa05205) | <0.0001 |
| Glioma (hsa05214) | <0.0001 |
| Chronic myeloid leukemia (hsa05220) | <0.0001 |
| Viral carcinogenesis (hsa05203) | <0.0001 |
| Renal cell carcinoma (hsa05211) | <0.0001 |
| Bacterial invasion of epithelial cells (hsa05100) | <0.0001 |
| Pancreatic cancer (hsa05212) | <0.0001 |
| Bladder cancer (hsa05219) | <0.0001 |
| Prostate cancer (hsa05215) | <0.0001 |
| Colorectal cancer (hsa05210) | <0.0001 |
| Non-small cell lung cancer (hsa05223) | <0.0001 |
| Endometrial cancer (hsa05213) | 0.00022 |
| Melanoma (hsa05218) | 0.00024 |
| Small cell lung cancer (hsa05222) | 0.00035 |
| Thyroid cancer (hsa05216) | 0.0011 |
| Acute myeloid leukemia (hsa05221) | 0.0029 |
| Hepatitis C (hsa05160) | 0.0037 |
| HTLV-I infection (hsa05166) | 0.044 |
3.4. Diagnostic accuracy of plasma miR-19a-3p, miR-19b-3p, and miR-92a-3p in SARS-CoV-2 infected patients
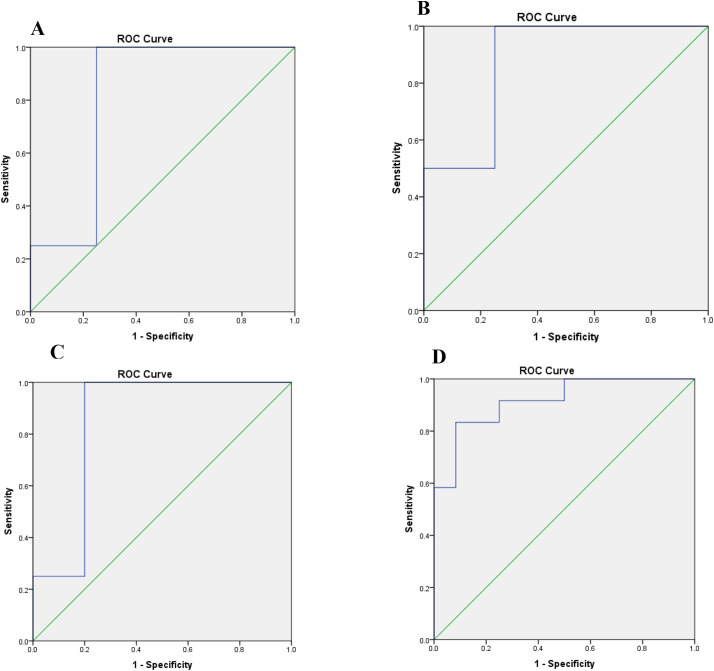
ROC curve analysis was applied to examine the diagnostic accuracy of the most statistically significant and differentially expressed plasma miRNAs: miR-19a-3p, miR-19b-3p, and miR-92a-3p. This analysis highlighted that miR-19a-3p, miR-19b-3p, and miR-92a-3p could serve as potential diagnostic biomarkers for distinguishing SARS-CoV-2 infected patients from healthy individuals with an AUC (the areas under the ROC curve) of 0.815 (P = 0.031) (Fig. 2 , Panel A), 0.875 (P = 0.012) (Fig. 2, Panel B), and 0.850 (P = 0.025) (Fig. 2, Panel C) respectively.
Fig. 2.
Receiver operating characteristics (ROC) curve analysis using miR-19a-3p, miR-19b-3p, and miR-92a-3p for discriminating SARS-CoV-2-infected patients. Plasma miR-19a-3p yielded an AUC of 0.815 (P = 0.031) with 88% sensitivity and 85% specificity in discriminating infected patients (Panel A). Plasma miR-19b-3p yielded AUC of 0.875 (P = 0.012) with 89% sensitivity and 86% specificity in discriminating infected patients (Panel B). Plasma miR-92a-3p yielded an AUC of 0.850 (P = 0.025) with 90% sensitivity and 87% specificity in discriminating infected patients (Panel C). Combined ROC analysis revealed a greater AUC of 0.917 (P = 0.0001) with 92% sensitivity and 89% specificity in discriminating infected patients (Panel D).
At the cut-off value greater than 0.834 for miR-19a-3p, the sensitivity and the specificity were 88% and 85%, respectively. At the cut-off value greater than 0.237 for miR-19b-3p, the sensitivity and the specificity were 89% and 86%, respectively. At the cut-off value greater than 0.47 for miR-92a-3p, the sensitivity and the specificity were 90% and 87%, respectively.
Combination ROC analyses resulted in an increased AUC of 0.917 (P = 0.0001) with 92% sensitivity and 89% specificity at a cut-off value greater than 0.52 indicating the additive effect in the diagnostic value of these 3 miRNAs (Fig. 2D).
3.5. Profiling miR-19a-3p, miR-19b-3p and miR-92a-3p expression in COVID-19 patients with different Cqs of RdRp gene
In a further step, we screened the expression levels of miR-19a-3p, miR-19b-3p and miR-92a-3p in a panel of COVID-19 patients characterized by different Cq values of RdRp at time of diagnosis (Table 5 ). Each of the indicated miRNAs was significanlty upregulated in each of the tested patients. Interestingly, even patients with Cq > 30 were characterized with elevated expression of the indicated miRNAs, suggessting that these miRNAs could serve as biomarkers even for the early stages of the infection.
Table 5.
Profiling plasma miR-19a-3p, miR-19b-3p and miR-92a-3p expression levels in different COVID-19 patients with different Cqs of RdRp gene.
| Patients | Cq of RdRp | miR-19a-3p |
miR-19b-3p |
miR-92a-3p |
|||
|---|---|---|---|---|---|---|---|
| Fold change | P-value | Fold change | P-value | Fold change | P-value | ||
| Patients (1–3) | 34 | 5.7 | 0.003 | 2.6 | 0.015 | 4.5 | 0.022 |
| Patients (4–6) | 32 | 6.5 | 0.0025 | 2.8 | 0.022 | 4.8 | 0.011 |
| Patients (7–9) | 31 | 6.6 | 0.002 | 2.5 | 0.032 | 4.2 | 0.032 |
| Patients (10−12) | 30 | 6.9 | 0.004 | 2.9 | 0.021 | 4.7 | 0.022 |
| Patients (13–15) | 28 | 7.5 | 0.001 | 3.5 | 0.011 | 6.7 | 0.001 |
| Patients (16, 18) | 24 | 8.3 | 0.001 | 4.1 | 0.033 | 9.5 | 0.014 |
| Patients (19, 21) | 20 | 9.2 | 0.005 | 5.5 | 0.041 | 15.8 | 0.001 |
| Patients (22, 24) | 17 | 9.9 | 0.002 | 6.2 | 0.019 | 18.2 | 0.011 |
| Patients (25, 27) | 15 | 10.8 | 0.001 | 6.4 | 0.032 | 18.8 | 0.033 |
4. Discussion
The ongoing outbreak of COVID-19 assures the necessity of early and accurate diagnosis of human SARS-CoV-2 infections in order to control its spread and emphasizes the urgent need to develope therapeutic tools to appropriately treat patients who have serious complications. In this study, we identified a set of miRNAs whose expression is deregulated in SARS-CoV-2 infected patients. Such miRNAs could hold a valuable diagnostic and therapeutic potential.
MicroRNAs are widely known to regulate the molecular mechanisms underlying various diseases due to their ability to potentially regulate nearly every aspect of cellular activity, including cell cycle, metabolism, apoptosis, viral infection and tumor development (Huang et al., 2011). Circulating miRNAs are extracellular serum/plasma miRNAs that could be involved in cell-cell communication, and thus might contribute to disease progression. Over the course of an infection, the infectious agent might trigger a significant change in the signature of cellular and/or circulating miRNAs, and consequently the latter could be used as biomarkers to follow disease progression. In the past decades, a wealth of studies have reported the benefit of miRNAs as circulating biomarkers for diagnosis or prognosis of wide array of infectious diseases, particularly viral diseases (Correia et al., 2017; Fayyad-Kazan et al., 2019). Recently, we have reported that circulating miR-29c, miR-30c, miR-193a-5p and miR-885-5p might act as potential biomarkers for HTLV-1 infection diagnosis (Fayyad-Kazan et al., 2013). Moreover, circulating miR-122, miR-22, and miR-34a have been associtated with liver injury in HIV-infected patients (Anadol et al., 2015). Furthermore, the expression profile of serum miR-29 has been described as potential biomarker to follow disease progression in patients with chronic hepatitis B virus infection (Huang et al., 2014). The present study highlights plasma miR-19a-3p, miR-19b-3p, and miR-92a-3p as potential biomarkers for diagnosis of SARS-CoV-2 infection. Although none of the indicated miRNAs have previously been shown to be significantly associated with viral infection of respiratory system, miR-92a, for instance, has been shown to be upregulated in serum of patients infected with hepatitis C virus (HCV) (Louten et al., 2015). Moreover, deregulated miR-19 expression has been associated with hepatitis B virus (HBV) infection (Li et al., 2010).
Remarkably, miR-19a-3p, miR-19b-3p, and miR-92a-3p were differentially expressed even in patients at early course of infection (Cq > 30). This high sensitivity represents a major advantage of this diagnositic strategy. Diagnosis of SARS-CoV-2 is typically made by polymerase chain reaction (PCR) against certain viral genes encoding different molecular components such as nucleocapsid (N), transmembrane (M), envelope (E), RNA-dependentRNA polymerase (RdRp) and envelope glycoproteins spike (S). Such regular PCR tests involve deep nasal or oral swabs that can be uncomfortable for some people, mainly in case of small children. Moreover, a major issue when performing the regular PCR test is the possibility of false-negative and false positive results, therefore results should be taken with some precautions. Forinstance, genetic diversity due to rapid evolution of SARS-CoV-2 have led to fFalse-negative results due to mutations occuring in the primer and probe target regions in the SARS-CoV-2 genome (Phan, 2020). On the other hand, an advantage of our proposed diagnostic strategy is that it doesnot involve invasive swabs. Moreover, since the targets of this startegy are human miRNAs but not viral genes, the possibility of having false negative results due to SARS-CoV-2 genome evolution is avoided.
Besides their diagnostic value, miRNAs are well known for their therapeutic potential, especially in viral diseases (Hemida et al., 2010). In fact, certain viruses encode their own miRNAs and employ them for replication and immune evasion. Other viruses that do not encode miRNAs may alter host cell miRNA signature in a way that favors their replication (Hemida et al., 2010). Intriguingly, SARS-CoV-1 has been shown to hijack host cellular miRNAs such as miR-17, miR-574-5p, and miR-214 to favor its replication and immune evasion (Qin et al., 2005). Moreover, the nucleocapsid and spike glycoproteins were shown to downregulate the expression of miR-223 and miR-98, respectively. This process allows the virus to create an environment that is optimal for its replication. Interestingly, restoration of normal miR-223 and miR-98 expression levels represents a promising novel therapeutic approach for treating SARS-CoV infection (Qin et al., 2005). In this context, a recent report has compared the miRNA signature in the peripheral blood of COVID-19 patients versus healthy donors and several miRNAs have been identified to be deregulated (Li et al., 2020). These deregulated miRNAs could interfere with the shaping of the immune responses (Li et al., 2020). In this study, we found that the deregulated miRNAs are invloved in various biological process, mainly viral carcinogensis, bacterial invasion of epithelial cells as well as in different cancers. Remarkably, Transforming growth factor beta (TGF-β) signalling pathway was identified among the biological processes that can be targeted by miR-19a-3p and miR-19b-3p. It is well established in the literature that TGF-β-mediated responses could be suppressed by miR-19a/b (Li et al., 2021; Souma et al., 2018; Wa et al., 2018). TGF-β signalling cascade plays an important immunosuppressive and anti-inflammatory role via inducing regulatory T cells, inhibiting B and T cells function as well as favoring M2 than M1 macrohage responses (Aschner and Downey, 2016). Therefore, the upregulated levels of miR-19a/b could be involved in the inflammatory storm seen in COVID-19 patients via inhibiting the immunosuppressive and anti-inflammatory role ensured by TGF-β signalling pathway. On the other hand, the mechanism by which miR-92a-3p could contribute to COVID-19 pathogenesis is less clear. However, it could be possible that miR-92a-3p plays a role during COVID-19 pathogenesis via its role in regulating endothelial cell functions such as control of vascular inflammation and angiogenesis (Nemecz et al., 2016). In this context, targeting these identified circulating miRNAs could help to control the undesired outcomes triggered by the viral infection.
5. Conclusion
In conclusion, we characterized the signature of circulating miRNAs in SARS-Cov-2–infected patients. We identified several miRNAs that could have diagnostic and/or therapeutic potential in case of SARS-Cov-2 infection.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the local ethics committee of the “Lebanese University”and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding
This work is supported by the Lebanese University .
Declaration of Competing Interest
None.
Acknowledgments
All participants are acknowledged in the authorship.
References
- Anadol E., Schierwagen R., Elfimova N., Tack K., Schwarze-Zander C., Eischeid H., Noetel A., Boesecke C., Jansen C., Dold L., Wasmuth J.-C., Strassburg C.P., Spengler U., Rockstroh J.K., Odenthal M., Trebicka J. Circulating MicroRNAs as a marker for liver injury in human immunodeficiency virus patients. Hepatology. 2015;61:46–55. doi: 10.1002/hep.27369. [DOI] [PubMed] [Google Scholar]
- Aschner Y., Downey G.P. Transforming growth factor-B: master regulator of the respiratory system in health and disease. Am. J. Respir. Cell Mol. Biol. 2016 doi: 10.1165/rcmb.2015-0391TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Correia C.N., Nalpas N.C., McLoughlin K.E., Browne J.A., Gordon S.V., MacHugh D.E., Shaughnessy R.G. Circulating microRNAs as potential biomarkers of infectious disease. Front. Immunol. 2017;8:118. doi: 10.3389/fimmu.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyad-Kazan H., Bitar N., Najar M., Lewalle P., Fayyad-Kazan M., Badran R., Hamade E., Daher A., Hussein N., ELDirani R., Berri F., Vanhamme L., Burny A., Martiat P., Rouas R., Badran B. Circulating miR-150 and miR-342 in plasma are novel potential biomarkers for acute myeloid leukemia. J. Transl. Med. 2013;11 doi: 10.1186/1479-5876-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyad-Kazan M., ElDirani R., Hamade E., El Majzoub R., Akl H., Bitar N., Fayyad-Kazan H., Badran B. Circulating miR-29c, miR-30c, miR-193a-5p and miR-885-5p: novel potential biomarkers for HTLV-1 infection diagnosis. Infect. Genet. Evol. 2019;74:103938. doi: 10.1016/j.meegid.2019.103938. [DOI] [PubMed] [Google Scholar]
- Hemida M.G., Ye X., Thair S., Yang D. Exploiting the therapeutic potential of MicroRNAs in viral diseases. Mol. Diagn. Ther. 2010;14:271–282. doi: 10.1007/bf03256383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Shen X.J., Zou Q., Wang S.P., Tang S.M., Zhang G.Z. Biological functions of microRNAs: a review. J. Physiol. Biochem. 2011 doi: 10.1007/s13105-010-0050-6. [DOI] [PubMed] [Google Scholar]
- Huang C., Zheng J.M., Cheng Q., Yu K.K., Ling Q.X., Chen M.Q., Li N. Serum microRNA-29 levels correlate with disease progression in patients with chronic hepatitis B virus infection. J. Dig. Dis. 2014;15:614–621. doi: 10.1111/1751-2980.12185. [DOI] [PubMed] [Google Scholar]
- Jopling C.L., Yi M.K., Lancaster A.M., Lemon S.M., Sarnow P. Molecular biology: modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science (80-.) 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- Kreth S., Hübner M., Hinske L.C. MicroRNAs as clinical biomarkers and therapeutic tools in perioperative medicine. Anesth. Analg. 2018;126:670–681. doi: 10.1213/ANE.0000000000002444. [DOI] [PubMed] [Google Scholar]
- Li L.M., Hu Z. Bin, Zhou Z.X., Chen X., Liu F.Y., Zhang J.F., Shen H.B., Zhang C.Y., Zen K. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res. 2010;70:9798–9807. doi: 10.1158/0008-5472.CAN-10-1001. [DOI] [PubMed] [Google Scholar]
- Li C., Hu X., Li L., Li J. Hui. Differential microRNA expression in the peripheral blood from human patients with COVID-19. J. Clin. Lab. Anal. 2020;34 doi: 10.1002/jcla.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Peng H., Qu L., Sommar P., Wang A., Chu T., Li X., Bi X., Liu Q., Gallais Sérézal I., Rollman O., Lohcharoenkal W., Zheng X., Eliasson Angelstig S., Grünler J., Pivarcsi A., Sonkoly E., Catrina S.B., Xiao C., Ståhle M., Mi Q.S., Zhou L., Xu Landén N. miR-19a/b and miR-20a promote wound healing by regulating the inflammatory response of keratinocytes. J. Invest. Dermatol. 2021;141:659–671. doi: 10.1016/j.jid.2020.06.037. [DOI] [PubMed] [Google Scholar]
- Louten J., Beach M., Palermino K., Weeks M., Holenstein G. MicroRNAs expressed during viral infection: biomarker potential and therapeutic considerations. Biomark. Insights. 2015;10:25–52. doi: 10.4137/BMI.S29512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemecz M., Alexandru N., Tanko G., Georgescu A. Role of MicroRNA in endothelial dysfunction and hypertension. Curr. Hypertens. Rep. 2016 doi: 10.1007/s11906-016-0696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Li X., Yang G., Fan J., Tang Y., Zhao J., Long X., Guo S., Zhao Z., Liu Y., Hu H., Xue H., Li Y. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J. Inf. Secur. 2020;81:e28–e32. doi: 10.1016/j.jinf.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect. Genet. Evol. 2020;81 doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Wang J., Wei Q., She M., Marasco W.A., Jiang H., Tu X., Zhu H., Ren L., Gao L., Guo L., Huang L., Yang R., Cong Z., Guo L., Wang Y., Liu Y., Sun L., Duan S., Qu J., Chen L., Tong W., Ruan L., Liu P., Zhang H., Zhang J., Zhang H., Liu D., Liu Q., Hong T., He W. An animal model of SARS produced by infection of Macaca mulatta with SARS coronavirus. J. Pathol. 2005;206:251–259. doi: 10.1002/path.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Souma K., Shichino S., Hashimoto S., Ueha S., Tsukui T., Nakajima T., Suzuki H.I., Shand F.H.W., Inagaki Y., Nagase T., Matsushima K. Lung fibroblasts express a miR-19a-19b-20a sub-cluster to suppress TGF-β-associated fibroblast activation in murine pulmonary fibrosis. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-34839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir ul Qamar M., Alqahtani S.M., Alamri M.A., Chen L.L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J. Pharm. Anal. 2020;10:313–319. doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tüfekci K.U., Öner M.G., Meuwissen R.L.J., Genç Ş. Methods in Molecular Biology (Clifton, N.J.) 2014. The role of MicroRNAs in human diseases; pp. 33–50. [DOI] [PubMed] [Google Scholar]
- Wa Q., Li L., Lin H., Peng X., Ren D., Huang Y., He P., Huang S. Downregulation of MIR-19a-3p promotes invasion, migration and bone metastasis via activating TGF-β signaling in prostate cancer. Oncol. Rep. 2018;39:81–90. doi: 10.3892/or.2017.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.R., Abd-Aziz N., Affendi S., Poh C.L. Role of microRNAs in antiviral responses to dengue infection. J. Biomed. Sci. 2020 doi: 10.1186/s12929-019-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]