Abstract
Background
Abrus precatorius is used in folk medicine across Afro-Asian regions of the world. Earlier, glucose lowering and pancreato-protective effects of Abrus precatorius leaf extract (APLE) was confirmed experimentally in STZ/nicotinamide-induced diabetic rats; however, the underlying mechanism of antidiabetic effect and pancreato-protection remained unknown.
Objective
This study elucidated antidiabetic mechanisms and pancreato-protective effects of APLE in diabetic rats.
Materials and Methods
APLE was prepared by ethanol/Soxhlet extraction method. Total phenols and flavonoids were quantified calorimetrically after initial phytochemical screening. Diabetes mellitus (DM) was established in adult Sprague-Dawley rats (weighing 120–180 g) of both sexes by daily sequential injection of nicotinamide (48 mg/kg; ip) and Alloxan (120 mg/kg; ip) over a period of 7 days. Except control rats which had fasting blood glucose (FBG) of 4.60 mmol/L, rats having stable FBG (16–21 mmol/L) 7 days post-nicotinamide/Alloxan injection were considered diabetic and were randomly reassigned to one of the following groups (model, APLE (100, 200, and 400 mg/kg, respectively; po) and metformin (300 mg/kg; po)) and treated daily for 18 days. Bodyweight and FBG were measured every 72 hours for 18 days. On day 18, rats were sacrificed under deep anesthesia; organs (kidney, liver, pancreas, and spleen) were isolated and weighed. Blood was collected for estimation of serum insulin, glucagon, and GLP-1 using a rat-specific ELISA kit. The pancreas was processed, sectioned, and H&E-stained for histological examination. Effect of APLE on enzymatic activity of alpha (α)-amylase and α-glucosidase was assessed. Antioxidant and free radical scavenging properties of APLE were assessed using standard methods.
Results
APLE dose-dependently decreased the initial FBG by 68.67%, 31.07%, and 4.39% compared to model (4.34%) and metformin (43.63%). APLE (100 mg/kg) treatment restored weight loss relative to model. APLE increased serum insulin and GLP-1 but decreased serum glucagon relative to model. APLE increased both the number and median crosssectional area (×106μm2) of pancreatic islets compared to that of model. APLE produced concentration-dependent inhibition of α-amylase and α-glucosidase relative to acarbose. APLE concentration dependently scavenged DPPH and nitric oxide (NO) radicals and demonstrated increased ferric reducing antioxidant capacity (FRAC) relative to standards.
Conclusion
Antidiabetic effect of APLE is mediated through modulation of insulin and GLP-1 inversely with glucagon, noncompetitive inhibition of α-amylase and α-glucosidase, free radical scavenging, and recovery of damaged/necro-apoptosized pancreatic β-cells.
1. Introduction
Diabetes mellitus (DM) is one of the metabolic syndromes characterized by chronic hyperglycemia culminating from dysregulated glucose metabolism secondary to defects in pancreatic β-cell function [1]. Pathologically, DM is a product of errors in glucose metabolism due to defects in pancreatic β-cell function which occasions either insulin insufficiency (type 1 DM) or insulin resistance (type 2 DM). Common symptoms of DM include polyphagia, polydipsia, blurred vision, chronic loss in bodyweight, gastroparesis, and polyuria [2]. Over time, unresolved hyperglycemia increases risk of microvascular complications of DM such as diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy [2]. These complications occur due to extensive oxidative stress and lipid peroxidation which are the byproducts of reactions between glucose and biological molecules [3]. Extensive glycosylation of biological molecules such as DNA, proteins, and lipids leads to generation of unstable biochemical intermediates which serve as sources of reactive oxygen species (ROS) and nitrogen species (RNS). ROS and RNS are key drivers of oxidant and peroxidative reactions in various cells and tissues [3].
Epidemiologically, it is reported that DM affects over 387 million people worldwide [4]. This provisional estimate is projected to increase to 592 million by the year 2035 [5]. Initially, DM was thought to be adult-onset, but now, DM is shown to be independent of age, which clearly shows the enormity of the threat posed by DM to global health.
Conventionally, treatment of DM (types 1 and 2) involves the use of antidiabetic agents, which are grouped as noninsulin (including the biguanides (e.g., metformin), sulfonylureas (e.g., glyburide), SGLT-2 inhibitors (e.g., dapagliflozin), bile acid sequestrants (e.g., colesevelam), amylin mimetic (e.g., pramlintide), dopamine-2 agonist (e.g., bromocriptine), DPP-4 inhibitors (e.g., sitagliptin), GLP-1RAs (e.g., exenatide), thiazolidinedione (e.g., rosiglitazone), and α-glucosidase inhibitors (e.g., Acarbose and voglibose)) and insulin (e.g., human insulin and analogs) therapies [6]. The conventional antidiabetic therapies have been proven effective despite the fact that the use of some of them is costive and may be associated with serious side effects [7]. Cost, side effects, and the general belief that conventional drugs are synthetic and for that matter unsafe compared to herbal medicines, which are thought to be “natural” and relatively safe have contributed to the renewed interest in the use of herbs and herb-derived products for the treatment and management of human diseases including DM [8, 9]. This paradigm is quiet common in poorer tropical countries of the world, where most people rely on herbal medicine to meet their primary health care needs [8]. Also, this may probably affirm the usual assertion that use of herbs predates modern medicine and that natural products continue to serve as templates for pharmaceutical semisynthesis of new drugs [10] including antidiabetic agents. Importantly, these herbs and herb-derived therapies are used as adjunctive therapy in a manner that reflects the belief systems and ethnobotanical heritage of most local communities across Afro-Asian regions of the world. For instance many medicinal plants with ethnobotanical claims for the treatment of diabetes mellitus such as Chrysobalanus orbicularis [11], Spondias mombin and Mangifera indica [12], Sesamum indicum [13], and Bryophyllum pinnatum [14] have all demonstrated α-amylase and α-glucosidase inhibitory effects which is considered vital for glycemic control. It is in response to this renewed interest in the use of herbs and herb-derived products as adjunctive or alternative therapy, that the World Health Organization (WHO) together with other interest groups has recommended incorporation of indigenous healing systems, particularly herbal medicine into healthcare systems of poorer countries [15]. This recommendation has necessitated the need to verify health claims on medicinal herbs commonly used by local people to treat human diseases [16, 17]. Interestingly, Abrus precatorius commonly called “jequirity pea” is a medicinal herb extensively used in folk medicine of many cultures across Afro-Asian regions of the world for the treatment of human diseases [18–20]. Several reports have highlighted the traditional uses of Abrus precatorius leaves in the treatment and management of diabetes mellitus [21–23]. Of note, extracts from leaves of Abrus precatorius have demonstrated efficacy against diabetes mellitus [1] and breast cancer [24], two human diseases that have contributed significantly to global disease burden [25]. Many studies have justified the ethnopharmacological uses and other medicinal claims of Abrus precatorius by demonstrating experimentally its biological properties including free radical scavenging [26], reno-protection [27], neuro-protection [28], immuno-modulatory [19], anti-inflammatory [29], antilipotoxicity [30], and antiplatelet aggregation [31] just to mention but a sample.
Previously, glucose lowering and pancreato-protective effects of APLE were demonstrated experimentally in STZ/nicotinamide-induced diabetic rats to confirm its use as an antidiabetic folk medicine by local people in the western region of Ghana [1]. However, the mechanism underlying the glucose lowering and pancreato-protective effects of APLE remained poorly elucidated. Presently, mechanisms underlying the glucose lowering and pancreato-protective effects of APLE such as modulation of insulin, glucagon, and GLP-1, inhibition of enzymatic activity of α-amylase and α-glucosidase, free radical scavenging, and recovery of pancreatic β-cell damage and necro-apoptosis were demonstrated in vivo and in vitro.
2. Materials and Methods
2.1. Drugs and Chemicals
Alloxan monohydrate (Sigma-Aldrich Co., St. Louis, MO, USA), nicotinamide (Sigma-Aldrich Co., St. Louis, MO, USA), acarbose (LGM Pharma, USA), metformin (Bristol laboratories Ltd.), gallic acid (Merck KGaA, USA), rutin (Acros organics, USA), ascorbic acid (Carl Roth, Germany), quercetin (Alfa Aesar, UK), p-nitropheynyl glucopyranoside (pNPG) (BBI Biotech, China), Rat Insulin ELISA kit, Rat Glucagon-ELISA kit, and Rat GLP-1 ELISA kit (Wuxi Donglin Sci & Tech Development Co. Ltd., China) were used in the study. All other solvents and chemicals used in the study were of analytical grade.
2.2. Collection, Identification, and Authentication of Abrus precatorius Leaves
Fresh leaves of A. precatorius were collected from an uncultivated farm land at Sefwi Asawinso'A' (Bibiani-Ahwiaso-Bekwai District, Western Region, Ghana). The leaves were identified and authenticated by a qualified botanist at the herbarium unit, School of Biological Sciences, University of Cape Coast, where a voucher specimen (CC3366) was deposited as previously reported [1].
2.3. Preparation of Abrus precatorius Leaf Extract (APLE)
APLE was prepared according to a previous method [1]. Briefly, shade-dried leaves of A. precatorius were mechanically milled into fine powder. The powdered leaves were stored in airtight plastic containers prior to use. A 120 g quantity of the powdered leaves was soaked in 700 mL of ethanol for 48 hours and covered with cheesecloth. The mixture was stirred at least 3 times per day. On the second day, the mixture was filtered through a filter paper fitted in a Buchner funnel. The filtrate was subjected to separation using a rotary evaporator mounted on a water bath. After retrieving ethanol using the rotary evaporator, the resultant dark-green syrupy liquid was dried in a desiccator for several weeks leaving a dark-green solid extract. The extract was labelled and stored in a desiccator prior to use. APLE was continuously prepared in order to obtain enough quantities for all experiments.
2.4. Phytochemical Screening of APLE
APLE was screened for the presence of phytocompounds as previously reported [1].
2.5. Determination of Total Phenolic Content (TPC) in APLE
Total phenolic content in APLE was determined by using the Folin Ciocalteu reagent method as previously described [32] with slight modifications. A dilute concentration of APLE (250 μL of 1 mg/mL) was mixed with 750 μL Folin Ciocalteu reagent (1 in 10 dilutions with water) and 750 μL of 7% Na2CO3. The mixtures were then made up to 5 mL with distilled water and incubated at room temperature for 2 hours for color development. Absorbance was then measured at 765 nm by using a spectrophotometer (UV-vis, T70 PG Instruments). After preparation of a standard gallic acid (0–300 μg/mL) curve, TPC in APLE was extrapolated from the gallic acid calibration curve and expressed in gallic acid equivalent (GAE). All experiments were conducted in triplicates.
2.6. Determination of Total Flavonoid Content (TFC) in APLE
Total flavonoid content in APLE was measured per a previously described method [33] with some few modifications. Briefly, 500 μL of Abrus precatorius plant extract (1 mg/mL) prepared in 50% ethanol was mixed with 500 μL of 10% aluminium chloride (AlCl3.6H2O) and 1500 μL of 10% sodium acetate (NaC2H3O2.3H2O). The reaction mixture was incubated at room temperature for 40 minutes, and absorbance was measured at 415 nm using a spectrophotometer (UV-vis, T70 PG Instruments). After preparation of a standard rutin (0-250 μg/mL) calibration curve, TFC in APLE was extrapolated from the rutin calibration curve and expressed in rutin equivalent (RE). All experiments were carried out in triplicate.
2.7. Ethical Approval
All protocols involving animals were initially reviewed and approved by the Kwame Nkrumah University of Science and Technology (KNUST) Institutional Review Board on Animal Experimentation (FPPS/PCOL/006/2019). Also, experiments involving rats were carried out in accordance with the European Union Directives (2010/63/EU) on the use and care of animals in scientific experimentation. Animal suffering and the number of animals were reduced as much as possible in order to comply with the requirements in the 3R (Refinement, Replacement, and Reduction) recommendations [34].
2.8. Acquisition and Care of Experimental Animals
Healthy 7-8-week-old adult Sprague-Dawley rats of both sexes (weighing between 120 and 180 g) were purchased from the Noguchi Medical Research Institute (NMRI), University of Ghana. Rats were later transported to the Animal House of the Department of Biomedical Sciences, University of Cape Coast, Cape Coast, Ghana. Rats were housed in stainless cages (34 cm × 47 cm × 18 cm) with dry wood shavings as bedding, fed with normal rodent chow (GAFCO, TEMA), and had unrestricted access to water. Rats were maintained under ambient conditions of temperature, humidity, and normal dark/light cycle. However, these conditions were varied to suit specific requirements of some experiments. Rats were allowed to habituate under these ambient and laboratory conditions for over two weeks before use in experiments.
2.9. Establishment of Alloxan/Nicotinamide-Induced Diabetes Mellitus in Rats
Experimental diabetes mellitus was established in overnight-fasted normoglycemic adult Sprague-Dawley rats according to a previously described method [1, 35] with some modification. Briefly, rats were sequentially injected with nicotinamide (48 mg/kg; ip) dissolved in normal saline solution and maintained on ice; then, 15 minutes later, Alloxan (120 mg/kg; ip) reconstituted in 100 mM citrate buffer (pH 4.5) solution was administered to rats daily for 7 days. Subsequently, rats were given 5% glucose (5 mL/kg; po) solution daily for 7 days. To confirm establishment of hyperglycemia in rats, blood was collected from overnight-fasted rats 3-7 days post-Alloxan/nicotinamide and 5% glucose treatments using the tail-tip amputation method. Tails of rats were first wiped clean with sterile cotton dipped in 10% ethanol. Fasting blood glucose (FBG) was measured by using URIT G26 glucometer (URIT Medical Electronic Co., Ltd.).
2.10. Experimental Design
Curative rather than prophylactic treatment approach was adopted in this study, where after establishment of experimental diabetes mellitus in rats, diabetic rats were subjected to various treatments. Figure 1 shows an outline of experiments carried out in this study. Rats having consistent FBG (11.1 mmol/L or > 250 mg/dL) upon multiple measurements were considered as having diabetes mellitus (hyperglycemia) [1, 35]. Confirmed diabetic rats were randomly assigned into five groups. Control rats were not exposed to Alloxan; instead, they were given normal saline. All groups were treated for a period of 18 days as shown below:
Figure 1.
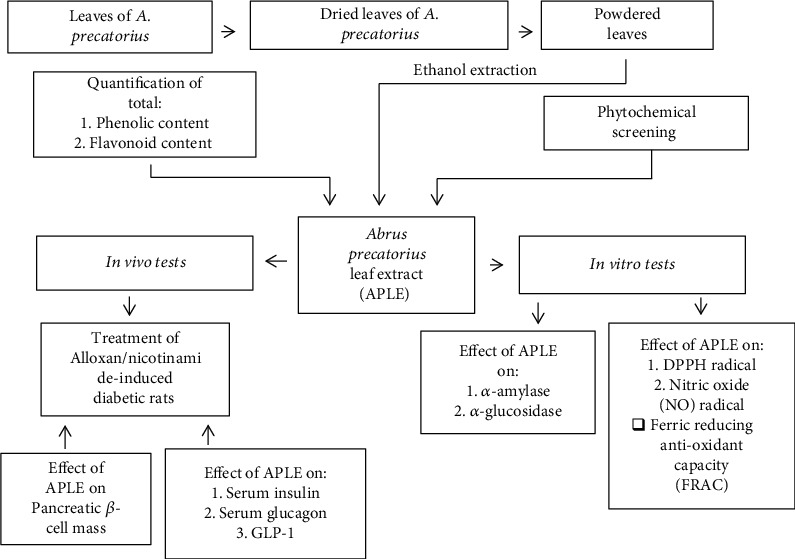
A schematic illustration of the study.
Control: normal saline (5 mL/rat/day; po) + rodent chow + water
Model: nicotinamide (48 mg/kg; ip) + Alloxan (120 mg/kg; ip) + rodent chow + water
Metformin: nicotinamide (48 mg/kg; ip) + Alloxan (120 mg/kg; ip) + metformin (300 mg/kg; po) + rodent chow + water
APLE (100 mg/kg): nicotinamide (48 mg/kg; ip) + Alloxan (120 mg/kg; ip) + APLE (100 mg/kg; po) + rodent chow + water
APLE (200 mg/kg): nicotinamide (48 mg/kg; ip) + Alloxan (120 mg/kg; ip) + APLE (200 mg/kg; po) + rodent chow + water
APLE (400 mg/kg): nicotinamide (48 mg/kg; ip) + Alloxan (120 mg/kg; ip) + APLE (400 mg/kg; po) + rodent chow + water
2.11. Measurement of Bodyweight
Bodyweight of rats was measured prior to the commencement of all animal experiments. Subsequently, bodyweight of rats in all groups was measured every 3 days over 18 days. Doses were adjusted to reflect changes in bodyweight every 3 days. Finally, bodyweight of rats in all groups were measured prior to their sacrifice.
2.12. Blood Collection, Preparation of Sera, and Isolation of Organs
After weighing rats on day 18, rats were sacrificed under deep chloroform anaesthesia. Blood samples were collected via cardiac puncture and then dispensed into labelled empty vacutainer tubes. Representative blood samples for each group were centrifuged at 3000 rpm (Eppendorf centrifuge 5702, 4°C) for 5 minutes. To obtain sera, the supernatant was collected into respective labelled sample tubes. Liver, kidney spleen, and pancreas were harvested, freshly weighed, and fixed in 10% formalin.
2.13. Measurement of Serum Insulin, Glucagon, and GLP-1
Serum insulin, glucagon, and GLP-1 levels were, respectively, measured by using rat insulin ELISA (Wuxi Donglin Sci & Tech Development Co. Ltd., China), rat glucagon ELISA (Wuxi Donglin Sci & Tech Development Co. Ltd., China), and rat GLP-1 ELISA (Wuxi Donglin Sci & Tech Development Co. Ltd., China) strictly according to manufacturer's instructions.
2.14. Determination of Median Area of Pancreatic Islets of Langerhans
After histological processing of representative pancreas, resultant photomicrographs for respective groups were scanned by using Amscope MD35 eyepiece camera as previously described [1]. Each photomicrograph was uploaded onto Adobe photoshop CS6 and subsequently superimposed on a stereological grid. The number of intersections overlying the stroma of pancreatic islets was counted. Crosssectional area of pancreatic islets of Langerhans of representative pancreas was determined by using Cavalieri method and point counting [36]. The crosssectional area was determined by the formula:
| (1) |
where
A represents crosssectional area of pancreatic islets of Langerhans
∑P represents the total number of intersections overlying the stroma of pancreatic islets
a/p represents the area per point of the stereological grid
M represents the linear magnification.
2.15. Extraction of α-Amylase from the Pancreas of Guinea Pigs
After 20 hours of starvation, a guinea pig was sacrificed under deep anesthesia, and the pancreas was surgically removed. The pancreas was trimmed free of fat and any other tissue and immediately washed in sodium phosphate buffer (pH 7.4). After weighing the pancreas, it was kept in a freezer at 4°C. The frozen pancreas was then homogenized (1 g of pancreas: 5 mL buffer) with ice cold, 0.1 M sodium phosphate buffer (pH of 7.4) by using a homogenizer (WiseTis HG-15D homogenizer) and then centrifuged at 4400 rpm for 30 minutes at 4°C. The supernatant was pipetted into separate microfuge tubes and stored in a freezer at -20°C. To confirm the extracted enzyme (α-amylase), a commercially available α-amylase was benchmarked with the extracted enzyme (α-amylase) using starch and iodine. Faint or disappearance of the blue-black coloration considered as an indication of α-amylase activity was compared to a control set up (no enzyme + starch + iodine) where there was a deep blue-black coloration.
2.16. Effect of APLE on α-Amylase Enzymatic Activity
Inhibition of alpha (α)-amylase enzymatic activity was assayed per a previous method [37] with some modifications. Briefly, APLE (125 μL) or acarbose at increasing concentrations (100-1500 μg/mL) was added to a set of labeled test tubes containing 125 μL of α-amylase solution in 20 mM sodium phosphate buffer (pH 6.9). The APLE or acarbose/α-amylase mixture was preincubated at 25°C for 10 minutes. Subsequently, 125 μL of 1% starch (prepared in 20 mM phosphate buffer, pH = 6.9) was added at selected time intervals. The reaction mixture in each case was incubated at 25°C for 10 minutes. Termination of each reaction was done at time intervals by adding 250 μL of 3, 5-dinitrosalicylic acid (DNSA) reagent and further heated in a water bath at 100°C for 5 minutes, then allowed to cool to room temperature. Each reaction was made up to 2.5 mL with distilled water and the absorbance measured at 540 nm using a spectrophotometer (UV-vis, T70 PG Instruments). A control set-up was prepared using the same procedure except that α-amylase was preincubated with distilled water instead of APLE. Alpha (α)-Amylase inhibitory activity was calculated as follows:
| (2) |
2.17. Mode of Inhibition of α-Amylase Enzymatic Activity by APLE
The mode of inhibition of α-amylase enzymatic activity by APLE was done per a previous method [38] with some modification. Briefly, 125 μL of APLE (5 mg/mL) was first preincubated with 125 μL of α-amylase solution in 20 mM phosphate at 25°C for 10 minutes in a set of labeled test tubes. Afterwards, 125 μL of starch solution at varying concentrations (0.3–5 mg/mL) was added to the reaction mixtures to start the reaction. The reaction mixture was then incubated at 25°C for 10 minutes before addition of 250 μL of 3, 5-dinitrosalicylic acid (DNSA) reagent to stop the reaction. The amount of product formed was assayed by measuring the absorbance at a wavelength of 540 nm using a spectrophotometer (UV-vis, T70 PG Instruments). The procedure was repeated for a control set up by preincubation of α-amylase with a buffer instead of APLE. Lineweaver-Burk plot from the plot of Michaeles-Menten was done using GraphPad prism version 8(Graph Pad Software, San Diego, CA, USA) for the estimation of Km and Vmax. The mode of inhibition of α-amylase enzymatic activity by APLE was deduced from the estimated Km and Vmax.
2.18. Extraction of α-Glucosidase from the Small Intestines of Guinea Pigs
Extraction of α-glucosidase from the intestines of guinea pigs was performed according to a previous method [39] with some modifications. Briefly, guinea pigs were first starved for 20 hours and then sacrificed under deep anesthesia. The small intestine located immediately above the cecum and immediately below the duodenum was surgically removed and thoroughly rinsed in ice-cold 0.02 M sodium phosphate buffer (pH 6.9) before dipping in fresh buffer solution. At a proportion of 1 g of intestine : 5 mL of buffer, the isolated intestine was homogenized (WiseTis HG-15D homogenizer, Germany) at 4400 rpm for 10 minutes at 2 minutes intermittent stoppages on ice. The homogenate was centrifuged (Eppendorf 5430R centrifuge) for 30 minutes at 4°C and the supernatant gently harvested. Aliquots were put in 2 mL cryotubes and kept at -20°C for immediate use in experiments. To confirm the extracted enzyme (α-glucosidase), a commercially available α-glucosidase was benchmarked with the extracted enzyme (α-glucosidase) by incubating 50 μL of 3 mM p-nitrophenyl glucopyranoside (pNPG) with 25 μL of commercially available α-glucosidase or 25 μL of extracted enzyme (α-glucosidase) at 25°C. Observation of a yellow p-nitrophenol product after 5minutes in each case was considered a confirmation of the activity of α-glucosidase.
2.19. Effect of APLE on α-Glucosidase Enzymatic Activity
The inhibitory effects of APLE and acarbose (a standard α-glucosidase inhibitor) were assessed per a previous method [40] with some modifications. Briefly, phosphate buffer (20 mM; pH = 6.9) was used to prepare p-nitropheynyl glucopyranoside (pNPG) (substrate for α-glucosidase). In separate experiments, α-glucosidase (50 μL) was preincubated with increasing concentrations of APLE (25 μL in each case), acarbose, or buffer for 10 minutes in each case. Afterwards, 25 μL of 3 mM p-nitrophenyl glucopyranoside was added to either APLE/α-glucosidase or acarbose/α-glucosidase preincubated mixture to start a reaction. The reaction mixture in each case was incubated at 25°C for 20 minutes. After 20 minutes of incubation, the reaction was stopped by adding 0.1 M Na2CO3 (1 mL). A control was prepared using the same procedure except that α-glucosidase was preincubated with a buffer instead of APLE or acarbose. The reaction product (a yellow-colored p-nitrophenol) after termination of the reaction was measured by using a spectrophotometer (UV-vis, T70 PG Instruments) at 405 nm for the determination of α-glucosidase activity. The results were expressed as percentage of the control. In each case, experiments were repeated three times. Percentage (%) inhibition of α-glucosidase activity was estimated using the formula:
| (3) |
2.20. Mode of α-Glucosidase Inhibition by APLE
The mode of inhibition of α-glucosidase enzymatic activity by APLE was conducted according to a previous procedure [41] with some modifications. Briefly, in one set of test tubes, exactly 25 μL of 5 mg/mL of APLE was preincubated with α-glucosidase solution (50 μL) at 25°C for 10 minutes. In a second set of tubes, α-glucosidase was preincubated with 50 μL of 20 mM phosphate buffer (pH = 6.9). Then, 25 μL of p-nitrophenyl glucopyranoside (pNPG) at varying concentrations (0.3–3.0 mg/mL) was added to both sets of test tubes to start the reaction. The reaction mixtures were then incubated at 25°C for 20 minutes after which Na2CO3 (1000 μL) was added to terminate the reaction. The amount of product released was measured spectrophotometrically using a p-nitrophenol standard curve and converted to reaction velocities, (v). A double reciprocal plot (1/v against 1/[S]) where [S] is substrate concentration was plotted. The mode of inhibition of α-glucosidase activity by APLE was determined by analysis of the Line weaver-Burk plot.
2.21. Assessment of 2, 2,-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
DPPH radical scavenging activity was carried out according to a previous method [42] with some modification. Briefly, a 2 mL reaction mixture was prepared by mixing DPPH (1.0 mL of 0.135 mM) prepared in methanol and 1.0 mL of varying concentrations of APLE (40, 80, 120, 160, and 200 μg/mL) or ascorbic acid. The reaction mixture was shaken vigorously and left in the dark at room temperature for 30 minutes. The absorbance was measured (UV-vis, T70 PG Instruments) at 517 nm against a blank. All tests were performed in triplicates. An equal amount of methanol and DPPH solution served as control. DPPH radical scavenging activity was estimated by using the formula:
| (4) |
2.22. Nitric Oxide (NO) Radical Scavenging Assay
NO radical scavenging activity of APLE and standards were estimated using Griess Illosvory reaction as previously described [43] with some few modifications. Briefly, Griess Illosvory reagent utilized 0.1% (w/v) napthyl ethylene diamine dihydrochloride instead of 5% 1-napthylamine. Varying concentrations (0-800 μg/mL) of both APLE and standards (gallic acid and ascorbic acid) prepared and made up to 167 μL were placed in labeled test tubes, and then, 667 μL of 10 mM sodium nitroprusside was added. Sodium phosphate buffer (167 μL, pH 7.4) was then added, and the mixture was incubated at 25°C for 150 minutes. Afterwards, 2000 μL sulfanilic acid reagent (0.33% in 20% glacial acetic acid) was added and allowed to stand for 5 minutes for the completion of the reaction of diazotization. Finally, a further 2000 μL of 0.1% napthyl ethylene diamine dihydrochloride was added, mixed, and was then allowed to stand for 30 minutes at 25°C. The nitrite concentration was measured (UV-vis, T70 PG Instruments) at 546 nm and was calculated with the control nitrite solution which had no APLE/or standards but having all other components of the reaction mixture. Sodium phosphate buffer was used as blank solution. Percentage (%) NO− scavenging ability of APLE and standards was calculated using the formula:
| (5) |
where Ac is absorbance of control and At is absorbance of test (APLE/standard).
2.23. Ferric Reducing Antioxidant Capacity (FRAC) Assay
FRAC was assayed per a previous method [44] with few modifications. Briefly, the reaction comprised 250 μL APLE/standard solution at different concentration (12.5–200 μg/mL), 625 μL of sodium phosphate buffer (0.2 M at pH 6.6), and 625 μL of 1% potassium ferric cyanide, [K3Fe(CN)6] into various test tubes. These were incubated for 20 minutes at 50°C to complete the reaction. Then, 625 μL of 10% trichloro acetic acid (TCA) solution was added into the test tubes. The total mixture was centrifuged at 3000 rpm for 10 minutes, after which 1800 μL supernatant was taken and mixed with 1800 μL of distilled water. Afterwards, 360 μL of 0.1% ferric chloride (FeCl3) solution was added and thoroughly mixed. The absorbance of the solution was measured at 700 nm using a spectrophotometer (UV-vis, T70 PG Instruments) against reaction blank. A typical blank solution containing the same solution mixture without APLE/or quercetin was incubated under similar conditions. Increased absorbance of the reaction mixture indicates increased reducing capacity. The experiment was repeated three times at each concentration. FRAC was measured as quercetin equivalent (QE).
2.24. Estimation of IC50 and EC50
To determine IC50 and EC50 of APLE with respective to its antioxidant, free radical scavenging, and inhibitory effect on α-amylase and α-glucosidase enzymatic activity, a series of increasing concentration of APLE was tested against the respective activities. After converting the concentrations to log10, it was plotted on the horizontal axis against the % maximal activities (antioxidant, free radical scavenging, and inhibitory effect on α-amylase and α-glucosidase enzymatic activity) on the vertical axis. The concentration of APLE that produced 50% of the maximal activities (antioxidant, free radical scavenging, and inhibitory effect on α-amylase and α-glucosidase enzymatic activity) assayed was determined graphically using GraphPad prism version 8 statistical software.
2.25. Statistical Analysis
Data obtained were presented as mean ± SD. Statistical analysis was done by using Graph Pad Prism Version 8 for Windows (Graph Pad Software, San Diego, CA, USA). Mean comparison between groups was done by using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests. P ≤ 0.05 was considered statistically significant in all analyses.
3. Results
3.1. Phytochemical Screening and Quantification of Total Phenols and Flavonoids in APLE
An average yield of 9.6% APLE was obtained from the initial powdered (120 g) leaves of Abrus precatorius. Standard phytochemical screening showed the presence of phenols, flavonoids, tannins, alkaloids, and saponins in APLE. From the calibration curve of rutin (standard), flavonoid content in APLE was estimated to be 220.29 μg/mL of rutin equivalent (RE) (Figure 2(a)). Also, from the calibration curve of gallic acid (standard), phenolic content in APLE was estimated to be 85.51 μg/mL of gallic acid equivalent (GAE) (Figure 2(b)).
Figure 2.
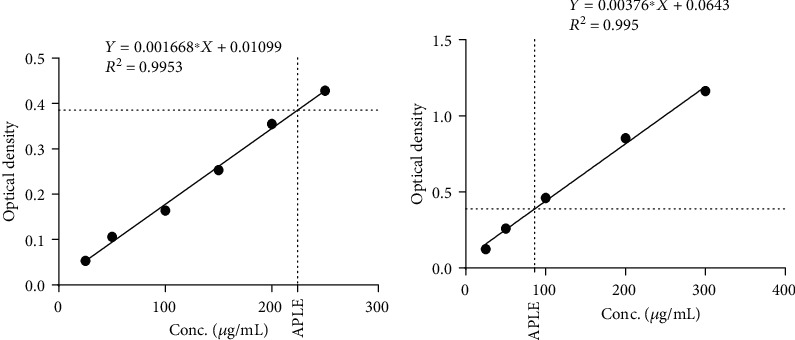
Quantification of total flavonoids and phenolics in APLE. (a) Calibration curve of rutin showing total flavonoid content in APLE. (b) Calibration curve of gallic acid showing total phenolic content in APLE. APLE: Abrus precatorius leaf extract.
3.2. APLE Restored Loss in Bodyweight Associated with Alloxan/Nicotinamide-Induced Diabetic Rats
Compared to control rats, model rats significantly (P ≤ 0.05) lost bodyweight. However, treatment of Alloxan/nicotinamide-induced diabetic rats with APLE, particularly APLE (100 mg/kg), significantly restored bodyweight loss relative to model rats (Table 1). Although there were differences in the organ weight/bodyweight ratios between control and model and also between model and APLE, these differences were statistically insignificant (P > 0.05).
Table 1.
Effect of APLE on organ weight/bodyweight ratios and bodyweight change.
| Organ weight/body weight ratio | Body weight (g) | ||||||
|---|---|---|---|---|---|---|---|
| Spleen | Liver | Kidney | Pancreas | Initial | Final | % change in body weight | |
| Control | 0.005 ± 3.7 × 10−5 | 0.043 ± 3.0 × 10−4 | 0.005 ± 3.2 × 10−4 | 0.004 ± 2.6 × 10−5 | 153.6 ± 14.5 | 154.5 ± 17.4 | 0.58 |
| Model | 0.005 ± 1.6 × 10−4 | 0.032 ± 1.0 × 10−3 | 0.004 ± 1.3 × 10−4 | 0.005 ± 1.6 × 10−4 | 167.4 ± 23.8 | 156.4 ± 8.0 | -6.57a |
| Metformin (mg/kg) | 0.005 ± 1.2 × 10−4 | 0.042 ± 1.0 × 10−3 | 0.004 ± 1.0 × 10−4 | 0.002 ± 5.5 × 10−4 | 167.2 ± 52.9 | 163.1 ± 11.2 | -0.02ns |
| APLE (mg/kg) | |||||||
| 100 | 0.006 ± 1.6 × 10−4 | 0.050 ± 1.0 × 10−3 | 0.006 ± 1.6 × 10−4 | 0.005 ± 1.4 × 10−4 | 138.5 ± 32.4 | 145.5 ± 62.6 | 5.10b |
| 200 | 0.006 ± 2.3 × 10−4 | 0.055 ± 2.0 × 10−3 | 0.006 ± 2.4 × 10−4 | 0.004 ± 1.4 × 10−4 | 146.6 ± 43.5 | 140.9 ± 21.5 | -3.86ns |
| 400 | 0.005 ± 2.3 × 10−4 | 0.031 ± 1.0 × 10−3 | 0.003 ± 1.3 × 10−4 | 0.005 ± 2.0 × 10−4 | 156.2 ± 23.0 | 154.6 ± 24.9 | -0.99ns |
∗Received normal saline; APLE: Abrus precatorius leaf extract (APLE); ns: not significant; aP ≤ 0.05 (model vs. control); bP ≤ 0.05 (APLE and metformin vs. model).
3.3. APLE Decreased Elevated Blood Glucose Levels of Alloxan/Nicotinamide-Induced Diabetic Rats
Sequential exposure of rats to Alloxan monohydrate (120 mg/kg; ip) and nicotinamide (48 mg/kg; ip) resulted in elevated blood glucose levels in model rats (diabetic rats) compared to control rats. Treatment of Alloxan/nicotinamide-induced diabetic rats with APLE (100, 200, and 400 mg/kg) for 18 days resulted in significant (P < 0.05) decrease in average blood glucose levels of diabetic rats. Over the 18-day treatment/observation period, control rats had a percentage decrease in mean blood glucose levels from the initial blood glucose level by 11.96% as against 4.3% by model rats (diabetic rats). Compared to model rats, APLE treatment, particularly APLE (100 mg/kg; po) produced 68.67% decrease in mean blood glucose levels from the initial blood glucose level of Alloxan/nicotinamide-induced diabetic rats over 18 days of treatment (Table 2).
Table 2.
Effect of APLE on elevated blood glucose levels of Alloxan/nicotinamide-induced diabetic rats.
| Treatment groups | Day of blood glucose peak | Blood glucose level after Alloxan/nicotinamide exposure (mmol/L) | Blood glucose level on day 18 (mmol/L) | Mean decrease in blood glucose | % decrease in blood glucose |
|---|---|---|---|---|---|
| ∗Control | 9 | 4.60 ± 0.282 | 4.05 ± 0.212 | 0.55 ± 0.07 | 11.96 |
| Model | 12 | 21.90 ± 9.050 | 20.95 ± 5.586 | 0.95 ± 3.464 | 4.34a |
| Metformin (300 mg/kg) | 12 | 18.45 ± 1.626 | 10.40 ± 5.515 | 8.05 ± 3.889 | 43.63b |
| APLE (mg/kg) | |||||
| 100 | 9 | 16.60 ± 4.52 | 5.20 ± 1.414 | 11.4 ± 3.106 | 68.67b |
| 200 | 12 | 16.90 ± 2.969 | 11.65 ± 1.242 | 5.25 ± 8.273 | 31.07b |
| 400 | 15 | 17.10 ± 2.828 | 16.35 ± 0.070 | 0.75 ± 2.758 | 4.39ns |
∗Received normal saline; aP ≤ 0.05 (model vs. control); bP ≤ 0.05 (APLE and metformin vs. model); APLE: Abrusprecatorius leaf extract; ns: not significant.
3.4. APLE Treatment Increased the Number and Median Crosssectional Area of Pancreatic Islets of Langerhans of Alloxan/Nicotinamide-Induced Diabetic Rats
The number of pancreatic islets of Langerhans was not different between control and model rats; however, median crosssectional area of pancreatic islets of model rats was decreased compared to that of control rats. Treatment of Alloxan/nicotinamide-induced diabetic rats with APLE significantly increased both the number and crosssectional area of pancreatic islets of Langerhans compared to model rats (Figure 3 and Table 3).
Figure 3.
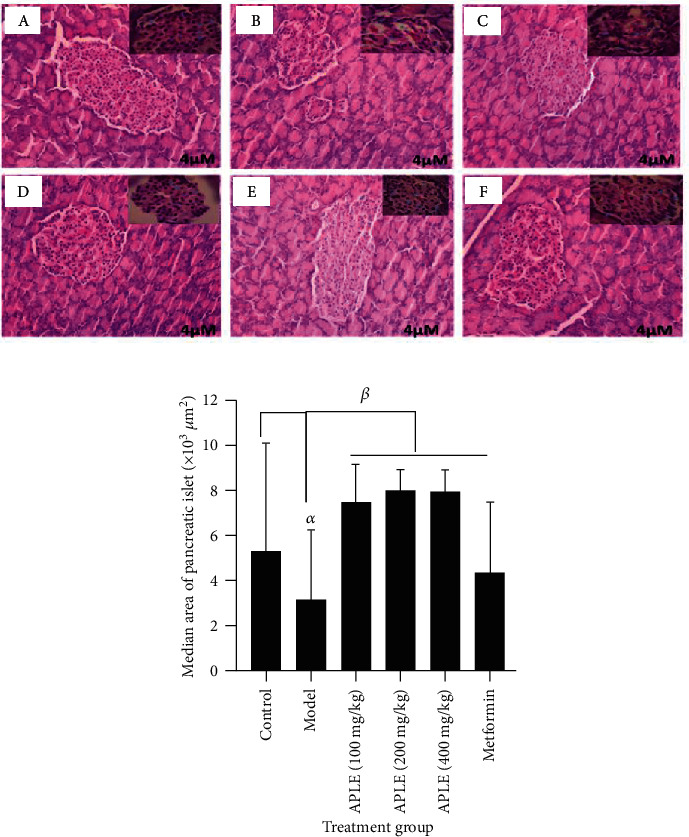
Effect of APLE and metformin on Alloxan/nicotinamide-induced pancreatic β-cell damage and necro-apoptosis in diabetic rats. (a) A histomicrograph of representative H&E-stained pancreatic islets of Langerhans showing (A) control, (B) model, (C) APLE (100 mg/kg po), (D) APLE (200 mg/kg; po), (E) APLE (400 mg/kg; po), and (F) metformin (300 mg/kg; po). (b) A bar graph showing the median area of pancreatic islets of Langerhans. Each bar is the mean ± SD median area of pancreatic islets of Langerhans. αP ≤ 0.05 (model vs. Control); βP ≤ 0.05 (APLE and metformin vs. model); APLE: Abrus precatorius leaf extract.
Table 3.
Effect of APLE on number of pancreatic islets and median pancreatic area of Alloxan/nicotinamide-induced diabetic rats.
| Treatment groups | Number of islets | Median area (×103μm2; Q1-Q3) | Average rank |
|---|---|---|---|
| Control | 16 | 5.299 (2.047-9.996) | 65.0 |
| Model | 16ns | 3.131 (1.445-6.142)a | 34.9 |
| Metformin (300 mg/kg) | 18ns | 4.336 (2.348-7.587)ns | 50.6 |
| APLE (mg/kg) | |||
| 100 | 27b | 7.467 (5.299-9.635)b | 76.0 |
| 200 | 25b | 7.949 (6.624-9.153)b | 83.2 |
| 400 | 35b | 7.949 (6.744-9.394)b | 80.3 |
a P ≤ 0.05 (model vs control); bP ≤ 0.05 (APLE and metformin vs. model); APLE: Abrus precatorius leaf extract; ns: not significant.
3.5. APLE Increased Serum Insulin and GLP-1 Levels Inversely with Glucagon in Alloxan/Nicotinamide-Induced Diabetic Rats
Sequential exposure of rats to Alloxan monohydrate (120 mg/kg; ip) and nicotinamide (48 mg/kg; ip) led to decrease in serum insulin in model rats (diabetic rats) compared to control rots. However, treatment of alloxan/nicotinamide-induced diabetic rats with APLE (100, 200, and 400 mg/kg) over a period of 18 days restored serum insulin levels even more than that of control rats. Surprisingly, APLE (200 mg/kg) had no effect on Alloxan/nicotinamide-induced decrease in serum insulin (Figure 4(a)). Serum glucagon levels decreased in Alloxan/nicotinamide-induced diabetic rats compared to that of control rats. Relative to Alloxan/nicotinamide-induced diabetic rats (model rats), APLE treatment, particularly APLE (400 mg/kg), significantly (P < 0.05) decreased serum glucagon levels (Figure 4(b)). Serum GLP-1 significantly (P < 0.05) decreased in Alloxan/nicotinamide-induced diabetic rats (model rats) compared to control rats. However, treatment of Alloxan/nicotinamide-induced diabetic rats (model rats) with APLE (100, 200, and 400 mg/kg) restored serum GLP-1 levels in a dose-related manner (Figure 4(c)).
Figure 4.
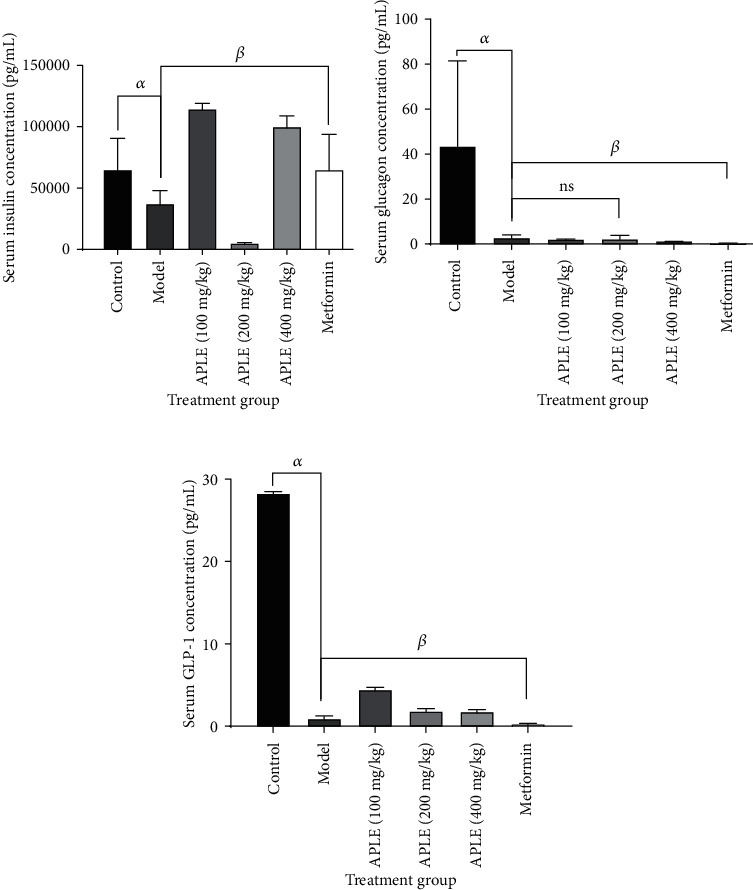
Effect of APLE on serum levels of insulin, glucagon, and GLP-1 of Alloxan/nicotinamide-induced diabetic rats. Each bar is the mean ± SD, n = 3. (a) Effect of APLE on serum insulin, (b) effect of APLE on serum glucagon, and (c) effect of APLE on serum GLP-1. αP ≤ 0.05 (model vs. control); βP ≤ 0.05 (APLE and metformin vs. model); ns: not significant; APLE: Abrus precatorius leaf extract; metformin (300 mg/kg; po).
3.6. APLE Concentration Dependently Decreased Enzymatic Activity of α-Amylase
At equivalent concentrations, both APLE and acarbose concentration dependently inhibited enzymatic activity of α-amylase; however, the concentration-% inhibition curve for APLE was shifted to the left of that of acarbose (Figure 5(a)). From the Lineweaver-Burk and Michaeles-Menten plots (Figures 5(b) and 5(c)), APLE decreased maximum velocity (Vmax) of α-amylase/substrate reaction relative to control but increased the Michaeles constant (Km) relative to control (Table 4). Respectively, the IC50 estimates for APLE and acarbose were 259 μg/mL and 297 μg/mL (Table 5).
Figure 5.
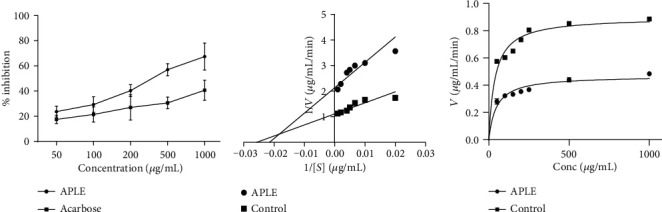
Effect of APLE on α-amylase enzymatic activity. Each plotted point is the mean ± SD, n = 3. (a) % inhibitory effect of APLE on α-amylase enzymatic activity, (b) Lineweaver-Burk plot showing the mode of inhibition of α-amylase enzymatic activity by APLE. (c) Michaeles-Menten plot showing effect of APLE on α-amylase kinetics (Vmax and Km). αP ≤ 0.05 (APLE vs. acarbose); APLE: Abrus precatorius leaf extract; Vmax: maximum velocity; Km: Michaeles constant.
Table 4.
Effect of APLE on kinetics of α-amylase and α-glucosidase.
| α-Amylase | α-Glucosidase | |||
|---|---|---|---|---|
| Vmax | Km | Vmax | Km | |
| Control | 0.8992 | 38.86 | 2.665 | 1.750 |
| APLE | 0.4685 | 46.52 | 2.577 | 1.901 |
APLE: Abrus precatorius leaf extract.
Table 5.
Estimated IC50 values for APLE and standards.
| Drugs and standards | IC50 (μg/mL) | |
|---|---|---|
| FRAC | APLE | 2.799∗ |
| Quercetin | 3.243∗ | |
|
| ||
| DPPH radical scavenging activity | APLE | 115.100 |
| Ascorbic acid | 107.900 | |
|
| ||
| NO radical scavenging activity | APLE | 1100.000 |
| Gallic acid | 1670.000 | |
| Ascorbic acid | 1236.000 | |
|
| ||
| α-Glucosidase enzymatic activity | APLE | 1090.000 |
| Acarbose | 1176.000 | |
|
| ||
| α-Amylase enzymatic activity | APLE | 297.000 |
| Acarbose | 259.100 | |
∗Values represent EC50; APLE: Abrus precatorius leaf extract; DPPH: 2, 2,-diphenyl-1-picrylhydrazyl; FRAC: ferric reducing antioxidant capacity; NO: nitric oxide.
3.7. APLE Decreased Enzymatic Activity of α-Glucosidase
At equimolar concentrations, both APLE and acarbose demonstrated concentration-dependent inhibitory effects on α-glucosidase enzymatic activity; however, the concentration-% inhibition curve for APLE was shifted to the left of that of acarbose (Figure 6(a)). From the Lineweaver-Burk and Michaeles-Menten plots (Figures 6(b) and 6(c)), APLE decreased maximum velocity (Vmax) of α-glucosidase/substrate reaction relative to control but increased Michaeles constant (Km) relative to control (Table 4). Respectively, the IC50 estimates for APLE and acarbose were 1176 μg/mL and 1090 μg/mL (Table 5).
Figure 6.
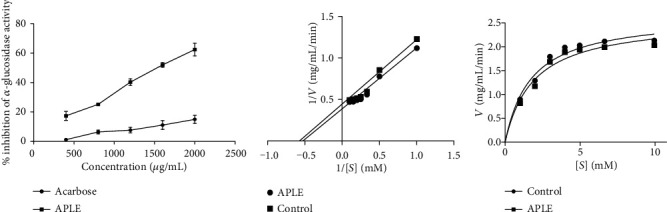
Effect of APLE on α-glucosidase enzymatic activity. Each plotted point is the mean ± SD, n = 3. (a) % inhibitory effect of APLE on α-glucosidase enzymatic activity, (b) Lineweaver-Burk plot showing the mode of inhibition of α-glucosidase enzymatic activity by APLE. (c) Michaelis-Menten plot showing effect of APLE on α-glucosidase kinetics (Vmax and Km). αP ≤ 0.05 (APLE vs. acarbose); APLE: Abrus precatorius leaf extract; Vmax: maximum velocity; Km: Michaeles constant.
3.8. APLE Increased DPPH and NO Radical Scavenging Activity as well as Demonstrating Ferric Reducing Antioxidant Capacity (FRAC)
In vitro APLE demonstrated concentration-dependent DPPH radical scavenging activity, but it was lower than that of ascorbic acid (Figure 7(a)). Compared to ascorbic acid and gallic acid, APLE demonstrated concentration-dependent scavenging activity on nitric oxide (NO) radical. While APLE and gallic acid morphologically demonstrated a flat concentration-response (% NO radical scavenging activity) curve, ascorbic acid showed a steep concentration-response (% NO radical scavenging activity) curve (Figure 7(b)). From the IC50 estimates, APLE had the lowest IC50 value relative to ascorbic acid and gallic acid (Table 5). At equimolar concentrations, quercetin demonstrated significant concentration-dependent ferric reducing antioxidant capacity relative to APLE (Figure 7(c)).
Figure 7.
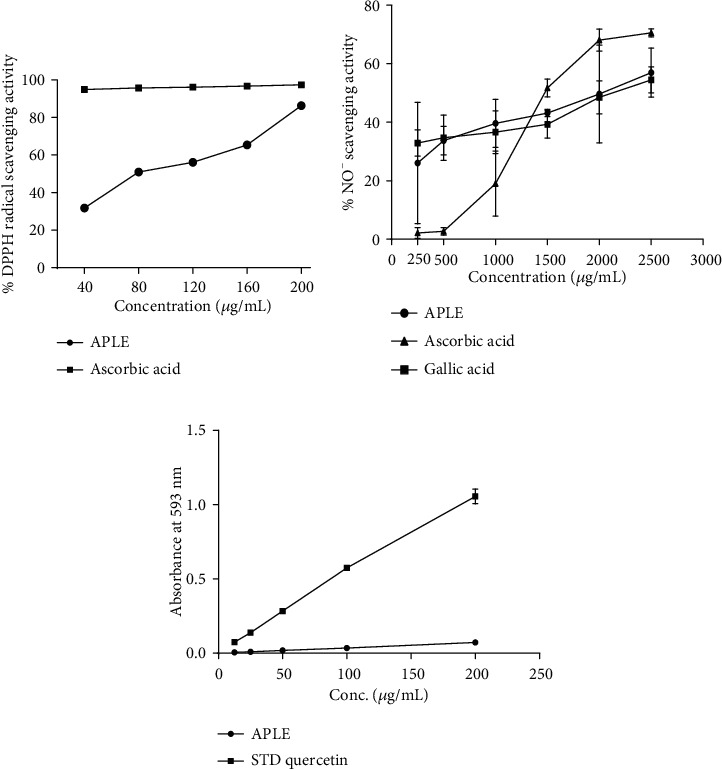
Free radical scavenging and antioxidant effects of APLE. (a) DPPH radical scavenging activity of APLE. (b) NO radical scavenging activity of APLE. (c) Ferric reducing antioxidant activity of APLE. αP ≤ 0.05 (APLE vs. ascorbic acid and quercetin); APLE: Abrus precatorius leaf extract; DPPH: 2, 2,-diphenyl-1-picrylhydrazyl; NO: nitric oxide.
4. Discussion
Herbs have been used in many capacities by mankind to improve human health and also serve as a source of natural templates for pharmaceutical semisynthesis of novel drugs. Many local communities across Afro-Asian regions of the world rely heavily on their ethnobotanical heritage to meet most of their primary healthcare needs. This study demonstrated that antidiabetic effect of APLE in experimental diabetes mellitus in rats is mediated through multiple mechanisms including inverse modulation of insulin and GLP-1 with glucagon, inhibition of α-amylase and α-glucosidase enzymatic activity, free radical scavenging, antioxidant, and recovery of necro-apoptosized pancreatic β-cells. As the present results corroborate earlier report on Abrus precatorius [1, 35], it further confirms folk claims on Abrus precatorius especially those made by the local communities in western Ghana, where the leaves are used to treat diabetes mellitus.
Alloxan was used as a diabetogenic agent in this study, in view of its specific pancreatic β-cell toxicity to establish experimental diabetes mellitus in rats. Upon exposure of Alloxan to rats, it undergoes phase 1 reaction; specifically, it is biotransformed by hepatic metabolic enzymes via reduction into dialuric acid. The dialuric acid is reoxidized back to Alloxan establishing a redox cycle leading to production of superoxide radicals (O2−), which dismutate to form hydrogen peroxide (H2O2). From the H2O2, reactive hydroxyl radicals (OH−) are formed through the Fenton reaction.
Resultant ROS induces increase in cytosolic calcium concentrations which in turn induces rapid destruction and necro-apoptosis of pancreatic β-cells. Extensive destruction of pancreatic β-cells occasions insulin insufficiency and hyperglycemic episode leads to glucose toxicity (gluco-toxicity). Expectedly, rats in model group (Alloxan/nicotinamide-induced diabetic rats) developed sustained hyperglycemia due to insulin insufficiency culminating from extensive destruction of pancreatic β-cells. However, treatment of diabetic rats with APLE over 18 days reversed the chronic hyperglycemia in diabetic rats relative to model rats (Table 2). To ascertain the mechanism by which APLE produced glucose lowering over the 18 days, serum concentrations of insulin, glucagon, and GLP-1 were measured across all groups by using a rat-specific ELISA kit. Physiologically, at any given time point, blood glucose concentration reflects a balance between glucose production (dietary sources of glucose, glycogenolysis, and gluconeogenesis) and utilization (uptake of glucose by insulin-responsive tissues and conversion of excess glucose to storage carbohydrate) primarily in response to secretion pattern and the actions of insulin and glucagon. While insulin decreases peripheral glucose concentration by increasing glucose utilization by insulin-responsive tissues such as the brain, muscles, liver, and other body cells as well as inhibition of glucagon secretion, glucagon on the other hand increases peripheral glucose levels by promoting break down of glycogen (glycogenolysis) and biosynthesis of glucose from noncarbohydrate sources (fatty acids, pyruvate, and amino acids, i.e., gluconeogenesis). Interestingly, APLE treatment reversed decreased insulin concentration inversely with glucagon in diabetic rats compared with model rats, indicating that APLE treatment improved utilization of peripheral glucose in an insulin-dependent manner. APLE-dependent increase in insulin inversely with glucagon confirms the already established observation that insulin inhibits glucagon secretion and action. To assess how APLE increased insulin but decreased glucagon in diabetic rats, the pancreatic islets of Langerhans was histologically examined; specifically, the number of islets and the median area of the islets were studied across groups. Of note, APLE treatment did not only recover partially damaged pancreatic β-cells but also increased the number and median crosssectional area of islets relative to that of model rats (Figure 3 and Table 3). Since insulin concentration in blood is directly related to pancreatic β-cell population and mass, it is possible that APLE-dependent increase in insulin inversely with glucagon was through recovery of damaged pancreatic β-cells as well as increase in the number of islets and mass of pancreatic β-cells, which enhanced insulin secretion and utilization of peripheral glucose by insulin-responsive tissues. Also, APLE produced increase in GLP-1 inversely with glucagon. GLP-1 is one of the incretins (INtestine seCRETion Insulin), and just like glucose-dependent insulinotropic polypeptide (GIP), they exert insulinotropic effect in response to the presence of glucose in the duodenum. GLP-1 and GIP are, respectively, produced by enteroendocrine L and K cells. These two hormones exert their insulinotropic effect by binding to and activating G-protein-coupled receptors (GIP receptor (GIPR) and GLP-1 receptor (GLP-1R)) in the plasma membrane of pancreatic β-cells. Binding and activation of the G-protein receptor leads to decoupling of the α-subunit of the G-protein and its transactivation of adenylate cyclase, which dephosphorylates ATP to cyclic AMP. Increase in cyclic AMP activates protein kinase A which mediates closure of K+ ion-gated channels. Subsequently, influx of Ca2+ via voltage-gated Ca2+ channels causes depolarization of β-cell membrane which eventually leads to secretion of insulin by pancreatic β-cells [45]. GLP-1 and GIP promote pancreatic β-cell proliferation, inhibit necro-apoptosis of pancreatic β-cells, thereby expanding pancreatic β-cell mass [46]. While GIP enhances postprandial glucagon response, GLP-1 suppresses postprandial glucagon response. Pancreato-protective effects of APLE could be due to enhanced release of GLP-1, since decrease in GLP-1 in diabetic rats corresponded with decreased number of islets as well as crosssectional area of pancreatic islets. Also, APLE-dependent increase in insulin could be related to GLP-1 mediation.
Enzymatically, carbohydrate digestion in humans begins in the mouth by rapid hydrolysis of both amylopectin and amylose in cooked starch by α-amylase, which is secreted by both salivary glands and the pancreas. Alpha (α)-amylase is an endoglycosidase which specifically hydrolyses internal α-1, 4 linkages yielding maltose, maltotriose, and α-dextrin. Unlike α-amylase, α-glucosidase is a brush border enzyme of duodenal enterocytes. Functionally, α-glucosidase hydrolyzes the terminal nonreducing (1-4) α-glucose residues of maltose to release a single α-glucose. These two enzymes serve as key targets for pharmacological modulation of carbohydrate digestion in people suffering from diseases related to errors in carbohydrate metabolism such as DM. Inhibition of these two enzymes results in significant delay in the release of glucose from disaccharides thereby reducing glucose availability and absorption. Indeed, among the available conventional oral hypoglycemic agents, α-glucosidase inhibitors (e.g., acarbose) enjoy therapeutic preference for the treatment of type 2 DM. Interestingly, APLE concentration-dependently inhibited these two enzymes, which reveals yet another mechanism by which APLE lowers postprandial blood glucose levels, and this observation corroborates an earlier study [47], which demonstrated that a triterpene ketone (lupenone) isolated from the leaves of Abrus precatorius exerted potent α-amylase inhibitory effect. The inhibitory effects of APLE against α-amylase and α-glucosidase mirror that of other medicinal plants known for their antidiabetic properties including Chrysobalanus orbicularis [11], Spondias mombin and Mangifera indica [12], Sesamum indicum [13], and Bryophyllum pinnatum [14].
Chronic hyperglycemia induces nonenzymatic glycosylation of various macromolecules leading to generation of unstable chemical species including ROS. ROS inhibits glyceraldehyde-3-phosphate dehydrogenase (GADPH) in the glycolytic pathway, thereby increasing upstream intermediates of GADPH. These glycolytic intermediates (glucose, fructose-6-phosphate, and glyceraldehyde-3-phosphate) are shunted into other biochemical pathways which are implicated in DM. Also, ROS is implicated in lipid peroxidation and oxidative stress. Oxidative stress induced by ROS as a result of chronic hyperglycemia plays a key role in the onset of various diabetic complications including insulin resistance and pancreatic β-cell dysfunction. In this study, free radical scavenging activity of APLE was assessed by using DPPH and NO assays, while antioxidant capacity of APLE was assessed using FRAC. DPPH is a stable free radical as a result of the delocalization of electrons all over the molecule. Delocalization of electrons in DPPH results in a deep violet color, and upon reduction by any hydrogen or electron donor, the violet color of DPPH fades and leads to formation of a pale-yellow hydrazine. The color change reflects shifting of wavelength in the visible spectra from 517 nm to 330 nm. As a result, free radical scavenging activity corresponds to reduction of DPPH, which can be quantified by measuring absorbance at 517 nm [48]. Similarly, NO partakes in a series of reactions leading to decrease in mitochondrial ATP and aconitase, which in turn induce increase in xanthine oxidase. Nitric oxide (NO) donors such as STZ promote reaction between superoxide (O2−) and hydrogen peroxide (H2O2), which yields reactive hydroxyl (OH−) and nitro radicals that cause DNA damage of pancreatic β-cells. Conversion of ferric (Fe3+) to ferrous (Fe2+) by donation of an electron by an electron donor (antioxidant agent) forms the basis of FRAC [49]. Therefore, FRAC assay provides a direct measure of the reducing or electron donating ability of an antioxidant. In this study, APLE demonstrated concentration-dependent scavenging activity against DPPH and NO and also demonstrated reducing capacity in the FRAC assay. These observations point to the ability of APLE to mop-up unstable chemical intermediates generated by Alloxan exposure to rats, thereby preventing ROS-mediated cell damage and necro-apoptosis, which accounted for extensive damage of pancreatic β-cells and attendant hyperglycemia in model rats. The antioxidant and free radical scavenging effects of APLE are attributable to the bioactive secondary plant metabolites identified in APLE, particularly the phenolic compounds (Figure 2). Phenolic compounds derived from plants exhibit many biological properties which account for their health benefits and a justification for their use in food and drug discovery industries. Phenolic compounds exert their biological effects by interacting with diverse cellular components including membrane transporters, protein kinases, catechol-O-methyltransferases, membrane-bound NADPH oxidases, xanthine oxidase, cyclo-oxygenases, lipoxygenases, and some transition metals [50–52]. Phenolic compounds exert antioxidant and free radical scavenging activities either directly or indirectly. Mostly, antioxidant effects of phenolic compounds are exerted indirectly by the ability of phenolic compounds to induce cellular events that lead to production of ROS-scavenging enzyme systems in vivo, while direct antioxidant effects of phenolic compounds are related to their ability to suppress the initiation step needed for generation of oxidant species or direct interaction with these unstable chemical species. Phenolic compounds derived from many plants have demonstrated inhibitory effects on activity of α-amylase and α-glucosidase [53–55], which supports the assertion that the inhibitory effects of APLE against activity of α-amylase and α-glucosidase observed in the present study could be due to the phenolic compounds detected in APLE. Also, tannins, saponins, and alkaloids were identified in APLE confirming a previous report [1]. Further, it is suspected that inhibitory effects of APLE on α-amylase and α-glucosidase enzymatic activity could be due to the combined effects of its phytoconstituents including tannins and saponins whose inhibitory effects against α-amylase and α-glucosidase have already been established [56–58].
Putting together this study has demonstrated that glucose lowering and pancreato-protective effects of APLE is mediated through multiple mechanisms including hormonal modulation, enzyme inhibition, free radical scavenging, antioxidant activity, and repair of damaged pancreatic β-cells. This study could have benefited from investigating the effect of APLE on the counterregulatory hormonal systems particularly, the catechol amines, and the stress hormone (cortisol) in gluconeogenesis (a major contributor to peripheral glucose) as well as effect of APLE on specific glucose transporters; nonetheless, the present results provide compelling basis for further mechanistic elucidation of antidiabetic effects of APLE.
5. Conclusion
Increase in insulin and GLP-1 inversely with glucagon, inhibition of α-amylase/α-glucosidase enzymatic activity, free radical scavenging, antioxidant, and pancreatic β-cell recovery underpin antidiabetic effects of Abrus precatorius leaf extract (APLE), and these pharmacological effects are attributable to phenolic and flavonoid contents of APLE. As this finding confirms folk use of APLE as an antidiabetic herbal medicine by local communities, it also lays a foundation for possible translational studies on APLE.
Acknowledgments
We specially acknowledge the traditional healers from Sefwi Asawinso Kitiwamu in the western region of Ghana for volunteering information on the traditional uses of Abrus precatorius.
Abbreviations
- APLE:
Abrus precatorius leaf extract
- AscAE:
Ascorbic acid equivalent
- DM:
Diabetes mellitus
- DPPH:
2, 2-diphenyl-1-picrylhydrazyl
- GADPH:
Glyceraldehyde-3-phosphate dehydrogenase
- QE:
Quercetin equivalent
- IR:
Insulin resistance
- ROS:
Reactive oxygen species.
Data Availability
All data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Authors' Contributions
AB conceived, designed, and wrote the manuscript. VYAB performed all chemistry-related experiments. DOA read and edited the final manuscript together with AB and VYAB for intellectual content. EOG performed experiments and analyzed data.
References
- 1.Boye A., Acheampong D. O., Gyamerah E. O., et al. Glucose lowering and pancreato-protective effects of Abrus Precatorius (L.) leaf extract in normoglycemic and STZ/nicotinamide-induced diabetic rats. Journal of ethnopharmacology. 2020;258, article 112918 doi: 10.1016/j.jep.2020.112918. [DOI] [PubMed] [Google Scholar]
- 2.Bettencourt-Silva R., Aguiar B., Sá-Araújo V., et al. Diabetes-related symptoms, acute complications and management of diabetes mellitus of patients who are receiving palliative care: a protocol for a systematic review. BMJ open. 2019;9(6, article e028604) doi: 10.1136/bmjopen-2018-028604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rehman K., Akash M. S. H. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: how are they interlinked? Journal of cellular biochemistry. 2017;118(11):3577–3585. doi: 10.1002/jcb.26097. [DOI] [PubMed] [Google Scholar]
- 4.Calvo P., Abadia B., Ferreras A., Ruiz-Moreno O., Verdes G., Pablo L. E. Diabetic macular edema: options for adjunct therapy. Drugs. 2015;75(13):1461–1469. doi: 10.1007/s40265-015-0447-1. [DOI] [PubMed] [Google Scholar]
- 5.Archvadze A., Kistauri A., Gongadze N., Makharadze T., Chirakadze K. Medical basis of diabetic neuropathy formation (review) Georgian Medical News. 2018;283:154–162. [PubMed] [Google Scholar]
- 6.Skyler J. S., Bakris G. L., Bonifacio E. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66(2):241–255. doi: 10.2337/db16-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang C., Hu S. Evaluating access to oral anti-diabetic medicines: a cross-sectional survey of prices, availability and affordability in Shaanxi Province, Western China. PloS one. 2019;14(10, article e0223769) doi: 10.1371/journal.pone.0223769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jokar N. K., Noorhosseini S. A., Allahyari M. S., Damalas C. A. Consumers' acceptance of medicinal herbs: an application of the technology acceptance model (TAM) Journal of Ethnopharmacology. 2017;207:203–210. doi: 10.1016/j.jep.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Chi G., Wang L., Chen B., et al. Polyoxometalates: study of inhibitory kinetics and mechanism against α-glucosidase. Journal of Inorganic Biochemistry. 2019;199:p. 110784. doi: 10.1016/j.jinorgbio.2019.110784. [DOI] [PubMed] [Google Scholar]
- 10.Boye A., Addo J. K., Acheampong D. O., et al. The hydroxyl moiety on carbon one (C1) in the monoterpene nucleus of thymol is indispensable for anti-bacterial effect of thymol. Heliyon. 2020;6(3, article e03492) doi: 10.1016/j.heliyon.2020.e03492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilobekemen Ekakitie L., Ojo O. A., Oyinloye B. E., Olaitan Ajiboye B. Antioxidant and inhibitory activities of enzymes linked to type II diabetes mellitus: the novel role of Chrysobalanus orbicularis leaf extract. Iranian Journal of Toxicology. 2020;14(3):179–186. doi: 10.32598/ijt.14.3.371.2. [DOI] [Google Scholar]
- 12.Ojo O. A., Afon A. A., Ojo A. B., Ajiboye B. O., Oyinloye B. E., Kappo A. P. Inhibitory effects of solvent-partitioned fractions of two Nigerian herbs (Spondias mombin Linn. and Mangifera indica L.) on α-amylase and α-glucosidase. Antioxidants. 2018;7(6):p. 73. doi: 10.3390/antiox7060073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talabi J., Adeyemi S., Awopetu S., Ajiboye B. O., Ojo O. A. Inhibitory effect of aqueous extracts of raw and roasted Sesamum indicum L. seeds on key enzymes linked to type-2 diabetes (α-amylase and α-glucosidase) and Alzheimer’s disease (acetylcholinesterase and butyrylcholinesterase) Potravinarstvo. 2018;12(1) [Google Scholar]
- 14.Ojo O. A., Ojo A. B., Ajiboye B. O., et al. Inhibitory effect of Bryophyllum pinnatum (Lam.) Oken leaf extract and their fractions on α-amylase, α-glucosidase and cholinesterase enzyme. Pharmacognosy Journal. 2018;10(3) [Google Scholar]
- 15.Asiimwe S., Kamatenesi-Mugisha M., Namutebi A., Borg-Karlsson A. K., Musiimenta P. Ethnobotanical study of nutri-medicinal plants used for the management of HIV/AIDS opportunistic ailments among the local communities of western Uganda. Journal of Ethnopharmacology. 2013;150(2):639–648. doi: 10.1016/j.jep.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Van Wyk B. E. A review of commercially important African medicinal plants. Journal of Ethnopharmacology. 2015;176:118–134. doi: 10.1016/j.jep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Pathak-Gandhi N., Vaidya A. D. Management of Parkinson’s disease in Ayurveda: medicinal plants and adjuvant measures. Journal of Ethnopharmacology. 2017;197:46–51. doi: 10.1016/j.jep.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Taur D. J., Patil R. Y. Mast cell stabilizing and antiallergic activity of Abrus precatorius in the management of asthma. Asian Pacific Journal of Tropical Medicine. 2011;4(1):46–49. doi: 10.1016/S1995-7645(11)60030-8. [DOI] [PubMed] [Google Scholar]
- 19.Bhutia S. K., Behera B., Nandini Das D., et al. Abrus agglutinin is a potent anti-proliferative and anti-angiogenic agent in human breast cancer. International Journal of Cancer. 2016;139(2):457–466. doi: 10.1002/ijc.30055. [DOI] [PubMed] [Google Scholar]
- 20.Mobin L., Saeed S. A., Ali R., Saeed S. G., Ahmed R. Antibacterial and antifungal activities of the polyphenolic fractions isolated from the seed coat of Abrus precatorius and Caesalpinia crista. Natural Product Research. 2018;32(23):2835–2839. doi: 10.1080/14786419.2017.1378217. [DOI] [PubMed] [Google Scholar]
- 21.Jouad H., Haloui M., Rhiouani H., El Hilaly J., Eddouks M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez-Boulemane) Journal of Ethnopharmacology. 2001;77(2-3):175–182. doi: 10.1016/S0378-8741(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 22.Osadebe P. O., Okide G. B., Akabogu I. C. Study on anti-diabetic activities of crude methanolic extracts of Loranthus micranthus (Linn.) sourced from five different host trees. Journal of Ethnopharmacology. 2004;95(2-3):133–138. doi: 10.1016/j.jep.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Abo K. A., Fred-Jaiyesimi A. A., Jaiyesimi A. E. Ethnobotanical studies of medicinal plants used in the management of diabetes mellitus in South Western Nigeria. Journal of Ethnopharmacology. 2008;115(1):67–71. doi: 10.1016/j.jep.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Sofi M. S., Sateesh M. K., Bashir M., Ganie M. A., Nabi S. Chemopreventive and anti-breast cancer activity of compounds isolated from leaves of Abrus precatorius L. 3 Biotech. 2018;8(8):p. 371. doi: 10.1007/s13205-018-1395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ssebuufu R. Double burden of disease: a global health challenge. The Annals of thoracic surgery. 2019;107(4):p. 1286. doi: 10.1016/j.athoracsur.2018.09.050. [DOI] [PubMed] [Google Scholar]
- 26.Elbashir S. M. I., Devkota H. P. Free radical scavenging, α-glucosidase inhibitory and lipase inhibitory activities of eighteen Sudanese medicinal plants. BMC complementary and alternative medicine. 2018;18(1):p. 282. doi: 10.1186/s12906-018-2346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falayi O. O., Oyagbemi A. A., Omobowale T. O., et al. Nephroprotective properties of the methanol stem extract of Abrus precatorius on gentamicin-induced renal damage in rats. Journal of Complementary and Integrative Medicine. 2018;16(3) doi: 10.1515/jcim-2017-0176. [DOI] [PubMed] [Google Scholar]
- 28.Garaniya N., Bapodra A. Ethno botanical and phytophrmacological potential of Abrus precatorius L.: a review. Asian Pacific Journal of Tropical Biomedicine. 2014;4(Suppl 1):S27–S34. doi: 10.12980/APJTB.4.2014C1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong Y. H., Park J. S., Kim D. H., Kang J. L., Kim H. S. Anti-inflammatory mechanism of lonchocarpine in LPS- or poly(I:C)-induced neuroinflammation. Pharmacological Research. 2017;119:431–442. doi: 10.1016/j.phrs.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Jiang Z. Z., Chen M., et al. Protective effect of total flavonoid C-glycosides from Abrus mollis extract on lipopolysaccharide-induced lipotoxicity in mice. Chinese Journal of Natural Medicines. 2014;12(6):461–468. doi: 10.1016/S1875-5364(14)60072-8. [DOI] [PubMed] [Google Scholar]
- 31.Kuo S. C., Chen S. C., Chen L. H., Wu J. B., Wang J. P., Teng C. M. Potent antiplatelet, anti-inflammatory and antiallergic isoflavanquinones from the roots of Abrus precatorius. Planta Medica. 1995;61(4):307–312. doi: 10.1055/s-2006-958089. [DOI] [PubMed] [Google Scholar]
- 32.Kupina S., Fields C., Roman M. C., Brunelle S. L. Determination of total phenolic content using the Folin-C assay: single-laboratory validation, first action 2017.13. Journal of AOAC International. 2018;101(5):1466–1472. doi: 10.5740/jaoacint.18-0031. [DOI] [PubMed] [Google Scholar]
- 33.Matić P., Sabljić M., Jakobek L. Validation of spectrophotometric methods for the determination of total polyphenol and total flavonoid content. Journal of AOAC International. 2017;100(6):1795–1803. doi: 10.5740/jaoacint.17-0066. [DOI] [PubMed] [Google Scholar]
- 34.Franco N. H., Sandøe P., Olsson I. A. S. Researchers’ attitudes to the 3Rs-An upturned hierarchy? PLoS One. 2018;13(8, article e0200895) doi: 10.1371/journal.pone.0200895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umamahesh B., Veeresham C. Antihyperglycemic and insulin secretagogue activities of Abrus precatorius leaf extract. Pharmacognosy research. 2016;8(4):303–308. doi: 10.4103/0974-8490.188881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altunkaynak B. Z., Altunkaynak E., Unal D., Unal B. A novel application for the cavalieri principle: a stereological and methodological study. The Eurasian journal of medicine. 2009;41(2):99–101. [PMC free article] [PubMed] [Google Scholar]
- 37.Zółtowska K. Alpha-amylase release by pancreatic acini isolated from guinea pigs infected with invasive eggs of Ascaris suum. Wiadomości Parazytologiczne. 1993;39(3):233–239. [PubMed] [Google Scholar]
- 38.Valentina P., Ilango K., Chander S., Murugesan S. Design, synthesis and α-amylase inhibitory activity of novel chromone derivatives. Bioorganic Chemistry. 2017;74:158–165. doi: 10.1016/j.bioorg.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Thilagam E., Parimaladevi B., Kumarappan C., Mandal S. C. α-Glucosidase and α-amylase inhibitory activity of Senna surattensis. Journal of Acupuncture and Meridian Studies. 2013;6(1):24–30. doi: 10.1016/j.jams.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Peng X., Zhang G., Liao Y., Gong D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chemistry. 2016;190:207–215. doi: 10.1016/j.foodchem.2015.05.088. [DOI] [PubMed] [Google Scholar]
- 41.Kazeem M. I., Adamson J. O., Ogunwande I. A. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed Research International. 2013;2013:6. doi: 10.1155/2013/527570.527570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okawa M., Kinjo J., Nohara T., Ono M. DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of flavonoids obtained from some medicinal plants. Biological & Pharmaceutical Bulletin. 2001;24(10):1202–1205. doi: 10.1248/bpb.24.1202. [DOI] [PubMed] [Google Scholar]
- 43.Bichler J., Cavin C., Simic T., et al. Coffee consumption protects human lymphocytes against oxidative and 3-amino-1-methyl-5H-pyrido[4,3-b]indole acetate (Trp-P-2) induced DNA-damage: results of an experimental study with human volunteers. Food and Chemical Toxicology. 2007;45(8):1428–1436. doi: 10.1016/j.fct.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 44.de Souza M. W., Moya H. D. The evaluation of reduction of Fe(III) in 3-hydroxy-4-nitroso-2,7-naphthalene disulphonic medium as an alternative ferric reducing activity power assay. Phytochemical Analysis. 2015;26(2):119–126. doi: 10.1002/pca.2543. [DOI] [PubMed] [Google Scholar]
- 45.Kamvissi V., Salerno A., Bornstein S. R., Mingrone G., Rubino F. Incretins or anti-incretins? A new model for the "entero-pancreatic axis". Hormone and Metabolic Research. 2015;47(1):84–87. doi: 10.1055/s-0034-1394374. [DOI] [PubMed] [Google Scholar]
- 46.Seino Y., Fukushima M., Yabe D. GIP and GLP-1, the two incretin hormones: similarities and differences. Journal of diabetes investigation. 2010;1(1-2):8–23. doi: 10.1111/j.2040-1124.2010.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yonemoto R., Shimada M., Gunawan-Puteri M. D., Kato E., Kawabata J. α-Amylase inhibitory triterpene from Abrus precatorius leaves. Journal of Agricultural and Food Chemistry. 2014;62(33):8411–8414. doi: 10.1021/jf502667z. [DOI] [PubMed] [Google Scholar]
- 48.Yeo J., Shahidi F. Critical re-evaluation of DPPH assay: presence of pigments affects the results. Journal of agricultural and food chemistry. 2019;67(26):7526–7529. doi: 10.1021/acs.jafc.9b02462. [DOI] [PubMed] [Google Scholar]
- 49.Ali H. M., Almagribi W., Al-Rashidi M. N. Antiradical and reductant activities of anthocyanidins and anthocyanins, structure-activity relationship and synthesis. Food Chemistry. 2016;194:1275–1282. doi: 10.1016/j.foodchem.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Valko M., Leibfritz D., Moncol J., Cronin M. T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Kang N. J., Shin S. H., Lee H. J., Lee K. W. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacology & Therapeutics. 2011;130(3):310–324. doi: 10.1016/j.pharmthera.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Mladenka P., Zatloukalová L., Filipský T., Hrdina R. Cardiovascular effects of flavonoids are not caused only by direct antioxidant activity. Free Radical Biology & Medicine. 2010;49(6):963–975. doi: 10.1016/j.freeradbiomed.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Kalita D., Holm D. G., LaBarbera D. V., Petrash J. M., Jayanty S. S. Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PloS one. 2018;13(1, article e0191025) doi: 10.1371/journal.pone.0191025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ombra M. N., d'Acierno A., Nazzaro F., et al. Alpha-amylase, α-glucosidase and lipase inhibiting activities of polyphenol-rich extracts from six common bean cultivars of Southern Italy, before and after cooking. International Journal of Food Sciences and Nutrition. 2018;69(7):824–834. doi: 10.1080/09637486.2017.1418845. [DOI] [PubMed] [Google Scholar]
- 55.Figueiredo-González M., Reboredo-Rodríguez P., González-Barreiro C., Carrasco-Pancorbo A., Cancho-Grande B., Simal-Gándara J. The involvement of phenolic-rich extracts from Galician autochthonous extra-virgin olive oils against the α-glucosidase and α-amylase inhibition. Food Research International. 2019;116:447–454. doi: 10.1016/j.foodres.2018.08.060. [DOI] [PubMed] [Google Scholar]
- 56.Trinh B. T. D., Staerk D., Jäger A. K. Screening for potential α-glucosidase and α-amylase inhibitory constituents from selected Vietnamese plants used to treat type 2 diabetes. Journal of Ethnopharmacology. 2016;186:189–195. doi: 10.1016/j.jep.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 57.Semaan D. G., Igoli J. O., Young L., Marrero E., Gray A. I., Rowan E. G. In vitro anti-diabetic activity of flavonoids and pheophytins from Allophylus cominia Sw . on PTP1B, DPPIV, alpha-glucosidase and alpha-amylase enzymes. Journal of Ethnopharmacology. 2017;203:39–46. doi: 10.1016/j.jep.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 58.Kim Y., Keogh J. B., Clifton P. M. Polyphenols and glycemic control. Nutrients. 2016;8(1) doi: 10.3390/nu8010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support the findings of this study are available from the corresponding author upon request.


