Abstract
Diseases or conditions where diaphragm muscle (DIAm) function is impaired, including chronic obstructive pulmonary disease, cachexia, asthma, and aging, are associated with an increased risk of pulmonary symptoms, longer duration of hospitalizations, and increasing requirements for mechanical ventilation. Vitamin D deficiency is associated with proximal muscle weakness that resolves following therapy with vitamin D3. Skeletal muscle expresses the vitamin D receptor (VDR), which responds to the active form of vitamin D, 1,25-dihydroxyvitamin D3 by altering gene expression in target cells. In knockout mice without skeletal muscle VDRs, there is marked atrophy of muscle fibers and a change in skeletal muscle biochemistry. We used a tamoxifen-inducible skeletal muscle Cre recombinase in Vdrfl/fl mice (Vdrfl/fl actin.iCre+) to assess the role of muscle-specific VDR signaling on DIAm-specific force, fatigability, and fiber type-dependent morphology. Vdrfl/fl actin.iCre+ mice treated with vehicle and Vdrfl/fl mice treated with tamoxifen served as controls. Seven days following the final treatment, mice were euthanized, the DIAm was removed, and isometric force and fatigue were assessed in DIAm strips using direct muscle stimulation. The proportion and cross-sectional areas of DIAm fiber types were evaluated by immunolabeling with myosin heavy chain antibodies differentiating type I, IIa and IIx, and/or IIb fibers. We show that in mice with skeletal muscle-specific VDR deletion, maximum specific force and residual force following fatigue are impaired, along with a selective atrophy of type IIx and/or IIb fibers. These results show that the VDR has a significant biological effect on DIAm function independent of systemic effects on mineral metabolism.
NEW & NOTEWORTHY Vitamin D deficiency and vitamin D receptor (VDR) polymorphisms are associated with adverse pulmonary and diaphragm muscle (DIAm)-associated respiratory outcomes. We used a skeletal muscle-specific tamoxifen-inducible VDR knockout to investigate DIAm dysfunction following reduced VDR signaling. Marked DIAm weakness and atrophy of type IIx and/or IIb fibers are present in muscle-specific tamoxifen-induced VDR knockout mice compared with controls. These results show that the VDR has a significant biological effect on DIAm function independent of systemic effects on mineral metabolism.
Keywords: fatigue, muscle fiber type, muscle-specific force, vitamin D, vitamin D receptor
INTRODUCTION
The diaphragm muscle (DIAm) is essential for inspiration and also generates high pressures required for expulsive efforts, such as coughing, sneezing, and defecation (1–3). Importantly, the DIAm comprises of muscle fibers that express different isoforms of myosin heavy chain (MyHC), including MyHCSlow, MyHC2A, MyHC2X, and MyHC2B that distinguish different muscle fiber types, i.e., type I, IIa, IIx, and IIb, respectively (4–7). The diversity of contractile and fatigue properties of DIAm fibers underlies the functional utility of DIAm motor units (1, 8–11). Recruitment of fatigue-resistant DIAm motor units comprising type I and IIa fibers is necessary for ventilatory efforts that require lower pressures but display a high duty cycle (2, 3, 6, 12–14). By contrast, recruitment of higher force, but fatigable DIAm motor units comprising type IIx and/or IIb fibers is necessary to accomplish higher force, expulsive/straining maneuvers that occur infrequently, and with a much lower duty cycle (2, 3, 6, 12–14). In conditions such as chronic obstructive pulmonary disease (COPD) (15–18), cachexia (19, 20), aging (1, 9, 10, 21, 22), and emphysema (23), maximum DIAm-specific force is reduced, concomitant with a selective atrophy of type IIx and/or IIb DIAm fibers (12, 24–26). Thus, ventilatory function of the DIAm is not directly compromised, instead there is an inability to perform airway defense activities, leading to respiratory illness (2, 12, 24).
In children and adults with asthma and COPD, serum deficiency (<10 ng/mL or <25 nM) of the active form of vitamin D [1,25-dihydroxyvitamin D; 1,25(OH)2D] is associated with exacerbated symptoms, increased duration of hospitalizations, increased requirements for mechanical ventilation, and increased morbidity in respiratory diseases (27–32). Previous work has shown that skeletal muscle expresses the vitamin D receptor (VDR), which binds the active form of vitamin D, 1,25(OH)2D to activate gene expression (33–36). Prolonged vitamin D-deficiency is necessary to reduce 1,25(OH)2D levels and there may also be effects on the VDR (37, 38). Ray et al. (39) reported that in a 10-wk-old mice fed a vitamin D-deficient diet for 6 wk, thereby reducing serum 1,25(OH)2D concentrations to ∼7 ng/mL, maximum DIAm-specific force decreased and fatigability increased. They also found that while relative expression of DIAm MyHC isoforms was unchanged, fiber atrophy was observed (39). Unfortunately, fiber-type specific changes in DIAm fiber cross-sectional areas were not assessed.
Altered VDR signaling also appears to play a prominent role in the outcome of respiratory illnesses. Polymorphisms of the skeletal muscle VDR have been associated with COPD and other chronic pulmonary diseases (40). Clinically, proximal (axial) muscle weakness is a key presentation in ∼30% of cases with vitamin D deficiency (41–43). In knockout mice without skeletal muscle VDR, there is atrophy of muscle fibers and a derangement of myogenic maturation, with neonatal MyHC isoform expression persisting into the eighth postnatal week (44). In mice, expression of the VDR peaks by 4 wk old and reduces thereafter (45), similar to the age-associated decline of VDR in humans (46). Our understanding of the fiber type-specific expression of VDR is incomplete, with skeletal muscle VDR knockout mice having abnormalities across fiber types (44). In humans, vitamin D deficiency is associated with selective atrophy of type II fibers (47), although there was no distinction between IIa and IIx and/or IIb fibers in this study.
In the present study, we bred mice expressing a tamoxifen (TAM)-inducible, skeletal muscle-specific Cre-recombinase (actin-iCre) with Vdrfl/fl mice to obtain Vdrfl/flactin-iCre positive (+) mice, which were subsequently treated with tamoxifen (TAM) to delete the VDR in skeletal muscle, including DIAm. We hypothesized that a knockout of DIAm VDR would reduce maximum specific force. We further hypothesized that VDR knockout would induce selective atrophy of type IIx and/or IIb DIAm fibers.
METHODS
Animals
Muscle-specific VDR knockout mice were generated by crossing VDRflox/flox mice (a gift from S. Kato) with actin-iCre+/− mice (Jackson Laboratories) (48). Male mice whose Vdr was flanked with loxP sites at exon 3 (Vdrfl/fl) were crossed with female actin.iCre mice from Jackson Laboratories (Stock No. 02750). To confirm successful transmission of the desired genotype, VDRfl/flactin.cre+, tails from littermates were clipped from 10-day-old mice and sent to Transnetyx (Cordova, TN) for PCR analysis of the wild-type Vdr (forward primer 5′- GTGCAGGGTGCAGGACCTCC-3′; reverse primer 5′- GCTTTATGTGCCTTCTATGCATATCTGAAAGC-3′); floxed Vdr (forward primer 5′- GCATACATTATACGAAGTTATCGGATCTGGG-3′; reverse primer 5′- GCCTTCTATGCATATCTGAAAGCTCTCTCTG-3′); and actin-iCre (forward primer 5′- TTAATCCATATTGGCAGAACGAAAACG-3′; reverse primer 5′-CAGGCTAAGTGCCTTCTCTACA-3′) genes. After weening, mice (n = 14 females, n = 17 males) were fed PicoLab Mouse Diet 20 5058 (LabDiet, St. Louis, MO) containing 3.4 IU/g Vitamin D3 and 0.81% calcium ad libitum and group-housed using a 12-h light/dark cycle. At 7–8 wk old, Vdrfl/fl actin.iCre+ mice were randomly assigned to receive tamoxifen (TAM) to induce expression of the Cre recombinase in skeletal muscles or vehicle to control for the effects of TAM. Vdrfl/fl mice receiving TAM were also used to control for the potential effects of the drug in the absence of Cre recombinase. Within each litter used, mice were assigned randomly to a group upon weaning. Tamoxifen (75 µL, 100 mg/kg body wt carried in 10% ethanol in corn oil) or vehicle (10% ethanol in corn oil) was administered intraperitoneally for 5 days (day 1 to day 5). Mice were euthanized and terminal experiments were performed 7 days after the last tamoxifen or vehicle injection. This timing of tamoxifen treatment has previously been employed to effectively induce selective Cre recombinase mutations in skeletal muscle (49, 50). All animal procedures were approved by the Mayo Clinic Institute Animal Care and Use Committee (IACUC No. A00004516-19) and complied with National Institutes of Health (NIH) and American Physiological Society guidelines.
Diaphragm Muscle Strip Preparation
Following euthanasia, blood was collected from mice by cardiac puncture and the DIAm was excised and placed in a tray of Rees-Simpson solution (containing, in mM: 135 Na+, 5 K+, 2 Ca2+, 1 Mg2+, 120 Cl−, and 25 HCO3−) bubbled with carbogen gas (95% O2-5% CO2) at room temperature. While in solution, two portions of the costal region of the right side of the DIAm were flash-frozen in liquid nitrogen for biochemical analyses. From the left costal region of the DIAm, two ∼2-mm wide strips were cut, stretched to 150% of resting length, which approximates the optimal length for isometric force generation by the DIAm (51), pinned on cork, and rapidly frozen in melting isopentane in a manner identical to past reports (1, 7, 10, 52–54). One of these DIAm strips was used for fiber type and cross-sectional area analyses, whereas the second DIAm strip was used for measurements of muscle-specific force and fatigue. In a subset of animals, the entire right DIAm was dissected and weighed.
Measurement of Mouse Serum 1,25(OH)2D
We quantified the concentration of 1,25(OH)2D in serum using methods identical to past reports (55). Briefly, a total of 50-μL mouse serum was added to 450 μL 1% (wt/vol) BSA solution in phosphate buffer (0.01 mM, pH 7.4). A total of 0.2 ng deuterated internal standard 1,25(OH)2D (Isosciences, 2 ng/mL solution) was added to the sample. After 15 min at 25°C, 500 μL of 0.2 N HCl was added. The sample was applied to a C18-OH solid phase extraction cartridge (Varian Instruments). The cartridge was washed once with 5 mL 70:30 methanol/water (vol/vol), and once with 5 mL 90:10 hexane/methylene chloride (vol/vol). Vitamin D metabolites were eluted with 5 mL 90:10 hexane/isopropyl alcohol (vol/vol). Eluants were dried under stream of nitrogen. A total of 250 μL 1,25(OH)2D antibody-bound beads (Immunodiagnostic Systems) were added to the tubes and allowed to bind 1α,25(OH)2D3 for 60 min at 25°C. The beads were transferred to a 96-well plate, washed once with buffer, and twice with deionized water. 1,25(OH)2D bound to the antibody beads was eluted with ethanol, and eluants were dried and derivatized with 4-phenyl-1,2,4,-triazoline-3,5-dione (PTAD) (Sigma; 250 μL of 200 μg/mL in acetonitrile). Derivatized vitamin D metabolites were separated by liquid chromatography at a flow rate of 0.4 mL/min on an XDB-C8, 2.1 × 50 mm column (Agilent) for 7.25 min with a methanol-H2O-ammonium formate (1 mM) gradient (60%–95%) and quantified using mass spectrometry.
RNA Isolation and Quantitative PCR of Skeletal Muscle VDR
One portion of the flash-frozen DIAm was crushed in a chilled mortar and pestle and homogenized in QIAZOL lysis reagent using a needle and syringe. QIAGEN miRNEASY Mini Kit was used to isolate total RNA from tissue homogenates and quantified by using a DU 7400 spectrophotometer (Beckman) to obtain absorbances at 260 nm and 280 nm. Synthesis of complementary DNA strands was achieved using a SuperScript III reverse transcriptase (Thermo Fisher), random primers, equal amounts of total RNA (100 ng). Primers for VDR (forward 5′-GCTTCCACRTCAACGCTATGA-3′; reverse 5′- ATGCGGCAATCTCCATTGAA-3′) (0.5 µM) were suspended with RT reactions in a SYBR Green PCR Master Mix (Applied Biosystems) for subsequent qPCR. Temperature cycles were regulated using an ABI ViiA-7 Real-Time PCR System (Thermo Fisher). Relative RNA transcripts were quantified as the inverse value 2Ct (Ct = the number of PCR cycles required to obtain VDR mRNA fluorescent signal cross threshold for each reverse-transcriptase reaction containing equal amounts of RNA).
Total Protein and Western Blot Analysis
A second portion of the flash-frozen DIAm was lysed and homogenized in lysis buffer containing Tris HCl 10% (vol/vol), glycerol 20% (vol/vol), sodium dodecyl sulfate 8% (wt/vol), dithiothreitol 2.3% (wt/vol), complete protease inhibitor cocktail tablet (1 tablet/10 mL volume), and EDTA 0.10% (vol/vol). Tissue homogenization was achieved by pulse sonication on ice using an Ultrasonic Processor (Thermo Scientific). Homogenates were collected for Western blot analysis. Protein was separated by SDS-PAGE on a 10% polyacrylamide gel, transferred on to a polyvinylidene difluoride membrane, blocked with Roche blocking reagent (Cat. No. 11500694001) and incubated with a monoclonal rat anti-VDR antibody (Thermo Scientific No.9A7) at 4°C overnight. The next day, membrane was incubated with a goat anti-rat conjugated with horseradish-peroxidase (Invitrogen A10549) and the blot was developed using a Roche Chemiluminescence kit (Cat. No. 11500694001). Prior to blocking, membrane was subjected to a Ponceau S stain to assess total protein transferred. VDR to total protein ratios were obtained using ImageJ software to calculate the density of the 49-kDa band and the total protein in Ponceau S stain. Data are reported as VDR/total protein in vehicle and TAM-treated mice.
Muscle-Specific Force and Fatigue
Methods for assessing DIAm isometric force and fatigue have been previously described (1, 5, 10, 23, 53, 56–59). Briefly, the DIAm strip was suspended in a tissue bath containing a gassed Rees-Simpson solution at 26°C with the costal margin clamped and the central tendon tied with silk and attached to a force transducer (6350, Cambridge Technology, MA). Optimal DIAm length (Lo) and supramaximal stimulus settings were established in a manner identical to those described in past rodent studies (11, 21, 52). Electrical field stimulation was achieved via platinum plate electrodes placed on either side of the muscle, with stimulation current provided using a stimulator (701C, Aurora Scientific, ON, Canada). Supramaximal (∼150 mA) stimulus pulses (0.05 ms duration) were delivered at 5, 10, 20, 30, 40, 50, 75, and 100 Hz in 1 s trains (11, 21, 52). Output of the force transducer data was digitized (1 kHz sampling rate) and recorded in LabChart software (ADInstuments, Dunedin, New Zealand). Specific force of the DIAm was calculated by normalizing force to the estimated cross-sectional area of the DIAm strip (muscle cross-sectional area = muscle strip weight (g)/(Lo (cm) × 1.056 g/cm3) and expressed as N/cm2. In past studies using adult rats and mice, no differences were observed between males and females in isometric force generation of the DIAm, so these groups were combined for analysis (1, 8–11).
Fatigue was assessed by repetitive stimulation of the DIAm at 40 Hz in 333 ms trains repeated each second [33% duty cycle, approximating the duty cycle of ventilation (11, 52, 60, 61)] for 120-s period. Fatigue was characterized by the force decline during the 120-s period of stimulation as well as the absolute residual force at the end of stimulation (1, 39, 56). A fatigue index was calculated as the ratio of the residual force after 120 s to the initial force.
Diaphragm Muscle Fiber Types
For DIAm fiber type and cross-sectional area analyses, 10 μm transverse sections were cut using a cryostat (Reichert Jung Frigocut 2800). Sections were fixed in acetone for 10 min before commencing fiber type analysis based on immunoreactivity to specific antibodies for different MyHC isoforms as previously described (7, 10, 11, 21, 22, 52, 62). Briefly, serial sections were blocked for 30 min in 10% goat serum and incubated overnight at 4°C in primary antibodies for the following MyHC isoforms: MyHCSlow (BA-F8, 1:3 dilution; Developmental Studies Hybridoma Bank, Iowa City, IA), MyHC2A (SC-71, 1:3 dilution; Developmental Studies Hybridoma Bank). Fluorescently conjugated secondary antibodies were then applied at a 1:200 dilution, using Cy3 to visualize MyHCSlow or MyHC2A in serial sections. The DIAm sections were then imaged using a ×20 oil-immersion objective (NA 1.0) on an Olympus FV2000 laser confocal microscope. Images were captured in a 1,024 × 1,024 pixel array, with similar acquisition parameters across preparations. We have previously validated the antibodies for MyHCSlow, MyHC2A, and MyHC2X as well as an antibody that immune reacts for all MyHC isoforms except MyHC2X (14, 63,64). Immunoreactivity for MyHCSlow and MyHC2A can be readily used to classify type I and IIa fibers in mice (5, 8). In alternate sections, we verified that the fibers showing no immunoreactivity for MyHCSlow and MyHC2A displayed immunoreactivity for MyHC2X. Immunoreactivity for the all-but-2× antibody was consistent with immunoreactivity patterns of the other specific antibodies and suggested trace amounts of the MyHC2B isoform in a few diaphragm fibers. This is consistent with single fiber studies in which we determined MyHC isoform expression by electrophoretic separation and Western analysis (14, 63, 64). Accordingly, we adopted the classification of type IIx and/or IIb diaphragm muscle fibers, in addition to the type I and IIa. For fiber-type proportions, we sampled three DIAm muscle fields per mouse in a systematically randomized fashion, ensuring no overlapping or assessment close to the thoracic or abdominal surface of the DIAm where edge effects in imaging may be apparent. The cross-sectional areas of DIAm fibers were determined using morphometric tools in ImageJ, where we sampled a minimum 15 muscle fibers of each type (type I, type IIa and/or type IIx, and/or IIb) from these fields, consistent with previous reports (1, 7, 8, 10, 11, 21, 52–54, 58).
Statistical Analysis
The expected effect size (Cohen’s d) was calculated with an a priori biologically relevant difference of 20% and equal variance, with the required sample size estimated using d = 1.3, α = 0.05, and β = 0.8. Based on previous data (1, 5), the number of animals required for adequate power when assessing force and fatigue data was n = 10 per group. Data points that were greater than twice the standard deviation from the mean were excluded from analysis. All data were assessed for normality with Shapiro–Wilk tests. Statistical analysis was done using Prism 8 (Graphpad, CA), with Student’s unpaired t tests, one-way ANOVA, and Tukey’s post hoc tests used to compare experimental groups. Two-way ANOVA with Bonferroni post hoc tests were used to compare experimental groups and factor (e.g., stimulation frequency or time). Significance was set as P < 0.05, all data are presented as means ± 95% confidence intervals (CI), unless otherwise stated. Within each experimental group, male and female values for primary outcome measures were compared using Student’s unpaired t tests (Table 1).
Table 1.
Properties of male and female Vdrfl/fl TAM, Vdrfl/fl actin iCre+ VEH and Vdrfl/fl actin iCre+ TAM mice
| Parameter | Vdrfl/flTAM (n) | Vdrfl/fl Actin iCre VEH (n) | Vdrfl/fl Actin iCre TAM (n) |
|---|---|---|---|
| DIAm vitamin D mRNA | na | ♀ (3): 4.2 × 10−9; ♂ (3): 9.2 × 10−10; P = 0.12 | ♀ (3): 4.1 × 10−10; ♂ (3): 4.1 × 10−10; P = 0.53 |
| Serum 1,25 vitamin D, pg/mL | ♀ (3): 22.3 ± 2.8; ♂ (5): 33.2 ± 9.4; P = 0.22 | ♀ (5): 24.5 ± 1.5; ♂ (5):35.3 ± 4.9; P = 0.13 | ♀ (5): 23.6 ± 2.9; ♂ (5): 52.8 ± 14.0; P = 0.08 |
| Max. DIAm specific force, N/cm2 | ♀ (4): 22.1 ± 2.1; ♂ (6): 24.1 ± 2.2; P = 0.53 | ♀ (5): 20.2 ± 1.3; ♂ (5): 23.4 ± 2.0; P = 0.27 | ♀ (5): 15.0 ± 3.1; ♂ (6): 15.2 ± 1.7; P = 0.95 |
| Residual force following fatigue, N/cm2 | ♀ (4): 7.7 ± 0.3; ♂ (6): 7.3 ± 0.8; P = 0.69 | ♀ (5): 6.3 ± 0.3; ♂ (5): 8.1 ± 0.9; P = 0.14 | ♀ (5): 5.1 ± 0.9; ♂ (6): 5.9 ± 0.7; P = 0.51 |
| Fatigue index | ♀ (4): 0.37 ± 0.02; ♂ (6): 0.36 ± 0.3; P = 0.74 | ♀ (5): 0.37 ± 0.01; ♂ (5): 0.40 ± 0.02; P = 0.21 | ♀ (5): 0.45 ± 0.02; ♂ (6): 0.46 ± 0.03; P = 0.79 |
| DIAm type I fiber cross-sectional area, µm2 | ♀ (4): 291 ± 25; ♂ (4): 361 ± 73; P = 0.39 | ♀ (5): 287 ± 31; ♂ (5): 334 ± 73; P = 0.57 | ♀ (3): 271 ± 36; ♂ (3): 293 ± 44; P = 0.72 |
| DIAm type IIa fiber cross-sectional area, µm2 | ♀ (4): 474 ± 73; ♂ (4): 444 ± 91; P = 0.81 | ♀ (4): 482 ± 92; ♂ (4): 431 ± 43; P = 0.64 | ♀ (3): 326 ± 75; ♂ (3): 284 ± 16; P = 0.61 |
| DIAm type IIx/b fiber cross-sectional area, µm2 | ♀ (4): 1,081 ± 128; ♂ (4): 1,220 ± 219; P = 0.60 | ♀ (4): 1,129 ± 100; ♂ (4): 1,163 ± 177; P = 0.87 | ♀ (3): 645 ± 84; ♂ (3): 691 ± 87; P = 0.73 |
All comparisons by Student’s unpaired t test, n represents no. of animals. All data presented as means ± SE. DIAm, diaphragm muscle; TAM, tamoxifen; VDR, vitamin D receptor.
RESULTS
Reduction of DIAm VDR in Tamoxifen-Treated Vdrfl/fl Actin iCre+ Mice
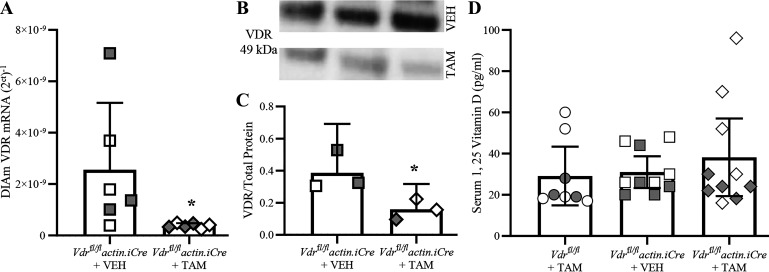
In the DIAm of TAM-treated Vdrfl/fl actin iCre+ mice, the expression of Vdr mRNA and VDR protein was reduced compared with vehicle-treated Vdrfl/fl actin iCre+ mice that received vehicle. There was an ∼87% reduction in Vdr mRNA assessed by mRNA qPCR in Vdrfl/fl actin iCre+ mice treated with TAM compared with Vdrfl/fl actin iCre+ mice treated with vehicle (P = 0.004, Mann–Whitney U test; Fig. 1A). Within each group assessed, we did not observe an effect of sex on the relative expression of VDR mRNA (Table 1). The relative abundance of total VDR protein within DIAm strips was also assessed (Fig. 1B). The ratio of VDR to total protein in DIAm was reduced by ∼59% in Vdrfl/fl actin iCre+ mice treated with TAM, compared with Vdrfl/fl actin iCre+ treated with vehicle mice (P = 0.047, Student’s unpaired t test; Fig. 1C). Taken together, our VDR message and protein analyses show effective knockdown of Vdr/VDR expression in DIAm.
Figure 1.
Vitamin D receptor (VDR) knockout in tamoxifen (TAM)-treated Vdrfl/fl actin iCre+ mice. A: scatterplot shows relative VDR mRNA transcripts plotted as (2Ct)−1, with Vdrfl/fl actin iCre+ TAM mice (diamonds; n = 6) having an ∼78% reduction in VDR mRNA relative to vehicle-treated controls (squares; n = 6). Unpaired Student’s t test, with *P < 0.05. B: Western blot analysis of the VDR (49 kDa) in homogenized diaphragm muscle (DIAm) tissue from TAM and vehicle-treated Vdrfl/fl actin iCre+ mice. C: scatterplot showing VDR to total protein is reduced by 59% in Vdrfl/fl actin iCre+ TAM (n = 3) compared with Vdrfl/fl actin iCre+ VEH mice (n = 3). Unpaired Student’s t test, with *P < 0.05. D: scatterplot shows that the serum concentration (pg/mL) of 1,25-dihydroxyvitamin D (1,25 vitamin D) is unchanged between Vdrfl/flTAM (circles; n = 8) and Vdrfl/fl actin iCre+ VEH (squares; n = 10) control mice and Vdrfl/fl actin iCre+ TAM mice (diamonds; n = 10; P = 0.59, One-way ANOVA). All data presented as means ± 95% confidence intervals, n represents no. of animals. Gray symbols denote female, open symbols denote males.
We did not observe any differences in serum concentration of 1,25-dihydroxyvitamin D between Vdrfl/fl mice treated with TAM (29.2 ± 14.2 pg/mL), Vdrfl/fl actin iCre+ mice treated with vehicle (31.0 ± 8.7 pg/mL), and Vdrfl/fl actin iCre+ mice treated with TAM (38.2 ± 18.8 pg/mL; F2,25 = 0.6, P = 0.57, one-way ANOVA; Fig. 1D). Within each group assessed, we did not observe an effect of sex on the serum level of 1,25-dihydroxyvitamin D (Table 1).
Skeletal Muscle VDR Knockout is Associated with Impaired Weight Gain in Adult Mice
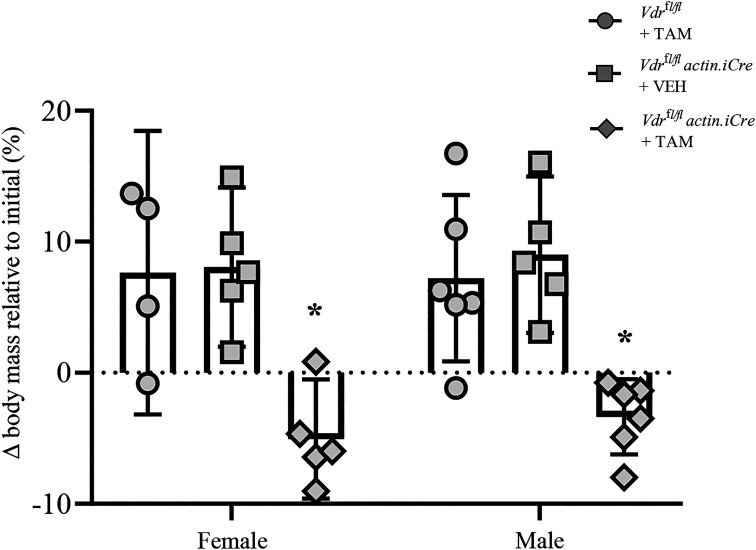
Immediately before dosing with TAM, there was no difference in body mass between Vdrfl/fl mice, Vdrfl/fl actin iCre+ mice treated with tamoxifen, and Vdrfl/fl actin iCre+ mice treated with vehicle (F2,25 = 1.3, P = 0.29; two-way ANOVA). However, there was a difference in body mass when stratified by sex, with males (28.5 ± 3.9 g) ∼28% heavier than females (20.4 ± 3.8 g; F2,25 = 9.7, P = 0.005; two-way ANOVA), with no interaction with genotype. Following the administration of TAM or vehicle to Vdrfl/fl actin iCre+ mice, there was a significant effect of genotype/treatment on weight gain over the 12 days until terminal experiments (F2,25 = 21.8, P < 0.0001; two-way ANOVA; Fig. 2). Post hoc Bonferroni tests revealed that compared with Vdrfl/fl actin iCre+ mice treated with vehicle or Vdrfl/fl mice treated with TAM, female Vdrfl/fl actin iCre+ mice treated with TAM gained ∼12% less weight (P < 0.002 compared with either female control group; Fig. 2) and male weight gain was reduced by ∼8% (P < 0.002 compared with either male control group; Fig. 2).
Figure 2.
Reduced weight gain in male and female skeletal muscle vitamin D receptor (VDR) knockout mice. Scatterplot shows that following the administration of TAM or vehicle to Vdrfl/fl actin iCre+ mice, there was a significant effect of genotype/treatment on weight gain over the 12 days until terminal experiments (P < 0.0001; two-way ANOVA). Post hoc Bonferroni tests revealed that compared with Vdrfl/fl mice treated with tamoxifen (TAM) (circles) or Vdrfl/fl actin iCre+ mice treated with vehicle (squares), female Vdrfl/fl actin iCre+ mice treated with TAM (diamonds) gained ∼12% less weight (*P < 0.002 compared with either female control group) and male weight gain was reduced by ∼8% (*P < 0.002 compared with either male control group). All data presented as means ± 95% confidence intervals. Each symbol represents one animal (n).
We observed no difference in right hemi-diaphragm mass between Vdrfl/fl mice (0.025 ± 0.012 g, n = 5), Vdrfl/fl actin iCre+ mice treated with tamoxifen (0.027 ± 0.004 g, n = 5), and Vdrfl/fl actin iCre+ mice treated with vehicle (0.025 ± 0.003 g, n = 5; F2,12 = 0.2, P = 0.85; one-way ANOVA).
Skeletal Muscle VDR Knockdown is Associated with Reduced DIAm Isometric Force Generation
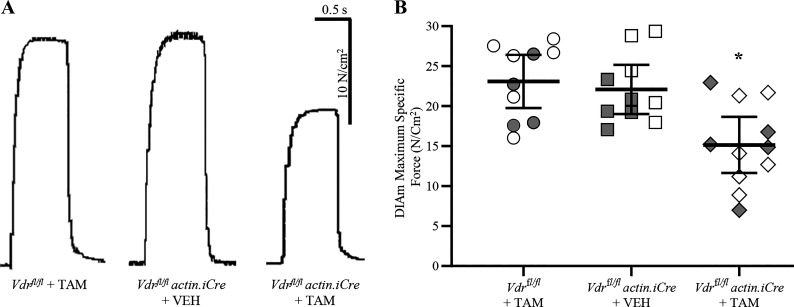
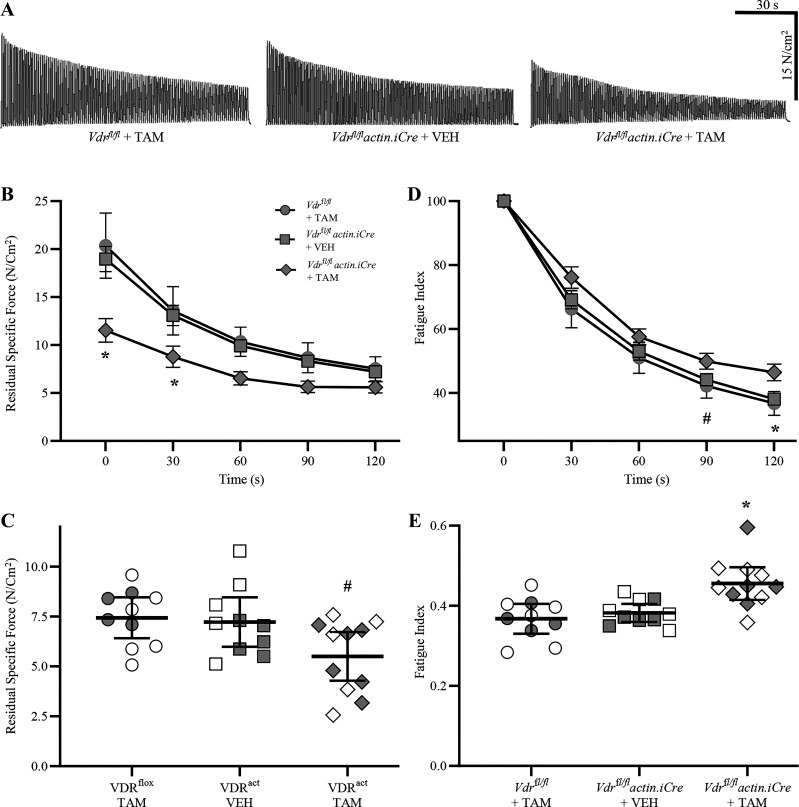
Isometric force generation of DIAm was assessed in Vdrfl/fl actin iCre+ mice treated with TAM, Vdrfl/fl actin iCre+ mice treated with vehicle, and Vdrfl/fl mice treated with TAM (Fig. 3). Compared with the Vdrfl/fl actin iCre+ mice treated with vehicle (5.1 ± 0.9 N/cm2) and Vdrfl/f mice treated with TAM (5.5 ± 1.3 N/cm2), there was an ∼40% reduction in the DIAm twitch force of Vdrfl/flactin iCre+ mice treated with TAM (3.1 ± 0.7 N/cm2; F2,28 = 7.0, P = 0.004, one-way ANOVA, Tukey’s post hoc tests P < 0.02 in both cases). We observed no difference in isometric forced generation of DIAm between the control groups, Vdrfl/fl actin iCre+ mice treated with vehicle, and Vdrfl/fl mice treated with TAM (P = 0.83).
Figure 3.
Reduced maximum diaphragm muscle (DIAm)-specific force in skeletal muscle vitamin D receptor (VDR) knockout mice. A: maximum isometric DIAm-specific force during tetanic contractions of Vdrfl/fl tamoxifen (TAM) and Vdrfl/fl actin iCre+ VEH control mice and Vdrfl/fl actin iCre+ TAM mice lacking VDR. B: there was an ∼33% reduction in the maximum DIAm tetanic force of Vdrfl/fl actin iCre+ TAM mice (diamond, n = 11) compared with Vdrfl/fl TAM (circle; n = 10) and Vdrfl/fl actin iCre+ VEH (square, n = 10) controls (P = 0.003, one-way ANOVA, Tukey’s post hoc tests, *P < 0.012 in both comparisons). All data presented as means ± 95% confidence intervals, n represents no. of animals. Gray symbols denote female, open symbols denote males.
Compared with the Vdrfl/fl actin iCre+ mice treated with vehicle (22.1 ± 4.1 N/cm2) and Vdrfl/fl mice treated with TAM (23.1 ± 3.3 N/cm2), there was an ∼33% reduction in the maximum DIAm tetanic force of Vdrfl/fl actin iCre+ mice treated with TAM (15.1 ± 3.6 N/cm2; F2,28 = 7.4, P = 0.003, one-way ANOVA, Tukey’s post hoc tests P < 0.012 in both cases; Fig. 3B). We observed no difference between the control groups, Vdrfl/fl actin iCre+ mice treated with vehicle, and Vdrfl/fl mice treated with TAM (P = 0.89). Within each group assessed, we did not observe an effect of sex on the DIAm maximum specific force (Table 1).
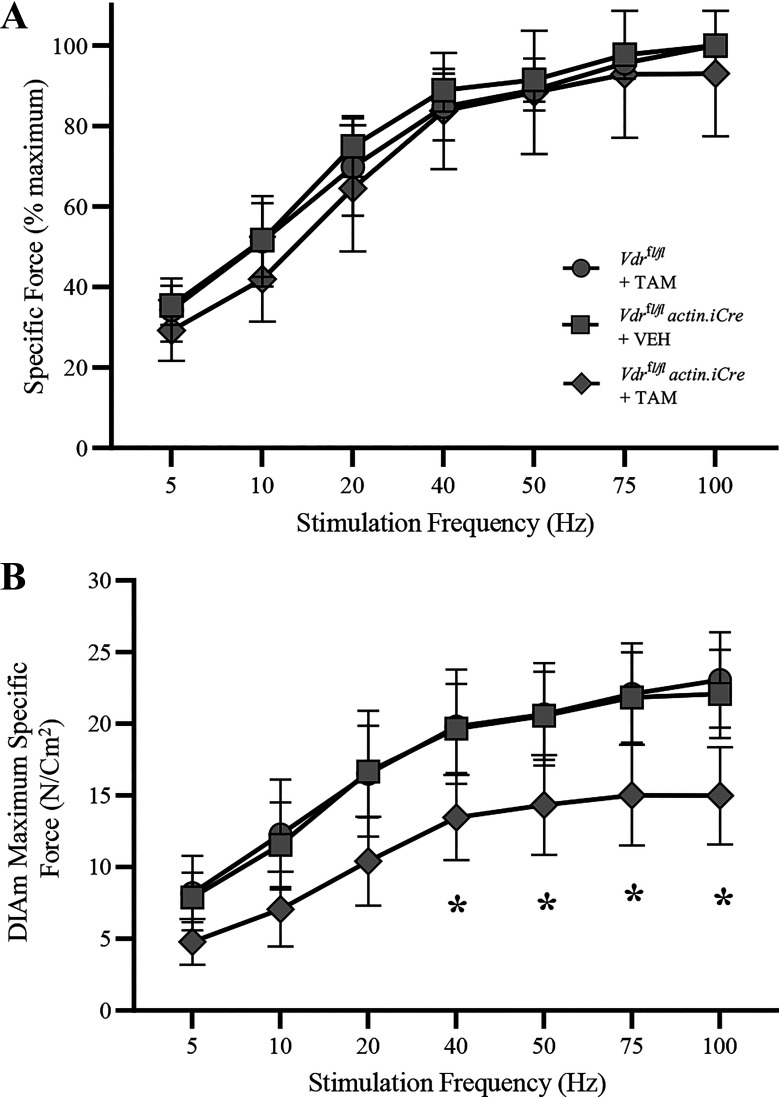
As expected, isometric tetanic force of the DIAm was dependent on stimulation frequency (F6,168 = 280.3, P < 0.0001, two-way ANOVA) in all animals, with maximum specific force occurring between ∼75–100 Hz stimulation in all mice (Fig. 4A). In the DIAm of Vdrfl/fl actin iCre+ mice treated with TAM, isometric specific force (N/cm2) was reduced compared with the Vdrfl/fl actin iCre+ mice treated with vehicle and Vdrfl/fl mice treated with TAM (F2,28 = 6.7, P < 0.004, two-way ANOVA), but the reduction was dependent on stimulation frequency (F6,168 = 241.4, P < 0.0001, two-way ANOVA; Fig. 4B). Bonferroni post hoc tests showed that in Vdrfl/fl actin iCre+ mice treated with TAM DIAm-specific force was reduced by ∼30% between 40 Hz and 100 Hz (P < 0.05 all relevant comparisons), compared with Vdrfl/fl actin iCre+ mice treated with vehicle and Vdrfl/fl mice treated with TAM (Fig. 4B). Taken together, these data on isometric force show a robust ∼33% reduction in maximum tetanic DIAm force in mice with skeletal muscle-specific VDR knockout.
Figure 4.
Altered diaphragm muscle (DIAm) force-stimulation frequency relationship in skeletal muscle vitamin D receptor (VDR) knockout mice. A: force frequency curves (% of max) illustrating isometric tetanic force increases with stimulation frequency in Vdrfl/fl tamoxifen (TAM) (circles, n = 10) and Vdrfl/fl actin iCre+ VEH (squares, n = 10) control mice and Vdrfl/fl actin iCre+ TAM (diamonds, n = 11) mice lacking VDR. B: in the DIAm of Vdrfl/fl actin iCre+ TAM mice, isometric specific force (N/cm2) was reduced compared with the Vdrfl/flTAM and Vdrfl/fl actin iCre+ VEH control mice (P < 0.0001) with stimulation frequency at or above 40 Hz (P < 0.05 all relevant comparisons). For all statistical comparisons, two-way ANOVA with Bonferroni post hoc tests was performed, *P < 0.05. All data presented as means ± 95% confidence intervals, n represents no. of animals. Gray symbols denote female, open symbols denote males.
Skeletal Muscle VDR Knockout is Associated with Altered DIAm Fatigue Properties
Fatigability of the DIAm was assessed both by the progressive decline in specific force during 120 s of repetitive stimulation as well as the residual force after the 120-s period (Fig. 5A). During the 120-s period of repeated stimulation, residual specific force declined with time (F2,62 = 217.4, P < 0.0001, two-way ANOVA) and genotype/treatment (F2,28 = 9.4, P < 0.001, two-way ANOVA; Fig. 5B). Bonferroni post hoc tests revealed that at 0 s and 30 s, residual specific force of Vdrfl/fl actin iCre+ mice treated with TAM was significantly lower than that of Vdrfl/fl actin iCre+ mice treated with vehicle and Vdrfl/fl mice treated with TAM (P < 0.05 in both cases) (Fig. 5B). The residual specific force following 120 s of contractions of Vdrfl/fl actin iCre+ mice treated with TAM (5.6 ± 1.2 N/cm2) was reduced compared with Vdrfl/fl mice treated with TAM (7.3 ± 1.3 N/cm2, P = 0.035) controls (F2,28 = 3.8, P = 0.04, one-way ANOVA; Fig. 5C), Tukey’s post hoc testing did not indicate differences between Vdrfl/fl actin iCre+ mice treated with vehicle and Vdrfl/fl mice treated with TAM (7.2 ± 1.2 N/cm2, P = 0.06). We observed no difference in the residual specific force between the Vdrfl/fl actin iCre+ mice treated with vehicle and Vdrfl/fl mice treated with TAM (P = 0.96, Tukey’s post hoc test; Fig. 5C). Within each group assessed, we did not observe an effect of sex on the residual DIAm-specific force (Table 1).
Figure 5.
Altered diaphragm muscle (DIAm) fatigue properties in skeletal muscle vitamin D receptor (VDR) knockout mice. A: the fatigability and residual specific force following 120 s of fatiguing contractions were assessed in all experimental groups. B: during 120 s of fatiguing contractions, DIAm specific force was affected by time (P < 0.0001) and genotype/treatment (P < 0.001), with DIAm-specific force of Vdrfl/fl actin iCre+ tamoxifen (TAM) mice significantly lower than that of Vdrfl/fl TAM and Vdrfl/fl actin iCre+ VEH controls (P < 0.05 in both cases). C: the residual specific force following 120 s of contractions of Vdrfl/fl actin iCre+ TAM mice was altered compared with control groups (P = 0.04), with post hoc tests showing an ∼20% reduction compared with Vdrfl/flTAM mice (P = 0.035), but not Vdrfl/fl actin iCre+ VEH mice (P = 0.06). D: the fatigue index during 120 s of fatiguing contraction was affected by time (P < 0.0001) and genotype/treatment (P = 0.04), with the DIAm fatigue index of Vdrfl/fl actin iCre+ TAM (diamonds, n = 11) mice significantly greater than that of Vdrfl/flTAM (circles, n = 10) at 90 and 120 s (P < 0.05) and Vdrfl/fl actin iCre+ VEH (squares, n = 10) control mice (P < 0.05) at 120 s (P < 0.05). E: the final DIAm fatigue index following 120 s of contractions of Vdrfl/fl actin iCre+ TAM mice increased by ∼19% compared with Vdrfl/fl TAM and Vdrfl/fl actin iCre+ VEH control mice (P = 0.001). For statistical comparisons in (B and D), two-way ANOVA with Bonferroni post hoc tests was performed. For statistical comparisons in (C and E), one-way ANOVA with Tukey’s post hoc tests was performed. *P < 0.005 comparing all relevant groups, #P < 0.05 between Vdrfl/flTAM and Vdrfl/fl actin iCre+ TAM groups. All data presented as means ± 95% confidence intervals, n represents no. of animals. Gray symbols denote female, open symbols denote males.
The fatigue index, reflecting this progressive decline in force across time was affected by genotype/treatment (F2,28 = 3.6, P = 0.04, two-way ANOVA; Fig. 5D). Bonferroni post hoc test revealed that at 120 s, the DIAm fatigue index of Vdrfl/fl actin iCre+ mice treated with TAM was significantly greater than that of Vdrfl/fl actin iCre+ mice treated with vehicle and Vdrfl/fl mice treated with TAM (P < 0.05) control groups (Fig. 5D). However, this was due primarily to the disproportionate reduction in initial specific force. The final DIAm fatigue index following 120 s of contractions of Vdrfl/fl actin iCre+ mice treated with TAM (0.46 ± 0.04) was increased by ∼19% compared with Vdrfl/fl actin iCre+ mice treated with vehicle (0.38 ± 0.02) and Vdrfl/fl mice treated with TAM (0.37 ± 0.03, P = 0.006) (F2,28 = 9.4, P = 0.001, one-way ANOVA; Fig. 5E). We observed no difference in the final fatigue index between the Vdrfl/fl actin iCre+ mice treated with vehicle and Vdrfl/flmice treated with TAM groups (P = 0.79, Tukey’s post hoc test; Fig. 5E). Within each group assessed, we did not observe an effect of sex on the DIAm fatigue index (Table 1). Overall, our fatigue data show a marginal ∼20% reduction in residual force following repetitive contractions of DIAm in mice with selective skeletal muscle knockdown of VDR.
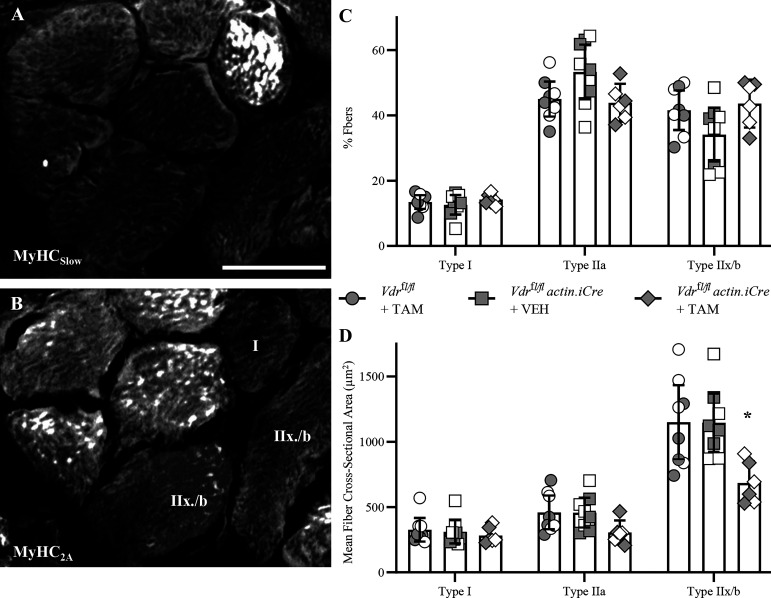
Skeletal Muscle VDR Knockout is Associated with DIAm Fiber Atrophy
Immunoreactivity of DIAm fibers to different MyHC isoforms was used to classify fibers as type I (MyHCSlow positive), type IIa (MyHC2A positive), or type II and/or IIb (devoid of MyHCSlow or MyHC2A reactivity) (Fig. 6A). In all animals, the proportion of type I fibers was less prevalent than IIa and IIx and/or IIb fibers (F2,38 = 102.1, P < 0.0001, two-way ANOVA; Fig. 6B). Importantly, the proportion of fiber types did not change across genotype treatment (F2,19 = 1.1, P = 0.36, two-way ANOVA) with ∼15% Type I, ∼45% Type Iia, and ∼40% type IIx and/or IIb, similar to past reports in mice (5).
Figure 6.
Fiber atrophy of type IIx and/or IIb diaphragm muscle (DIAm) fibers in skeletal muscle vitamin D receptor (VDR) knockout mice. A and B: immunoreactivity of serial sections labeled with myosin heavy chain (MyHC)Slow and MyHC2A isoforms, respectively, to classify DIAm fibers as type I, type Iia, or type II and/or IIb. C: there was no difference in the relative fiber type % of Vdrfl/fl tamoxifen (TAM) (circles, n = 8), Vdrfl/fl actin iCre+ VEH (squares, n = 8), or Vdrfl/fl actin iCre+ TAM (diamonds, n = 6) mice (P = 0.36). D: the cross-sectional areas of DIAm fibers were influenced by genotype/treatment group (P = 0.002), with post hoc assessment showing an ∼40% reduction in the cross-sectional area of type IIx and/or IIb DIAm fibers of Vdrfl/fl actin iCre+ TAM mice compared with Vdrfl/fl TAM (P < 0.0001) and Vdrfl/fl actin iCre+ VEH (P < 0.0001) controls. For all statistical comparisons, two-way ANOVA with Bonferroni post hoc tests was performed, *P < 0.05. All data presented as means ± 95% confidence intervals, n represents no. of animals. Gray symbols denote female, open symbols denote males. Scale bar: 20 µm.
The type-specific cross-sectional areas of DIAm was heavily influenced by fiber type, with IIx and or IIb fibers markedly larger than type I and IIa fibers (F2,38 = 167.6, P < 0.0001, two-way ANOVA; Fig. 6C). The cross-sectional areas were also influenced by genotype/treatment group (F2,19 = 47.6, P = 0.002, two-way ANOVA), with post hoc Benferroni assessment showing an ∼40% reduction in the cross-sectional area of type IIx and/or IIb DIAm fibers of Vdrfl/fl actin iCre+ mice treated with TAM (686 ± 167 µm2) compared with Vdrfl/flmice treated with TAM (1,150 ± 286 µm2; P < 0.0001) and Vdrfl/fl actin iCre+ mice treated with vehicle (1,146 ± 223 µm2; P < 0.0001) controls (Fig. 6C). Within each group and fiber-type assessed, we did not observe an effect of sex on the mean fiber cross-sectional area (Table 1).
DISCUSSION
We observed four major findings in the present study: 1) tamoxifen-induced reduction of VDR mRNA reliably reduces VDR protein levels in DIAm of adult mice; 2) VDR knockout reduces maximum twitch and tetanic isometric DIAm force compared with control mice; 3) VDR knockout reduces residual force following repetitive contractions compared with control mice; and 4) VDR knockout is associated with a selective atrophy of type IIx and/or IIb DIAm fibers compared with controls. Taken together, these findings support a major effect of VDR knockout on the cross-sectional area and specific force (force per cross-sectional area) of type IIx and/or IIb DIAm fibers. Selective atrophy and weakness of type IIx and or IIb DIAm fibers is likely to impair the generation of higher force airway clearance and straining maneuvers. However, it is important to note that these deficits in maximum force generation do not necessarily lead to impairments in ventilatory pressures (i.e., eupnea). For example, in muscular dystrophy models, impaired DIAm force generation is observed without any reduction in peak inspiratory pressures (65), suggesting compensatory activation of other chest wall musculature may ameliorate functional impairments in various afflictions (2, 12, 52, 66–68). The lower residual force after repeated activation indicates increased fatigability of type I and IIa DIAm fibers, which may impair ventilatory endurance during exercise and increase the risk of ventilatory failure.
The inducible knockout VDR mice (VDRact TAM) exhibited a robust ∼87% reduction in VDR mRNA and an ∼60% reduction in VDR protein compared with control Vdrfl/fl mice treated with vehicle. These results are equivalent to the reductions in VDR observed in other noninducible skeletal muscle-specific VDR mice (69). Whole DIAm tissue comprises many cell types (endothelium, collagen) that continue to express relatively normal VDR levels, thus an ∼60% knockdown is expected. Notably, inducible knockout VDR mice do not have VDR deficiency during postnatal development, a time of rapid motor unit (motor neurons and the skeletal muscle fibers they innervate) growth and VDR levels in rodents (2, 45, 57, 63, 70–75), thus avoiding many confounders when assessing muscle fiber properties such as fiber-type proportions and cross-sectional areas. Following knockout of VDR, we observed a reduced rate of weight gain in Vdrfl/fl actin iCre mice treated with TAM compared with the control groups, Vdrfl/flmice treated with TAM, and Vdrfl/fl actin iCre mice treated with vehicle, although we did not observe a difference in body mass at the terminal experiments between any experimental group. These results are similar to past reports in other murine VDR models. In mice where VDR deletion was specific to skeletal muscle, no weight difference between VDR knockdown and control groups was present (69), whereas in scenarios of whole body knockdown, body mass was reduced compared with controls (44, 76). Thus, the effects of blunted growth (body mass gains) may have been caused by possible extra-skeletal expression of the Actin-Cre recombinase gene. We do not attribute any of our reported changes to reduction in serum concentrations of 1,25(OH)2D, as the VDR knockdown mice had levels commensurate with those in the control groups. Furthermore, we found the effects of VDR knockdown to be similar in female and male mice for the major outcome measures of the present study, although we were not powered to detect differences between the sexes per se.
Reduced skeletal muscle force is prevalent in VDR-deficient mice (77, 78). In mice deficient in vitamin D, maximum DIAm-specific force is decreased (39), a result similar to the present study where we observed an ∼33% reduction following VDR knockdown. Reduced DIAm-specific force is present in many conditions, including COPD (15–18), cachexia (19, 20), emphysema (23), and aging sarcopenia (1, 9, 10, 21, 22). In human sarcopenia, there are known vitamin D deficiencies that may contribute to morbidity (79–82). In the DIAm, the prime deficit observed in humans with sarcopenia is an impairment in pressures generated during expulsive maneuvers (i.e., maximal activation of DIAm) that protect the airway and prevent aspirations and infections (83, 84), a phenomenon also observed in rodent aging models (11, 85). These behaviors are subserved largely by the recruitment of more fatigable motor units comprising type IIx and/or IIb DIAm fibers, which produce the greatest specific force (14). Our results show that in scenarios where maximum force requiring maneuvers are impaired (e.g., COPD, cachexia, emphysema, and sarcopenia), special attention must be paid to ensuring adequate concentrations of 1, 25-dihydroxyvitamin D.
In prior studies of vitamin D-deficient mice, fatigability of DIAm was shown to increase, with reduced residual specific force following 120 s of repeated contractions (39). Here, in mice with skeletal muscle-specific knockout of VDR we observe a similar phenomenon, with increased fatigue index, and a reduction in the residual force following fatigue. This residual force is important, as muscle endurance is closely related to this residual force capacity (1, 12). Along with COPD (15, 16, 18), this would make vitamin D deficiency (itself associated with COPD) (86, 87), one of the few conditions of frank DIAm weakness that was not exclusive to type IIx and/or IIb fibers. Indeed, decreased endurance has dire consequences for ventilation during eupnea and exercise, where recruitment of motor units innervating fatigue-resistant type I and IIa DIAm fibers is sufficient to generate necessary intrathoracic pressure for ventilation (2, 3, 11, 16). In these scenarios, motor units comprising higher-force producing but fatigable type IIx and/or IIb fibers are recruited to compensate, however, this is usually insufficient as the incessant nature of breathing obviated fatigue-resistant fibers. These failures result in profound dyspnea, a common symptom prevalent in both COPD and vitamin D deficiency (16, 86–88). In the present study, we are not certain as to whether fatiguability per se is altered due to VDR knockdown. However, the decrease in residual specific force following continued activations for 120 s may be sufficient to compromise ventilatory performance of type I and IIa DIAm fibers, consistent with the presence of VDR on multiple skeletal muscle fiber types in mice (44, 45, 89), a phenomena consistent with human reports (90).
Previously, we and others have shown that young adult wild-type mouse DIAm comprises a mixture of muscle fiber types, with ∼20% type I, ∼40% type Iia, and ∼40% type IIx and or IIb (5, 8, 39). In our experimental cohort, VDR knockout did not alter the proportion of fiber types (based on MyHC immunolabeling) within the DIAm. In previous work with vitamin D-deficient mice, expression of MyHC was similarly unchanged compared with controls (39). We observed an ∼40% reduction in the cross-sectional area of type IIx and/or IIb DIAm fibers. This is consistent with previous reports in a variety of skeletal muscles of humans and mice with vitamin D deficiency (47, 91–94), including DIAm (39), where type II fibers were shown to have atrophied. In DIAm, the not all type II fibers have similar function, with type IIa fibers being highly fatigue resistant and generating less specific force compared with IIa and IIb fibers, which are highly fatiguable and produce much greater forces (14).
In scenarios where vitamin D deficiency is systemic, the effect on skeletal muscle is highly variable, with weakness and atrophy present in both slow and fast fibers or exclusive to one or the other, depending on the location (39, 69, 95). In the present study, we selectively investigate the effect of reduced vitamin D on skeletal muscle in adults, using an inducible VDR knockout. Here we find that VDR knockout leads to DIAm atrophy, with more pronounced effects on type IIx and/or IIb fibers. Future studies will be centered on probing how vitamin D interacts with muscle contractile proteins and other elements within skeletal muscle to maintain effective contractile function.
GRANTS
Funding for this project was provided by the National Institutes of Health Grants AG-057052, AG-044615 (to G. C. Sieck); DK-125252 and DK-107870 (to R. Kumar) and Grant from the Fred C. and Katherine B. Andersen Foundation (to R. Kumar). C. J. Reynolds was supported by NIH Nephrology and Hypertension Training Grant T32 DK-007013.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.F., C.J.R., R.K., and G.C.S. conceived and designed research; M.J.F., L.L.L., T.A.C., C.J.R., and A.D.B. performed experiments; M.J.F., L.L.L., T.A.C., C.J.R., A.D.B., and R.K. analyzed data; M.J.F., L.L.L., T.A.C., C.J.R., R.K., and G.C.S. interpreted results of experiments; M.J.F., L.L.L., T.A.C., and G.C.S. prepared figures; M.J.F. and G.C.S. drafted manuscript; L.L.L., T.A.C., C.J.R., A.D.B., and R.K. edited and revised manuscript; M.J.F., L.L.L., T.A.C., C.J.R., A.D.B., R.K., and G.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Ravinder J. Singh, Y. H. Fang, and B. Macken for assistance in the completion of this project.
REFERENCES
- 1.Fogarty MJ, Mantilla CB, Sieck GC. Impact of sarcopenia on diaphragm muscle fatigue. Exp Physiol 104: 1090–1099, 2019. doi: 10.1113/EP087558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fogarty MJ, Sieck GC. Evolution and functional differentiation of the diaphragm muscle of mammals. Compr Physiol 9: 715–766, 2019. doi: 10.1002/cphy.c180012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol (1985) 66: 2539–2545, 1989. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- 4.Fogarty MJ, Mantilla CB, Sieck GC. Breathing: motor control of diaphragm muscle. Physiology (Bethesda) 33: 113–126, 2018. doi: 10.1152/physiol.00002.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol 48: 881–887, 2013. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sieck GC, Fournier M, Enad JG. Fiber type composition of muscle units in the cat diaphragm. Neurosci Lett 97: 29–34, 1989. doi: 10.1016/0304-3940(89)90134-1. [DOI] [PubMed] [Google Scholar]
- 7.Sieck GC, Fournier M, Prakash YS, Blanco CE. Myosin phenotype and SDH enzyme variability among motor unit fibers. J Appl Physiol (1985) 80: 2179–2189, 1996. doi: 10.1152/jappl.1996.80.6.2179. [DOI] [PubMed] [Google Scholar]
- 8.Fogarty MJ, Brandenburg JE, Sieck GC. Diaphragm neuromuscular transmission failure in a mouse model of an early-onset neuromotor disorder. J Appl Physiol (1985) 130: 708–720, 2021. doi: 10.1152/japplphysiol.00864.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fogarty MJ, Gonzalez Porras MA, Mantilla CB, Sieck GC. Diaphragm neuromuscular transmission failure in aged rats. J Neurophysiol 122: 93–104, 2019. doi: 10.1152/jn.00061.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogarty MJ, Marin Mathieu N, Mantilla CB, Sieck GC. Aging reduces succinate dehydrogenase activity in rat type IIx/IIb diaphragm muscle fibers. J Appl Physiol (1985) 128: 70–77, 2020. doi: 10.1152/japplphysiol.00644.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khurram OU, Fogarty MJ, Sarrafian TL, Bhatt A, Mantilla CB, Sieck GC. Impact of aging on diaphragm muscle function in male and female Fischer 344 rats. Physiol Rep 6: e13786, 2018. doi: 10.14814/phy2.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogarty MJ, Sieck GC. Diaphragm muscle adaptations in health and disease. Drug Discov Today Dis Models 29–30: 43–52, 2019. doi: 10.1016/j.ddmod.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol 59: 1055–1066, 1988. doi: 10.1152/jn.1988.59.3.1055. [DOI] [PubMed] [Google Scholar]
- 14.Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol (1985) 89: 695–703, 2000. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- 15.Levine S, Nguyen T, Kaiser LR, Rubinstein NA, Maislin G, Gregory C, Rome LC, Dudley GA, Sieck GC, Shrager JB. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J Respir Crit Care Med 168: 706–713, 2003. doi: 10.1164/rccm.200209-1070OC. [DOI] [PubMed] [Google Scholar]
- 16.Mantilla CB, Sieck GC. Neuromotor control in chronic obstructive pulmonary disease. J Appl Physiol (1985) 114: 1246–1252, 2013. doi: 10.1152/japplphysiol.01212.2012. [DOI] [PubMed] [Google Scholar]
- 17.Ottenheijm CA, Heunks LM, Hafmans T, van der Ven PF, Benoist C, Zhou H, Labeit S, Granzier HL, Dekhuijzen PN. Titin and diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173: 527–534, 2006. doi: 10.1164/rccm.200507-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottenheijm CA, Heunks LM, Sieck GC, Zhan WZ, Jansen SM, Degens H, de Boo T, Dekhuijzen PN. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 172: 200–205, 2005. doi: 10.1164/rccm.200502-262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts BM, Ahn B, Smuder AJ, Al-Rajhi M, Gill LC, Beharry AW, Powers SK, Fuller DD, Ferreira LF, Judge AR. Diaphragm and ventilatory dysfunction during cancer cachexia. FASEB J 27: 2600–2610, 2013. doi: 10.1096/fj.12-222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts BM, Frye GS, Ahn B, Ferreira LF, Judge AR. Cancer cachexia decreases specific force and accelerates fatigue in limb muscle. Biochem Biophys Res Commun 435: 488–492, 2013. doi: 10.1016/j.bbrc.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott JE, Omar TS, Mantilla CB, Sieck GC. Diaphragm muscle sarcopenia in Fischer 344 and Brown Norway rats. Exp Physiol 101: 883–894, 2016. doi: 10.1113/EP085703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gosselin LE, Johnson BD, Sieck GC. Age-related changes in diaphragm muscle contractile properties and myosin heavy chain isoforms. Am J Respir Crit Care Med 150: 174–178, 1994. doi: 10.1164/ajrccm.150.1.8025746. [DOI] [PubMed] [Google Scholar]
- 23.Lewis MI, Zhan WZ, Sieck GC. Adaptations of the diaphragm in emphysema. J Appl Physiol (1985) 72: 934–943, 1992. doi: 10.1152/jappl.1992.72.3.934. [DOI] [PubMed] [Google Scholar]
- 24.Fogarty MJ, Sieck GC. Spinal cord injury and diaphragm neuromotor control. Expert Rev Respir Med 14: 453–464, 2020. doi: 10.1080/17476348.2020.1732822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantilla CB, Sieck GC. Neuromuscular adaptations to respiratory muscle inactivity. Respir Physiol Neurobiol 169: 133–140, 2009. doi: 10.1016/j.resp.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan WZ, Sieck GC. Adaptations of diaphragm and medial gastrocnemius muscles to inactivity. J Appl Physiol (1985) 72: 1445–1453, 1992. doi: 10.1152/jappl.1992.72.4.1445. [DOI] [PubMed] [Google Scholar]
- 27.Dayal D, Kumar S, Sachdeva N, Kumar R, Singh M, Singhi S. Fall in vitamin D levels during hospitalization in children. Int J Pediatr 2014: 291856, 2014. doi: 10.1155/2014/291856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta A, Sjoukes A, Richards D, Banya W, Hawrylowicz C, Bush A, Saglani S. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med 184: 1342–1349, 2011. doi: 10.1164/rccm.201107-1239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, Coolen J, Mathieu C, Decramer M, Lambrechts D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 65: 215–220, 2010. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 30.Malinovschi A, Masoero M, Bellocchia M, Ciuffreda A, Solidoro P, Mattei A, Mercante L, Heffler E, Rolla G, Bucca C. Severe vitamin D deficiency is associated with frequent exacerbations and hospitalization in COPD patients. Respir Res 15: 131, 2014. doi: 10.1186/s12931-014-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol 44: 981–988, 2009. doi: 10.1002/ppul.21089. [DOI] [PubMed] [Google Scholar]
- 32.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr 58: 563–567, 2004. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 33.Capiati D, Benassati S, Boland RL. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J Cell Biochem 86: 128–135, 2002. doi: 10.1002/jcb.10191. [DOI] [PubMed] [Google Scholar]
- 34.Doroudi M, Chen J, Boyan BD, Schwartz Z. New insights on membrane mediated effects of 1alpha,25-dihydroxy vitamin D3 signaling in the musculoskeletal system. Steroids 81: 81–87, 2014. doi: 10.1016/j.steroids.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Ryan ZC, Craig TA, Folmes CD, Wang X, Lanza IR, Schaible NS, Salisbury JL, Nair KS, Terzic A, Sieck GC, Kumar R. 1α,25-Dihydroxyvitamin D3 regulates mitochondrial oxygen consumption and dynamics in human skeletal muscle cells. J Biol Chem 291: 1514–1528, 2016. doi: 10.1074/jbc.M115.684399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan ZC, Craig TA, Wang X, Delmotte P, Salisbury JL, Lanza IR, Sieck GC, Kumar R. 1alpha,25-dihydroxyvitamin D3 mitigates cancer cell mediated mitochondrial dysfunction in human skeletal muscle cells. Biochem Biophys Res Commun 496: 746–752, 2018. doi: 10.1016/j.bbrc.2018.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab 25: 543–559, 2011. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, Jurutka PW. Molecular mechanisms of vitamin D action. Calcif Tissue Int 92: 77–98, 2013. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 39.Ray AD, Personius KE, Williamson DL, Dungan CM, Dhillon SS, Hershberger PA. Vitamin D3 intake modulates diaphragm but not peripheral muscle force in young mice. J Appl Physiol (1985) 120: 1124–1131, 2016. doi: 10.1152/japplphysiol.00643.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hopkinson NS, Li KW, Kehoe A, Humphries SE, Roughton M, Moxham J, Montgomery H, Polkey MI. Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr 87: 385–390, 2008. doi: 10.1093/ajcn/87.2.385. [DOI] [PubMed] [Google Scholar]
- 41.Al-Said YA, Al-Rached HS, Al-Qahtani HA, Jan MM. Severe proximal myopathy with remarkable recovery after vitamin D treatment. Can J Neurol Sci 36: 336–339, 2009. doi: 10.1017/s0317167100007083. [DOI] [PubMed] [Google Scholar]
- 42.Fluss J, Kern I, de Coulon G, Gonzalez E, Chehade H. Vitamin D deficiency: a forgotten treatable cause of motor delay and proximal myopathy. Brain Dev 36: 84–87, 2014. doi: 10.1016/j.braindev.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Rasheed K, Sethi P, Bixby E. Severe vitamin d deficiency induced myopathy associated with rhabydomyolysis. N Am J Med Sci 5: 334–336, 2013. doi: 10.4103/1947-2714.112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, Kato S, Matsumoto T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 144: 5138–5144, 2003. doi: 10.1210/en.2003-0502. [DOI] [PubMed] [Google Scholar]
- 45.Girgis CM, Mokbel N, Cha KM, Houweling PJ, Abboud M, Fraser DR, Mason RS, Clifton-Bligh RJ, Gunton JE. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 155: 3227–3237, 2014. doi: 10.1210/en.2014-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell WW, Johnson CA, McCabe GP, Carnell NS. Dietary protein requirements of younger and older adults. Am J Clin Nutr 88: 1322–1329, 2008. doi: 10.3390/nu8060359. [DOI] [PubMed] [Google Scholar]
- 47.Boland R. Role of vitamin D in skeletal muscle function. Endocr Rev 7: 434–448, 1986. doi: 10.1210/edrv-7-4-434. [DOI] [PubMed] [Google Scholar]
- 48.Nakamichi Y, Udagawa N, Horibe K, Mizoguchi T, Yamamoto Y, Nakamura T, Hosoya A, Kato S, Suda T, Takahashi N. VDR in osteoblast-lineage cells primarily mediates vitamin D treatment-induced increase in bone mass by suppressing bone resorption. J Bone Miner Res 32: 1297–1308, 2017. doi: 10.1002/jbmr.3096. [DOI] [PubMed] [Google Scholar]
- 49.Harfmann BD, Schroder EA, Kachman MT, Hodge BA, Zhang X, Esser KA. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet Muscle 6: 12, 2016. doi: 10.1186/s13395-016-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCarthy JJ, Srikuea R, Kirby TJ, Peterson CA, Esser KA. Inducible Cre transgenic mouse strain for skeletal muscle-specific gene targeting. Skelet Muscle 2: 8, 2012. [Erratum in Skelet Muscle 2: 22, 2012]. doi: 10.1186/2044-5040-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prakash YS, Fournier M, Sieck GC. Effects of prenatal undernutrition on developing rat diaphragm. J Appl Physiol (1985) 75: 1044–1052, 1993. doi: 10.1152/jappl.1993.75.3.1044. [DOI] [PubMed] [Google Scholar]
- 52.Khurram OU, Fogarty MJ, Rana S, Vang P, Sieck GC, Mantilla CB. Diaphragm muscle function following mid-cervical contusion injury in rats. J Appl Physiol (1985) 126: 221–230, 2019. doi: 10.1152/japplphysiol.00481.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sieck GC, Lewis MI, Blanco CE. Effects of undernutrition on diaphragm fiber size, SDH activity, and fatigue resistance. J Appl Physiol (1985) 66: 2196–2205, 1989. doi: 10.1152/jappl.1989.66.5.2196. [DOI] [PubMed] [Google Scholar]
- 54.Sieck GC, Zhan WZ, Prakash YS, Daood MJ, Watchko JF. SDH and actomyosin ATPase activities of different fiber types in rat diaphragm muscle. J Appl Physiol (1985) 79: 1629–1639, 1995. doi: 10.1152/jappl.1995.79.5.1629. [DOI] [PubMed] [Google Scholar]
- 55.Ryan ZC, Ketha H, McNulty MS, McGee-Lawrence M, Craig TA, Grande JP, Westendorf JJ, Singh RJ, Kumar R. Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci USA 110: 6199–6204, 2013. doi: 10.1073/pnas.1221255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fogarty MJ, Enninga EAL, Ibirogba ER, Ruano R, Sieck GC. Impact of congenital diaphragmatic hernia on diaphragm muscle function in neonatal rats. J Appl Physiol (1985) 130: 801–812, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sieck GC, Fournier M, Blanco CE. Diaphragm muscle fatigue resistance during postnatal development. J Appl Physiol (1985) 71: 458–464, 1991. doi: 10.1152/jappl.1991.71.2.458. [DOI] [PubMed] [Google Scholar]
- 58.Watchko JF, Sieck GC. Respiratory muscle fatigue resistance relates to myosin phenotype and SDH activity during development. J Appl Physiol 75: 1341–1347, 1993. doi: 10.1152/jappl.1993.75.3.1341. [DOI] [PubMed] [Google Scholar]
- 59.Zhan WZ, Watchko JF, Prakash YS, Sieck GC. Isotonic contractile and fatigue properties of developing rat diaphragm muscle. J Appl Physiol (1985) 84: 1260–1268, 1998. doi: 10.1152/jappl.1998.84.4.1260. [DOI] [PubMed] [Google Scholar]
- 60.Seven YB, Mantilla CB, Zhan WZ, Sieck GC. Non-stationarity and power spectral shifts in EMG activity reflect motor unit recruitment in rat diaphragm muscle. Respir Physiol Neurobiol 185: 400–409, 2013. doi: 10.1016/j.resp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sieck GC, Trelease RB, Harper RM. Sleep influences on diaphragmatic motor unit discharge. Exp Neurol 85: 316–335, 1984. doi: 10.1016/0014-4886(84)90143-2. [DOI] [PubMed] [Google Scholar]
- 62.Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve 21: 887–895, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 63.Geiger PC, Cody MJ, Macken RL, Bayrd ME, Fang YH, Sieck GC. Mechanisms underlying increased force generation by rat diaphragm muscle fibers during development. J Appl Physiol (1985) 90: 380–388, 2001. doi: 10.1152/jappl.2001.90.1.380. [DOI] [PubMed] [Google Scholar]
- 64.Geiger PC, Cody MJ, Sieck GC. Force-calcium relationship depends on myosin heavy chain and troponin isoforms in rat diaphragm muscle fibers. J Appl Physiol (1985) 87: 1894–1900, 1999. doi: 10.1152/jappl.1999.87.5.1894. [DOI] [PubMed] [Google Scholar]
- 65.Burns DP, Murphy KH, Lucking EF, O'Halloran KD. Inspiratory pressure-generating capacity is preserved during ventilatory and non-ventilatory behaviours in young dystrophic mdx mice despite profound diaphragm muscle weakness. J Physiol 597: 831–848, 2019. doi: 10.1113/JP277443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katagiri M, Young RN, Platt RS, Kieser TM, Easton PA. Respiratory muscle compensation for unilateral or bilateral hemidiaphragm paralysis in awake canines. J Appl Physiol (1985) 77: 1972–1982, 1994. doi: 10.1152/jappl.1994.77.4.1972. [DOI] [PubMed] [Google Scholar]
- 67.O'Halloran KD, Burns DP. Breathing with neuromuscular disease: does compensatory plasticity in the motor drive to breathe offer a potential therapeutic target in muscular dystrophy? Respir Physiol Neurobiol 265: 49–54, 2019. doi: 10.1016/j.resp.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 68.Romer SH, Seedle K, Turner SM, Li J, Baccei ML, Crone SA. Accessory respiratory muscles enhance ventilation in ALS model mice and are activated by excitatory V2a neurons. Exp Neurol 287: 192–204, 2017. doi: 10.1016/j.expneurol.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 69.Girgis CM, Cha KM, So B, Tsang M, Chen J, Houweling PJ, Schindeler A, Stokes R, Swarbrick MM, Evesson FJ, Cooper ST, Gunton JE. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J Cachexia Sarcopenia Muscle 10: 1228–1240, 2019. doi: 10.1002/jcsm.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fogarty MJ, Mu EW, Noakes PG, Lavidis NA, Bellingham MC. Marked changes in dendritic structure and spine density precede significant neuronal death in vulnerable cortical pyramidal neuron populations in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Acta Neuropathol Commun 4: 77, 2016. doi: 10.1186/s40478-016-0347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fournier M, Alula M, Sieck GC. Neuromuscular transmission failure during postnatal development. Neurosci Lett 125: 34–36, 1991. doi: 10.1016/0304-3940(91)90124-c. [DOI] [PubMed] [Google Scholar]
- 72.Geiger PC, Bailey JP, Mantilla CB, Zhan WZ, Sieck GC. Mechanisms underlying myosin heavy chain expression during development of the rat diaphragm muscle. J Appl Physiol (1985) 101: 1546–1555, 2006. doi: 10.1152/japplphysiol.00221.2006. [DOI] [PubMed] [Google Scholar]
- 73.Kanjhan R, Fogarty MJ, Noakes PG, Bellingham MC. Developmental changes in the morphology of mouse hypoglossal motor neurons. Brain Struct Funct 221: 3755–3786, 2016. doi: 10.1007/s00429-015-1130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sieck GC, Prakash YS, Han YS, Fang YH, Geiger PC, Zhan WZ. Changes in actomyosin ATP consumption rate in rat diaphragm muscle fibers during postnatal development. J Appl Physiol (1985) 94: 1896–1902, 2003. doi: 10.1152/japplphysiol.00617.2002. [DOI] [PubMed] [Google Scholar]
- 75.Watchko JF, Brozanski BS, O'Day TL, Guthrie RD, Sieck GC. Contractile properties of the rat external abdominal oblique and diaphragm muscles during development. J Appl Physiol (1985) 72: 1432–1436, 1992. doi: 10.1152/jappl.1992.72.4.1432. [DOI] [PubMed] [Google Scholar]
- 76.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 16: 391–396, 1997. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 77.Girgis CM, Cha KM, Houweling PJ, Rao R, Mokbel N, Lin M, Clifton-Bligh RJ, Gunton JE. Vitamin D receptor ablation and vitamin D deficiency result in reduced grip strength, altered muscle fibers, and increased myostatin in mice. Calcif Tissue Int 97: 602–610, 2015. doi: 10.1007/s00223-015-0054-x. [DOI] [PubMed] [Google Scholar]
- 78.Nakamura S, Sato Y, Kobayashi T, Kaneko Y, Ito E, Soma T, Okada H, Miyamoto K, Oya A, Matsumoto M, Nakamura M, Kanaji A, Miyamoto T. Vitamin D protects against immobilization-induced muscle atrophy via neural crest-derived cells in mice. Sci Rep 10: 12242, 2020. doi: 10.1038/s41598-020-69021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conzade R, Grill E, Bischoff-Ferrari HA, Ferrari U, Horsch A, Koenig W, Peters A, Thorand B. Vitamin D in relation to incident sarcopenia and changes in muscle parameters among older adults: the KORA-Age Study. Calcif Tissue Int 105: 173–182, 2019. doi: 10.1007/s00223-019-00558-5. [DOI] [PubMed] [Google Scholar]
- 80.Girgis CM, Baldock PA, Downes M. Vitamin D, muscle and bone: integrating effects in development, aging and injury. Mol Cell Endocrinol 410: 3–10, 2015. doi: 10.1016/j.mce.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 81.Scott D, Blizzard L, Fell J, Ding C, Winzenberg T, Jones G. A prospective study of the associations between 25-hydroxy-vitamin D, sarcopenia progression and physical activity in older adults. Clin Endocrinol (Oxf) 73: 581–587, 2010. doi: 10.1111/j.1365-2265.2010.03858.x. [DOI] [PubMed] [Google Scholar]
- 82.Sohl E, van Schoor NM, de Jongh RT, Visser M, Deeg DJ, Lips P. Vitamin D status is associated with functional limitations and functional decline in older individuals. J Clin Endocrinol Metab 98: E1483–E1490, 2013. doi: 10.1210/jc.2013-1698. [DOI] [PubMed] [Google Scholar]
- 83.Chong CP, Street PR. Pneumonia in the elderly: a review of the epidemiology, pathogenesis, microbiology, and clinical features. South Med J 101: 1141–1145, 2008. doi: 10.1097/SMJ.0b013e318181d5b5. [DOI] [PubMed] [Google Scholar]
- 84.Polkey MI, Harris ML, Hughes PD, Hamnegard CH, Lyons D, Green M, Moxham J. The contractile properties of the elderly human diaphragm. Am J Respir Crit Care Med 155: 1560–1564, 1997. doi: 10.1164/ajrccm.155.5.9154857. [DOI] [PubMed] [Google Scholar]
- 85.Greising SM, Mantilla CB, Sieck DC, Sieck GC. Transdiaphragmatic pressure measurements reveal age-related diaphragm muscle dysfunction during non-ventilatory behaviors. FASEB J 27: 719.7, 2013. doi: 10.1096/fasebj.27.1_supplement.719.7. [DOI] [Google Scholar]
- 86.Ghosh AJ, Moll M, Hayden LP, Bon J, Regan E, Hersh CP; COPDGene Investigators. Vitamin D deficiency is associated with respiratory symptoms and airway wall thickening in smokers with and without COPD: a prospective cohort study. BMC Pulm Med 20: 123, 2020. doi: 10.1186/s12890-020-1148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mulrennan S, Knuiman M, Walsh JP, Hui J, Hunter M, Divitini M, Zhu K, Cooke BR, Musk AWB, James A. Vitamin D and respiratory health in the Busselton Healthy Ageing Study. Respirology 23: 576–582, 2018. doi: 10.1111/resp.13239. [DOI] [PubMed] [Google Scholar]
- 88.Phillips DB, Collins SE, Stickland MK. Measurement and interpretation of exercise ventilatory efficiency. Front Physiol 11: 659, 2020. doi: 10.3389/fphys.2020.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Srikuea R, Zhang X, Park-Sarge OK, Esser KA. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: potential role in suppression of myoblast proliferation. Am J Physiol Cell Physiol 303: C396–C405, 2012. doi: 10.1152/ajpcell.00014.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stahelin HB, Dick W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochemical Journal 33: 19–24, 2001. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- 91.Floyd M, Ayyar DR, Barwick DD, Hudgson P, Weightman D. Myopathy in chronic renal failure. Q J Med 43: 509–524, 1974. [PubMed] [Google Scholar]
- 92.Polly P, Tan TC. The role of vitamin D in skeletal and cardiac muscle function. Front Physiol 5: 145, 2014. doi: 10.3389/fphys.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis 20: 187–192, 2005. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 94.Sorensen OH, Lund B, Saltin B, Lund B, Andersen RB, Hjorth L, Melsen F, Mosekilde L. Myopathy in bone loss of ageing: improvement by treatment with 1 alpha-hydroxycholecalciferol and calcium. Clin Sci (Lond) 56: 157–161, 1979. doi: 10.1042/cs0560157. [DOI] [PubMed] [Google Scholar]
- 95.Schubert L, DeLuca HF. Hypophosphatemia is responsible for skeletal muscle weakness of vitamin D deficiency. Arch Biochem Biophys 500: 157–161, 2010. doi: 10.1016/j.abb.2010.05.029. [DOI] [PubMed] [Google Scholar]