Abstract
Studies in humans and animal models with spinal cord injury (SCI) have demonstrated that medications targeting serotonin receptors may decrease the susceptibility to central sleep-disordered breathing (SDB). We hypothesized that mirtazapine would decrease the propensity to develop hypocapnic central sleep apnea (CSA) during sleep. We performed a single-blind pilot study on a total of 10 men with SDB (7 with chronic SCI and 3 noninjured) aged 52.0 ± 11.2 yr. Participants were randomly assigned to either mirtazapine (15 mg at bedtime) or a placebo for at least 1 wk, followed by a 7-day washout period before crossing over to the other intervention. Split-night studies included polysomnography and induction of hypocapnic CSA using a noninvasive ventilation (NIV) protocol. The primary outcome was CO2 reserve, defined as the difference between eupneic and end of NIV end-tidal CO2 (PETCO2) preceding induced hypocapneic CSA. Secondary outcomes included controller gain (CG), other ventilatory parameters, and SDB severity. CG was defined as the ratio of change in minute ventilation (V̇e) between control and hypopnea to the change in CO2 during sleep. CO2 reserve was significantly widened on mirtazapine than placebo (−3.8 ± 1.2 vs. −2.0 ± 1.5 mmHg; P = 0.015). CG was significantly decreased on mirtazapine compared with placebo [2.2 ± 0.7 vs. 3.5 ± 1.9 L/(mmHg × min); P = 0.023]. There were no significant differences for other ventilatory parameters assessed or SDB severity between mirtazapine and placebo trials. These findings suggest that the administration of mirtazapine can decrease the susceptibility to central apnea by reducing chemosensitivity and increasing CO2 reserve; however, considering the lack of changes in apnea-hypopnea index (AHI), further research is required to understand the significance of this finding.
NEW & NOTEWORTHY To our knowledge, this research study is novel as it is the first study in humans assessing the effect of mirtazapine on CO2 reserve and chemosensitivity in individuals with severe sleep-disordered breathing. This is also the first study to determine the potential therapeutic effects of mirtazapine on sleep parameters in individuals with a spinal cord injury.
Keywords: central sleep apnea, CO2 reserve, mirtazapine, sleep disordered-breathing, spinal cord injury
INTRODUCTION
Mechanical therapies such as positive airway pressure (PAP) are the treatment modality most commonly prescribed to alleviate sleep-disordered breathing (SDB) (1). However, alternative therapies are needed owing to PAP intolerance and suboptimal adherence to therapy, especially in individuals living with spinal cord injury (SCI) (2, 3).
The effectiveness of pharmaceutical treatments for SDB has been inconsistent, and as of yet, there is no best medication for treating this disease (4). Within the brainstem, serotonin (5-HT) acts to modulate respiratory circuitry and ventilatory behavior (5). There are a variety of 5-HT receptor subtypes acting throughout the brain that can influence respiratory drive (6), central CO2 chemoreception (7–10), and hypoxia-induced respiratory plasticity (11). Moreover, dilator motor neuron postsynaptic serotonin receptor subtypes are potential new targets for the treatment of SDB (12, 13). This is of particular importance in the treatment of SDB in SCI, especially considering that tetraplegia is considered a risk factor for central sleep apnea (CSA) (14). Animal studies have shown that drugs that stimulate 5-HT1A receptors can counteract respiratory abnormalities in conscious rats after SCI (15) and stimulate both hypoglossal and phrenic nerves (16). We have also recently discovered that the administration of the 5-HT1A receptor agonist (buspirone) decreased the susceptibility to develop hypocapnic central sleep apnea by reducing chemosensitivity and hence increasing CO2 reserve in individuals with chronic SCI (17). However, buspirone administration was not associated with improvement in SDB severity.
In contrast to buspirone, mirtazapine has a longer half-life and sedative features, which could alter the arousability during sleep (18). These features make mirtazapine a desirable agent, particularly in individuals with SCI who have mixed etiology of SDB, low arousal threshold, and fragmented sleep (19). Mirtazapine is an antidepressant that promotes 5-HT1 activity and inhibits 5-HT2/5-HT3 activity in the central and peripheral nervous system, ultimately promoting serotonin release in the brain (20, 21). In a rodent model, mirtazapine was shown to reduce the expression of central sleep apnea (CSA) by 50% during both rapid eye movement (REM) and non-REM (NREM) sleep (22). These findings have been replicated in humans suffering from SDB, with data suggesting that apnea-hypopnea index (AHI) reductions of 50% or greater are achievable (23, 24). Considering that 5-HT receptors are also located within the peripheral nervous system, the dose of mirtazapine may be an essential factor in its effectiveness (23). At ordinary therapeutic doses, it may predominately act by increasing brainstem serotonin or blocking peripheral 5-HT3 receptors rather than blocking central 5-HT2 receptors, resulting in enhanced genioglossus activity. In contrast, at higher doses, the upper airway musculature may become depressed (25).
We hypothesized that mirtazapine would reduce the propensity of patients with SDB to develop hypocapnic central apnea during sleep.
METHODS
Subjects
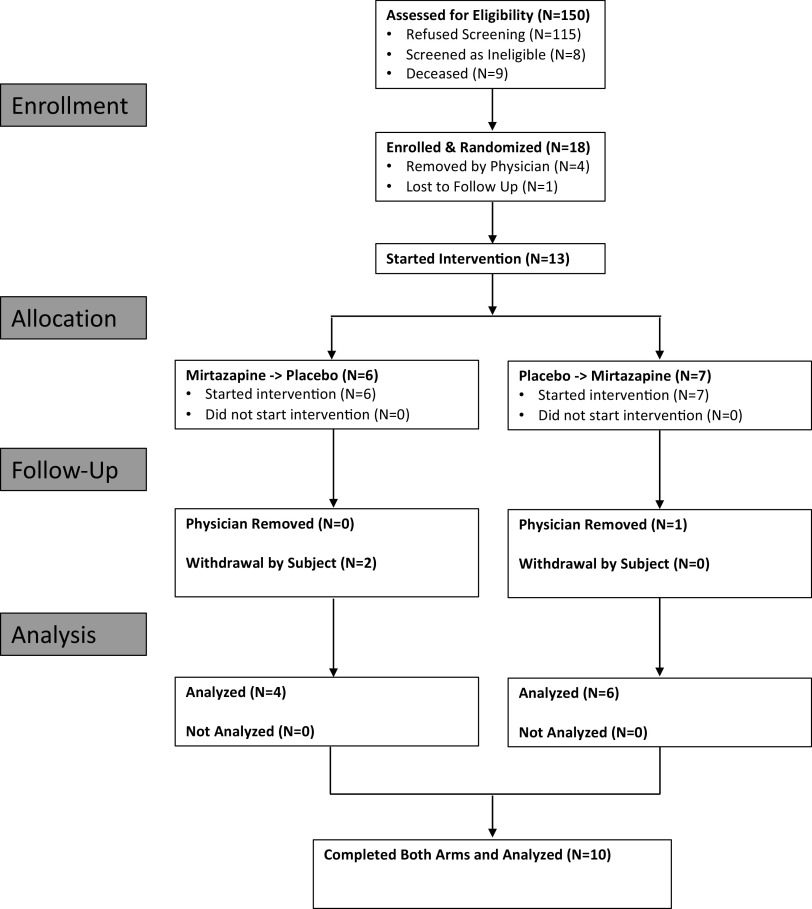
The experimental protocol was approved by the Human Investigation Committee of Wayne State University and the Veterans Affairs Medical Center. This study iswas retrospectively registered on clinicaltrials.gov under the identifier NCT04799782. This study was also part of a larger study entitled “Role of Enhancing Serotonin Receptors Activity for Sleep Apnea Treatment in Patients with SCI (REST-SCI)” registered on clinicaltrials.gov under the identifier NCT02458469. Participants were recruited through advertisement of the study at the John D. Dingell VA Medical Center and the Detroit Medical Center. In addition, individuals who had previously participated in any of our prior research studies were also contacted and invited to participate if they fit the inclusion criteria. Participants provided written informed consent before inclusion in the investigation, and inclusion criteria were as follows: nonventilator-dependent, chronic SCI (>6 mo postinjury) American Spinal Injury Association (ASIA) grade A, B, C, or D, from cervical (C1-C7) to thoracic levels (T1-T6), and noninjured individuals. Exclusion criteria included: 1) <18 yr of age; 2) pregnant or lactating females; 3) tracheostomy tube in place; 4) advanced heart failure, peripheral vascular disease, or stroke; 5) history of head trauma resulting in neurological symptoms or loss of consciousness; 6) advanced lung, liver, or chronic kidney disease; and 7) Class 3 obesity [body mass index (BMI) >40 kg/m2]. Figure 1 displays the progress of all participants through the trial in the form of a CONSORT flowchart.
Figure 1.
CONSORT flow diagram displaying the progress of all participants through each phase of the trial.
Randomization and Study Design
All participants underwent a full polysomnography (PSG) to assess the severity and presence of SDB based on the American Academy of Sleep Medicine (AASM) criteria (26). Eligible participants were then randomized to mirtazapine versus placebo arms. This investigation was a single-blinded randomized crossover pilot physiological study. Each participant was provided with either mirtazapine (15 mg at bedtime daily) or a placebo for at least 1 wk (split-night study performed on night 6 of intervention) followed by a 1-wk washout period before beginning the other intervention. The participants were instructed to take the medication immediately before bed and were blinded to the treatment group. The order in which participants received the mirtazapine or placebo was randomized.
During overnight sleep studies, both the intervention (which provided apneic threshold and gain measurements) and polysomnography (PSG) [which provided apnea-hypopnea index (AHI) and other measures of SDB] were performed during the same split-night study. The intervention measurements were always performed first, and a short period was left (at least 3 min) after PAP was removed before beginning the PSG data collection to allow for normal sleep to be recorded. If insufficient sleep was achieved during the PSG portion (<2 h), the participant was brought back to the laboratory the following night, given a dose of medication, and the PSG was repeated. Participants were instructed to restrict their sleep to 4–6 h of sleep the night before the split-night study and to abstain from consuming caffeine, using tobacco products, and drinking alcohol the day before their split-night study.
Polysomnography
Participants completed overnight in-laboratory polysomnography (PSG) test using the Comet Polysomnography System (Grass Technologies, Warwick, RI) or the SomnoStar z4 Sleep System (Vyaire Medical, Mettawa, IL). All participants remained in a supine position throughout the night and during all measurements. AASM recommended criteria were used to score respiratory events such as apneas and hypopneas that occurred during the PSG portion of the split-night study (26). A pneumotachometer (Hans Rudolph, Model 3700 A, Shawnee, KS) connected to a tight-fitting nasal mask was used to measure airflow. The integration of the pneumotachograph flow signal was used to determine the tidal volume (VT), and a modified lead II electrode placement was used for ECG analysis. Oxygen saturation () was measured using a Datex-Ohmeda Biox 3740 Pulse Oximeter (Louisville, CO) or SomnoStar z4 Sleep System. End-tidal CO2 (PETCO2) and end-tidal O2 (PETO2) were measured using the GEMINI O2 and CO2 Monitor (CWE, Ardmore, PA).
Apneic Threshold Determination
Noninvasive ventilation protocol.
The OmniLab Advanced + Titration System (Philips Respironics, Murrysville, PA) was used to provide positive airway pressure (PAP) within the mask and thus eliminate obstructive respiratory events. Following this, hyperventilation was induced by increasing inspiratory pressure for at least 2 min, resulting in hypopnea or central apnea. Noninvasive ventilation (NIV) was terminated during expiration to the baseline expiratory PAP for a minimum of 3 min. If necessary, higher pressure support (1–2 cmH2O) was used to produce central apnea. If a central apnea occurred, the trial was repeated at lower pressure support (1–2 cmH2O) to provide the closest PETCO2 to the AT.
Hypercapneia protocol.
For any participants exhibiting spontaneous central apnea during sleep, a 40% CO2 gas with a flow rate of 0.5 L/min was added to the mask. If required, the CO2 flow rate was increased by 0.5 L/min in subsequent trials until central apneas were abolished. At least 2 min of recovery time was provided in between trials, and if any breaths followed arousal or if the PETCO2 signal did not adequately plateau, they were excluded.
Data Analysis
To assess baseline ventilatory parameters (described in Table 2), 10 breaths from both wakefulness and stable NREM sleep (no apneas within the preceding 2 min) were measured using the PowerLab acquisition system (ADInstruments, Model 16SP, Colorado Springs, CO). If participants displayed an apnea-hypopnea index (AHI) greater than or equal to 5 events/h of sleep, this was considered SDB. To be classified as central SDB, a central apnea index (CAI) greater than or equal to 5 central events/h of sleep must also have been observed. The control period used for hyperventilation trials (Fig. 2) was the average of five breaths immediately preceding the onset of mechanical ventilation. The average of the last five NIV breaths before the ventilator was turned back to the baseline expiratory PAP was used to assess hyperventilation. To determine changes in PETCO2 (ΔPETCO2), the difference between the control period and the last five NIV breaths was calculated. Individual breaths were excluded if the PETCO2signal did not adequately plateau. Apneic threshold (AT) was defined as the PETCO2at which the apnea occurred and was measured at the end of the hyperventilation trial. The CO2 reserve was defined as ΔPETCO2 between the central apnea onset and the control breaths. To be considered an apnea, the nadir breath during the recovery period must have displayed a maximum flow of less than 0.05 L/s. Steady-state plant gain (PG) was calculated as the ratio of the PETCO2 to minute ventilation (V̇e) for all breaths during the control period of the valid trials. The controller gain (CG) was calculated as the V̇e of the breaths during the control period of the trial divided by the change in PETCO2 between the mechanical ventilation and baseline breaths. Trials were excluded if they occurred during wake or REM sleep or if arousal or respiratory event occurred within 1 min of hyperventilation inducement or termination. One participant who had CSA during the placebo trials was given a CO2 gas mixture until CSA was eliminated. This same subject was able to complete the NIV protocol during the mirtazapine-intervention split night study. Therefore, in this participant who required CO2 administration to eliminate CSA, the CO2 reserve was defined as ΔPETCO2 between the control period (i.e., stable NREM sleep after central apnea was eliminated) and the last five breaths before central apnea occurred. Another participant had their AT calculated using interpolation after poor sleep prevented them from achieving an apnea during the trials. Linear regression was used to determine the relationship between ΔPETCO2 and percent change in V̇e compared with the control period and the first three breaths post mechanical ventilation trial. The resulting linear regression equation was used to determine the value of ΔPETCO2 when V̇e is zero. The researcher scoring the PSG analysis was blinded; however, they were not blinded during the analysis of physiological parameters.
Table 2.
Ventilatory parameters of participants after 6 days of placebo vs. mirtazapine treatment
| Placebo | Mirtazapine | |
|---|---|---|
| V̇e, L/min | 7.2 ± 2.1 | 8.4 ± 2.5 |
| VT, L | 0.6 ± 0.2 | 0.6 ± 0.2 |
| f, breaths/min | 12.8 ± 2.3 | 13.6 ± 3.1 |
| VMAX Insp, L/s | 0.4 ± 0.1 | 0.4 ± 0.1 |
| TI, s | 2.0 ± 0.5 | 2.0 ± 0.4 |
| TE, s | 2.8 ± 0.6 | 2.6 ± 0.8 |
| TTot, s | 4.8 ± 0.9 | 4.6 ± 1.1 |
| TI/TTot | 0.4 ± 0.1 | 0.4 ± 0.1 |
| PETCO2, mmHg | 40.6 ± 4.2 | 40.3 ± 4.8 |
| , % | 96.4 ± 1.5 | 96.2 ± 1.4 |
Values are presented as means ± SD; n = 10 participants. Measures were determined during non-REM stable sleep during normal breathing on room air. f, respiratory rate; PETCO2, end-tidal CO2 partial pressure; REM, rapid eye movement; , oxygen saturation; TI, inspiratory duration; TE, expiratory duration; TTot, breath duration; TI/ttot, fractional inspiratory time; V̇e, minute ventilation; VMAX insp, maximal inspiratory pressure; VT, tidal volume.
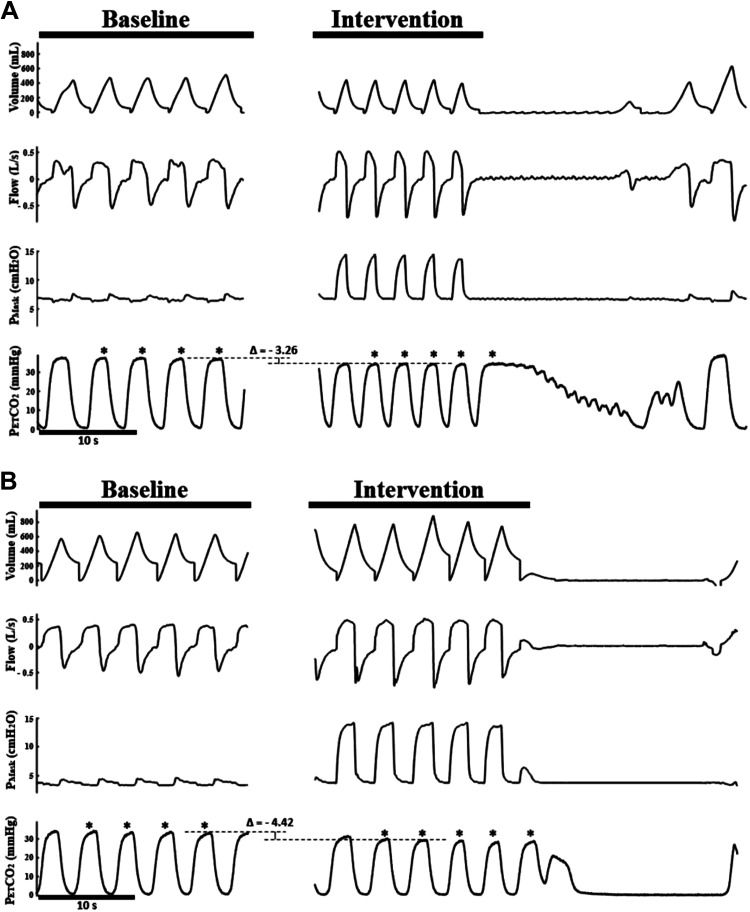
Figure 2.
Representative polygraphs from the NIV protocol depicting the difference in CO2 reserve in an SCI subject between the placebo (A) and mirtazapine (B) arms. Eupneic PETCO2 is represented by the upper dashed line of the bracket, and the AT PETCO2 is represented by the lower dashed line of the bracket. The breaths used to calculate these values are denoted with asterisks. The CO2 reserve was wider in the mirtazapine arm than the placebo arm. AT PETCO2, end-tidal CO2 partial pressure at apneic threshold; NIV, noninvasive ventilation; Pmask, mask pressure; SCI, spinal cord injury.
Statistical Analysis
A priori power analysis of this study indicated that 10 participants were required. The sample size was determined based on an analysis of variance with type 1 error 0.05, with minimal expected power of 0.8 to detect an effect size of 1 mmHg (clinically significant value) and a standard deviation of 1 mmHg for CO2 reserve.
Demographic characteristics were summarized as mean and standard deviation (SD) or frequency and percentage scores, as applicable. A paired-samples t test was used to examine the change in CO2 reserve, CG, plant gain (PG), as well as SDB and ventilatory parameters between the two treatment groups (mirtazapine vs. placebo). All analyses were considered statistically significant at P ≤ 0.05. Due to one participant’s CO2 reserve being determined using interpolation and another requiring CO2 to eliminate CSA, their results could not be included in the analysis for controller gain and AT PETCO2 (n = 8).
RESULTS
We studied 10 participants who had SDB (defined as AHI ≥ 5 events/h), seven of which had chronic SCI (see Table 1 for a summary of baseline demographics). Figure 2 displays representative polygraphs of a single participant for the placebo and mirtazapine arms. The difference in PETCO2 values between the dashed lines on the two nights provides a visual indication of how the CO2 reserve was determined for each participant.
Table 1.
Participant demographics at baseline
| Demographic Characteristics | Group (n = 10) |
|---|---|
| n | 10 |
| Age, yr | 52.0 ± 11.18 |
| BMI, kg/m2 | 25.7 ± 4.8 |
| Gender, M/F | 10/0 |
| NC, cm | 40.2 ± 2.8 |
| Time since injury, y (n = 7) | 5.3 ± 6.8 |
| Level of injury | |
| Cervical | 4 |
| Thoracic | 3 |
| Not injured | 3 |
| ASIA score | |
| A | 3 |
| C | 2 |
| D | 2 |
| ESS | 11.0 ± 5.2 |
| FSS | 27.8 ± 14.6 |
| PSQI (n = 9) | 8.3 ± 3.9 |
| AHI, events/h | 33.6 ± 23.3 |
| CAI, events/h | 10.6 ± 17.2 |
| ODI, events/h | 14.2 ± 16.7 |
Values are presented as means ± SD. Note: One subject did not complete the baseline PSQI.
AHI, apnea-hypopnea index; ASIA, American Spinal Injury Association; BMI, body mass index; CAI, central apnea index; ESS, Epworth sleepiness scale; FSS, fatigue severity scale; NC, neck circumference; ODI, oxygen desaturation index; PSQI, Pittsburgh sleep quality index.
Baseline Ventilation
A number of ventilatory parameters were assessed at baseline during NREM sleep and are displayed in Table 2 None of the parameters assessed were found to be significantly different between placebo and mirtazapine treatments.
Physiological Outcomes
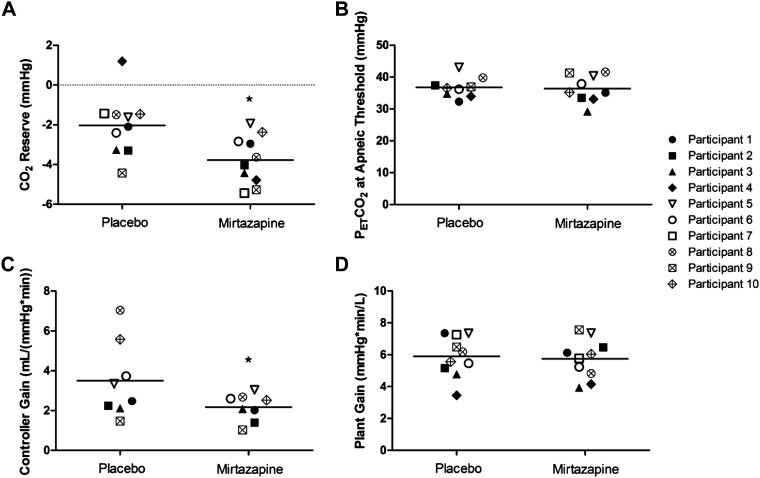
Figure 2 depicts the widening of CO2 reserve in a participant on mirtazapine compared with placebo. In addition, for participants who underwent NIV trials to induce hypocapnic central apnea, there was a significant decrease in their PAP level requirement during those trials for mirtazapine compared with placebo nights, respectively (6.6 ± 2.9 vs. 8.8 ± 3.7 mmHg; P = 0.044). Figure 3A depicts a significant widening of CO2 reserve on mirtazapine compared with placebo (−3.8 ± 1.2 vs. −2.0 ± 1.5 mmHg; P = 0.015). Figure 3B shows a summary of AT PETCO2 between the different interventions. There were no significant changes in AT PETCO2 for mirtazapine compared with placebo [36.4 ± 4.2 mmHg vs. 36.8 ± 3.2 mmHg; P = not significant (NS)]. Figure 3C shows that mirtazapine significantly decreased CG compared with placebo (2.2 ± 0.7 vs. 3.5 ± 1.9 L/(mmHg × min); P = 0.023). However, PG was not significantly different for mirtazapine than placebo (5.7 ± 1.2 vs. 5.9 ± 1.3 mmHg × min/L; P = NS) (Fig. 3D).
Figure 3.
Effect of mirtazapine on CO2 reserve (n = 10) (A), PETCO2 at apneic threshold (n = 9) (B), controller gain (n = 8) (C), and plant gain (n = 10) (D). *P < 0.05 vs. placebo using paired t test, presented as means ± SD. Solid symbols denote participants with cervical SCI, hollow symbols denote participants with thoracic SCI, x-marked symbols denote able-bodied participants. AT PETCO2, end-tidal CO2 partial pressure at apneic threshold; SCI, spinal cord injury.
Clinical Outcomes
A summary of the indices of SDB between each intervention arm is highlighted in Table 3. For the entire group analysis, AHI was not significantly different for mirtazapine compared with placebo (46.4 ± 22.5 vs. 47.8 ± 20.6 events/h; P = NS), and there were also no significant differences for the central-apnea index (CAI), obstructive-apnea index (OAI), or oxygen-desaturation index (ODI) (Table 3).
Table 3.
Sleep-disordered breathing parameters of participants after 6 days of placebo vs. mirtazapine treatment
| Placebo | Mirtazapine | |
|---|---|---|
| TRT, min | 247.0 ± 138.6 | 187.0 ± 65.3 |
| TST, min | 201.5 ± 124.2 | 148.8 ± 58.1 |
| Sleep efficiency, % | 81.3 ± 15.0 | 79.2 ± 14.6 |
| Sleep inset latency, min | 11.4 ± 19.6 | 5.7 ± 9.1 |
| N1 sleep time, % sleep time | 47.5 ± 17.5 | 58.4 ± 20.1 |
| N2 sleep time, % sleep time | 46.4 ± 20.1 | 30.0 ± 17.1 |
| N3 sleep time, % sleep time | 1.4 ± 4.2 | 2.6 ± 7.4 |
| REM sleep time, % sleep time | 4.8 ± 5.3 | 9.0 ± 11.5 |
| Arousal index, arousals/h | 51.5 ± 20.9 | 53.0 ± 17.7 |
| AHI, events/h | 47.8 ± 20.6 | 46.4 ± 22.5 |
| OAI, apneas/h | 14.1 ± 18.8 | 12.8 ± 15.7 |
| CAI, apneas/h | 4.8 ± 8.3 | 3.5 ± 5.5 |
| ODI, desaturations/h | 18.3 ± 17.9 | 21.2 ± 20.6 |
Values are presented as means ± SD; n = 10 participants. AHI, apnea-hypopnea index; CAI, central apnea index; OAI, obstructive apnea index; ODI, oxygen desaturation index; REM, rapid eye movement; TRT, total recording time; TST, total sleep time.
DISCUSSION
Summary of Findings
The novel findings of this study are as follows: 1) Administration of a standard dose of mirtazapine (15 mg) for 1 wk was associated with a widening of the CO2 reserve and decreased controller gain. 2) These alterations did not translate into an improvement in SDB severity or improvement in sleep quality with mirtazapine treatment.
Mirtazapine and Sleep-Disordered Breathing
Our finding that a 1-wk treatment period with mirtazapine was able to reduce CG and increase CO2 reserve in individuals with SDB, including chronic SCI, is the first of its kind. The efficacy of mirtazapine in treating SDB was initially studied using a rodent model and showed positive results (22), with a 50%–60% reduction in CSA observed. Despite this finding, there have been few studies and mixed results in human cohorts. The first reported trial in humans was a case study from Castillo et al. (24) in which they provided elderly male suffering from severe SDB (consisting of predominately CSA) with 15 mg of mirtazapine for a 3-mo period. Following treatment, there was a significant decrease in AHI (54.9 to 9.3 events/h) and both a reduction in daytime sleepiness and improvement in self-reported daytime functioning. These positive findings were replicated in a randomized, double-blind placebo-controlled crossover trial assessing both males and females with moderate SDB (23). Following 7 days with 15 mg of mirtazapine, mean AHI was significantly reduced from 22.3 to 11.4 events/h, arousal index was significantly reduced from 41.1 to 28.1 events/h, and sleep efficiency significantly increased from 82.9% to 90.1% compared with a placebo (23). Furthermore, it has also been established that mirtazapine can enhance sleep continuity and architecture in patients suffering from depression (27).
In contrast, Marshall et al. (28) carried out two randomized placebo-controlled trials investigating the effect of mirtazapine on patients with SDB and found no significant changes in any index of sleep or sleep apnea. The objective of their study was to assess the effect of differing mirtazapine doses, ranging from 0 to 45 mg/day for 2 wk. Not only did they see a significant worsening of AHI with the 15 mg/day and 30 mg/day doses (puzzlingly not with the 45 mg dose, however), but they also discontinued the trial due to significant weight gain within their participants, which is a known risk factor for SDB (29). The objective of their second study was to assess mirtazapine (15 mg dose) for a longer 4-wk period. None of the treatment groups resulted in any improvement in indices of SDB, leading the authors to conclude that mirtazapine is not an effective treatment for managing sleep apnea (28).
Due to the paucity of literature examining the efficacy of mirtazapine in regard to sleep and SDB, comparisons can be made with other pharmacological 5-HT receptor modulators. One such example is buspirone, a serotonin receptor agonist that augments central 5-HT1 activity (30). Increasing activity of 5-HT1 receptors seems to result in an increase in ventilation (31), which has been observed to stabilize breathing and reduce indices of SDB in both animal and human models (15, 30–33). Furthermore, recent evidence suggests that buspirone can blunt chemoreceptor activity (controller gain) in systolic heart failure patients, leading to a reduction in loop gain and, subsequently, a reduction in central sleep apneas throughout a night (34, 35). Recent data from our group indicate that buspirone can also reduce controller gain and widen CO2 reserve in an SCI cohort (17). Although this current study was underpowered to detect changes in indices of SDB, we did observe a reduction in chemosensitivity and a widening of the CO2 reserve following 7 days of mirtazapine treatment. Individuals with OSA have a narrower CO2 reserve and higher chemoreflex sensitivity than healthy individuals, which may contribute to ventilatory instability and an increased propensity to develop central SDB (36).
Furthermore, our group has previously demonstrated that individuals with cervical SCI have a narrower CO2 reserve and increased PG compared with able-bodied individuals (14) (including ∼40% of participants that did not have CSA) as well as increased chemosensitivity (37). Furthermore, we have also noted that individuals with cervical SCI exhibit long-term facilitation, a serotonin-dependent central nervous system phenomenon most likely acting via the 5-HT2A receptor on phrenic motor neurons (38). This supports a potential role for medications that modulate 5-HT in treating SDB (especially in individuals with SCI) due to their stimulatory effect on dilator motor neurons, respiratory output, and their role in stimulating both hypoglossal and phrenic nerves leading to respiratory plasticity or what is known as long-term facilitation (LTF). In addition, the hypocapnic apneic threshold is unmasked during a state of sleep, and thus the width of the CO2 reserve can be a significant determinant of breathing instability (39). For example, with a narrowed CO2 reserve, only a minor perturbation in PETCO2 levels can result in central apnea from occurring. Therefore, modalities that can enlarge the CO2 reserve and reduce CG may be advantageous for reducing CSA onset. In this study, we noticed that despite the widening of CO2 reserve, there was no difference in eupneic PETCO2 either between the mirtazapine night and placebo night or between subjects. However, the hypocapnic ventilatory response was less in the mirtazapine arm than the placebo arm. Therefore, mirtazapine may mitigate the propensity to hypocapnic central apnea. It must be emphasized that the purpose of this pilot study was to assess the physiological effects of mirtazapine on individuals with SDB; thus the effectiveness of mirtazapine for the treatment of central apnea in SCI or non-SCI individuals requires further testing in a clinical trial.
Mechanistic Action of Mirtazapine Treatment
Several mechanisms have been proposed for how mirtazapine may positively influence the physiology to elicit beneficial changes to sleep. Mirtazapine has been shown to inhibit both presynaptic a2-adrenoreceptors and postsynaptic 5-HT2 and 5-HT3 receptors (20, 21). Furthermore, mirtazapine seems to be a high-affinity antagonist at 5-HT2A, 5-HT2C, and 5-HT3 receptor sites, resulting in increased postsynaptic 5-HT1 activity within the brain and reduced 5-HT2 and 5-HT3 postsynaptic activity in the central and peripheral nervous systems (22). The antagonism of the presynaptic a2-receptors located on serotonergic neurons increases serotonin release, with an overall stimulation of respiration and suppression of apnea. In addition, animal studies have shown that increasing central serotonin release can enhance genioglossus and other upper airway musculature activity (25, 40, 41) and that the removal of serotonergic input can increase the likelihood of airway obstruction (42). There is also evidence to suggest that mirtazapine can improve sleep quality at doses of 15–30 mg (27, 43, 44). The 5-HT2 receptor may be connected to sleep initiation/maintenance (27), so the inhibition of these sites by mirtazapine (20) could be the cause of sleep architecture changes. An alternative explanation is that mirtazapine elicits a sedative effect by acting as a potent antagonist to histamine receptors (27, 28). Reducing sleep fragmentation and improving architecture may directly contribute to apnea suppression during NREM sleep (45).
Despite the promising mechanistic properties observed in animal models, it is currently unclear why these do not consistently result in improvements in humans. Disparate findings between studies may result from populations that differ phenotypically in some aspect that is important to the medication’s effect on SDB (28). To our knowledge, this is the first study to assess the effect of mirtazapine on SDB parameters in a population including patients with SCI. Although a reduced CG and widened CO2 reserve are promising findings, we are unable to explain why these did not result in a significant reduction of AHI or CAI. There is evidence that individuals with SCI (most notably at the cervical level) are more likely to develop central SDB (14); however it is currently unclear how 5-HT neurons mediate this process. Further investigations exploring the mechanistic action of 5-HT modulators in an SCI population are warranted.
It is also possible that differences in the dose or duration of treatment could affect the different outcomes observed, considering that dose-specific differences have been previously noted for both OSA severity (28) and sedation level (46). We chose to study a daily 15 mg dose to be consistent with most prior studies assessing mirtazapine in relation to sleep (23, 24, 27, 28). Although this dose is commonly used, equivocal results have been observed on its ability to alter SDB severity. Further incongruity arises when considering that Marshall et al. (28) observed a worsening of OSA with a clear dose-response relationship between 15 mg and 30 mg, but not 45 mg. Future studies should continue to evaluate differing doses to determine the optimal level to elicit benefits.
Clinical Implications
Our findings suggest that mirtazapine is associated with decreased CG and narrowing of the CO2 reserve and hence mitigates the propensity to central apnea. Therefore, mirtazapine could be a viable treatment for reducing central SDB in patients with chronic SCI. In addition, the observed significant decrease in PAP level requirement on mirtazapine versus placebo may indicate some beneficial therapeutic effect on upper airway mechanics in individuals with SDB. Given the paucity of effective pharmacological treatment for central apnea (47), mirtazapine may be a candidate drug given its relatively rapid onset of action, high response and remission rates, and a generally favorable side-effect profile (48). Nevertheless, the potential side effect on weight gain observed in prior investigations must be assessed further as this could have negative implications in the SCI population.
Limitations
The most significant limitation of our study was the small sample size. Although we used a crossover design to attempt to mitigate the impact of this, this study was not virtually powered to detect changes in SDB parameters. This makes it somewhat difficult to make inferences on whether changes in CO2 reserve and CG would have any clinical significance in the SCI population we studied. We would expect these changes in CG and CO2 reserve to result in reduced CAI or AHI; however, this was not the case. This study was primarily designed as a novel physiological pilot study to determine whether a future clinical trial within this population would be warranted. In addition, there was heterogeneity in our population due to variations in injury severity (n = 3 ASIA A; n = 2 ASIA C; n = 2 ASIA D; n = 3 noninjured) and level (n = 4 cervical; n = 3 thoracic; n = 3 noninjured). Physiological differences caused by these injury variations may have reduced the ability to detect changes in ventilatory parameters or SDB indices.
However, despite the variation, the groups seem to have manifested similarly following mirtazapine administration, as shown in Fig. 3. Furthermore, the time since SCI occurrence was variable within our cohort (median: 7 yr, range: 1–27 yr). Guidelines for conducting clinical trials within patients with SCI indicate that the most dramatic improvements occur over the first 9 mo (49). However, a completely stable baseline may not be achieved until 18 mo after SCI (dependent on severity and level of the injury). Considering that SCI had occurred at least 18 mo prior in all but one participant (1 yr since injury), we believe that our sample displayed an adequate functional baseline. Only male participants participated in this study, so at present, the conclusions can only be applied to this demographic. Another factor that must be considered is medication compliance. We provided a telephone reminder to take the medications three times weekly.
In addition, we asked participants to self-report their medication intake and required them to bring the medication container to the laboratory on the night of testing (enabling us to count any remaining pills). Despite these measures, there is a small possibility that they may have forgotten and/or misplaced the medication. It is also important to acknowledge that sleep restriction can alter sleep physiology (50). Participants were asked to mildly restrict their sleep the night preceding the split-night study to increase the likelihood of them remaining asleep during the instrumentation involved with the intervention, so it is possible that this may have impacted the results. Despite this, however, we believe that sleep restriction would be a small effect on this protocol given the same protocol was applied on both arms of the study. Furthermore, this protocol is consistent with how clinical and physiological sleep laboratories perform sleep studies (17, 51). Finally, it is worth noting that the SDB of this cohort displayed a greater obstructive component than the central component. Future investigations should endeavor to study SCI participants with a greater central component of SDB to see if this would result in AHI changes.
Conclusions
In conclusion, our data showed that mirtazapine reduced chemosensitivity and widened the CO2 reserve in individuals with SDB and chronic SCI. Future studies using a greater sample size and a population with a more significant central apnea component should be performed to gain a better understanding of the clinical significance of mirtazapine treatment on SDB.
GRANTS
The study was funded by the US Department of Veterans Affairs Career Development Award 1IK2CX000547 and Merit Review 1I01CX001040, by the National Heart, Lung, and Blood Institute Grant R01HL130552, and in part by a grant from the Detroit Medical Center Foundation.
DISCLAIMERS
The opinions expressed in this article reflect those of the authors and do not necessarily represent the official views of the Veterans Affairs . This was not an industry-supported study.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S. conceived and designed research; S.M. and E.K. performed experiments; J.L.P., S.M., B.A., and A.S. analyzed data; J.L.P., S.M., S.V., E.K., B.A., M.S.B., and A.S. interpreted results of experiments; J.L.P., S.M., and S.V. prepared figures; J.L.P. and S.M. drafted manuscript; J.L.P., S.M., S.V., M.S.B., and A.S. edited and revised manuscript; J.L.P., S.M., S.V., E.K., B.A., M.S.B., and A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge and thank all of the veterans and volunteers from the Sleep Research Laboratory that helped with this study, and both Dr. George and Cheryl Lang, who assisted with the recruitment of participants.
REFERENCES
- 1.Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, Boehlecke B, Brown TM, Coleman J, Friedman L, Kapen S, Kapur VK, Kramer M, Lee-Chiong T, Owens J, Pancer JP, Swick TJ, Wise MS; American Academy of Sleep Medicine. Practice param4 eters for the use of continuous and bilevel positive airway pressure de4 vices to treat adult patients with sleep4related breathing disorders. Sleep 29: 375–380, 2006. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 2.Sankari A, Badr MS. Does auto-PAP work in patients with acute quadriplegia and sleep-disordered breathing? Thorax 74: 217–218, 2019. doi: 10.1136/thoraxjnl-2018-212729. [DOI] [PubMed] [Google Scholar]
- 3.Sankari A, Martin JL, Bascom AT, Mitchell MN, Badr MS. Identification and treatment of sleep-disordered breathing in chronic spinal cord injury. Spinal Cord 53: 145–149, 2015. doi: 10.1038/sc.2014.216. [DOI] [PubMed] [Google Scholar]
- 4.Aurora RN, Chowdhuri S, Ramar K, Bista SR, Casey KR, Lamm CI, Kristo DA, Mallea JM, Rowley JA, Zak RS, Tracy SL. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep 35: 17–40, 2012. doi: 10.5665/sleep.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 6.Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med 9: 542–548, 2003. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci 5: 401–402, 2002. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- 8.Nattie EE, Li A, Richerson GB, Richerson G, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol 556: 235–253, 2004. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol 566: 543–557, 2005. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol 85: 2224–2235, 2001. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- 11.Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol 42: 171–188, 1980. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- 12.Mendelson WB, Maczaj M, Holt J. Buspirone administration to sleep apnea patients. J Clin Psychopharmacol 11: 71–72, 1991. doi: 10.1097/00004714-199102000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Veasey SC. Serotonin agonists and antagonists in obstructive sleep apnea. Am J Respir Med 2: 21–29, 2003. doi: 10.1007/BF03256636. [DOI] [PubMed] [Google Scholar]
- 14.Sankari A, Bascom AT, Chowdhuri S, Badr MS. Tetraplegia is a risk factor for central sleep apnea. J Appl Physiol (1985) 116: 345–353, 2014. doi: 10.1152/japplphysiol.00731.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng YD, Bingaman M, Taveira-DaSilva AM, Pace PP, Gillis RA, Wrathall JR. Serotonin 1A receptor agonists reverse respiratory abnormalities in spinal cord-injured rats. J Neurosci 23: 4182–4189, 2003. doi: 10.1523/JNEUROSCI.23-10-04182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richmonds CR, Hudgel DW. Hypoglossal and phrenic motoneuron responses to serotonergic active agents in rats. Respir Physiol 106: 153–160, 1996. doi: 10.1016/s0034-5687(96)00079-5. [DOI] [PubMed] [Google Scholar]
- 17.Maresh S, Prowting J, Vaughan S, Kruppe E, Alsabri B, Yarandi H, Badr MS, Sankari A. Buspirone decreases susceptibility to hypocapnic central sleep apnea in chronic SCI patients. J Appl Physiol (1985) 129: 675–682, 2020. doi: 10.1152/japplphysiol.00435.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis R, Wilde MI. Mirtazapine: a review of its pharmacology and therapeutic potential in the management of major depression. CNS Drugs 5: 389–402, 1996. doi: 10.2165/00023210-199605050-00007. [DOI] [PubMed] [Google Scholar]
- 19.Rizwan A, Sankari A, Bascom AT, Vaughan S, Badr MS. Nocturnal swallowing and arousal threshold in individuals with chronic spinal cord injury. J Appl Physiol (1985) 125: 445–452, 2018. doi: 10.1152/japplphysiol.00641.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Boer T. The pharmacologic profile of mirtazapine. J Clin Psychiatry 57: 19–25, 1996. [PubMed] [Google Scholar]
- 21.DeVane CL. Differential pharmacology of newer antidepressants. J Clin Psychiatry 59: 85–93, 1998. [PubMed] [Google Scholar]
- 22.Carley DW, Radulovacki M. Mirtazapine, a mixed-profile serotonin agonist/antagonist, suppresses sleep apnea in the rat. Am J Respir Crit Care Med 160: 1824–1829, 1999. doi: 10.1164/ajrccm.160.6.9902090. [DOI] [PubMed] [Google Scholar]
- 23.Carley DW, Olopade C, Ruigt GS, Radulovacki M. Efficacy of mirtazapine in obstructive sleep apnea syndrome. Sleep 30: 35–41, 2007. doi: 10.1093/sleep/30.1.35. [DOI] [PubMed] [Google Scholar]
- 24.Castillo JL, Menendez P, Segovia L, Guilleminault C. Effectiveness of mirtazapine in the treatment of sleep apnea/hypopnea syndrome (SAHS). Sleep Med 5: 507–508, 2004. doi: 10.1016/j.sleep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Berry RB, Koch GL, Hayward LF. Low-dose mirtazapine increases genioglossus activity in the anesthetized rat. Sleep 28: 78–84, 2005. doi: 10.1093/sleep/28.1.78. [DOI] [PubMed] [Google Scholar]
- 26.Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, Troester MT, Vaughn BV. AASM Scoring Manual Updates for 2017 (Version 2.4). J Clin Sleep Med 13: 665–666, 2017. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winokur A, Sateia MJ, Hayes JB, Bayles-Dazet W, MacDonald MM, Gary KA. Acute effects of mirtazapine on sleep continuity and sleep architecture in depressed patients: a pilot study. Biol Psychiatry 48: 75–78, 2000. doi: 10.1016/s0006-3223(00)00882-9. [DOI] [PubMed] [Google Scholar]
- 28.Marshall NS, Yee BJ, Desai AV, Buchanan PR, Wong KKH, Crompton R, Melehan KL, Zack N, Rao SG, Gendreau RM, Kranzler J, Grunstein RR. Two randomized placebo-controlled trials to evaluate the efficacy and tolerability of mirtazapine for the treatment of obstructive sleep apnea. Sleep 31: 824–831, 2008. doi: 10.1093/sleep/31.6.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol (1985) 99: 1592–1599, 2005. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 30.Garner SJ, Eldridge FL, Wagner PG, Dowell RT. Buspirone, an anxiolytic drug that stimulates respiration1-3. Am Rev Respir Dis 139: 946–950, 1989. doi: 10.1164/ajrccm/139.4.946. [DOI] [PubMed] [Google Scholar]
- 31.Mendelson WB, Martin JV, Rapoport DM. Effects of buspirone on sleep and respiration. Am Rev Respir Dis 141: 1527–1530, 1990. doi: 10.1164/ajrccm/141.6.1527. [DOI] [PubMed] [Google Scholar]
- 32.Andaku DK, Mercadante MT, Schwartzman JS. Buspirone in Rett syndrome respiratory dysfunction. Brain Dev 27: 437–438, 2005. doi: 10.1016/j.braindev.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 33.El-Khatib MF, Kiwan RA, Jamaleddine GW. Buspirone treatment for apneustic breathing in brain stem infarct. Respir Care 48: 956–958, 2003. [PubMed] [Google Scholar]
- 34.Borrelli C, Giannoni A, Mirizzi G, Emdin M, Passino C. P3525 buspirone effect on chemoreflex and central apneas in heart failure. Eur Heart J 40: ehz745.0389, 2019. doi: 10.1093/eurheartj/ehz745.0389. [DOI] [Google Scholar]
- 35.Borrelli C, Mirizzi G, Passino C, Bramanti F, Iudice G, Benelli E, Agazio A, Grotti F, Emdin M, Giannoni A. Buspirone is effective on central apneas in patients with systolic heart failure. J Am Coll Cardiol 71: A941, 2018. doi: 10.1016/S0735-1097(18)31482-7. [DOI] [Google Scholar]
- 36.Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med 181: 189–193, 2010. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bascom AT, Sankari A, Badr MS. Spinal cord injury is associated with enhanced peripheral chemoreflex sensitivity. Physiol Rep 4: e12948, 2016. doi: 10.14814/phy2.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sankari A, Bascom AT, Riehani A, Badr MS. Tetrapelgia is associated with enhanced peripheral chemoreflex sensitivity and ventilatory long term facilitation. J Appl Physiol (1985) 119: 1183–1193, 2015. doi: 10.1152/japplphysiol.00088.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dempsey JA, Smith CA, Przybylowski T, Chenuel B, Xie A, Nakayama H, Skatrud JB. The ventilatory responsiveness to CO2 below eupnoea as a determinant of ventilatory stability in sleep. J Physiol 560: 1–11, 2004. doi: 10.1113/jphysiol.2004.072371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bayliss DA, Viana F, Talley EM, Berger AJ. Neuromodulation of hypoglossal motoneurons: cellular and developmental mechanisms. Respir Physiol 110: 139–150, 1997. doi: 10.1016/s0034-5687(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 41.Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett 143: 164–168, 1992. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- 42.Veasey SC, Panckeri KA, Hoffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep-disordered breathing. Am J Respir Crit Care Med 153: 776–786, 1996. doi: 10.1164/ajrccm.153.2.8564132. [DOI] [PubMed] [Google Scholar]
- 43.Radhakishun FS, van den Bos J, van der Heijden BC, Roes KC, O'Hanlon JF. Mirtazapine effects on alertness and sleep in patients as recorded by interactive telecommunication during treatment with different dosing regimens. J Clin Psychopharmacol 20: 531–537, 2000. doi: 10.1097/00004714-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Thase ME. Antidepressant treatment of the depressed patient with insomnia. J Clin Psychiatry 60: 28–31, 1999. [PubMed] [Google Scholar]
- 45.Carley D, Trbovic S, Monti D, Radulovacki M. Effects of sleep fragmentation and clonidine administration on apnea in the rat. Research Communications in Psychology Psychiatry and Behavior 20: 95–112, 1995. [Google Scholar]
- 46.Kasper S, Praschak-Rieder N, Tauscher J, Wolf R. A risk-benefit assessment of mirtazapine in the treatment of depression. Drug Saf 17: 251–264, 1997. [Erratum in Drug Saf 18: 123, 1998]. doi: 10.2165/00002018-199717040-00005. [DOI] [PubMed] [Google Scholar]
- 47.Panossian LA, Avidan AY. Review of sleep disorders. Med Clin North Am 93: 407–425, 2009. doi: 10.1016/j.mcna.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Alam A, Voronovich Z, Carley JA. A review of therapeutic uses of mirtazapine in psychiatric and medical conditions. Prim Care Companion CNS Disord 15: PCC.13r01525, 2013. doi: 10.4088/PCC.13r01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J, Dobkin BH, Havton LA, Ellaway PH, Fehlings MG, Privat A, Grossman R, Guest JD, Kleitman N, Nakamura M, Gaviria M, Short D. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45: 190–205, 2007. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- 50.Webb WB, Agnew HW. The effects on subsequent sleep of an acute restriction of sleep length. Psychophysiology 12: 367–370, 1975. doi: 10.1111/j.1469-8986.1975.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 51.Ginter G, Sankari A, Eshraghi M, Obiakor H, Yarandi H, Chowdhuri S, Salloum A, Badr MS. Effect of acetazolamide on susceptibility to central sleep apnea in chronic spinal cord injury. J Appl Physiol (1985) 128: 960–966, 2020. doi: 10.1152/japplphysiol.00532.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]