Abstract
Muscle atrophy and decline in muscle strength appear very rapidly with prolonged disuse or mechanical unloading after acute hospitalization or experimental bed rest. The current study analyzed data from short-, medium-, and long-term bed rest (5–120 days) in a pooled sample of 318 healthy adults and modeled the mathematical relationship between muscle strength decline and atrophy. The results show a logarithmic disuse-induced loss of strength and muscle atrophy of the weight-bearing knee extensor muscles. The greatest rate of muscle strength decline and atrophy occurred in the earliest stages of bed rest, plateauing later, and likely contributed to the rapid neuromuscular loss of function in the early period. In addition, during the first 2 wk of bed rest, muscle strength decline is much faster than muscle atrophy: on day 5, the ratio of muscle atrophy to strength decline as a function of bed rest duration is 4.2, falls to 2.4 on day 14, and stabilizes to a value of 1.9 after ∼35 days of bed rest. Positive regression revealed that ∼79% of the muscle strength loss may be explained by muscle atrophy, while the remaining is most likely due to alterations in single fiber mechanical properties, excitation-contraction coupling, fiber architecture, tendon stiffness, muscle denervation, neuromuscular junction damage, and supraspinal changes. Future studies should focus on neural factors as well as muscular factors independent of atrophy (single fiber excitability and mechanical properties, architectural factors) and on the role of extracellular matrix changes. Bed rest results in nonuniform loss of isometric muscle strength and atrophy over time, where the magnitude of change was greater for muscle strength than for atrophy. Future research should focus on the loss of muscle function and the underlying mechanisms, which will aid in the development of countermeasures to mitigate or prevent the decline in neuromuscular efficiency.
NEW & NOTEWORTHY Our study contributes to the characterization of muscle loss and weakness processes reflected by a logarithmic decline in muscle strength induced by chronic bed rest. Acute short-term hospitalization (≤5 days) associated with periods of disuse/immobilization/prolonged time in the supine position in the hospital bed is sufficient to significantly decrease muscle mass and size and induce functional changes related to weakness in maximal muscle strength. By bringing together integrated evaluation of muscle structure and function, this work identifies that 79% of the loss in muscle strength can be explained by muscle atrophy, leaving 21% of the functional loss unexplained. The outcomes of this study should be considered in the development of daily countermeasures for preserving neuromuscular integrity as well as preconditioning interventions to be implemented before clinical bed rest or chronic gravitational unloading (e.g., spaceflights).
Keywords: aging, bed rest immobilization, mechanical unloading, muscle atrophy, neuromuscular efficiency
INTRODUCTION
Prolonged bed rest is associated with health deficits and functional impairment (1). With a persistency beyond the acute period of inactivity accompanied by long recovery periods (2), physical deconditioning has gained socioeconomic importance in contemporary societies, increasingly raising scientific debates about time courses and underlying mechanisms (3).
One of the most prominent clinical hazards of bed rest is physical inactivity and mechanical unloading, manifested by muscle atrophy and loss of muscle function requiring extensive physical rehabilitation (4). Prolonged periods of bed rest result in a significant atrophy of musculoskeletal mass and volume in the weight-bearing leg musculature such as the quadriceps femoris (5–8). Moreover, bed rest has a substantial impact on muscle function established in a decline in performance (9, 10). Numerous studies using gold standard technologies for monitoring atrophic effects [magnetic resonance imaging (MRI) and computed tomography (CT)] and muscle strength decreases (isokinetic or isometric torque measures) have shown that bed rest degradation is progressive but not linear. Thereby, recent findings clustered the periods of disuse in three subsections: acute periods of 1–10 days, the mid-term interval of 11 days up to 1 mo and long-term periods >1 mo (11). Studies in adults documented that skeletal muscle size and function are already reduced after a few days of bed rest (12) and continue to decline with the length of exposure (13) until an asymptotic saturation is reached. Declines after short periods are of high clinical relevance as patients are hospitalized on average for 7 days (14). With advancement in understanding the cellular and neuronal mechanisms involved in the effect of disuse on skeletal muscle atrophy and strength deficits, signaling pathways and the plasticity of the central nervous system have been studied to understand their regulatory role in these processes (2).
Neurostructural changes during phases of disuse have been described elsewhere (15–17); however, a systematic review of bed rest-related decline of skeletal muscle mass and strength with the intent to describe the proportionality for the factors muscle atrophy and function has not yet been compiled. Scientific evidence manifests discrepancies in the magnitude and degradation rates between atrophy and the functional decline of skeletal muscles as a response to bed rest (18–20). Numerically established degeneration rates for the knee extensors differed by 5% to 80% dependent on the duration of disuse (6, 18, 21–23). With the emphasis on current socioeconomic challenges of the 21st century associated with hospitalization and disuse, the number of bed rest studies has increased in recent years, and the density of publications allows a systematic analysis and synthesis of the data for a conclusive statement. Although duration of bed rest is a leading factor in the magnitude of decline in muscle mass and strength, it would be interesting to see the pure effect of bed rest after also controlling for age, sex, and bed rest modality (horizontal vs. head-down tilt). In addition, it is important to determine the effect of bed rest on muscle strength and muscle mass and their interrelationship as major determinants of health outcomes (24). Therefore, our aim was to systematically review and synthesize the existing literature on muscle atrophy and strength decline of the upper leg musculature in humans in vivo in relation to the duration of bed rest.
MATERIALS AND METHODS
Search Strategy
Following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, our comprehensive search included the following electronic databases from inception to April 15, 2020: PubMed/MEDLINE (NLM), Embase, Scopus, Web of Science, and Google Scholar. The search aimed to identify all available research that investigated muscle mass and size (volume and cross-sectional area of knee extensor muscles, respectively) and function (isometric maximal voluntary contraction of knee extensor muscles) outcomes following bed rest intervention in healthy adult populations. The search was executed independently by two researchers, R.R. and U.M. The search string was created with two sections: the first encompassed synonyms for bed rest while the second was composed of synonyms for the topic of muscle (Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.13604447). To ensure that at least one search term within one section was included in the results, all synonyms were connected with the operator “OR” and both sections were connected with the operator “AND.” Furthermore, truncations and adjacency searching were used to find variations of the corresponding term and to restrict the results to specific ordered terms. Database searching was performed with no restrictions (“All field/All text” search) except in Scopus where the search was restricted to “Title, Abstract, Keywords.” Manuscripts written in English and including humans with specific deviations of keyword combinations comprising “bed rest,” “muscle mass,” and “muscle function” were included (for details, see Supplemental Table S1).
Inclusion and Exclusion Criteria
All studies were screened for eligibility with regard to our inclusion and exclusion criteria, which were based on the PICOS principle (i.e., population extraction, intervention, comparison intervention, outcome measures, and study design information) (25, 26). Studies were considered relevant if 1) subjects were healthy adults (aged 18–50 yr), 2) the study design allowed comparisons between pre- and postbed rest values, and 3) muscle mass and size and/or strength of the weight-bearing body regions of the leg were assessed pre- and postbed rest.
Studies were not considered relevant if 1) participants had received a substance previously shown to result in muscle loss, or 2) the manuscript was not written in the English language. Additionally, the quality of reports was determined using the Physical Evidence Database (PEDro) scale, which is based on the Delphi list (27) (Supplemental Table S2; see https://doi.org/10.6084/m9.figshare.12287501). Studies with a score <3 were excluded from this review. For each of the 11 items of the PEDro scale, 2 researchers (R.R. and U.M.) assessed the studies independently. In four cases, a third reviewer (M.N.) evaluated the study to find a consensus.
Participants
All participants in the included bed rest studies were healthy at baseline. Data related to drug, dietary, exercise, or any other interventions were excluded as they could bias the outcomes (28–30). Populations with chronic diseases, illnesses, neurological disorders, orthopedic dysfunction, or any other medical problem were excluded from analysis. Participants aged >50 were not included as age- or menopause-induced endocrine adaptations secondary to bed rest could have negatively affected the sample’s homogeneity (31, 32). No exclusion was made in regard to the baseline level of physical condition beyond being healthy. Included studies had to report outcomes relating to muscle mass and size and/or function. For completeness of reporting the current state of the evidence base and to avoid introducing selection bias, no exclusion was made based on type of outcome or amount of data. The study population is described in Table 1.
Table 1.
Characteristics of included trials reporting bed-rest-induced changes in muscle mass and size and muscle function
| Study (Ref.) | Average Characteristics of Included Participants (n, Sex, Age, Height, Mass) | Bed Rest Modality, Bed Rest Duration, Days Between Baseline and Post Measurement | Assessment Method (Parameter and Units) | Relative Change at the End of Bed Rest |
|
|---|---|---|---|---|---|
| Atrophy of the knee extensor muscles | Knee extension strength | ||||
| Mulder et al. (33) | Healthy adults, n = 10 (0 women), age 30 ± 6 yr, height 179 ± 5 cm, mass 78 ± 5 kg | 6° Head-down tilt bed rest, 5 days, 5 days | 1.5T MRI (m. quadriceps femoris CSA in mm2); MVIC at a 90° knee angle | –2.2 % | –8.6 % |
| Rittweger et al. (34) | Healthy adults, n = 12 (0 women), age 34 ± 7 yr, height 179 ± 7 cm, mass 76 ± 6 kg | 6° Head-down tilt bed rest, 5 days, 5 days | MVIC at a 90° knee angle | NA | –4.1 % |
| Dirks et al. (12) | Healthy adults, n = 10 (0 women), age 23 ± 1 yr, height 181 ± 2 cm, mass 75 ± 2 kg | Horizontal bed rest, 7 days, 7 days | Computed tomography (knee extensors: m. quadriceps femoris CSA in mm2) | –3.0 % | NA |
| Dirks et al. (35) | Healthy adults, n = 10 (0 women), age 27 ± 1 yr, height 181 ± 3 cm, mass 78 ± 5 kg | Horizontal bed rest, 7 days, 7 days | Computed tomography (knee extensors: m. quadriceps femoris CSA in mm2) | –1.1% | NA |
| Ferrando et al. (36) | Healthy young adults, n = 5 (0 women), age 23 ± 3 yr | Horizontal bed rest, 7 days, 7 days | 0.6T MRI (thigh volume in cm3 | –3.0 % | NA |
| McDonnell et al. (37) | Healthy adults, n = 11 (0 women), age 24 ± 2 yr, height 180 ± 7 cm, mass 73 ± 12 kg | Horizontal bed rest, 10 days, 10 days | Computed tomography (knee extensors: m. quadriceps femoris CSA in mm2); MVIC at 60 °knee flexion | –3.6 % | –14.5 % |
| Bamman et al. (38) | Healthy adults, n = 8 (0 women), age 30 ± 2 yr, mass 178 ± 2 cm, mass 72 ± 4 kg | 6° Head-down tilt bed rest, 14 days, 14 days | MVIC at a 60° rad of knee flexion | NA | –14.5 % |
| Pisot et al. (10)a | Healthy young adults, n = 7 (0 women), age 23 ± 3 yr, height 177 ± 7 cm, mass 75 ± 9 kg | Horizontal bed rest, 14 days, 14 days | 1.5T MRI (m. quadriceps femoris volume in cm3); MVIC at a 110° knee angle with hip fixed at 90° | –6.1 % | –9.8 % |
| Akima et al. (22) | Healthy adults n = 4 (0 women), age 20 ± 2 yr, height 171 ± 5 cm, mass 67 ± 11 kg | 6° Head-down tilt bed rest, 20 days, 21 days | 1.0T MRI (knee extensors: m. quadriceps femorisb; volume in cm3 and CSA in cm2); MVIC at a 90° rad of knee flexion | –7.5 % | –16.8 % |
| Akima et al. (39) | Healthy adults, n = 6 (0 women), age 23 ± 3 yr, height 170 ± 8 cm, mass 67 ± 14 kg | 6° Head-down tilt bed rest, 20 days, 20-23 days | 0.2T MRI (knee extensors: m. quadriceps femoris; volume in cm3) | –10.3 % | NA |
| Kawakami et al. (19) | Healthy adults, n = 5 (0 women) | 6° Head-down tilt bed rest, 20 days, 20 days | MRI (m. quadriceps femoris; CSA in cm2) | –10.2 % | NA |
| Kawakami et al. (19) | Healthy adults, n = 4 (0 women), age 18-28 yr, height 172 ± 5 cm, mass 68 ± 11 kgc | 6° Head-down tilt bed rest, 20 days, 20 days | 1.0T MRI (m. quadriceps femoris CSA in cm2); MVIC with hip angle at 80° and knee angle at 90° | –7.8 % | –10.2 % |
| Kubo et al. (6) | Healthy adults, n = 6 (0 women), age 24 ± 5 yr, height 172 ± 5 cm, mass 68 ± 11 kg | 6° Head-down tilt bed rest, 20 days, 21 days | 0.5T MRI (m. quadriceps femoris CSA); MVIC with hip and knee angles both 80° | –7.5 % | –19.2 % |
| Kubo et al. (7) | Healthy adults, n = 8 (0 women), age 24 ± 4 yr, height 172 ± 9 cm, mass 69 ± 13 kg | 6° Head-down tilt bed rest, 20 days, 20 days | MRI (m. quadriceps femoris volumed); MVIC with hip and knee angles both 80° | –10.4 % | –20.4 % |
| Shinohara et al. (40) | Healthy adults, n = 6 (0 women), age 23 ± 3 yr, height 170 ± 8 cm, mass 67 ± 14 kg | 6° Head-down tilt bed rest, 20 days, 20 days | MVIC at a 90° knee angle and 80° hip angle | NA | –17.6 % |
| Suzuki et al. (41) | Healthy adults, n = 11 (5 women), mean age 21 yr, height NA, mass NA | Horizontal bed rest, 20 days, 20 days | Max isokinetic torque at 90° knee and hip angles | NAe | –15.8 % |
| Liphardt et al. (42) | Healthy adults, n = 11 (0 women), age 35 ± 8 yr, height 176 ± 6 cm, mass 70 ± 8 kg | 6° Head-down tilt bed rest, 21 days, 21 days | 1.5T MRI (knee extensors: m. quadriceps femoris CSA in cm2) | –9.9 % | NA |
| Germain et al. (43) | Healthy adults, n = 6 (0 women), age 34 ± 2 yr, height 178 ± 1 cm, mass 79 ± 3 kg | 6° Head-down tilt bed rest, 28 days, 28 days | MVIC at 0° × s−1, the lower leg was fixed at an angle of 105° compared with full extension (0°) | NA | –12.0 % |
| Alkner and Tesch (44)f | Healthy adults, n = 9 (0 women), age 32 ± 4 yr, height 173 ± 3 cm, mass 72 ± 5 kg | 6° Head-down tilt bed rest, 29 days, 29 days | 1.0T MRI (knee extensors: m. quadriceps femoris; volume in cm3) | –9.8 % | NA |
| Berry et al. (45) | Healthy adults (professional divers), n = 6 (0 women), age 31.5 yr (range: 27–36 yr) | 6° Head-down tilt bed rest, 30 days, 31–32 days | 0.5T MRI (m. quadriceps femoris CSA in cm2) | –11.0 % | NA |
| Dudley et al. (46) | Healthy adults, n = 7g | 6° Head-down tilt bed rest, 30 days, R + 1 = 31days | MVIC with hip angle at 100° | NA | –24.0 % |
| Greenleaf et al. (47) | Healthy adults, n = 5 (0 women), age 36 ± 1 yr, height 178 ± 2 cm, mass 75 ± 5 kg | 6° Head-down tilt bed rest, 30 days, 30 days | MVIC during knee extension at 100°/s | NA | –16 % |
| Schneider et al. (48)h | Healthy twins, n = 15 (7 women), age 34 ± 7 yr, height 179 ± 7 cm, mass 76 ± 6 kg | 6° Head-down tilt bed rest 30 days, 28 days | MVIC at a 60° knee angle | NA | –13.0 % |
| Berg et al. (18) | Healthy adults, n = 10 (0 women), age 25 ± 5 yr, height 180 ± 9 cm, mass 71 ± 8 kg | Horizontal bed rest, 35 days, 35 days CSA; 36 days MVIC | Computed tomography (knee extensors: m. quadriceps femoris CSA in cm2); MVIC with hip and knee angles at 90°; 15° reclined back support from vertical | –9.5 | –16.9 % |
| Gogia et al. (49) | Healthy adults, n = 9 (0 women), age 29 ± 10 yri | Horizontal bed rest, 35 days, 35 days | MVIC at 60 °s/s from 0° to 180° extension | NA | –19 % |
| Krainski et al. (20) | Healthy adults n = 9 (1 women), age 33 ± 13 yr, height 174 ± 11 cm, mass 71 ± 10 kg | 6° Head-down tilt bed rest, 35 days, 35 days | 1.5 T MRI (m. quadriceps femoris volume in ml); max isokinetic torque at 60°/s | –8.9 % | –13.7 % |
| Berg et al. (23) | Healthy adults, n = 7 (0 women), age 28 ± 2 yr, height 176 ± 3 cm, mass 74 ± 9 kg | 6° Head-down tilt bed rest, 42 days, 37 days CSA; 44 days MVIC | 1.5T MRI (m. quadriceps femoris CSA in cm2); MVIC at 95° knee and 180° hip angle | –14.2 % | –25.8 % |
| Ferretti et al. (50) | Healthy adults, n = 7 (0 women), age 28 ± 1 yr, height 176 ± 1 cm, mass 74 ± 3 kg | 6° Head-down tilt bed rest, 42 days, 37 days | 1.5T MRI (thigh CSA in cm2) | –13.4 | NA |
| Ferretti et al. (51) | Healthy adults, n = 7 (0 women), age 28 ± 1 yr, height 176 ± 1 cm, mass 75 ± 9 kg | 6° Head-down tilt bed rest, 42 days, 47 days | 1.5T MRI (thigh CSA in cm2) | –17.0 % | NA |
| Belavy et al. (52) | Healthy adults, n = 10 (0 women),j age 33 ± 7 yr, height 185 ± 7 cm, mass 79 ± 10 kg | Horizontal bed rest, 56 days, 56 days | 1.5T MRI (knee extensors: m. quadriceps femoris volume in cm3) | –14.4 %k | NA |
| Mulder et al. (5)l | Healthy adults, n = 10 (0 women), age 33 ± 7 yr, height 185 ± 8 cm, mass 80 ± 11 kg | Horizontal bed rest, 56 days, 56 days | 1.5T MRI (m. quadriceps femoris CSA in cm2); MVIC with hip angle at 115° and knee angle at 90° | –14.2 % | –14.8 % |
| Arbeille et al. (53)m | Healthy adults, n= 8 (8 women), age 34 ± 1 yr, height 163 ± 2 cm, mass 56 ± 1 kg | 6° Head-down tilt bed rest, 60 days, 57 days | 1.5T MRI (knee extensors: m. quadriceps femoris volume in cm3) | –21.2 % | NA |
| Lee et al. (54) | Healthy adults, n = 8 (8 women), age 34 ± 1 yr, height 163 ± 2 cm, mass 56 ± 1 kg | 6° Head-down tilt bed rest, 60 days, 66 days | Max isokinetic torque between 20 and 95°; 60°/s | NA | –31.8 % |
| Miokovic et al. (55) | Healthy adults, n = 9 (0 women), age 31 ± 8 yr, height 181 ± 6 cm, mass 81 ± 5 kg | 6° Head-down tilt bed rest, 60 days, 55/56 daysn | 1.5T MRI (m. rectus femoris volume in cm3) | –14.6 % | NA |
| Mulder et al. (8) | Healthy adults, n = 9 (0 women), age 31 ± 8 yr, height 181 ± 6 cm, mass 81 ± 5 kg | 6° Head-down tilt bed rest, 60 days, 55/56 days MRI, 56 days MVIC | 1.5T MRI (m. quadriceps femoris CSA in cm2); MVIC with hip angle at 130° and knee angle at 60° | –13.7 % | –21.5 % |
| Trappe et al. (30) | Healthy adults, n = 8 (8 women), age 34 ± 1 yr, height 163 ± 2 cm, mass 56 ± 1 kg | 6° Head-down tilt bed rest, 60 days, 57 dayso | 1.5T MRI (knee extensors: m. quadriceps femoris volume in cm3) | –21.2% | NAp |
| Ploutz-Snyder et al. (56) | Healthy adults, n = 8 (1 woman), age 37 ± 8 yr, height NA, mass 79 ± 10 kg | 6° Head-down tilt bed rest, 70 days, 70 days | 1.5T MRI (m. quadriceps femoris CSA in cm2); max isokinetic torque at 60°/s | –9.6 % | –22.8 % |
| Alkner et al. (57)q | Healthy adults, n = 9 (0 women), age 32 ± 4 yr, height 173 ± 3 cm, mass 72 ± 5 kg | 6° Head-down tilt bed rest, 90 days, 89 days MRI; R + 1 = 91 days MVIC | 1.0T MRI (knee extensors: m. quadriceps femoris volume in cm3); MVIC at a 90° rad of knee flexionr | –18.0 % | –45 % |
| LeBlanc et al. (58) | Healthy adults, n = 8 (0 women), age 32 ± 12 yr, height 173 ± 7 cm, mass 74 ± 7 kg | Horizontal bed rest, 119 days, 126 days | 0.36-1.0T MRI (m. quadriceps femoris); max isokinetic torque at 60°/s and 180°/s | –15.0 % | –30.7 % |
| Shackelford et al. (59)s | Healthy adults, n = 8 (3 women), age 32 ± 9 yr, height 171 ± 9 cm, mass 74 ± 9 kg | Horizontal bed rest, 119 days, 119 days | 1.5T MRI (m. quadriceps femoris; volume); max isokinetic torque at 60°/s; range 10-95° | –15.8 % | –35.0 % |
aThis study shows a trend toward greater muscle atrophy and strength decline in old (–8.4%; –12.8%) vs. young adults (–6.1%; –9.8%). n, no. of subjects; MRI, magnetic resonance imaging; CSA, cross-sectional area; MVIC, maximal voluntary isometric contraction, T, tesla. bRF, rectus femoris; VL, vastus lateralis; VI, vastus intermedius; VM, vastus medialis. cAge and body characteristics are reported for total sample (n = 9). dRelative change reported only. eCross-sectional area decline of –10.4% reported by dual energy X-ray absorption (DEXA); reported in footnotes due to the fact that knee extensors cannot be extracted by DEXA alone and therefore changes were not included in the calculations in RESULTS. fThis paper reports on data from the initial 29 days of the 90-day bed rest campaign published in Alkner and Tesch (44) and Alkner et al. (57). gOther data not reported. hThis study shows a trend toward greater muscle strength decline in women (–16%) vs. men (–10%) after 28 days of bed rest. iAge reported for a total sample (n = 15). jMissing data on BR1 and BR28 due to magnetic resonance imaging (MRI) scanner failure and movement artifacts during data acquisition. kAuthors measured with MRI also on days 14, 28, and 42 with volumetric decline of –6.4, –9.1, and –12.0%, respectively. lStudy addressed muscle size and strength during a 56-day bed rest on 8 time points (6 during the bed rest) that might affect the outcome measures and was therefore removed from the calculations. mThis paper reports data that were published also in Trappe et al. (30). nAuthors measured with MRI also on day 27/28 with volumetric decline of –8.4%. oAuthors measured with MRI also on day 29 with volumetric decline of –16.8%. pMuscle strength was measured on a specifically designed flywheel inertial ergometer adapted for the 6° head-down tilt experiments (21). Pre-Post (R + 2) isometric force declined for –33.7%. For the regression analysis, muscle strength values were taken from Lee et al. (54), which belong to the same bed rest campaign. qReported also in Alkner and Tesch (21). rAuthors reported maximal voluntary isometric contraction (MVIC) also at a 120° rad of knee flexion where knee extension strength decline was –60.1%. sThis study shows a trend toward greater muscle strength decline in women (–21%) vs. men (–13%) after 119 days of bed rest.
Data Extraction and Assessment of Reviewer Agreement
After screening, relevant considered articles were assessed for eligibility based on their full texts. At this stage, we extracted information about 1) population characteristics, 2) bed rest and interventional characteristics, 3) primary outcome measures, 4) methods, and 5) the main results of the study. When effects were assessed at multiple time points during periods of bed rest, only the very last time point was included in the calculation. In case of incomplete raw data availability, we contacted the corresponding author of the manuscript or extrapolated the data from figures, if the authors could not be reached. All studies were assessed for inclusion in this systematic review independently by two researchers (R.R. and U.M.) based on the aforementioned extracted information. In case of disagreements about inclusion or exclusion of a study, a third reviewer (M.N.) was consulted. The judgement of the two researchers was consistent each time except once. There were no periods of reconciliation between the two researchers and the third reviewer. The extracted data from the included studies are presented in Supplemental Table S2 and Table 1.
Outcome Measures
The primary outcome measure was absolute knee extensors muscles mass and size established by volume (cm3) or cross-sectional area (cm2) via MRI or CT (61), as well as maximal voluntary isometric knee extensor contraction established isokinetically or isometrically by its monoarticular torque (62). We pooled the aforementioned spectrum of methods due to their high validity and reliability minimizing the selection bias (63–66). Other methodological approaches and technologies for estimating muscle size and strength were not considered in this review. Mixed measurements for knee extensor strength, which included muscle groups different from the knee extensors, were excluded from the analysis. To ensure maximum homogeneity and test reproducibility (61) we used the changes in muscle mass and size indicated for m. rectus femoris, the m. vastus lateralis, intermedius, and medialis (22, 62). If these changes were reported separately in the included manuscript, we calculated the sum and used this value for further analysis. The cross-sectional area (CSA) is equivalent to the muscle size and volume to muscle mass and thus they are not analogues but proportional to each other.
Synthesis of Results
For each study, percent changes {[(MEANpost − MEANpre)/MEANpre] × 100} of muscle strength, and muscle mass and size were calculated. In case of multiple assessment methods, the minimum and maximum mean value of each method are reported.
Statistical Analyses
In most cases data were extracted from trials reporting baseline and follow-up means, standard deviations, and total number of included participants. To extract the necessary data from manuscripts presenting the results in graphs only, the Web Plot Digitizer software (Version 3.10, Austin, TX) was used. In some cases, a transformation from means ± SE to means ± SD had to be performed manually. In cases of uncertainty of published data, additional explanation of the employed protocols and/or parameters was requested from manuscripts’ corresponding authors. Coding of outcome measures was performed by two reviewers (U.M. and R.R.) and approved by an expert (M.N.). Due to the nature of bed rest studies (low number of participants and large SD; e.g., Ref. 19), the priori decision was made to summarize the results of muscle atrophy and strength decline as relative deltas. A nonlinear regression analysis using logarithmic function was performed between days of bed rest and muscle atrophy as well as strength decline. Finally, the linear regression analysis between muscle atrophy and strength decline as a function of bed rest duration was applied only with data from studies that reported both modalities together. Both linear and nonlinear regression analyses controlled for age, sex, bed rest modality (horizontal vs. head-down tilt bed rest), and duration. R software version 4.0.3 for Windows (R Foundation for Statistical Computing, Vienna, Austria) was used for all regression and curve-fitting analyses. All statistical decisions were accepted at P ≤ 0.05.
RESULTS
Search Results
A total of 40 of the initial 4,258 studies were included in this systematic review. We evaluated the full texts of 108 studies (for the complete search process, see Fig. 1). After assessing the suitability of these articles based on our inclusion and exclusion criteria, we excluded studies that performed postmeasurements 2 days after the end of bed rest (n = 2); assessed changes in muscle strength in a multiarticular movement paradigm, gait assessment, or jump (n = 44); assessed changes in muscle strength and size in muscles other than knee extensors (n = 15); and assessed changes in muscle size using technologies other than MRI or CT (n = 7). Four additional studies had to be excluded due to poor study quality (PEDro score <4). Sixteen articles examined the effect of bed rest on both muscle atrophy and strength. A further 14 articles reported outcome measures of muscle atrophy (volume or size) and 10 studies reported on muscle strength.
Figure 1.
Summary of knee extensor muscle atrophy and strength decline as a function of bed rest duration.
The study characteristics are presented in Table 1. The mean age of included bed rest participants ranged from 20 to 37 yr and the number of participants included in these studies was 318 (41 women), with a range of 4 to 15 subjects per article. Bed rest modality varied across included trials; 28 of the included trials used 6° head-down tilt bed rest modality, 11 carried out horizontal bed rest, and one study did not specify the bed rest position. The majority of included bed rest studies can be classified as strict bed rest (24/7), but only a few reported an average of 30 min per day sitting in a wheelchair for toileting or showering (41, 67). In the current systematic review, we report results from short-, medium-, and long-term bed rest studies that varied from 5 to 120 days (Table 1).
Bed Rest Effects on Muscle Atrophy and Strength Parameters
Knee extensor muscle mass and size parameters show a nonlinear decline (see Fig. 1) with 58% explained variance (Supplemental Table S3: see https://doi.org/10.6084/m9.figshare.13604456). The muscle atrophy was fitted with the following curve [ln(abs(y(Muscle atrophy))) = 1.184 + 0.117 × X1(Bed rest duration) − 0.014 × X2(Mean age) − 0.003 × X(Sex) + 0.634 × X4(Bed rest modality)]. Calculated examples of muscle mass and size decrease of extra short-term (<1 wk; 5 days), short-term (1–2 wk; 10 days), medium-term (3–5 wk; 35 days), and long-term (6 wk onwards; 120 days) bed rest show a −1.2, −5.0, −11.8, and −18.6% decline, respectively.
Studies evaluating knee extensor strength parameters also showed a nonlinear descent trajectory, but almost twice as large as the descent trajectory of muscle mass and size parameters (Figs. 1 and 2). The curve-fitting model yielded 61% explained variance (Supplemental Table S4; see https://doi.org/10.6084/m9.figshare.13604459). The muscle strength decline was fitted with the following curve [ln(abs(y(Muscle strength decline))) = 3.303 + 0.015 × X1(Bed rest duration) − 0.037 × X2(Mean age) − 0.003 × X3(Sex) + 0.168 × X4(Bed rest modality)]. Calculated examples of muscle strength loss of extra short-term (<1 wk; 5 days), short-term (1–2 wk; 10 days), medium-term (3–5 wk; 35 days), and long-term (6 wk onwards; 120 days) bed rest show a −3.6, −9.9, −21.2, and −32.4% decline, respectively.
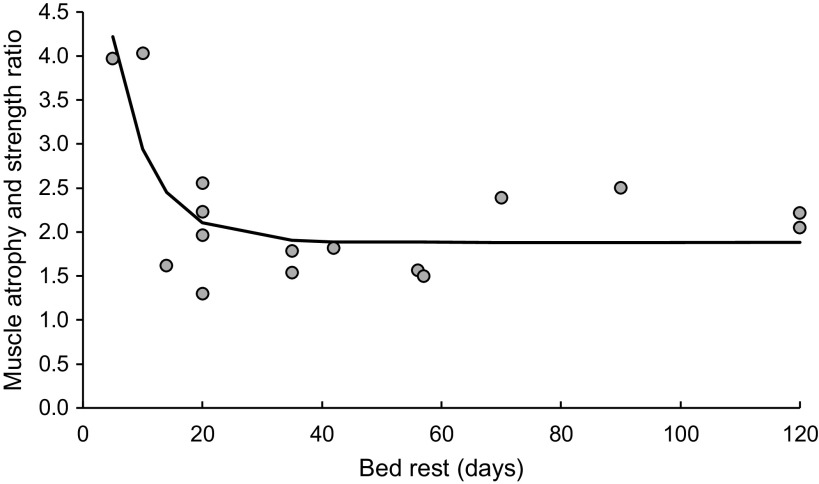
Figure 2.
The ratio of muscle strength to atrophy decline as a function of bed rest duration.
The muscle atrophy and strength decline ratio, as a function of bed rest duration, is shown in Fig. 2, with individual values of muscle atrophy and strength decline ratio as well as best-fitting curve [y = 1.882 + (7.021–1.882) e(−0.1576 × x)]. Over the first 2 wk of bed rest, the decline in muscle strength is much faster than that of muscle atrophy, it reaches a maximum of 4.2 on the 5th day, falling to 2.4 on the 14th day and stabilizing at a value of 1.9 after ∼35 days of bed rest.
Regression between the Decline in Muscle Strength and Muscle Atrophy
Sixteen studies reported both outcome modalities, muscle mass/size and strength, in the same study and were, due to consistency in bed rest protocols, further inserted into the regression analysis (Fig. 3). Our model revealed that 74% (P < 0.001) of variance of muscle strength decline can be explained with muscle atrophic processes. Furthermore, while controlling for additional covariates (age, sex, bed rest modality, and duration) the model revealed that 79% [F(5, 10) = 12.254, P = 0.001, adjusted r2 = 0.790] of variance can be explained. Due to several measurement points during bed rest that might affect the outcome measures, the study from Mulder et al. (5) was excluded (see discussion).
Figure 3.
Relationship between the muscle strength decline and muscle atrophy, including only studies that reported both parameters together.
DISCUSSION
The current study affords important insights into the disuse-related, nonuniform decline in strength and mass/size of weight-bearing muscles in healthy adults. Both muscle atrophy and strength demonstrated a nonlinear logarithmic decrease until an asymptotic saturation is reached after ∼3 mo of chronic bed rest. Higher deterioration rates coupled with earlier onsets have been manifested for maximal isometric strength as compared with muscle atrophy numerically factorized by 2 for synergistically acting knee extensors. In the first 2 wk of bed rest, the decline in muscle strength is much faster than that of muscle atrophy, with short-term 5-day bed rest showing a fourfold greater decline in muscle strength compared with muscle atrophy.
Greatest rate of changes in muscle strength and size occurred in the earliest stages of disuse/mechanical unloading, as noted by others (68, 69). After the initial period(s) the rate of change plateaus. These suggest a greater contribution of neuromuscular function loss to declines in strength in the initial period(s) of limb unloading, while changes in muscle size dominate in the later stages (68). Further investigation of the mechanisms underlying the bed rest-induced muscle atrophy and muscle strength decline is needed.
This review systematically analyzed the numerical impact of various types and duration of bed rest on muscle atrophy and strength parameters in healthy individuals. Our mathematical model is valid for the weight-bearing musculature located at the upper leg region (thigh muscles) characterized by the underlying data set pooling 318 subjects among various periods of bed rest ranging from 5 to 120 days. With reference to Clark (70), the transferability to other body regions or contractile classes is limited by the phenotype (21), fiber composition (71), function [i.e., extensors or flexors (72)], and the muscles’ task specificity (70, 73, 74). Bed rest trials are highly standardized experimental approaches with an evidence level of I and II biased by a limited number of subjects (≤15) and an emphasis on male individuals (Table 1). With a high impact on evidence-based medicine and space research, this restriction funded on single studies prevents however from generalization. Therefore, this systematic review which collects and aggregates the data of 30 yr disuse research within a frame of 5 to 120 days of bed rest confinement, consolidates a broader overview of bed rest-related effects cumulated in mathematical degradation function.
Atrophy of the Knee Extensor Muscles after Bed Rest
Data show a progressive atrophy of the weight-bearing knee extensor muscles with an increasing length of bed rests (Fig. 1). The decline in muscle mass and size is logarithmic. This finding is in general agreement with clinical (75) and spaceflight induced atrophy, which was reported by Edgerton et al. (76) and LeBlanc et al. (77) but is in contrast to Mulder et al. (5), who demonstrated linear decline of quadriceps femoris CSA during 56 days of bed rest. The latter authors postulated that their finding could be slightly biased by the hypertrophic stimulus of eight consecutive maximal muscle strength assessments during the course of 56 days (5). To support our findings, Šimunič et al. (78) found in their randomized control trial a nonlinear decline in three thigh muscles during 35-day bed rest where significant decline was detected after the 7th day (–4.5%) and reached –15.2% at the 35th day of bed rest. Taking into consideration our calculations with relative deltas, the trend of bed rest-induced atrophy of knee extensor muscles is in accordance with the underlying cellular and subcellular adaptations (38). Using histochemical investigations, the authors modeled a logarithmic time course of myofiber atrophy, which is proportional to changes in muscle CSA despite the effect on muscle fiber distribution and changes in isoforms (38). Consistently, the immediate onset of muscle atrophy in the early phase and later plateau with continuous exposure to unloading is therefore indicated in our systematic review. Taking four discrete time points in the logarithmic function of our model (Fig. 1), the expected muscle atrophy of the knee extensor muscles in healthy adults could be estimated at 2%, 5%, 12%, and 19% after 5, 10, 35, and 120 days of bed rest confinement, respectively.
Reduced Maximal Strength of Knee Extensor Muscles after Bed Rest
On average, the loss of muscle strength was on average twofold greater than that of muscle mass and cross-sectional area. With reference to atrophic effects (4.1), the reduction of muscle strength was on average twofold greater than muscle atrophy, following a nonlinear, logarithmic function (Fig. 1). Despite the delta between the muscle atrophy and strength decline, the mathematical model for muscle strength is based on a logarithmic estimate and, thus, proportional to muscle atrophy (Fig. 3). Conclusively, the decrease in torque could not be derived solely from structural changes reflected by atrophy as an equivalent of gradually reduced contractile material (70). The discrepancy between the loss of muscle quantity and function after bed rest must be further explained by a decrease in “muscle quality,” tendon alterations, and neural factors, muscle architecture change (79), reduced neural drive (23, 60, 80–82), alterations in tendon mechanical properties (83), muscle fiber atrophy and force reduction (72, 84–87), fiber phenotype shift toward fast twitch phenotypes only after prolonged bedrest (84), reduction in myofibrillar content (86), impaired calcium release (88), and more. Additionally, as the extracellular matrix contributes to lateral force transmission (via costameres that enable nonmyofascial force transmission), changes in the extracellular matrix with unloading may contribute to the nonuniform force loss (89–91). Although these factors are essential to understand mechanisms and dynamics of muscle deterioration the detailed analysis of these is beyond the scope of this paper. However, the neuronal factors have been specified by an increased coactivation of antagonistic muscles in voluntary movement (92) increasing the vector force, which counteracts the movement direction as well as adaptations on the spinal circuitries and supraspinal level within the central nervous system (93, 94). Sensory input is processed and integrated differently into motor programs after periods of disuse (93), and the excitability of interneurons of the motor cortex and spinal alpha motoneurons is modulated in response to immobilization (94). The aforementioned factors may cause cumulative effects (71, 72), which may account for the particularly steep slope portion in muscle strength (21, 93).
The Ratio between Muscle Atrophy and Strength Decline and Proposed Underlying Mechanisms
We found a linear trend of relative muscle mass/size and strength loss described by the factor 2 (Fig. 2). The ratio between muscle atrophy and strength decline is higher in early phase of bed rest (e.g., 4.3-fold greater after 5 days of bed rest) as compared with chronic bed rest exposure (e.g., 1.8- and 1.7-fold greater after 35 and 120 days of bed rest, respectively; Fig. 2). Our systematic review is in general agreement with the majority of other bed rest trials, which report that muscle strength is compromised to a greater extent than muscle size following bed rest (6, 7, 10, 18, 19, 21–23, 56, 58). Evidence-based explanations for higher declines in muscle function (84) are alterations in neural drive and changes in muscle fiber properties, such as thin filament density (95) yielding lower force per cross-sectional area (84). Obviously, these changes in muscle microarchitecture are not detected when using macroscopic-level imaging methods such as dual-energy X-ray absorptiometry or MRI. Interestingly, Mulder et al. (5) addressed changes in muscle size and strength during a 56-day bed rest measured at eight different time points (6 during the bed rest). They reported linear and equal decline in muscle strength and size. However, frequent strength assessment during the bed rest was recognized as a countermeasure against disuse, likely providing a hypertrophic and neuronal stimulus. For this reason, we excluded this study from a summary calculation and graphical representation.
It is noteworthy that the “gold standard” criteria have been utilized as a prerequisite of study enrollment (61, 62). Muscle size and mass assessment were established by the MRI (61) outcomes: cross-sectional area (cm2) and volume (cm3). Monoarticular MVC expressed as knee extensor joint torque has been selected for muscle strength (62). Thus the quality control of our systematically pooled results is based on high reliability coupled with a minimized selection bias of in-between studies. Although isometric and dynamic knee extensor torque at differing joint angles and angular speeds included in our set of pooled data are evidence-based predictors for clinically relevant lower extremity functions (96), the quantitative transferability in multiarticular dynamic conditions is restricted (97). A prospect for future studies shall therefore combine the effects of muscle strength parameters in multiarticular dynamics, which could presumably reflect a better measure of daily function of individuals after physical inactivity.
It is important to mention limitations of the current work. First, female subjects were underrepresented among the total of 326 participants with 41 subjects, which is why sex-specific conclusions are based on a small sample. Second, dependent on their topography and function (21), muscles are more or less apt to undergo atrophy in response to unloading or disuse (71, 72). With reference to Belavý et al. (96), soleus and gastrocnemii exhibit the greatest rates and magnitude of atrophy, followed by vasti, biceps femoris, other posterior calf musculature, semimembranosus, anterior tibial muscles, adductor magnus, and quadratus femoris. Whether this is due to inherent differences in phenotype or anti-gravity function that may influence protein metabolism remains to be proven. Considering such evidence, it becomes apparent that distinctions in their histology and molecular muscle characteristics requires care in transferring the rates and extent of atrophy in the knee extensors to other skeletal muscles of the lower extremities (21), not to mention lumbar or thoracic regions (96). Third, aside from atrophic differences for muscle groups, the degree of atrophy varies between disuse models: within the pool of models proposed to examine disuse, including horizontal (e.g., Refs. 10, 18) and head-down tilt bed rest (e.g., Refs. 33, 56), lower limb suspension (83, 98–100), limb immobilization (101, 102), microgravity exposure (103, 104), and head-out dry and water immersion (105, 106), for the purpose of the present systematic review, we summarized the effects of bed rest models only given the consistency and reliability concomitant with the relevance of bed rest in healthy adult population. The healthy population of younger adults will differ substantially from the healthy older (10) and clinical population. In addition to choosing to include both bed rest modalities (horizontal and head-down tilt), two other studies (41, 67) deviated from strict bed rest (24/7) and allowed 30 min per day of sitting in a wheelchair for toileting or showering. This should be kept in mind when interpreting the results. Finally, the small sample size and the large interindividual variability (e.g., Ref. 19) of the responses raise the question of the relevance of a meta-analytic summary of the results, but at the same time open new perspectives for the investigation of individual-specific mechanisms of bed rest-induced deterioration.
Conclusions and Future Prospects
This systematic literature review revealed a significant time-dependent muscle atrophy and decrease in muscle strength after bed rest confinement. Higher deterioration rates and magnitudes coupled with earlier onsets were found for the decline in strength to exceed by twofold that of muscle mass and size. With reference to the steep slope dynamics immediately upon the onset of bed rest, an emphasis of future research on the early biomarkers of neuromuscular alterations underlying the nonuniform strength loss would appear to be called for. Addressing cellular and subcellular adaptations of contractile material besides neuroplasticity within brain regions, it would be of benefit to understand the mechanisms underlying the effects of disuse affecting the corticospinal tract and spinal circuitries. These observations highlight the concept that with chronic inactivity, skeletal muscle becomes intrinsically weaker and that some of this functional loss resides in the muscle itself but also a significant portion seems accounted for by neural changes operating at the central and peripheral levels. Future studies should evaluate the reported effects on bedridden individuals, assess to what extent this can be mitigated by preconditioning programs, countermeasures during bed rest, as well as the types and duration of recovery necessary to successfully regain motor function.
GRANTS
This study was supported by a bilateral project between Slovenia and Germany (Slovenian Research Agency BI-DE/19-20-003 and German DAAD ID 57448338). The authors also acknowledge financial support from the Slovenian Research Agency (Research Core Funding No. P5-0381).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
U.M., M.N., and R.R. conceived and designed research; U.M. and R.R. analyzed data; U.M., M.N., B.S., and R.R. interpreted results of experiments; U.M. prepared figures; U.M. and R.R. drafted manuscript; U.M., M.N., B.S., R.P., and R.R. edited and revised manuscript; U.M., M.N., B.S., R.P., and R.R. approved final version of manuscript.
REFERENCES
- 1.Schefold JC, Wollersheim T, Grunow JJ, Luedi MM, Z'Graggen WJ, Weber‐Carstens S. Muscular weakness and muscle wasting in the critically ill. J Cachexia Sarcopenia Muscle 11: 1399–1412, 2020. doi: 10.1002/jcsm.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavy-Le Traon A, Heer M, Narici MV, Rittweger J, Vernikos J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986-2006). Eur J Appl Physiol 101: 143–194, 2007. doi: 10.1007/s00421-007-0474-z. [DOI] [PubMed] [Google Scholar]
- 3.Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol 88: 774–787, 2000. doi: 10.1152/jappl.2000.88.2.774. [DOI] [PubMed] [Google Scholar]
- 4.Greenleaf JE, Quach DT. Recovery After Prolonged Bed-Rest Deconditioning. NASA Technical Reports Server, 2003.
- 5.Mulder ER, Stegeman DF, Gerrits K, Paalman M, Rittweger J, Felsenberg D, De Haan A. Strength, size and activation of knee extensors followed during 8 weeks of horizontal bed rest and the influence of a countermeasure. Eur J Appl Physiol 97: 706–715, 2006. doi: 10.1007/s00421-006-0241-6. [DOI] [PubMed] [Google Scholar]
- 6.Kubo K, Akima H, Kouzaki M, Ito M, Kawakami Y, Kanehisa H, Fukunaga T. Changes in the elastic properties of tendon structures following 20 days bed-rest in humans. Eur J Appl Physiol 83: 463–468, 2000. doi: 10.1007/s004210000309. [DOI] [PubMed] [Google Scholar]
- 7.Kubo K, Akima H, Ushiyama J, Tabata I, Fukuoka H, Kanehisa H, Fukunaga T. Effects of 20 days of bed rest on the viscoelastic properties of tendon structures in lower limb muscles. Brit J Sports Med 38: 324–330, 2004. doi: 10.1136/bjsm.2003.005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulder ER, Horstman AM, Stegeman DF, De Haan A, Belavý DL, Miokovic T, Armbrecht G, Felsenberg D, Gerrits KH. Influence of vibration resistance training on knee extensor and plantar flexor size, strength, and contractile speed characteristics after 60 days of bed rest. J Appl Physiol 107: 1789–1798, 2009. doi: 10.1152/japplphysiol.00230.2009. [DOI] [PubMed] [Google Scholar]
- 9.Coker RH, Hays NP, Williams RH, Wolfe RR, Evans WJ. Bed rest promotes reductions in walking speed, functional parameters, and aerobic fitness in older, healthy adults. J Gerontol A Biol Sci Med Sci 70: 91–96, 2015. doi: 10.1093/gerona/glu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pisot R, Marusic U, Biolo G, Mazzucco S, Lazzer S, Grassi B, Reggiani C, Toniolo L, di Prampero PE, Passaro A, Narici M, Mohammed S, Rittweger J, Gasparini M, Gabrijelcic Blenkus M, Simunic B. Greater loss in muscle mass and function but smaller metabolic alterations in older compared with younger men following 2 wk of bed rest and recovery. J Appl Physiol 120: 922–929, 2016. doi: 10.1152/japplphysiol.00858.2015. [DOI] [PubMed] [Google Scholar]
- 11.Parry SM, Puthucheary ZA. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem Physiol Med 4: 16, 2015. doi: 10.1186/s13728-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dirks ML, Wall BT, van de Valk B, Holloway TM, Holloway GP, Chabowski A, Goossens GH, van Loon LJ. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes 65: 2862–2875, 2016. doi: 10.2337/db15-1661. [DOI] [PubMed] [Google Scholar]
- 13.Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol 95: 2185–2201, 2003. doi: 10.1152/japplphysiol.00346.2003. [DOI] [PubMed] [Google Scholar]
- 14.Eurostat. Hospital Discharges and Length of Stay Statistics. Luxembourg City, Luxembourg: Eurostat, 2016. [Google Scholar]
- 15.Marusic U, Grosprêtre S. Non-physical approaches to counteract age-related functional deterioration: Applications for rehabilitation and neural mechanisms. Eur J Sport Sci 18: 639–649, 2018. doi: 10.1080/17461391.2018.1447018. [DOI] [PubMed] [Google Scholar]
- 16.Lundbye-Jensen J, Nielsen JB. Central nervous adaptations following 1 wk of wrist and hand immobilization. J Appl Physiol 105: 139–151, 2008. doi: 10.1152/japplphysiol.00687.2007. [DOI] [PubMed] [Google Scholar]
- 17.Liepert J, Tegenthoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol 97: 382–386, 1995. doi: 10.1016/0924-980X(95)00194-P. [DOI] [PubMed] [Google Scholar]
- 18.Berg HE, Eiken O, Miklavcic L, Mekjavic IB. Hip, thigh and calf muscle atrophy and bone loss after 5-week bedrest inactivity. Eur J Appl Physiol 99: 283–289, 2007. doi: 10.1007/s00421-006-0346-y. [DOI] [PubMed] [Google Scholar]
- 19.Kawakami Y, Akima H, Kubo K, Muraoka Y, Hasegawa H, Kouzaki M, Imai M, Suzuki Y, Gunji A, Kanehisa H, Fukunaga T. Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. Eur J Appl Physiol 84: 7–12, 2001. doi: 10.1007/s004210000330. [DOI] [PubMed] [Google Scholar]
- 20.Krainski F, Hastings JL, Heinicke K, Romain N, Pacini EL, Snell PG, Wyrick P, Palmer MD, Haller RG, Levine BD. The effect of rowing ergometry and resistive exercise on skeletal muscle structure and function during bed rest. J Appl Physiol 116: 1569–1581, 2014. doi: 10.1152/japplphysiol.00803.2013. [DOI] [PubMed] [Google Scholar]
- 21.Alkner B, Tesch PA. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur J Appl Physiol 93: 294–305, 2004. doi: 10.1007/s00421-004-1172-8. [DOI] [PubMed] [Google Scholar]
- 22.Akima H, Kubo K, Kanehisa H, Suzuki Y, Gunji A, Fukunaga T. Leg-press resistance training during 20 days of 6 degrees head-down-tilt bed rest prevents muscle deconditioning. Eur J Appl Physiol 82: 30–38, 2000. doi: 10.1007/s004210050648. [DOI] [PubMed] [Google Scholar]
- 23.Berg HE, Larsson L, Tesch PA. Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol 82: 182–188, 1997. doi: 10.1152/jappl.1997.82.1.182. [DOI] [PubMed] [Google Scholar]
- 24.LeBlanc A, Rowe R, Evans H, West S, Shackelford L, Schneider V. Muscle atrophy during long duration bed rest. Int J Sports Med 18: S283–S285, 1997. doi: 10.1055/s-2007-972726. [DOI] [PubMed] [Google Scholar]
- 25.Stern C, Jordan Z, McArthur A. Developing the review question and inclusion criteria. Am J Nursing 114: 53–56, 2014. doi: 10.1097/01.NAJ.0000445689.67800.86. [DOI] [PubMed] [Google Scholar]
- 26.Akers J. Systematic reviews: CRD's Guidance for Undertaking Reviews in Health Care. York, UK: Centre for Reviews and Dissemination, 2009. [Google Scholar]
- 27.Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, Knipschild PG. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 51: 1235–1241, 1998. doi: 10.1016/S0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 28.Salanova M, Schiffl G, Püttmann B, Schoser B, Blottner D. Molecular biomarkers monitoring human skeletal muscle fibres and microvasculature following long‐term bed rest with and without countermeasures. J Anat 212: 306–318, 2008. doi: 10.1111/j.1469-7580.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact 7: 33, 2007. [PubMed] [Google Scholar]
- 30.Trappe T, Burd NA, Louis E, Lee G, Trappe S. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxf) 191: 147–159, 2007. doi: 10.1111/j.1748-1716.2007.01728.x. [DOI] [PubMed] [Google Scholar]
- 31.Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci 58: M911–M916, 2003. doi: 10.1093/gerona/58.10.M911. [DOI] [PubMed] [Google Scholar]
- 32.Agostini D, Donati Zeppa S, Lucertini F, Annibalini G, Gervasi M, Ferri Marini C, Piccoli G, Stocchi V, Barbieri E, Sestili P. Muscle and bone health in postmenopausal women: Role of protein and vitamin D supplementation combined with exercise training. Nutrients 10: 1103, 2018. doi: 10.3390/nu10081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulder E, Clément G, Linnarsson D, Paloski WH, Wuyts FP, Zange J, Frings-Meuthen P, Johannes B, Shushakov V, Grunewald M, Maassen N, Buehlmeier J, Rittweger J. Musculoskeletal effects of 5 days of bed rest with and without locomotion replacement training. Eur J Appl Physiol 115: 727–738, 2015. doi: 10.1007/s00421-014-3045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rittweger J, Bareille M-P, Clément G, Linnarsson D, Paloski WH, Wuyts F, Zange J, Angerer O. Short-arm centrifugation as a partially effective musculoskeletal countermeasure during 5-day head-down tilt bed rest—results from the BRAG1 study. Eur J Appl Physiol 115: 1233–1244, 2015. doi: 10.1007/s00421-015-3120-1. [DOI] [PubMed] [Google Scholar]
- 35.Dirks ML, Smeets JS, Holwerda AM, Kouw IW, Marzuca-Nassr GN, Gijsen AP, Holloway GP, Verdijk LB, van Loon LJ. Dietary feeding pattern does not modulate the loss of muscle mass or the decline in metabolic health during short-term bed rest. Am J Physiol Endocrinol Metab 316: E536–E545, 2019. doi: 10.1152/ajpendo.00378.2018. [DOI] [PubMed] [Google Scholar]
- 36.Ferrando AA, Stuart CA, Brunder DG, Hillman GR. Magnetic resonance imaging quantitation of changes in muscle volume during 7 days of strict bed rest. Aviat Space Environ Med 66: 976–981, 1995. [PubMed] [Google Scholar]
- 37.McDonnell AC, Eiken O, Frings-Meuthen P, Rittweger J, Mekjavic IB. The LunHab project: Muscle and bone alterations in male participants following a 10 day lunar habitat simulation. Exp Physiol 104: 1250–1261, 2019. doi: 10.1113/EP087482. [DOI] [PubMed] [Google Scholar]
- 38.Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, Greenisen MC. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol 84: 157–163, 1998. doi: 10.1152/jappl.1998.84.1.157. [DOI] [PubMed] [Google Scholar]
- 39.Akima H, Ushiyama JI, Kubo J, Fukuoka H, Kanehisa H, Fukunaga T. Effect of unloading on muscle volume with and without resistance training. Acta Astronaut 60: 728–736, 2007. doi: 10.1016/j.actaastro.2006.10.006. [DOI] [Google Scholar]
- 40.Shinohara M, Yoshitake Y, Kouzaki M, Fukuoka H, Fukunaga T. Strength training counteracts motor performance losses during bed rest. J Appl Physiol 95: 1485–1492, 2003. doi: 10.1152/japplphysiol.01173.2002. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki Y, Kashihara H, Takenaka K, Kawakubo K, Makita Y, Goto S, Ikawa S, Gunji A. Effects of daily mild supine exercise on physical performance after 20 days bed rest in young persons. Acta Astronaut 33: 101–111, 1994. doi: 10.1016/0094-5765(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 42.Liphardt AM, Bolte V, Eckstein F, Wirth W, Brüggemann GP, Niehoff A. Response of thigh muscle cross‐sectional area to 21‐days of bed rest with exercise and nutrition countermeasures. Trans Sports Med 3: 93–106, 2020. doi: 10.1002/tsm2.122. [DOI] [Google Scholar]
- 43.Germain P, Güell A, Marini JF. Muscle strength during bedrest with and without muscle exercise as a countermeasure. Eur J Appl Physiol 71: 342–348, 1995. doi: 10.1007/BF00240415. [DOI] [PubMed] [Google Scholar]
- 44.Alkner B, Tesch PA. Efficacy of a gravity‐independent resistance exercise device as a countermeasure to muscle atrophy during 29‐day bed rest. Acta Physiol Scand 181: 345–357, 2004. doi: 10.1111/j.1365-201X.2004.01293.x. [DOI] [PubMed] [Google Scholar]
- 45.Berry P, Berry I, Manelfe C. Magnetic resonance imaging evaluation of lower limb muscles during bed rest–a microgravity simulation model. Aviat Space Environ Med 64: 212–218, 1993. [PubMed] [Google Scholar]
- 46.Dudley GA, Duvoisin MR, Convertino VA, Buchanan P. Alterations of the in vivo torque-velocity relationship of human skeletal muscle following 30 days exposure to simulated microgravity. Aviat Space Environ Med 60: 659–663, 1989. [PubMed] [Google Scholar]
- 47.Greenleaf J, Bernauer E, Ertl A, Bulbulian R, Bond M. Isokinetic strength and endurance during 30-day 6 degrees head-down bed rest with isotonic and isokinetic exercise training. Aviat Space Environ Med 65: 45–50, 1994. [PubMed] [Google Scholar]
- 48.Schneider SM, Lee SM, Feiveson AH, Watenpaugh DE, Macias BR, Hargens AR. Treadmill exercise within lower body negative pressure protects leg lean tissue mass and extensor strength and endurance during bed rest. Physiol Rep 4: e12892, 2016. doi: 10.14814/phy2.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gogia P, Schneider V, LeBlanc A, Krebs J, Kasson C, Pientok C. Bed rest effect on extremity muscle torque in healthy men. Arch Phys Med Rehab 69: 1030–1032, 1988. [PubMed] [Google Scholar]
- 50.Ferretti G, Antonutto G, Denis C, Hoppeler H, Minetti AE, Narici MV, Desplanches D. The interplay of central and peripheral factors in limiting maximal O2 consumption in man after prolonged bed rest. J Physiol 501: 677–686, 1997. doi: 10.1111/j.1469-7793.1997.677bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferretti G, Berg HE, Minetti AE, Moia C, Rampichini S, Narici MV. Maximal instantaneous muscular power after prolonged bed rest in humans. J Appl Physiol 90: 431–435, 2001. doi: 10.1152/jappl.2001.90.2.431. [DOI] [PubMed] [Google Scholar]
- 52.Belavý DL, Miokovic T, Armbrecht G, Rittweger J, Felsenberg D. Resistive vibration exercise reduces lower limb muscle atrophy during 56-day bed-rest. J Musculoskelet Neuronal Interact 9: 225–235, 2009. [PubMed] [Google Scholar]
- 53.Arbeille P, Kerbeci P, Capri A, Dannaud C, Trappe SW, Trappe TA. Quantification of muscle volume by echography: comparison with MRI data on subjects in long-term bed rest. Ultrasound Med Biol 35: 1092–1097, 2009. doi: 10.1016/j.ultrasmedbio.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Lee SM, Schneider SM, Feiveson AH, Macias BR, Smith SM, Watenpaugh DE, Hargens AR. WISE-2005: Countermeasures to prevent muscle deconditioning during bed rest in women. J Appl Physiol 116: 654–667, 2014. doi: 10.1152/japplphysiol.00590.2013. [DOI] [PubMed] [Google Scholar]
- 55.Miokovic T, Armbrecht G, Felsenberg D, Belavý DL. Heterogeneous atrophy occurs within individual lower limb muscles during 60 days of bed rest. J Appl Physiol 113: 1545–1559, 2012. doi: 10.1152/japplphysiol.00611.2012. [DOI] [PubMed] [Google Scholar]
- 56.Ploutz-Snyder LL, Downs M, Goetchius E, Crowell B, English KL, Ploutz-Snyder R, Ryder JW, Dillon EL, Sheffield-Moore M, Scott JM. Exercise training mitigates multisystem deconditioning during bed rest. Med Sci Sports Exerc 50: 1920–1928, 2018. doi: 10.1249/MSS.0000000000001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alkner BA, Norrbrand L, Tesch PA. Neuromuscular adaptations following 90 days bed rest with or without resistance exercise. Aerosp Med Hum Perform 87: 610–617, 2016. doi: 10.3357/AMHP.4383.2016. [DOI] [PubMed] [Google Scholar]
- 58.LeBlanc AD, Schneider VS, Evans HJ, Pientok C, Rowe R, Spector E. Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol 73: 2172–2178, 1992. doi: 10.1152/jappl.1992.73.5.2172. [DOI] [PubMed] [Google Scholar]
- 59.Shackelford L, LeBlanc A, Driscoll T, Evans H, Rianon N, Smith S, Spector E, Feeback D, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol 97: 119–129, 2004. doi: 10.1152/japplphysiol.00741.2003. [DOI] [PubMed] [Google Scholar]
- 60.Gondin J, Guette M, Maffiuletti N, Martin A. Neural activation of the triceps surae is impaired following 2 weeks of immobilization. Eur J Appl Physiol 93: 359–365, 2004. doi: 10.1007/s00421-004-1225-z. [DOI] [PubMed] [Google Scholar]
- 61.Wade RC, Gorgey AS. Anthropometric prediction of skeletal muscle cross-sectional area in persons with spinal cord injury. J Appl Physiol 122: 1255–1261, 2017. doi: 10.1152/japplphysiol.01042.2016. [DOI] [PubMed] [Google Scholar]
- 62.Sleivert GG, Wenger HA. Reliability of measuring isometric and isokinetic peak torque, rate of torque development, integrated electromyography, and tibial nerve conduction velocity. Arch Phys Med Rehabil 75: 1315–1321, 1994. doi: 10.1016/0003-9993(94)90279-8. [DOI] [PubMed] [Google Scholar]
- 63.Miyatani M, Kanehisa H, Kuno S, Nishijima T, Fukunaga T. Validity of ultrasonograph muscle thickness measurements for estimating muscle volume of knee extensors in humans. Eur J Appl Physiol 86: 203–208, 2002. doi: 10.1007/s00421-001-0533-9. [DOI] [PubMed] [Google Scholar]
- 64.Mitsiopoulos N, Baumgartner R, Heymsfield S, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 85: 115–122, 1998. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 65.Tingart MJ, Apreleva M, Lehtinen JT, Capell B, Palmer WE, Warner JJ. Magnetic resonance imaging in quantitative analysis of rotator cuff muscle volume. Clin Orthop Relat Res 415: 104–110, 2003. doi: 10.1097/01.blo.0000092969.12414.e1. [DOI] [PubMed] [Google Scholar]
- 66.Sanada K, Kearns CF, Midorikawa T, Abe T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol 96: 24–31, 2006. doi: 10.1007/s00421-005-0061-0. [DOI] [PubMed] [Google Scholar]
- 67.Akima H, Kuno S, Suzuki Y, Gunji A, Fukunaga T. Effects of 20 days of bed rest on physiological cross-sectional area of human thigh and leg muscles evaluated by magnetic resonance imaging. J Gravit Physiol 4: S15–21, 1997. [PubMed] [Google Scholar]
- 68.Campbell M, Varley-Campbell J, Fulford J, Taylor B, Mileva KN, Bowtell JL. Effect of immobilisation on neuromuscular function in vivo in humans: a systematic review. Sports Med 49: 931–950, 2019. doi: 10.1007/s40279-019-01088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wall BT, van Loon LJ. Nutritional strategies to attenuate muscle disuse atrophy. Nutr Rev 71: 195–208, 2013. doi: 10.1111/nure.12019. [DOI] [PubMed] [Google Scholar]
- 70.Clark BC. In vivo alterations in skeletal muscle form and function after disuse atrophy. Med Sci Sports Exerc 41: 1869–1875, 2009. doi: 10.1249/MSS.0b013e3181a645a6. [DOI] [PubMed] [Google Scholar]
- 71.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol 68: 1–12, 1990. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- 72.Fitts RH, Riley DR, Widrick JJ. Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol 89: 823–839, 2000. doi: 10.1152/jappl.2000.89.2.823. [DOI] [PubMed] [Google Scholar]
- 73.Droppert P. A review of muscle atrophy in microgravity and during prolonged bed rest. J Brit Interplanetary Soc 46: 83–86, 1993. [PubMed] [Google Scholar]
- 74.Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc 29: 197–206, 1997. doi: 10.1097/00005768-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 75.Gruther W, Benesch T, Zorn C, Paternostro-Sluga T, Quittan M, Fialka-Moser V, Spiss C, Kainberger F, Crevenna R. Muscle wasting in intensive care patients: ultrasound observation of the M. quadriceps femoris muscle layer. J Rehab Med 40: 185–189, 2008. doi: 10.2340/16501977-0139. [DOI] [PubMed] [Google Scholar]
- 76.Edgerton VR, Zhou MY, Ohira Y, Klitgaard H, Jiang B, Bell G, Harris B, Saltin B, Gollnick PD, Roy RR, Et A. Human fiber size and enzymatic properties after 5 and 11 days of spaceflight. J Appl Physiol 78: 1733–1739, 1995. doi: 10.1152/jappl.1995.78.5.1733. [DOI] [PubMed] [Google Scholar]
- 77.LeBlanc A, Rowe R, Schneider V, Evans H, Hedrick T. Regional muscle loss after short duration spaceflight. Aviat Space Environ Med 66: 1151–1154, 1995. [PubMed] [Google Scholar]
- 78.Šimunič B, Koren K, Rittweger J, Lazzer S, Reggiani C, Rejc E, Pišot R, Narici M, Degens H. Tensiomyography detects early hallmarks of bed-rest-induced atrophy before changes in muscle architecture. J Appl Physiol 126: 815–822, 2019. doi: 10.1152/japplphysiol.00880.2018. [DOI] [PubMed] [Google Scholar]
- 79.Narici M, Cerretelli P. Changes in human muscle architecture in disuse-atrophy evaluated by ultrasound imaging. J Gravit Physiol 5: P73–74, 1998. [PubMed] [Google Scholar]
- 80.Mulder ER, Gerrits K, Kleine B, Rittweger J, Felsenberg D, De Haan A, Stegeman DF. High-density surface EMG study on the time course of central nervous and peripheral neuromuscular changes during 8 weeks of bed rest with or without resistive vibration exercise. J Electromyogr Kinesiol 19: 208–218, 2009. doi: 10.1016/j.jelekin.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 81.Zamparo P, Minetti A, Di P. Interplay among the changes of muscle strength, cross-sectional area and maximal explosive power: theory and facts. Eur J Appl Physiol 88: 193–202, 2002. doi: 10.1007/s00421-002-0691-4. [DOI] [PubMed] [Google Scholar]
- 82.Kramer A, Kümmel J, Gollhofer A, Armbrecht G, Ritzmann R, Belavy D, Felsenberg D, Gruber M. Plyometrics can preserve peak power during 2 months of physical inactivity: an RCT including a one-year follow-up. Front Physiol 9: 633, 2018. doi: 10.3389/fphys.2018.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Boer MD, Maganaris CN, Seynnes OR, Rennie MJ, Narici MV. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower‐limb suspension in young men. J Physiol 583: 1079–1091, 2007. doi: 10.1113/jphysiol.2007.135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed‐rest and resistance exercise. J Physiol 557: 501–513, 2004. doi: 10.1113/jphysiol.2004.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brocca L, Longa E, Cannavino J, Seynnes O, de Vito G, McPhee J, Narici M, Pellegrino MA, Bottinelli R. Human skeletal muscle fibre contractile properties and proteomic profile: adaptations to 3 weeks of unilateral lower limb suspension and active recovery. J Physiol 593: 5361–5385, 2015. doi: 10.1113/JP271188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Larsson L, Li X, Berg HE, Frontera WR. Effects of removal of weight-bearing function on contractility and myosin isoform composition in single human skeletal muscle cells. Pflugers Arch 432: 320–328, 1996. doi: 10.1007/s004240050139. [DOI] [PubMed] [Google Scholar]
- 87.Rejc E, Floreani M, Taboga P, Botter A, Toniolo L, Cancellara L, Narici M, Šimunič B, Pišot R, Biolo G, Passaro A, Rittweger J, Reggiani C, Lazzer S. Loss of maximal explosive power of lower limbs after 2 weeks of disuse and incomplete recovery after retraining in older adults. J Physiol 596: 647–665, 2018. doi: 10.1113/JP274772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fraysse B, Desaphy JF, Pierno S, De Luca A, Liantonio A, Mitolo CI, Camerino DC. Decrease in resting calcium and calcium entry associated with slow-to-fast transition in unloaded rat soleus muscle. FASEB J 17: 1–19, 2003. doi: 10.1096/fj.02-1012fje. [DOI] [PubMed] [Google Scholar]
- 89.Sharafi B, Blemker SS. A mathematical model of force transmission from intrafascicularly terminating muscle fibers. J Biomech 44: 2031–2039, 2011. doi: 10.1016/j.jbiomech.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sinha U, Malis V, Csapo R, Narici M, Sinha S. Magnetic resonance imaging based muscle strain rate mapping during eccentric contraction to study effects of unloading induced by unilateral limb suspension. Eur J Transl Myol 30: 139–143, 2020. doi: 10.4081/ejtm.2019.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li R, Narici MV, Erskine RM, Seynnes OR, Rittweger J, Pišot R, Šimunič B, Flück M. Costamere remodeling with muscle loading and unloading in healthy young men. J Anat 223: 525–536, 2013. doi: 10.1111/joa.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ritzmann R, Freyler K, Kümmel J, Gruber M, Belavy DL, Felsenberg D, Gollhofer A, Kramer A, Ambrecht G. High intensity jump exercise preserves posture control, gait, and functional mobility during 60 days of bed-rest: an RCT including 90 days of follow-up. Front Physiol 9: 1713, 2018. doi: 10.3389/fphys.2018.01713, 10.3389/fpls.2018.01713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lundbye-Jensen J, Nielsen JB. Immobilization induces changes in presynaptic control of group Ia afferents in healthy humans. J Physiol 586: 4121–4135, 2008. doi: 10.1113/jphysiol.2008.156547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leukel C, Taube W, Rittweger J, Gollhofer A, Ducos M, Weber T, Lundbye-Jensen J. Changes in corticospinal transmission following 8 weeks of ankle joint immobilization. Clin Neurophysiol 126: 131–139, 2015. doi: 10.1016/j.clinph.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 95.Riley DA, Bain JL, Thompson JL, Fitts RH, Widrick JJ, Trappe SW, Trappe TA, Costill DL. Decreased thin filament density and length in human atrophic soleus muscle fibers after spaceflight. J Appl Physiol 88: 567–572, 2000. doi: 10.1152/jappl.2000.88.2.567. [DOI] [PubMed] [Google Scholar]
- 96.Belavý DL, Ohshima H, Rittweger J, Felsenberg D. High-intensity flywheel exercise and recovery of atrophy after 90 days bed-rest. BMJ Open Sport Exerc Med 3: e000196, 2017. doi: 10.1136/bmjsem-2016-000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kramer A, Kümmel J, Mulder E, Gollhofer A, Frings-Meuthen P, Gruber M. High-intensity jump training is tolerated during 60 days of bed rest and is very effective in preserving leg power and lean body mass: an overview of the cologne RSL study. PLoS One 12: e0169793, 2017. doi: 10.1371/journal.pone.0169793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adams G, Hather B, Dudley G. Effect of short-term unweighting on human skeletal muscle strength and size. Aviat Space Environ Med 65: 1116–1121, 1994. [PubMed] [Google Scholar]
- 99.Deschenes MR, Giles JA, McCoy RW, Volek JS, Gomez AL, Kraemer WJ. Neural factors account for strength decrements observed after short-term muscle unloading. Am J Physiol Regul Integr Comp Physiol 282: R578–R583, 2002. doi: 10.1152/ajpregu.00386.2001. [DOI] [PubMed] [Google Scholar]
- 100.de Boer MD, Seynnes OR, di Prampero PE, Pisot R, Mekjavic IB, Biolo G, Narici MV. Effect of 5 weeks horizontal bed rest on human muscle thickness and architecture of weight bearing and non-weight bearing muscles. Eur J Appl Physiol 104: 401–407, 2008. doi: 10.1007/s00421-008-0703-0. [DOI] [PubMed] [Google Scholar]
- 101.Toussaint L, Meugnot A. Short-term limb immobilization affects cognitive motor processes. J Exp Psychol Learn Mem Cogn 39: 623–632, 2013. doi: 10.1037/a0028942. [DOI] [PubMed] [Google Scholar]
- 102.Pileggi CA, Hedges CP, D'Souza RF, Durainayagam BR, Markworth JF, Hickey AJ, Mitchell CJ, Cameron-Smith D. Exercise recovery increases skeletal muscle H2O2 emission and mitochondrial respiratory capacity following two-weeks of limb immobilization. Free Rad Biol Med 124: 241–248, 2018. doi: 10.1016/j.freeradbiomed.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 103.Vico L, Hargens A. Skeletal changes during and after spaceflight. Nat Rev Rheumatol 14: 229–245, 2018. doi: 10.1038/nrrheum.2018.37. [DOI] [PubMed] [Google Scholar]
- 104.Mulavara AP, Peters BT, Miller CA, Kofman IS, Reschke MF, Taylor LC, Lawrence EL, Wood SJ, Laurie SS, Lee SM, Buxton RE, May-Phillips TR, Stenger MB, Ploutz-Snyder LL, Ryder JW, Feiveson AH, Bloomberg JJ. Physiological and functional alterations after spaceflight and bed rest. Med Sci Sports Exerc 50: 1961–1980, 2018. doi: 10.1249/MSS.0000000000001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Demangel R, Treffel L, Py G, Brioche T, Pagano AF, Bareille MP, Beck A, Pessemesse L, Candau R, Gharib C, Chopard A, Millet C. Early structural and functional signature of 3‐day human skeletal muscle disuse using the dry immersion model. J Physiol 595: 4301–4315, 2017. doi: 10.1113/JP273895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Watenpaugh DE. Analogs of microgravity: head-down tilt and water immersion. J Appl Physiol 120: 904–914, 2016. doi: 10.1152/japplphysiol.00986.2015. [DOI] [PubMed] [Google Scholar]