Abstract
Duchenne muscular dystrophy (DMD) is characterized by a progressive replacement of muscle by fat and fibrous tissue, muscle weakness, and loss of functional abilities. Impaired vasodilatory and blood flow responses to muscle activation have also been observed in DMD and associated with mislocalization of neuronal nitric oxide synthase mu (nNOSμ) from the sarcolemma. The objective of this study was to determine whether the postcontractile blood oxygen level-dependent (BOLD) MRI response is impaired in DMD and correlated with established markers of disease severity in DMD, including MRI muscle fat fraction (FF) and clinical functional measures. Young boys with DMD (n = 16, 5–14 yr) and unaffected controls (n = 16, 5–14 yr) were evaluated using postcontractile BOLD, FF, and functional assessments. The BOLD response was measured following five brief (2 s) maximal voluntary dorsiflexion contractions, each separated by 1 min of rest. FFs from the anterior compartment lower leg muscles were quantified via chemical shift-encoded imaging. Functional abilities were assessed using the 10 m walk/run and the 6-min walk distance (6MWD). The peak BOLD responses in the tibialis anterior and extensor digitorum longus were reduced (P < 0.001) in DMD compared with controls. Furthermore, the anterior compartment peak BOLD response correlated with function (6MWD ρ = 0.87, P < 0.0001; 10 m walk/run time ρ = −0.78, P < 0.001) and FF (ρ = −0.52, P = 0.05). The reduced postcontractile BOLD response in DMD may reflect impaired microvascular function. The relationship observed between the postcontractile peak BOLD response and functional measures and FF suggests that the BOLD response is altered with disease severity in DMD.
NEW & NOTEWORTHY This study examined the postcontractile blood oxygen level-dependent (BOLD) response in boys with Duchenne muscular dystrophy (DMD) and unaffected controls, and correlated this measure to markers of disease severity. Our findings indicate that the postcontractile BOLD response is impaired in DMD after brief muscle contractions, is correlated to disease severity, and may be valuable to implement in future studies to evaluate treatments targeting microvascular function in DMD.
Keywords: blood oxygenation level dependent, Duchenne muscular dystrophy, magnetic resonance imaging, microvascular, skeletal muscle
INTRODUCTION
Duchenne muscular dystrophy (DMD) is an X-linked recessive disorder affecting approximately one in 3,600–6,000 male births, and is characterized by progressive muscle degeneration, loss of functional abilities, and reduced life expectancy (1). DMD is caused by a mutation in the gene encoding dystrophin (2), a key protein within the dystrophin-glycoprotein complex that not only provides structural stabilization, but also localizes neuronal nitric oxide synthase mu (nNOSμ) to the sarcolemma to signal nitric oxide (NO) production (3–6). NO derived from nNOS plays an important role in the regulation of blood flow in contracting skeletal muscle by inhibiting the vasoconstrictor response to activation of α-adrenergic receptors (7). In dystrophic muscle, nNOS mislocalization may result in inadequate blood flow relative to demand during muscle contraction (termed functional ischemia), attenuating oxygen and nutrient supply and blunting removal of accumulated metabolites (8, 9). This impaired vasodilatory response during and after muscle contraction accelerates damage after contractions (10) and causes exaggerated fatigue during and after exercise in mdx mice, a common model of DMD (11, 12). Thus, development of effective treatment strategies may need to target both the vascular/microvascular and cellular impairments associated with dystrophin deficiency (13).
The postcontractile blood oxygen level-dependent (BOLD) response may offer a method to noninvasively infer microvascular function using MRI. Although BOLD has been extensively used to study the brain’s neural activity and response after a stimulus, it is increasingly being applied to skeletal muscle to examine the response following muscle contractions (14–19). A transient increase in BOLD signal intensity is elicited within muscle after performing a brief muscle contraction (e.g., 1–2 s), reflecting increased small vessel oxygen saturation and blood volume in response to muscle contraction (18). Thus, the postcontractile change in skeletal muscle BOLD signal intensity may provide a measure of peripheral microvascular health. However, in order to be a reflection of microvascular function and obtain a maximum BOLD response, a minimum intensity of contraction must be performed. Wigmore et al. (20) observed that the peak BOLD response increased with contraction intensity up to ∼70% of maximal voluntary contraction. Another factor potentially influencing the postcontractile BOLD response is altered muscle structure and composition, such as fatty tissue infiltration, which may influence the intramuscular pressure during muscle contractions. During a maximal isometric contraction, the intramuscular pressure results in the compression of small vessels, ejecting blood from the venous circulation and restricting blood flow in the arterioles, and following a contraction there is a refilling of the small vessels and a transient increase in blood flow and volume, reflecting the postcontractile BOLD response observed (16). Indeed, the magnitude of the postcontractile BOLD response has been shown to vary across a spectrum of health and disease; where it is decreased in older adults (17), in patients with peripheral artery disease (21), and in individuals with diabetes (16, 22), whereas it is greater in endurance-trained adults compared with sedentary adults (23). Although postcontractile BOLD has not yet been applied to patients with DMD, it has been shown to effectively detect differences between a dystrophic mouse model (mdx) and wild-type mice (24).
The purpose of this study was to evaluate the postcontractile BOLD response as a potential marker to infer microvascular function in boys with DMD, and to examine its relationship with known markers of disease severity. We have previously shown increases in MRI fat fraction (FF) to be a sensitive marker of disease severity and progression in DMD (25–27). Here, we compare postcontractile BOLD response measurements with muscle FF measured with chemical shift-encoded MRI (also known as Dixon imaging) and other standard ambulation measures of functional ability in a group of boys with DMD and unaffected controls. Reproducibility of the BOLD response was also assessed. We hypothesize that 1) boys with DMD would have a reduced postcontractile peak BOLD response compared with unaffected controls and 2) the postcontractile BOLD response would be associated with muscle FF and functional assessments of walking/running performance.
METHODS
Subjects
Boys with DMD (n = 16) and unaffected controls (n = 16) participated in a 1) MRI protocol to evaluate the postcontractile BOLD response and fat fraction and 2) clinical functional tests. All subjects were between the ages of 5 and 14 yr and ambulatory, defined as the ability to walk for at least 75 m without an external assistive device. All participants were confirmed to have DMD based on 1) clinical history with features before the age of five, 2) physical examination, 3) elevated serum creatine kinase level, and 4) absence of dystrophin expression, as determined by immunostaining or Western blot (<2%) and/or DNA confirmation of dystrophin mutation. Subjects taking PDE5a inhibitors (e.g., sildenafil citrate or tadalafil), or other medications known to modulate blood flow or muscle metabolism or control subjects who were participating in sport specific training 2 times or more per week were excluded. This study was approved by the institutional review board at the University of Florida and conducted in compliance with the Health Insurance Portability and Accountability Act (HIPAA). Informed written consent and assent were obtained from a guardian and subject, respectively, before participation. The postcontractile BOLD responses were collected from a total of 32 subjects and a subset of subjects from each group were asked to repeat the BOLD protocol to examine reproducibility (n = 4 controls; n = 7 DMD).
MR Imaging Acquisition
All MR images were acquired using a 3-Tesla whole body scanner (Achieva Quasar Dual or Ingenia Elition MRI, Philips, Best, The Netherlands) at the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) Facility at the University of Florida. Each participant was positioned supine with the right leg in a slightly flexed position with the right foot secured to a custom-built exercise apparatus for isometric dorsiflexion contractions (footplate fixed at 110°). The participants legs and hips were also secured using straps and supports adjacent to each of the legs and torso region to minimize accessory muscle movement. A transmit/receive radiofrequency knee coil (InVivo, Gainesville, Florida) was centered over the midbelly of the calf, corresponding to the largest cross section of the tibialis anterior (TA). Initial survey (gradient echo) images with minimum echo time (TE) = 3.54 ms, repetition time (TR) = 7.22 ms, and 12 slices (2.5 mm-thick and 7.5 mm-spacing) (27) with a field of view (FOV) 250 × 250 × 43 mm3 and a 20° flip angle, 512 data points per signal average and 1 signal average were acquired to locate muscle groups for further MRI sequences. Following the survey scan, a three-point chemical shift encoded imaging (Dixon) sequence was run using a fast field echo (FFE) pulse sequence with a 20° flip angle at 3 different echo times (TR/TE = 430/8.06 ms, 9.21 ms, 10.36 ms) over 25 axial slices (4-mm slice thickness, 1 mm gap) with a 20° flip angle using mDixon (Philips) and FOV adjusted depending on subject anatomy (28, 29). Water and fat maps were reconstructed using the standard Philips in-line processing (mDixon) with the fat map derived using a 7 peak fat model (30).
After images were obtained at rest, BOLD images were acquired during an isometric dorsiflexion exercise protocol recruiting the TA and extensor digitorum longus (EDL). Participants were asked to perform 5 maximal voluntary contractions (MVCs), where each isometric contraction was 2 s in duration and separated by 1 min of rest. Force measures were obtained for each contraction using a force transducer (Interface Force Measurement Solutions; INF-USB2 Load Cell) mounted to the underside of the footplate. The force measurement was sampled at 200 Hz (5 ms) and recorded on a separate computer. Force was measured at rest and during each of the 5 maximal contractions. Dynamic BOLD images were acquired using a single shot echo planar imaging (ssEPI) sequence with a 90° flip angle, TR of 1,000 ms, TE of 35 ms, 1 signal average, eight 10 mm-thick slices with no gap, with fat suppression (spectral presaturation with inversion recovery, SPIR), and an optimized FOV (∼140 × 140 × 80 mm3; acquisition/reconstruction matrix ∼80 × 80). The slices were acquired sequentially in the distal to proximal direction. As a result, 360 dynamic scans were acquired over a total of 6 min, with a brief maximal isometric dorsiflexion contraction performed once each minute.
MR Data Analysis
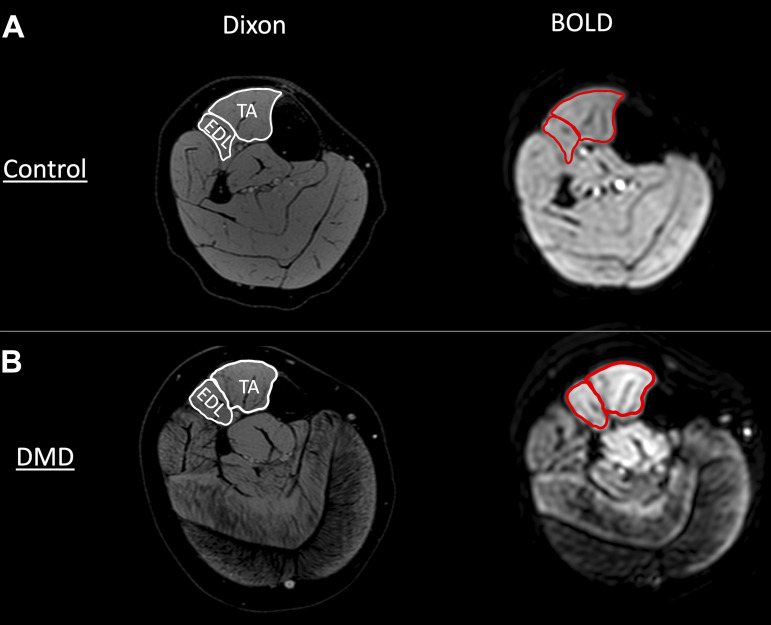
Initially, the images acquired were examined by experienced investigators to ensure they met quality assurance guidelines, including evaluating motion artifacts precluding the identification of muscle boundaries, ringing or aliasing artifacts contaminating the area of interest, and signal dropout in muscles of interest. In order to minimize any potential movement artefacts from the measures, a registration alignment procedure (“Register Virtual Stack Slices”) using ImageJ software was utilized (https://imagej.net/Register_Virtual_Stack_Slices). The TA and EDL were traced using the water images generated by mDixon images and then copied to the ssEPI images using OsiriX software (Fig. 1). The TA and EDL regions of interest (ROI’s) were inspected to ensure that only the muscle was included during the entire BOLD sequence (with no significant shifts due to movement artefacts), and that large blood vessels (e.g., anterior tibial artery/vein) were not included in the ROI. The high-resolution water/fat maps and in- and out-of-phase dixon images provided an opportunity to visualize the structure and location of the large vessels, which were excluded from the ROI’s (Fig. 1).
Figure 1.
Representative high-resolution water Dixon image used for drawing of region of interest (ROI) for blood oxygen level-dependent analysis (BOLD), and corresponding echo planar image (image 1/360) acquired to measure dynamic BOLD response in a control (A) and a participant with Duchenne muscular dystrophy (DMD; B). EDL, extensor digitorum longus; TA, tibialis anterior.
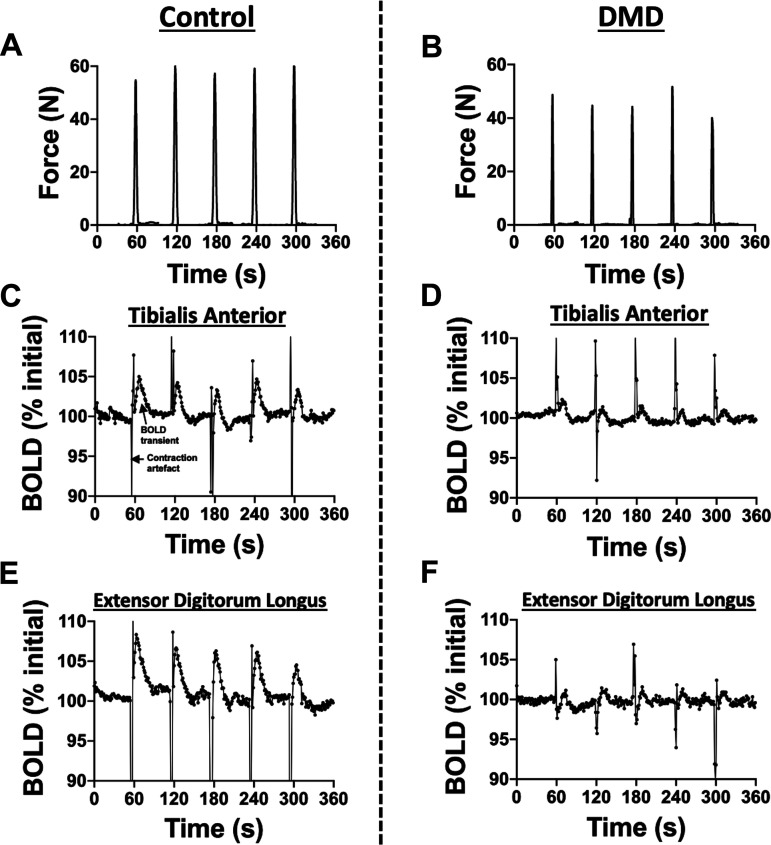
The BOLD responses that corresponded with the highest force contractions (>70% MVC) without excessive subject motion were averaged together, typically incorporating two to four postcontractile responses (17, 20) (Fig. 2). Force measurements were analyzed using MATLAB with a Gaussian-weighted moving average filter with a smoothing factor to remove MRI radio-frequency pulse generated artefact from the raw force data signal. Force data was converted to Newtons (N) based on the linear calibration of the force transducer.
Figure 2.
Example force (control: A; DMD: B) and postcontractile blood oxygen level-dependent (BOLD) responses in a control and in a participant with Duchenne muscular dystrophy (DMD) of the tibialis anterior (control: C; DMD: D) and extensor digitorum longus (control: E; DMD: F). Five isometric maximal voluntary dorsiflexion contractions were performed separated by 1 min of rest.
To ensure maximal contractions were performed during the contractions, an investigator was in the MR suite during the protocol and they observed the subject and contractions during the protocol to encourage a maximal volitional effort. Also, before going into the magnet, practice contractions were performed outside the magnet and standardized instructions were given before starting the protocol for the participant to become familiar with the set-up and contractions required. There was at least 15 min separating the warm-up/practice contractions with the protocol. Finally, a minimum specific force (N/nonfat area) value was required to be obtained for the data to be utilized. The minimum value was determined for each group (controls and DMD) based on the lower 95% confidence interval of the group mean of the peak specific force, and then a minimum of 70% of this value was used as the threshold for acceptable effort, since this intensity has been shown to be the minimum force required to reach a maximum peak BOLD response (20). That is, if at least 70% of the lower 95% confidence interval of the specific force was not reached, then the subject was excluded. This analysis resulted in the exclusion of 1 control subject who had a disproportionately low force relative to his muscle size, suggesting he may not have performed maximal effort during any of his contractions.
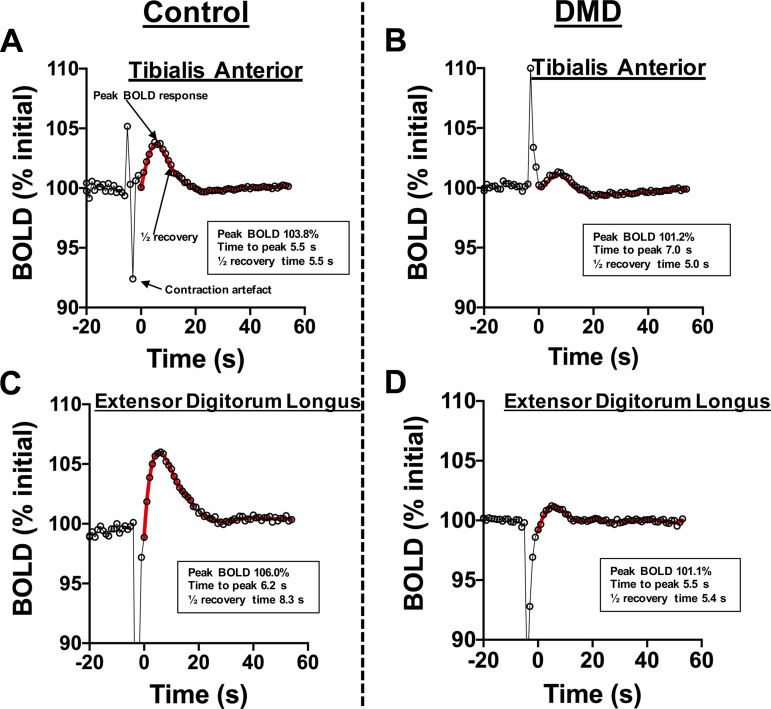
The BOLD response analysis included time to peak (TTP) as determined from time of the first point after the contraction (t = 0) to the maximum signal intensity following the contraction, the half-recovery time or time for the signal intensity to decrease to half of the maximum intensity, and the peak BOLD response (% change from initial), determined as a change in signal intensity relative to the signal intensity at rest. These parameters were determined after fitting the BOLD response using a six to nine order polynomial fitting algorithm similar to that described previously (Fig. 3) (16, 31, 32). To account for any drift of the BOLD signal intensity during the entire experiment, potentially due to instrument heating/stability, linear correction was performed and the signal intensity was normalized to a baseline value set to 100% signal intensity (SI) at rest. In an additional analysis, to account for the potential impact of muscle fat due to, for example, lack of BOLD response in the fat tissue or the fat content resulting in altered intramuscular pressure, we also normalized BOLD response of the anterior compartment (TA and EDL) relative to the fraction of the fat free area of that region. This was accomplished by dividing the peak BOLD response (percent change in SI) by fat free mass (i.e., 1-FF), to better reflect the BOLD response relative to muscle mass.
Figure 3.
Postcontractile BOLD responses of each contraction and the responses are averaged for an overall average BOLD response of the tibialis anterior (control: A; DMD: B) and extensor digitorum longus (control: C; DMD: D). Contraction artifacts were omitted from the BOLD analysis. The peak BOLD response, time to peak, and half-recovery time were evaluated after fitting the postcontractile BOLD response using a polynomial fitting algorithm. BOLD, blood oxygen level-dependent; DMD, Duchenne muscular dystrophy.
FF from the Dixon images was determined by drawing regions of interest for the TA and EDL on the vendor generated fat/water maps (28, 33). Care was taken to avoid inclusion of extramuscular tissue such as bone, subcutaneous fat, large blood vessels, or other muscles in the outlined segment. Three consecutive slices in or near the center of the muscle belly were analyzed for each muscle, and the pixel-by-pixel FF values were averaged to give a mean FF for each muscle (27, 28, 34). Trained analyzers selected 3 contiguous slices for analysis using predefined anatomic landmarks that ensured slice selection consistency among participants. The slices selected were the most distal slice in which the popliteus muscle was first visible and the 2 slices distal to it, which corresponded to the largest girth of the lower leg and the location of the BOLD measures (27, 28, 34).
Functional Assessments
Clinical function tests were performed by each participant within 24 h post MRI acquisitions and included a 10-m walk/run (10-m W/R) and 6-min walk test (6MWT). The functional tests have been described previously (25, 35–37), and incorporate simple standardized instructions with appropriate rest breaks, performed by trained evaluators. Briefly, for the 10-m W/R subjects were instructed to stand at the beginning of a measured 10 m length of an indoor walkway marked with tape, and starting with hands by their side told to walk or run as fast as they can from the start to finish. The 10-m W/R were repeated three times, and the fastest time was utilized for analysis and a maximal time of 45 s to complete each test was allowed. Also, the 6MWT was performed indoors along a walkway marked with a 25-m tape-line. A cone was positioned at each end of the course. Each subject was instructed to walk at a self-selected pace to cover as much distance as possible, over 6 min. Two functional evaluators monitored the test to ensure the subject stayed on the marked path without stopping. One evaluator walked behind the subject to provide encouragement, and one observer timed, tallied the laps, and calculated the distance traveled.
Statistical Analysis
Welch’s unpaired two-tailed t test was used to compare DMD and unaffected controls with the demographics, fat fraction, cross-sectional area, force, and BOLD response parameters. Spearman’s correlation was performed to examine the relationship between BOLD response between the anterior compartment (AC; weighted average of the TA and EDL based on muscle size) and functional measures and corresponding FF in the same region as the BOLD measures. Statistical analysis was performed using MATLAB (R2019a) with Statistics Toolbox, The MathWorks, Inc., Natick, Massachusetts and GraphPad Prism v8.0.0 for Mac OS, GraphPad Software, San Diego, California. For all comparisons, P ≤ 0.05 was considered significant. Data are reported as means ± SD.
RESULTS
Demographics
Participants with DMD and unaffected control subjects were of similar age (P = 0.497) and body weight (P = 0.216), and DMD subjects were shorter (P < 0.001) than controls (Table 1). Two participants with DMD were corticosteroid naïve, and the remainder were being administered corticosteroids. All participants (n = 32) completed the MRI protocol, and 7 of the DMD subjects and 4 of the control subjects repeated the MRI protocol to examine test-to-test reproducibility. One control subject was excluded due to uncertainty whether maximal effort was performed during the contractions (due to excessively low specific force) and one DMD subject was excluded due to noncompliance with the contraction protocol. Of the remaining 30 subjects, force values were not accurately obtained in 5 of the subjects due to technical difficulties of the force acquisitions in the bore of the magnet resulting in excessive noise to the force traces. All subjects completed the functional testing, with the exception of one participant with DMD who declined to participate in the 6MWD exercise. Overall, the protocol was well tolerated by both DMD and control participants without any adverse events being reported.
Table 1.
Demographics of participants with Duchenne muscular dystrophy and unaffected controls
| Controls |
DMD |
P Value |
|
|---|---|---|---|
| n | 16 | 16 | NA |
| Age, yr | 9.0 ± 2.7 | 9.0 ± 2.1 | 0.497 |
| Range, yr | 5.8–14.9 | 5.5–12.3 | NA |
| Corticosteroids, n | NA | 14/16 | NA |
| Weight, kg | 33.0 ± 12.7 | 29.7 ± 11.3 | 0.216 |
| Height, cm | 135.1 ± 18.7 | 119.2 ± 6.3 | <0.001 |
Welch’s unpaired t test was used to compare DMD and unaffected controls. Values are expressed as means ± SD. DMD, Duchenne muscular dystrophy; NA, not applicable.
Postcontractile BOLD Response in DMD Versus Controls
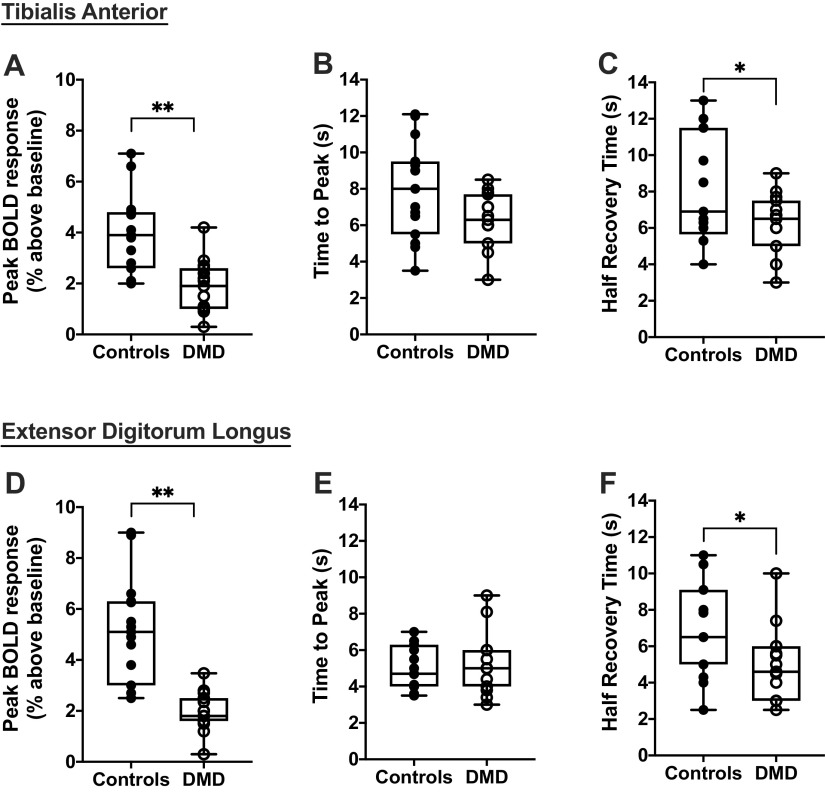
We observed a positive BOLD response (signal intensity increased relative to rest) following isometric dorsiflexion contractions in all participants (Fig. 2). The magnitude of the response was reduced (P < 0.001) in the TA and EDL of DMD compared with controls (Fig. 4). The half recovery time was shorter (P < 0.05) in the TA and EDL of DMD than controls, whereas the time to peak was not different between groups (Fig. 4). Furthermore, when we examined the peak BOLD response after contractions relative to the nonfat region, we also observed clear differences (P = 0.002) between DMD and controls in the TA (controls 4.4 ± 1.7%; DMD: 2.3 ± 1.1%) and EDL (controls 5.6 ± 2.2%; DMD: 2.3 ± 0.8%).
Figure 4.
Parameters of the postcontractile blood oxygen level-dependent (BOLD) response were compared between unaffected controls (n = 15) and participants with Duchenne muscular dystrophy (DMD; n = 15), including the peak BOLD response (A), time to peak (B), and the half recovery time (C) for the tibialis anterior and the peak BOLD response (D), time to peak (E), and half recovery time (F) for the extensor digitorum longus. Welch’s unpaired two-tailed t test was used to compare DMD and unaffected controls. Box and whisker plots represent median, 10/90 percentile, and minimum/maximum. *P < 0.05, **P < 0.001.
We obtained force measures in the majority of subjects (13 controls and 12 DMD) during the muscle contractions with the MR imaging acquisition, and we did not observe a significant difference in the peak force or specific force (normalized to nonfat muscle cross-sectional area) obtained during the isometric dorsiflexion contractions between controls and DMD (Table 2). Notably, when we only included the subjects with valid force data in our BOLD comparisons (i.e., the 5 subjects without accurate force data were excluded), our main findings were the same; the peak BOLD response and half time to recovery were reduced in DMD compared with controls for both the TA and EDL.
Table 2.
Force measurements in control and subjects with DMD during isometric dorsiflexion contractions
| Controls |
DMD |
P Value |
|
|---|---|---|---|
| n | 13 | 12 | NA |
| MVC, N | 71.3 (47.8, 94.8) | 53.1 (41.3, 64.9) | 0.300 |
| MVC, N/cm2 | 17.2 (12.8, 21.7) | 13.6 (10.0, 17.2) | 0.148 |
| MVC, % | 90.5 (85.1, 95.8) | 91.1 (85.6, 96.6) | 0.821 |
Maximum voluntary contraction (MVC) was defined as the maximum force obtained during isometric dorsiflexion contractions and was normalized to the nonfat cross-sectional area of the anterior compartment of the lower leg (tibialis anterior and extensor digitorum longus). The average relative Welch’s unpaired two-tailed t test was used to compare DMD (n = 15) and unaffected controls (n = 15). Values are expressed as means (lower and upper 95% confidence interval; C95).
DMD, Duchenne muscular dystrophy; EDL, extensor digitorum longus; NA, not applicable; TA, tibialis anterior; 6MWD, 6-min walk distance.
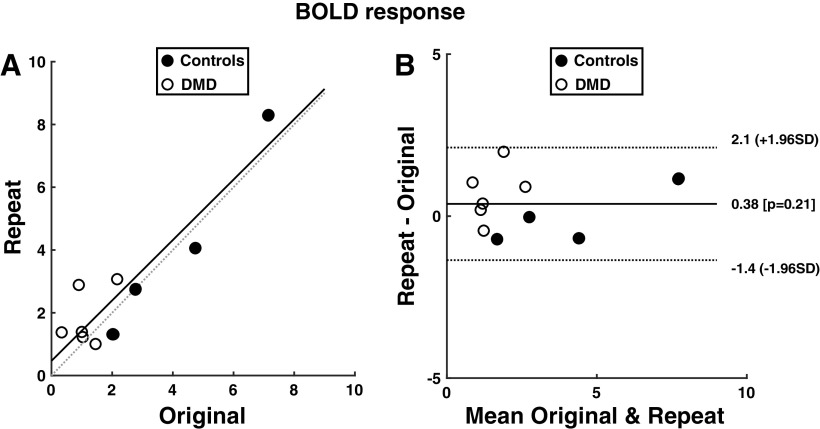
Reproducibility of BOLD and Force Measures
To test reproducibility of the postcontractile BOLD response following maximal dorsiflexion contractions, several of the subjects repeated this portion of the MRI protocol (n = 4 controls, n = 7 DMD). Adequate reproducibility of the TA was observed when evaluated using Bland–Altman plots, with intraclass correlation coefficients (ICC) of 0.94 for the peak BOLD response (Fig. 5), 0.88 for the time to peak, and 0.96 for the half recovery time. Similarly, the BOLD response was observed to be reproducible for the EDL analysis (peak BOLD response ICC = 0.96, time to peak ICC = 0.82, and half recovery time = 0.87). Also, the maximum force levels were reproducible between testing sessions (ICC 0.98), with no systematic differences (P > 0.05) between the testing sessions (test 1: 66 ± 55 N; test 2: 62 ± 42 N; coefficient of variation (CV) = 7.6 ± 5.9 N). Also, the force produced during the contractions within the BOLD protocol was similar among the 5 contractions in both DMD and controls, indicating recovery between contractions was adequate and no fatigue was observed with the BOLD protocol. For example, in DMD, compared with the initial contraction, the peak force levels were, on average, 98.6% (contraction 2), 102.9% (contraction 3), 99.2% (contraction 4), and 100.1% (contraction 5) in the subsequent contractions. Similarly, the forces observed in control subjects were 101.4% (contraction 2), 94.7% (contraction 3), 98.2% (contraction 4), and 99.4% (contraction 5) relative to the initial contraction during the BOLD protocol.
Figure 5.
A: correlation plot with initial (x-axis) and repeated (y-axis) of BOLD responses of the tibialis anterior muscle from both control (n = 4) and Duchenne muscular dystrophy (DMD; n = 7) subjects that repeated the BOLD protocol (ICC = 0.94). B: Bland–Altman plot showing limits of agreement (±1.96SD) and bias (0.21) of the (absolute) peak BOLD response. BOLD, blood oxygen level-dependent.
We also tested the BOLD multislice acquisition versus a single slice acquisition in 3 control subjects due to the potential of inflow effects with the multislice acquisition due to the possible contribution of blood entering the slice and magnetization transfer effects. We observed no differences when comparing these scans in the peak BOLD response when matching the slices for analysis of the AC (multislice acquisition: 103.8 ± 1.1%; single slice acquisition: 103.7 ± 1.2%), time to peak (multislice acquisition: 5.7 ± 1.5 s; single slice acquisition: 5.0 ± 1.0 s), and half recovery time (multislice acquisition: 7.3 ± 4.9 s; single slice acquisition: 6.3 ± 4.0 s).
Relationship of BOLD Response to Function and Fat Fraction
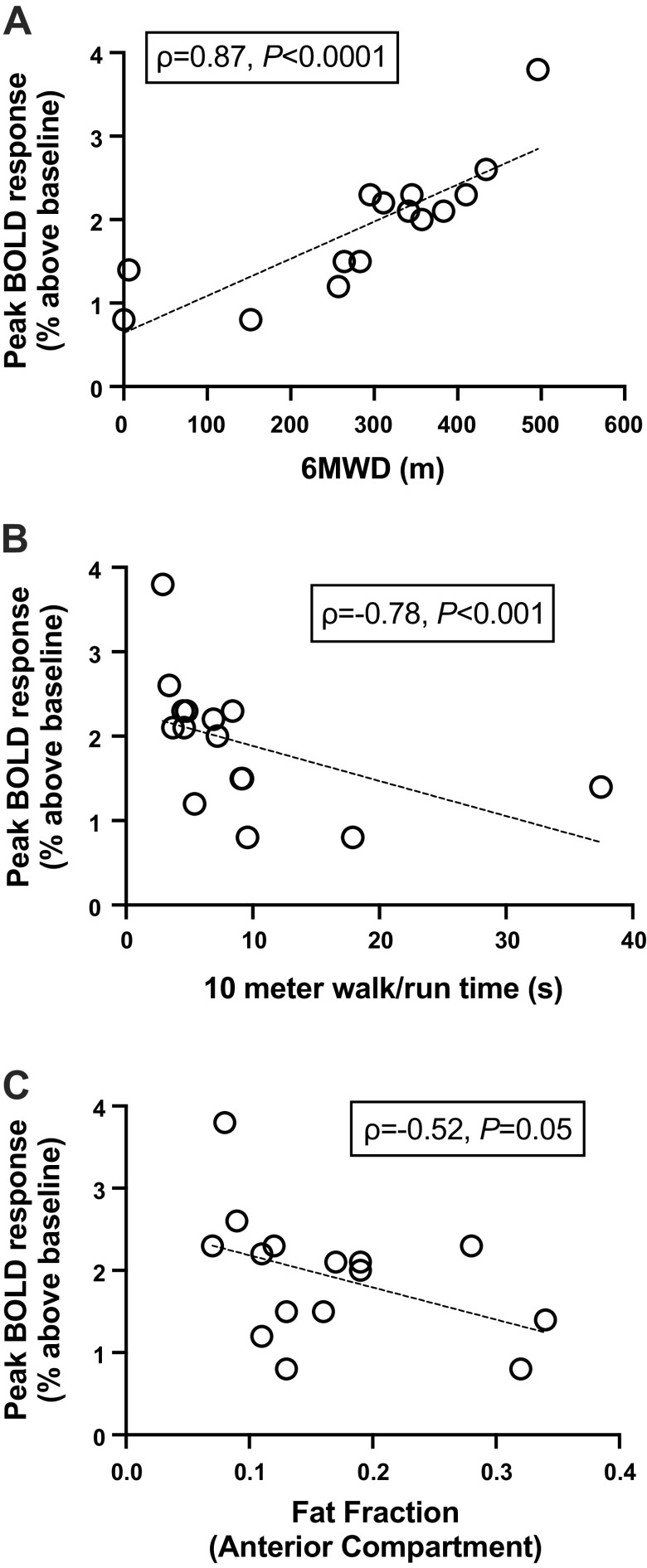
Participants with DMD had greater fatty tissue infiltration in the TA and EDL and lower functional scores on standard clinical walking/running tests compared with unaffected controls (Table 3). The peak BOLD response of the AC was correlated with these indices of disease severity in DMD (Fig. 6). A positive correlation was observed between the peak BOLD response and 6MWD (ρ = 0.87, P < 0.0001, Fig. 6). Furthermore, there was a negative correlation between the peak BOLD response and the time to complete the 10-m W/R (ρ = −0.78, P < 0.001) and AC FF (ρ = −0.52, P = 0.05, Fig. 6). Specific force obtained during the maximal contractions was not significantly correlated to the AC peak BOLD response in DMD (ρ = −0.41, P = 0.15). In contrast to DMD, the peak BOLD response of the AC in control subjects was not correlated to 10-m W/R (ρ = 0.40, P = 0.15), 6MWD (ρ = 0.10, P = 0.73), and AC fat fraction (ρ = −0.32, P = 0.12).
Table 3.
Clinical functional measures and muscle fat fraction of participants with Duchenne muscular dystrophy and unaffected controls
| Controls | DMD | P Value | |
|---|---|---|---|
| Functional test | |||
| 6MWD, m | 514 (469, 558) | 389 (203, 375) | <0.0001 |
| 10 m walk/run, s | 2.8 (2.6, 3.0) | 9.0 (3.7, 14.3) | <0.0001 |
| Muscle CSA, cm2 | |||
| TA | 3.25 (2.57, 3.92) | 3.26 (2.73, 3.78) | 0.430 |
| EDL | 1.43 (1.16, 1.70) | 1.54 (1.16, 1.92) | 0.602 |
| Fat fraction, % | |||
| TA | 8.9 (7.4, 10.5) | 16.3 (11.6, 21.0) | <0.0001 |
| EDL | 7.7 (6.6, 8.7) | 17.1 (11.2, 23.0) | <0.0001 |
Values are expressed as mean (lower and upper 95% confidence interval; C95). Welch’s unpaired two-tailed t test was used to compare DMD (n = 15) and unaffected controls (n = 15). CSA, cross-sectional area; DMD, Duchenne muscular dystrophy; EDL, extensor digitorum longus; TA, tibialis anterior; 6MWD, 6-min walk distance.
Figure 6.
Correlation between the postcontractile peak BOLD response of the anterior compartment (weighted average of the tibialis anterior and extensor digitorum longus) vs. the 6-min walk distance (6MWD; ρ = 0.87, P < 0.0001, n = 15; A), 10-m walk/run time (ρ = −0.78, P < 0.001, n = 15; B), and fat fraction of the anterior compartment (ρ = −0.52, P = 0.05; C) in participants with DMD. Data were analyzed using Spearman’s correlation analyses. BOLD, blood oxygen level-dependent; DMD, Duchenne muscular dystrophy.
DISCUSSION
This study evaluated the postcontractile BOLD response in ambulatory participants with DMD and unaffected controls between 5 to 14 yr of age. We observed that the peak BOLD response after maximal isometric dorsiflexion contractions was impaired in participants with DMD compared with unaffected controls with no differences in force of contractions. These findings are consistent with the notion of impaired microvascular function in DMD, possibly due to lack of nNOSμ. Furthermore, we observed that the peak postcontractile BOLD response was correlated with functional walking/running performance and FF of the AC of the lower leg muscles (TA, EDL).
The application of BOLD to skeletal muscle to infer microvascular function is only beginning to be exploited with clinical applications (14–19, 38). The muscle BOLD response is affected by changes in oxygen saturation and blood volume of small vessels (18), and reflects the balance between oxygen delivery and utilization; therefore a positive BOLD response (increase in signal relative to rest) indicates that the delivery of oxygen is in excess of oxygen consumption. Vascular responses after a single brief contraction have been proposed to arise primarily from rapid arteriole vasodilation (39) and a number of factors can modulate the postcontractile BOLD response, including local arterial vasodilatory mechanisms, comprising of both endothelial dependent and independent processes, vasoconstriction modulation, sympathetic responses, and mitochondrial function (18, 40). Importantly, the BOLD response is dependent on contraction intensity and requires an increase in intramuscular pressure causing occlusion of the vasculature, which then establishes the postcontractile BOLD response (16). The peak BOLD response after muscle contraction increases approximately linearly until ∼60% of MVC and plateaus at ∼70% MVC (20). Therefore, it is critical that a near maximal volitional effort during the contractions is made to obtain an accurate measure of the peak BOLD response. In order to help verify subjects were providing a maximal effort, specific force levels (relative to muscle cross-sectional area) were utilized in this study to ensure adequate intensity of contractions were performed. Our approach resulted in reproducible BOLD responses, with comparable reproducibility to that observed previously using a similar protocol in adults (41). For example, in that study, the ICC for the TA muscle at MVC condition was 0.88 (P = 0.005), whereas we observed an ICC of 0.94 for the TA and the Bland–Altman plots revealed repeatability coefficients (RCs) of 1.4 for MVC conditions in the TA muscle, whereas we observed an average of 1.8. Some previous studies have utilized a single slice acquisition approach, which would avoid the potential of inflow effects (16, 18). In this study, we utilized a multislice acquisition with an EPI sequence, TR 1 s, and acquired the axial slices in a distal to proximal direction; we expected this sequence and parameters would have a relatively small effect of inflow effects in multislice acquisitions (42, 43). In order to confirm this, we directly compared the BOLD response in multislice versus single slice in controls (n = 3) and observed no differences in peak BOLD response, time to peak, and half recovery time.
To the best of our knowledge, this is the first study to show that the postcontractile BOLD response is reduced in DMD compared with unaffected controls. Previously, we have observed an impaired BOLD response in dystrophic mice following brief stimulated contractions, and this response was improved following the administration of a phosphodiesterase type 5a (PDE5a) inhibitor (24). PDE5 inhibitors prolong the half-life of cGMP, a downstream target of NO in vascular smooth muscle, which may partly compensate for the lack of subsarcolemma localization of nNOSμ in dystrophic muscle (3–6). Lack of nNOSμ has been attributed to impair vascular function due to an inability to blunt sympathetic vasoconstriction post exercise (9), and detriments in vascular function have been previously described during and following exercise in dystrophic mice models and boys with DMD (5, 8, 12). Furthermore, Nelson et al. (8) observed blunted brachial artery blood flow in DMD compared with controls after 7 min of handgrip contractions, and more recently, it was observed that changes in blood flow estimated using power Doppler sonography in the anterior forearms and TA following 1 min of maximal contractions was reduced in Duchenne and Becker muscular dystrophy (D/BMD) (44). Our findings are consistent with these previously reported impairments and suggest that the postcontractile peak BOLD response may provide a quantitative index of microvascular function with high spatial resolution that can be reproducibility administered to participants with DMD, including young subjects.
Along with microvascular function influencing the postcontractile BOLD response, it is also possible that the fat content of the muscle could affect the BOLD response by, for example, altering the intramuscular pressure. An increase in muscle fat fraction in DMD is a common characteristic of disease progression in DMD (45), with the increase in fat fraction in DMD reflecting predominantly extracellular fat stores, although intracellular fat content also tends to be elevated when overall muscle fat fraction is evaluated in DMD (46). However, both the TA and EDL (and hence the AC) fat fraction are relatively low in DMD compared with other muscles (46), and there were 4 DMD subjects with fat fraction levels at or below the mean of control subjects (0.09). Even in these DMD subjects with low fat fraction comparable with controls, the peak BOLD response was reduced (P = 0.02) in DMD (2.5 ± 1.1%) compared with controls (4.3 ± 1.6%). This suggests that factors other than fat fraction (and associated potential reductions in intramuscular pressure) contribute to the impairment in peak BOLD response observed in DMD.
The effect of corticosteroids on microvascular function in DMD is not clear, and may depend on a number of factors including dosing regimen (daily vs. weekly), corticosteroid type (Emflaza vs. prednisone), and length of time the participants have been administered corticosteroids (47). Previous studies have generally shown corticosteroids to have beneficial effects on clinical functional tests, ambulation, and muscle strength (plantar flexion and knee extension) in DMD and this is generally attributed to reduced inflammation (34). Although studies examining the effects of corticosteroids on microvascular function in DMD are limited, other conditions characterized by inflammation, such as inflammatory rheumatic diseases, have shown improvements in vascular function following corticosteroid interventions (48). However, there is also considerable evidence to suggest that corticosteroids can have detrimental effects on microvascular function in certain circumstances, such as impairing vascular reactivity by altering 11 β-hydroxysteroid dehydrogenase expression in vascular endothelial and smooth muscles (49, 50). The majority of participants with DMD in this study were taking corticosteroids long-term (greater than 6 mo). The 2 DMD subjects who were not taking corticosteroids in this study did not have an altered BOLD response compared with those on corticosteroids; both participants not taking corticosteroids (ages 6 and 9 yr; 6 min walk distance of 295 m and 345 m) had an AC peak BOLD response of 102.3%, versus the DMD group average of 101.9%, range of 100.8%–103.8%), but clearly more research is needed in this area to examine age and drug interactions.
As well as the peak BOLD response being impaired in DMD, we observed the half time to recover following the peak BOLD response was shorter in DMD than controls. Consistent with this, some studies have observed that a reduced postcontractile peak BOLD response coincides with a faster half recovery time (e.g., Ref. 23). We also observed a positive relationship between the peak BOLD response and half recovery time, although this was not a strong relationship (ρ = 0.27, P = 0.04). Other studies have also shown evidence that the peak BOLD response and the half recovery time may not be tightly coupled. For example, Larsen et al. (51) observed a similar peak BOLD response but a longer half recovery time following eccentric contractions compared with baseline. The different half recovery times may reflect alterations in the balance between oxygen delivery and utilization. It has been well established that oxygen consumption in muscle begins without any appreciable delay during muscle contractions (52), and oxygen is also utilized to resynthesize phosphocreatine (PCr) after contractions (53). Therefore, it is likely that muscle oxygen utilization also occurs during and after the brief contractions implemented in this study (54), and oxygen utilization has previously been incorporated as an important component of a model of the postcontractile BOLD response (18). The faster decrease of the BOLD signal intensity after reaching peak values may reflect a relatively greater oxygen utilization than delivery, and faster off-transient kinetics of measures reflecting the balance between oxygen delivery and utilization (e.g., microvascular PO2) after muscle contractions have been observed under conditions in which microvascular circulation is impaired (55). Therefore, the faster recovery time could indicate a relatively reduced oxygen delivery in DMD compared with controls after the peak BOLD response.
Notably, there was considerable heterogeneity of the BOLD responses among participants with DMD, as well as controls. In this study we observed a significant relationship between the peak postcontractile BOLD response and functional walking/running performances and muscle FF in DMD, suggesting the BOLD response measure is associated with disease severity. As the disease progresses, there are several structural changes that occur that may contribute to an impaired BOLD response, including fibrosis and altered capillary density (56, 57). Several factors may also contribute to the heterogeneity of the postcontractile BOLD response among controls, including physical activity patterns and fitness levels, consistent with previous reports of the postcotractile BOLD response being related to endurance performance and physical activity level (14, 23). In controls, we did not observe a significant relationship between peak BOLD response and 10-m W/R, 6-min walk distance, and fat fraction, but this may be due to the relative homogenous group of control subjects (e.g., limited range for 10-m W/R = 2.1–3.1 s; 6-min walk distance = 400–666 m; fat fraction: 0.05–0.15). Despite a relationship observed in this study with the peak BOLD response and ambulatory function in DMD, further larger cross-sectional and longitudinal studies should be performed to better understand how the BOLD response changes with disease progression in DMD.
Limitations and Future Directions
This study focused on the TA and EDL muscles during dorsiflexion contractions, and this model was straightforward to interpret since the TA and EDL are the primary muscles recruited during this contraction. The TA and EDL are also relatively spared muscles in DMD, have less fatty tissue infiltration, and allow for adequate muscle to be sampled over a wide range of ages. However, muscles involved in other movements may be affected differently, such as the soleus, gastrocnemius, or vastus lateralis muscles, which tend to be affected earlier and to a greater extent (45), and owing to this, the impairments we observed in this study may underestimate the impairments in other muscles. Also, our analysis was limited to the muscle midbelly, but there is considerable heterogeneity along the length of the muscles and the proximal and distal ends of the muscles are often more affected (58). Furthermore, there are other relatively minor dorsiflexor muscles of the ankle and foot that should be considered to be examined using the BOLD response in future studies (59). Overall, future studies should consider incorporating other muscles and examining different regions within muscles. Also, other approaches of examining the BOLD response have been implemented, such as investigating the response after cuff occlusion (60, 61). For example, the BOLD response was observed to be different after cuff occlusion in sedentary than athletes of college age (61). Since examining the hyperemic response after occlusion likely has different contributing mechanisms than after contractions (e.g., may be more independent of nNOS), this approach may provide additional insight into the deficits of the BOLD response observed in this study in DMD.
Summary
To the best of our knowledge, this was the first study to examine the postcontractile BOLD response in children. We showed the feasibility of implementing this dorsiflexion technique in unaffected controls and in boys with DMD, including as young as 5 yr of age, and with adequate test-to-test reproducibility. Overall, our findings demonstrate that the peak BOLD response is impaired in DMD compared with unaffected controls after brief muscle contractions. Furthermore, the postcontractile peak BOLD response was related to clinical function performance and muscle FF, markers of disease severity in DMD. Our data provide support that the postcontractile BOLD response may be a valuable tool to evaluate interventions aimed at improving vascular function in DMD.
GRANTS
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR070101). Also, a portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and the State of Florida. T. Taivassalo is supported by the US Department of Defense project W81XWH1910330. A. Batra was also supported by a training award from the Wellstone Muscular Dystrophy Cooperative Center (U54 AR052646).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.L., G.A.W., and S.C.F. conceived and designed research; C.L., A.S., H.C.R., A.B., H.A., and S.C.F. performed experiments; C.L., M.G.B., A.M.R., and S.C.F. analyzed data; C.L., T.T., M.G.B., and S.C.F. interpreted results of experiments; C.L. and S.C.F. prepared figures; C.L., T.T., M.G.B., A.M.R., and S.C.F. drafted manuscript; C.L., T.T., M.G.B., A.S., H.C.R., A.B., H.A., G.A.W., K.V., and S.C.F. edited and revised manuscript; C.L., T.T., M.G.B., A.S., H.C.R., A.B., H.A., A.M.R., G.A.W., K.V., and S.C.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants and families for participation in this study, and we are appreciative of the MR technologists and research staff for assisting in data collection and analysis.
REFERENCES
- 1.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C; DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 9: 77–93, 2010. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51: 919–928, 1987. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 3.Adams ME, Odom GL, Kim MJ, Chamberlain JS, Froehner SC. Syntrophin binds directly to multiple spectrin-like repeats in dystrophin and mediates binding of nNOS to repeats 16-17. Hum Mol Genet 27: 2978–2985, 2018. doi: 10.1093/hmg/ddy197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heydemann A, McNally E. NO more muscle fatigue. J Clin Invest 119: 448–450, 2009. doi: 10.1172/jci38618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL, Duan D. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest 119: 624–635, 2009. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Percival JM. Perspective: spectrin-like repeats in dystrophin have unique binding preferences for syntrophin adaptors that explain the mystery of how nNOSμ localizes to the sarcolemma. Front Physiol 9: 1369, 2018. doi: 10.3389/fphys.2018.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA 95: 15090–15095, 1998. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson MD, Rader F, Tang X, Tavyev J, Nelson SF, Miceli MC, Elashoff RM, Sweeney HL, Victor RG. PDE5 inhibition alleviates functional muscle ischemia in boys with Duchenne muscular dystrophy. Neurology 82: 2085–2091, 2014. doi: 10.1212/WNL.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci USA 97: 13818–13823, 2000. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rando TA. Role of nitric oxide in the pathogenesis of muscular dystrophies: a “two hit” hypothesis of the cause of muscle necrosis. Microsc Res Tech 55: 223–235, 2001. doi: 10.1002/jemt.1172. [DOI] [PubMed] [Google Scholar]
- 11.Asai A, Sahani N, Kaneki M, Ouchi Y, Martyn JAJ, Yasuhara SE. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS One 2: e806, 2007. doi: 10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, Campbell KP. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature 456: 511–515, 2008. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Yue Y, Li L, Hakim CH, Zhang K, Thomas GD, Duan D. Dual AAV therapy ameliorates exercise-induced muscle injury and functional ischemia in murine models of Duchenne muscular dystrophy. Hum Mol Genet 22: 3720–3729, 2013. doi: 10.1093/hmg/ddt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurley DM, Williams ER, Cross JM, Riedinger BR, Meyer RA, Abela GS, Slade JM. Aerobic exercise improves microvascular function in older adults. Med Sci Sports Exerc 51: 773–781, 2019. doi: 10.1249/MSS.0000000000001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer RA, Towse TF, Reid RW, Jayaraman RC, Wiseman RW, McCully KK. BOLD MRI mapping of transient hyperemia in skeletal muscle after single contractions. NMR Biomed 17: 392–398, 2004. doi: 10.1002/nbm.893. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez OA, Copenhaver EA, Chance MA, Fowler MJ, Towse TF, Kent-Braun JA, Damon BM. Postmaximal contraction blood volume responses are blunted in obese and type 2 diabetic subjects in a muscle-specific manner. Am J Physiol Heart Circ Physiol 301: H418–H427, 2011. doi: 10.1152/ajpheart.00060.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonson A, Noble KE, Meyer RA, Rozman MR, Foley KT, Slade JM. Age reduces microvascular function in the leg independent of physical activity. Med Sci Sports Exerc 49: 1623–1630, 2017. doi: 10.1249/MSS.0000000000001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Towse TF, Slade JM, Ambrose JA, DeLano MC, Meyer RA. Quantitative analysis of the postcontractile blood-oxygenation-level-dependent (BOLD) effect in skeletal muscle. J Appl Physiol (1985) 111: 27–39, 2011. doi: 10.1152/japplphysiol.01054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wigmore D, Propert K, Kent-Braun J. Blood flow does not limit skeletal muscle force production during incremental isometric contractions. Eur J Appl Physiol 96: 370–379, 2006. doi: 10.1007/s00421-005-0037-0. [DOI] [PubMed] [Google Scholar]
- 20.Wigmore DM, Damon BM, Pober DM, Kent-Braun JA. MRI measures of perfusion-related changes in human skeletal muscle during progressive contractions. J Appl Physiol (1985) 97: 2385–2394, 2004. doi: 10.1152/japplphysiol.01390.2003. [DOI] [PubMed] [Google Scholar]
- 21.Ledermann H-P, Schulte A-C, Heidecker H-G, Aschwanden M, JäGer KA, Scheffler K, Steinbrich W, Bilecen D. Blood oxygenation level-dependent magnetic resonance imaging of the skeletal muscle in patients with peripheral arterial occlusive disease. Circulation 113: 2929–2935, 2006. doi: 10.1161/CIRCULATIONAHA.105.605717. [DOI] [PubMed] [Google Scholar]
- 22.Slade JM, Towse TF, Gossain VV, Meyer RA. Peripheral microvascular response to muscle contraction is unaltered by early diabetes but decreases with age. J Appl Physiol (1985) 111: 1361–1371, 2011. doi: 10.1152/japplphysiol.00009.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Towse TF, Slade JM, Meyer RA. Effect of physical activity on MRI-measured blood oxygen level-dependent transients in skeletal muscle after brief contractions. J Appl Physiol (1985) 99: 715–722, 2005. doi: 10.1152/japplphysiol.00272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batra A, Vohra RS, Chrzanowski SM, Hammers DW, Lott DJ, Vandenborne K, Walter GA, Forbes SC. Effects of PDE5 inhibition on dystrophic muscle following an acute bout of downhill running and endurance training. J Appl Physiol (1985) 126: 1737–1745, 2019. doi: 10.1152/japplphysiol.00664.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnard AM, Willcocks RJ, Finanger EL, Daniels MJ, Triplett WT, Rooney WD, Lott DJ, Forbes SC, Wang D-J, Senesac CR, Harrington AT, Finkel RS, Russman BS, Byrne BJ, Tennekoon GI, Walter GA, Sweeney HL, Vandenborne K. Skeletal muscle magnetic resonance biomarkers correlate with function and sentinel events in Duchenne muscular dystrophy. PloS One 13: e0194283–e0194283, 2018. doi: 10.1371/journal.pone.0194283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forbes S, Willcocks R, Triplett W, Rooney W, Lott D, Wang D, Pollaro J, Senesac C, Daniels M, Finkel R, Russman B, Byrne B, Finanger E, Tennekoon G, Walter G, Sweeney H, Vandenborne K. Magnetic resonance imaging and spectroscopy assessment of lower extremity skeletal muscles in boys with Duchenne muscular dystrophy: a multicenter cross sectional study. PLoS One 9: e106435, 2014. doi: 10.1371/journal.pone.0106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willcocks RJ, Rooney WD, Triplett WT, Forbes SC, Lott DJ, Senesac CR, Daniels MJ, Wang D-J, Harrington AT, Tennekoon GI, Russman BS, Finanger EL, Byrne BJ, Finkel RS, Walter GA, Sweeney HL, Vandenborne K. Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large Duchenne muscular dystrophy cohort. Ann Neurol 79: 535–547, 2016. doi: 10.1002/ana.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Triplett WT, Baligand C, Forbes SC, Willcocks RJ, Lott DJ, DeVos S, Pollaro J, Rooney WD, Sweeney HL, Bönnemann CG, Wang D-J, Vandenborne K, Walter GA. Chemical shift-based MRI to measure fat fractions in dystrophic skeletal muscle. Magn Reson Med 72: 8–19, 2014. doi: 10.1002/mrm.24917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willcocks RJ, Triplett WT, Forbes SC, Arora H, Senesac CR, Lott DJ, Nicholson TR, Rooney WD, Walter GA, Vandenborne K. Magnetic resonance imaging of the proximal upper extremity musculature in boys with Duchenne muscular dystrophy. J Neurol 264: 64–71, 2017. doi: 10.1007/s00415-016-8311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eggers H, Brendel B, Duijndam A, Herigault G. Dual-echo Dixon imaging with flexible choice of echo times. Magn Reson Med 65: 96–107, 2011. doi: 10.1002/mrm.22578. [DOI] [PubMed] [Google Scholar]
- 31.Towse TF, Childs BT, Sabin SA, Bush EC, Elder CP, Damon BM. Comparison of muscle BOLD responses to arterial occlusion at 3 and 7 Tesla. Magn Reson Med 75: 1333–1340, 2016. doi: 10.1002/mrm.25562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Towse TF, Elder CP, Bush EC, Klockenkemper SW, Bullock JT, Dortch RD, Damon BM. Post-contractile BOLD contrast in skeletal muscle at 7T reveals inter-individual heterogeneity in the physiological responses to muscle contraction. NMR Biomed 29: 1720–1728, 2016. doi: 10.1002/nbm.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forbes SC, Arora H, Willcocks RJ, Triplett WT, Rooney WD, Barnard AM, Alabasi U, Wang D-J, Lott DJ, Senesac CR, Harrington AT, Finanger EL, Tennekoon GI, Brandsema J, Daniels MJ, Sweeney HL, Walter GA, Vandenborne K. Upper and lower extremities in Duchenne muscular dystrophy evaluated with quantitative MRI and proton MR spectroscopy in a multicenter cohort. Radiology 295: 616–625, 2020. doi: 10.1148/radiol.2020192210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arpan I, Willcocks RJ, Forbes SC, Finkel RS, Lott DJ, Rooney WD, Triplett WT, Senesac CR, Daniels MJ, Byrne BJ, Finanger EL, Russman BS, Wang DJ, Tennekoon GI, Walter GA, Sweeney HL, Vandenborne K. Examination of effects of corticosteroids on skeletal muscles of boys with DMD using MRI and MRS. Neurology 83: 974–980, 2014. doi: 10.1212/WNL.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akima H, Lott D, Senesac C, Deol J, Germain S, Arpan I, Bendixen R, Lee Sweeney H, Walter G, Vandenborne K. Relationships of thigh muscle contractile and non-contractile tissue with function, strength, and age in boys with Duchenne muscular dystrophy. Neuromuscul Disord 22: 16–25, 2012. doi: 10.1016/j.nmd.2011.06.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arpan I, Forbes SC, Lott DJ, Senesac CR, Daniels MJ, Triplett WT, Deol JK, Sweeney HL, Walter GA, Vandenborne K. T2 mapping provides multiple approaches for the characterization of muscle involvement in neuromuscular diseases: a cross-sectional study of lower leg muscles in 5–15-year-old boys with Duchenne muscular dystrophy. NMR Biomed 26: 320–328, 2013. doi: 10.1007/s00415-016-8311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald CM, Henricson EK, Abresch RT, Florence JM, Eagle M, Gappmaier E, Glanzman AM, Spiegel R, Barth J, Elfring G, Reha A, Peltz S; PTC124‐GD‐007‐DMD Study Group. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve 48: 343–356, 2013. doi: 10.1002/mus.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobi B, Bongartz G, Partovi S, Schulte A-C, Aschwanden M, Lumsden AB, Davies MG, Loebe M, Noon GP, Karimi S, Lyo JK, Staub D, Huegli RW, Bilecen D. Skeletal muscle BOLD MRI: From underlying physiological concepts to its usefulness in clinical conditions. J Magn Reson Imaging 35: 1253–1265, 2012. doi: 10.1002/jmri.23536. [DOI] [PubMed] [Google Scholar]
- 39.Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol (1985) 96: 639–644, 2004. doi: 10.1152/japplphysiol.00769.2003. [DOI] [PubMed] [Google Scholar]
- 40.Utz W, Jordan J, Niendorf T, Stoffels M, Luft FC, Dietz R, Friedrich MG. Blood oxygen level-dependent MRI of tissue oxygenation: relation to endothelium-dependent and endothelium-independent blood flow changes. Arterioscler Thromb Vasc Biol 25: 1408–1413, 2005. doi: 10.1161/01.ATV.0000170131.13683.d7. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez OA, Louie EA, Copenhaver EA, Damon BM. Repeatability of a dual gradient-recalled echo MRI method for monitoring post-isometric contraction blood volume and oxygenation changes. NMR Biomed 22: 753–761, 2009. doi: 10.1002/nbm.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao J-H, Liu H-L. Inflow effects on functional MRI. Neuroimage 62: 1035–1039, 2012. doi: 10.1016/j.neuroimage.2011.09.088. [DOI] [PubMed] [Google Scholar]
- 43.Howseman AM, Grootoonk S, Porter DA, Ramdeen J, Holmes AP, Turner R. The effect of slice order and thickness on fMRI activation data using multislice echo-planar imaging. Neuroimage 9: 363–376, 1999. doi: 10.1006/nimg.1998.0418. [DOI] [PubMed] [Google Scholar]
- 44.Dietz AR, Connolly A, Dori A, Zaidman CM. Intramuscular blood flow in Duchenne and Becker muscular dystrophy: quantitative power Doppler sonography relates to disease severity. Clin Neurophysiol 131: 1–5, 2020. doi: 10.1016/j.clinph.2019.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rooney WD, Berlow YA, Triplett WT, Forbes SC, Willcocks RJ, Wang DJ, Arpan I, Arora H, Senesac C, Lott DJ, Tennekoon G, Finkel R, Russman BS, Finanger EL, Chakraborty S, O'Brien E, Moloney B, Barnard A, Sweeney HL, Daniels MJ, Walter GA, Vandenborne K. Modeling disease trajectory in Duchenne muscular dystrophy. Neurology 94: e1622–e1633, 2020. doi: 10.1212/WNL.0000000000009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lott DJ, Forbes SC, Mathur S, Germain SA, Senesac CR, Sweeney HL, Walter GA, Vandenborne K. Assessment of intramuscular lipid and metabolites of the lower leg using magnetic resonance spectroscopy in boys with Duchenne muscular dystrophy. Neuromuscul Disord 24: 574–582, 2014. doi: 10.1016/j.nmd.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quattrocelli M, Zelikovich AS, Salamone IM, Fischer JA, McNally EM. Mechanisms and clinical applications of glucocorticoid steroids in muscular dystrophy. J Neuromuscul Dis 8: 39–52, 2021. doi: 10.3233/JND-200556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arida A, Protogerou AD, Kitas GD, Sfikakis PP. Systemic inflammatory response and atherosclerosis: the paradigm of chronic inflammatory rheumatic diseases. Int J Mol Sci 19: 1890, 2018. doi: 10.3390/ijms19071890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol 157: 545–559, 2007. doi: 10.1530/EJE-07-0455. [DOI] [PubMed] [Google Scholar]
- 50.Yang S, Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol 2: 1–12, 2004. doi: 10.2174/1570161043476483. [DOI] [PubMed] [Google Scholar]
- 51.Larsen RG, Hirata RP, Madzak A, Frøkjær JB, Graven-Nielsen T. Eccentric exercise slows in vivo microvascular reactivity during brief contractions in human skeletal muscle. J Appl Physiol (1985) 119: 1272–1281, 2015. doi: 10.1152/japplphysiol.00563.2015. [DOI] [PubMed] [Google Scholar]
- 52.Kindig CA, Kelley KM, Howlett RA, Stary CM, Hogan MC. Assessment of O2 uptake dynamics in isolated single skeletal myocytes. J Appl Physiol (1985) 94: 353–357, 2003. doi: 10.1152/japplphysiol.00559.2002. [DOI] [PubMed] [Google Scholar]
- 53.Meyerspeer M, Boesch C, Cameron D, Dezortová M, Forbes SC, Heerschap A, Jeneson JAL, Kan HE, Kent J, Layec G, Prompers JJ, Reyngoudt H, Sleigh A, Valkovič L, Kemp GJ, Baligand C, Carlier PG, Chatel B, Damon B, Heskamp L, Hájek M, Jooijmans M, Krssak M, Reichenbach J, Schmid A, Slade J, Vandenborne K, Walter GA, Willis D; Experts' Working Group on PMRSoSM. 31P magnetic resonance spectroscopy in skeletal muscle: Experts’ consensus recommendations. NMR Biomed 34: e4246–e4246, 2021. doi: 10.1002/nbm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slade JM, Towse TF, Delano MC, Wiseman RW, Meyer RA. A gated 31P NMR method for the estimation of phosphocreatine recovery time and contractile ATP cost in human muscle. NMR Biomed 19: 573–580, 2006. doi: 10.1002/nbm.1037. [DOI] [PubMed] [Google Scholar]
- 55.Behnke BJ, Kindig CA, McDonough P, Poole DC, Sexton WL. Dynamics of microvascular oxygen pressure during rest-contraction transition in skeletal muscle of diabetic rats. Am J Physiol Heart Circ Physiol 283: H926–H932, 2002. doi: 10.1152/ajpheart.00059.2002. [DOI] [PubMed] [Google Scholar]
- 56.Latroche C, Matot B, Martins-Bach A, Briand D, Chazaud B, Wary C, Carlier PG, Chrétien F, Jouvion G. Structural and functional alterations of skeletal muscle microvasculature in dystrophin-deficient mdx mice. Am J Pathol 185: 2482–2494, 2015. doi: 10.1016/j.ajpath.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen F, Guigand L, Goubault-Leroux I, Wyers M, Cherel Y. Microvessel density in muscles of dogs with golden retriever muscular dystrophy. Neuromuscul Disord 15: 154–163, 2005. doi: 10.1016/j.nmd.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Chrzanowski SM, Baligand C, Willcocks RJ, Deol J, Schmalfuss I, Lott DJ, Daniels MJ, Senesac C, Walter GA, Vandenborne K. Multi-slice MRI reveals heterogeneity in disease distribution along the length of muscle in Duchenne muscular dystrophy. Acta Myol 36: 151–162, 2017. [PMC free article] [PubMed] [Google Scholar]
- 59.Stacy MR, Qiu M, Papademetris X, Caracciolo CM, Constable RT, Sinusas AJ. Application of BOLD magnetic resonance imaging for evaluating regional volumetric foot tissue oxygenation: a feasibility study in healthy volunteers. Eur J Vasc Endovasc Surg 51: 743–749, 2016. doi: 10.1016/j.ejvs.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schulte A-C, Aschwanden M, Bilecen D. Calf muscles at blood oxygen level-dependent MR imaging: aging effects at postocclusive reactive hyperemia. Radiology 247: 482–489, 2008. doi: 10.1148/radiol.2472070828. [DOI] [PubMed] [Google Scholar]
- 61.Stacy MR, Caracciolo CM, Qiu M, Pal P, Varga T, Constable RT, Sinusas AJ. Comparison of regional skeletal muscle tissue oxygenation in college athletes and sedentary control subjects using quantitative. Physiol Rep 4: e12903, 2016. doi: 10.14814/phy2.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]