Abstract
Low skeletal muscle capillarization is associated with impaired glucose tolerance (IGT); however, aerobic exercise training with weight loss (AEX + WL) increases skeletal muscle capillarization and improves glucose tolerance in adults with IGT. Given that the expression of angiogenic growth factors mediates skeletal muscle capillarization, we sought to determine whether angiogenic growth factor levels are associated with low capillarization in those with IGT versus normal glucose tolerance (NGT) or to the benefits of AEX + WL in both groups. Sixteen overweight or obese men 50–75 yr of age completed 6 mo of AEX + WL with oral glucose tolerance tests and vastus lateralis muscle biopsies for measurement of muscle vascular endothelial growth factor (VEGF), placental growth factor (PlGF), soluble fms-like tyrosine kinase receptor-1 (sFlt-1), and basic fibroblast growth factor (bFGF). At baseline, all growth factor levels were numerically lower in IGT than NGT, but these did not reach statistical significance (P = 0.06–0.33). Following AEX + WL, aerobic capacity [maximal oxygen consumption (V̇o2max)] increased by 16%, whereas body weight and 120-min postprandial glucose levels decreased by 10% and 15%, respectively (P ≤ 0.001 for all). There was a main effect of AEX + WL to increase VEGF (0.095 ± 0.016 vs. 0.114 ± 0.018 ng/µg, P < 0.05), PlGF (0.004 ± 0.001 vs. 0.005 ± 0.001 ng/µg, P < 0.05), and sFlt-1 (0.216 ± 0.029 vs. 0.264 ± 0.036 ng/µg, P < 0.01), with overall increases driven by the IGT group. These results suggest that 6 mo of AEX + WL increases skeletal muscle angiogenic growth factor levels in obese older adults with IGT and NGT, which may contribute to our previous findings that AEX + WL increases capillarization to improve glucose tolerance in those with IGT.
NEW & NOTEWORTHY Skeletal muscle capillarization is lower in adults with impaired glucose tolerance than normal controls. This may, in part, be attributable to differential expression of angiogenic growth factors in skeletal muscle. Using a 6-mo aerobic exercise intervention with ∼10% body weight loss (AEX + WL), we show that the expression of angiogenic growth factors tends to be lower in adults with impaired glucose tolerance compared with normal controls and that AEX + WL increased expression of angiogenic growth factors in all participants.
Keywords: angiogenesis, impaired glucose tolerance, vascular endothelial growth factor
INTRODUCTION
Low skeletal muscle capillarization is linked to insulin resistance in people with impaired glucose tolerance (IGT) and type 2 diabetes mellitus (T2DM), in part due to reduced surface area for diffusion of glucose and insulin in skeletal muscle. Aerobic exercise training and weight loss (AEX + WL) are recommended as interventions to improve glucose tolerance and prevent progression to T2DM (1, 2). We have previously shown reduced capillarization in people with IGT versus normal glucose tolerance (NGT) (3, 4) and that AEX (with or without WL) increases skeletal muscle capillarization in overweight older adults, contributing to improvements in insulin sensitivity and glucose tolerance (3, 5). Skeletal muscle expression of angiogenic growth factors plays an important role in maintaining and increasing capillarization (6), yet little is known about potential differences in skeletal muscle-derived angiogenic growth factors in insulin-resistant older adults before and after AEX + WL.
The angiogenic potential of skeletal muscle is regulated largely by the expression of angiogenic growth factors, such as vascular endothelial growth factor (VEGF). VEGF binds to its receptor (VEGFR-2) on endothelial cells and is a critical regulator of angiogenesis. Indeed, muscle-specific VEGF knockout in mice reduces capillary density and aerobic fitness and attenuates responses to exercise training (6, 7), and endothelial-specific knockout of VEGF in mice results in embryonic lethality (8). Furthermore, muscle-derived VEGF appears necessary for endothelial and flow-mediated angiogenesis, supporting an essential role of skeletal muscle VEGF in exercise-induced angiogenesis (9). To date, little is known about the skeletal muscle expression of VEGF in sedentary adults with IGT and obesity. Although most studies show that an acute bout of muscle contraction or a passive leg movement increases the muscle expression of VEGF in healthy subjects (10–12), mixed results have been reported on the effects of exercise training on the skeletal muscle VEGF protein expression, at rest or in response to acute exercise, and its association with skeletal muscle capillarization (10, 13, 14). For example, a study of exercise training in young and older women showed similar training-induced increases in capillary contacts per fiber in both groups; however, older women had greater interstitial muscle VEGF levels at rest, whereas younger women had greater increases in interstitial muscle VEGF levels following submaximal exercise (13), suggesting that exercise-induced changes in VEGF may not always predict capillary adaptations. An exercise training intervention in young and older men resulted in increases in resting interstitial VEGF after training, and submaximal acute exercise-induced changes in VEGF levels directly correlated with capillary contacts per fiber (14). Another study from the same group showed that muscle VEGF protein levels were similar between lean and obese individuals at baseline and after exercise, yet capillary density was lower in groups of obese versus lean individuals (15). Thus, although VEGF is a potent angiogenic factor, it is not yet clear whether it is the sole contributor to low capillarization in muscle. This necessitates the investigation of VEGF and other factors as potential contributors to low capillarization in aging, obesity, and impaired glucose tolerance. Growth factors such as placental growth factor (PlGF) and basic fibroblast growth factor (bFGF) are expressed by skeletal muscle (16, 17) and have distinct roles in promoting angiogenesis (18, 19). These angiogenic factors may work in tandem with antiangiogenic factors such as soluble fms-like tyrosine kinase receptor (sFlt-1), a membrane-free form of VEGFR-2 that scavenges VEGF and PlGF (20–22), which likely works as another level of control to prevent unchecked angiogenesis.
AEX + WL is a primary form of prevention and treatment for IGT and T2DM (1) and improves skeletal muscle capillarization (3), potentially through the alteration of growth factor expression in skeletal muscle. However, it is currently unclear whether skeletal muscle angiogenic growth factor expression is reduced in IGT compared with NGT or whether AEX + WL increases angiogenic growth factor expression in obese older adults. Therefore, this study tested the hypothesis that the skeletal muscle expression of angiogenic growth factors is lower in obese older adults with IGT versus NGT and that 6 mo of AEX + WL increases the skeletal muscle expression of these growth factors.
MATERIALS AND METHODS
Participants
Participants (n = 19) were overweight or obese men between the ages of 50 and 75 yr who were also classified as sedentary (<20 min moderate/vigorous exercise for 2 or fewer days per week) and either with IGT (n = 9) or NGT (n = 10). Although participants were recruited without the knowledge of glucose tolerance status, the IGT and the NGT groups were formed after the baseline oral glucose tolerance test and before any analysis. The participants had no evidence of cardiovascular disease or overt T2DM. Prescreening procedures included medical history questionnaires, physical examination, and treadmill-based exercise testing. All participants were nonsmokers and free from cancer and other diseases (hematological, pulmonary, renal, or thyroid). Those with poorly controlled hypertension, dyslipidemia, or anemia were excluded. In addition, participants were of stable weight (no recent changes >2 kg) and not currently taking β-blockers, statins, steroids, or diabetes-related medications. Alcohol consumption was reported as less than two drinks per day. Subject characteristics from some subjects were previously reported as part of other studies (23, 24). Study methods were approved by the Institutional Review Board at the University of Maryland School of Medicine and conformed with the Declaration of Helsinki. All participants provided written informed consent.
Pretesting Protocols
Before baseline testing, all participants received 6–8 wk of counseling from a registered dietitian regarding a weight-maintaining “therapeutic lifestyle changes” diet (25) to control for any potential effects of dietary composition on outcomes. Participants were required to be weight stable (±2 kg) before baseline testing. Nutrient intake was controlled for 2 days before testing by an isocaloric diet provided to each participant (3). Participants did not perform any moderate-vigorous exercise during the 2-day period. This was repeated for postintervention tests; however, testing was performed 36–48 h following the final exercise bout.
Body Composition
Weight (nearest 0.1 kg) and height (nearest 0.1 cm) were measured using an electronic scale and wall-mounted stadiometer, respectively. Body mass index (BMI) was calculated by dividing weight (kg) by height (m2). Percent body fat was measured using dual-energy X-ray absorptiometry (Prodigy, Lunar Radiation Corp., Madison, WI).
Maximal Oxygen Consumption
As previously described (3), maximal oxygen consumption (V̇o2max) was measured by indirect calorimetry (Quark, Cosmed, Chicago, IL) and determined during a graded exercise test on a motorized treadmill. Participants walked at a constant speed with incremental increases in grade (starting at 0%) every 2 min until maximal effort was achieved. V̇o2max was defined according to standard physiological criteria, namely, a respiratory exchange ratio >1.1 or no change in oxygen consumption (V̇o2) with an increase in workload.
Oral Glucose Tolerance Test, Lipid Profiles, and Blood Pressure
Following a 12-h fast, participants completed a 2-h oral glucose tolerance test (OGTT). Blood samples were taken from a catheter in the antecubital vein before and 30, 60, 90, and 120 min after ingestion of a beverage containing 75 g of glucose. Blood samples were centrifuged, and plasma was stored at −80°C until needed for further analysis. A glucose analyzer (YSI 2300 STAT Plus, YSE, Yellow Springs, OH) was used to measure glucose levels in plasma. The trapezoidal method was used to calculate glucose area under the curve (GAUC). Participants were classified as having IGT or NGT by the American Diabetes Association (26) criteria. Plasma lipids were analyzed in fasting samples using an enzymatic assay (UniCel DxC880i; Beckman Coulter, Brea, CA), and high-density lipoprotein cholesterol was measured in the supernatant after precipitation with dextran sulfate. Low-density lipoprotein cholesterol was calculated as total cholesterol − (triglyceride/5 + high-density lipoprotein cholesterol). Brachial blood pressure was measured after 15 min of seated rest.
Muscle Biopsies and Processing
A Bergström needle (Stille, Solna, Sweden) was used for biopsies of the vastus lateralis (∼12–13 cm above the patella on the anterolateral aspect of the right leg) as previously described (27). Muscle tissue was immediately freeze-clamped with tongs frozen in liquid nitrogen and stored at −80°C. Frozen samples were then lyophilized for 48 h and dissected free of connective tissue, fat, and vascular cells before homogenization. Skeletal muscle was homogenized in ice-cold Tris lysis buffer (150 mM NaCl, 20 mM Tris at pH 7.5, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, protease inhibitor, and phosphatase inhibitors I and II; Meso Scale Discovery, Gaithersburg, MD). Following homogenization, samples were kept on ice for 20 min and then centrifuged at 4°C for 10 min at 20,000 g. The supernatant was collected and stored at −80°C.
Measurement of Angiogenic Growth Factors
Muscle concentrations of VEGF, bFGF, sFlt-1, and PlGF were measured with multiplex ultrasensitive sandwich immunoassays (Meso Scale Discovery, Gaithersburg, MD) following the manufacturer’s instructions. The mean of triplicate measures was used for statistical analyses. Prepared plates were then read on a Meso Scale Discovery SECTOR Imager 2400. To control for interassay variability, all samples from any subject were analyzed on the same plate.
Aerobic Exercise Training and Weight Loss
Following baseline testing, participants completed 6 mo of weekly meetings with a registered dietitian with counseling to follow the therapeutic lifestyle changes diet (25) and achieve more than 5% weight loss over the 6-mo period by inducing an ∼500 kcal/day deficit. In addition, participants performed thrice weekly AEX on motorized treadmills supervised by exercise physiologists at the Baltimore Veterans Affairs Medical Center, Geriatric Research, Education, and Clinical Center (GRECC) exercise facility. The 6-mo AEX training program began with three 20-min sessions/week of aerobic exercise at 50% V̇o2max and then progressed during the first 4–6 wk to three 45-min sessions/week of aerobic exercise at 60%–70% V̇o2max, a level maintained for >4 additional months. Aerobic exercise was primarily composed of treadmill walking, but in some instances, participants reached a light jog by the end of the program.
Statistical Analysis
Data are reported as means ± SE, and all analyses were completed using IBM SPSS v22 (IBM, Armonk, NY). Repeated-measures ANOVA was used to test for the effects of AEX + WL (repeated within-group: baseline vs. 6-mo) and group (between-group: IGT vs. NGT). Because we hypothesized differences in skeletal muscle growth factor levels between the IGT and the NGT groups at specific time points, the decision was made a priori to conduct pairwise comparisons. Statistical significance was established at P ≤ 0.05.
RESULTS
Participant Characteristics
Participant characteristics, body composition, and aerobic fitness before and after the intervention are summarized in Table 1. There were no baseline differences in age, weight, BMI, V̇o2max, fasting plasma glucose, HDL cholesterol, LDL cholesterol, or blood pressure between groups. Triglyceride levels were 46% higher in the IGT group compared with the NGT group at baseline (P = 0.01), and the IGT group had 65% higher baseline 120-min postprandial glucose levels compared with the NGT group (P = 0.01).
Table 1.
Subject characteristics and responses to 6-mo aerobic exercise training and weight loss
| Group | Baseline | 6-Mo AEX + WL | ANOVA P Values |
|||
|---|---|---|---|---|---|---|
| AEX + WL | IGT vs. NGT | Interaction | ||||
| Age, yr | IGT | 58 ± 3 | ||||
| NGT | 61 ± 3 | |||||
| Weight, kg | IGT | 102 ± 4.4 | 90.9 ± 4.2* | <0.001 | 0.291 | 0.140 |
| NGT | 100.4 ± 4.2 | 91.2 ± 3.9* | ||||
| BMI, kg/m2 | IGT | 31.3 ± 1.1 | 28.3 ± 1.1* | <0.001 | 0.824 | 0.594 |
| NGT | 31.4 ± 1.1 | 28.9 ± 1.1* | ||||
| Body fat, % | IGT | 33.7 ± 1.6 | 29.3 ± 1.9* | <0.001 | 0.274 | 0.585 |
| NGT | 31.3 ± 1.6 | 26.2 ± 1.9* | ||||
| V̇o2max, L/min | IGT | 2.25 ± 0.20 | 2.72 ± 0.22* | <0.001 | 0.193 | 0.549 |
| NGT | 2.70 ± 0.18 | 3.01 ± 0.20* | ||||
| V̇o2max, mL/kg/min | IGT | 22.4 ± 1.8 | 30.1 ± 2.4* | 0.001 | 0.183 | 0.425 |
| NGT | 26.6 ± 2.4 | 33 ± 2.1* | ||||
| Fasting glucose, mg/dL | IGT | 99 ± 2 | 98 ± 3 | 0.412 | 0.178 | 0.800 |
| NGT | 95 ± 2 | 93 ± 3 | ||||
| 120-min postprandial glucose, mg/dL | IGT | 179 ± 9† | 130 ± 14* | 0.025 | 0.007 | 0.004 |
| NGT | 109 ± 8 | 116 ± 13 | ||||
| Glucose AUC, mg/dL/120 min | IGT | 20,460 ± 1,045† | 17,833 ± 1,184* | 0.006 | 0.092 | 0.016 |
| NGT | 16,536 ± 1,045 | 16,327 ± 1,184 | ||||
| Triglycerides, mg/dL | IGT | 139 ± 11† | 116 ± 14 | 0.089 | 0.016 | 0.575 |
| NGT | 95 ± 10 | 83 ± 14 | ||||
| Total cholesterol, mg/dL | IGT | 178 ± 11 | 165 ± 12 | 0.041 | 0.652 | 0.807 |
| NGT | 170 ± 10 | 160 ± 11 | ||||
| HDL cholesterol, mg/dL | IGT | 38 ± 2 | 40 ± 3 | 0.015 | 0.247 | 0.204 |
| NGT | 41 ± 2 | 46 ± 3* | ||||
| LDL cholesterol, mg/dL | IGT | 112 ± 9 | 105 ± 10 | 0.041 | 0.710 | 0.575 |
| NGT | 111 ± 9 | 98 ± 9 | ||||
| Systolic BP, mmHg | IGT | 124 ± 5 | 114 ± 4 | 0.02 | 0.980 | 0.807 |
| NGT | 123 ± 5 | 115 ± 3 | ||||
| Diastolic BP, mmHg | IGT | 77 ± 2 | 69 ± 3* | 0.059 | 0.654 | 0.122 |
| NGT | 75 ± 2 | 75 ± 3 | ||||
Data are means ± SE. AEX + WL, aerobic exercise training with weight loss; AUC, area under the curve; BP, blood pressure; BMI, body mass index; HDL, high-density lipoprotein; IGT, impaired glucose tolerance (n = 9); LDL, low-density lipoprotein; NGT, normal glucose tolerance (n = 10); V̇o2max, maximal oxygen consumption.
*Significant difference from baseline (P ≤ 0.01).
†Significant difference compared with NGT group at time point (P < 0.05). Bold font represents statistically significant P values.
Cardiometabolic Responses to Aerobic Exercise Training with Weight Loss
After 6-mo AEX + WL, participants in both groups had reduced body weight by 10% (P < 0.001), reduced body fat by 4.7% (P < 0.001), and increased V̇o2max (L/min) by 16%. Total cholesterol and LDL cholesterol decreased, whereas HDL cholesterol increased after the intervention (−7%, −9%, +9%, respectively, P < 0.05 for all). There was a significant AEX + WL × group interaction effect (P < 0.004) on 120-min postprandial glucose levels that decreased by 27% in the IGT group (P < 0.001) but not in the NGT group. Systolic blood pressure decreased by 8% after AEX + WL (P = 0.04), but there was no significant effect on diastolic blood pressure.
Angiogenic Growth Factors
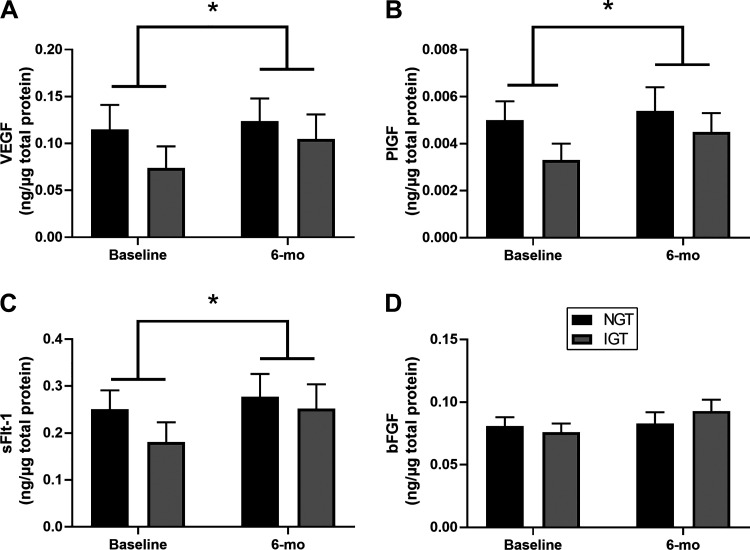
Skeletal muscle levels of angiogenic growth factors are presented in Fig. 1. At baseline, levels of all growth factors were numerically lower in the IGT group than the NGT group; however, none of these reached statistical significance (PlGF, P = 0.06; VEGF, P = 0.12; sFlt-1, P = 0.12; bFGF, P = 0.33). Likewise, after AEX + WL, there were no statistically significant AEX + WL × group interaction effects on any of the growth factors. There was a main effect of AEX + WL to increase VEGF by 20%, PlGF by 22%, and sFlt-1 by 25% (P < 0.05 for all; Fig. 1, A–C). There were no significant effects of AEX + WL on bFGF levels. In post hoc analyses, only the IGT group significantly increased muscle levels of VEGF, PlGF, and sFlt-1 (P < 0.05 for all).
Figure 1.
Changes in the muscle expression of angiogenic proteins in those with normal glucose tolerance (NGT; black bar) and impaired glucose tolerance (IGT; gray bar) before and after 6 mo of aerobic exercise training and weight loss (AEX + WL). There was a significant effect of AEX + WL to increase vascular endothelial growth factor (VEGF; A), placental growth factor (PlGF; B), and soluble fms-like tyrosine kinase-1 (sFlt-1; C) but not basic fibroblast growth factor (bFGF; D). Data are presented as means + SE. *Significant main effect of exercise training (P < 0.05).
DISCUSSION
The primary finding of this study is that 6-mo AEX + WL increased skeletal muscle expression of VEGF and PlGF in overweight-obese older men, which is consistent with our previous report of angiogenesis and increased skeletal muscle capillarization in response to AEX + WL (3). sFLT-1 (a scavenger of VEGF and PlGF in circulation) also significantly increased following the intervention. These findings occurred in tandem with cardiometabolic improvements including increases in aerobic capacity and reductions in body fat percentage, systolic blood pressure, and 120-min postprandial glucose levels. Furthermore, following AEX + WL, the mean 120-min postprandial glucose level in the IGT group decreased to fall within the range for NGT, and half of the IGT participants reverted to NGT status after the intervention. At baseline, we hypothesized that skeletal muscle expression of angiogenic growth factors would be lower in subjects with IGT compared with subjects with NGT. Although there were no significant differences in growth factor expression between groups at baseline, the expression of VEGF and PlGF was numerically lower in the IGT versus the NGT group at baseline; thus, we cannot rule out a contribution of these factors to the low capillarization previously associated with IGT.
Vascular Endothelial Growth Factor and Soluble Fms-Like Tyrosine Kinase Receptor-1
Concordant with our hypothesis, 6 mo of AEX + WL increased muscle VEGF, and these changes were accompanied by a significant decrease in 120-min postprandial glucose levels in those with IGT. This is the first study to our knowledge that has assessed the changes in skeletal muscle VEGF protein levels in those with IGT after 6 mo of AEX + WL. Given the well-established role of VEGF in promoting capillarization, these findings are in line with our previous findings, illustrating that exercise training improves capillarization and insulin sensitivity (3, 5). Studies consistently show that acute exercise increases skeletal muscle VEGF in young men (10, 11, 15, 28). Because of this, it can be difficult to distinguish the effects of acute and chronic exercise. Although a definitive time course of skeletal muscle VEGF levels from 0 to 48 h after exercise is not available to our knowledge, there is evidence that interstitial VEGF protein levels decline substantially within a few hours of a bout of exercise (28) and that skeletal muscle VEGF mRNA levels return to baseline levels within 24 h after a bout of exercise (29). It is, therefore, likely that the timing of the 6-mo muscle biopsy in the present study (36–48 h after the final training session) represents a response to training as opposed to an acute bout of exercise; however, we cannot make a definitive conclusion at this time.
Sex and aging also appear to play a role, as one study showed that healthy older women have greater resting muscle VEGF but attenuated exercise training responses compared with their younger counterparts (13), whereas another study in healthy young and older men showed that VEGF levels were similar between groups, as well as before and after exercise training (14). Limited data in a pathological state showed that muscle VEGF mRNA and protein, as well as capillarization, increased after 8 wk of aerobic knee-extensor training in older men and women with heart failure (30). Considering that glucose intolerance specifically targets the microvasculature (31), it stands to reason that those with IGT may be more susceptible to the proangiogenic benefits of exercise training. Our data support this, as the increase in skeletal muscle VEGF was 42% in the IGT group versus only 8% in the NGT group. Furthermore, the increase in VEGF across groups was of similar magnitude and direction as the 15% increase in capillary density previously demonstrated following AEX + WL in previously sedentary, older men and women with IGT (3).
Some studies have shown that changes in skeletal muscle VEGF dialysate do not correlate with or predict changes in muscle capillarization (15, 32). These findings, along with others illustrating a temporal control of capillarization by both pro- and antiangiogenic factors following exercise (33), were the rationale for assessment of sFlt-1, an antiangiogenic factor that competitively binds available VEGF. Interestingly, we showed that exercise training increased muscle sFlt-1 by 40% in IGT and 10% in NGT, concordant with our hypothesis. Although our sample did not include anyone with T2DM, the present results are similar to those from an animal study of diet-induced T2DM in which skeletal muscle from diabetic animals had lower basal sFlt-1 protein levels than that from normal controls (34). When the animals were subjected to hind limb ischemia, skeletal muscle sFlt-1 levels increased in both groups; however, the magnitude of the increase was greater in the diabetic animals (34). It is possible that increased production of sFlt-1 by skeletal muscle following AEX + WL, or hind limb ischemia, serves to prevent maladaptive angiogenesis, as increased levels of sFlt-1 and Flt-1 have been shown to reduce detectible (free and presumably bioactive) VEGF protein levels. Other studies using animal models and knocking out negative regulators of VEGF show that uncontrolled activation of VEGF promotes aberrant angiogenesis (35), and microvascular complications such as diabetic retinopathy and diabetic nephropathy are characterized by increased vascularization (36). Thus, it is possible that the increased expression of skeletal muscle sFlt-1 after AEX + WL could also act to limit the effects of soluble VEGF isoforms on these peripheral tissues in a protective manner. It is not yet known whether muscle expression of sFlt-1 sequesters VEGF primarily within muscle interstitium or systemically; however, considering that skeletal muscle VEGF is loaded into vesicles (12), muscle VEGF may be protected from local, but not systemic, antagonistic binding by sFlt-1, and we speculate that this is one way in which skeletal muscle promotes site-specific angiogenesis in skeletal muscle.
Placental Growth Factor
The AEX + WL intervention also led to increases in skeletal muscle PlGF expression. Although there were numerical increases in both groups, the largest increase (∼36%) was seen in the IGT group. PlGF is known to indirectly enhance angiogenesis through binding with VEGFR-1 (37). VEGFR-1 activation independent of other stimuli is not thought to bring about robust enhancements in angiogenesis (38); however, PlGF binding of VEGFR-1 allows VEGF to primarily bind with VEGFR-2 (39) to stimulate the proangiogenic PLCγ-PKC-MAPK pathway and von Willebrand factor (vWF) secretion (38). This combined activation of both receptors is expected to lead to significant enhancements in angiogenesis (38–40). Our laboratory has previously shown increases in circulating PlGF levels following acute exercise in both endurance-trained and sedentary healthy young men (41). However, there is conflicting evidence on the exact role that PlGF plays in exercise-induced angiogenesis (37, 41). Specifically, Gigante et al. (42) reported that although mice with PlGF knockout showed an expected rise in capillary density following an exercise training protocol, PlGF knockout mice did exhibit lower capillary density when untrained. Given the proangiogenic role of PlGF and our finding of increased skeletal muscle PlGF expression after AEX + WL, it seems likely that an increase in PlGF is part of the AEX + WL-induced proangiogenic response. However, the ability of this increase in PlGF to directly enhance angiogenesis remains unclear. More research is needed to fully elucidate the role of PlGF in exercise training-induced angiogenesis.
Basic Fibroblast Growth Factor
Although we detected significant increases in PlGF and VEGF, bFGF was not significantly different between groups or following AEX + WL. Few studies have assessed skeletal muscle expression of bFGF, all of which are in animal models. In a rat model of diabetes mellitus, skeletal muscle bFGF mRNA was significantly lower in diseased versus control rats, whereas treatment with insulin restored muscle bFGF expression (43, 44). One other study showed that 12 wk of resistance exercise reduced skeletal muscle bFGF protein levels in mice (45). Skeletal muscle bFGF does elicit angiogenesis in vitro, and skeletal muscle transfection with bFGF enhances endothelial cell migration through regulation of cathepsin L (46). Circulating bFGF also has angiogenic potential, and several studies have demonstrated that both arterial and intravenous infusion of bFGF result in increased collateral circulation and enhance limb salvage after hind limb ischemia (47–50). Although bFGF is a proangiogenic factor, there is currently little evidence to support AEX training-induced increases in basal muscle bFGF protein levels. Our results suggest that skeletal muscle bFGF expression may not be a primary factor in the low capillarization observed in IGT or in the mechanisms by which AEX + WL elicits angiogenesis in those with IGT.
The present study was not without limitations. Although our group has previously demonstrated increases in skeletal muscle capillarization after AEX + WL (3), capillarization data were only available for 7 of the 19 participants in the present report (5 IGT and 2 NGT). Although these data are generally supportive of an increase in capillarization after the intervention and of a relationship between skeletal muscle growth factor levels and capillarization, we deemed this small sample insufficient for publication. In terms of participant selection, future studies in this area may benefit from using a more pathologically diverse population, such as those with cardiovascular disease or overt T2DM. This may further uncover how exercise training enhances angiogenesis in a disease state. The relatively small sample size of the present study could have hidden differences between groups at baseline; however, the sample size was still appropriate for detecting significant changes induced by AEX + WL in the overall group. The study was not designed to distinguish any independent effects of obesity or age; however, groups were similar in terms of age and obesity. The present study also included only men. Future studies could focus on investigating any sex differences that exist in insulin resistance and possible divergent effects with AEX + WL. Furthermore, the addition of a control group and separate AEX and WL groups might have strengthened the results and provided an insight into the contribution of either intervention alone compared with their combined effect. Finally, even though great care was taken to microdissect the muscle tissue to remove obvious nonmuscle tissues, there is still a possibility that some nonmuscle cells remained and contributed to our findings. However, this is unlikely and does not negate the significance of our results.
Conclusions
Six months of AEX + WL increases muscle expression of the angiogenic factors VEGF and PlGF and the antiangiogenic factor sFlt-1 but not bFGF in overweight or obese older men. The increases in angiogenic factors occurred alongside improved glucose tolerance, loss of body fat percentage, and increased aerobic fitness, which extends previous findings that AEX + WL improves glucose tolerance, in part, as a result of increased muscle capillarization. Furthermore, these results provide evidence that certain protein candidates may be part of the mechanisms regulating skeletal muscle capillarization in older adults with NGT and IGT.
GRANTS
This research was supported by a VA Advanced Research Career Development Award (to J.B.B.); a VA Merit Review Award (to A.S.R.); NIH K01-AG-021457 (to Lyndon J. Joseph); a Paul B. Beeson Patient-Oriented Research Career Development Award in Aging, NIH K23-AG040775, and American Federation for Aging Research (to S.J.P.); a VA Career Development Award (to S.J.P.); a VA Senior Research Career Scientist Award (to A.S.R.); the University of Maryland Claude D. Pepper Center (NIH P30-AG028747); the Mid-Atlantic Nutrition Obesity Research Center (P30-DK072488); and the Baltimore Veterans Affairs Medical Center Geriatric Research, Education and Clinical Center (GRECC).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.B.B., A.S.R., and S.J.P. conceived and designed research; J.B.B., A.S.R., and S.J.P. performed experiments; W.S.E., J.M.H., and S.J.P. analyzed data; W.S.E., J.M.H., and S.J.P. interpreted results of experiments; W.S.E. prepared figures; W.S.E. and J.M.H. drafted manuscript; W.S.E., J.B.B., J.M.H., A.S.R., and S.J.P. edited and revised manuscript; W.S.E., J.B.B., J.M.H., A.S.R., and S.J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the subjects who participated in this study and appreciate the contribution of Dr. Lyndon J. Joseph to the study.
REFERENCES
- 1.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Aunola S, Cepaitis Z, Moltchanov V, Hakumäki M, Mannelin M, Martikkala V, Sundvall J, Uusitupa M; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343–1350, 2001. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 3.Prior SJ, Blumenthal JB, Katzel LI, Goldberg AP, Ryan AS. Increased skeletal muscle capillarization after aerobic exercise training and weight loss improves insulin sensitivity in adults with IGT. Diabetes Care 37: 1469–1475, 2014. doi: 10.2337/dc13-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prior SJ, McKenzie MJ, Joseph LJ, Ivey FM, Macko RF, Hafer-Macko CE, Ryan AS. Reduced skeletal muscle capillarization and glucose intolerance. Microcirculation 16: 203–212, 2009. doi: 10.1080/10739680802502423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prior SJ, Goldberg AP, Ortmeyer HK, Chin ER, Chen D, Blumenthal JB, Ryan AS. Increased skeletal muscle capillarization independently enhances insulin sensitivity in older adults after exercise training and detraining. Diabetes 64: 3386–3395, 2015. doi: 10.2337/db14-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol 587: 1755–1767, 2009. doi: 10.1113/jphysiol.2008.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olfert IM, Howlett RA, Wagner PD, Breen EC. Myocyte vascular endothelial growth factor is required for exercise-induced skeletal muscle angiogenesis. Am J Physiol Regul Integr Comp Physiol 299: R1059–R1067, 2010. doi: 10.1152/ajpregu.00347.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development 126: 1149–1159, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Uchida C, Nwadozi E, Hasanee A, Olenich S, Olfert IM, Haas TL. Muscle-derived vascular endothelial growth factor regulates microvascular remodelling in response to increased shear stress in mice. Acta Physiol (Oxf) 214: 349–360, 2015. doi: 10.1111/apha.12463. [DOI] [PubMed] [Google Scholar]
- 10.Hoier B, Nordsborg N, Andersen S, Jensen L, Nybo L, Bangsbo J, Hellsten Y. Pro- and anti-angiogenic factors in human skeletal muscle in response to acute exercise and training. J Physiol 590: 595–606, 2012. doi: 10.1113/jphysiol.2011.216135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellsten Y, Rufener N, Nielsen JJ, Høier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294: R975–R982, 2008. doi: 10.1152/ajpregu.00677.2007. [DOI] [PubMed] [Google Scholar]
- 12.Hoier B, Prats C, Qvortrup K, Pilegaard H, Bangsbo J, Hellsten Y. Subcellular localization and mechanism of secretion of vascular endothelial growth factor in human skeletal muscle. FASEB J 27: 3496–3504, 2013. doi: 10.1096/fj.12-224618. [DOI] [PubMed] [Google Scholar]
- 13.Gavin TP, Kraus RM, Carrithers JA, Garry JP, Hickner RC. Aging and the skeletal muscle angiogenic response to exercise in women. J Gerontol A Biol Sci Med Sci 70: 1189–1197, 2015. doi: 10.1093/gerona/glu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavin TP, Ruster RS, Carrithers JA, Zwetsloot KA, Kraus RM, Evans CA, Knapp DJ, Drew JL, McCartney JS, Garry JP, Hickner RC. No difference in the skeletal muscle angiogenic response to aerobic exercise training between young and aged men. J Physiol 585: 231–239, 2007. doi: 10.1113/jphysiol.2007.143198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavin TP, Stallings IH, Zwetsloot KA, Westerkamp LM, Ryan NA, Moore RA, Pofahl WE, Hickner RC. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol (1985) 98: 315–321, 2005. doi: 10.1152/japplphysiol.00353.2004. [DOI] [PubMed] [Google Scholar]
- 16.Guthridge M, Wilson M, Cowling J, Bertolini J, Hearn MT. The role of basic fibroblast growth factor in skeletal muscle regeneration. Growth Factors 6: 53–63, 1992. doi: 10.3109/08977199209008871. [DOI] [PubMed] [Google Scholar]
- 17.Silva AT, Rouf F, Semola OA, Payton ME, Lovern PC. Placental growth factor levels in quadriceps muscle are reduced by a Western diet in association with advanced glycation end products. Am J Physiol Heart Circ Physiol 317: H851–H866, 2019. doi: 10.1152/ajpheart.00511.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med 8: 831–840, 2002. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 19.Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation 92: II365–II371, 1995. doi: 10.1161/01.CIR.92.9.365. [DOI] [PubMed] [Google Scholar]
- 20.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 90: 10705–10709, 1993. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kendall RL, Wang G, DiSalvo J, Thomas KA. Specificity of vascular endothelial cell growth factor receptor ligand binding domains. Biochem Biophys Res Commun 201: 326–330, 1994. doi: 10.1006/bbrc.1994.1705. [DOI] [PubMed] [Google Scholar]
- 22.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem 269: 25646–25654, 1994. doi: 10.1016/S0021-9258(18)47298-5. [DOI] [PubMed] [Google Scholar]
- 23.Blumenthal JB, Gitterman A, Ryan AS, Prior SJ. Effects of exercise training and weight loss on plasma fetuin-A levels and insulin sensitivity in overweight older men. J Diabetes Res 2017: 1492581, 2017. doi: 10.1155/2017/1492581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan AS, Katzel LI, Prior SJ, McLenithan JC, Goldberg AP, Ortmeyer HK. Aerobic exercise plus weight loss improves insulin sensitivity and increases skeletal muscle glycogen synthase activity in older men. J Gerontol A Biol Sci Med Sci 69: 790–798, 2014. doi: 10.1093/gerona/glt200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Cholesterol Education Panel. Your Guide to Lowering Your Cholesterol With TLC. Bethesda: U.S. Department of Health and Human Services, 2005. [Google Scholar]
- 26.American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 42: S13–S28, 2019. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 27.Hennessey JV, Chromiak JA, Della VS, Guertin J, MacLean DB. Increase in percutaneous muscle biopsy yield with a suction-enhancement technique. J Appl Physiol (1985) 82: 1739–1742, 1997. doi: 10.1152/jappl.1997.82.6.1739. [DOI] [PubMed] [Google Scholar]
- 28.Höffner L, Nielsen JJ, Langberg H, Hellsten Y. Exercise but not prostanoids enhance levels of vascular endothelial growth factor and other proliferative agents in human skeletal muscle interstitium. J Physiol 550: 217–225, 2003. doi: 10.1113/jphysiol.2002.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen L, Pilegaard H, Neufer PD, Hellsten Y. Effect of acute exercise and exercise training on VEGF splice variants in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 287: R397–R402, 2004. doi: 10.1152/ajpregu.00071.2004. [DOI] [PubMed] [Google Scholar]
- 30.Gustafsson T, Bodin K, Sylvén C, Gordon A, Tyni-Lenné R, Jansson E. Increased expression of VEGF following exercise training in patients with heart failure. Eur J Clin Invest 31: 362–366, 2001. doi: 10.1046/j.1365-2362.2001.00816.x. [DOI] [PubMed] [Google Scholar]
- 31.Nazimek-Siewniak B, Moczulski D, Grzeszczak W. Risk of macrovascular and microvascular complications in Type 2 diabetes: results of longitudinal study design. J Diabetes Complications 16: 271–276, 2002. doi: 10.1016/s1056-8727(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 32.Olfert IM, Breen EC, Mathieu-Costello O, Wagner PD. Skeletal muscle capillarity and angiogenic mRNA levels after exercise training in normoxia and chronic hypoxia. J Appl Physiol 91: 1176–1184, 2001. doi: 10.1152/jappl.2001.91.3.1176. [DOI] [PubMed] [Google Scholar]
- 33.Olenich SA, Gutierrez-Reed N, Audet GN, Olfert IM. Temporal response of positive and negative regulators in response to acute and chronic exercise training in mice. J Physiol 591: 5157–5169, 2013. doi: 10.1113/jphysiol.2013.254979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res 101: 948–956, 2007. doi: 10.1161/CIRCRESAHA.107.160630. [DOI] [PubMed] [Google Scholar]
- 35.Tessneer KL, Pasula S, Cai X, Dong Y, McManus J, Liu X, Yu L, Hahn S, Chang B, Chen Y, Griffin C, Xia L, Adams RH, Chen H. Genetic reduction of vascular endothelial growth factor receptor 2 rescues aberrant angiogenesis caused by epsin deficiency. Arterioscler Thromb Vasc Biol 34: 331–337, 2014. [Erratum in Arterioscler Thromb Vasc Biol 34: e7, 2014]. doi: 10.1161/ATVBAHA.113.302586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Min W, Yamanaka N. Three-dimensional analysis of increased vasculature around the glomerular vascular pole in diabetic nephropathy. Virchows Arch A Pathol Anat Histopathol 423: 201–207, 1993. doi: 10.1007/BF01614771. [DOI] [PubMed] [Google Scholar]
- 37.De Falco S. The discovery of placenta growth factor and its biological activity. Exp Mol Med 44: 1–9, 2012. doi: 10.3858/emm.2012.44.1.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibuya H, Sakai K, Kabir-Salmani M, Wachi Y, Iwashita M. Polymerization of insulin-like growth factor-binding protein-1 (IGFBP-1) potentiates IGF-I actions in placenta. J Cell Physiol 226: 434–439, 2011. doi: 10.1002/jcp.22349. [DOI] [PubMed] [Google Scholar]
- 39.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, VandenDriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 7: 575–583, 2001. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 40.Rahimi N. Vascular endothelial growth factor receptors: molecular mechanisms of activation and therapeutic potentials. Exp Eye Res 83: 1005–1016, 2006. doi: 10.1016/j.exer.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landers-Ramos RQ, Jenkins NT, Spangenburg EE, Hagberg JM, Prior SJ. Circulating angiogenic and inflammatory cytokine responses to acute aerobic exercise in trained and sedentary young men. Eur J Appl Physiol 114: 1377–1384, 2014. doi: 10.1007/s00421-014-2861-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gigante B, Tarsitano M, Cimini V, De Falco S, Persico MG. Placenta growth factor is not required for exercise-induced angiogenesis. Angiogenesis 7: 277–284, 2004. doi: 10.1007/s10456-004-4179-1. [DOI] [PubMed] [Google Scholar]
- 43.Karpen CW, Spanheimer RG, Randolph AL, Lowe WL. Tissue-specific regulation of basic fibroblast growth factor mRNA levels by diabetes. Diabetes 41: 222–226, 1992. doi: 10.2337/diab.41.2.222. [DOI] [PubMed] [Google Scholar]
- 44.Xiang J, Zhao Y, Chen J, Zhou J. Expression of basic fibroblast growth factor, protein kinase C and members of the apoptotic pathway in skeletal muscle of streptozotocin-induced diabetic rats. Tissue Cell 46: 1–8, 2014. doi: 10.1016/j.tice.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Kim JS, Yoon DH, Kim HJ, Choi MJ, Song W. Resistance exercise reduced the expression of fibroblast growth factor-2 in skeletal muscle of aged mice. Integr Med Res 5: 230–235, 2016. doi: 10.1016/j.imr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung JH, Im EK, Jin TW, Lee SM, Kim SH, Choi EY, Shin MJ, Lee KH, Jang Y. Cathepsin L derived from skeletal muscle cells transfected with bFGF promotes endothelial cell migration. Exp Mol Med 43: 179–188, 2011. doi: 10.3858/emm.2011.43.4.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang HT, Feng Y. bFGF increases collateral blood flow in aged rats with femoral artery ligation. Am J Physiol Heart Circ Physiol 278: H85–H93, 2000. doi: 10.1152/ajpheart.2000.278.1.H85. [DOI] [PubMed] [Google Scholar]
- 48.Yang HT, Deschenes MR, Ogilvie RW, Terjung RL. Basic fibroblast growth factor increases collateral blood flow in rats with femoral arterial ligation. Circ Res 79: 62–69, 1996. doi: 10.1161/01.res.79.1.62. [DOI] [PubMed] [Google Scholar]
- 49.Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000. doi: 10.1152/ajpheart.2000.278.6.H1966. [DOI] [PubMed] [Google Scholar]
- 50.Lee SL, Pevec WC, Carlsen RC. Functional outcome of new blood vessel growth into ischemic skeletal muscle. J Vasc Surg 34: 1096–1102, 2001. doi: 10.1067/mva.2001.117889. [DOI] [PubMed] [Google Scholar]