Abstract
Right ventricular (RV) functional adaptation to afterload determines outcome in pulmonary hypertension (PH). RV afterload is determined by the dynamic interaction between pulmonary vascular resistance (PVR), characteristic impedance (Zc), and wave reflection. Pulmonary vascular impedance (PVZ) represents the most comprehensive measure of RV afterload; however, there is an unmet need for an easier bedside measurement of this complex variable. Although a recent study showed that Zc and wave reflection can be estimated from RV pressure waveform analysis and cardiac output, this has not been validated. Estimations of Zc and wave reflection coefficient (λ) were validated relative to conventional spectral analysis in an animal model. Zc, λ, and the single-beat ratio of end-systolic to arterial elastance (Ees/Ea) to estimate RV-pulmonary arterial (PA) coupling were determined from right heart catheterization (RHC) data. The study included 30 pulmonary artery hypertension (PAH) and 40 heart failure with preserved ejection fraction (HFpEF) patients [20 combined pre- and postcapillary PH (Cpc-PH) and 20 isolated postcapillary PH, (Ipc-PH)]. Also included were 10 age- and sex-matched controls. There was good agreement with minimal bias between estimated and spectral analysis-derived Zc and λ. Zc in PAH and Cpc-PH groups exceeded that in the Ipc-PH group and controls. λ was increased in Ipc-PH (0.84 ± 0.02), Cpc-PH (0.87 ± 0.05), and PAH groups (0.85 ± 0.04) compared with controls (0.79 ± 0.03); all P values were <0.05. λ was the only afterload parameter associated with RV-PA coupling in PAH. In the PH-HFpEF group, RV-PA uncoupling was independent of RV afterload. Our findings indicate that Zc and λ derived from an RV pressure curve can be used to improve estimation of RV afterload. λ is the only afterload measure associated with RV-PA uncoupling in PAH, whereas RV-PA uncoupling in PH-HFpEF appears to be independent of afterload consistent with an inherent abnormality of the RV myocardium.
NEW & NOTEWORTHY Pulmonary vascular impedance (PVZ) represents the most comprehensive measure of right ventricle (RV) afterload; however, measurement of this variable is complex. We demonstrate that characteristic impedance (Zc) and a wave reflection coefficient, λ, can be derived from RV pressure waveform analysis. In addition, RV dysfunction in left heart disease is independent of its afterload. The current study provides a platform for future studies to examine the pharmacotherapeutic effects and prognosis of different measures of RV afterload.
Keywords: heart failure with preserved ejection fraction, pulmonary arterial hypertension, right ventricle-pulmonary artery coupling, vascular impedance, wave reflection
INTRODUCTION
An increased pulmonary vascular resistance (PVR) defines pulmonary vascular disease and associated right ventricular (RV) afterload (1). However, PVR relies on the simplification of complex pressure and flow waves into mean values, thus ignoring the pulsatile component of RV hydraulic work (2). In reality, the opposition to pulmonary arterial (PA) flow actually results from the dynamic and oscillating interaction between resistance, compliance, and wave reflection (3). This information is captured by pulmonary vascular impedance (PVZ), representing pulsatile pressure divided by pulsatile flow (2, 4). Although PVZ offers a comprehensive assessment of RV afterload in patients with pulmonary hypertension (PH), it is uncommonly measured, and its clinical relevance remains unclear (4).
An impedance calculation requires a spectral analysis of synchronized pressure and flow waves ideally measured in the same location (5). Pulmonary arterial impedance at 0 Hz (Z0) is the ratio of mean PA pressure (mPAP) to mean pulmonary blood flow (cardiac output, CO) and thus parallels “total” PVR (TPR). This parameter is mainly determined by the small resistance vessels and left atrial pressure. As frequency increases, the impedance is affected by more proximal elements of the arterial tree. At high frequencies, the PAP-flow ratio is decreased and oscillates around a constant value called characteristic impedance (Zc). As an alternative to calculating Zc in the frequency domain using spectral analysis, it is also possible to calculate Zc in the time domain as the slope of the PAP-flow relationship in early systole (6). Conceptually, Zc represents an inertance to compliance ratio, and as blood inertance can be considered constant, Zc is, therefore, inversely proportional to the compliance of the proximal PA tree (6). Wave reflection increases with stiffer PA walls, and therefore, a so-called wave reflection index, λ, can be calculated as (Z0 − Zc/Z0 + Zc) (7). Because PA resistance and compliance share an inverse hyperbolic relationship (8), this index incorporates both steady-state (predominantly distal vasculature) and pulsatile (proximal vessel) components of hydraulic load. Thus, it may be a more complete measure of the opposition to PA flow than Z0 or Zc alone and, therefore, more descriptive of RV afterload. To our knowledge, there has been no previous study specifically evaluating the effects of the λ coefficient on RV-PA coupling in patients with PH.
Clinical determination of Zc using spectral analysis is technically demanding because of the need for instantaneous PA pressure and flow measurements. However, previous data suggest that it is possible to derive Zc from a method developed for estimating CO from a RV pressure curve (9). This approach uses RV pressure waveform derivates to calculate a variable that when multiplied by a Zc-based conversion factor yields flow in L/min (6). When actual CO is known, Zc can be calculated as a ratio of estimated to measured CO, and λ then can be derived. However, although values for Zc and λ determined by this method have been published, accuracy and precision relative to spectral analysis remain unknown (9).
This study was designed to 1) validate an RV pressure-based method for estimation of Zc using PVZ spectra constructed from archived experimental data and 2) apply this method to characterize differences in Zc and λ between pulmonary artery hypertension (PAH) and PH-heart failure with preserved ejection fraction (PH-HFpEF) using data recorded during standard right heart catheterization. We hypothesize that the additive effects of increased resistive and pulsatile afterload encountered in PAH and PH-HFpEF associated with combined pre- and postcapillary PH (Cpc-PH) would allow for an improved evaluation of RV functional adaptation to increased afterload.
METHODS
RV Pressure-Based Estimation of Pulmonary Vascular Impedance and the Wave Reflection Coefficient
Validity of deriving Zc and λ from the RV pressure waveform as described previously (10, 11) was first assessed using archived recordings of RV and PA pressures and PA flow. Data had been acquired from 11 anesthetized Yorkshire swine (50–55 kg; 7 male; ∼4 mo of age) under a protocol approved by the Animal Care and Use Committee of Cornell University Medical College (No. 9912-653 A) and in compliance with the National Institutes of Health (NIH) guide for the Care and Use of Laboratory Animals. This algorithm incorporates RV pressure waveform derivates (Fig. 1) and heart rate to calculate a variable, which when multiplied by a Zc-based conversion factor generates an estimate of CO (eCO) (9):
Figure 1.
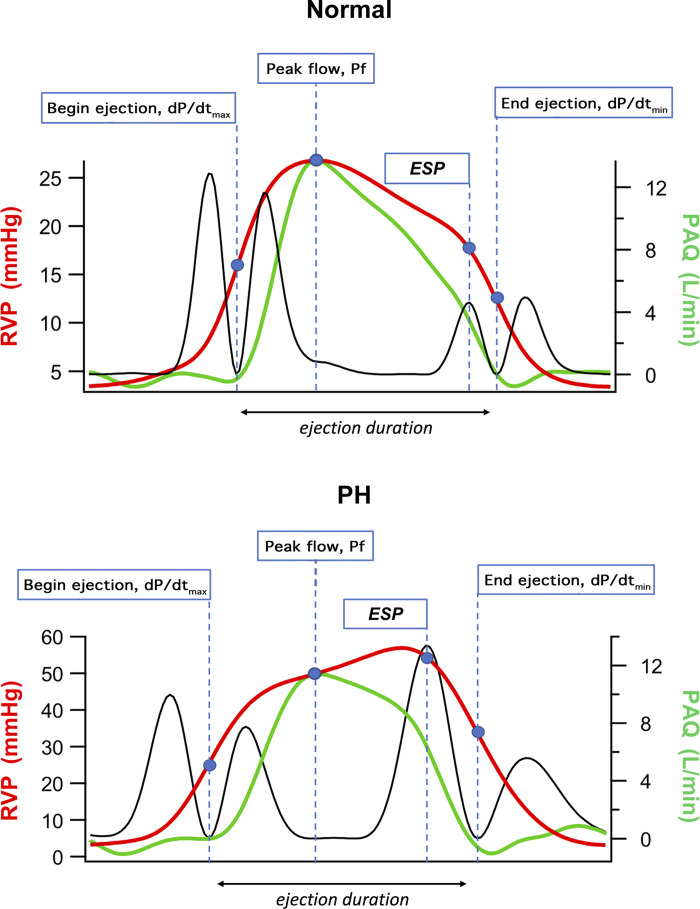
Right ventricle pressure-based estimation of pulmonary vascular impedance in a control and pulmonary-hypertensive animal model. Black solid lines represent the second derivative squared of the right ventricle pressure waveform. ESP, end-systolic pressure; PAQ, pulmonary artery blood flow measured by using electromagnetic flow probe around the main pulmonary artery root; Pf, right ventricle pressure at time of peak blood flow; PH, pulmonary hypertension; RVP, right ventricle pressure.
Pf represents RV pressure at the time of peak flow, and ejection – duration (ED) is the time interval between RV pressure at dP/dtmax and dP/dtmin. From the eCO and simultaneous measurement of CO by thermodilution (TD CO), Zc can be estimated as the ratio of eCO to TD CO. The wave reflection coefficient, λ, can be calculated as follows (7):
Animals were initially sedated with ketamine (20–25 mg/kg, intramuscular), and then anesthesia was induced with isoflurane via a face mask. The trachea was intubated, and the lungs were ventilated with isoflurane in oxygen. End-tidal carbon dioxide concentration was continuously monitored, and the minute ventilation was adjusted to maintain normocapnia. The electrocardiogram was monitored, and femoral venous and arterial sheaths were placed percutaneously. Via the arterial sheath, systemic blood pressure was measured with a 5-Fr micromanometer catheter (Millar Instruments Inc., Dallas, TX). To allow for administration of a range of isoflurane doses later in the experiment without imposing the risk of inadequate anesthesia, after establishing vascular access, pentobarbital was administered (15 mg/kg load over 7–10 min followed by a 2-mg·kg−1·h−l infusion), and isoflurane concentration was reduced. A median sternotomy was then performed, the heart was suspended in a pericardial cradle, and two 5-Fr micromanometer catheters (Millar Instruments Inc.) were inserted into the RV free wall via small stab wounds that were closed by purse-string sutures. One micromanometer was positioned to measure RV pressure, and the other was advanced into the PA just distal to the pulmonic valve for measurement of proximal PA pressure. Proximal PA flow was measured with an electromagnetic probe (Carolina Medical Electronics, King, NC). After intrathoracic preparation, estimated blood losses were replaced in a 3:1 ratio with lactated Ringer’s solution, and an infusion rate of 5–7 mL·kg−1·h−l was maintained. Throughout the surgical preparation and experimental protocol, animals were kept normothermic by using a water-circulating heating blanket and warming lights. For each animal, 10-s apneic segments of synchronized RV pressure, PA pressure, and PA flow recorded at 200 Hz before and during administration of high-dose isoflurane to alter RV-PA coupling (12) were analyzed (two data points per animal). Z0 and Zc were derived from RV pressure wave forms as described earlier. For frequency-domain analysis of input impedance, PA pressure and flow waveforms were resolved into a Fourier series, and the pressure/flow ratio at each frequency was calculated. The ratio at 0 Hz (Z0) was regarded as a parallel to TPR, with Zc defined as the mean of values between 3 and 15 Hz. Values for λ were then calculated as (Z0 − Zc)/(Z0 + Zc) (7).
Clinical Study Population and Design
We enrolled consecutive patients who underwent clinically indicated diagnostic RHC studies between April 2019 and October 2020. The study protocol was approved by our Institutional Review Board (IRB 2000024783), and all patients consented to having their clinical and investigative data used for research purposes.
The study included 40 consecutive patients with PH-HFpEF and 30 consecutive patients with PAH. Only patients with interpretable RV pressure waveforms were included in the study (Supplemental Fig. S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.14258285). All patients with PH-HFpEF had a left ventricular ejection fraction (EF) ≥50% with no evidence of moderate or severe left-sided valvular heart disease on echocardiography (13). In addition, patients with PH-HFpEF had resting supine mean pulmonary artery pressures (mPAP) >20 mmHg and pulmonary artery wedge pressures (PAWP) >15 mmHg on RHC study (1). The PH-HFpEF group was further classified as isolated postcapillary PH (Ipc-PH) if the PVR was <3 Wood unit (WU) or as combined pre- and postcapillary PH (Cpc-PH) if the PVR was ≥3 WU (1). All patients with PAH had resting supine mean pulmonary artery pressure (mPAP) >20 mmHg with a PAWP <15 mmHg and PVR ≥3 WU on RHC study (1). Once we identified our PH-HFpEF and PAH cohort, we then selected 10 age- and sex-matched controls from the records of patients with suspected PH who exhibited normal resting supine hemodynamics on RHC (1). Overall, the study sample consisted of 80 subjects (Supplemental Fig. S1). The diagnostic association and details on PAH-targeted pharmacotherapy for subjects with PAH are described in Supplemental Table S1. The diagnostic association of control subjects is described in Supplemental Table S2.
MEASUREMENTS
Right Heart Hemodynamic Assessment
RHC was performed in the supine position with a 7.5-Fr Swan-Ganz catheter (Edwards LifeSciences, Irvine, CA) inserted percutaneously under fluoroscopic and ultrasound guidance into the internal jugular vein. A zero reference was obtained at the midthoracic level (14), and right atrial (RA), RV, PA, and PA wedge pressures (PAWP) were measured. Cardiac output (CO) was determined using the thermodilution method. PVR was calculated as (mPAP − PAWP)/CO and expressed in WU. Stroke volume (SV) was calculated as CO divided by the heart rate. CO and SV were indexed to body surface area to obtain both cardiac index and SV index. PA compliance was calculated as the ratio of SV to PA pulse pressure and expressed in mL/mmHg. The DPG was calculated as the difference between diastolic PA pressure and PAWP (15, 16). Pulmonary vascular distensibility, α, was determined by nonlinear squares fitting (i.e., least square fitting) of the following equation (44):
where R0 is the total pulmonary resistance, calculated as (mPAP/CO) and expressed in WU, and α is the percent increase of resistive vessel diameter per mmHg of transmural vascular pressure. In the current study, we measured PAWP, mPAP, and CO at rest. We did not measure the extravascular pressure, but as it remains constant, we assumed that the relative change in transmural vascular pressure remained constant as well.
RV Function Assessment
RV end-systolic elastance (Ees) was determined using the single-beat method as described previously (17) and detailed further in the Supplemental Data (Supplemental Fig. S2). Briefly, the signal average of a series of RV pressure waveforms was created and its second derivative squared to produce upright peaks corresponding to different events in the cardiac cycle (Supplemental Fig. S2). These peaks were then used as timing markers to define the “up and down” pressure segments for predicting Pmax based on a distribution function (the 4-parameter Weibull peak fit) within SigmaPlot (version 13, Systat Software, Inc., San Jose, CA). In addition, end-systolic pressure (ESP) defined as the point of maximal time varying elastance was approximated. Ees was then determined as (Pmax − RV ESP)/SV index. Pulmonary artery (PA) elastance (Ea) was calculated as RV ESP/SV index, and the RV-PA coupling was expressed as Ees/ Ea.
Statistical Analysis
Unless otherwise stated, values are presented as means and standard deviation. For method validation studies, estimated and measured Zc, Z0, and λ were compared using linear regression to define correlation and Bland–Altman plots to define accuracy and precision of estimated data. Measurements were considered interchangeable when the average difference between them (bias) was <10% of the mean of all values, and the overall error defined as the bias standard deviation × (1.96/mean of all data) was ≤30%. These criteria are consistent with previously reported method-comparison studies in swine (18). For clinical studies, comparisons of baseline characteristics, resting supine right heart hemodynamics, and RV-specific contractile and afterload parameters among the four groups were performed using one-way ANOVA with Bonferroni’s post hoc correction. Chi-square tests were used to analyze dichotomous variables. Univariate analysis was performed to determine the predictors of RV-PA coupling. A probability value of <0.05 was considered significant. Statistical analyses were performed using GraphPad Prism v7 (GraphPad Software, LLC, La Jolla, CA) and SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Method Validation
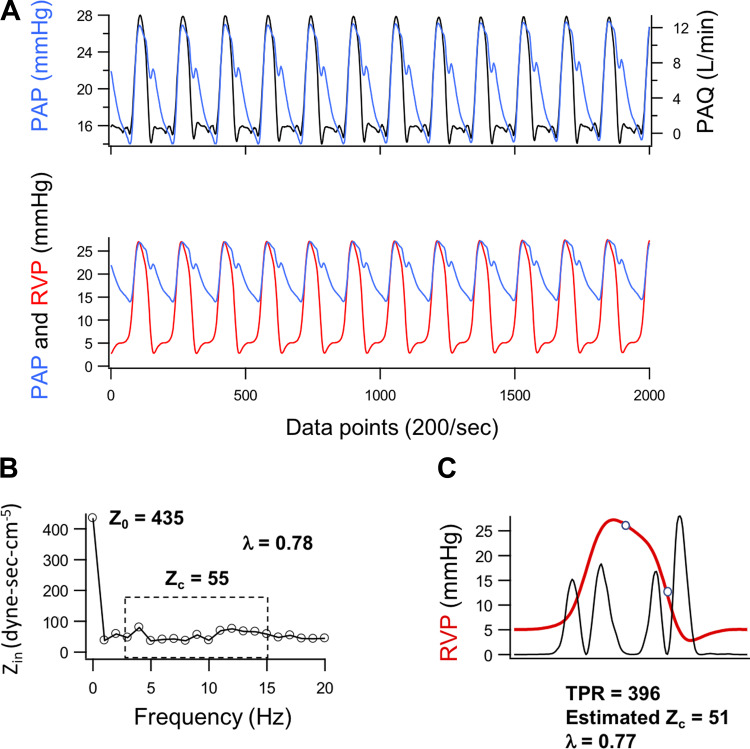
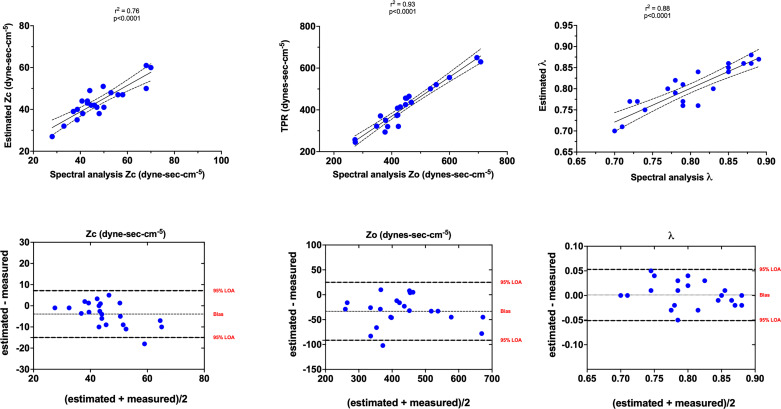
An example of data used for validation of the pressure-based method to estimate Zc is shown in Fig. 2. Overall, there was strong correlation between frequency-domain analysis and estimates of Zc (r2 = 0.76, P < 0.0001), Z0 (r2 = 0.93, P < 0.0001), and λ (r2 = 0.88, P < 0.0001) (Fig. 3). Bland–Altman analysis indicated that estimates of Z0 and Zc tended, on average, to underestimate measured values by ∼8% with overall mean errors of 13% and 15%, respectively (Fig. 3). However, since λ is determined by the relationship between Z0 and Zc, the values derived from RV pressure waveform estimates exhibit no bias and only 6% error relative to those derived from frequency-domain analysis.
Figure 2.
Method validation for the estimation of pulmonary vascular impedance (Z0), characteristic impedance (Zc), and a derived wave reflection coefficient (λ) in a Yorkshire swine model (7 males). A: examples of 10-s recordings of proximal pulmonary arterial pressure (PAP), pulmonary arterial blood flow (PAQ), and right ventricular pressure (RVP) used in validation of the pressure-based method for estimating frequency-independent pulmonary vascular impedance (Z0), characteristic impedance (Zc), and a derived wave reflection coefficient (λ). Data segments were acquired at a sampling rate of 200 Hz from anesthetized open-chest swine during apnea and superimposed to show the temporal relationship between PAP and other measurements. Total pulmonary resistance (TPR) was calculated as an estimate of Z0 from mean PAP and mean PAQ (i.e., cardiac output) averaged over the 10-s interval (TPR = mean PAP/cardiac output). B: the derived input impedance (Zin) spectrum providing reference values for Z0 and Zc calculated as the mean of values from 3 to 15 Hz. C: the signal average of RVP over the interval (red) with event markers derived from the second derivative of RVP (black, arbitrary units) superimposed. The open circles represent the pressures at peak PAQ and end-ejection as described in Fig. 1.
Figure 3.
Top: Pearson’s correlation between spectral analysis measured and right ventricle pressure-based estimation of characteristic impedance (Zc), frequency-independent input impedance (Z0), and total pulmonary resistance (TPR) as an estimate and wave reflection coefficients (λ). Data presented as means and 95% confidence intervals of the linear regression functions. Bottom: Bland–Altman plot showing the mean difference between methods (bias) and 95% limits of agreement (LOA) between the estimated and measured Zc, Z0, and λ.
Demographic and Clinical Characteristics
There was no significant difference in the age, sex, and hemoglobin concentration between the four groups. Patients with Ipc-PH had greater body mass index compared with controls and patients with PAH. The baseline characteristics, medications, and comorbidities are summarized in Table 1. The diagnostic association for PAH and controls is described in Supplemental Tables S1 and S2.
Table 1.
Baseline characteristics and hemodynamic assessment
| Controls | Ipc-PH | Cpc-PH | PAH | |
|---|---|---|---|---|
| n | 10 | 20 | 20 | 30 |
| Characteristics | ||||
| Age, yr | 65 ± 10 | 57 ± 13 | 69 ± 11 | 59 ± 16 |
| BMI, kg/m2 | 28 ± 4 | 39 ± 11ad | 32 ± 8 | 27 ± 6 |
| Female sex, n (%) | 6 (60) | 14 (70) | 15 (75) | 23 (76) |
| Hemoglobin, g/dL | 12.7 ± 1.3 | 11.5 ± 2.8 | 12.5 ± 1.9 | 12.9 ± 2.1 |
| Comorbidities, n (%) | ||||
| Systemic hypertension | 7 (70) | 12 (60) | 9 (45) | 12 (40) |
| Diabetes | 2 (20) | 6 (30) | 3 (15) | 3 (10) |
| Atrial fibrillation | 0 | 2 (10) | 9 (45) | 2 (7) |
| Obstructive sleep apnea | 1 (14) | 10 (50) | 7 (35) | 6 (20) |
| Coronary artery disease | 2 (20) | 2 (10) | 3 (15) | 0 |
| Medications, n (%) | ||||
| β-adrenergic receptor blocker | 2 (20) | 6 (30) | 12 (66) | 6 (20) |
| Calcium channel blocker | 3 (30) | 7 (35) | 3 (17) | 5 (16) |
| ACE inhibitor or ARB | 3 (30) | 7 (35) | 6 (33) | 5 (17) |
| Diuretics | 4 (40) | 12 (60) | 13 (72) | 15 (52) |
| Right heart catheterization | ||||
| SpO2, % | 96 ± 2 | 94 ± 5 | 93 ± 7 | 95 ± 4 |
| MvO2, % | 74 ± 5 | 65 ± 9 | 61 ± 8ad | 67 ± 5 |
| Right atrial pressure, mmHg | 5 ± 3 | 12 ± 3ad | 14 ± 3ad | 8 ± 2 |
| Stroke volume index, mL/m2 | 39.5 ± 5.4 | 47.5 ± 16.7 | 35.9 ± 11.3b | 39.7 ± 12.1 |
| Cardiac index, L/min/m2 | 2.99 ± 0.6 | 3.29 ± 0.71 | 2.52 ± 0.76b | 2.96 ± 1.0 |
| mPAP, mmHg | 18 ± 1 | 34 ± 6a | 54 ± 10ab | 42 ± 11ab |
| PAWP, mmHg | 9 ± 3 | 20 ± 3ad | 23 ± 5ad | 10 ± 3 |
| PVR, WU | 1.8 ± 0.6 | 1.9 ± 0.6 | 6.7 ± 2.2ab | 6.7 ± 3.0ab |
| TPR, WU | 3.3 ± 0.7 | 4.9 ± 1.0a | 11.5 ± 3.2abd | 8.8 ± 4.3ab |
| DPG, mmHg | 3 ± 1 | 3 ± 4 | 13 ± 6ab | 16 ± 7abc |
| PA compliance, mL/mmHg | 5.4 ± 3.1 | 3.9 ± 0.9a | 1.4 ± 0.5ab | 2.1 ± 1.0ab |
| PV distensibility, %/mmHg | 1.32 ± 0.45 | 0.95 ± 0.43a | 0.42 ± 0.17ab | 0.33 ± 0.18ab |
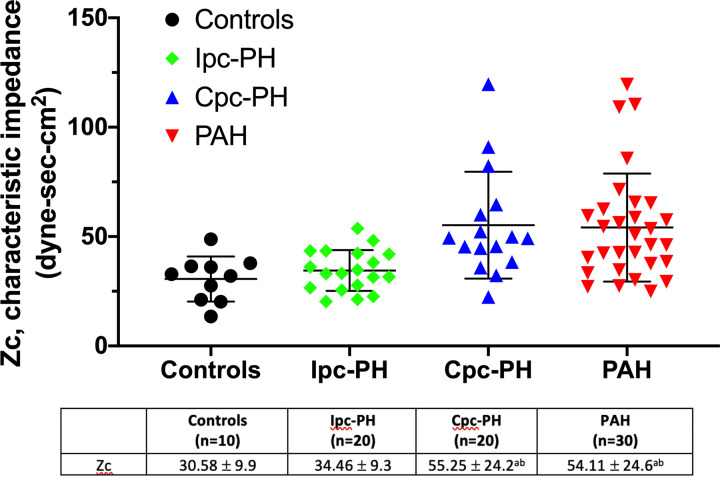
| Zc, dyne·s·cm−5 | 30.58 ± 9.9 | 34.46 ± 9.3 | 55.25 ± 24.2ab | 54.11 ± 24.6ab |
| Wave reflection coefficient, λ | 0.79 ± 0.03 | 0.84 ± 0.02a | 0.87 ± 0.05a | 0.85 ± 0.04a |
| RV functional assessment | ||||
| Ees, mmHg/mL/m2) | 0.36 ± 0.2 | 0.28 ± 0.13 | 0.58 ± 0.31b | 0.53 ± 0.3b |
| Ea, mmHg/mL/m2) | 0.31 ± 0.1 | 0.51 ± 0.3 | 1.04 ± 0.3ab | 0.84 ± 0.4ab |
| RV-PA coupling (Ees/Ea) | 1.11 ± 0.4 | 0.66 ± 0.5a | 0.58 ± 0.3a | 0.67 ± 0.4a |
Data presented as n (%) or means ± SD. ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; Cpc-PH, combined pre- and postcapillary pulmonary hypertension; DPG, diastolic pressure gradient; Ea, arterial elastance; Ees, end-systolic elastance; Ipc-PH, isolated post-capillary pulmonary hypertension; mPAP, mean pulmonary artery pressure; MvO2, mixed venous oxygen saturation; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PAWP, pulmonary artery wedge pressure; PV, pulmonary vascular; PVR, pulmonary vascular resistance; RV, right ventricle; SpO2, peripheral oxygen saturation; TPR, total pulmonary resistance; WU, Wood unit; Zc, characteristic impedance.
aP < 0.05 vs. controls; bP < 0.05 vs. Ipc-PH; cP < 0.05 vs. Cpc-PH; dP < 0.05 vs. PAH.
Conventional Resting Right Heart Catheterization Data
The resting right heart catheterization data are summarized in Table 1. Patients with PH-HFpEF had greater RA pressure compared with control and PAH groups. Patients with Cpc-PH and PAH had similarly elevated mPAP compared with control and Ipc-PH groups. By study design, PH-HFpEF groups exhibited the greatest PAWP compared with other groups, whereas PAH and Cpc-PH groups exhibited the greatest PVR compared with other groups. PA compliance and pulmonary vascular distensibility, α, were similarly reduced in Cpc-PH and PAH groups compared with other groups.
RV Functional Assessment
The single-beat estimation of RV-PA coupling is summarized in Table 1. Cpc-PH and PAH groups exhibited greater RV contractility (Ees) compared with Ipc-PH group. Cpc-PH and PAH groups had similarly increased RV afterload (Ea) compared with other groups. The resting RV-PA coupling ratio was significantly lower in PH-HFpEF and PAH groups compared with controls.
Characteristic Impedance
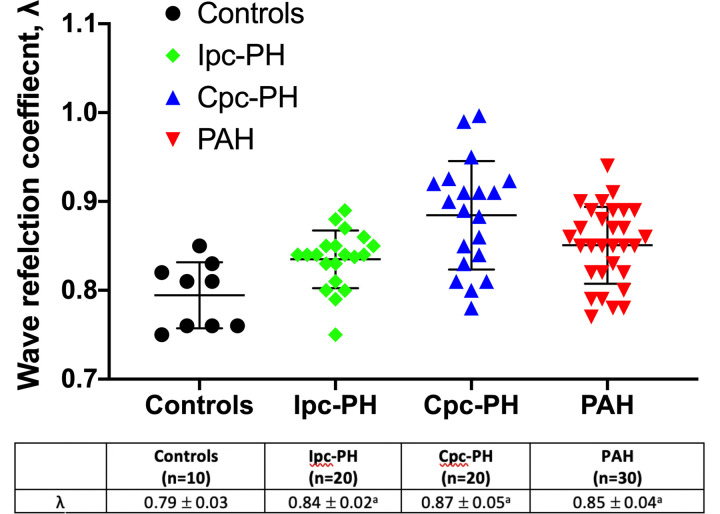
RV pressure waveform estimations of λ and Zc across the groups are summarized in Table 1 and in Figs. 4 and 5, respectively. The wave reflection coefficient index, λ, was significantly greater in the PH-HFpEF and PAH groups compared with controls (Table 1 and Fig. 4). The Cpc-PH and PAH groups demonstrated higher Zc compared with other groups (Table 1 and Fig. 5). There was no difference in Zc between the Ipc-PH group and controls. On univariate analysis, λ was associated with RV-PA coupling in the PAH group (Supplemental Table S3). In contrast, there was no significant association between the various RV afterload parameters and RV-PA coupling in the PH-HFpEF group (Supplemental Table S3).
Figure 4.
Wave reflection coefficient, λ, between controls, Ipc-PH, Cpc-PH, and PAH is shown. Ipc-PH, Cpc-PH, and PAH groups demonstrated increased λ compared with controls. Data presented as means ± SD. Cpc-PH, combined pre-and post-capillary pulmonary hypertension; Ipc-PH, isolated post-capillary pulmonary hypertension; PAH, pulmonary arterial hypertension. aP < 0.05 vs. controls. Statistical comparison of wave reflection coefficient, λ, between the groups was performed using one-way ANOVA with Bonferroni’s post hoc correction.
Figure 5.
Characteristic impedance, Zc, between controls, Ipc-PH, Cpc-PH, and PAH is shown. The Cpc-PH and PAH groups demonstrated similarly increased Zc compared with controls and the Ipc-PH group. Data presented as means ± SD. Cpc-PH, combined pre-and postcapillary pulmonary hypertension; Ipc-PH, isolated postcapillary pulmonary hypertension; PAH, pulmonary arterial hypertension. aP < 0.05 vs. controls; bP < 0.05 vs. Ipc-PH. Statistical comparison of characteristic impedance, Zc, between the groups was performed using one-way ANOVA with Bonferroni’s post hoc correction.
DISCUSSION
The present results show that Zc can be derived from RV pressure waveform analysis and that a wave reflection coefficient, λ, can be calculated from TPR and Zc. We demonstrated that Zc was similarly increased in the Cpc-PH and PAH groups, whereas λ was increased in both PH-HFpEF groups and the PAH group. However, although an increase in λ was associated with RV-PA uncoupling in the PAH group, it was not in the PH-HFpEF group. The absence of afterload determinants of RV-PA coupling in the PH-HFpEF group suggests a predominant role of RV cardiomyopathy in these patients.
As recently summarized, there are several valid estimations of RV afterload (4). The first is maximum wall tension, which is difficult to obtain due to the irregular shape and regionally inhomogeneous contraction of the RV. The second is hydraulic power (WTOT), which is the sum of oscillatory power (WOSC) and steady power (WST = mPAP × CO). This measure requires the integration of synchronous pressure and flow waves, which is not clinically practical either. The third is arterial elastance (Ea), or end-systolic pressure (ESP) divided by SV measured on a pressure-volume loop, which corresponds to a measurement of afterload as “seen” by the ventricle. This measure does not integrate all forces that oppose PA flow but rather represents afterload pressure at end-systolic elastance (Ees) and is therefore convenient to assess RV contractile adaptation to increased afterload. The fourth and most comprehensive, although least accessible to clinicians, is pulmonary vascular impedance (PVZ), which incorporates the three components of the RV hydraulic load—resistance, compliance, and wave reflections (2).
Unlike PA compliance where only 20% is stored within the main and proximal PAs (19), Zc is an inherent property of the proximal PA and is dependent on blood viscosity, proximal PA diameter, and the elasticity of its vessel wall (20, 21). Because the hemoglobin concentration and presumably blood viscosity across the groups were similar, the increase in Zc observed in the Cpc-PH and PAH groups is, therefore, related to a less distensible proximal PA. Increased PA stiffness in the Cpc-PH and PAH groups has been attributed to pulmonary vascular remodeling with medial and intimal thickening of the arteries along with the loss of elastin and excess collagen deposition (22, 23). Unlike the Cpc-PH group, the Ipc-PH group did not exhibit an increase in Zc, suggesting the lack of advanced proximal PA remodeling.
A PVZ spectrum is derived by plotting pressure/flow ratio as a function of increasing frequency. However, although this “frequency domain” analysis is generally regarded as the reference standard, it is technically challenging to generate and complex to interpret, thus not readily applicable in clinical settings. As a less complex alternative to PVZ spectral analysis, time domain methods have been described to simply estimate Z0 as mean PA pressure/cardiac output (mPAP/CO) and derive Zc from proximal PA pulsatile pressure and flow velocity or diameter (20, 24). Time domain analysis of PA pressure waveforms can also help to examine the timing, extent, and magnitude of wave reflection (25, 26). In the current study, algorithms previously developed to estimate cardiac output from the RV pressure waveform contour were adapted to estimate Zc when actual cardiac output is known (9–11). In this method, if the value of Zc is unknown but assumed to be constant, Zc can be determined empirically as the ratio of estimated cardiac output to the measured cardiac output (by thermodilution) (9). Although the values for Zc and λ determined by this method have been published, to our knowledge, it has not been validated against the reference standard spectral analysis method. In this study, we validated an RV pressure-based method for estimation of Zc and λ. Our method is applicable for the evaluation of patients undergoing clinically indicated right heart catheterization, during which RV pressure waveforms are commonly recorded and CO routinely measured.
The pulmonary circulation with its extensive network branching vessels has a larger surface area over a shorter distance compared with the systemic circulation (27). The systemic circulation, made up primarily of the aorta and its side branches, generates a positive pressure wave reflection that opposes the ejected blood from the left ventricle (7). In contrast, the PA branches early and rapidly creating an “open-end”-type reflection site located approximately at the end of the lobar PAs (28). During RV systole, the acceleration of blood from the RV is accompanied by a large forward propagating compression wave that is reflected as a negative retrograde expansion wave from these reflection sites (27). This negative retrograde wave serves to further augment the forward moving compression wave generated with RV systole (27). In patients with PH with abnormal pulmonary vasculature, there is an additional positive retrograde pressure wave reflection that can impede RV ejection (27). In addition, alterations in PA compliance could also mitigate the favorable effect of the aforementioned negative retrograde waves and further compromise RV function (27).
Previous studies have examined the effects of increased PA wave reflection on RV dysfunction in chronic thromboembolic pulmonary hypertension (CTEPH) (29) and in a mixed cohort of PH (30). In these studies, RV dysfunction was described in the context of reduced RV ejection fraction by cardiac magnetic resonance imaging (MRI) (31) or by reduced tricuspid annular plane systolic excursion (TAPSE) on echocardiogram (30). Kussmaul et al. (21) examined the impact of PVZ on RV function during acute transient myocardial ischemia in patients with known coronary artery disease undergoing percutaneous angioplasty. As in our Ipc-PH cohort, the authors observed that during acute PAWP elevation (induced by balloon angioplasty-induced ischemia), there was an increase in PA wave reflection but the characteristic impedance, Zc, was unchanged. The authors concluded that increased PA wave reflection contributed to reduced RV stroke volume and resulted in RV-PA uncoupling. However, during transient myocardial ischemia, reduced left ventricular (LV) systolic function and altered LV diastolic compliance could also account for the reduced RV stroke volume through RV-LV systolic and diastolic interdependence (32). To our knowledge, the current study is the first to integrate the static and pulsatile components of afterload on RV function in patients with PAH and PH-HFpEF.
Why is λ increased in patients with PAH and PH-HFpEF? Wave reflections represent a major determinant of the phase between very low (i.e., resistance) and high (i.e., proximal PA compliance) frequencies and, therefore, indicate a more global measure of the hydraulic load experienced by the RV. Normally, the high capacitance and low resistance of the pulmonary vascular bed limit the slow return of small, reflected pressure and flow waves from the distal vasculature (25, 33). These retrograde waves return to the RV outflow tract following the closure of the pulmonic valve (i.e., during RV diastole) and have little impact on RV systolic function allowing for optimization of RV-PA coupling (2, 34). In patients with PAH and PH-HFpEF, the ensuing vascular remodeling increases the pulsatile and resistive components, which generate retrograde waves of greater amplitude and velocity. These arrive in the proximal PA while the RV is still ejecting. This late phase, reflective component adds to the incident or backward pressure that the still-ejecting RV must overcome, unfavorably impacting the ejecting RV and resulting in RV-PA uncoupling (2, 34). In the current study, although both PH-HFpEF groups and PAH group exhibited increased λ, RV-PA uncoupling was associated with increased λ in PAH only (Supplemental Table S3). A recent cardiac MRI study showed that the patients with PH-HFpEF exhibited a similar degree of diffuse RV fibrosis compared with patients with PAH (31). However, although the diffuse RV fibrosis and the resulting RV dysfunction encountered in patients with PAH was related to its afterload, there was no association between RV myocardial fibrosis and RV afterload in patients with PH-HFpEF. In addition, Fayyaz et al. (22) demonstrated that the severity of pulmonary arterial and venous remodeling seen in PH-HFpEF was not different among those with and without RV dysfunction on echocardiogram. These findings, along with results from the current study, suggest that RV dysfunction in PH-HFpEF is mediated by load-independent mechanisms. Unlike PAH, patients with HFpEF have increased prevalence of ischemic heart disease, atrial fibrillation, microvascular inflammation, and capillary rarefaction (35, 36), all of which may contribute to their intrinsic RV myocardial dysfunction. Given the central role of RV dysfunction in determining exercise capacity and outcomes in PH-HFpEF (37–42), this may in part explain the paucity of success seen with primary RV afterload reduction therapy in this patient population (43, 44).
Prior studies have demonstrated that in PH-associated left heart disease, RV-PA coupling was driven by abnormal increase in RV afterload (38, 40, 45, 46). These studies, however, examined the effects of afterload on various load-dependent measures of RV contractile function, including the tricuspid annular plane systolic excursion (TAPSE) and echocardiography-derived RV fractional area change. Consequently, these measures are insufficient to identify the contribution of abnormal afterload on RV function. In the current study, we examined the effects of various components of RV afterload on Ees/Ea and showed that RV-PA uncoupling in the PH-HFpEF group is independent of its afterload.
Limitations
Our PAH cohort consisted of patients who were already on established PAH-targeted pharmacotherapy. However, PAH pharmacotherapy would not have significantly affected the PVZ results. An increase in Zc reflects abnormal remodeling of the proximal PA vessel wall. The current available PAH therapy primarily corrects the imbalance between pulmonary vasoconstriction and vasodilation without inherent reverse remodeling properties. In addition, as we described earlier, increases in λ are contingent on abnormal distal pulmonary vascular compliance and resistance. Data from three prior pivotal PAH trials showed that PAH therapy (oral sildenafil, subcutaneous treprostinil, and intravenous epoprostenol) had little effect on reducing RV pulsatile afterload (47). Accordingly, it is unlikely that PAH-targeted therapy would have significantly influenced the PVZ results in the current study.
The present study limited the evaluation of PVZ to Z0 and Zc, thereby apparently ignoring the contribution of low-frequency impedance to RV hydraulic load. Previous reports of PVZ spectra show a significant increase in low-frequency impedance added to Z0 and Zc (2, 48, 49) and that this augmentation is of prognostic relevance (50). However, in the present study, Zc represents a lumped measurement of low- and high-frequency impedance adjusted from a RV pressure-curve-derived prediction of flow, rather than based on pressure/flow moduli at frequencies higher than 4–10 Hz or more (24, 51). Therefore, the wave reflection coefficient, λ, was an indirect estimate rather than a quantification of wave reflection per se.
Conclusions
Increased arterial load-induced RV failure is an important determinant of functional capacity and outcome, but simple methods for its bedside determination are not yet agreed upon in expert consensus documents (4, 8). In this study, we showed that it is possible to quantify Zc and λ during routine, clinically indicated RHC using RV pressure waveforms. Unlike PVR, which solely represents “static” afterload, Zc and λ have the added advantage of quantifying and integrating the pulsatile components of RV afterload imposed during the cardiac cycle and, therefore, provide a more accurate description of afterload. Although both the PH-HFpEF groups and the PAH group exhibited increased λ, only the Cpc-PH and PAH groups showed abnormal Zc, suggesting that the latter two PH phenotypes are associated with advanced remodeling of the proximal PA. In addition, we also demonstrated that in the PH-HFpEF group, RV systolic dysfunction is an intrinsic RV myocardial pathology that is independent of its afterload. In the PAH group, however, increase in λ is associated with RV-PA uncoupling. Given the pivotal role of RV (mal)adaptation to its pulmonary vascular load, the current assessment of Zc and λ provides a future platform to examine the prognostic significance of these measures in all forms of PH. In addition, in the PAH population, the current methodology can help examine the effects of PAH-targeted therapy on the various components of RV afterload obtained during clinically indicated right heart catheterization. In the PH-HFpEF population, given the significant prognostic value of RV function, the current study provides a platform for preclinical and clinal studies alike to examine pharmacotherapeutic strategies directed toward augmentation of RV inotropy and not simply isolated RV afterload reduction therapy.
SUPPLEMENTAL DATA
Supplemental Figs S1 and S2 and Tables S1, S2, and S3: https://doi.org/10.6084/m9.figshare.14258285.
GRANTS
This work was supported by Grant 2T32GM086287-11 (to A.E.) and Grant 2T32HL007778-26 (to H.O.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.O., P.M.H., and I.S. conceived and designed research; P.J., A.E., M.C., P.M.H., and I.S. performed experiments; H.O., R.N., A.E., P.M.H., and I.S. analyzed data; H.O., R.N., A.E., P.M.H., and I.S. interpreted results of experiments; P.M.H. and I.S. prepared figures; H.O., R.N., P.M.H., and I.S. drafted manuscript; H.O., R.N., P.M.H., and I.S. edited and revised manuscript; H.O., P.J., R.N., A.E., M.C., P.M.H., and I.S. approved final version of manuscript.
REFERENCES
- 1.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 53: 1801913, 2019. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kussmaul WG, Noordergraaf A, Laskey WK. Right ventricular-pulmonary arterial interactions. Ann Biomed Eng 20: 63–80, 1992. doi: 10.1007/BF02368506. [DOI] [PubMed] [Google Scholar]
- 3.Sniderman AD, Fitchett DH. Vasodilators and pulmonary arterial hypertension: the paradox of therapeutic success and clinical failure. Int J Cardiol 20: 173–181, 1988. doi: 10.1016/0167-5273(88)90261-6. [DOI] [PubMed] [Google Scholar]
- 4.Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, Hemnes AR, Kawut SM, Kline JA, Kolb TM, Mathai SC, Mercier O, Michelakis ED, Naeije R, Tuder RM, Ventetuolo CE, Vieillard-Baron A, Voelkel NF, Vonk-Noordegraaf A, Hassoun PM; American Thoracic Society Assembly on Pulmonary Circulation. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. an official American thoracic society research statement. Am J Respir Crit Care Med 198: e15–e43, 2018. doi: 10.1164/rccm.201806-1160ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergel DH, Milnor WR. Pulmonary vascular impedance in the dog. Circ Res 16: 401–415, 1965. doi: 10.1161/01.res.16.5.401. [DOI] [PubMed] [Google Scholar]
- 6.Dujardin JP, Stone DN, Forcino CD, Paul LT, Pieper HP. Effects of blood volume changes on characteristic impedance of the pulmonary artery. Am J Physiol 242: H197–H202, 1982. doi: 10.1152/ajpheart.1982.242.2.H197. [DOI] [PubMed] [Google Scholar]
- 7.Vlachopoulos C, O’Rourke M, Nichols WW. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. London, UK: Taylor & Francis Group, 2011. [Google Scholar]
- 8.Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, Kawut SM, Langleben D, Lumens J, Naeije R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J 53: 1801900, 2019. doi: 10.1183/13993003.01900-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karamanoglu M, Bennett T, Stahlberg M, Splett V, Kjellstrom B, Linde C, Braunschweig F. Estimation of cardiac output in patients with congestive heart failure by analysis of right ventricular pressure waveforms. Biomed Eng Online 10: 36, 2011. doi: 10.1186/1475-925X-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karamanoglu M, Bennett TD. A right ventricular pressure waveform based pulse contour cardiac output algorithm in canines. Cardiovasc Eng 6: 83–92, 2006. doi: 10.1007/s10558-006-9014-4. [DOI] [PubMed] [Google Scholar]
- 11.Karamanoglu M, McGoon M, Frantz RP, Benza RL, Bourge RC, Barst RJ, Kjellstrom B, Bennett TD. Right ventricular pressure waveform and wave reflection analysis in patients with pulmonary arterial hypertension. Chest 132: 37–43, 2007. doi: 10.1378/chest.06-2690. [DOI] [PubMed] [Google Scholar]
- 12.Heerdt PM, Gandhi CD, Dickstein ML. Disparity of isoflurane effects on left and right ventricular afterload and hydraulic power generation in swine. Anesth Analg 87: 511–521, 1998. doi: 10.1097/00000539-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 40: 3297–3317, 2019. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs G, Avian A, Olschewski A, Olschewski H. Zero reference level for right heart catheterisation. Eur Respir J 42: 1586–1594, 2013. doi: 10.1183/09031936.00050713. [DOI] [PubMed] [Google Scholar]
- 15.Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, Maurer G, Lang IM. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 143: 758–766, 2013. doi: 10.1378/chest.12-1653. [DOI] [PubMed] [Google Scholar]
- 16.Naeije R, Vachiery JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 41: 217–223, 2013. doi: 10.1183/09031936.00074312. [DOI] [PubMed] [Google Scholar]
- 17.Heerdt PM, Kheyfets V, Charania S, Elassal A, Singh I. A pressure-based single beat method for estimation of right ventricular ejection fraction: proof of concept. Eur Respir J 55: 1901635, 2020. doi: 10.1183/13993003.01635-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berlin DA, Manoach S, Oromendia C, Heerdt PM. Automated expiratory ventilation assistance through a small endotracheal tube can improve venous return and cardiac output. Intensive Care Med Exp 7: 6, 2019. doi: 10.1186/s40635-018-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saouti N, Westerhof N, Helderman F, Marcus JT, Stergiopulos N, Westerhof BE, Boonstra A, Postmus PE, Vonk-Noordegraaf A. RC time constant of single lung equals that of both lungs together: a study in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol 297: H2154–H2160, 2009.doi: 10.1152/ajpheart.00694.2009. [DOI] [PubMed] [Google Scholar]
- 20.Heerdt PM, Pleimann BE. Nitric oxide synthesis inhibition and right ventricular systolic function in swine. J Cardiothorac Vasc Anesth 10: 909–914, 1996. doi: 10.1016/s1053-0770(96)80055-3. [DOI] [PubMed] [Google Scholar]
- 21.Kussmaul WG 3rd, Wieland J, Altschuler J, Laskey WK. Pulmonary impedance and right ventricular-vascular coupling during coronary angioplasty. J Appl Physiol (1985) 74: 161–169, 1993. doi: 10.1152/jappl.1993.74.1.161. [DOI] [PubMed] [Google Scholar]
- 22.Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, Frantz RP, Jenkins SM, Redfield MM. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation 137: 1796–1810, 2018. doi: 10.1161/CIRCULATIONAHA.117.031608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schafer M, Myers C, Brown RD, Frid MG, Tan W, Hunter K, Stenmark KR. Pulmonary arterial stiffness: toward a new paradigm in pulmonary arterial hypertension pathophysiology and assessment. Curr Hypertens Rep 18: 4, 2016.[26733189]doi: 10.1007/s11906-015-0609-2. [DOI] [PubMed] [Google Scholar]
- 24.Huez S, Brimioulle S, Naeije R, Vachiery JL. Feasibility of routine pulmonary arterial impedance measurements in pulmonary hypertension. Chest 125: 2121–2128, 2004. doi: 10.1378/chest.125.6.2121. [DOI] [PubMed] [Google Scholar]
- 25.Castelain V, Herve P, Lecarpentier Y, Duroux P, Simonneau G, Chemla D. Pulmonary artery pulse pressure and wave reflection in chronic pulmonary thromboembolism and primary pulmonary hypertension. J Am Coll Cardiol 37: 1085–1092, 2001. doi: 10.1016/S0735-1097(00)01212-2. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama Y, Nakanishi N, Hayashi T, Nagaya N, Sakamaki F, Satoh N, Ohya H, Kyotani S. Pulmonary artery reflection for differentially diagnosing primary pulmonary hypertension and chronic pulmonary thromboembolism. J Am Coll Cardiol 38: 214–218, 2001. doi: 10.1016/S0735-1097(01)01365-1. [DOI] [PubMed] [Google Scholar]
- 27.Hollander EH, Wang JJ, Dobson GM, Parker KH, Tyberg JV. Negative wave reflections in pulmonary arteries. Am J Physiol Heart Circ Physiol 281: H895–H902, 2001. doi: 10.1152/ajpheart.2001.281.2.H895. [DOI] [PubMed] [Google Scholar]
- 28.Bouwmeester JC, Belenkie I, Shrive NG, Tyberg JV. Wave reflections in the pulmonary arteries analysed with the reservoir-wave model. J Physiol 592: 3053–3062, 2014. doi: 10.1113/jphysiol.2014.273094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukumitsu M, Westerhof BE, Ruigrok D, Braams NJ, Groeneveldt JA, Bayoumy AA, Marcus JT, Meijboom LJ, de Man FS, Westerhof N, Bogaard HJ, Noordegraaf AV. Early return of reflected waves increases right ventricular wall stress in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol 319: H1438–H1450, 2020. doi: 10.1152/ajpheart.00442.2020. [DOI] [PubMed] [Google Scholar]
- 30.Arkles JS, Opotowsky AR, Ojeda J, Rogers F, Liu T, Prassana V, Marzec L, Palevsky HI, Ferrari VA, Forfia PR. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med 183: 268–276, 2011. doi: 10.1164/rccm.201004-0601OC. [DOI] [PubMed] [Google Scholar]
- 31.Patel RB, Li E, Benefield BC, Swat SA, Polsinelli VB, Carr JC, Shah SJ, Markl M, Collins JD, Freed BH. Diffuse right ventricular fibrosis in heart failure with preserved ejection fraction and pulmonary hypertension. ESC Heart Fail 7: 253–263, 2020. doi: 10.1002/ehf2.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clapham KR, Singh I, Capuano IS, Rajagopal S, Chun HJ. MEF2 and the right ventricle: from development to disease. Front Cardiovasc Med 6: 29, 2019. doi: 10.3389/fcvm.2019.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Bos GC, Westerhof N, Randall OS. Pulse wave reflection: can it explain the differences between systemic and pulmonary pressure and flow waves? A study in dogs. Circ Res 51: 479–485, 1982. doi: 10.1161/01.res.51.4.479. [DOI] [PubMed] [Google Scholar]
- 34.Ha B, Lucas CL, Henry GW, Frantz EG, Ferreiro JI, Wilcox BR. Effects of chronically elevated pulmonary arterial pressure and flow on right ventricular afterload. Am J Physiol Heart Circ Physiol 267: H155–H165, 1994. doi: 10.1152/ajpheart.1994.267.1.H155. [DOI] [PubMed] [Google Scholar]
- 35.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 131: 550–559, 2015. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 37.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 37: 3293–3302, 2016. doi: 10.1093/eurheartj/ehw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerges M, Gerges C, Pistritto AM, Lang MB, Trip P, Jakowitsch J, Binder T, Lang IM. Pulmonary hypertension in heart failure. epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med 192: 1234–1246, 2015. doi: 10.1164/rccm.201503-0529OC. [DOI] [PubMed] [Google Scholar]
- 39.Goliasch G, Zotter-Tufaro C, Aschauer S, Duca F, Koell B, Kammerlander AA, Ristl R, Lang IM, Maurer G, Mascherbauer J, Bonderman D. Outcome in heart failure with preserved ejection fraction: the role of myocardial structure and right ventricular performance. PLoS One 10: e0134479, 2015. doi: 10.1371/journal.pone.0134479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 35: 3452–3462, 2014. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh I, Oliveira RKF, Naeije R, Rahaghi FN, Oldham W, Systrom DM, Waxman AB. Pulmonary vascular distensibility and early pulmonary vascular remodeling in pulmonary hypertension. Chest 156: 724–732, 2019. doi: 10.1016/j.chest.2019.04.111. [DOI] [PubMed] [Google Scholar]
- 42.Singh I, Rahaghi FN, Naeije R, Oliveira RKF, Systrom DM, Waxman AB. Right ventricular-arterial uncoupling during exercise in heart failure with preserved ejection fraction: role of pulmonary vascular dysfunction. Chest 156: 933–943, 2019. doi: 10.1016/j.chest.2019.04.109. [DOI] [PubMed] [Google Scholar]
- 43.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E; RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309: 1268–1277, 2013. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vachiery JL, Delcroix M, Al-Hiti H, Efficace M, Hutyra M, Lack G, Papadakis K, Rubin LJ. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J 51: 1701886, 2018. doi: 10.1183/13993003.01886-2017. [DOI] [PubMed] [Google Scholar]
- 45.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 37: 183–188, 2001. doi: 10.1016/S0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 46.Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Temporelli PL, Arena R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol 305: H1373–H1381, 2013.doi: 10.1152/ajpheart.00157.2013. [DOI] [PubMed] [Google Scholar]
- 47.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 125: 289–297, 2012. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haneda T, Nakajima T, Shirato K, Onodera S, Takishima T. Effects of oxygen breathing on pulmonary vascular input impedance in patients with pulmonary hypertension. Chest 83: 520–527, 1983. doi: 10.1378/chest.83.3.520. [DOI] [PubMed] [Google Scholar]
- 49.Laskey WK, Ferrari VA, Palevsky HI, Kussmaul WG. Pulmonary artery hemodynamics in primary pulmonary hypertension. J Am Coll Cardiol 21: 406–412, 1993. doi: 10.1016/0735-1097(93)90682-Q. [DOI] [PubMed] [Google Scholar]
- 50.Hunter KS, Lee PF, Lanning CJ, Ivy DD, Kirby KS, Claussen LR, Chan KC, Shandas R. Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. Am Heart J 155: 166–174, 2008. doi: 10.1016/j.ahj.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin FC, Guzman PA, Brin KP, Maughan WL, Brinker JA, Traill TA, Weiss JL, Weisfeldt ML. Effect of nitroprusside on hydraulic vascular loads on the right and left ventricle of patients with heart failure. Circulation 67: 1330–1339, 1983. doi: 10.1161/01.cir.67.6.1330. [DOI] [PubMed] [Google Scholar]