Abstract
Antigen accumulation in lymph nodes (LNs) is critical for vaccine efficacy, but understanding of vaccine biodistribution in humans or large animals remains limited. Using the rhesus macaque model, we employed a combination of positron emission tomography (PET) and fluorescence imaging to characterize the whole-animal to tissue-level biodistribution of a subunit vaccine comprised of an HIV envelope trimer protein nanoparticle (trimer-NP) and lipid-conjugated CpG adjuvant (amph-CpG). Following immunization in the thigh, PET imaging revealed vaccine uptake primarily in inguinal and iliac LNs, reaching distances up to 17 cm away from the injection site. Within LNs, trimer-NPs exhibited striking accumulation on the periphery of follicular dendritic cell (FDC) networks in B cell follicles. Comparative imaging of soluble Env trimers (not presented on nanoparticles) in naïve or previously-immunized animals revealed diffuse deposition of trimer antigens in LNs following primary immunization, but concentration on FDCs in pre-immunized animals with high levels of trimer-specific IgG. These data demonstrate the capacity of nanoparticle or “albumin hitchhiking” technologies to concentrate vaccines in genitourinary tract-draining LNs, which may be valuable for promoting mucosal immunity.
Keywords: vaccines, HIV, nanoparticles, PET imaging, fluorescence imaging, non-human primates
INTRODUCTION
Unlike oral or systemically-administered drugs, the majority of licensed vaccines are administered as parenteral injections into the muscle or skin, and are thought to act in a local, regionalized manner at the lymph nodes draining the injection site. Primary immune responses require antigen to be delivered to lymph nodes, where antigen-specific activation of naïve T cells and B cells is orchestrated. Both T and B cell responses are sensitive to the amount of antigen accumulating in lymph nodes [1-3]. In addition, preferential transport of vaccine adjuvants into lymphatics rather than the blood circulation is critical to avoid systemic side effects [3]. Thus, biodistribution plays a key role in both the safety and efficacy of vaccines. Responding T cells and B cells are also programmed in lymph nodes in a tissue-specific manner: lymphocytes activated in gut-associated lymphoid tissues are induced to express gut homing adhesion and chemokine receptors, activation in skin-draining lymph nodes leads to skin-homing lymphocytes, and activation in lung-draining lymph nodes primes lymphocytes that traffic to the respiratory mucosa [4-6]. This tissue-specific programming extends also to lymphocyte effector functions such as class switching of B cells to produce IgA in mucosa-draining lymph nodes [7-9]. Thus, targeting vaccines to lymph nodes draining mucosal sites may be an effective strategy for optimal protection at these sites of pathogen entry [10].
The transport and localization of vaccines within lymph nodes also has significant implications for the resulting immune response. Antigens and adjuvant compounds carried into lymph can be captured by macrophages in the subcapsular sinus or medullary regions of the lymph node, transported to follicular dendritic cells (FDCs) localized within B cell follicles, or trafficked into collagen conduits that enter the follicles or the deeper T cell regions of the lymph node [11-13]. Intranodal antigen trafficking is particularly important in the humoral immune response: Activated B cells enter germinal centers (GCs) where they will cyclically proliferate, undergo somatic hypermutation of their antigen receptors, and interact with follicular helper T cells (Tfh). Antigen must be efficiently captured in the GC to support affinity maturation of the antibody response, which can persist for weeks to months following a single immunization [14,15].
These issues have motivated increasing efforts to understand factors impacting immunogen and adjuvant biodistribution. Physical size plays an important role in dictating whether parenterally-injected compounds clear from tissues by entering blood versus lymphatic vessels [16,17]. Thus, vaccines formulated as particulates [18-21], modified to bind to lymph-trafficking albumin [3], or engineered to bind tightly to the common vaccine adjuvant alum [22,23] show reduced dissemination into the blood and enhanced lymph node accumulation. Within lymph nodes, particulate antigens that are recognized by natural IgM or trigger spontaneous complement activation are shuttled in a complement-dependent manner to the FDC network, in a manner also dependent on particle size [24,25], while soluble glycoproteins can be captured by interfollicular macrophages and dendritic cells (DCs) via scavenger receptors [26]. We recently reported that nanoparticle forms of densely-glycosylated HIV Env immunogens are recognized by mannose binding lectin, leading to complement activation through the lectin pathway and subsequent rapid trafficking to FDCs within lymph nodes [27]. However, strategies for lymph node targeting and modulation of antigen trafficking have to date been primarily studied in small animal models, and insight into vaccine biodistribution in large animals that would more closely approximate the setting of human immunization, especially on a whole-animal level, remains limited.
Here we combined dual positron emission tomography (PET), whole tissue light sheet microscopy, and traditional histology methods to visualize the fate of HIV Env subunit vaccines from the whole-animal to microscopic levels in rhesus macaques, the most relevant preclinical model for HIV vaccine development. We analyzed the biodistribution of a vaccine incorporating two distinct technologies for targeting immunogen and adjuvant to regional lymph nodes: linkage of a molecular adjuvant, CpG DNA, to an albumin-binding lipid moiety (to exploit “albumin hitchhiking” for lymph node targeting [3,28]), and formulation of a stabilized HIV Env gp140 trimer, either as a soluble trimer or as a self-assembled trimer-nanoparticle, favoring LN trafficking through physical size. Using PET imaging, we traced vaccine localization following injection at a subcutaneous site selected to favor vaccine accumulation in inguinal and iliac lymph nodes that drain the vaginal tract and rectum [29,30], of interest for promoting mucosal immunity to HIV. Guided further by ex vivo PET analysis of necropsied lymph nodes, we further imaged the localization of fluorescently-labeled antigen and adjuvant in draining lymph nodes in both intact whole lymph nodes and sectioned tissues, to analyze the localization of vaccine at both early (two days post immunization) and later (7 days p.i.) time points, to gain insights into how the physical form of immunogen and adjuvant influence localization within lymphoid tissues.
MATERIALS AND METHODS
Immunogen synthesis
MD39, a BG505 SOSIP trimer, was prepared as previously described [31,32]. Briefly, trimer genes containing C-terminal His-tags were co-transfected with human furin at a 2:1 trimer:furin DNA ratio using 293fectin into Freestyle 293-F cells (ThermoFisher). Supernatants were harvested five days post transfection by centrifugation and purified by affinity chromatography using HisTrap HP columns (GE Healthcare) followed by size-exclusion chromatography (SEC) using a S200 Increase column (GE Healthcare). Trimer molecular weight was confirmed by SEC multi-angle light-scattering (SECMALS) using DAWN HELEOS II and Optilab T-rEX instruments (Wyatt Technology). MD39-NPs, a nanoparticulate fusion of MD39 and ferritin from the hyperthermophilic archaeal anaerobe Pyrococcus furiosus, were produced and purified by lectin chromatography and SEC as previously described [27]. Particle formation was assessed by SECMALS and by staining MD39-8mer with 2% uranyl formate, gridding and imaging by negative stain electron microscopy on a Philips CM100 TEM with a Soft Imaging Systems MegaView III CCD and SIA model 12C CCD cameras.
For PET/MRI imaging, MD39-ferritin was labeled with copper-chelating agent DOTA-NHS-ester (Macrocyclics) in 0.1M sodium phosphate buffer (pH 7.3) previously treated with Chelex 100 chelating resin (BioRad), and allowed to react overnight at 4°C. The labeled product was purified using a Sephadex G-25 PD-10 Desalting column (GE) and stored at −80°C until use. For fluorescence imaging, MD39-ferritin or soluble MD39 were labeled with AlexaFluor647 antibody labeling kits (Thermo Fisher Scientific) and unreacted dye was removed according to the manufacturer’s instructions. Labeling conditions were optimized to achieve a mean labeling of ~1.1 AF647 dyes per MD39 trimer in each case.
Adjuvant synthesis
Amph-CpG.
DOTA-labeled amph-CpG 7909 adjuvant used for PET/MRI imaging studies (5'--Diacyl--T-s-dC-dG-T-s-dC-dG-T-s-T-s-T-s-T-s-dG-s-T-s-dC-dG-T-s-T-s-T-s-T-s-dG-s-T-s-dC-dG-T-s-dT--C6Amino--DOTA-3', where ‘s’ denotes phosphorothioate linkages) was prepared by solid phase synthesis by OligoFactory (Holliston, MA). The lipid moiety used to produce amph-CpG 7909 was bisstearamide CEP diacyl monomer, provided by Berry and Associates (Dexter, MI). The product was stored at −80°C until use. Sim ilarly, 3’-terminal amine-modified amph-CpG7909 used for fluorescence imaging (5'--Diacyl--T-s-dC-dG-T-s-dC-dG-T-s-T-s-T-s-T-s-dG-s-T-s-dC-dG-T-s-T-s-T-s-T-s-dG-s-T-s-dC-dG-T-s-dT--C6Amino-3’) was also synthesized by OligoFactory. This material was labeled with NHS-rhodamine (Thermo Fisher Scientific) according to the manufacturer’s instructions, then purified using a PD-10 desalting column (GE).
Saponin Nanoparticles
The saponin nanoparticle adjuvant used for antigen comparison and boost immunization studies was an ISCOM-like nanoparticle comprised of self-assembled cholesterol, phospholipid, and Quillaja saponin prepared as previously described [27]. All synthesis was performed under sterile conditions with sterile reagents. Briefly, 10 mg each of cholesterol (Avanti Polar Lipids 700000) and DPPC (Avanti Polar Lipids 850355) were dissolved separately in 20% MEGA-10 (Sigma D6277) detergent at a final concentration of 20 mg/ml and 50 mg Quil-A saponin (InvivoGen vacquil) was dissolved in deionized water at a final concentration of 100 mg/ml. Next, DPPC solution was added to cholesterol followed by addition of Quil-A saponin in rapid succession and the volume was brought up with PBS for a final concentration of 1 mg/ml cholesterol and 2 % MEGA-10. The solution was allowed to equilibrate at 25°C overnight, followed by 5 days of dialysis against PBS using a 10k MWCO membrane. The adjuvant solution was then filter sterilized using a 0.2 μm Supor syringe filter, concentrated using 50k MWCO centricon filters, and further purified by FPLC using a Sephacryl S-500 HR size exclusion column. Each adjuvant batch was finally characterized by negative stain TEM and DLS to confirm uniform morphology and size and validated for low endotoxin by Limulus Amebocyte Lysate assay (Lonza QCL-1000). Final adjuvant concentration was determined by cholesterol quantification (Sigma MAK043), assuming lipids and saponin incorporated into the particles in the same molar ratios as initially added to the synthesis. Doses of saponin adjuvant are reported in terms of the mass of saponin.
Radiochemistry
64Cu-chloride obtained from the University of Wisconsin-Madison was neutralized with previously Chelex®-treated 1M ammonium acetate buffer (Sigma) and the pH was adjusted to approximately 5.5. Radioactivity was measured using a Capintec CRC®-25R dose calibrator. An aliquot of 64Cu-chloride (~370 MBq) was mixed with DOTA-MD39 nanoparticles (53 μg, 1.06 mg mL−1) in ammonium acetate buffer (pH 5.5) and incubated for 1 h at 40°C in a circulating water bath. The reaction mixture was then loaded on to Zeba® desalting spin columns (40 kD MWCO, Thermo Fisher) and eluted with phosphate buffered saline (PBS, Thermo Scientific). The purity of eluted 64Cu-DOTA-MD39 nanoparticle sample was determined by instant thin layer chromatography (iTLC). As ferritin itself has the potential to bind to metal ions, we carried out initial tests of 64Cu binding to MD39-ferritin particles with vs. without DOTA labeling, and found that DOTA-MD39-ferritin bound to 8.5 μCi/μg protein, while MD39-ferritin bound only 1.8 μCi/μg protein. Thus, while there is some binding by ferritin itself, it was only ~20% of the labeling that could be achieved using DOTA tagging. An aliquot of 64Cu-DOTA-MD39 NP (25 μg mL−1 per administered dose) was mixed with AlexaFluor647-MD39-NP (25 μg mL−1), amph-CpG7909 (125 μg mL−1), and rhodamine-amph-CpG7909 (125 μg mL−1) to prepare the vaccine formulation (38 – 148 MBq in 1 mL of PBS). Amph-CpG-DOTA was loaded with 64Cu by the same procedure. Then an aliquot of 64Cu-DOTA-amph-CpG (125 μg mL−1 per administered dose) was mixed with rhodamine-amph-CpG7909 (125 μg mL−1), MD39-NP (25 μg mL−1), and AlexaFluor647-MD39-NP (25 μg mL−1) to prepare the vaccine formulation (125 – 147 MBq in 1 mL of PBS). All formulations were performed under standard aseptic conditions.
PET imaging studies
PET imaging study immunizations.
All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of UT Health San Antonio and followed National Institutes of Health guidelines for animal care. Indian origin rhesus macaques, Macaca mulatta, were anesthetized with ketamine hydrochloride (10 mg kg−1, I.M.) and maintained with isoflurane (1-3%, inhaled) mixed with 100% oxygen using an isoflurane vaporizer. Endotracheal intubation was performed following anesthesia induction. Animals breathed spontaneously throughout the procedure(s). Freshly prepared vaccine formulations were administered subcutaneously in 1mL of PBS midline at the anterior upper thigh 5 cm below the groin of anesthetized macaques prior to imaging. Group 1 (MD39-NP PET imaging, n=5) received 50 μg total MD39-NP (25 μg 64Cu-DOTA-MD39-NP + 25 μg AlexaFluor647-MD39-NP) and 250 μg total amph-CpG (125 μg unlabeled amph-CpG + 125 μg rhodamine-amph-CpG). Group 2 (amph-CpG PET imaging, n=3) received 50 μg total MD39-NP (25 μg unlabeled MD39-NP + 25 μg AlexaFluor647-MD39-NP) and 250 μg total amph-CpG (125 μg 64Cu-DOTA-amph-CpG + 125 μg rhodamine-amph-CpG). The animal’s body was immobilized in dorsal recumbency within a vacuum sealed veterinary positioner. Radioactive fiducial markers (0.5 – 2 MBq 64Cu standards) were attached to the animal positioner to assist the PET/MRI co-registration. Body temperature was maintained with a warm air blanket covering the animal (3M Bair hugger Model 505 warming unit). Continuous physiological parameter monitoring was performed to include measurements of end-tidal PCO2, electrocardiogram, heart rate, and respiratory rate within the 3T MRI Scanner as well as visual assessment for respiration, movement, and mucosal coloration in the PET Scanner. Upon completion of the PET scan, animals were allowed to fully recover from anesthesia and returned to their housing. All animals underwent 24 hr post vaccine injection (p.i) PET scans and some of the animals underwent scans at 3, 48, and 72 hr p.i. as well.
MRI Imaging.
All MRI studies were performed on a Siemens 3.0T TIM-Trio MRI scanner (Siemens Healthcare, Malvern, PA, USA) with large 6-channel body matrix phased-array coil and 12-channel spine matrix phased-array coil. The upper and lower body 3D images were acquired separately to cover the whole length of the animal with about 30% overlap between the two image sets. Two to three common fiducial markers were included in each set to assist the PET/MRI co-registration and image merging. The structural T1 images were acquired with a 3D Flash sequence (repetition time/echo time 6.33/1.51 ms, flip angle 10 deg, matrix 512 × 512, coronal, field of view 500 x 250 mm, slice thickness 1.2 mm, 128 slices without gap, 6 averages, accelerate factor 2 GRAPPA, and scan time 366 s).
In vivo PET Imaging.
Immediately after the MRI session, animals in the positioner were transferred to the PET scanner bed while maintaining their position. All whole-body PET images were acquired on a CTI EXACT HR+ scanner (Knoxville, TN). PET acquisitions were performed in 3D mode in an axial field of view of 15.5 cm with 63 - 2.5 mm contiguous slices. Emission data were corrected for decay, dead time, scatter, random coincidences and measured photon attenuation (with 68Ge/68Ga transmission scans) using the scanner software (ECAT version 7.2, CTI PET Systems, Knoxville, TN). Corrected image data were reconstructed using OSEM with 4 iterations and 16 subsets, applying a matrix size of 256×256 and a 5 mm FWHM standard Gaussian filter. All acquired image data were archived on an XNAT-powered data archival system for post-acquisition data analysis.
Necropsy, Ex vivo PET Imaging, and Tissue Preservation.
At the end of the last PET imaging session, animals were humanely euthanized with 100 mg kg−1 euthanasia solution (Euthasol®, i.v.) and the necropsy was performed by an experienced veterinary pathologist. Relevant harvested tissues (e.g. lymph nodes) were re-scanned using the PET imaging protocol described above. Isolated tissues were preserved in freshly prepared paraformaldehyde-lysine-periodate fixative (PLP) and stored at 4°C for further analysis by optical microscopy.
PET/MRI Data Analysis.
PET and MRI data analyses and PET/MRI co-registrations were performed using Multi-Image Analysis GUI (MANGO, Research Imaging Institute, UT Health San Antonio). Decay-corrected (to vaccine injection time) PET images were normalized by injected doses and body weight to produce Standardized Uptake Value (SUV) maps. Regions of Interest (ROIs) were drawn on target tissues by placing spherical outlines, free hand outline drawing, and/or by signal thresholding of tissue contours on the SUV maps. Statistical calculations were performed on these ROIs to determine SUVmax, SUVmean, and SUVsum. Three-dimensional (3D) image data were presented as color-coded maximum intensity projections (MIPs) of the SUVmax maps. Distal lymph node analysis was performed by drawing 3D ROIs for the injection site and most distal lymph node with significant detectable signal, which were then projected to a 3D surface-rendered PET image. A straight line was drawn connecting the SUVmax points of the injection site and the relevant lymph node on the 3D image to measure distance traveled by the vaccine.
Fluorescent immunogen immunization studies
Fluorescent antigen immunization studies were carried out at the Yerkes National Primate Research Center (YNPRC) at Emory University. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Emory University and followed National Institutes of Health guidelines for animal care. For soluble MD39 trimer vs. MD39-NP comparisons, groups of rhesus macaques were immunized on day 0 with 50 μg AlexaFluor647-conjugated MD39 soluble trimer and 187.5 μg saponin adjuvant in the right and left inner thighs (same location as the PET imaging studies), or 50 μg AlexaFluor647-MD39-NP and 187.5 μg saponin adjuvant in right and left inner thighs. Animals were sacrificed and lymph nodes collected for analysis two or seven days post immunization. For studies assessing the impact of pre-existing antibodies on immunogen trafficking, a group of macaques previously immunized with a related SOSIP trimer according to the schedule shown in Figure S8A were immunized with 50 μg AlexaFluor647-MD39 soluble trimer and 187.5 μg saponin adjuvant in the right and left inner thighs. Animals were sacrificed and lymph nodes collected for analysis two or seven days post immunization.
Ex vivo tissue fixation
All NHP LNs were harvested and immediately placed in PLP buffer (pH 7.4 50 mM PBS + 100 mM lysine, 1% paraformaldehyde, 2 mg/mL sodium periodate) for fixation. After 4-5 days at 4°C, tissues were washed and stored in PBS with 0.0 5% sodium azide at 4°C until imaging.
Whole organ fluorescence measurement
Total antigen signal within LNs was measured by placing the tissues directly on the glass scanning surface of a Typhoon FLA 9500 biomolecular imager (GE Healthcare Life Sciences) and imaging using a 635 nm excitation laser and a ≥665 nm long-pass filter. The integrated signal density corresponding to AlexaFluor647-MD39 in each LN was calculated using ImageJ and plotted using GraphPad Prism 8.
Whole organ clarification
Selected LNs were clarified via a hybrid protocol, merging aspects of iDISCO [33] and CUBIC [34] organ-clearing methods. The LNs were first delipidated based on the iDISCO methanol incubation protocol: Tissues were washed in water for 1 hour, then 20% methanol in water for 2 hours. A series of step increases in methanol percentage (40%, 60%, 100%, 100%) followed, each step for 2 hours. The LNs were then placed into 2:1 MeOH:DCM overnight, and the next day were rehydrated with the following series of methanol solutions for 2 hours each: 100%, 100%, 80%, 60%, 40%, 20%, 0%, 0%. Next, the LNs were placed into 10-20 mL of a 1:1 mixture of CUBIC-R solution for 1 day, followed by at least 20 mL of undiluted CUBIC-R for 2 days or as long as needed for adequate clarification. Larger organs were moved into a fresh 20 mL of CUBIC-R solution to ensure that the refractive index of the solution would not be significantly lowered by residual water in the tissue.
Light sheet microscopy
Clarified LNs were imaged in CUBIC-R solution using a LaVision Ultramicroscope II Light Sheet Microscope at 1.25x optical zoom. The AlexaFluor647-labeled antigen was imaged using the 640 nm laser and the rhodamine-labeled adjuvant was imaged using the 561 nm laser at 100 ms exposure time on an Andor Neo camera. Snapshots and movies were generated using the 3D viewer in the FIJI package of ImageJ.
Immunofluorescence staining
Selected LNs were embedded in 3% low melting temperature agarose (Sigma-Aldrich), and then sliced into 350 μm-thick sections using a vibratome. The slices were blocked and permeabilized overnight in PBS with 5% mouse serum, 5% rat serum, and 0.2% Triton-X-100, followed by staining for 3 days at 37 °C with 1:100 dilutions of BrilliantViolet421-labeled mouse anti-human CD35 clone E11 (BD Biosciences) and AlexaFluor488-labeled mouse anti-Ki67 clone B56 (BD Biosciences) in the same buffer as the blocking/permeabilization step. Stained slices were then washed for 3 days at room temperature with PBS containing 0.2% Tween-20, and then mounted onto glass slides with Prolong Glass antifade mountant (Thermo Fisher Scientific).
Confocal microscopy
Imaging was performed on either a Leica SP8 or an Olympus FV1200 laser scanning confocal microscope using 10x objectives. Images were analyzed using ImageJ.
Statistics.
Data were statistically analyzed by one-way or two-way ANOVA or by unpaired t-test as indicated in the figure captions using GraphPad Prism.
RESULTS
Inner thigh immunization leads to prominent vaccine delivery to inguinal and iliac lymph nodes
We first used PET imaging to visualize the biodistribution behavior of an HIV Env trimer immunogen and molecular adjuvant in rhesus macaques, where each component of the vaccine was prepared in a form designed to promote lymph node targeting. MD39, a hyperstabilized gp140 SOSIP trimer, was fused to an archaeal ferritin, forming a nanoparticle (MD39-NP) presenting eight MD39 trimers [27] with a size (~30 nm diam.) promoting lymphatic trafficking [35]. As an adjuvant, we prepared an amphiphilic lipid-functionalized single-stranded CpG DNA, using a sequence (CpG 7909) selected for stimulation of Toll-like receptor 9 in NHPs and humans [36-38]. This amph-CpG molecule was designed to traffic to lymphatics via binding of the phospholipid tail to endogenous albumin present in interstitial fluid, and in prior studies showed efficient lymph node targeting in mice [3,28]. To enable parallel PET and fluorescence imaging, MD39-NP and amph-CpG were labeled with DOTA as a chelator for 64Cu or with a fluorescent dye. MD39-NP was labeled using N-hydroxysuccinimide dye/DOTA, while amph-CpG was labeled at the 3’ terminus of the CpG with a single DOTA or rhodamine molecule (Figure S1A-B). DOTA-labeled molecules were then radiolabeled with 64Cu with greater than 95% purity determined by iTLC (95.40-99.70%, see Methods). 64Cu-loaded DOTA-MD39-NPs were stable for at least 24 hr, as evidenced by no drop in radiochemical purity by iTLC and no change in particle size by DLS (Figure S1C). We expected that amph-CpG-DOTA could be less stable in vivo, despite stabilization of the oligonucleotide backbone by phosphorothioate linkages. To assess stability of this conjugate, we incubated 64Cu-loaded amph-CpG-DOTA in rhesus serum for 24 hr at 37°C and then re-measured radiochemical purity by iTLC. This analysis showed a loss of ~40% of the intact conjugate with release of 64Cu or hydrolysis of the DOTA (Figure S1D). However, because free 64Cu or 64Cu/DOTA are rapidly cleared in vivo, we did not expect this degradation to impact interpretation of the PET scans, and we chose an injected dose to ensure sufficient intact amph-CpG signal for quantification.
For in vivo imaging, the injected dose (ID) ranged from 38.48-148.74 MBq for 64Cu-DOTA-MD39-NP and 125.06-147.26 MBq for 64Cu-DOTA-amph-CpG (Table S1). As illustrated in Figure 1A, one group of animals was immunized with 64Cu-DOTA-labeled MD39-NP mixed with fluorescent MD39-NP and fluorescent amph-CpG, while a second group received 64Cu-DOTA-labeled amph-CpG mixed with fluorescent vaccine. A secondary goal of this study was to evaluate the ability of vaccines to be targeted to putative reproductive tract/rectum-draining lymph nodes that might promote mucosal immunity. Thus, we administered vaccines subcutaneously in the upper inner thigh of the right leg to assess the potential of targeting vaccine to inguinal, iliac, and possibly deeper internal lymph nodes. All animals underwent whole-body PET and MRI scans at 24 hr, followed by an ex vivo post-necropsy PET scan to obtain 64Cu signals from isolated lymph nodes and other tissues. Initial pilot immunizations tracking 64Cu signal over 3 days revealed accurately quantifiable PET signals for scans up to 48 hr; at 72 hr the rapid decay of 64Cu led to low total signals that were unreliable for quantification. Thus, we confined the majority of our analysis to 24-48 hr post injection, and necropsied animals after 48 hr PET scans for ex vivo imaging (Figure 1A). Dual MRI and PET scanning was performed in order to generate co-registered images that were used to isolate, identify, and quantify regions of interest (ROIs) corresponding to lymph nodes and other tissues (Figure S2). Following quarantine to allow radioactive decay, the isolated tissues were processed for subsequent histology and whole-tissue fluorescence imaging.
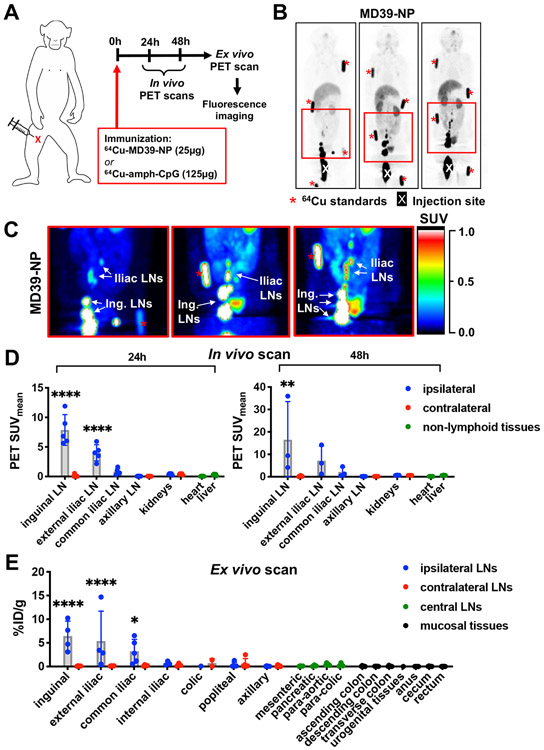
Figure 1. Accumulation of MD39 trimer-nanoparticle immunogen in inguinal and iliac lymph nodes of macaques.
A) Immunization scheme for PET and MRI imaging studies. Group 1 (n=5) received 50 μg total MD39-NP (25 μg 64Cu-DOTA-MD39-NP + 25 μg AF647-MD39-NP) and 250 μg total amph-CpG7909 (125 μg unlabeled amph-CpG7909 + 125 μg rhodamine-amph-CpG7909). Group 2 (n=3) received 50 μg total MD39-NP (25 μg unlabeled MD39-NP + 25 μg AF647-MD39-NP) and 250 μg total amph-CpG7909 (125 μg 64Cu-DOTA-amph-CpG7909 + 125 μg rhodamine-amph-CpG7909). B) Maximum Intensity Projections (MIPs) of representative in vivo PET scan at 24h showing 64Cu-DOTA-MD39-NP signal. “X” denotes injection site, red asterisks indicate fiducial markers. C) Pseudocolor images of boxed areas shown in (B), highlighting drainage to iliac and inguinal lymph nodes (LNs). D) Radiolabeled MD39-NP signal from in vivo PET scan of whole animals at 24h (n=5) and 48h (n=3) quantified as mean standardized uptake value (SUVmean) of gated ROIs; statistical significance compared to liver. E) Radiolabeled MD39-NP signal from ex vivo PET scan of isolated tissues post-necropsy at 48h quantified as percent injected dose (%ID) normalized to excised tissue weight (n=5); statistical significance compared to non-draining left axillary lymph node. All statistical significance determined by ordinary one-way ANOVA followed by Dunnett’s post-hoc test (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
We first imaged the biodistribution of PET-labeled MD39-NPs co-administered with amph-CpG adjuvant. As shown in Figure 1B, at 24 hr post immunization, a substantial pool of MD39-NPs remained in the vicinity of the injection site (approximately 60% of the injected dose). However, clear preferential accumulation of the Env nanoparticles was also observed in ipsilateral inguinal and iliac lymph nodes (Figure 1B-D and Videos S1 and S2). No antigen signal was detected in contralateral inguinal/iliac LNs, and signals were much lower in systemic tissues such as the gut, heart, liver, or kidneys and not above background in more distal nodes (e.g., axillary) (Figure 1B-D, Figure S3A and Videos S1 and S2). At 48 hr post immunization, the qualitative biodistribution of MD39-NPs remained unchanged (Figure S4A). Antigen signals decayed or remained approximately constant in the lymph nodes showing antigen accumulation, and further accumulation at more distal LNs was not detected (Figure 1D, Figure S3B). Ex vivo PET scans on isolated tissues at 48 hr were in concordance with the in vivo imaging observations, with significant uptake of the nanoparticle antigen detected in multiple inguinal, external iliac, and common iliac LNs (Figure 1F). In terms of total injected dose, ex vivo PET scans revealed mean accumulations of 2-4% of the antigen in the proximal draining lymph nodes (Figure S3C-D).
We next visualized dissemination of DOTA-labeled amph-CpG adjuvant using PET imaging. Amph-CpG appeared to clear from the immunization site more rapidly than the Env trimer nanoparticles, with only 25% of the injected dose remaining at the injection site 24 hr after immunization (Figure 2A). Clear accumulation in inguinal and iliac lymph nodes was visualized in the live animals (Figure 2B-C, Figure S5A, and Videos S3 and S4). The albumin-binding adjuvant also trafficked further than MD39-NPs, with adjuvant signal detected in common and internal iliac LNs, and even low levels of signal in para-aortic nodes up to 17 cm away from the immunization site (Figure 2D and Figure S6). This more distal dissemination of amph-CpG up the LN chain was confirmed by ex vivo imaging of tissues at 48 hr (Figure 2F). Similar to the findings from imaging MD39-NP, 2-5% of the injected dose accumulated in the proximal draining nodes (Figure S5B-C). At 48 hr post immunization, the qualitative biodistribution of amph-CpG remained unchanged (Figure S4B). Altogether, these data demonstrate that s.c. inner thigh immunization was effective for rapidly delivering vaccine antigen and adjuvant to inguinal and iliac lymph nodes.
Figure 2. Accumulation of amph-CpG adjuvant in inguinal and iliac lymph nodes of macaques.
A) Percent injected dose (%ID) of 64Cu-DOTA-MD39-NP (n=5) and 64Cu-DOTA-amph-CpG (n=3) remaining at site of injection (SOI) at 24h, determined from in vivo PET scan. Statistical significance determined by Welch’s t-test (*p=0.0266). B) Maximum Intensity Projections (MIPs) of in vivo PET scan at 24h showing 64Cu-DOTA-amph-CpG signal in macaques with region of interest highlighted. “X” denotes injection site, red asterisks indicate fiducial markers. C) Zoomed-in pseudocolor image of 64Cu-DOTA-amph-CpG signal in boxed area shown in (B), highlighting drainage to iliac and inguinal LNs. D) Average distances traveled by 64Cu-DOTA-MD39-NP or 64Cu-DOTA-amph-CpG, determined by calculating distance from SOI to the most distal LN with significant PET signal. Statistical significance determined by Welch’s t-test (*p=0.0390). E) Radiolabeled amph-CpG signal from in vivo PET scan of whole animals at 24h (n=4) and 48h (n=2) quantified as mean standardized uptake value (SUVmean) of gated ROIs; statistical significance compared to liver. F) Radiolabeled amph-CpG signal from ex vivo PET scan of isolated tissues post-necropsy at 48h quantified as percent injected dose (%ID) normalized to excised tissue weight; statistical significance compared to non-draining left axillary lymph node. Statistical significance determined by ordinary one-way ANOVA followed by Dunnett’s post-hoc test (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001) (n=3).
HIV Env trimer nanoparticles concentrate in the follicles of macaque lymph nodes
In the experiments described above, each of the radiolabeled vaccines was co-administered with fluorescently labeled MD39-NP and amph-CpG to permit subsequent fluorescent imaging. We thus next examined the uptake of MD39-NP and amph-CpG within tissues recovered from the PET-imaged NHPs to define their localized biodistributions within the draining lymph nodes. We selected iliac and inguinal LNs that were positive for antigen/adjuvant signal based on both ex vivo PET scans and fluorescence signals collected with a high-throughput flatbed Typhoon fluorescence scanner. Vaccine fluorescence signals showed the same patterns of LN accumulation as observed for ex vivo PET signals for both MD39-NP and amph-CpG, indicating consistent trafficking behavior from PET- or fluorophore-labeled vaccine (Figure S7A-D). Additionally, there was a strong correlation between the fluorescence signal of MD39-NP and amph-CpG in all lymph nodes (Figure 3A). We first sectioned selected LNs that showed median levels of antigen accumulation by ex vivo PET into 100 μm thick slices and immunostained for CD35, a marker characteristic of follicular dendritic cells (FDCs) to identify B cell follicles, and imaged slices by confocal microscopy. Env trimer NPs and amph-CpG showed very distinct patterns of accumulation in draining nodes: While the CpG adjuvant signal localized in the deeper parenchyma of the lymph nodes and surrounding some follicles, MD39-NP were observed in a half-moon-like distribution ringing the FDC networks of follicles (Figure 3B-C). As expected from the PET imaging, no signal for either antigen or adjuvant were detected in contralateral LNs (Figure 3B). We previously observed MD39-NP concentration in follicles in mice [27], but in mice the trimer nanoparticles accumulated more uniformly across the FDC network, whereas in NHPs we observed peripheral accumulation around the rim of the follicles.
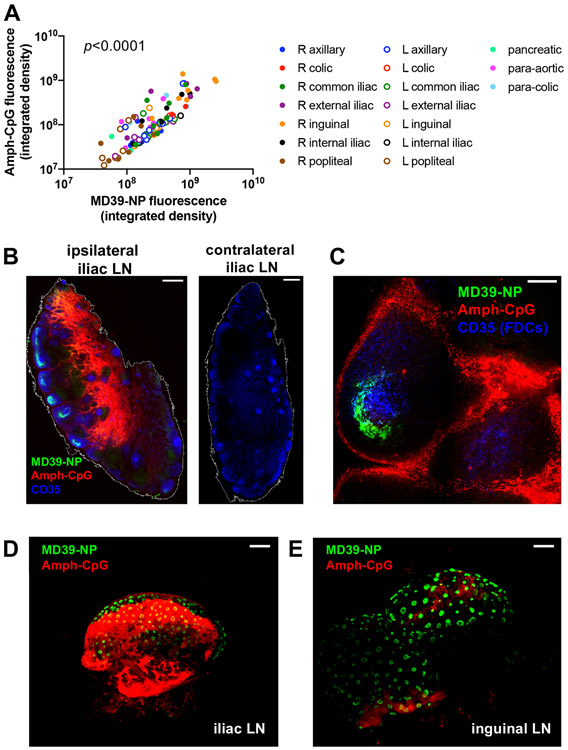
Figure 3. Distribution and localization of MD39-NP immunogen and amph-CpG adjuvant within lymph nodes.
A) Correlations between AF647-MD39-NP and Rho-amph-CpG fluorescence signal in individual lymph nodes measured on a Typhoon flatbed scanner (p<0.0001, Pearson’s coefficient r2=0.5893). B) Comparison of right and left external iliac LNs from a single animal. Scale bar represents 500 μm. C) Higher magnification view of a follicle from the same right external iliac LN shown in panel B. Scale bar represents 100 μm. D-E) Maximum projections of representative whole cleared iliac (D) and inguinal (E) LNs as imaged by light sheet microscopy. Scale bars represent 1 mm.
To gain further insight into antigen and adjuvant distribution within the tissues, we established methods to clear whole macaque lymph nodes for whole-tissue imaging by light sheet microscopy. In preparation for imaging the selected samples, we evaluated a variety of published tissue clearing protocols for their capacity to enable high signal-to-noise imaging in the wavelengths used for the antigen and adjuvant dye labels with minimal autofluorescence on fixed NHP LN tissues. We found that an optimal protocol involved removing lipids with organic solvents [33], followed by rehydrating the tissue and raising the refractive index of the solution to match the tissue [34]. As shown for representative iliac (Figure 3D, Video S5) and inguinal (Figure 3E, Video S5) lymph nodes, MD39-NPs showed a striking localization in cup-like morphologies, the three-dimensional equivalent of the half-moon patterns observed around the periphery of follicles in the 2D tissue sections. Env NPs were observed in hundreds of follicles across a single lymph node. By contrast, amph-CpG was distributed widely in the LNs and exhibited localization in the lymph node interior/paracortex in some nodes, although also with greater variation by tissue. Altogether, these data suggest that Env NP immunogens are rapidly concentrated in follicles following immunization in NHPs, while CpG adjuvant disperses more broadly in macaque LN tissues.
Env trimer-nanoparticles exhibit greater LN accumulation and follicular targeting than soluble trimers following primary immunization
Given the striking concentration of MD39-NPs observed both in tissue sections and cleared whole LN organs, we next sought to compare the trafficking of the ferritin particle fusion immunogen with free MD39 trimers. Groups of macaques were immunized with AlexaFluor-labeled MD39 or MD39-NP at an equivalent molar dose of Env trimer, combined with an ISCOM-like saponin adjuvant we have previously found elicits potent humoral responses in NHPs [14,39]. Two or seven days later, animals were necropsied and lymph nodes were collected for analysis (Figure 4A). Total antigen accumulation in LNs was first assessed by imaging whole fixed LNs on a flatbed laser fluorescence scanner. As NHPs show a variable number of lymph nodes at a given draining site (e.g., inguinal or iliac nodes), we pooled the LNs from each draining site to measure the average total antigen accumulation in distinct drainage areas. This analysis revealed that antigen accumulation was readily detectable in inguinal and iliac LNs: On day 2, the amount of MD39 and MD39-NP in iliac LNs, along with MD39-NP in inguinal LNs, was significantly greater than that detected in non-draining axillary LNs (Figure 4B). By day 7, antigen signal decayed by ~80% in draining LNs (Figure 4B).
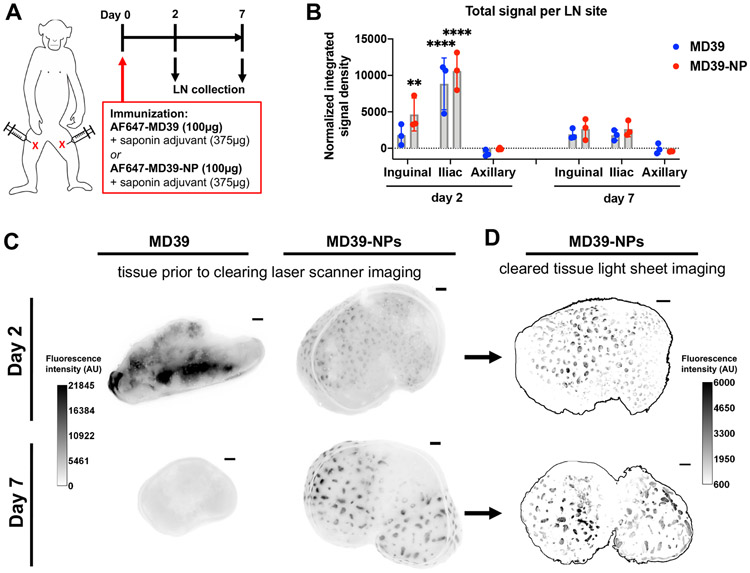
Figure 4. Env trimer nanoparticles but not free trimer exhibit follicular concentration following a prime immunization.
A) Immunization timeline: Rhesus macaques (n=3/group) were immunized on day 0 with a total dose of 100 μg AF647-MD39 + 375 μg saponin adjuvant or 100 μg AF647-MD39-NP + 375 μg saponin adjuvant, administered subcutaneously in both left and right upper inner thighs. Animals were sacrificed and LNs collected two and seven days after immunization. B) Quantification of total fluorescent signal within fixed LNs from each collection site as measured using a fluorescent laser flatbed scanner. Statistical significance compared to non-draining axillary lymph node determined by two-way ANOVA followed by Dunnett’s post- hoc test (**p<0.01, ****p<0.0001). C, D) Comparison of LNs from MD39-NP-receiving animals before and after clarification imaged by laser scanner prior to tissue clearing (C) and the LNs imaged after tissue clearing by light sheet microscopy (D), respectively. Light sheet microscopy images are maximum projections through an entire LN stack. Scale bars represent 1 mm.
In addition to an integrated measure of total antigen signal, the laser scanner also provided a low-resolution image of the pattern of antigen accumulation in the whole tissues. As shown in Figure 4C, free MD39 trimer showed a diffuse, inhomogeneous pattern of signal in the fixed tissues, which decayed from day 2 to day 7. By contrast, a clearly punctate pattern of MD39-NP antigen signal was observed at both time points (Figure 4C). To gain further insight, we next carried out tissue clearing and imaged the same LNs by light sheet microscopy. Strikingly, cleared LNs from animals receiving free trimer showed near-zero antigen signal above background (Video S6). This finding suggests the majority of antigen signal in these lymph nodes may have been localized in lymph fluid in the peripheral sinuses, where it would not be immobilized during fixation and thus would be extracted during the tissue clearing treatment. By contrast, the cup-like follicular concentration of MD39-NPs was observed here as in the prior study (Video S6 and Figure 4D).
To complement the cleared tissue imaging, we also carried out confocal microscopy of 100 μm-thick sections of LN tissues to detect low levels of antigen at higher resolution. Samples were first imaged by the flatbed fluorescence scanner for a low-resolution view of trimer signal, followed by sectioning for confocal imaging of the same samples. As shown in Figure 5A, two days post immunization, LNs exhibiting diffuse MD39 trimer signal via laser scanner imaging showed detectable antigen signal in the LN parenchyma when imaged at high resolution, albeit at low levels, and dispersed through interfollicular areas, reminiscent of prior reports of trimer distribution in mouse LNs [26,27]. By day 7, soluble trimer signal was decreased but could be detected in FDC networks, and Ki67 staining revealed the initiation of germinal centers (Figure 5B; note Figure 5B(iii) highlighting antigen signal as an isolated channel in false color). In contrast, MD39-NP was detected ringing CD35+ follicles at both time points, mirroring the findings from the previous study (Figure 5C-D). Notably, the trimer nanoparticles accumulated on the side of follicles opposite the prominent Ki67 staining marking areas of B cell proliferation in the dark zone of developing germinal centers, suggesting concentration in the light zone of GCs (Figure 5D). Thus, Env trimer nanoparticles, but not soluble trimer, efficiently accumulated in follicles as early as 2 days post immunization.
Figure 5. Env trimer nanoparticles accumulate in the periphery of the FDC network of follicles while soluble Env trimer is dispersed through lymph nodes following primary immunization.
(A-D) Representative lymph nodes positive for antigen signal were imaged by flatbed fluorescence scanner (i) and then sliced as 100 μm sections for confocal imaging. Shown are example whole slices or selected follicles (ii) and isolated antigen fluorescence channel in false color (iii). A) Iliac LN 2 days post immunization with MD39 trimer. Scale bars: (i) 2.5 mm, (ii) 1 mm, (iii) and inset 100 μm. B) Iliac LN 7 days post immunization with MD39 trimer. Scale bars: (i) 2.5 mm, (ii) and (iii) 100 μm. C) Iliac LN 2 days post immunization with MD39-NP. Scale bars: (i) 2.5 mm, (ii) and (iii) 100 μm. D) Iliac LN 7 days post immunization with MD39-NP. Scale bars: (i) 2.5 mm, (ii) 1 mm, (iii) and inset 100 μm.
Soluble Env trimers exhibit follicular targeting in animals with pre-existing anti-Env immunoglobulin
The striking follicular trafficking of MD39-NP mirrors our recent findings of follicular trafficking of the same nanoparticle in mice, which we showed was dependent on mannose binding lectin recognition and complement deposition on the particles [27]. As immune complexes have also been shown to be transported to the FDC network in mice mediated by antibody-triggered complement deposition [24,40], we hypothesized that even soluble Env trimers might exhibit concentration in follicles of animals that had pre-existing antibodies against the immunogen. To test this, we utilized a group of rhesus macaques that had previously been immunized three times with BG505 SOSIP Olio6CD4 KO [14,41], a stabilized Env SOSIP trimer highly related in sequence to MD39. We first confirmed these animals had high levels of circulating IgG that recognized MD39 trimer by ELISA (Figure S8). We anticipated that the presence of high antigen-specific antibody titers in these animals would lead to immediate immune complex formation upon injection of soluble MD39, and potentially induce follicular homing of the immunogen as observed with the MD39-NP. These animals were immunized with AlexaFluor-labeled MD39 combined with saponin adjuvant and draining LNs were harvested 2 or 7 days post injection (Figure 6A). Similar to the earlier studies, we analyzed antigen uptake in lymph nodes first by fluorescence scanner imaging of fixed tissues, followed by tissue clearing and whole-organ light sheet imaging.
Figure 6. Soluble Env trimer immunization in pre-immune animals leads to rapid antigen concentration in follicles.
A) Immunization timeline: Rhesus macaques (n=3/group) were primed or boosted on day 0 with a total dose of 100 μg AF647-MD39 + 375 μg saponin adjuvant, administered subcutaneously in both left and right upper inner thighs. Boost animals were previously immunized with BG505 SOSIP Olio6 and exhibited pre-existing circulating MD39-specific IgG. Animals were sacrificed and LNs collected two and seven days after immunization. B) Quantification of total fluorescent signal from fixed LNs at each collection site as measured using a fluorescent laser flatbed scanner. Dots represent pooled total fluorescence from one animal at the indicated site (minus naïve signal). Statistical significance determined by two-way ANOVA followed by Sidak’s or Dunnett’s post-hoc test (*p<0.05, ****p<0.0001). C) Comparison of LNs from MD39 boost animals before and after clarification, imaged by laser scanner (left) and light sheet microscopy (right), respectively. Light sheet microscopy images are maximum projections through an entire LN stack. Scale bars represent 1 mm.
As observed with the earlier experiments in naïve animals, substantial antigen signal accumulated in inguinal and iliac LNs by day 2. Figure 6B shows quantified signal in animals that were previously immunized and boosted (‘post-boost’) compared to signal in naïve animals immunized only once with MD39 (‘post-prime’, i.e. soluble trimer data from Figure 4B). At day 2, both post-prime and post-boost animals exhibited high levels of MD39 in iliac lymph nodes, while boosted animals also showed significant levels of antigen in inguinal LNs. Antigen levels in inguinal and iliac LNs decayed by day 7 but were still readily detectable above background (Figure 6B). Whole-tissue images from the fluorescence scanner revealed that antigen-positive LNs from previously immunized animals, both 2 and 7 days post-boost with soluble trimer, were characterized by the same punctate pattern of antigen concentration previously observed only for MD39-NP (Figure 6C). Antigen concentration in follicles was also revealed across entire lymph nodes by light sheet imaging of the cleared tissues in a pattern similar to that observed for MD39-NP, at both 2 and 7 days post-boost (Figure 6C, Video S7). Thus, in agreement with findings from small animals, soluble HIV Env immunogens captured in immune complexes in vivo are also rapidly transported to B cell follicles.
DISCUSSION
The biodistribution of subunit vaccines at the organismal and tissue levels controls many facets of the outcome of immunization, influencing safety and the magnitude of the immune response. Further, priming of T and B cell responses in lymphoid tissues draining mucosal sites programs homing of memory and plasma cells to the mucosal portals of pathogen entry [42]. For HIV, prior studies have suggested that vaccine delivery to iliac lymph nodes draining the genitourinary tract could lead to establishment of antigen-specific cytotoxic T cells in the vaginal and rectal tissues [43], and achieve enhanced protection from mucosal SIV challenge in macaques [10]. These findings are consistent with more recent analyses of lymphatic drainage in macaques suggesting that iliac lymph nodes drain the vaginal and rectal mucosa [29]. However, attempting to concentrate vaccine near deep iliac lymph nodes through needle injection is non-trivial.
These considerations motivated us to assess whether formulations that promote efficient trafficking of antigens/adjuvant into lymphatics could enable vaccine transport to key genitourinary tract-draining mucosal lymph nodes from an easily accessible subcutaneous injection site. Using a combination of whole animal PET and tissue-level fluorescence imaging in the rhesus macaque model, we assessed the impact of two distinct technology platforms for promoting lymph node targeting of vaccines, protein nanoparticles and albumin-binding molecular conjugates, on the fate of an HIV Env trimer immunogen and the molecular adjuvant CpG, respectively. Protein particles displaying antigen are being intensively studied for their capacity to enhance B cell triggering and augment the humoral immune response in preclinical models [27,44-48] and nanoparticle immunogens, including ferritin particles, are being assessed in humans (clinicaltrials.gov Identifiers: NCT03547245, NCT03186781, NCT04579250, NCT03814720). PET imaging revealed trafficking of both antigen and adjuvant to inguinal and iliac lymph nodes extending up the lymphatic chain from the injection site. Histology and whole cleared tissue imaging on individual lymph nodes in the drainage path revealed that while both Env nanoparticles and amph-CpG accumulated in draining lymph nodes, they exhibited very distinct distributions, with CpG distributing in the parenchyma and surrounding follicles, while the trimer NP immunogen exhibited a striking accumulation around the periphery of B cell follicles. Such follicular targeting was not detected for the equivalent soluble trimer in naïve animals, but was recapitulated in animals with pre-existing high levels of antigen-specific antibodies administered the soluble trimer.
Increasing the number of lymph nodes receiving a vaccine could be envisioned to enhance the immune response by allowing more independent germinal centers to develop and enabling antigen-specific cells to develop without intense competition for cytokines and other factors within a single lymph node. In small animals, lymph-targeted vaccines have been shown to distribute substantial distances through lymphatics (reaching from injection sites near the tail to axillary lymph nodes an entire body length away) [3,28], and small nanoparticles administered parenterally can reach the thoracic duct and hence the systemic circulation [49]. However, much less is known about vaccine biodistribution in larger animals closer in physiology to humans. In macaques, liposomal antigens and antigens administered with alum or oil-in-water emulsion adjuvants have been reported to exhibit a relatively restricted biodistribution following i.m. or s.c. immunization [50,51]: For example, when fluorescently-labeled liposomal Env antigens were administered s.c. over the quadriceps, histological analysis focused on the inguinal and iliac lymph nodes as the expected immediate primary and secondary draining sites revealed vaccine drainage almost exclusively to the inguinal LN, with no uptake in iliac nodes above background.
PET imaging is well suited to provide insight into vaccine trafficking as it combines high sensitivity, reasonable resolution, and very low background. A recent study analyzed the biodistribution of an mRNA vaccine expression in macaques, where mRNA encoding a PET reporter gene was complexed with cationic lipids to form ~320 nm diam. nanoparticles and administered in the quadriceps of macaques [52]. This analysis revealed transfected cells in the injection site, inguinal, iliac, and para-aortic lymph nodes at an early timepoint of 4 hr, suggesting direct transfection of cells at these sites. The most distal lymph nodes reached by this vaccine were 9.2 cm away from the injection site. Similarly, in the present study PET imaging revealed the nanoparticle Env immunogen accumulated in numerous inguinal and iliac lymph nodes extending ~10 cm from the injection site. The albumin-binding CpG adjuvant trafficked further up the lymphatic chain, reaching lymph nodes 17 cm from the immunization site. Antigen was largely undetectable outside of the draining lymphatic chain and immunization site. Variable numbers of lymph nodes are detected at each inguinal and iliac region in macaques, but we found significant vaccine uptake in at least half a dozen lymph nodes per animal. Ex vivo PET scanning greatly facilitated the identification and analysis of vaccine+ lymph nodes during necropsies; ensuring that all lymph nodes are identified and isolated in a typical animal necropsy can be extremely challenging.
To allow tracking of MD39-NPs at the tissue level in parallel to whole-animal PET imaging, we mixed DOTA-labeled MD39-NPs with fluorophore-labeled particles, to allow the dosing ratio of the PET agent and fluorescence tracer to be tightly controlled. This does raise the possibility that DOTA-labeled particles and fluorophore-labeled particles have somewhat different biodistributions due to the different labels used. However, as shown in Figure S7C-D, the PET and fluorescence signals in lymph nodes for both MD39-NP and amph-CpG were highly correlated at the tissue level. Further, we have observed in our prior studies that fluorophore labeling did not alter the trafficking of ferritin nanoparticles in mouse lymph nodes [27], and hence we expect the labeling had minimal impact here.
Most notably, we observed that within individual lymph nodes, the Env trimer nanoparticle immunogen localized to dozens to hundreds of follicles, suggesting that antigen would be readily available to support ongoing germinal center reactions. We recently reported similar localization of the same ferritin-Env trimer particle immunogen to B cell follicles in mice, and showed this trafficking was driven by mannose binding lectin-mediated recognition of Env glycans and subsequent complement deposition on the particles [27]. A key question was whether follicular localization of such particulate immunogens would also be active in macaques, which are genetically much closer to humans, and how it would manifest in macaque lymphoid tissues that are much larger than mice and contain many more follicles. We recently reported initial findings with a two-component Env trimer protein nanoparticle, which exhibited follicular localization following vaccination in non-human primates [53]. Here we found that whole-tissue, cleared tissue, and histological imaging all showed that while soluble Env trimer was dispersed at low levels through the lymph node without notable localization in follicles, Env trimer-ferritin NP exhibited clear concentration in cup-like morphologies around many follicles in each lymph node (in many cases, exceeding 100 follicles). Preferential trafficking of the NP Env immunogen to follicles in naïve animals is consistent with a role for MBL-mediated recognition of these heavily glycosylated particles as observed in mice. This pattern of trimer accumulation was also observed when animals with pre-existing high levels of trimer-specific antibody were immunized with fluorescent soluble trimer. In the latter case, FDC trapping of antigen is expected to be mediated by immune complex (IC) formation and complement deposition on ICs, as described in mouse models [40]. This pattern of antigen accumulation mirrors our observations in a previous study where macaques were immunized with either slow-release osmotic pumps or through “extended dosing” immunization (where a given dose of vaccine is administered through injections spread over 2 weeks) [14]– we expect in these slow delivery immunizations that antigen capture on the FDC network is also mediated by immune complex formation.
CONCLUSIONS
Altogether, these results demonstrate that in large animals, subunit vaccines access a significant pool of lymph nodes from a common s.c. immunization site, and can reach internal lymph nodes many cm away from the actual site of immunization within 24 hr. Further formulation of antigens in a nanoparticle form alters the trafficking of antigen within lymph nodes and can lead to pronounced accumulation in B cell follicles. Such follicular localization is associated with enhanced germinal center responses and the production of long-lived plasma cells in mice [27]. This follicular localization that was found to formally depend on mannose binding lectin-mediated recognition of dense glycosylation of the particulate immunogens in mice is relevant for diverse antigens ranging from HIV to influenza to SARS-CoV-2 [54], due to the prevalence of glycosylation on viral spike proteins that are important protective antigens.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NIH (award P01AI048240 to RMR, SCK, PTF, and DJI, award UM1 AI144462 to DJI and WRS), the Ragon Institute of MGH, MIT, and Harvard, the U. S. Army Research Office through the Institute for Soldier Nanotechnologies at MIT, under Cooperative Agreement Number W911NF-18-2-0048, and the Koch Institute Support (core) Grant P30-CA14051.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
WRS is an inventor on pending patents related to the design of the MD39 and MD39-NP immunogens. DJI is an inventor on patents related to the amph-CpG adjuvant that are licensed to Elicio Therapeutics. DJI is a consultant and holds equity in Elicio Therapeutics.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- [1].Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F, Persistent Antigen and Germinal Center B Cells Sustain T Follicular Helper Cell Responses and Phenotype, Immunity. 38 (2013) 596–605. 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- [2].Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, Hu JK, Kumari S, Crampton J, Baldeon AD, Sanders RW, Moore JP, Crotty S, Langer R, Anderson DG, Chakraborty AK, Irvine DJ, Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination, Proceedings Of The National Academy Of Sciences Of The United States Of America. 113 (2016) E6639–E6648. 10.1073/pnas.1606050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Egeren DSV, Park C, Irvine DJ, Structure-based programming of lymph-node targeting in molecular vaccines, Nature. (2014) 1–15. 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH, Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells, Nature. 424 (2003) 88–93. 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- [5].Habtezion A, Nguyen LP, Hadeiba H, Butcher EC, Leukocyte Trafficking to the Small Intestine and Colon, Gastroenterology. 150 (2018) 1–15. 10.1053/j.gastro.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mani V, Bromley SK, Äijö T, Mora-Buch R, Carrizosa E, Warner RD, Hamze M, Sen DR, Chasse AY, Lorant A, Griffith JW, Rahimi RA, McEntee CP, Jeffrey KL, Marangoni F, Travis MA, Lacy-Hulbert A, Luster AD, Mempel TR, Migratory DCs activate TGF-β to precondition naïve CD8 +T cells for tissue-resident memory fate, Science (New York, N.Y.) 366 (2019) eaav5728–15. 10.1126/science.aav5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reboldi A, Arnon TI, Rodda LB, Atakilit A, Sheppard D, Cyster JG, IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches., Science. 352 (2016) aaf4822–aaf4822. 10.1126/science.aaf4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee A-Y, Chang S-Y, Kim J-I, Cha H-R, Jang MH, Yamamoto M, Kweon M-N, Dendritic cells in colonic patches and iliac lymph nodes are essential in mucosal IgA induction following intrarectal administrationvia CCR7 interaction, Eur J Immunol. 38 (2008) 1127–1137. 10.1002/eji.200737442. [DOI] [PubMed] [Google Scholar]
- [9].Tezuka H, Abe Y, Asano J, Sato T, Liu J, Iwata M, Ohteki T, Prominent Role for Plasmacytoid Dendritic Cells in Mucosal T Cell-Independent IgA Induction, Immunity. 34 (2011) 247–257. 10.1016/j.immuni.2011.02.002. [DOI] [PubMed] [Google Scholar]
- [10].Lehner T, Wang Y, Cranage M, Bergmeier LA, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, Klavinskis L, Jones I, Doyle C, Ward R, Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques., Nature Medicine. 2 (1996) 767–775. [DOI] [PubMed] [Google Scholar]
- [11].Cyster JG, B cell follicles and antigen encounters of the third kind., Nature Immunology. 11 (2010) 989–996. 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- [12].Gonzalez SF, Lukacs-Kornek V, Kuligowski MP, Pitcher LA, Degn SE, Kim Y-A, Cloninger MJ, Martinez-Pomares L, Gordon S, Turley SJ, Carroll MC, Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes, Nature Immunology. 11 (2010) 427–434. 10.1038/ni.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, Mebius RE, von Andrian UH, Carroll MC, Conduits mediate transport of low-molecular-weight antigen to lymph node follicles, Immunity. 30 (2009) 264–276. 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cirelli KM, Carnathan DG, Nogal B, Martin JT, Rodriguez OL, Upadhyay AA, Enemuo CA, Gebru EH, Choe Y, Viviano F, Nakao C, Pauthner MG, Reiss S, Cottrell CA, Smith ML, Bastidas R, Gibson W, Wolabaugh AN, Melo MB, Cossette B, Kumar V, Patel NB, Tokatlian T, Menis S, Kulp DW, Burton DR, Murrell B, Schief WR, Bosinger SE, Ward AB, Watson CT, Silvestri G, Irvine DJ, Crotty S, Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance, Cell. 177 (2019) 1153–1171.e28. 10.1016/j.cell.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, García-Sastre A, Compans R, Pulendran B, Programming the magnitude and persistence of antibody responses with innate immunity, Nature. 470 (2011) 543–547. 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Trevaskis NL, Kaminskas LM, Porter CJH, From sewer to saviour — targeting the lymphatic system to promote drug exposure and activity, Nature Reviews Drug Discovery. 14 (2015) 781–803. 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- [17].Schudel A, Francis DM, Thomas SN, Material design for lymph node drug delivery, Nature Reviews Materials. 293 (2019) 1–14. 10.1038/s41578-019-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Marcandalli J, Fiala B, Ols S, Perotti M, de van der Schueren W, Snijder J, Hodge E, Benhaim M, Ravichandran R, Carter L, Sheffler W, Brunner L, Lawrenz M, Dubois P, Lanzavecchia A, Sallusto F, Lee KK, Veesler D, Correnti CE, Stewart LJ, Baker D, Loré K, Perez L, King NP, Induction of Potent Neutralizing Antibody Responses by a Designed Protein Nanoparticle Vaccine for Respiratory Syncytial Virus, Cell. 176 (2019) 1420–1431.e17. 10.1016/j.cell.2019.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lynn GM, Sedlik C, Baharom F, Zhu Y, Ramirez-Valdez RA, Coble VL, Tobin K, Nichols SR, Itzkowitz Y, Zaidi N, Gammon JM, Blobel NJ, Denizeau J, Rochere P, Francica BJ, Decker B, Maciejewski M, Cheung J, Yamane H, Smelkinson MG, Francica JR, Laga R, Bernstock JD, Seymour LW, Drake CG, Jewell CM, Lantz O, Piaggio E, Ishizuka AS, Seder RA, Peptide–TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens, Nat Biotechnol. 38 (2020) 1–19. 10.1038/s41587-019-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF, Nanoparticles target distinct dendritic cell populations according to their size, European Journal Of Immunology. 38 (2008) 1404–1413. 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- [21].Reddy S, van der Vlies A, Simeoni E, Angeli V, Randolph G, O’Neil C, Lee L, Swartz M, Hubbell J, Exploiting lymphatic transport and complement activation in nanoparticle vaccines, 25 (2007) 1159–1164. [DOI] [PubMed] [Google Scholar]
- [22].Moyer TJ, Kato Y, Abraham W, Chang JYH, Kulp DW, Watson N, Turner HL, Menis S, Abbott RK, Bhiman JN, Melo MB, Simon HA, la Mata SH-D, Liang S, Seumois G, Agarwal Y, Li N, Burton DR, Ward AB, Schief WR, Crotty S, Irvine DJ, Engineered immunogen binding to alum adjuvant enhances humoral immunity, Nature Medicine. 26 (2020) 1–33. 10.1038/s41591-020-0753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu TY-H, Singh M, Miller AT, Gregorio ED, Doro F, D’Oro U, Skibinski DAG, Mbow ML, Bufali S, Herman AE, Cortez A, Li Y, Nayak BP, Tritto E, Filippi CM, Otten GR, Brito LA, Monaci E, Li C, Aprea S, Valentini S, Calabró S, Laera D, Brunelli B, Caproni E, Malyala P, Panchal RG, Warren TK, Bavari S, O’Hagan DT, Cooke MP, Valiante NM, Rational design of small molecules as vaccine adjuvants, Sci Transl Med. 6 (2014) 263ra160–263ra160. 10.1126/scitranslmed.3009980. [DOI] [PubMed] [Google Scholar]
- [24].Link A, Zabel F, Schnetzler Y, Titz A, Brombacher F, Bachmann MF, Innate immunity mediates follicular transport of particulate but not soluble protein antigen., 188 (2012) 3724–3733. 10.4049/jimmunol.1103312. [DOI] [PubMed] [Google Scholar]
- [25].Zhang Y-N, Lazarovits J, Poon W, Ouyang B, Nguyen LNM, Kingston BR, C.O. WCW 0000-0001-5435-4785, Nanoparticle Size Influences Antigen Retention and Presentation in Lymph Node Follicles for Humoral Immunity, Nano Letters. 19 (2019) 1–10. 10.1021/acs.nanolett.9b02834. [DOI] [PubMed] [Google Scholar]
- [26].Park C, Arthos J, Cicala C, Kehrl JH, The HIV-1 envelope protein gp120 is captured and displayed for B cell recognition by SIGN-R1(+) lymph node macrophages., ELife. 4 (2015) e06467. 10.7554/elife.06467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tokatlian T, Read BJ, Jones CA, Kulp DW, Menis S, Chang JYH, Steichen JM, Kumari S, Allen JD, Dane EL, Liguori A, Sangesland M, Lingwood D, Crispin M, Schief WR, Irvine DJ, Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers, Science. 363 (2019) eaat9120. 10.1126/science.aat9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moynihan KD, Holden RL, Mehta NK, Wang C, Karver MR, Dinter J, Liang S, Abraham W, Melo MB, Zhang AQ, Li N, Gall SL, Pentelute BL, Irvine DJ, Enhancement of Peptide Vaccine Immunogenicity by Increasing Lymphatic Drainage and Boosting Serum Stability., Cancer Immunology Research. 6 (2018) 1025–1038. 10.1158/2326-6066.cir-17-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Smedley J, Turkbey B, Bernardo ML, Prete GQD, Estes JD, Griffiths GL, Kobayashi H, Choyke PL, Lifson JD, Keele BF, Tracking the luminal exposure and lymphatic drainage pathways of intravaginal and intrarectal inocula used in nonhuman primate models of HIV transmission., PLoS ONE. 9 (2014) e92830. 10.1371/journal.pone.0092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Park JM, Charnsangavej C, Yoshimitsu K, Herron DH, Robinson TJ, Wallace S, Pathways of Nodal Metastasis from Pelvic Tumors: CT Demon- stration1, Radiographics. 14 (1994) 1309–1321. 10.1148/radiographics.14.6.7855343. [DOI] [PubMed] [Google Scholar]
- [31].Sok D, Briney B, Jardine JG, Kulp DW, Menis S, Pauthner M, Wood A, Lee EC, Le KM, Jones M, Ramos A, Kalyuzhniy O, Adachi Y, Kubitz M, MacPherson S, Bradley A, Friedrich GA, Schief WR, Burton DR, Priming HIV-1 broadly neutralizing antibody precursors in human Ig loci transgenic mice, Science (New York, NY). (2016) 1–10. 10.1126/science.aah3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Steichen JM, Kulp DW, Tokatlian T, Escolano A, Dosenovic P, Stanfield RL, McCoy LE, Ozorowski G, Hu X, Kalyuzhniy O, Briney B, Schiffner T, Garces F, Freund NT, Gitlin AD, Menis S, Georgeson E, Kubitz M, Adachi Y, Jones M, Mutafyan AA, Yun DS, Mayer CT, Ward AB, Burton DR, Wilson IA, Irvine DJ, Nussenzweig MC, Schief WR, HIV Vaccine Design to Target Germline Precursors of Glycan-Dependent Broadly Neutralizing Antibodies, Immunity. (2016) 1–15. 10.1016/j.immuni.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M, iDISCO: A Simple, Rapid Method to Immunolabel Large Tissue Samples for Volume Imaging, Cell. 159 (2014) 896–910. 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- [34].Kubota SI, Takahashi K, Nishida J, Morishita Y, Ehata S, Tainaka K, Miyazono K, Ueda HR, Whole-Body Profiling of Cancer Metastasis with Single-Cell Resolution., Cell Reports. 20 (2017) 236–250. 10.1016/j.celrep.2017.06.010. [DOI] [PubMed] [Google Scholar]
- [35].Irvine DJ, Swartz MA, Szeto GL, Engineering synthetic vaccines using cues from natural immunity., Nature Materials. 12 (2013) 978–990. 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stewart VA, McGrath S, Krieg AM, Larson NS, Angov E, Smith CL, Brewer TG, Heppner DG, Activation of Innate Immunity in Healthy Macaca mulatta Macaques by a Single Subcutaneous Dose of GMP CpG 7909: Safety Data and Interferon-Inducible Protein-10 Kinetics for Humans and Macaques, Clinical and Vaccine Immunology. 15 (2008) 221–226. 10.1128/cvi.00420-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kwissa M, Amara RR, Robinson HL, Moss B, Alkan S, Jabbar A, Villinger F, Pulendran B, Adjuvanting a DNA vaccine with a TLR9 ligand plus Flt3 ligand results in enhanced cellular immunity against the simian immunodeficiency virus., Journal of Experimental Medicine. 204 (2007) 2733–2746. 10.1084/jem.20071211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cooper CL, Davis HL, Morris ML, Efler SM, Krieg AM, Li Y, Laframboise C, Adhami MJA, Khaliq Y, Seguin I, Cameron DW, Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine, Vaccine. 22 (2004) 3136–3143. 10.1016/j.vaccine.2004.01.058. [DOI] [PubMed] [Google Scholar]
- [39].Escolano A, Gristick HB, Abernathy ME, Merkenschlager J, Gautam R, Oliveira TY, Pai J, West AP, Barnes CO, Cohen AA, Wang H, Golijanin J, Yost D, Keeffe JR, Wang Z, Zhao P, Yao K-H, Bauer J, Nogueira L, Gao H, Voll AV, Montefiori DC, Seaman MS, Gazumyan A, Silva M, McGuire AT, Stamatatos L, Irvine DJ, Wells L, Martin MA, Bjorkman PJ, Nussenzweig MC, Immunization expands B cells specific to HIV-1 V3 glycan in mice and macaques, Nature. 570 (2019) 1–23. 10.1038/s41586-019-1250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Phan TG, Grigorova I, Okada T, Cyster JG, Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells, Nature Immunology. 8 (2007) 992–1000. 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- [41].Kulp DW, Steichen JM, Pauthner M, Hu X, Schiffner T, Liguori A, Cottrell CA, Havenar-Daughton C, Ozorowski G, Georgeson E, Kalyuzhniy O, Willis JR, Kubitz M, Adachi Y, Reiss SM, Shin M, Val N, Ward AB, Crotty S, Burton DR, Schief WR, Structure-based design of native-like HIV-1 envelope trimers to silence non-neutralizing epitopes and eliminate CD4 binding, Nature Communications. 8 (2017) 1–14. 10.1038/s41467-017-01549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lycke N, Recent progress in mucosal vaccine development: potential and limitations, Nature Reviews Immunology. 12 (2012) 592–605. 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- [43].Klavinskis LS, Bergmeier LA, Gao L, Mitchell E, Ward RG, Layton G, Brookes R, Meyers NJ, Lehner T, Mucosal or targeted lymph node immunization of macaques with a particulate SIVp27 protein elicits virus-specific CTL in the genito-rectal mucosa and draining lymph nodes., Journal of Immunology (Baltimore, Md : 1950). 157 (1996) 2521–2527. [PubMed] [Google Scholar]
- [44].Brouwer PJM, Antanasijevic A, Berndsen Z, Yasmeen A, Fiala B, Bijl TPL, Bontjer I, Bale JB, Sheffler W, Allen JD, Schorcht A, Burger JA, Camacho M, Ellis D, Cottrell CA, Behrens A-J, Catalano M, del Moral-Sánchez I, Ketas TJ, Labranche C, van Gils MJ, Sliepen K, Stewart LJ, Crispin M, Montefiori DC, Baker D, Moore JP, Klasse P-J, Ward AB, King NP, Sanders RW, Enhancing and shaping the immunogenicity of native-like HIV-1 envelope trimers with a two-component protein nanoparticle, Nature Communications. 10 (2019) 1–17. 10.1038/s41467-019-12080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jardine JG, Ota T, Sok D, Pauthner M, Kulp DW, Kalyuzhniy O, Skog PD, Thinnes TC, Bhullar D, Briney B, Menis S, Jones M, Kubitz M, Spencer S, Adachi Y, Burton DR, Schief WR, Nemazee D, HIV-1 VACCINES. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen., Science (New York, NY). 349 (2015) 156–161. 10.1126/science.aac5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kanekiyo M, Joyce MG, Gillespie RA, Gallagher JR, Andrews SF, Yassine HM, Wheatley AK, Fisher BE, Ambrozak DR, Creanga A, Leung K, Yang ES, Boyoglu-Barnum S, Georgiev IS, Tsybovsky Y, Prabhakaran MS, Andersen H, Kong W-P, Baxa U, Zephir KL, Ledgerwood JE, Koup RA, Kwong PD, Harris AK, McDermott AB, Mascola JR, Graham BS, Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses, Nature Immunology. 20 (2019) 1–18. 10.1038/s41590-018-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sliepen K, Ozorowski G, Burger JA, Montfort T, Stunnenberg M, Labranche C, Montefiori DC, Moore JP, Ward AB, Sanders RW, Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity, Retrovirology. 12 (2015) 1–5. 10.1186/s12977-015-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yassine HM, Boyington JC, McTamney PM, Wei C-J, Kanekiyo M, Kong W-P, Gallagher JR, Wang L, Zhang Y, Joyce MG, Lingwood D, Moin SM, Andersen H, Okuno Y, Rao SS, Harris AK, Kwong PD, Mascola JR, Nabel GJ, Graham BS, Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection, Nature Medicine. 21 (2015) 1065–1070. 10.1038/nm.3927. [DOI] [PubMed] [Google Scholar]
- [49].Kourtis IC, Hirosue S, de Titta A, Kontos S, Stegmann T, Hubbell JA, Swartz MA, Peripherally Administered Nanoparticles Target Monocytic Myeloid Cells, Secondary Lymphoid Organs and Tumors in Mice, PLoS ONE. 8 (2013) e61646. 10.1371/journal.pone.0061646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ols S, Yang L, Thompson EA, Pushparaj P, Tran K, Liang F, Lin A, Eriksson B, Hedestam GBK, Wyatt RT, Loré K, Route of Vaccine Administration Alters Antigen Trafficking but Not Innate or Adaptive Immunity, Cell Reports. 30 (2020) 3964–3971.e7. 10.1016/j.celrep.2020.02.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liang F, Lindgren G, Sandgren KJ, Thompson EA, Francica JR, Seubert A, Gregorio ED, Barnett S, O’Hagan DT, Sullivan NJ, Koup RA, Seder RA, Loré K, Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake., Sci Transl Med. 9 (2017). 10.1126/scitranslmed.aal2094. [DOI] [PubMed] [Google Scholar]
- [52].Lindsay KE, Bhosle SM, Zurla C, Beyersdorf J, Rogers KA, Vanover D, Xiao P, x000ED nga MA, Shirreff LM, Pitard B, Baumhof P, Villinger F, Santangelo PJ, Visualization of early events in mRNA vaccine delivery in non-human primates via PET-CT and near-infrared imaging, Nature Biomedical Engineering. 196 (2019) 1–13. 10.1038/s41551-019-0378-3. [DOI] [PubMed] [Google Scholar]
- [53].Martin JT, Cottrell CA, Antanasijevic A, Carnathan DG, Cossette BJ, Enemuo CA, Gebru EH, Choe Y, Viviano F, Fischinger S, Tokatlian T, Cirelli KM, Ueda G, Copps J, Schiffner T, Menis S, Alter G, Schief WR, Crotty S, King NP, Baker D, Silvestri G, Ward AB, Irvine DJ, Targeting HIV Env immunogens to B cell follicles in nonhuman primates through immune complex or protein nanoparticle formulations, Npj Vaccines. 5 (2020) 72. 10.1038/s41541-020-00223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Watanabe Y, Allen JD, Wrapp D, McLellan JS, Crispin M, Site-specific glycan analysis of the SARS-CoV-2 spike, Science. 369 (2020) 330–333. 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.