EDITORIAL
Trends toward improved outcomes of orthotopic heart transplant (OHT) recipients have unfortunately slowed in recent years (1). Major survival gains in the early period after transplantation have largely stemmed from improvements in immunosuppression regimens and decreased rates of clinically significant graft rejection. More recently, attention has shifted attention toward later critical determinants of post-transplant morbidity, including coronary allograft vasculopathy (CAV). CAV, a multifactorial, pan-arterial disease characterized by intimal hyperplasia of the epicardial coronary arteries and microvasculature, remains a leading cause of death and re-transplantation after the first year (2). Given the frequently asymptomatic and insidious nature of the disease in the denervated heart, both non-invasive and invasive screening approaches have been employed for early detection. Perfusion positron emission tomography (PET) has emerged as a robust non-invasive method for CAV screening. PET parameters including myocardial perfusion imaging and myocardial blood flow quantification are associated with cardiovascular outcomes (3). Additionally, multiparametric cardiac PET evaluation in OHT recipients that included parameters largely associated with left ventricular function and hemodynamics provide superior test characteristics over standard myocardial perfusion imaging (MPI) assessment (4). However, the incremental benefit of a more general assessment of cardiopulmonary performance, cardiopulmonary transit time (CPTT), had not been established in this population.
CPTT is a simple measure which represents the time needed for blood to travel between the right and left ventricles and is easily obtained from first-pass, dynamic imaging sequences. CPTT is inherently a nonspecific measure of overall cardiac performance and is affected by a variety of pathologies including ventricular failure, valvular disease, diastolic dysfunction, pulmonary hypertension, among others. The concept of “circulation time” was first explored more than 90 years ago (5). More recent work has demonstrated its potential as a “non-invasive right heart catheterization”, as circulation time (measured by computed tomography (CT) or magnetic resonance imaging (MRI)) has been associated with several invasively measured and derived hemodynamic parameters (6).
Within this context, Harms et al. retrospectively assessed the utility of CPTT using 13N-ammonia PET among comprehensively phenotyped heart transplant recipients at a single, large, academic center (7). Importantly, standard of care at this institution involves yearly screening for CAV, alternating PET and invasive coronary angiography. The systematic, routine use of coronary angiography in their institutional protocol guards against referral bias in analyses relating CPTT to invasively defined CAV. CPTT is relatively straightforward to measure as the difference in midpoint time between the radiotracer peak activity in the blood pools of the left and right ventricles. Thus, CPTT can be calculated from dynamic cardiac PET imaging and can routinely be evaluated during myocardial blood flow quantification if a right ventricular (in addition to the left ventricular) input function is also specified. Of note, the authors also multiply CPTT by heart rate given its influence on transit time, and thus CPTT is reported as beats rather than time.
The authors measured and dichotomized CPTT for 94 participants more than 10 years from transplant on average. A “prolonged CPTT” (>17.75 beats) was defined by receiver operating characteristics for major adverse cardiovascular events and was present in 20% of participants. Therefore, the relationships between dichotomized CPTT and adverse clinical characteristics may in part be self-fulfilling prophecies. As such, prolonged CPTT was associated with worse left ventricular function and stress myocardial blood flow. While rest myocardial blood flow and flow reserve were also lower, these differences were not statistically significant. Using invasive hemodynamics, CPTT was also a marker of impaired cardiac output and elevated filling pressures. Analysis of CPTT as a continuous measure reassuringly reflected some of these findings in limited analyses. CPTT, expressed both as a dichotomous and continuous predictor variable, was associated with major adverse events after adjusting for a few clinical and PET based variables, including the PET-CAV score. The prognostic value of CPTT beyond comprehensive clinical risk factors or hemodynamic measurements from right heart catheterization remains unknown, however, as further adjustment in this analysis was precluded by the small number of events.
Taken together, these findings suggest that a prolonged CPTT derived from PET can be used to identify OHT patients with reduced cardiac output, elevated filling pressures, and at high risk for poor outcomes. Though this is a small single-center study, the authors describe a novel PET biomarker which is measured noninvasively from data that are already been obtained during a typical perfusion PET and can be added to the evaluation of OHT recipients. Analysis with invasive data suggest that CPTT can be used to identify patients at the highest risk of decompensation. These features make CPTT an appealing imaging biomarker that can be easily and broadly implemented in the evaluation of OHT recipients.
Although the findings of this study are currently generalizable only to OHT recipients who are many years out from their transplant, the associations between CPTT and hemodynamic parameters such as cardiac index and filling pressures are especially interesting observations that should generate further investigation. These findings raise the question of whether the relationships that CPTT has with these hemodynamic parameters are applicable to different populations and/or disease conditions. For example, the utility of CPTT as a marker of allograft rejection in the early transplant period warrants investigation. Future studies are also needed to evaluate the role of CPTT in patients with heart failure with reduced (HFrEF) or preserved ejection fraction (HFpEF) undergoing PET imaging, as has been done in MRI (8). These studies could also evaluate whether CPTT correlates with parameters of diastolic function and filling pressures on echocardiography, such as tissue velocities and E/e’ ratios, and its utility beyond these parameters. With this additional investigation into CPTT, PET may be able to provide useful information about systolic and diastolic markers for undifferentiated patients undergoing PET MPI for evaluation of chest pain, dyspnea, or worsening exercise tolerance, which could potentially inform diagnosis, management, risk stratification, and recommendations for future testing.
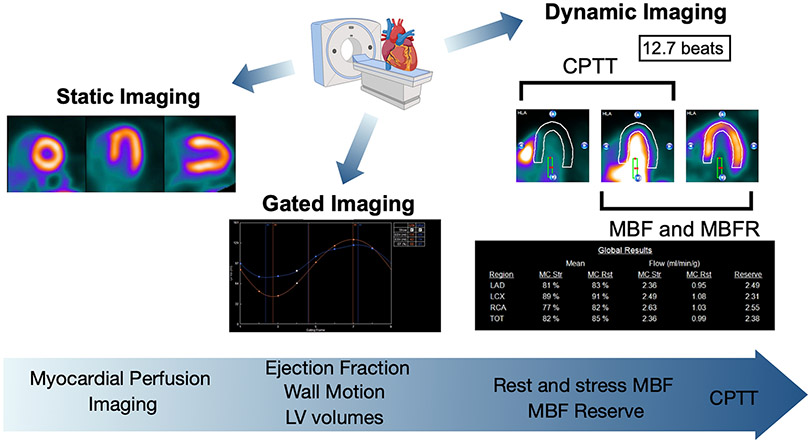
PET has become a powerful tool for the evaluation of OHT recipients and is the noninvasive modality of choice for the monitoring of CAV given its ability to provide diagnostic and prognostic information at a low radiation exposure (Figure). CPTT is a novel and simple imaging biomarker that can be easily measured in this patient cohort and can identify patients at the highest risk for poor outcomes. Future studies will determine the utility and applicability of CPTT to other patient populations.
Figure. Perfusion PET imaging post-heart transplant.
Static and gated imaging provide traditional measures of cardiac perfusion and function. Perfusion PET also allows for dynamic imaging which allows for quantification of myocardial blood flow (MBF), MBF reserve (MBFR), and CPTT. Representative images highlight the different data obtained with perfusion PET. Created using 4DM software (INVIA, MI) and www.Biorender.com.
Acknowledgments
DISCLOSURES
SS is supported by the Doris Duke Charitable Foundation (Physician Scientist Fellowship Award 2020061), the Measey Foundation, and the American Society of Nuclear Cardiology (Institute for the Advancement of Nuclear Cardiology award). MG is supported by K08HL136890, R01HL108119, and the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- (1).Khush KK, Cherikh WS, Chambers DC, Harhay MO, Hayes D Jr., Hsich E et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report - 2019; focus theme: Donor and recipient size match. J Heart Lung Transplant 2019;38:1056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:996–1008. [DOI] [PubMed] [Google Scholar]
- (3).Mc Ardle BA, Davies RA, Chen L, Small GR, Ruddy TD, Dwivedi G et al. Prognostic value of rubidium-82 positron emission tomography in patients after heart transplant. Circ Cardiovasc Imaging 2014;7:930–7. [DOI] [PubMed] [Google Scholar]
- (4).Bravo PE, Bergmark BA, Vita T, Taqueti VR, Gupta A, Seidelmann S et al. Diagnostic and prognostic value of myocardial blood flow quantification as non-invasive indicator of cardiac allograft vasculopathy. Eur Heart J 2018;39:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Blumgart HL, Yens OC. STUDIES ON THE VELOCITY OF BLOOD FLOW: I. The Method Utilized. J Clin Invest 1927;4:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Selzer A, Dunlap RW, Wray HW, Russell J. A critical appraisal of the circulation time test. Arch Intern Med 1968;122:491–5. [PubMed] [Google Scholar]
- (7).Harms HJ, Bravo PE, Bajaj NS, Zhou W, Gupta A, Tran T et al. Cardiopulmonary Transit Time: A Novel PET Imaging Biomarker of In-Vivo Physiology for Risk Stratification of Heart Transplant Recipients. J Nucl Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Cao JJ, Li L, McLaughlin J, Passick M. Prolonged central circulation transit time in patients with HFpEF and HFrEF by magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 2018;19:339–46. [DOI] [PubMed] [Google Scholar]