Abstract
Ketamine has emerged as a novel treatment for common psychiatric conditions such as Major Depressive Disorder (MDD) and anxiety disorders, many of which can be initiated and exacerbated by psychological stress. Sex differences in the frequency of both anxiety and depressive disorders are well known and could be due in part to sex differences in neuroendocrine responses to stress. Ketamine is an MDD treatment and is itself known to modulate the hormonal response to stress, specifically corticosterone. Though differential behavioral responses to ketamine between the sexes have been reported in animals, it is not clear if the acute effect of ketamine on corticosterone differs by sex, or what role this could play in the subsequent behavioral response. Here we test whether a single injection of (R,S)-ketamine (30 mg/kg, i.p.), administered either with or without unpredictable chronic stress (UCS), has different sustained effects on open field (OFT), elevated zero maze (EZM) or forced swim test (FST) behavior in female versus male C57BL/6J mice. We also test whether plasma corticosterone may play a predictive role in those responses. In the OFT (24 hours post-injection), ketamine increased center square exploration in males but not females. In contrast, in the FST (72 hours post-injection), females showed a trend toward a decrease in immobility after ketamine whereas males were not strongly modulated. These behavioral effects of ketamine were stronger in the presence of UCS than in unstressed animals. UCS animals also showed lower corticosterone after injection than unstressed animals, and in the presence of UCS ketamine increased corticosterone; these effects were similar in both sexes. Corticosterone post-injection did not predict subsequent behavioral measures. These findings complement a growing preclinical literature suggesting both stress-dependency and sex differences in OFT and FST behavioral responses to ketamine, and these differences are not mediated by corticosterone.
Keywords: major depression, chronic mild stress, psychosocial stress, corticosterone, open field, forced swim
LAY SUMMARY
In humans it is known that major depression and anxiety disorders, which can be caused or made worse by exposure to psychological stress, occur roughly twice as frequently in women than in men, but the underpinnings of these effects are not well characterized. In the current study we explored how sex interacts with stress and ketamine (a rapidly acting antidepressant) by assessing both open field and forced swim behavior in mice after chronic mild stress. We report the novel finding that male mice exhibit greater exploration of the aversive center square in the open field after ketamine, whereas females trended toward lower immobility (often interpreted as an antidepressant-like effect) in the forced swim test after this drug, and these effects were amplified by prior stress exposure.
INTRODUCTION
There is now ample evidence that the anesthetic drug ketamine can rapidly improve symptoms of Major Depressive Disorder (MDD) in human patients when used at sub-anesthetic doses (Berman et al., 2000; Krystal, Abdallah, Sanacora, Charney, & Duman, 2019; Wei, Chang, & Hashimoto, 2020; Zarate et al., 2006). However, potential sex differences in the effects of this drug are not well understood. One recent randomized study of treatment resistant depression has suggested that there may not be significant sex differences in the therapeutic properties of ketamine in humans (Freeman et al., 2019), but a growing preclinical literature has found a number of sex differences in FST behavior in mice and rats that were given this drug. Several of these studies have suggested that female mice and rats are more behaviorally responsive than males to ketamine, showing benefits at lower doses (Carrier & Kabbaj, 2013; Franceschelli, Sens, Herchick, Thelen, & Pitychoutis, 2015; Sarkar & Kabbaj, 2016) and with more rapid onset but a shorter duration (Franceschelli et al., 2015). Potential sex differences in OFT behavior after a single injection of ketamine are somewhat less well studied than FST behavior [but see: (Franceschelli et al., 2015; Kara, Agam, Anderson, Zitron, & Einat, 2017; Thelen, Sens, Mauch, Pandit, & Pitychoutis, 2016)].
Psychological stress is well known to be a causal or exacerbating factor in MDD and various anxiety disorders (Bonde et al., 2016; Costa e Silva & Steffen, 2019; Hosang, Shiles, Tansey, McGuffin, & Uher, 2014; Lautarescu, Craig, & Glover, 2020). It is also well established that women are roughly twice as likely as men to be diagnosed with MDD, but the hormonal and neurochemical basis (among other potential factors) for this discrepancy, including sex differences in response to various antidepressant drugs, is not well understood (Albert, 2015; Baxter et al., 2014; Whiteford et al., 2013). Women also suffer from anxiety disorders, such as specific phobias and generalized anxiety disorder, at higher rates than men (Baxter et al., 2014; McLean, Asnaani, Litz, & Hofmann, 2011). As with MDD, the neurochemical and neurohormonal factors that may give rise to sex differences in anxiety disorders, including their stress-sensitivity, are not well characterized at this time.
Any sex differences in ketamine’s effects on FST or OFT behaviors could be mediated by neuroendocrine factors. For example, it has been shown that ovariectomized female rats no longer exhibit favorable responses to ketamine, suggesting sex hormones such as estrogen and progesterone may be necessary for the therapeutic effects of this drug in females (Carrier & Kabbaj, 2013). Another candidate molecule for modulating the behavioral effects of ketamine in rodents is the adrenal corticosteroid, corticosterone. Ketamine has been shown to acutely boost corticosterone in the absence of chronic stress (Fahringer, Foley, & Redgate, 1974; Radford, Park, & Choi, 2018; Kudo, Kudo, Matsuki, & Ishihara, 1993; Nistico et al., 1978). Stress itself alters hypothalamic-pituitary-adrenal (HPA) axis dynamics, and so any difference in the effect of ketamine in stressed or non-stressed states could be mediated by its interaction with glucocorticoids.
In a previous study (Fitzgerald, Yen, & Watson, 2019) we found that a single injection of low-dose ketamine (30 mg/kg, i.p.) decreased immobility in the forced swim test (FST) 24 hours after injection in unpredictable chronic stress (UCS)-exposed male mice, but not in unstressed animals. The goal of the current study was to determine whether ketamine has sex- and stress- specific effects on various behaviors using several classic measures of exploratory behavior (OFT, EZM) as well as the FST, and whether differential stimulation of corticosterone could explain these differences.
METHODS
Subjects
Sixty-four (n=8 per sex/stress/drug cohort) experimentally naïve adult (8–9 weeks old upon arrival) C57BL/6J mice (32 females and 32 males) were obtained from The Jackson Laboratory (Bar Harbor, ME). Starting the day of arrival and throughout the experiments, mice were either group housed (unstressed mice) or single housed (unpredictable chronic stress (UCS)) in cages within a humidity- and temperature-controlled vivarium, and kept on a 12:12 hr light/dark cycle (lights on at 6 am) with ad libitum access to food and water. On the day of arrival at the facility, all mice were subjected to a five-minute locomotion test in a 30-cm square box under dim lighting conditions (30 lux) to assess basal locomotion. Mice were then divided into groups of eight (as noted above) that were matched for total distance traveled, to control for baseline variability in locomotion. All experiments were carried out in the daytime and during the light phase. All procedures were conducted at the University of Michigan and were performed in accordance with the guidelines and regulations set forth by the National Institutes of Health and the University of Michigan, with approval from its Institutional Animal Care and Use Committee (Protocol number: PRO00007803).
Unpredictable chronic stress
Mice were chronically subjected to one of two behavioral procedures: unstressed/standard procedures or unpredictable chronic stress (UCS). Unstressed procedures consisted of group housing of the mice (4 per cage) on the day of arrival in the vivarium and throughout experimentation, handling (i.e., allowing the mouse to explore the experimenter’s gloved hand and covered forearm) for ~30 seconds a day for the first five days after arrival, and maintenance of standard levels of cage enrichment with a single package of nesting material (Enviropak, Lab Supply, Fort Worth, TX).
In contrast, mice subjected to UCS were single housed on the day of arrival and throughout the experiments, were not handled by the experimenter in the first five days (and throughout the experiments, except when necessary), and their cages were not enriched with an Enviropak and were instead given two white nestlets. These UCS mice also received one stressor/hassle a day at a random time, beginning the day after arrival and lasting for 14 consecutive days (see S1 Table in Fitzgerald et al. (2019) for a description and the sequence of these stressors), completed the day of the ketamine injection and one day prior to the start of behavioral testing. These stressors consisted of a randomized sequence of: cage tilting (20 degrees, two hours), homecage bedding change, cage change, turning ambient lights on and off repeatedly for three hours, replacement of nest with two still-intact nestlets, placing mouse in novel empty cage for one hour, and placing divider in homecage for four hours. All mice received the same stressors at the same time, in the same sequence. Male and female mice were housed in separate rooms and stressed at different times in the same room. The experimental timeline is shown in Figure 1; all mice were weighed two days prior to injection with ketamine or vehicle.
Figure 1.
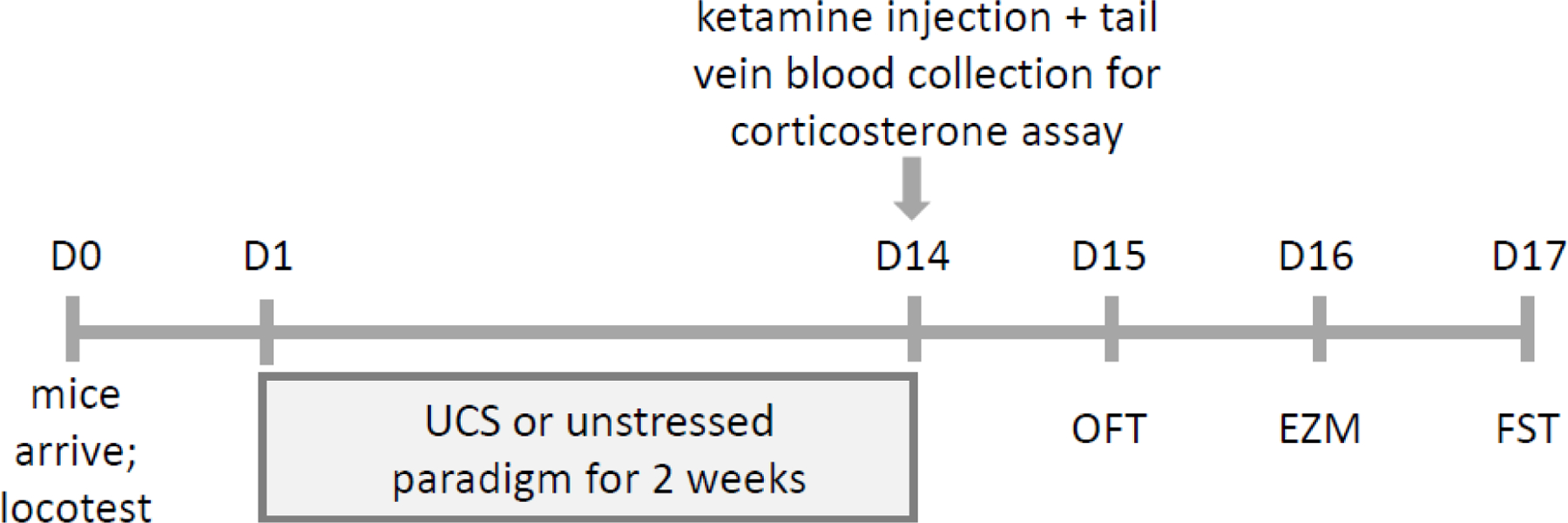
Experimental timeline. Mice arrived at the facility on Day 0. On Day 1, mice were subjected to either unstressed or unpredictable chronic stress (UCS) conditions composed of scheduled but varying daily hassles. Following this two-week period of stress or no stress, all mice received a single injection (Day 14) of either vehicle (0.9% saline, i.p.) or 30 mg/kg ketamine. Thirty minutes after injection, tail vein blood was collected for corticosterone measurement. Twenty-four hours later (Day 15), mice were given an open field test (OFT), followed by an elevated zero maze (EZM) test on Day 16, and the forced swim test (FST) on Day 17.
Drug
(R,S)-ketamine hydrochloride (Ketalar, Par Pharmaceutical, Chestnut Ridge, NY) was stored in darkness at room temperature, and for administration was diluted in 0.9% physiological saline solution (vehicle) to a concentration of 3.0 mg/ml and injected intraperitoneally (i.p.), at a volume of 10 ml/kg to reach an injected dose of 30 mg/kg.
Corticosterone measurement
Thirty minutes after the ketamine or vehicle injection, mice were warmed for 1–2 minutes under an infrared lamp to dilate the tail veins. Each mouse was secured in a tail vein restrainer (Braintree Scientific, Cambridge, MA), tail vein identified, and the area was cleaned with ethanol and incised using a razor blade. The tail vein was accessed using a small-gauge needle, and blood was collected using Microvette collection tubes (Braintree Scientific, Cambridge, MA) within 60 seconds of tail vein incision. Pressure was applied to achieve hemostasis before returning the mouse to its homecage. The blood samples were later centrifuged at 4000 rpm for 10 minutes to separate plasma. Corticosterone was measured from plasma using the DetectX Corticosterone Enzyme Immunoassay Kit (Arbor Assays, Ann Arbor, MI). One male mouse and two female mice had inadequate sample volume for analysis.
Behavioral testing
Beginning 24 hours after ketamine injection, mice were subjected to daily behavioral tests in the following order: open field test (OFT), elevated zero maze (EZM), and forced swim test (FST); Figure 1. Forced swim was performed last in the sequence to eliminate the possibility that this most stressful of the tests would affect the behavior of the mice in the OFT or EZM. The experimenter (SKK) was blind to the drug treatment groups, and all behavior was also scored in an automated fashion (see below). On each testing day, mice were allowed to acclimate to the testing room in their home cages for at least 30 minutes prior to testing. The open field consisted of a square box with sides 72 cm in length and walls 36 cm high. For analysis, the center region of the box floor was defined as a 36 cm square. The EZM (San Diego Instruments, Inc., San Diego, CA) was a circular track with outside wall diameter 61 cm, inside wall diameter 51 cm, and half of the track enclosed on both sides by 15-cm walls. Each mouse was allowed to explore the open field or elevated zero maze for five minutes under lighting conditions of 200 lux. At the end of testing, each mouse was immediately removed from the box or maze and returned to its homecage. The box or maze was cleaned with 50% ethanol solution between animals.
For the FST, up to four mice were tested simultaneously in a set of clear Plexiglas cylindrical tanks, 20 cm in diameter, filled halfway with water at 23–25ºC. Opaque plastic dividers were placed between the forced swim tanks to block the animals’ view of one another. Ambient white lighting (200 lux) was present in the room during acclimation and throughout testing. Each trial lasted six minutes, but behavior was only scored in the last five minutes. At the end of the trial, the mouse was immediately removed from the tank, dried, and returned to its homecage. EthoVision version 11.5 was used for behavioral analysis (Noldus Information Technology, Leesburg, VA). For the FST, we defined “immobile” behavior in EthoVision as comprising bin-by-bin changes in mouse image pixelation of 0–10%.
Analysis and statistics
We analyzed the data with conventional parametric statistics (GraphPad Prism, GraphPad Software, La Jolla, CA). Two-way (stress x drug) or three-way (sex x stress x drug) analysis of variance (ANOVA) was used to assess general main effects and interactions (α = 0.05). When there was a main effect of sex in the three-way ANOVA, we analyzed each sex separately with two-way ANOVA, followed by Tukey’s post-hoc test when appropriate. Results are shown as mean ± standard error of the mean (SEM).
RESULTS
To address how a single injection of ketamine influences behavior in stressed and unstressed animals of both sexes, 32 female and 32 male C57BL/6J mice underwent the experimental protocol shown in Figure 1. Half of the mice were exposed to a two-week protocol of unpredictable chronic stress (UCS) and half were not stressed. Following completion of the stress protocol, mice were injected once with either 30 mg/kg (i.p.) ketamine or vehicle solution (0.9% saline). There were 8 mice per sex, drug and stress-specific group. Outcome measures included a measure of corticosterone from tail vein blood 30 minutes after ketamine injection, to measure the effect of ketamine on the final product of HPA axis activation. Other outcome measures were OFT, EZM, and FST behavioral tests at 1, 2, and 3 days, respectively, post-injection.
Open field test
There were sex differences in open field behavior independent of stress or drug group, with females showing greater general activity and exploratory behavior (Figure 2). Females traveled a greater distance in the arena than males [main effect of sex: F(1,56) = 13.80, p < 0.01; Figure 2A] and in the center square [F(1,56) = 20.89, p < 0.01; Figure 2B], and females showed increased frequency in the center relative to males [F(1,56) = 18.29, p < 0.01; Figure 2C] and duration [F(1,56) = 14.20, p < 0.01; Figure 2D].
Figure 2.
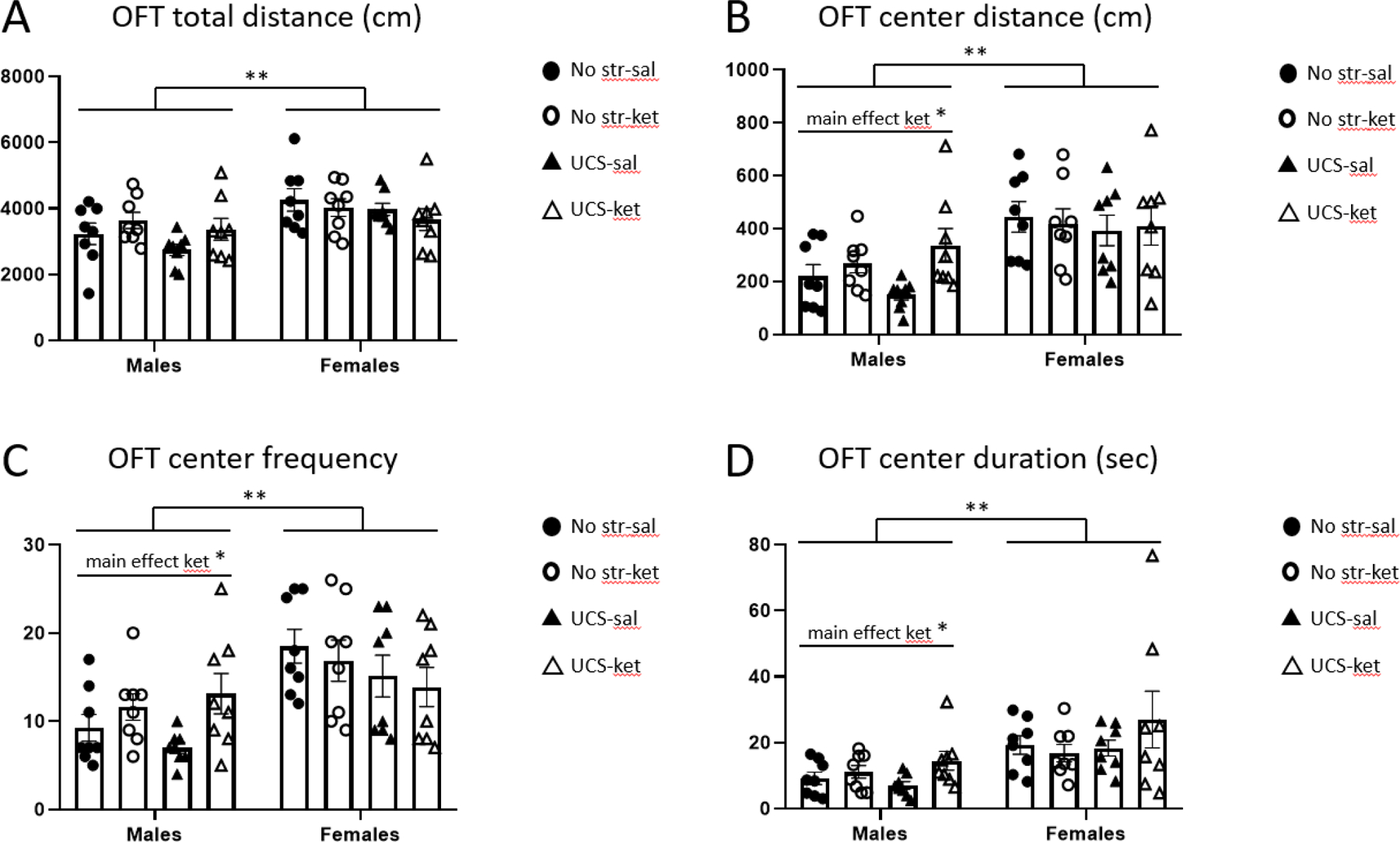
Male mice exhibited greater exploration of the center square when given ketamine after UCS in the open field test. Behavior (24 hours post-injection) was automatically scored in EthoVision and parsed into: A, total distance traveled; B, center square distance; C, center square frequency; D, center square duration. Females were more active than males in all four measures. UCS-exposed males exhibited a significant, or trending, behavioral modulation after ketamine for measures B-D (see Table 1). Abbreviations: no stress saline (No str-sal), no stress ketamine (No str-ket), unpredictable chronic stress saline (UCS-sal), unpredictable chronic stress ketamine (UCS-ket). Error bars: ± standard error of mean (SEM). Three-way ANOVA, main effect of sex: ** p < 0.01, Two-way ANOVA, main effect of drug: * p < 0.05.
For OFT center distance, center frequency, and center duration, there were main effects of ketamine to increase exploration in males, irrespective of stress, but not in females [for males: center distance [F(1,28) = 7.27, p < 0.05]; center frequency [F(1,28) = 7.05, p < 0.05]; center duration [F(1,28) = 5.31, p < 0.05]]. Post hoc tests in males (Table 1) showed that ketamine significantly increased center distance in the UCS but not the unstressed groups; the same trend was seen for center frequency and duration. The effect of ketamine on these three OFT measures in UCS males was substantial: center distance (saline mean = 149.2 cm, ketamine mean = 336.4 cm; 125.4% increase), center frequency (saline = 7.0, ketamine = 13.1; 87.5% increase), center duration (saline = 7.0 sec, ketamine = 14.5 sec; 106.5% increase).
Table 1.
Post hoc comparison between ketamine and vehicle for various behavioral outcome measures. In the open field test (OFT) and forced swim test (FST), a two-way (stress by drug) ANOVA was calculated for each sex, followed by Tukey’s post-hoc test whose p values are displayed here.
| Tukey’s post-hoc comparison between ketamine and vehicle for various outcome measures | ||
|---|---|---|
| No Stress p value | UCS p value | |
| Fig 2a: OFT total distance | 0.73 (males) | 0.39 (males) |
| Fig 2b: OFT center distance | 0.86 (males) | 0.025 (males) |
| Fig 2c: OFT center frequency | 0.72 (males) | 0.053 (males) |
| Fig 2d: OFT center duration | 0.91 (males) | 0.067 (males) |
| Fig 4: FST immobility | 0.84 (females) | 0.19 (females) |
In summary, in the OFT females were more active than males, and males but not females showed increased center square exploration after ketamine treatment, which was most pronounced in the UCS group.
Elevated zero maze
In the EZM (Figure 3), there were no sex differences and no significant modulation of any measure by ketamine. For EZM total distance traveled (Figure 3A), there was no modulation of behavior by sex, stress, or drug (each p > 0.05). There was an overall effect of UCS to decrease open arm exploration compared to no-stress, seen as main effects of stress on open arm distance [F(1,56) = 4.60, p < 0.05; Figure 3B], open arm frequency [F(1,56) = 7.45, p < 0.01; Figure 3C], and open arm duration [F(1,56) = 6.92, p < 0.05; Figure 3D].
Figure 3.
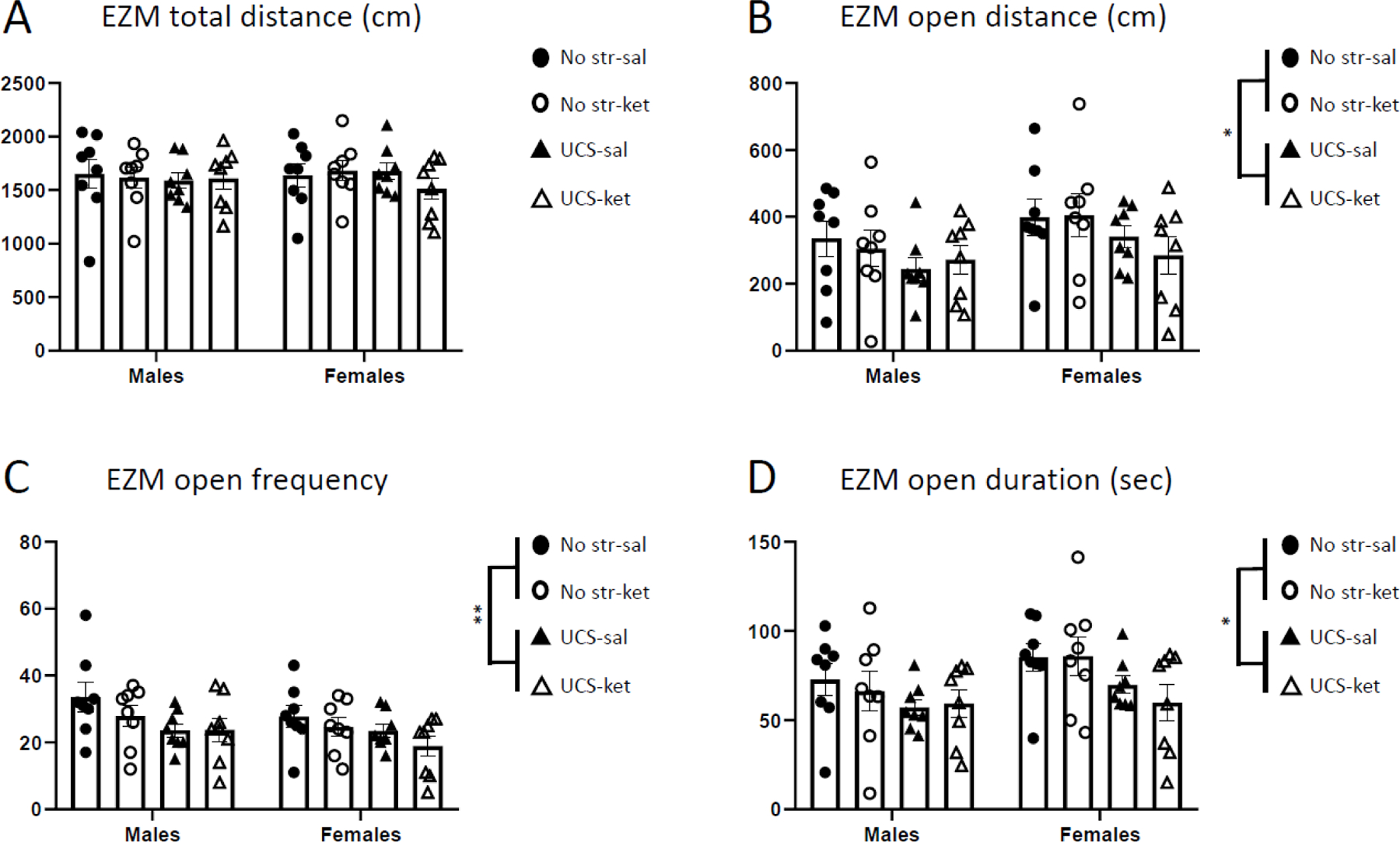
Ketamine did not modulate behavior in the elevated zero maze in either sex. Behavior (48 hours post-injection) was automatically scored by computer and parsed into: A, total distance traveled; B, open arm distance; C, open arm frequency; D, open arm duration. UCS-exposed animals exhibited decreases in measures B-D, irrespective of ketamine or sex. Error bars: ± SEM. Three-way ANOVA, main effect of stress: * p < 0.05, ** p < 0.01.
In summary, UCS exposure tended to decrease open arm exploration in the EZM by suppressing open arm distance, frequency, and duration.
Forced swim test
In the FST, females had less immobility than males, when combining all stress and drug groups [main effect of sex: F(1,56) = 38.66, p < 0.01] (Figure 4). Two-way ANOVA within each sex revealed that male mice did not exhibit modulation of immobility as a function of stress or drug. In contrast, stress trended toward a decrease in immobility in the females [main effect of stress: F(1,28) = 3.28, p = 0.081], and females also showed a trend toward decreased immobility after ketamine independent of stress [main effect of drug: F(1,28) = 4.14, p = 0.052]. UCS, ketamine-treated female mice showed the lowest immobility of all the groups.
Figure 4.
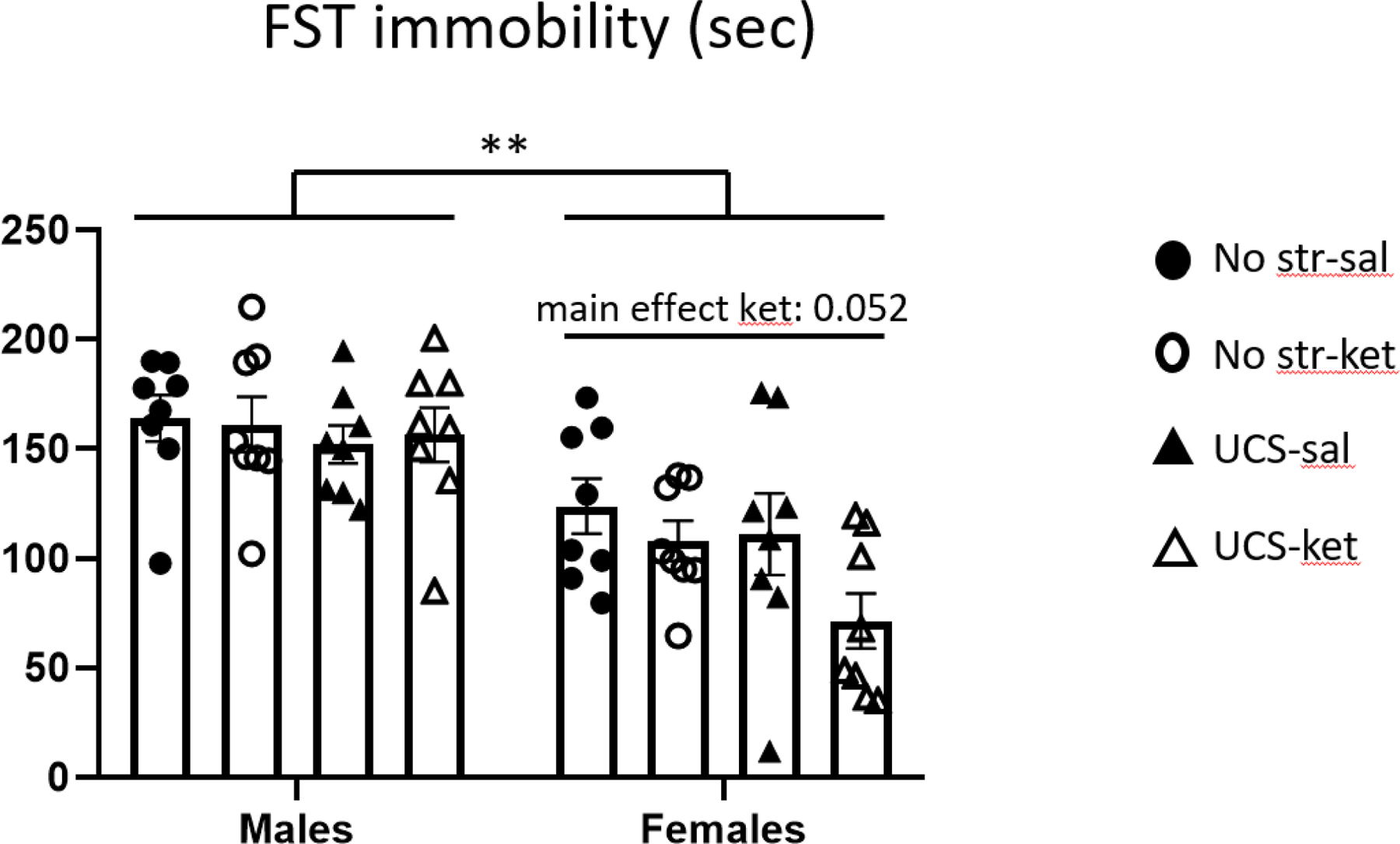
Female mice trended toward a decrease in immobility after ketamine in the forced test. Immobility behavior (72 hours post-injection) was automatically scored. Females showed lower immobility than males. Females, especially those exposed to UCS, showed a trend toward an therapeutic-like response to ketamine. Error bars: ± SEM. Three-way ANOVA, main effect of sex: ** p < 0.01, Two-way ANOVA, main effect of drug: * p = 0.052.
In summary, in an FST three days post-injection, there was a tendency for a decrease in immobility for stressed females given ketamine, but not in males given ketamine.
Plasma corticosterone
To understand how stress and ketamine differentially affect the corticosterone response to acute injection, tail vein blood was collected 30 minutes after ketamine or vehicle injection (Figure 5), in the same mice for which behavior is described above. There were no sex differences in corticosterone. A three-way ANOVA (sex x stress x drug) revealed a main effect of stress, indicating that the UCS-exposed mice showed lower levels of corticosterone than unstressed mice after injection [F(1,53) = 7.33, p < 0.01]. There was also a stress x drug interaction [F(1,53) = 5.30, p < 0.05]. Separate two-way ANOVAs for the no-stress and UCS groups separately showed no significant main effects or interaction for unstressed mice (each p > 0.05), whereas in UCS animals there was a trend toward ketamine increasing corticosterone [main effect of drug: F(1,26) = 3.64, p = 0.068]. Corticosterone was much lower in stressed mice given saline injection than in the unstressed mice given saline, suggesting habituation of the stress response in the group that had been repeatedly stressed. Among stressed mice, the ketamine-injected mice had higher mean corticosterone than saline-injected mice, while the opposite was true for the unstressed groups. Upon Pearson correlation testing, there was no consistent animal-by-animal relationship found between corticosterone and any of the subsequent behavioral measures, either in the full sample or when analyzing the two sexes separately. Thus, plasma corticosterone measured 30 minutes post-injection was lower in stressed mice and did not predict subsequent behavior.
Figure 5.
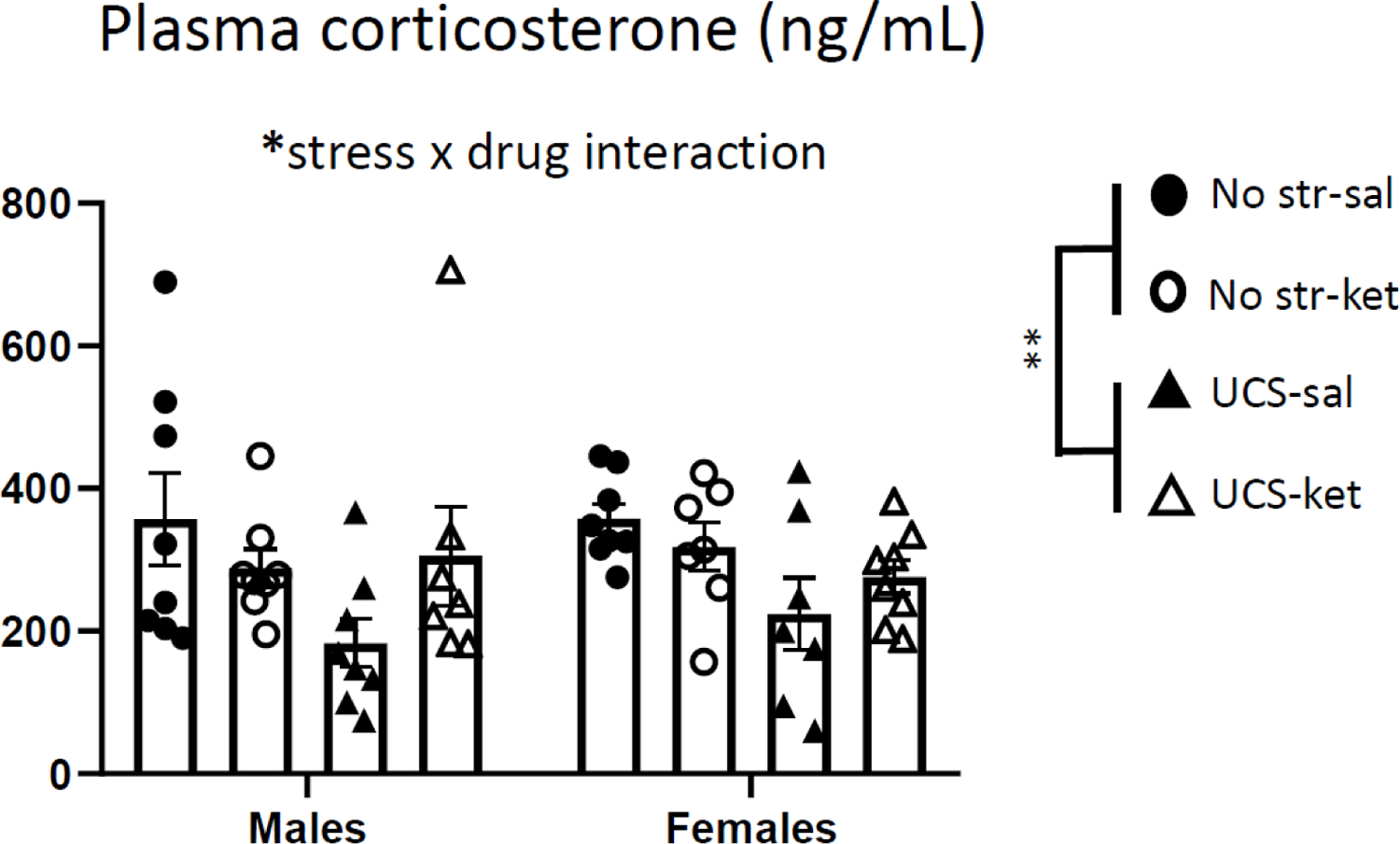
Chronic stress and ketamine interacted to modulate the corticosterone response to acute injection stress. Plasma corticosterone was measured 30 minutes after ketamine or vehicle injection, by tail vein puncture. Prior UCS exposure suppressed corticosterone irrespective of sex. There was also a significant stress by drug interaction. Error bars: ± SEM. Three-way ANOVA, main effect of stress: ** p < 0.01, stress x drug interaction: * p < 0.05.
DISCUSSION
In this study, we investigated the effects of sex and chronic stress history on corticosterone and subsequent behavior in response to a single subanesthetic dose of ketamine in C57BL/6J mice. We found contrasting effects of sex, stress, and ketamine on behavior in the three different tests. Specifically, ketamine caused increased center exploration in the OFT in males only, regardless of stress history. In both sexes, stress decreased open arm exploration in the EZM, which was not reversed by ketamine. Ketamine tended to decrease immobility in females in the FST, a finding that is often interpreted as an antidepressant-like response. Finally, stress history modulated the effect of ketamine on corticosterone in a sex-independent manner, with ketamine tending to increase corticosterone in chronically stressed mice.
The effects of ketamine to increase center exploration in the OFT in males and to reduce immobility in the FST in females were strongest in the UCS group. This finding is in line with our previous studies (Fitzgerald et al., 2019; Polis, Fitzgerald, Hale, & Watson, 2019) which showed that increased activity after ketamine in the FST tended to only be observed in C57BL/6J mice in the presence of UCS but not in unstressed animals. While in those previous studies we actually found that unstressed mice showed an opposing response to ketamine relative to stress-exposed animals, the data we report here at least show that stress can amplify the therapeutic effect of this drug as assessed by the FST (and the OFT) relative to unstressed animals. It should be noted that the sex of the experimenter, as well as brand of ketamine, are potential sources of variability in behavioral studies of this drug (Chapman, Benedict, & Schiöth, 2018; Fitzgerald et al., 2019; Georgiou et al., 2018). In this study the FST and OFT experiments were carried out by a female experimenter while stress was carried out by a male experimenter – whereas in our previous study, both were carried out by a male. We further suggest that the relatively modest effects that UCS produced in our three behavioral tests is consistent with the relative stress resilience of the C57BL/6J strain (Mehta & Schmauss, 2011; Millstein & Holmes, 2007; Shanks et al., 1990). We also point out that since the daily hassles in UCS mice did not extend into the three days of behavioral testing, although these tests themselves are stressors, the behavioral modulation (or lack thereof) that we observed after chronic stress may be attributed to stress cessation.
We are not the first research group to suggest that there are sex differences in FST mobility behavior of rodents in response to low dose ketamine. For example, a pair of studies in Sprague Dawley rats found that, in the dosage range of 2.5–10 mg/kg, females respond to lower doses than males in the FST, with or without chronic stress (Carrier & Kabbaj, 2013; Sarkar & Kabbaj, 2016). Likewise, data in C57BL/6J mice from another group that used a similar dosage range has shown that females respond at a lower dose than males do 24 hours post-injection (Franceschelli et al., 2015). Perhaps surprisingly, in an experiment using chronic mild stress this group also found that males that had been given 10 mg/kg still responded at the 7-day timepoint, whereas females did not (Franceschelli et al., 2015). Thus the existing literature suggests that females show decreased immobility after ketamine at lower doses, but that the duration of action may be longer in males. This latter finding may stand in contrast to our current result that showed females trended toward a decrease in immobility 72 hours (i.e., 3 days) post-injection but males did not, although we used a higher dose (30 mg/kg) and did not test at the 7-day timepoint. We used 30 mg/kg as our standard dose here because we have previously shown that in male C57BL/6J mice, this dose (but not 10 mg/kg) has mobility enhancing properties in the 24-hour post-injection FST in UCS-exposed (but not unstressed) mice (Fitzgerald et al., 2019; Polis et al., 2019).
Here we present the novel finding that stressed males (but not females) given 30 mg/kg ketamine showed approximately twice as much investigation of the center square as vehicle animals in the OFT. While these results could be confounded by generalized increases in locomotion in males given ketamine, there was no significant effect of ketamine on total distance traveled in the OFT. Previous studies have examined sex differences in response to ketamine in the OFT at different time points. In C57BL/6J mice, 10 mg/kg ketamine was found to induce an acute (30 minutes after injection) reduction in center square exploration in female mice irrespective of stress, whereas males were not affected by this drug or stress (Franceschelli et al., 2015). In a subsequent study, this group found that chronic ketamine can reduce center square exploration in the OFT in female mice without doing so in males (Thelen et al., 2016). A different group of researchers found that chronic administration of this drug to mice from the ICR strain did not modulate center square exploration in either males or females (Kara et al., 2017). Thus the effect of ketamine on open field exploration, may depend on the dose, timing, and chronicity of exposure in addition to the mouse strain. From our study, we conclude not that one sex is more or less sensitive to ketamine, but that their specific behavioral sensitivities to ketamine differ. The time point at which the specific tests are run, and the ketamine dose, are important variables. The sex differences in behavioral sensitivity to ketamine shown in this study could be due to fundamentally different effects of this compound on neurotransmitter signaling or neuroplasticity (Thelen et al., 2019).
In this study there was a dissociation between the effects of UCS and ketamine on two different tests of novelty exploration, the OFT and EZM, which are often thought of as similar tests of anxiety-like behavior. We observed that UCS exposure produced decreased exploration of the open arms at the 48-hour post-injection timepoint in the EZM, irrespective of ketamine and sex. We saw no similar overall effect of UCS on OFT behavior, and there were no ketamine effects in the EZM, unlike in the OFT. While the tests were done at different time points, we think this finding likely reflects important differences in the neural substrates underlying performance in these two tests. It should also be noted that the mice exhibited relatively greater avoidance of the OFT center square than the EZM open arms. While this could be related to the inherent geometry of the two mazes, another possibility is that the timing of the two tests relative to injection and tail vein puncture, with OFT carried out first, influenced these behavioral outcomes.
In our measurement of plasma corticosterone 30 minutes post-injection, we found that stressed mice had lower corticosterone after a saline injection relative to unstressed mice. This, most likely, represents a blunted HPA axis response to the injection itself in the stressed mice, although we did not carry out baseline corticosterone measurement prior to injection. This could reflect neuroendocrine habituation of these mice to a novel stressor after UCS, or another mechanism; for example, changes in noradrenergic tone as a result of chronic stress. Ketamine-injected mice showed similar corticosterone levels in both stressed and unstressed groups. The mean corticosterone level was higher in stressed mice after ketamine injection as compared to saline, implying stimulation of the HPA axis by ketamine in this group. It could be that this effect was not seen in the unstressed mice due to a “ceiling” effect; that is, maximal stimulation of the HPA axis by the saline injection alone. It may also be that ketamine acutely affects the HPA axis differently in stressed and non-stressed animals. These findings do not specifically support the idea that acute ketamine-induced corticosterone changes mediate the behavioral effects of ketamine. This lack of mediation is particularly supported by the lack of correlation between post-injection corticosterone and the subsequent behavioral measures. However, these findings do support differences in HPA axis regulation in the UCS animals compared to the unstressed condition. While we also find that ketamine affects behavior differently in UCS and unstressed animals, the role of central HPA axis activity or glucocorticoids in this phenomenon remains to be explored.
The sex differences we observed in the effect of ketamine on behavior could suggest that ketamine modulates different neural circuitry in males and females. Alternatively, it could modulate similar neural circuits but this circuitry is sexually dimorphic in its behavioral output. In either case, these findings potentially have translational relevance to our understanding of human affective disorders. These data may also suggest that any putative sex differences in the therapeutic properties of ketamine are not mediated by upstream, acute effects on HPA axis responsivity, as we found this to be similar in our male and female animals. The possibility of sex differences in the modulation of neural circuitry underlying anxiety disorders versus MDD should be addressed in future clinical studies as it may be relevant to the clinical use of this drug (Chen, Huang, & Lin, 2014; Coyle & Laws, 2015; Niciu et al., 2014). Our data also lend support to a growing body of evidence suggesting that the time delay between administration of ketamine and the onset and continuation of its therapeutic-like effects is of critical importance and may differ across sexes (Fitzgerald et al., 2019; Franceschelli et al., 2015).
In conclusion, here we have shown novel, stress-dependent sex differences in behavior in response to a single injection of a subanesthetic dose ketamine in C57BL/6J mice. We find that male mice exhibited an increase in center square exploration after ketamine in the OFT (24 hours post-injection) which was more pronounced in the stressed group, whereas females showed a trend toward decreased immobility in the FST (72 hours post-injection) which was also more pronounced in the stressed group. Ketamine appeared to stimulate corticosterone in the UCS group, while this was not seen in unstressed animals perhaps due to maximal stimulation by the injection itself. The ketamine-stimulated corticosterone did not predict subsequent behavior. While future studies may further elucidate the neural mechanisms underlying these effects, such as sex-specific modulation of glutamatergic signaling in medial prefrontal cortex (Thelen et al., 2019), it should be noted that pharmacokinetic differences may play a role (Saland & Kabbaj, 2018). Our findings here describing stress-sensitivity reinforce that the effects of antidepressant medications may differ depending on stress history, dovetailing with our previous work (Fitzgerald et al., 2019; Polis et al., 2019). More generally, the discrepancies reported in the literature on the behavioral effects of ketamine in rodents—as a function of sex, strain, chronic stress, dose, behavioral test, testing lag—suggest that the field should seek clearer, more consistent biological measures of ketamine efficacy.
Our data on a single injection of this drug also complement an emerging literature on rodent ketamine sex differences (or similarities, including investigations of females instead of just males) with respect to: chronic dosing of ketamine (Thelen et al., 2016), ketamine enantiomers (Chang et al., 2018), drug self-administration or addictive behaviors (Schoepfer, Strong, Saland, Wright, & Kabbaj, 2019; Wright, Hagarty, Strong, Schoepfer, & Kabbaj, 2019; Wright & Kabbaj, 2018), hyperactivity (McDougall et al., 2019; Wilson et al., 2005, 2007), neurohormonal regulation (Dossat, Wright, Strong, & Kabbaj, 2018; Picard, Takesian, Fagiolini, & Hensch, 2019; Saland, Schoepfer, & Kabbaj, 2016; Wright, Strong, Addonizio, Brownstein, & Kabbaj, 2017), fear conditioning (Mastrodonato et al., 2018), and synaptic mechanisms (Sarkar & Kabbaj, 2016; Strong et al., 2017; Strong, Wright, & Kabbaj, 2019). These previous studies, as well as our data here and the literature described earlier in the Discussion, highlight a variety of sex differences in response to ketamine in mice and rats, and collectively underscore the importance of gaining a greater understanding of the neural mechanisms that mediate these sex differences and how they may translate to human psychopathology.
ACKNOWLEDGEMENTS
We thank Anuska Martinez Argueda, Jessica Babel, Andrew Drumheller, Tiffany Lapworth, Benjamin Stefadu, Brookelyn Wheeler, and Yuhuan Ye for assistance with this study.
Funding sources:
BW: NIH K08 MH107662, Collaboration Grant from The Kavli Neuroscience Innovators at University of Michigan, Neuroscience Fellows at the University of Michigan, Frances and Kenneth Eisenberg Scholar Award, The Taubman Emerging Scholars Award at the University of Michigan, The Pritzker Neuropsychiatric Disorders Research Consortium
JSS: NIH K08 MH116267, NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation, Collaboration Grant from The Kavli Neuroscience Innovators at University of Michigan
PF: University of Michigan Depression Center STAR Award
AG: National Institute on Drug Abuse (NIDA) T32 DA07268
JM: National Institute on Drug Abuse (NIDA) R01 DA044961, Frances and Kenneth Eisenberg Scholar Award, The Taubman Emerging Scholars Award at the University of Michigan
Footnotes
GEOLOCATION INFORMATION
All authors are located in Ann Arbor, Michigan, USA. This is where all research was carried out.
DECLARATION OF INTEREST
The authors have no financial conflicts of interest.
DATA AVAILABILITY STATEMENT
We will make all data associated with this study freely available upon publication acceptance.
REFERENCES
- Albert PR (2015, July 1). Why is depression more prevalent in women? Journal of Psychiatry and Neuroscience, Vol. 40, pp. 219–221. 10.1503/jpn.150205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AJ, Scott KM, Ferrari AJ, Norman RE, Vos T, & Whiteford HA (2014). Challenging the myth of an “epidemic” of common mental disorders: Trends in the global prevalence of anxiety and depression between 1990 and 2010. Depression and Anxiety, 31(6), 506–516. 10.1002/da.22230 [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, & Krystal JH (2000). Antidepressant Effects of Ketamine in Depressed Patients. In Biol Psychiatry (Vol. 47). [DOI] [PubMed] [Google Scholar]
- Bonde JP, Utzon-Frank N, Bertelsen M, Borritz M, Eller NH, Nordentoft M, … Rugulies R (2016). Risk of depressive disorder following disasters and military deployment: Systematic review with meta-analysis. British Journal of Psychiatry. 10.1192/bjp.bp.114.157859 [DOI] [PubMed] [Google Scholar]
- Carrier N, & Kabbaj M (2013). Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 10.1016/j.neuropharm.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Chang L, Toki H, Qu Y, Fujita Y, Mizuno-Yasuhira A, Yamaguchi JI, … Hashimoto K (2018). No sex-specific differences in the acute antidepressant actions of (R)-ketamine in an inflammation model. International Journal of Neuropsychopharmacology, 21(10), 932–937. 10.1093/ijnp/pyy053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CD, Benedict C, & Schiöth HB (2018). Experimenter gender and replicability in science. Science Advances. 10.1126/sciadv.1701427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Huang MC, & Lin SK (2014). Gender differences in subjective discontinuation symptoms associated with ketamine use. Substance Abuse: Treatment, Prevention, and Policy, 9(1), 1–7. 10.1186/1747-597X-9-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa e Silva JA, & Steffen RE (2019, November 1). Urban environment and psychiatric disorders: a review of the neuroscience and biology. Metabolism: Clinical and Experimental, Vol. 100. 10.1016/j.metabol.2019.07.004 [DOI] [PubMed] [Google Scholar]
- Coyle CM, & Laws KR (2015, May 1). The use of ketamine as an antidepressant: A systematic review and meta-analysis. Human Psychopharmacology, Vol. 30, pp. 152–163. 10.1002/hup.2475 [DOI] [PubMed] [Google Scholar]
- Dossat AM, Wright KN, Strong CE, & Kabbaj M (2018). Behavioral and biochemical sensitivity to low doses of ketamine: Influence of estrous cycle in C57BL/6 mice. Neuropharmacology, 130, 30–41. 10.1016/j.neuropharm.2017.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahringer EE, Foley EL, & Redgate ES (1974). Pituitary Adrenal Response to Ketamine and the Inhibition of the Response by Catecholaminergic Blockade1. In Neuroendocrinology (Vol. 14). [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Yen JY, & Watson BO (2019). Stress-sensitive antidepressant-like effects of ketamine in the mouse forced swim test. PLoS ONE, 14(4). 10.1371/journal.pone.0215554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, & Pitychoutis PM (2015). Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience. 10.1016/j.neuroscience.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Freeman MP, Papakostas GI, Hoeppner B, Mazzone E, Judge H, Cusin C, … Fava M (2019). Sex differences in response to ketamine as a rapidly acting intervention for treatment resistant depression. Journal of Psychiatric Research, 110, 166–171. 10.1016/j.jpsychires.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou P, Zanos P, Jenne C, Highland J, Gerhard D, Duman R, & Gould T (2018). Human experimenter sex modulates mouse behavioral responses to stress and to the antidepressant ketamine. Biological Psychiatry, 83(9, supplement), S277. 10.1016/j.biopsych.2018.02.715 [DOI] [Google Scholar]
- Hosang GM, Shiles C, Tansey KE, McGuffin P, & Uher R (2014). Interaction between stress and the BDNF Val66Met polymorphism in depression: A systematic review and meta-analysis. BMC Medicine. 10.1186/1741-7015-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara NZ, Agam G, Anderson GW, Zitron N, & Einat H (2017). Lack of effect of chronic ketamine administration on depression-like behavior and frontal cortex autophagy in female and male ICR mice. Behavioural Brain Research, 317, 576–580. 10.1016/j.bbr.2016.09.056 [DOI] [PubMed] [Google Scholar]
- Kennett Radford CD, Thomas Park UY, & Choi KH (2018). Effects of Subanesthetic Intravenous Ketamine Infusion on Corticosterone and Brain-Derived Neurotrophic Factor in the Plasma of Male Sprague-Dawley Rats, AANA Journal, October 2018. In AANA Journal (Vol. 86). Retrieved from www.aana.com/aanajournalonline [PubMed] [Google Scholar]
- Krystal JH, Abdallah CG, Sanacora G, Charney DS, & Duman RS (2019, March 6). Ketamine: A Paradigm Shift for Depression Research and Treatment. Neuron, Vol. 101, pp. 774–778. 10.1016/j.neuron.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo M, Kudo T, Matsuki A, & Ishihara H (1993). [Effects of ketamine on pituitary-adrenal axis in rats]. Masui, 42(4), 552–556. [PubMed] [Google Scholar]
- Lautarescu A, Craig MC, & Glover V (2020). Prenatal stress: Effects on fetal and child brain development. In International Review of Neurobiology (Vol. 150, pp. 17–40). 10.1016/bs.irn.2019.11.002 [DOI] [PubMed] [Google Scholar]
- Mastrodonato A, Martinez R, Pavlova IP, LaGamma CT, Brachman RA, Robison AJ, & Denny CA (2018). Ventral CA3 Activation Mediates Prophylactic Ketamine Efficacy Against Stress-Induced Depressive-like Behavior. Biological Psychiatry, 84(11), 846–856. 10.1016/j.biopsych.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Park GI, Ramirez GI, Gomez V, Adame BC, & Crawford CA (2019). Sex-dependent changes in ketamine-induced locomotor activity and ketamine pharmacokinetics in preweanling, adolescent, and adult rats. European Neuropsychopharmacology, 29(6), 740–755. 10.1016/j.euroneuro.2019.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, & Hofmann SG (2011). Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. Journal of Psychiatric Research, 45(8), 1027–1035. 10.1016/j.jpsychires.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta M, & Schmauss C (2011). Strain-Specific Cognitive Deficits in Adult Mice Exposed to Early Life Stress. Behavioral Neuroscience, 125(1), 29–36. 10.1037/a0021952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millstein RA, & Holmes A (2007). Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neuroscience and Biobehavioral Reviews, Vol. 31, pp. 3–17. 10.1016/j.neubiorev.2006.05.003 [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, Richards EM, … Zarate CA (2014). Clinical predictors of ketamine response in treatment-resistant major depression. Journal of Clinical Psychiatry, 75(5). 10.4088/JCP.13m08698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistico G, Pisanti N, Rotiroti D, Preziosi P, Cuocolo R, De Martino G, & Nistico GM (1978). EFFECTS OF ALTHESIN AND KETAMINE ON RESTING AND STRESS STIMULATED ADRENOCORTICAL ACTIVITY IN RATS. Br.J. Anaesth, 50, 891. [DOI] [PubMed] [Google Scholar]
- Picard N, Takesian AE, Fagiolini M, & Hensch TK (2019). NMDA 2A receptors in parvalbumin cells mediate sex-specific rapid ketamine response on cortical activity. Molecular Psychiatry, 24(6), 828–838. 10.1038/s41380-018-0341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polis AJ, Fitzgerald PJ, Hale PJ, & Watson BO (2019, December 30). Rodent ketamine depression-related research: Finding patterns in a literature of variability. Behavioural Brain Research, Vol. 376. 10.1016/j.bbr.2019.112153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saland SK, & Kabbaj M (2018). Sex Differences in the Pharmacokinetics of Low-dose Ketamine in Plasma and Brain of Male and Female Rats. Journal of Pharmacology and Experimental Therapeutics, 367(3), 393–404. 10.1124/jpet.118.251652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saland SK, Schoepfer KJ, & Kabbaj M (2016). Hedonic sensitivity to low-dose ketamine is modulated by gonadal hormones in a sex-dependent manner. Scientific Reports. 10.1038/srep21322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, & Kabbaj M (2016). Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats. Biological Psychiatry, 80(6), 448–456. 10.1016/j.biopsych.2015.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer KJ, Strong CE, Saland SK, Wright KN, & Kabbaj M (2019). Sex- and dose-dependent abuse liability of repeated subanesthetic ketamine in rats. Physiology and Behavior, 203, 60–69. 10.1016/j.physbeh.2017.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Griffiths J, Zalcman S, Zacharko RM, Anisman H, Griffiths J, … Anisman H (1990). Mouse Strain Differences in Plasma Corticosterone Following Uncontrollable Footshock. In Pharmacology Biochemistry & Behavior (Vol. 36). [DOI] [PubMed] [Google Scholar]
- Strong CE, Schoepfer KJ, Dossat AM, Saland SK, Wright KN, & Kabbaj M (2017). Locomotor sensitization to intermittent ketamine administration is associated with nucleus accumbens plasticity in male and female rats. Neuropharmacology, 121, 195–203. 10.1016/j.neuropharm.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong Caroline E., Wright KN, & Kabbaj M (2019). Sex and individual differences in alcohol intake are associated with differences in ketamine self-administration behaviors and nucleus accumbens dendritic spine density. ENeuro, 6(6). 10.1523/ENEURO.0221-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen C, Flaherty E, Saurine J, Sens J, Mohamed S, & Pitychoutis PM (2019). Sex Differences in the Temporal Neuromolecular and Synaptogenic Effects of the Rapid-acting Antidepressant Drug Ketamine in the Mouse Brain. Neuroscience, 398, 182–192. 10.1016/j.neuroscience.2018.11.053 [DOI] [PubMed] [Google Scholar]
- Thelen C, Sens J, Mauch J, Pandit R, & Pitychoutis PM (2016). Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behavioural Brain Research. 10.1016/j.bbr.2016.06.041 [DOI] [PubMed] [Google Scholar]
- Wei Y, Chang L, & Hashimoto K (2020, March 1). A historical review of antidepressant effects of ketamine and its enantiomers. Pharmacology Biochemistry and Behavior, Vol. 190. 10.1016/j.pbb.2020.172870 [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, … Vos T (2013). Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. The Lancet, 382(9904), 1575–1586. 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- Wilson C, Cone K, Kercher M, Hibbitts J, Fischer J, Van Lake A, & Sumner J (2005). Naloxone increases ketamine-induced hyperactivity in the open field in female rats. Pharmacology Biochemistry and Behavior, 81(3), 530–534. 10.1016/j.pbb.2005.03.018 [DOI] [PubMed] [Google Scholar]
- Wilson C, Kercher M, Quinn B, Murphy A, Fiegel C, & McLaurin A (2007). Effects of age and sex on ketamine-induced hyperactivity in rats. Physiology and Behavior, 91(2–3), 202–207. 10.1016/j.physbeh.2007.02.010 [DOI] [PubMed] [Google Scholar]
- Wright KN, Hagarty DP, Strong CE, Schoepfer KJ, & Kabbaj M (2019). Sex-Dependent Ketamine Addiction-Like Behavior Profile Following Exposure to Chronic Mild Stress. Chronic Stress, 3, 247054701983261. 10.1177/2470547019832613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KN, & Kabbaj M (2018, October 1). Sex differences in sub-anesthetic ketamine’s antidepressant effects and abuse liability. Current Opinion in Behavioral Sciences, Vol. 23, pp. 36–41. 10.1016/j.cobeha.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KN, Strong CE, Addonizio MN, Brownstein NC, & Kabbaj M (2017). Reinforcing properties of an intermittent, low dose of ketamine in rats: effects of sex and cycle. Psychopharmacology, 234(3), 393–401. 10.1007/s00213-016-4470-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, … Manji HK (2006). A Randomized Trial of an N-methyl-D-aspartate Antagonist in Treatment-Resistant Major Depression. Arch Gen Psychiatry, 856–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We will make all data associated with this study freely available upon publication acceptance.


