Abstract
In the field of tissue regeneration and tissue engineering, many years ago, various nano to macroscopic-sized materials have been used to reduce inflammation and restore damaged tissue. Whether it is safe to study the regeneration of all tissues based on the biological mechanisms of an organism composed of cells is still debated, and studies using extracellular vesicles derived from cells have become popular in the past decade. It has been reported that exosomes with a size of 100 nm or less, which plays an important role in cell–cell communication, contain various factors, such as proliferation, anti-inflammatory, and growth factors. In addition, the payload of exosomes varies depending on the parent cell and the recipient cell, and a technology to differentiate the selective payload must treat specific diseases. In this review, we examined the current trends in research using exosomes derived from cells or tissues and analyzed various research reports on factors that can affect tissue regeneration.
Keywords: Mesenchymal stem cell, Extracellular vesicle, Regeneration, Engineering, Medicine
Introduction
Extracellular vesicles (EVs) are released from cellular membranes under both physiological and pathological conditions [1]. The main function of EVs is to aid the communication of the parental cells with the recipient cells [1, 2]. EVs can be classified in several ways depending on their size and the cellular exocytic pathway they employ [3]. In this review, we attempted to demonstrate the trend of research in the field of tissue regeneration and engineering based on the EV biogenesis mechanism.
In the past decade, it has been reported that exosomes are involved in multivesicular body (MVB) maturation through intracellular endosome networks, and exosomes with a size of 30–100 nm are released when several endoplasmic reticulum and plasma membranes are fused [4]. Microvesicles or ectosomes, which are 100–1000 nm in size, sprout outward and are produced by the disruption of the plasma membrane [5, 6]. Apoptotic bodies and oncosomes, which are 100–2000 nm in size, are released by malignant cells [1]. Ectosomes are difficult to distinguish from exosomes, but they are produced during apoptosis and promote inflammation and apoptosis. It has a phospholipid postatidy-l serine on its surface, and affects the transfer of apoptotic substances between cells [1]. Oncosomes have been reported as special vesicles containing tumor-causing factors secreted from cancer cells, and are being studied in tumor microenvironments and diagnostic applications. Thus, various classifications of extracellular vesicles constantly have updated according to their size and function [3].
They consist of lipids that form plasma membranes that protect the vesicle contents and facilitate their migration to target cells [6]. Unlike microvesicles and apoptotic bodies, exosomes are generated by MVB maturation. At this time, numerous proteins involved in the intraluminal vesicle (ILV) formation process from the germination of all intracellular endosomes cooperate with the ESCRT complex [7, 8]. In addition, MVB is transported to the cell membrane by riding microtubules, and ILV is released from the cell.
Recent research on exosomes has focused on the mechanisms of biogenesis, biological activities that mediate cellular signals at long distances, the characterization of payloads, exosomes as biomarkers, and exosomes as therapeutics (Fig. 1) [9, 10]. In addition, advances in mouse and human genetics and exosome assay techniques have shifted the extracellular vesicle class from a constitutive class of secreted vesicles to being recognized as regulated bioactive mediators of cell biology, immunology, and inflammation [11, 12]. Recently, the EV-engineering field has been widely discussed as a therapeutic agent for diseases [13], diagnostic techniques [14], and target delivery systems [9]. The usage ability of exosomes obtained from biological sources, such as stem cells, urine, and plasma, is being evaluated [11, 13, 15]. In contrast, cellular studies conducted with tumor cells have also shown that PD-L1 induces the disruption of the immune system. The study related to the mechanisms of immunological function and specific molecules for regenerating tissue damaged by tumors is also of great interest [16]. When tumor-derived exosomes were compared with normal cell-derived exosomes, it was revealed that the factor that lowers the inflammatory function of T cells is PD-L1 on the surface of tumor-derived exosomes [16–18]. It has been reported that exosomes generated from cancer cells communicate with each other through a mechanism similar to that of normal cells, and there are also reported that their function varies depending on the difference in loads of harmful substances [19]. Research on the mechanism of skin regeneration by immune cells has also been reported as an interesting topic in the field of understanding communication between cells by discovering important biomarkers through miRNA profiling of exosomes generated by the surrounding cells [20, 21]. Thus, we believe that the study of exosomes organically active in vivo can be expected in the field of tissue regeneration clinically and that it is a valuable study through understanding and proof of biological mechanisms in molecular biology.
Fig. 1.
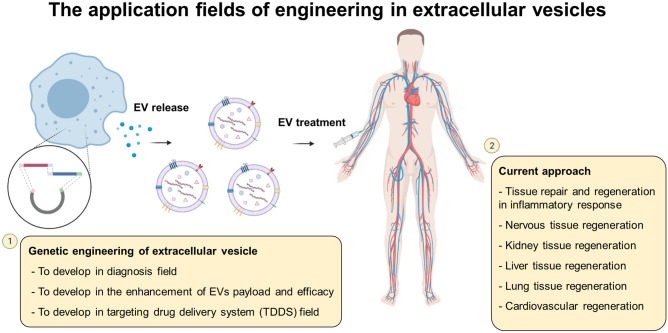
The application fields of engineering in extracellular vesicles. The genetic engineering of extracellular vesicles and the current approach are summarized. This image was drawn by D.J.P and Y.J.S. in the Adobe Photoshop 7.0 software
Basics of EV biology
EV biogenesis and secretion
The role of MVB maturation
EV biogenesis is regulated by the role of MVB, which redirects endocytosed proteins to their destinations (cells or organ etc.) [5]. Materials are internalized by endocytosis on the cell surface and transported through early and late endosomes (Fig. 2) [7, 22]. During MVB maturation, some substances that need to be separated move to the center of the cell, and unnecessary proteins are degraded by lysosomal binding [23]. EV secretion is important for the formation of ILVs in MVB [24]. They are surrounded by lipids that form a plasma membrane that protects the contents of the vesicle and facilitates their migration to target cells [25]. Recently, the classification of substances using the endosomal sorting complex request for transport (ESCRT)-dependent or -independent mechanism has been reported [8]. Briefly, ESCRT contains the cytoplasmic complex of ESCRT 0, I, II, III, and Vps4 [7, 8]. The ESCRT-complex gathers on the MVB membrane surface during maturation, and MVB moves to the plasma membrane through microtubules to release EVs.
Fig. 2.
The image of the release mechanism of extracellular vesicles (EVs) from the cells and composition of EVs. Additionally, the images of EV release from the cells using transmission electron microscopy (TEM). The TEM images show multivesicular bodies (MVB, red arrow), exosomes (30–100 nm, green arrow), and microvesicles (100–1000 nm, blue arrow). This image was drawn by D.J.P and Y.J.S. in the Adobe Photoshop 7.0 software
EV composition
In the EV studies reported in the past decade, when EVs are transferred from parental cells to recipient cells, their activity is maintained, and it has been demonstrated that they carry several types of macromolecules [1, 3]. Exosomes contain proteins and nucleic acids (DNA and RNA), lipids, and membrane-binding proteins derived from parental cells, and it is hypothesized to be due to biological causes rather than a random occurrence [1]. Representative hypotheses are related to the function and characteristics of EVs as mediators of communication between cells and their ability to transport to target cells [2]. Therefore, understanding the pathways leading to the development of exosomes and their internalization within the target cell represents an important goal in the current EV research field. Furthermore, as studies revealing the intrinsic biomolecules of exosomes are relevant to disease conditions and treatment studies, the discovery of new EV biomarkers is also considered a potential study. In the following section, the composition of representative biomolecules in EVs is presented, and the known mechanisms are described.
First, the proteins inherent in EVs contain a complex set of proteins derived from the parent cell (cytoplasm, nucleus, mitochondria, and membrane-bound proteins) [9]. Several proteomic studies have determined EV properties and activity by defining unique EV properties specifically related to parental cells [26]. Vesicle-specific proteins are often used as markers, such as specific heat stress proteins (HSPs), as well as several proteins related to the biogenesis process of EVs, such as tetraspanins (CD9, CD63, and CD81), major histocompatibility complex (MHC) molecules, and proteins of ESCRT complexes (Alix and TSG101) [27, 28]. The most characterized in EV protein classification are those mediated by members of the ESCRT family, but increasing evidence suggests that several ESCRT-independent pathways may also be involved [29].
Next, the presence of DNA molecules in EVs has been widely reported [30]. Mitochondria and genomic DNA was found in EVs derived from ds-DNA, and those isolated from the cell culture medium and biological fluids (human and mouse) are found inside EVs rather than outside [31, 32]. The presence of genomic DNA in EVs reflects the mutational status of parental tumor cells for genes such as P53, KRAS, and EGFR. They strongly support the diagnostic molecules in EVs and their potential role in the clinic [3, 31, 32]. Therefore, it was hypothesized that DNA payloads in EVs could be fluidly packaged by cell-type-specific mechanisms. However, it has been reported that EV shows a random distribution rather than an even amount of DNA distribution because of a specific mechanism; therefore, further research is needed [3, 31].
Finally, all cells have been reported to release EVs rich in both coding and noncoding RNAs, such as microRNAs (miRNAs), ribosomal RNAs (rRNAs), and circular RNAs (circRNAs) [28, 33]. In various studies of RNA analysis in exosomes, RNA is capable of selective transport through exosomes [33–35]. The report on RNAs or others in exosomes is an important study to discover new biomarkers and target substances that can target damaged tissues [36, 37]. Interest in miRNA studies, which is known as a major cause of gene and protein expression, has increased, and it has been reported that the miRNA profile of released exosomes cannot be randomly generated under certain immune response conditions [33, 38].
Cell–cell communication and payload in EVs
According to a recent report, all cell types have been reported to carry useful substances and communicate through EVs (Fig. 3). This has a strong influence not only on clinical research but also on the discovery of new biomarkers through mechanism analysis [39]. These cell-to-cell communication studies have been reported in studies of cancer and normal cells [19]. tumor-derived EVs play a key role in the crosstalk between malignant and transformed cells and the immune system, and they reported that cancer cells are used to suppress immune surveillance [16]. In addition, PD-L1 is found on the surface of EVs derived from tumor cells, which inhibit T cell activity, which is a study on tumor cell-derived EVs [17].
Fig. 3.
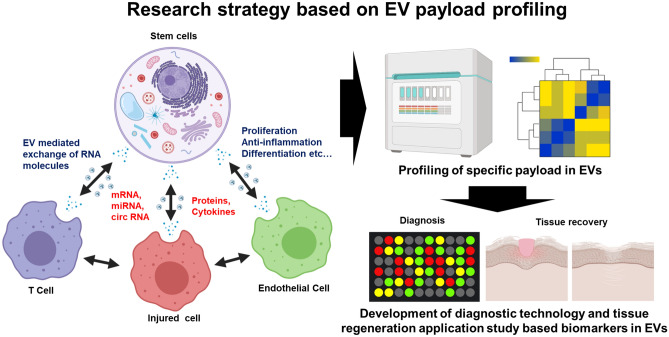
Research strategy based on EV payload profiling after cell–cell communication in the cellular microenvironment. New molecules generated by cell–cell communication can be used as special biomarkers and applied in the applied research and diagnostic tests of damaged tissue repair. This image was drawn by D.J.P and Y.J.S. in the Adobe Photoshop 7.0 software
Tissue regeneration using exosomes can be accomplished after determining that communication between the donor and recipient cells is associated [40]. However, the development of a platform that induces the transfer of substances between cells that contribute to exosomes and recipient cells based on intercellular communication has been reported, but it is a future task to resolve the uncertain mechanism in the industrial aspect. The development of a platform for trafficking exosomes by structurally synthesizing fluorescence directly to specific substances by analyzing the payload of exosomes generated from donor cells and delivering them to target recipient cells is also an interesting study [41, 42]. The transfer of substances by intercellular membrane binding of exosomes has already been revealed by a number of reports, and a study of profiling of newly generated exosomes from recipient cells are required [35, 36, 43]. In addition, it has been reported that in the communication between cells, analysis of receptors or membrane proteins required for exosomes to bind to membranes can also affect the payload [44, 45]. The importance of intercellular communication has been mainly reported in the field of tumor metastasis research, but now it is actively reported in the study of growth promotion and mechanism analysis of normal cells [37, 46, 47]. Therefore, research on cell-to-cell communication is increasing with interest in the effect of EVs on tumor progression, revealing the factors responsible for the crosstalk between blood tumor cells and bone marrow microenvironments, and extracellular vesicles, especially exosomes [48].
Current approach of stem cell-derived EVs in tissue regenerative medicine
Study on tissue repair and regeneration in an inflammatory response
Studies on tissue repair and regeneration have been considered to be mediated by direct intercellular interactions and the release of secretory factors [49, 50]. Various studies on EVs are presented in Table 1. In the 1980s, the products of multivesicular endosomes released extracellular vesicles from the surface of mature reticulocytes [51], and it was revealed that inflammation studies using exosomes are closely related to communication between cells [52]. Since exosomes can mediate immune stimulation and response, it is necessary to differentiate their relevance through payload profiling of EVs by comparing control and disease models to characterize cell sources, release mechanisms, and payloads [12, 53]. For example, EVs isolated from infected macrophages after bacterial or viral infection provide protection in the presence of APC and T cells in a mouse model, showing that EVs modulate immune responses in a simple model [54–57]. It has been reported that EV of DC cells due to wounds in skin tissue can affect wound healing, and proliferative substances such as EGFR, which affects wound healing, are inherent [46].
Table 1.
The list of fields in which EV has applications and a description of the methods, target disease, and related molecules
| Purpose of study | Cells/Animal | Source of EVs | EV isolation method | Target disease/organ | Related molecules | References |
|---|---|---|---|---|---|---|
| Wound healing | Wounded Mouse model | Plasma fluid | Ultracentrifuge (100 k × g, 70 min) | Immune response/skin | TBC1D3 | [54] |
| Parasite infection | Toxoplasma gondii | Medium | Ultracentrifuge (100 k × g, 1 h) | Immune response | Anti-TAg IgA, IFN-γ | [55] |
| Bacterial infection | M. bovis BCG, M. tuberculosis H37RV (Mtb) | Medium | Ultracentrifuge (100 k × g, 1 h) Filtration (0.22 μm) | Immune response | CD4, CD8, IL-12, CD83, CD86, CD69 IFN-γ | [56] |
| Viral infection | NPC tumor line | Medium | Ultracentrifuge (100 k × g, 1 h) | Immune response | LMP1, gal 9 | [57] |
| Biological study (crosstalk) | Wound-edge tissue cell (mouse) | Medium | Ultracentrifuge (245 k × g, 2 h) | Immune response/skin | hnRNPA2B1 | [58] |
| Atopic dermatitis | Adipose tissue-MSC (human) | Medium | Filtration (0.22 μm) | Immune response/skin | IL-4, IL-5, IL-13, TNF-α, IFN-γ, IL-17, and TSLP | [59] |
| Cardiovascular | Cardiosphere-derived cells | Medium | Ultracentrifuge | Myocardial infarction/hearts | MHC-I(+), MHC-II(−), CD80(−), CD86(−) | [64] |
| Cardiovascular | Cardiosphere-derived cells | Medium | Ultracentrifuge (100 k × g, 90 min), Filtration (0.22 μm) | Myocardial infarction/Heart | ephrin A3 and PTP1b | [66] |
| Cardiovascular | Cardiosphere-derived cells | Medium | ExoQuick‐TC Kit | Myocardial infarction/Heart | miR-146a | [67] |
| Regeneration | Adipose‐MSC | Medium | ExoQuick‐TC Kit | Liver fibrosis | miRNA‐181‐5p, miR-67 | [69] |
| Regeneration | Bone marrow-MSC | Medium | Ultracentrifuge (100 k × g, 4 h) | The brain | miR-124 | [69] |
| Regeneration | Human renal tubular cells | Medium | Ultracentrifuge (100 k × g, 70 min), Filtration | Hypoxic/kidney | HLA-A1 | [70] |
| Regeneration | Human neural stem cell | Medium | Ultracentrifuge, Filtration (0.22 μm) | Ischemic stroke | integrin α 2b (CD41b), integrin β-1 (CD29) | [71] |
| Regeneration | Pluripotent Stem Cell-Derived NSC and MSC | Medium | Ultracentrifuge, Filtration (0.22 μm) | Brain | Th17, Treg, functional M2 | [72] |
Research on tissues damaged by inflammatory reactions has focused on successful tissue repair. Myeloid progenitor cells, such as monocytes and macrophages, occur in the early stages of the inflammatory reaction in the wound bed. In this study, they evaluated the ability of keratinocyte-derived EVs to reduce the inflammatory response and tissue repair through conversion to fibroblast-like cells [58]. A large amount of bp RNA was found in EVs derived from the edge of wounded tissue, and factors related to wound healing were identified through miRNA analysis.
In addition, a study has shown that exosomes from adipose tissue-derived MSCs (ASCs) improve atopic dermatitis (AD) inflammation in vivo [59]. As a therapeutic point of ASC-derived exosomes in this study, the regeneration of tissues damaged by AD was suggested as ASC-derived exosomes to deliver ceramides to synthetic substances. They showed that ASC-derived exosomes can enhance the epidermal permeability barrier function, consistent with a marked increase in ceramide and a decrease in the immune response during AD. Thus, the results of this study suggest that stem cell-derived EVs are a promising cell-free alternative to the current limited EV treatment for AD with potentially harmful side effects.
Study on cardiovascular regeneration
Cardiovascular disease is the leading cause of death worldwide. Damage to the cardiovascular system leads to the loss of cardiomyocytes and is replaced by a fibrous skin wound bed. The lack of cardiomyocytes then reduces heart contraction, leading to pathological diastole and mechanical dysfunction, leading to heart failure [60, 61]. Therefore, recently, several strategies for improving cardiac function have led to cellular and non-cellular therapies. Tissue engineering technology using the latest EV as a diagnostic test technology is used to develop a targeted drug delivery system that recognizes disease markers or delivers a target ability of the EV surface [61–63]. In an experiment using a mouse model, it was confirmed that after MI, administration of EVs derived from murine heart bulbs rich in miR-451 inhibited myocardial cell apoptosis [64]. Furthermore, the administration of EVs predominantly derived from human stem cells also attenuated cardiac remodeling and improved cardiac function in preclinical studies of MI [64, 65]. In fact, the delivery of MSC-derived exosomes to the mouse heart reduced ROS and promoted the viability of cardiomyocytes after ischemia–reperfusion injury, resulting in decreased infarct size and improved cardiac function [66, 67]. Therefore, the delivery of miRNAs or ligands related to cell regeneration materials through the development of exosomes targeting the heart will be an attractive study, and if the molecular mechanism of exosomes is understood, the potential will be improved.
Study on nerve, kidney, liver, and lung tissue regenerative therapy
EVs are a treatment that allows tissue regeneration in all major organs. The treatment effect and differentiation can be increased while minimizing the selective side effects of the parent cell type and payload inside the EV. As in the previously reported studies, exosomes have demonstrated regenerative properties by reducing inflammation and cell death while promoting proliferation and angiogenesis. In addition, adipose mesenchymal stem cells overexpressing miR-181-5p were engineered to target hepatic stellate cells and reduce liver damage in a mouse model of liver fibrosis [68]. Furthermore, studies have reported the induction of neurogenesis by bypassing the blood–brain barrier after ischemic injury in a murine photo thrombosis model using modified EVs to effectively deliver miR-124 [19, 69]. Human renal tubular cells and stem cell-derived EVs have been reported to prevent kidney damage in a rat model of bilateral renal ischemia [70]. Human neural stem cell-derived EVs have a neuroprotective effect and have been reported to significantly improve tissue and functional levels in both a mouse model of thromboembolic stroke and a pig model of ischemic stroke [71, 72]. In this study, since the factors in EVs showed improvement in tissue regeneration, it can be considered that exosome-mediated tissue regeneration has potential. Most of the bioactive molecules secreted by MSCs also exert a paracrine effect on surrounding cells in the tissue microenvironment, providing therapeutic effects [73, 74]. In other words, the released factors in MSC-derived EVs contribute to tissue regeneration.
In contrast, studies focusing on various lung diseases, idiopathic pulmonary fibrosis, acute respiratory distress syndrome, and tracheal bill dysplasia have been conducted for various therapeutic purposes using MSCs. MSC-derived EV overexpression helped relieve inflammation and repair of epithelial alveoli in a mouse model of miR-30B-3P acute lung injury [20]. In addition, the systemic administration of MSCs to patients with acute respiratory distress syndrome reduces respiratory, hemodynamic, and multi-organ failure [75]. Understanding the biology of EVs will potentially advance their therapeutic applications in various tissue regenerative therapies.
Potential of EVs as therapeutic in various engineering fields
Engineering to develop in the enhancement of EV payload and efficacy
Exosomes act as an alternative to cell-free therapy in the field of cell therapy, but they face challenges when transitioning to clinical research [12, 76]. Table 2 summarizes recent studies on engineering approaches for EVs. In many preclinical studies, the characteristics of heterogeneous and homogeneous stability and immune efficacy have not been completely evaluated according to cell therapy [77]. The expectation of preclinical studies using exosomes is increasing through studies showing disease improvement effects in allogeneic and self-treatment. What is selected as a complementary point of exosome research is that the development and yield of technology to obtain exosomes that can be effectively used for tissue regeneration is low [10]. Thus, it is necessary to develop a technology to increase the number of exosomes produced by cells in an in vitro environment.
Table 2.
Factors required for EV engineering applications
| Cell type | Tools | Detail | Related factors | References | Year |
|---|---|---|---|---|---|
| A. Study on improvement and performance of EV | |||||
| MSCs | Iron oxide based PLGA-PEI nanoparticles | regulates autophagy mechanism | Rab7, Becilin-1, ATG5, STX17 | [78] | 2020 |
| U87 (glioblastoma cells), A549 (basal epithelial cells) | High frequency acoustic irradiation | Regulates a Ca2 + -dependent mechanism | ALIX, CD63, TSG101, syntenin-1, flotillin-1, Rab27a | [79] | 2020 |
| MDA-MB-231 cells | Genetic transfection | Described biological pathway to EV generation by Ca2 + -dependent mechanism | Munc13-4, Rab27, a-SMA | [80] | 2018 |
| RAW264.7 cells | Calcium phosphate (CaP) particles | Stimulated EV release by the release of Ca2 + into the cytosol | Ca2+ content | [82] | 2018 |
| Salmon head kidney leuko-cytes | Genetic transfection | Involved autophagy mechanism | ALIX, LC3B, MHC-Iib, flotillin-1 | [83] | 2018 |
| Mouse embryonic fibroblasts | Transient electrical stimulus | Involved by heat shock response at the nanopore site during CNP transfection | PTEN, miR-128, CD47, CDX-CD47, CREKA-CD47 | [92] | 2020 |
| Target disease | Tools | Detail | Biomarkers | References | Year |
|---|---|---|---|---|---|
| B. Application study on diagnosis with EV | |||||
| Colon cancer (CRC) | Serum | Disease detection using biomarker sensitivity and specificity is important in noninvasive analysis | miR-23a, miR-1246, let-7a, miR-1229, miR-150, miR-223, and miR-21 | [97] | 2020 |
| lung cancer | Blood, urine, breast milk | The exploration of biomolecule transport, circulation stability, tumor specificity and other properties of exosomes is important | miR-660-5p, miR-29a, miR-21, miR-494, miR-193a-3p, miR-210-3p, miR-5100, miR-23a, miR-9 | [14] | 2020 |
| Essential thrombocythemia (ET) | Bone marrow tissue | By demonstrating the potential function of circRNA and its interaction with specific miRNAs, circRNA-based ET diagnostic studies were performed | circDAP3, circASXL1, and circRUNX1 | [101] | 2020 |
| Cardiovascular diseases (SCAD) | Serum | SCAD diagnosis using miRNA of EV in serum | miR-942-5p, miR-149-5p, and miR-32-5p | [102] | 2020 |
| Purpose | Tools | Detail | References | Year |
|---|---|---|---|---|
| C. Application study on targeting delivery with EV | ||||
| Delivery molecules to EV | Iron oxide nanoparticles (IONPs)–incorporated MSCs (IONP-MSCs) | When IONP-NV was injected into the infarcted heart, rapid transition from the inflammatory stage to the recovery stage was induced and apoptosis was reduced | [107] | 2020 |
| Biomanufacturing platform of cancer‐targeted exosomes | EV engineering | The targeted delivery ability of mAb-exosomes developed and validated in this study provides a powerful drug delivery strategy for cancer treatment | [110] | 2020 |
Recently, our research team has developed a technology that can increase the number of EVs generated from stem cells by approximately 100 times by using iron oxide nanoparticles with a positive surface charge [78]. It was found that the components of the obtained exosomes contained miRNA factors related to proliferation, anti-inflammatory, and ROS reduction.
In addition, the generation of cells was stimulated by high-frequency sounds without harming cell viability [79]. Interestingly, it has been shown that the enrichment of calcium ions in the extracellular environment stimulates the ESCRT pathway, whether it can control the exosome production. As a result, the production rate of exosomes was concentrated by 8–10 times, and the time was reduced by 2.1 times.
Finally, various studies have been proposed to improve the yield of exosomes [76]. For example, studies on chemical (ionomycin [80], intracellular calcium [81, 82]), extracellular DNA [83], liposomes [84], proteomics-based strategies [85], pH [86], and mechanical stimulation [87] have been reported. Studies have also been conducted to induce cellular hypoxia [88], cytoskeletal protein alteration [89], and studies have been conducted on gene overexpression [90], exposure to heat, oxidative photodynamic, or radiation stress [91]. Recently, a cell nano perforation technique has also been reported for processing high concentrations of exosomes [92]. Recently, a method for obtaining exosomes from a medium using three-dimensional culturing genetically modified cells or stem cells using a bioreactor has also been devised [93, 94]. Therefore, we believe that research to improve the yield of exosomes requires the development of various methods for clinical application in the field of tissue regeneration.
Engineering to develop in diagnostic field
In many studies, scientists have developed a technology to diagnose biomarkers found in the early stages of the disease from urine or blood derived from the body, or plasma of the wound area [13, 95, 96]. They are also considered that small microvesicles, which released a byproduct of cells, are now called exosomes, and are measuring biomarkers derived from tumor cells by looking for markers of diseases circulating in the blood in exosomes [97–99]. Currently, a diagnostic test using exosomes obtained from liquid biopsies of blood and urine has been reported by the FDA [14]. In other words, the evolution of the disease can be evaluated by the payload and concentration of exosomes, and it is to prove that an engineered liquid biopsy-based on exosomes is a powerful tool for analyzing cancer metastasis or disease progression [14, 100].
The Petr Prikryl group studied EVs isolated from urine as a non-invasive biomarker source. An attempt was made to find a biomarker capable of early diagnosis of immune diseases caused by ANCA-associated vasculitis (AAV) [15]. By analyzing protein differences in EVs using LC–MS, they proved that they represent important regulation of Golgi enzymes such as MAN1A1, which may be involved in T cell activation by N-glycan glycosylation, thereby evaluating a biomarker for the onset and diagnosis of AAV.
Next, the Qiang Wang group, which used a clinical approach, tried identifying and diagnose lesions by analyzing the circRNA profile of exosomes derived from the bone marrow of ET patients in relation to essential thrombocythemia (ET) development [101]. At this time, the circRNA profile was significantly changed in the bone marrow-derived exosomes of ET patients, of which circDAP3, circASXL1, and circRUNX1 tended to decrease in ET patients. Thus, this study provides insights into the role of circRNA in ET pathogenesis and reported that circRNA, as well as miRNAs involved in cellular communication, will provide a predictable basis for predictive diagnosis and treatment of their potential functions [102].
EV engineering to develop a targeted drug delivery system
The limitations in the clinical application of stem cells and bio-derived exosomes are due to their low yield and random targeting ability [103]. Numerous researchers have developed techniques that can target tumors or inflammatory-damaged sites to regenerate tissues by modifying the surface of exosomes [104, 105]. The important points to be considered for creating an exosome-based drug delivery system are (1) immunogenicity and cytotoxicity of exosomes, (2) clarification of separation and purification methods, and (3) target properties. For example, the composition of exosomes derived from stem and cancer cells clearly shows that they have different payloads [106]. In other words, the molecules were released differently payload from the cell type, and the efficacy was different in the recipient cells without toxicity [107, 108]. In the MISEV2018, there has been a standard approach to study exosomes and a suggested separation and characterization method to extract exosomes of less than 100 nm [109]. Furthermore, released exosomes cannot target a specific destination because of the presence of receptors and membrane-binding proteins on the surface [103, 110, 111]. To target destinations, studies reported through engineering modifications of proteins such as CRISPR/CAS9 or aptamers have been introduced [112–114]. To verify the efficiency of targeted drug delivery, the histone deacetylase inhibitor lomidepsin was loaded into mAb-exosomes, which showed increased anticancer toxicity in vitro. This was demonstrated by evaluating the cancer-targeting ability of targeted exosomes in tumor transplant animal models and evaluating the anticancer efficacy of romidepsin [110].
CRISPR-Cas9 technology, which is widely used in cell and animal experiments, presents obstacles to its clinical application [113]. The researchers confirmed that the CRISPR-Cas9-sgRNA and Cas9 protein can be packaged into EVs that exist as sgRNA and Cas9 protein complexes of sgRNA: Cas9 ribonucleoprotein [111, 112]. However, the effect of deletion of the target gene by Cas9 is not shown in recipient cells, even if the exosome helps deliver Cas9 to the recipient cell. To remove the target gene, even in the recipient cell, we developed an artificial exosome in which the CD63-Cas9 protein of the exosome was fused, and the efficiency of effectively removing the target gene from the recipient cell was verified by fluorescence signal and gene expression. In this way, it can be said that complex studies using CRISPR/Cas9 technology and exosomes are also precious [112].
Studies have also been reported on the development of devices capable of generating EV-targeted tumors [115], and studies have also been reported to evaluate their effectiveness by incorporating drugs [116, 117]. They suggested that the stability of exosomes should be ascertained when the drug is loaded, and that evaluation of the efficacy released at the destination is important [118, 119]. Studies of regenerating damaged tissues using genetically engineered stem cell-derived exosomes have been mainly conducted in miRNA-based mass transfer studies and evaluated their target targeting ability [110, 112]. It has been reported that the effect is more than about 10 times that of naive exosomes when the payload is increased or when the number of exosomes is treated at high concentrations in damaged tissue [105]. Therefore, we believe that for industrial applications of exosomes, there will be a huge impact when a strategy to amplify function and a biotechnology approach that can increase yield are combined.
Conclusion and perspectives
Studies on the use of stem cell-derived exosomes in regenerative medicine have been reported in all major organs and tissues [61, 63, 65]. Unlike cell therapy, non-cell therapy has no side effects, and the field of EV study improves originality by communication between parental and recipient cells [19, 37, 45]. This implies that research on exosomes could lead to findings that could help us treat various diseases and regenerate tissues [47, 49]. One of the applications of EVs, which are secreted into all biological fluids occurring in various cell types and tissues, in the field of biomaterials, is drug delivery and improvement of regenerative medicine [53, 71, 74]. However, previously reports showed excellent therapeutic potential efficacy with application in EVs through bioengineering techniques such as cargo loading and surface modification [84, 85]. The low EV yield and the lack of the ability to target specific organs or molecules are two disadvantages associated with the use of EV; however, these limitations can be overcome by international regulation [77, 78]. In addition, clinical research using EVs will be accelerated by the development of 3D culture technology and increasing the yield of EVs by the use of bioreactors [93, 94]. The view of scientists on EVs is constantly revising standards to establish optimized standards, thus in a few years, the heterogeneous characteristics of EVs will become clear [35, 40, 43]. Therefore, we evaluated the potential of therapeutic potential in the EV field, including various tissue regeneration-engineering techniques. We expect that the use of exosomes in the field of tissue regeneration will have be beneficial and have a considerable positive impact.
Acknowledgements
This research was supported by a Grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant No.: HI19C1334). and by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (No. NRF-2020R1A2C1009789).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27:172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lázaro-Ibáñez E, Sanz-Garcia A, Visakorpi T, Escobedo-Lucea C, Siljander P, Ayuso-Sacido A, et al. Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: apoptotic bodies, microvesicles, and exosomes. Prostate. 2014;74:1379–1390. doi: 10.1002/pros.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of exosome composition. Cell. 2019;177:428–445e18. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. [DOI] [PMC free article] [PubMed]
- 7.Jackson CE, Scruggs BS, Schaffer JE, Hanson PI. Effects of inhibiting VPS4 support a general role for ESCRTs in extracellular vesicle biogenesis. Biophys J. 2017;113:1342–1352. doi: 10.1016/j.bpj.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 9.Joshi BS, de Beer MA, Giepmans BG, Zuhorn IS. Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano. 2020;14:4444–4455. doi: 10.1021/acsnano.9b10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buratta S, Tancini B, Sagini K, Delo F, Chiaradia E, Urbanelli L, et al. Lysosomal exocytosis, exosome release and secretory autophagy: the autophagic- and endo-lysosomal systems go extracellular. Int J Mol Sci. 2020;21:2576. doi: 10.3390/ijms21072576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pisano S, Pierini I, Gu J, Gazze A, Francis LW, Gonzalez D, et al. Immune (cell) derived exosome mimetics (IDEM) as a treatment for ovarian cancer. Front Cell Dev Biol. 2020;8:553576. doi: 10.3389/fcell.2020.553576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X, Xie F, Wang L, Zhang L, Zhang S, Fang M, et al. The function and clinical application of extracellular vesicles in innate immune regulation. Cell Mol Immunol. 2020;17:323–334. doi: 10.1038/s41423-020-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho BS, Kim JO, Ha DH, Yi YW. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res Ther. 2018;9:187. doi: 10.1186/s13287-018-0939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu K, Zhang C, Du T, Gabriel ANA, Wang X, Li X, et al. Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed Pharmacother. 2021;134:111111. doi: 10.1016/j.biopha.2020.111111. [DOI] [PubMed] [Google Scholar]
- 15.Prikryl P, Satrapova V, Frydlova J, Hruskova Z, Zima T, Tesar V, et al. Mass spectrometry-based proteomic exploration of the small urinary extracellular vesicles in ANCA-associated vasculitis in comparison with total urine. J Proteomics. 2021;233:104067. doi: 10.1016/j.jprot.2020.104067. [DOI] [PubMed] [Google Scholar]
- 16.Shenoy GN, Bhatta M, Loyall JL, Kelleher RJ, Jr, Bernstein JM, Bankert RB. Exosomes represent an immune suppressive T cell checkpoint in human chronic inflammatory microenvironments. Immunol Invest. 2020;49:726–743. doi: 10.1080/08820139.2020.1748047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177:414–427.e13. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan Z, Gao X, Dong Y, Zhao Y, Chen X, Yang G, et al. Exosome-mediated cell-cell communication in tumor progression. Am J Cancer Res. 2018;8:1661–1673. [PMC free article] [PubMed] [Google Scholar]
- 20.Cho KHT, Xu B, Blenkiron C, Fraser M. Emerging roles of miRNAs in brain development and perinatal brain injury. Front Physiol. 2019;10:227. doi: 10.3389/fphys.2019.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi X, Wei X, Lv H, An Y, Li L, Lu P, et al. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp Cell Res. 2019;383:111454. doi: 10.1016/j.yexcr.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 22.Scott CC, Vacca F, Gruenberg J. Endosome maturation, transport and functions. Semin Cell Dev Biol. 2014;31:2–10. doi: 10.1016/j.semcdb.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 23.Xia L, Gu W, Zhang M, Chang YN, Chen K, Bai X, et al. Endocytosed nanoparticles hold endosomes and stimulate binucleated cells formation. Part Fibre Toxicol. 2016;13:63. doi: 10.1186/s12989-016-0173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodman PG, Futter CE. Multivesicular bodies: co-ordinated progression to maturity. Curr Opin Cell Biol. 2008;20:408–414. doi: 10.1016/j.ceb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 26.Keerthikumar S, Gangoda L, Liem M, Fonseka P, Atukorala I, Ozcitti C, et al. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget. 2015;6:15375–15396. doi: 10.18632/oncotarget.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurley JH, Odorizzi G. Get on the exosome bus with ALIX. Nat Cell Biol. 2012;14:654–655. doi: 10.1038/ncb2530. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Hernandez D, Gutiérrez-Vázquez C, Jorge I, López-Martín S, Ursa A, Sánchez-Madrid F, et al. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem. 2013;288:11649–11661. doi: 10.1074/jbc.M112.445304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno-Gonzalo O, Fernandez-Delgado I, Sanchez-Madrid F. Post-translational add-ons mark the path in exosomal protein sorting. Cell Mol Life Sci. 2018;75:1–19. doi: 10.1007/s00018-017-2690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289:3869–3875. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai J, Wu G, Jose PA, Zeng C. Functional transferred DNA within extracellular vesicles. Exp Cell Res. 2016;349:179–183. doi: 10.1016/j.yexcr.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm (Vienna) 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 33.Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Ströbel T, Erkan EP, et al. miR-1289 and "zipcode"-like sequence enrich mRNAs in microvesicles. Mol Ther Nucleic Acids. 2012;1:e10. doi: 10.1038/mtna.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulkarni B, Gondaliya P, Kirave P, Rawal R, Jain A, Garg R, et al. Exosome-mediated delivery of miR-30a sensitize cisplatin-resistant variant of oral squamous carcinoma cells via modulating Beclin1 and Bcl2. Oncotarget. 2020;11:1832–1845. doi: 10.18632/oncotarget.27557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gidlöf O, Evander M, Rezeli M, Marko-Varga G, Laurell T, Erlinge D. Proteomic profiling of extracellular vesicles reveals additional diagnostic biomarkers for myocardial infarction compared to plasma alone. Sci Rep. 2019;9:8991. doi: 10.1038/s41598-019-45473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang G, Lin G, Zhu Y, Duan W, Jin D. Emerging technologies for profiling extracellular vesicle heterogeneity. Lab Chip. 2020;20:2423–2437. doi: 10.1039/D0LC00431F. [DOI] [PubMed] [Google Scholar]
- 37.Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49:347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Romero N, Esteban-Rubio S, Rackov G, Carrión-Navarro J, Belda-Iniesta C, Ayuso-Sacido A. Extracellular vesicles compartment in liquid biopsies: clinical application. Mol Aspects Med. 2018;60:27–37. doi: 10.1016/j.mam.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Delpech JC, Herron S, Botros MB, Ikezu T. Neuroimmune crosstalk through extracellular vesicles in health and disease. Trends Neurosci. 2019;42:361–372. doi: 10.1016/j.tins.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raimondo S, Giavaresi G, Lorico A, Alessandro R. Extracellular vesicles as biological shuttles for targeted therapies. Int J Mol Sci. 2019;20:1848. doi: 10.3390/ijms20081848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antes TJ, Middleton RC, Luther KM, Ijichi T, Peck KA, Liu WJ, et al. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J Nanobiotechnology. 2018;16:61. doi: 10.1186/s12951-018-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quiroz-Baez R, Hernández-Ortega K, Martínez-Martínez E. Insights into the proteomic profiling of extracellular vesicles for the identification of early biomarkers of neurodegeneration. Front Neurol. 2020;11:580030. doi: 10.3389/fneur.2020.580030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson BC, Maragh S, Ghiran IC, Jones JC, DeRose PC, Elsheikh E, et al. Measurement and standardization challenges for extracellular vesicle therapeutic delivery vectors. Nanomedicine (Lond) 2020;15:2149–2170. doi: 10.2217/nnm-2020-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonda A, Kabagwira J, Senthil GN, Wall NR. Internalization of exosomes through receptor-mediated endocytosis. Mol Cancer Res. 2019;17:337–347. doi: 10.1158/1541-7786.MCR-18-0891. [DOI] [PubMed] [Google Scholar]
- 45.Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 46.Tintut Y, Demer LL. Exosomes: nanosized cellular messages. Circ Res. 2015;116:1281–1283. doi: 10.1161/CIRCRESAHA.115.306324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 48.Morrissey SM, Yan J. Exosomal PD-L1: roles in tumor progression and immunotherapy. Trends Cancer. 2020;6:550–558. doi: 10.1016/j.trecan.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Verrilli MA, Court FA. Exosomes: mediators of communication in eukaryotes. Biol Res. 2013;46:5–11. doi: 10.4067/S0716-97602013000100001. [DOI] [PubMed] [Google Scholar]
- 50.Gutiérrez-Vázquez C, Villarroya-Beltri C, Mittelbrunn M, Sánchez-Madrid F. Transfer of extracellular vesicles during immune cell–cell interactions. Immunol Rev. 2013;251:125–142. doi: 10.1111/imr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol. 2013;200:367–371. doi: 10.1083/jcb.201212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bianco NR, Kim SH, Morelli AE, Robbins PD. Modulation of the immune response using dendritic cell-derived exosomes. Methods Mol Biol. 2007;380:443–55. [DOI] [PubMed]
- 53.Zhou Y, Zhou W, Chen X, Wang Q, Li C, Chen Q, et al. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm Sin B. 2020;10:1563–1575. doi: 10.1016/j.apsb.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qin S, Dorschner RA, Masini I, Lavoie-Gagne O, Stahl PD, Costantini TW, et al. TBC1D3 regulates the payload and biological activity of extracellular vesicles that mediate tissue repair. FASEB J. 2019;33:6129–6139. doi: 10.1096/fj.201802388R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beauvillain C, Ruiz S, Guiton R, Bout D, Dimier-Poisson I. A vaccine based on exosomes secreted by a dendritic cell line confers protection against T. gondii infection in syngeneic and allogeneic mice. Microbes Infect. 2007;9:1614–22. doi: 10.1016/j.micinf.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Giri PK, Schorey JS. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS One. 2008;3:e2461. [DOI] [PMC free article] [PubMed]
- 57.Keryer-Bibens C, Pioche-Durieu C, Villemant C, Souquère S, Nishi N, Hirashima M, et al. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer. 2006;6:283. doi: 10.1186/1471-2407-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou X, Brown BA, Siegel AP, El Masry MS, Zeng X, Song W, et al. Exosome-mediated crosstalk between keratinocytes and macrophages in cutaneous wound healing. ACS Nano. 2020;14:12732–12748. doi: 10.1021/acsnano.0c03064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin KO, Ha DH, Kim JO, Crumrine DA, Meyer JM, Wakefield JS, et al. Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de novo synthesis of ceramides in atopic dermatitis. Cells. 2020;9:680. doi: 10.3390/cells9030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.From the American Association of Neurological Surgeons, American Society of Neuroradiology, Cardiovascular Interventional Radiology Society of Europe, Canadian Interventional Radiology Association, Congress of Neurological Surgeons, European Society of Minimally Invasive Neurological Therapy, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13:612–32. [DOI] [PubMed]
- 61.Hashimoto H, Olson EN, Bassel-Duby R. Therapeutic approaches for cardiac regeneration and repair. Nat Rev Cardiol. 2018;15:585–600. doi: 10.1038/s41569-018-0036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bellin G, Gardin C, Ferroni L, Chachques JC, Rogante M, Mitrečić D, et al. Exosome in cardiovascular diseases: a complex world full of hope. Cells. 2019;8:166. doi: 10.3390/cells8020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin Y, Anderson JD, Rahnama LMA, Gu SV, Knowlton AA. Exosomes in disease and regeneration: biological functions, diagnostics, and beneficial effects. Am J Physiol Heart Circ Physiol. 2020;319:H1162–H1180. doi: 10.1152/ajpheart.00075.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38:201–211. doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baruah J, Wary KK. Exosomes in the regulation of vascular endothelial cell regeneration. Front Cell Dev Biol. 2020;7:353. doi: 10.3389/fcell.2019.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 67.Ibrahim AGE, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014;2:606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21:2491–2502. doi: 10.1111/jcmm.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dominguez JM, Dominguez JH, Xie D, Kelly K. Human extracellular microvesicles from renal tubules reverse kidney ischemia-reperfusion injury in rats. PLoS One. 2018;13:e0202550. doi: 10.1371/journal.pone.0202550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Webb RL, Kaiser EE, Jurgielewicz BJ, Spellicy S, Scoville SL, Thompson TA, et al. Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke. 2018;49:1248–1256. doi: 10.1161/STROKEAHA.117.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Webb RL, Kaiser EE, Scoville SL, Thompson TA, Fatima S, Pandya C, et al. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl Stroke Res. 2018;9:530–539. doi: 10.1007/s12975-017-0599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 74.Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Goncalves RM. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol. 2018;9:2837. doi: 10.3389/fimmu.2018.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simonson OE, Mougiakakos D, Heldring N, Bassi G, Johansson HJ, Dalen M, et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl Med. 2015;4:1199–1213. doi: 10.5966/sctm.2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics. 2019;9:1015–1028. doi: 10.7150/thno.30853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Preciado S, Muntion S, Sanchez-Guijo F. Improving hematopoietic engraftment: Potential role of mesenchymal stromal cell-derived extracellular vesicles. Stem Cells. 2021;39:26–32. doi: 10.1002/stem.3278. [DOI] [PubMed] [Google Scholar]
- 78.Park DJ, Yun WS, Kim WC, Park JE, Lee SH, Ha S, et al. Improvement of stem cell-derived exosome release efficiency by surface-modified nanoparticles. J Nanobiotechnology. 2020;18:178. doi: 10.1186/s12951-020-00739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ambattu LA, Ramesan S, Dekiwadia C, Hanssen E, Li H, Yeo LY. High frequency acoustic cell stimulation promotes exosome generation regulated by a calcium-dependent mechanism. Commun Biol. 2020;3:553. doi: 10.1038/s42003-020-01277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Messenger SW, Woo SS, Sun Z, Martin TFJ. A Ca2+-stimulated exosome release pathway in cancer cells is regulated by Munc13-4. J Cell Biol. 2018;217:2877–2890. doi: 10.1083/jcb.201710132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Savina A, Furlán M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 82.Shyong YJ, Chang KC, Lin FH. Calcium phosphate particles stimulate exosome secretion from phagocytes for the enhancement of drug delivery. Colloids Surf B Biointerfaces. 2018;171:391–397. doi: 10.1016/j.colsurfb.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 83.Iliev D, Strandskog G, Nepal A, Aspar A, Olsen R, Jørgensen J, et al. Stimulation of exosome release by extracellular DNA is conserved across multiple cell types. FEBS J. 2018;285:3114–3133. doi: 10.1111/febs.14601. [DOI] [PubMed] [Google Scholar]
- 84.Emam SE, Ando H, Abu Lila AS, Shimizu T, Ukawa M, Okuhira K, et al. A novel strategy to increase the yield of exosomes (extracellular vesicles) for an expansion of basic research. Biol Pharm Bull. 2018;41:733–742. doi: 10.1248/bpb.b17-00919. [DOI] [PubMed] [Google Scholar]
- 85.Novo D, Heath N, Mitchell L, Caligiuri G, MacFarlane A, Reijmer D, et al. Mutant p53s generate pro-invasive niches by influencing exosome podocalyxin levels. Nat Commun. 2018;9:5069. doi: 10.1038/s41467-018-07339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Z, Maruyama K, Sakisaka Y, Suzuki S, Tada H, Suto M, et al. Cyclic stretch force induces periodontal ligament cells to secrete exosomes that suppress IL-1 beta production through the inhibition of the NF-kappa B signaling pathway in macrophages. Front Immunol. 2019;10:1310. doi: 10.3389/fimmu.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sinha S, Hoshino D, Hong NH, Kirkbride KC, Grega-Larson NE, Seiki M, et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol. 2016;214:197–213. doi: 10.1083/jcb.201601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Böker KO, Lemus-Diaz N, Rinaldi Ferreira R, Schiller L, Schneider S, Gruber J. The impact of the CD9 tetraspanin on lentivirus infectivity and exosome secretion. Mol Ther. 2018;26:634–647. doi: 10.1016/j.ymthe.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jabbari N, Nawaz M, Rezaie J. Ionizing radiation increases the activity of exosomal secretory pathway in MCF-7 human breast cancer cells: a possible way to communicate resistance against radiotherapy. Int J Mol Sci. 2019;20:3649. doi: 10.3390/ijms20153649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4:69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel DB, Santoro M, Born LJ, Fisher JP, Jay SM. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol Adv. 2018;36:2051–2059. doi: 10.1016/j.biotechadv.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. doi: 10.1016/j.biomaterials.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O'Brien D, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551:55–64. doi: 10.1016/j.gene.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40:172–186. doi: 10.1016/j.tips.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 97.Xiao YW, Zhong JN, Zhong BY, Huang JY, Jiang LX, Jiang Y, et al. Exosomes as potential sources of biomarkers in colorectal cancer. Cancer Lett. 2020;476:13–22. doi: 10.1016/j.canlet.2020.01.033. [DOI] [PubMed] [Google Scholar]
- 98.He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mege D, Panicot-Dubois L, Ouaissi M, Robert S, Sielezneff I, Sastre B, et al. The origin and concentration of circulating microparticles differ according to cancer type and evolution: a prospective single-center study. Int J Cancer. 2016;138:939–948. doi: 10.1002/ijc.29837. [DOI] [PubMed] [Google Scholar]
- 100.Santoni G, Morelli MB, Amantini C, Battelli N. Urinary markers in bladder cancer: an update. Front Oncol. 2018;8:362. doi: 10.3389/fonc.2018.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Q, Yu G, He H, Zheng Z, Li X, Lin R, et al. Differential expression of circular RNAs in bone marrow-derived exosomes from essential thrombocythemia patients. Cell Biol Int. 2021;45:869–881. doi: 10.1002/cbin.11534. [DOI] [PubMed] [Google Scholar]
- 102.Zhang P, Liang T, Chen Y, Wang X, Wu TL, Xie ZX, et al. Circulating exosomal miRNAs as novel biomarkers for stable coronary artery disease. Biomed Res Int. 2020;2020:3593962. doi: 10.1155/2020/3593962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang LY, Yang X, Wang SB, Chen H, Pan HY, Hu ZM. Membrane derived vesicles as biomimetic carriers for targeted drug delivery system. Curr Top Med Chem. 2020;20:2472–2492. doi: 10.2174/1568026620666200922113054. [DOI] [PubMed] [Google Scholar]
- 104.Saleh AF, Lazaro-Ibanez E, Forsgard MA, Shatnyeva O, Osteikoetxea X, Karlsson F, et al. Extracellular vesicles induce minimal hepatotoxicity and immunogenicity. Nanoscale. 2019;11:6990–7001. doi: 10.1039/C8NR08720B. [DOI] [PubMed] [Google Scholar]
- 105.Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 2017;6:1324730. doi: 10.1080/20013078.2017.1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Popowski K, Lutz H, Hu S, George A, Dinh PU, Cheng K. Exosome therapeutics for lung regenerative medicine. J Extracell Vesicles. 2020;9:1785161. doi: 10.1080/20013078.2020.1785161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee JR, Park BW, Kim J, Choo YW, Kim HY, Yoon JK, et al. Nanovesicles derived from iron oxide nanoparticles-incorporated mesenchymal stem cells for cardiac repair. Sci Adv. 2020;6:eaaz0952. [DOI] [PMC free article] [PubMed]
- 108.Shi X, Cheng Q, Hou T, Han M, Smbatyan G, Lang JE, et al. Genetically engineered cell-derived nanoparticles for targeted breast cancer immunotherapy. Mol Ther. 2020;28:536–547. doi: 10.1016/j.ymthe.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ting PJ, Lin CH, Huang FL, Lin MC, Hwang KP, Huang YC, et al. Epidemiology of acute otitis media among young children: a multiple database study in Taiwan. J Microbiol Immunol Infect. 2012;45:453–458. doi: 10.1016/j.jmii.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 110.Si Y, Kim S, Zhang E, Tang Y, Jaskula-Sztul R, Markert JM, et al. Targeted exosomes for drug delivery: biomanufacturing, surface tagging, and validation. Biotechnol J. 2020;15:e1900163. doi: 10.1002/biot.201900163. [DOI] [PubMed] [Google Scholar]
- 111.Costafreda MI, Abbasi A, Lu H, Kaplan G. Exosome mimicry by a HAVCR1-NPC1 pathway of endosomal fusion mediates hepatitis A virus infection. Nat Microbiol. 2020;5:1096–1106. doi: 10.1038/s41564-020-0740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ye Y, Zhang X, Xie F, Xu B, Xie P, Yang T, et al. An engineered exosome for delivering sgRNA:Cas9 ribonucleoprotein complex and genome editing in recipient cells. Biomater Sci. 2020;8:2966–2976. doi: 10.1039/D0BM00427H. [DOI] [PubMed] [Google Scholar]
- 113.Kim D, Le QV, Wu Y, Park J, Oh YK. Nanovesicle-mediated delivery systems for CRISPR/Cas genome editing. Pharmaceutics. 2020;12:1233. doi: 10.3390/pharmaceutics12121233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shao J, Zaro J, Shen Y. Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. Int J Nanomedicine. 2020;15:9355–9371. doi: 10.2147/IJN.S281890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang J, Li W, Zhang L, Ban L, Chen P, Du W, et al. Chemically edited exosomes with dual ligand purified by microfluidic device for active targeted drug delivery to tumor cells. ACS Appl Mater Interfaces. 2017;9:27441–27452. doi: 10.1021/acsami.7b06464. [DOI] [PubMed] [Google Scholar]
- 116.Burgio S, Noori L, Marino Gammazza A, Campanella C, Logozzi M, Fais S, et al. Extracellular vesicles-based drug delivery systems: a new challenge and the exemplum of malignant pleural mesothelioma. Int J Mol Sci. 2020;21:5432. doi: 10.3390/ijms21155432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gudbergsson JM. Extracellular vesicles for targeted drug delivery: triumphs and challenges. Future Med Chem. 2020;12:1285–1287. doi: 10.4155/fmc-2020-0117. [DOI] [PubMed] [Google Scholar]
- 118.Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patil SM, Sawant SS, Kunda NK. Exosomes as drug delivery systems: a brief overview and progress update. Eur J Pharm Biopharm. 2020;154:259–269. doi: 10.1016/j.ejpb.2020.07.026. [DOI] [PubMed] [Google Scholar]