Abstract
Ischemic diseases are conditions associated with the restriction or blockage of blood supply to specific tissues. These conditions can cause moderate to severe complications in patients, and can lead to permanent disabilities. Since they are blood vessel-related diseases, ischemic diseases are usually treated with endothelial cells or endothelial progenitor cells that can regenerate new blood vessels. However, in recent years, mesenchymal stem cells (MSCs) have shown potent bioeffects on angiogenesis, thus playing a role in blood regeneration. Indeed, MSCs can trigger angiogenesis at ischemic sites by several mechanisms related to their trans-differentiation potential. These mechanisms include inhibition of apoptosis, stimulation of angiogenesis via angiogenic growth factors, and regulation of immune responses, as well as regulation of scarring to suppress blood vessel regeneration when needed. However, preclinical and clinical trials of MSC transplantation in ischemic diseases have shown some limitations in terms of treatment efficacy. Such studies have emphasized the current challenges of MSC-based therapies. Treatment efficacy could be enhanced if the limitations were better understood and potentially resolved. This review will summarize some of the strategies by which MSCs have been utilized for ischemic disease treatment, and will highlight some challenges of those applications as well as suggesting some strategies to improve treatment efficacy.
Keywords: Ischemic diseases, Mesenchymal stem cells, Blood regeneration and angiogenesis
Introduction
Ischemic conditions are associated with the restriction or blockage of blood supply to specific tissues, resulting in health complications, many of which can lead to permanent disabilities. Depending on the tissues where ischemic events take place, the lack of oxygen supply is the main factor which defines the damaging consequences. The lack of nutrition delivery and the inability to remove metabolic wastes that accumulate inside the cells can further trigger cell death and tissue necrosis. Ischemia occurs in metabolically dynamic tissues, such as the heart and brain, and may cause severe irreversible damage and even mortality [1]. Other organs, such as the liver and kidney, can also be affected by ischemia due to injury. The most commonly seen types of ischemia in the elderly appears in the limbs, particularly lower limbs, due to peripheral artery diseases (PAD) associated with aging, smoking and diabetes [2]. The current available treatments for ischemia predominantly include the administration of medicines or physical procedures to replenish blood supply to the affected tissues and to prevent further tissue damage. However, treatments for ischemia are invasive and ineffective, especially when severe ischemia has occurred or admission to the hospital is delayed.
In recent years, cell therapy using mesenchymal stem cells (MSCs) has been proposed as a safe and effective treatment that can be used to improve ischemic conditions. Several clinical trials that have used MSCs for transplantation as a treatment modality have shown improvement in patients with different types of ischemia [3–5]. In ischemic conditions, MSCs are known to function as an immunomodulator, helping to prevent inflammation and tissue damage. MSCs can also generate growth factors that support the regeneration of the vascular network to reconnect the blood supply to the affected tissues, which is the main purpose for utilizing MSC-based therapy. Indeed, the use of MSC transplantation in the treatment of ischemic heart condition has been shown to reduce heart infarction size, improve heart function with increased left ventricle ejection fraction (LVEF), and increase overall recovery and clinical outcomes [4]. In critical limb ischemia (CLI), intramuscular injection of MSCs was found to increase ankle-branchial index (ABI) and ankle pressure at 6-month follow-up, which indicated the improvement of blood flow into the lower limbs [6]. For ischemic brain or stroke conditions, long-term follow-up after MSC treatment showed no negative effects with intravenous application of bone marrow (BM)-derived MSCs (BM-MSCs), and to some extent, showed improved functional outcomes and survival rates [7, 8]. These data demonstrate that MSCs can be safely used to treat ischemic conditions in clinical trials, thereby proposing MSC transplantation as an effective cell therapy in future trials.
However, large-scale trials of MSC-based therapies have been limited in number, hence the controversy over how effective it really is as a therapy. There are data showing that while MSC treatment appeared to be safe, no significant outcome improvements were observed [9]. There has been suggestion that other factors, such as cell dosages, cell sources and individual health profiles, might need to be considered in future trials. The efficacy of MSC-based therapy for ischemic conditions has also been a subject of discussion in the literature. Some have proposed that the variation in efficacy could be due to various factors which can negatively impact the implanted MSCs as well as the host response in vivo [10–12]. These factors might include low survival rate of MSCs in vivo, inefficient cell migration to target tissues, and/or rejection by the host immune system [10–12]. Furthermore, the lack of information on the local ischemic environment associated with different individuals makes it challenging to identify the interaction between MSCs and host tissues, leading to suboptimal selection of MSCs or mis-preparation of suitable MSCs sources for application. Therefore, MSCs with optimal functionality may not be in the final product, leading to ineffective treatment efficacy. Indeed, understanding the interaction between donor cells and host tissues is vitally important for selection of optimal MSC characteristics which presumably would benefit the majority of treated individuals. In this review, we will discuss the changes that occur in the tissue environment following an ischemic event, and the potential of MSCs in modifying these changes in order to improve the ischemic conditions. We will also review the challenges that MSCs face in the in vivo environment, and the factors that impact MSC implant, survival and function in vivo. Understanding the above will help to gain a deeper understanding on the ischemic environment in order to target where the key problems lie, as well as to resolve the problems in a concerted effort.
Current status of ischemic disease treatment by MSC transplantation
The treatment efficacy of some ischemic diseases by MSC transplantation
Myocardial infarction
Myocardial infarction (MI), commonly known as a heart attack, is a disease condition in which disruption of blood flow to a part of the heart causes damage to the heart muscles. About 30% of people have atypical symptoms of this disease, and 5% of people over 75 years have had MI with little or no history of symptoms. Therapeutic efforts to treat MI have included stem cell therapy. In 2018, Kim et al. reported using autologous BM-MSCs to treat MI [13]. In this clinical trial, the authors treated 14 patients with BM-MSCs and compared them to 12 patients in the control group. All treatments were performed after 1 month of percutaneous coronary intervention. After 12 months of follow-up, the authors suggested that intracoronary administration of autologous MSCs significantly improved the LVEF in patients with anterior acute MI [13].
In a phase I, controlled, open-label and dose-escalating study, Penn et al. (2012) treated 19 patients with MI [14] by injection of allogeneic BM-MSCs into the patient’s myocardium. The results showed that at the high dose of MSCs (50 million MSCs), MSC transplantation could significantly improve the myocardial damage, ejection fraction and stroke volume 4 months later [14].
In a randomized placebo-controlled trial using autologous MSCs from BM, Mathiasen et al. investigated the treatment efficacy after 6 months of treatment [15] and 4 years of follow-up [16]. In this study, 60 patients were enrolled, with 40 in the treatment group and 20 in the placebo group. After 6 months, there were 51 patients (33 patients in treatment group and 18 in placebo group) who completed the follow-up. Compared to patients in the placebo group, there were significant improvements (p < 0.0001) in LVEF, left ventricular end systolic volume (LVESV), stroke volume and myocardial mass in patients of the treatment group. Furthermore, no serious adverse events were recorded [15]. Additionally, these results were maintained until 12 months after transplantation. After four years of follow-up, there were significantly fewer hospitalizations for angina in the MSC treatment group as compared to the placebo group, and again, no serious adverse events were identified [16].
Recently, there was a clinical trial which compared the treatment efficacy of chronic ischemic cardiomyopathy using allogeneic MSCs from umbilical cord (UC) (i.e. UC-MSCs), autologous mononuclear cells (MNCs) from BM (i.e. BM-MNCs), or placebo (control) [17]. In this study, 44 patients were placed into three groups (control: 16 patients, UC-MSC treatment: 26, and BM-MNC treatment: 12). After 12 months of follow-up, the results showed that significant improvements in LVEF, stroke volume, necrotic myocardium, and 6-min walking test were observed in patients treated with UC-MSCs versus those treated with BM-MNCs or placebo. While BM-MSC transplantation did improve LVEF and stroke volume, it did not decrease myocardium necrosis [17]. Additionally, in a previous study comparing the effects of BM-MSCs and BM-MNCs for the treatment of dilated cardiomyopathy, Xiao et al. [18] showed that after 1-year of follow-up, LVEF, New York Heart Association (NYHA) Functional Classification, and myocardial perfusion were significantly improved in the group receiving BM-MSCs but not BM-MNCs.
Contrary to the above reports, Qayyum et al. [19] suggested that there were no significant changes in patients with chronic ischemic heart diseases treated with autologous MSCs from adipose tissues, compared to control. In this phase II, randomized, double-blinded and placebo-controlled trial, the authors investigated the effects of intramyocardial injection of autologous MSCs and compared them to control. With respect to all criteria related to the improvement of disease, there were no significant differences found between the treatment and control groups. The authors also confirmed that there were no changes in functional parameters or amount of scar tissue in patients treated with stem cells as compared to those in the placebo group [19]. These results were different from other studies using MSCs to treat MI. However, it could be that Qayyum et al. used a different kind of MSCs compared to other studies. Thus, perhaps the source of MSCs can strongly affect treatment efficacy. Moreover, the dose of cells can also directly affect the treatment outcome, as has been well-established in the study by Florea et al. [20]. In their study, they investigated the effects of 2 doses of allogenic BM-MSCs (20 million and 100 million cells) on ischemic cardiomyopathy. The results revealed that only the 100 million dose of MSCs could increase ejection fraction [20].
The difference in treatment efficacy between autologous versus allogeneic MSC transplantation was also observed [21]. Hare et al. found that although there was no difference in severe adverse effects from autologous versus allogeneic MSC transplantation, the efficacy of treatment was different. In autologous MSC transplantation, the patients showed improvement in their 6-min walk test and MLHFQ scores, when compared to baseline values. However, this was not observed in patients receiving allogeneic MSC transplanation; moreover, their LV end-diastolic volume was reduced. Hare et al. also suggested that at the low dose of MSCs (20 million cells), there was a greater reduction in LV volume and an increased EF compared with the higher doses of MSCs (100 and 200 million cells) [21].
Ischemic stroke
Ischemic stroke is the most common type of stroke which results from the blockage of an artery supplying blood to the brain. This condition causes brain damage due to the reduction of oxygen to the brain. Currently, mechanical thrombolysis and stenting are strategies that are used to treat acute ischemic stroke [22]. There are certain thrombolysis-inducing drugs that are used to open the blockage of thrombosis [23, 24]. MSCs have also been investigated as a therapeutic platform for treatment of ischemic stroke. Unlike the strategies at present, MSC transplantation is considered as a novel strategy that not only triggers angiogenesis but also promotes brain regeneration after damage.
Honmou et al. treated 12 patients with stroke by transplantation of autologous stem cells expanded in autologous serum-supplemented medium [25]. MSC infusion was performed at 36–133 days post-stroke. In all the patients, no central nervous system tumors, abnormal cell growth, neurological deterioration, venous thromboembolism, systemic malignancy, or systemic infection was observed. The median daily rate of the National Institutes of Health Stroke Scale change was 0.36 during the first week post-infusion, compared to 0.04 from the first day of testing before infusion. The mean lesion volume was also reduced by more than 20% post-infusion [25].
Steinberg et al. (2018) treated 18 patients with chronic ischemic stroke using allogenic modified BM-MSCs [26]. There were 16 of 18 patients who completed 24 months of treatment and displayed significant improvements in the European Stroke Scale (ESS) score (p < 0.05), National Institutes of Health Stroke Scale (NIHSS) score (p < 0.01), Fugl-Meyer (F-M) total score (p < 0.01), and F-M Motor Scale score (p < 0.01) [26]. Similar to that study, Levy et al. (2019) evaluated allogenic BM-MSCs in a phase I/II clinical trial of 36 patients (15 patients in phase I and 21 patients in phase II). The phase I clinical study showed that intravenous transfusion of allogenic MSCs was safe, and the phase II showed that all behavioral endpoints were significantly increaesd over the 12 months of follow-up [27].
In recent publications, Jaillard et al. reported on the efficacy of autologous MSC transplantation for subacute ischemic stroke [28]. In this clinical trial, 16 patients received autologous BM-MSCs while15 patients received placebo control. Treatment efficacy was followed up after 6 months and 2 years of treatment. Although there were no treatment effects on the Barthel Index, the NIHSS, or modified-Rankin scores, there were significant (p < 0.05) improvements in the motor-NIHSS, motor Fugl-Meyer score, and task-related fMRI activities of MI-4a and MI-4p [28].
Critical limb ischemia (CLI)
Similar to other ischemic diseases, CLI has also been treated using MSC transplantation. MSCs from various sources, including adipose tissue, bone marrow, umbilical cord and placenta, have all been evaluated as treatment for this disease in the clinic.
In a report by Lu et al. (2019), the authors compared the treatment efficacy of BM-MSCs and BM-MNCs in the treatment of CLI and foot ulcers in patients with diabetes [29]. In the study, 41 patients were randomized into 3 groups: a group receiving autologous BM-MSCs, a group receiving autologous BM-MNCs, and a group receiving saline (control). The results showed that treatment with BM-MSCs significantly improved ulcer healing and recurrence rate compared to treatment with BM-MNCs or with saline. Moreover, compared to the BM-MNC group, the BM-MSC group showed a longer period of limb salvage and blood flow improvement, out to 9 months [29]. In a phase II study with 18 patients, Gupta et al. (2017) also investigated the effects of doses of allogenic BM-MSCs on CLI ischemia. The results of their study suggested that intramuscular injection of BM-MSCs, at a dose of 2 million cells per kg, showed superior clinical benefit [30].
Allogenic umbilical cord-derived MSCs have also been used to treat CLI [31, 32]. Huang et al. conducted 2 clinical trials to evaluate the use of UC-MSCs to treat CLI. After 6 months of follow-up, they found that rest pain, pain-free walking distance, and ulcers were significantly improved in the patients [32]. Moreover, these effects were related to the anti-inflammatory mechanisms and immunomodulation [31]. Allogenic MSCs from placenta have also been evaluated in the treatment of 4 diabetic patients with CLI [33]; the results demonstrated that there were no serious adverse events related to MSC injection during the 24 weeks. The clinical ischemic features improved after 24 weeks of treatment with a significant decrease in resting pain and limb coldness, and a significant increase in scores for pain-free walking distance [33].
MSC transplantation for ischemic diseases are superior to other kinds of stem cells
Besides MSCs, different types of adult stem cells included CD133+ cells, mononuclear cells from bone marrow, CD34+ cells, and cardiac stem cells, also were clinically used to treat some ischemic diseases.
CD133+ cells are defined as progenitor or stem cells known as a response for the angiogenesis [36] or protect against stroke [37]. However, some clinical studies represented some contradictory results. In 2014, Nasseri et al. used autologous CD133+ cells from bone marrow to treat ischemic myocardium in 60 patients [38]. They showed that autologous CD133+ cell transplantation did not affect the global left ventricular function and clinical symptoms. Indeed, there were no differences in 6-min walking distance, Minnesota Living with Heart Failure score, or Candian Cardiovascular Society class between treatment groups with CD133+ cells and placebo [38]. These results agreed with a pilot clinical study with 5 patients of ischemic myocardium that Forcillo et al. performed in 2013 [39]. However, it was different from Stamm et al. that intramycocardial delivery of CD133+ cells from bone marrow is safe and provides beneficial effects on the ischemic heart disease patients [40]. Stamm et al. showed that both LV ejection fraction and the perfusion of infarcted myocardium had been improved more in patients treated with CD133+ cells but not in control [40]. In phase II/III, randomized, double-blind, placebo-controlled trial recently published, Naseri et al. (2018) compared the treatment efficacy of patients with myocardial infarction with placebo and followed up for 18 months [41]. They also suggested that bone marrow-derived CD133+ cell transplantation increased left ventricular ejection fraction, improved decreased systolic wall thickening, significantly decreased non-viable segments compared to placebo after 6 and 18 months of transplantation [41]. These contradictory results suggested that some more extensive studies should be performed with some meta-analysis to provide evidence about CD133+ cell transplantation's treatment efficacy for ischemic myocardium.
In the non-expanded and purified form of stem cells from bone marrow, mononuclear cells (MNCs) also were used to treat some ischemic diseases. Some clinical studies also compared the treatment efficacy of MSCs versus MNCs [29, 34]. Heldman et al. (2014) reported a comparative study of ischemic cardiomyopathy treatment using autologous MSCs and autologous MNCs from bone marrow [34]. There were 19 patients treated with MSCs, 19 patients treated with MNCs, and 10 patients in placebo in this study. The results showed both patients transplanted with MSCs or MNCs improved the Minnesota Living With Heart Failure score compared to placebo. However, the 6-min walk distance only increased in patients transplanted with MSCs compared to placebo (p = 0.03); infarct size and regional myocardial function also only improved in patients with MSCs (p = 0.004), but not in MNCs, compared to placebo (p = 0.03) [34]. In another study, Lu et al. investigated long-term outcomes of transplantation of bone marrow-derived MSCs and compared to treatment efficacy of bone marrow-derived MNCs to treat critical limb ischemia and foot ulcer with diabetes [29]. Lu et al. suggested only in patients with MSC transplantation improved the hazard ratio for amputation, ulcer healing, and recurrence rate but not in patients with MNC transplantation. These primary investigations showed that MSC transplantation could be better than MNC transplantation in some ischemic diseases. However, more further studies should be performed to confirm these observations.
In another approach for heart regeneration after myocardial infarction, the cardiac stem cells were used to treat some patients with myocardial infarction [42]. The autologous cardiac stem cells were isolated and expanded by the cardiosphere culture. Although there was a reduction in scar mass, increased viable heart mass in the patients treated with cardiac stem cells, end-diastolic volume, end-systolic volume, and LVEF did not improve compared to the control [42]. However, different from this study, Chugh et al. confirmed transplantation of autologous cardiac stem cells provided positive results in ischemic cardiomyopathy patients. Patients transplanted with cardiac stem cells showed a marked increase in LVEF and regional EF; reduction of infarct size, and increased in viable tissues compared to control [43].
Like CD133+ stem cells, CD34+ stem cells contain a population of endothelial progenitor cells [36, 44] that can participate in the angiogenesis to regenerate the blood vessels. Some studies showed that CD34 could also express on the very small embryonic-like stem cells [45, 46], which display the huge potential to differentiate into various cells. CD34+ stem cells were used to treat some patients with angina [47]. In a recent publication, Velagapudi et al. performed a meta-analysis of randomized controlled trials with 269 patients treated ischemic heart disease by CD34+ stem cell transplantation. The analysis showed that the administration of autologous CD34+ cells decreased the risk of all-cause mortality, reduced angina frequency, and improved the exercise time compared to the control [48].
Although CD133+ cells, CD34+ cells, mononuclear cells, and cardiac stem cell transplantation can benefit ischemic diseases. These stem cells usually highly expressed human leukocyte antigen [49, 50]; they should be used as autologous sources. That is the main reason that usage of these stem cells is limited. Contrastly, MSCs did not express HLA class II and displayed the high potency of immune modulation [51, 52]. Therefore, MSCs are preferred to use to treat ischemic diseases more than other kinds of stem cells.
Treatment efficacy of MSC transplantation in ischemic diseases varies between kinds of MSCs
To date, various kinds of MSCs are used to treat ischemic diseases clinically. Some recent clinical studies introduced in Table 1 showed that both autologous and allogeneic MSCs were used in clinical treatment for ischemic diseases. Hare et al. compared the treatment efficacy of allogenic versus autologous bone marrow-derived mesenchymal stem cells for ischemic cardiomyopathy [21]. In this randomized study, there were 30 patients treated and follow-up to 13 months. Because the sample size is small, some results were non-significant differences between allogeneic and autologous bone marrow-derived MSC transplanted patients. The 1-year incidence of SAEs was 33.3% (n = 5) in allogeneic group while accounted for 53.3% (n = 8) in the autologous group (p = 0.46). Only patients with autologous MSCs improved the 6-min walk test and MLHFQ score, but not in allogenic MSC transplanted patients. The allogeneic MSC transplantation reduced the LV end-diastolic volumes. Both autologous and allogeneic MSC transplantation significant reduced mean EED by − 33.21% (p < 0.001) [21].
Table 1.
Clinical applications of mesenchymal stem cells for ischemic diseases
| Diseases | Phase | No. of patients | Sources of MSCs | Types of transplantation | Results | References |
|---|---|---|---|---|---|---|
| Myocardial infarction | Phase I, open label | 19 | Allogenic MSCs from bone marrow | Myocardium |
Safe and well tolerated At dose of > = 50 million of cells, the myocardial damage is significant improved, improved in ejection fraction and stroke volume 4 months later |
[14] |
| Stroke | 12 | Autologous MSCs from bone marrow | IV |
Safe Lesion volume reduced by > 20% at 1 week after infusion The median daily rate of National Institutes of Health Stroke Scale change was 0.36 during the first week post-infusion |
[25] | |
| Ischemic heart failure | Randomized, double-blind, placebo-controlled trial | 60 | Autologous MSCs from bone marrow | Intramyocardial injections |
Left ventricular end-systotic volume significant reduced in treatment group, but not in placebo Left ventricular ejection fraction, stroke volume and myocardial mass are also improved Amount of scar tissue is reduced, quality of life is increased in the treatment group, but not in placebo group |
[15, 16] |
| Chronic ischemic cardiomyopathy | Controlled, randomized trial | 44 | Autologous mononuclear cells from bone marrow; allogenic umbilical cord | Intramyocardial injections |
UC-MSCs are better than BM-MNCs in treatment efficacy LVEF, stroke volume improved in UC-MSC group Necrotic myocardium significant reduced in UC-MSC group 6-min walking test signficantly increased in the UC-MSC group |
[17] |
| Subacute ischemic stroke | Controlled, randomized trial | 31 | Autologous bone marrow-derived MSCs | IV |
No treatment effects on the Barthel Index, NIHSS, modified Rankin score Significant improvement in motor-NIHSS, motor-Fugl-Meyer score, task-related fMRI activity in MI-4a and MI-4p Safe and feasible Motor |
[28] |
| Myocardial infarction | Controlled trial | 26 | Autologous bone marrow-derived MSCs | Intracoronary delivery | Autologous BM-MSC was tolerable and safe with significant improvement in LVEF at 4 months and 12 months after transplantation | [13] |
| Chronic ischemic heart disease | Phase II, randomized, double blinded, placebo controlled trial | 60 | Autologous MSCs from adipose tissue | Intramyocardial injections |
Non-significant differences between stress, rest values between control and treatment groups LVEF, myocardial mass, stroke volume, left ventricle end-diastolic volume, end-systolic volume changed non-signficantly between control and treatment groups Scar tissue was unchanged between 2 groups of control and treatment |
[19] |
| Ischemic cardiomyopathy | Blinded trial | 30 | Allogenic MSCs from bone marrow | Transendocardial injection | All patients with dose of 20 and 100 million of cells reduced scar size, but only patients with 100 million cells increased ejection fraction | [20] |
| Ischemic cardiomyopathy | Phase ½ randomized trial | 30 | Allogenic and autologous MSCs from bone marrow | Transendocardial injection |
Both allogenic and autologous MSCs were both associated with low rates of SAEs, including immunologic reactions MSC injection affected patient functional capacity, quality of life, and ventricular remodeling |
[21] |
| Ischemic cardiomyopathy | Phase 1 and 2 randomized, blinded, placebo-controlled study | 65 | Autologous MSCs and MNCs from bone marrow | Transendocardial injection |
The 1-year incidence of SAEs are similar in groups of MSCs, MNCs and placebo Both MSCs and MNCs transplantation improved the Minnesota Living With Heart Failture score; however, the 6-min walk distance, infarct size, regional myocardial funtion only improved in the group of MSC transplantation, but not in MNCs or placebo |
[34] |
| Ischemic stroke | Phase 1/2a, open label, single-arm | 18 | Allogenic-modified bone marrow derived MSCs | Administered to the target sites |
There are 1 treatment emergent adverse event No patients withdrew due to adverse events 88.9% patients got headache 16/18 patients completed 24 months of follow-up with significant improvements from the baseline for European Stroke Scale (ESS) score, p < 0.05); National Institutes of Health Stroke Scale (NIHSS) score, p < 0.01, Fugl-Meyer (F-M) total score, 19.4 p < 0.01); and F-M motor scale score, p < 0.01 No significant changes in the modified Rankin Scale score |
[26] |
| Chronic stroke | Phase I/II | 15 in Phase I and 21 in phase II | Allogenic bone marrow-derived MSCs | IV | All behavioral end points showed significant gains over 12 months of follow-up | [27] |
| Critical limb ischemia and foot ulcer | Pilot study with control | 41 | Autologous BM-MSCs and BM-MNCs | Intramuscularly injection |
Patients with BM-MSCs, but not in patients with BM-MNCs, were significant different in ulcer healing and recurrence rate compared to control at 9 months of follow-up Treatment of BMMSCs, leaded to longer time of limb blood flow and ulcerative healing, reduced ulcer recurrence and amputation in 9 months |
[29] |
| Critical limb ischemia | Phase II, prospective, nonrandomized, open-label | 18 | Allogenic BM-MSCs | Intramuscularly injection | Admimistration of BM-MSCs at a dose of 2 million cell per kg showed the clinical benefit | [30] |
| Critical limb ischemia | Pilot study | 26 | Allogenic UC-MSCs | Intramuscularly injection |
No patients underwent lower limb amputation, not adverse effects related to transplantation Rest pain, pain free walking distance and uclers significantly improved at the end of the 6-months follow-up |
[32] |
| Critical limb ischemia | Pilot study | 4 | Allogenic MSCs from placenta | Intramuscularly injection |
The clinical ischemic features of patients were improved 24 weeks after treatment with MSCs The scores of resting pain and limb coldness significantly decreased Pain free walking distance significant increased from the baseline to 24 wks |
[33] |
| Critical limb ischemia | Pilot study | 15 | Autologous MSCs from adipose tissue | Intramuscularly injection |
Multiple injection of AD-MSCs cause no complications during 6 months follow-up Clinical approvemet was recorded in 66.7% pateitns At 6 months, significant improvement in pain rating scales, and claudication walking distance were recorded |
[35] |
Some kinds of MSCs were used in clinical treatment for ischemic diseases included bone marrow-derived MSCs, adipose-derived MSCs, umbilical cord-derived MSCs, and placenta-derived MSCs. However, there is no comparative study about the treatment efficacy of these sources of MSCs reported. Almost all reports suggested that MSC transplantation improved clinical symptoms of ischemic diseases [13, 14, 16, 17, 20, 21, 25–30, 32, 33, 35]. A study using the autologous MSCs from adipose tissue in chronic ischemic heart disease treatment showed a non-significantly improvement between treatment groups and the control [19]. However, autologous MSCs from adipose tissue could significant improve critical limb ischemia [35]. These observations suggested that the treatment efficacy can depend on different factors such as sources of MSCs, quality and dose of MSCs, kind of ischemic disease, and stage of ischemic disease…
The biology of ischemia
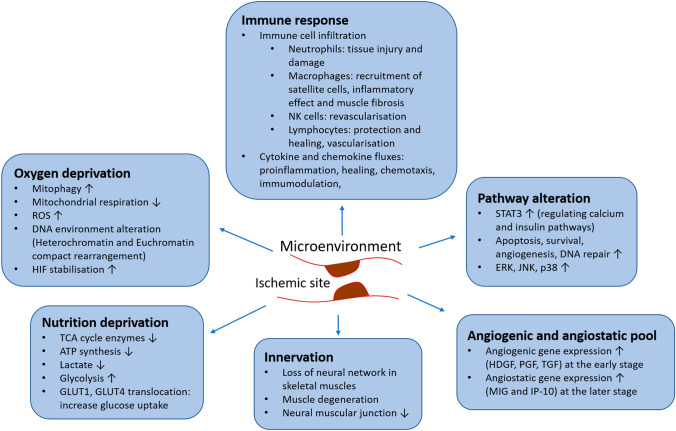
Ischemia creates a sudden decrease and loss of blood supply to the affected area, which results in several consequences to the microenvironment, directly affecting the survival of the cells in the surrounding tissues. Depending on the degree and time of restriction, the consequences brought by ischemia can be reversible or irreversible. At the cell level, the ischemic condition results in changes in oxygen supply and nutrition deprivation, forcing the cells to turn on different mechanisms to deal with the environment (Fig. 1).
Fig. 1.
Changes in the microenvironment surrounding ischemic site. These changes create the deprivation in oxygen and nutrition, leading to cell pathway alteration. Immune and nerve system in the local area are also affected and activated, resulting various degrees of tissue damage. The changes in angiogenic and angiostatic pool is modified to help the local tissue to adapt to the new condition
Nutrition deprivation
The reduction in oxygen supply can affect the ischemic condition at various degrees, depending on the need for oxidative metabolism in the specific tissues. The decrease in oxygen supply firstly lowers metabolism that requires oxygen, such as oxidative phosphorylation and beta-oxidation, resulting in a decrease in ATP synthesis. For instance, energy metabolism-related genes were among those downregulated in ischemic limb mouse models [53]. In rat ischemic heart, the low blood flow has been shown to reduce 84% of oxygen consumption, leading to 51% reduction of ATP and peak lactate production at 20 min of the ischemic event [54]. The increase in lactate production was found to be a result of ischemia due to the reduction in oxidative phosphorylation and increase in glycolysis and glycogen breakdown [55].
The attempt for ischemia-affected cells to increase glycolysis during the ischemic condition (to replenish energy requirement) is supported by multiple mechanisms. It has been suggested that ischemic conditions may increase anaerobic glycolysis as a result of reduced oxygen to maintain the levels of ATP and to help improve the survival of hepatocytes [56]. These data suggest that switching from aerobic metabolism to anaerobic metabolism is one of the strategies of cells at the ischemic site to maintain the energy pool that is needed for them to function. However, at the same time, when the blood supply is reduced, the decrease in glucose supply at the affected area also causes the cells to have short glycolysis. In a study investigating blood glucose in the subcutaneous tissue and skeletal muscles of patients with CLI, it was shown that a reduction in blood perfusion resulted in the decrease of glucose concentration in both the subcutaneous and intramuscular catheters. However, there was a reduction of lactate concentration only in the intramuscular measurement [57]. A similar glucose reduction was also found in ischemic models in rats [58], suggesting that reduced glucose concentration is a sensitive indicator of ischemic condition [59].
While glucose is a clear indicator, other metabolic pathways are also expected to be affected by the decrease in blood supply during ischemia. In a recent study that investigated the transcriptome in the skeletal muscle of CLI patients, it was found that the expression of succinate dehydrogenase (the enzyme involved in mitochondrial respiration and TCA cycle) was reduced [60]. In prolonged ischemia, the production of lactate and pyruvate decreases gradually until ischemia is completed [54, 55]. At the cell level, in order to enhance the rate of glycolysis, GLUT1 and GLUT4 expression was translocated to different compartments of myocytes and to the cardiac capillary to enhance the uptake of glucose [61, 62]. Particularly, it has been shown in rats with ischemic hearts that GLUT1 was translocated from the cardiac tissue into the capillary endothelium to enhance glucose absorption to cardiac cells [62]. In a similar model, GLUT4 was found to be translocated to the plasma membrane of cardiac myocytes during heart ischemia [61], or to the sarcolemma and T tubules of myocardiocytes to maximize the transport of glucose into the cells [62]. These data suggest that the reduction in blood supply to the ischemic tissues affect the cells to perform different mechanisms to adapt to the short supply of glucose and to maintain the energy levels for normal functioning.
Although there is knowledge about glycolysis and oxidative phosphorylation in ischemic conditions, there is still limited information about amino acid metabolism that has either been published or studied. In areas that need high levels of amino acids for physical activity, such as skeletal muscles, the requirement of amino acids is crucial to maintain tissue function and recovery. For example, resistance exercises recapitulate a small degree of reduced blood flow that occurs in ischemic muscles, and it was found that amino acid intake played an important role in protein recovery of the leg muscle [63]. Thus, a supply of amino acids at the ischemic site appears to be essential for the prevention of ischemic damage. Indeed, it has been shown in animal ischemic heart models that treatment with glutamine induces a protective effect to the cardiac muscle following ischemia and reperfusion, due to the production of O-linked N-acetyl-glucosamine (O-GlcNAc), the key player in the cardiac protection mechanism [64–66]. The involvement of O-GlcNAc in the activation of hexosamine biosynthesis pathway is the main driving factor in cardioprotection and is most likely to be related to ATP levels and other endogenous stress-activated pathways rather than protein synthesis [64, 66]. This suggests that the recovery mechanism for ischemic condition relies greatly on the ability to maintain energy and suppress stress induction that potentially leads to apoptosis. Glutamine and glutamate also play an important role in controlling action potential duration (APD), an indicator of electrical measure of cardiac function, to exert cardiac protection [64]. Thus, it is important to understand needs of tissues for amino acid metabolism during ischemia. It may also be important to know which amino acids are critically required or changed at the onset of the ischemic event and during prolonged periods of ischemia.
The above evidence suggest that a reduction in nutrition supply greatly affects the cells at the ischemic tissues. Hence, nutrition supply is an important factor for consideration in treatment, especially when using cell-based therapy. Ensuring that nutrition is affordable for donor cells to function and enhancing the ability of donor cells to adapt to limited nutrition supply in the ischemic environment are key factors to achieving success in cell-based therapy for ischemia.
Oxygen deprivation
The reduction of oxygen supply directly affects the respiration of cells. Mitochondria are known as the site of metabolism and respiration. Thus, the reduction in oxygen supply due to ischemic events poses potential damage to the function of the mitochondria. In fact, mitophagy was found to increase at day 14 after a hindlimb ischemic event in mice, indicating evidence of mitochondrial damage [67]. In a study that analyzed the gene expression profile of gastrocnemius muscle, both respiration capacity and expression of the genes associated with cellular respiration and mitochondrial inner membrane significantly decreased in patients with CLI [60]. Particularly, genes that are involved in the formation of the electron transport chain, such as cytochrome c oxidase (COX) proteins, ATP synthase, and ubiquinol-cytochrome C reductase (UQCR), were found to be reduced in the skeletal muscle of CLI patients compared to healthy adults [60]. Following the reduction in mitochondrial function and respiration, reactive oxygen species (ROS) were shown to be increased as a result of electron transport chain failure during the development of ischemia [68]. ROS were detected in ischemic hearts in rat models and the increase in ROS production was found to be correlated with an increase in necrosis and cell death, which could be reduced by the use of antioxidants, such as manganese superoxide dismutase (MnSOD) [68, 69].
However, the precise mechanisms and the functions of ROS during the ischemic period remain unresolved questions. The reperfusion of blood flow and replenishment of oxygen supply to the ischemic hearts were shown to result in a burst of ROS production [70, 71], which could further damage the cells at the ischemic site. Cells of the ischemic area are prone to ROS production, but it is not known whether the use of antioxidants can successfully inhibit the necrotic cascade. In this case, saving the mitochondria from being dysfunctional is the key factor to efficiently restore cell respiration and prevent the consequences of ischemia. In fact, the reduction in oxygen and nutrition supply can affect cells at the genomic level. Deprivation of oxygen and nutrition could cause alterations in the DNA environment of cardiomyocytes during a period of hypoxic culture, resulting in the restructuring of chromatins in which heterochromatin and euchromatin are compacted and rearranged in the cell nuclei [72]. Even in vitro, culture of cardiac cells in hypoxic conditions was found to lead to a cascade of reactions, including restricted access to histones and redistribution of polyamides to the nucleus, resulting in the inhibition of transcription [72]. In fact, the compaction of chromatin during hypoxia is correlated with a wide suppression of gene expression [73], suggesting that the lack of oxygen supply in the cell environment can cause remarkable changes in cell growth and behavior.
The lack of oxygen supply creates a hypoxic condition that triggers the stabilization of hypoxic induced factors (HIF), which play an important role in the regulation of metabolism and redox homeostasis [74]. HIF has been shown to have positive effects on rescuing cardiac muscle in ischemia via ischemic preconditioning of the heart, a condition with repetitive ischemic-reperfusion events that trigger cardiac protection [75]. Endothelial cell expression of HIF was found to reduce the inflammation response of renal cells, and only HIF-2α acted on vascular cell adhesion molecule-1 (Vcam1) to inhibit the infiltration of neutrophils during the recovery phase, which was necessary to protect the kidney from ischemic injury [76]. The enhanced HIF expression by adenovirus into ischemic limbs of diabetic mice showed that HIF-1α plays an important role in the recovery of the vessels and increases the recruitment of angiogenic cells, suggesting that HIF-1α is an important factor in response to the ischemic condition in diabetes [77]. Understanding the changes in oxygen supply at the ischemic tissues and how cells adapt to the hypoxic environment can be useful to predict the outcomes of the ischemic condition and help to devise strategies to support the cells in overcoming the challenges of low oxygen supply.
Pathway alterations
The reduction in oxygen and nutrition supply following ischemia can cause cellular pathway alterations to adapt to the new conditions and to rescue the ischemic injury. Depending on the tissues where the injury has occurred, different cellular pathways may be predominant in the response to the ischemic event. In repetitive ischemic reperfusion (I/R) periods of pig hearts, it was shown that repetitive I/R plays an important role in cardioprotection of the hearts. During examinations at the onset of ischemia, Pavo et al. showed that I/R affected a wide range of cellular pathways, including calcium and insulin signaling and adipocytokine pathways, as early as 5 h after the repetitive I/R events, which were regulated by the overexpression of STAT3 of the PI3K/Akt and the JAK/STAT pathways [78]. STAT3 signaling has also been shown to be important in the regulation of satellite cell recruitment in muscle injury and promoting angiogenesis in experimental peripheral arterial disease (PAD), a pathogenic cause leading to hind limb ischemia [79, 80]. Besides, pathways related to apoptosis, survival, angiogenesis, DNA repair, oxidative stress and metabolism were also observed in both distal and remote areas of the ischemic site after cycles of repetitive I/R [78]. These pathways were suggested to play an important role in protecting cardiac muscles from further injury; some of these pathways induce cardioprotection by applying repetitive I/R. The ERK, JNK, and p38 pathways were found to be elevated in I/R in rat spinal cord after hind limb ischemia, in which the activation of ERK and JNK by phosphorylation was correlated with the production of ROS [81].
Even though these results may require further validation (since the authors could not measure ROS directly), they have raised the possibility that ROS could be important signals for the activation of tissue survival and angiogenesis pathways via other remote tissues [82]. In response to ischemia, the rat brain has been shown to have an enhanced Notch1 signaling pathway, the pathway that is involved in the recruitment of precursor neural cells, as well as in the selection and stabilization of tip and stalk cells in angiogenesis [83–86]. Therefore, Notch signaling has been identified to be critically associated with the pathogenesis of brain ischemia and can be targeted to boost the recovery of ischemic and stroke conditions. Different chemicals have been examined to enhance Notch signaling as a potential therapeutic target in brain ischemia [87, 88]. The data suggest that cell signaling alterations play an important role in preventing tissue injury and promoting the protection of the ischemic areas. Understanding the function of different pathways can efficiently enhance the recovery of ischemic conditions which can be targeted for therapeutic purposes.
Immune response
The involvement of immunity is a prominent response of the body when ischemia is triggered. Multiple immune responses are known to occur at the ischemic site; some of these responses include cytokine and chemokine production, followed by immune cell infiltration and activation. Depending on the type of reaction, an immune response can either cause damage to the tissues or induce protection to prevent tissue injury. Both innate and adaptive immune responses have been shown to play pivotal roles in the progression and recovery of ischemic tissues [89, 90]. Immune cell infiltration to the affected area occurs within hours after heart ischemia occurs, and is also associated with the influx of cytokines and chemokines [91]. In ischemic heart, it was found that during the onset of MI, immune cells (such as neutrophils, M2-macrophages, natural killer (NK) cells and T-cells) were significantly recruited to the affected area [91, 92]. Among these cells, neutrophils were those that presented at the early stage, while macrophages were the most abundant cells in the affected area after 3 days of MI [91, 92]. Macrophages were also involved in the recruitment of satellite cells that are important for the regeneration of damaged muscles [93].
Along with immune cell infiltration, the ischemic heart also experiences fluxes of cytokines and chemokines from both the local tissues and the serum, which dynamically change with time during the prolonged period [91]. It was found that the expression of cytokines and chemokines was accelerated as early as 6 h after femoral artery ligation in mice and peaked after the first day ischemia took place [53]. The presence of cytokines (such as TNF-α, IL-6, IL-4 and IL-10) and chemokines (such as CXCR7, MIP-2 (macrophage inflammatory protein 2 or also named CXCL2), and CCR2) are known to exert both pro-inflammatory and protective function during the ischemic period [91, 92]. Circulating chemokines, such as MIP-2, were also found to be involved in the injury of lung, a distant tissue, in mice with hindlimb ischemia [94]. On the other hand, the addition of regulatory T (Treg) cells was shown to induce protection against tissue injury in mice with ischemic kidneys [95]. In this case, the expression of PD-1 ligand on Treg cells was responsible for their ability to protect the kidneys from damage; furthermore, the blockage of PD-1 ligand prior to kidney ischemia resulted in increased kidney injury [95]. The role of different chemokines and cytokines requires further investigation to unravel if they are required for cell–cell interaction in the ischemic area. Several studies have indicated the role of chemotactic interaction of immune cells in the recruitment of angiogenic cells, which improved the recovery of ischemic tissues. For example, the chemotactic axis of CCR7 with CCL19 and CCL21 could promote the accumulation of CD4+ T cells, which are essential for arterial remodeling in the mouse limb ischemia model [96]. These evidence suggest that the immune response is not only a local effect on the ischemic site but is also a systemic effect.
The damaging and protecting roles of immune cell infiltration during ischemia can be interchangeable, depending on the time when the cells are recruited to the ischemic sites. For example, the accumulation of neutrophils has been known to be damaging to the ischemic heart, potentially resulting in I/R injury. Moreover, other immune cells, such as macrophages and lymphocytes, could mediate protective and healing effects [97–99]. In the ischemic limb, lymphocytes and NK cells exerted their healing effects via the revascularization process [100, 101], while macrophages exerted their inflammatory effects, which often resulted in muscle fibrosis after ischemia [102].
It is noted that the expression profiles of immune cells are critical in the determination of whether an injury or a protective effect might occur in the ischemic tissues. For instance, the expression of PHD3 (prolyl-4-hydroxylase domain enzyme 3) on macrophages was shown to be correlated with increased fibrosis in skeletal muscles of the ischemic hindlimb, and that the depletion of PHD3 significantly rescued the damaging effect [102]. However, the transmembrane protein, toll-like receptor 4 (TLR-4), has been shown to be important for the accumulation of neutrophils in the extracellular muscle space during the ischemic condition, which is correlated with tissue injury [103]. Several studies have described the remodeling role of immune cells during the ischemic event. Particularly, neutrophils are responsible for both tissue injury during ischemia and tissue remodeling after ischemia. The infiltration of neutrophils can form plagues, with neutrophil accumulation on the arterial walls; moreover, the neutrophils help in vessel rupture in the later stages by interacting with circulating immune cells and vascular cells [104].
The accumulation of immune cells in the ischemic area also plays an important role in the cell-to-cell interaction, an important factor for the remodeling and the recovery process [89, 90]. Of note, the presence of immune cells with the secretion of cytokines and chemokines into the ischemic area are critical in the chemotactic process, which is essential for the recruitment of myoblast precursor cells and vascular precursor cells to the ischemic site [53, 97]. In mouse models, the role of CD8+ T cells in the ischemic area has been investigated, which showed that CD8+ T cells play an important role in the recruitment of CD4+ T cells, regulation of immune response, and support of blood flow recovery to the ischemic site via the expression of IL-16 [100]. Even though the precise mechanism for this effect has not yet been elucidated, the results of the study in mice have suggested the importance of the immune response in the healing of ischemia. Similarly, the presence of NK cells and CD4+ T cells have been shown to be correlated with improved arteriogenesis in mice with ischemic limbs [101, 105]. The depletion of CD8+ T cells, CD4+ T cells and NK cells can result in severe impairment of the limbs and can significantly impair arterial and collateral genesis [100, 101, 105]. The activation of CD4+ T cells, especially Treg cells, was shown to be critical for wound healing in mice with MI, which was suggested to be related to the ability to induce collagen secretion from other cells in the affected area [106]. Even though the precise mechanisms of these phenomena have not been well-understood, the activation of T cells during ischemic events was found to play an important role in promoting neovascularization and angiogenesis in ischemic limbs via the co-stimulation of other cell types, such as monocytes and vascular smooth muscle cells (VSMCs) [107, 108]. In particular, the stimulation of CD14+ monocytes by T cells was found to lead to enhanced expression of pro-angiogenic and pro-arteriogenic factors, such as vascular endothelial growth factor (VEGF), interleukin (IL)-6, tumor necrosis factor alpha (TNF-α), and platelet-derived growth factor (PDGF)-BB [108]. This effect may be due to the ability of monocytes to produce monocyte chemoattractant protein (MCP)-1 which, in turn, recruits macrophages that induce the expression of TNF-α and VEGF [109].
Thus, as an important factor during the ischemic event, the immune response can contribute broadly to both injury and recovery of the condition. The acceleration of immune responses is dependent not only on the onset and severity of the condition but also on the medical history and genetic profiles of different individuals. While other factors are important, genetic profiles have appeared to be critical for the prognosis of any ischemic-related complications and for development of efficient treatments for different individuals. Specifically, it will be important to understand how different people with different genetic profiles progress during ischemia and how they adapt to different types of treatments. Ideally, a therapeutic target that includes immune modulation and adaptation will be promising for a broad range of patients.
Innervation
Nerve distribution is important for the growth, development and regeneration of active muscles, especially skeletal muscles. The level of impact of an ischemic event in muscle, in turn, can result in the loss of the neural network. It has been shown that the degree of ischemic injury may be associated with neurological pathology and recovery of the ischemic tissue, where neurodegeneration around the ischemic site may correspond with the severity of muscle atrophy [110, 111]. Furthermore, the prolonged damage of the neural network often results in severe muscle degeneration. For example, long-term damage in replanted limbs in rats was shown to have a significant impact on the recovery of skeletal muscles since the damage reduced the muscle weight and resulted in smaller muscle cells [111]. Accordingly, it was noticed that the reduction in arterial flow did not lead to significant changes in muscle atrophy, and only denervation ischemia caused severe muscle degeneration [111]. In mice, denervation of motor units and neuromuscular junction appeared a few days after an ischemic event and lasted for 56 days after ischemia, leading to muscle degeneration [67]. The remodeling of subsynaptic nuclei at the neural muscular junction and mitochondria along the muscle fibers was found to play an important role in the repair of denervation and, therefore, was critical for muscle regeneration [67]. When it comes to ischemia with nerve damage, it has been shown that mice with ischemia accompanied with pain showed lower muscle weight and decreased physical activity [110], suggesting that the preservation of the neural network plays an important role in the ischemic condition. The latter needs to be considered when searching for appropriate treatments. Understanding the neural conditions in each ischemic case may be important for the control of ischemic damage. Moreover, treatment of an ischemic disease may need to be considered in combination with treatment of denervation to potentially gain the best outcome.
Angiogenic and angiostatic pool
Reduced blood supply causes the expression of various transcriptional factors to be influenced by changes in the living environment in ischemia. The production of several growth factors, including angiogenic and angiostatic factors, results in new vascular formation at the ischemic injured site to replenish blood flow. The induction of these factors after an ischemic event plays an important role in the balance between the formation and maturation of new blood vessels, since neovascularization without a proper maturation process can result in a more severe condition when a rupture occurs in the newly formed vessels. The expression of angiogenic genes, such as hepatoma-derived growth factor (HDGF), placental growth factor (PGF), and transforming growth factor (TGF), were among those that were highly expressed in the ischemic limb site after the ligation of femoral artery [53]. However, the expression of genes that are known as angiogenic factors, such as angiopoietin 1 (ANG1), vascular endothelial growth factor B and C (VEGF-B and C), and fibroblast growth factor (FGF), was not detected or increased, while angiostatic-related genes, such as monokine induced by IFN-ϒ (MIG) and IFN-ϒ-inducible protein-10, were significantly upregulated at the later stage of ischemia [53]. This evidence raises questions about how angiogenic homeostasis is maintained during the ischemic condition to support the recovery of the subjects. In fact, it is known that at the early stage of ischemia, the production of angiogenic factors is regulated so that the process of vessel cell recruitment and maturation are maintained to ensure the stable formation of mature vessels without the overgrowth of neo-vessels [112]. Therefore, the constitution and concomitant levels of angiogenic and angiostatic proteins in the ischemic environment are critical for the recovery outcome. Ensuring the replenishment of angiogenic and angiostatic factors in ischemic tissues is, therefore, an important criterion to achieve success in cell therapy.
Mechanisms of MSC transplantation for ischemia
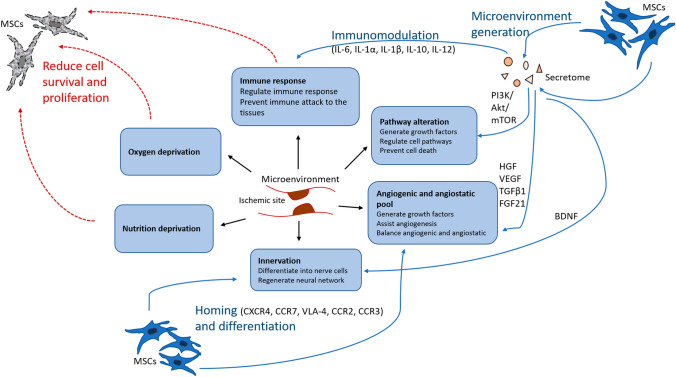
MSCs hold great potential for therapeutic clinical applications since they possess specific unique characteristics that cannot be found in other cell types. Importantly, MSCs can be expanded unlimitedly and stably in vitro, allowing them to be used in large quantity with low variation, thus meeting the consistency criterion required for large-scale applications [113]. Other characteristics that potentiate MSCs for therapeutic use include their ability to home to target tissues, to differentiate, to generate the microenvironment, and to immunomodulate. For conditions such as ischemia, these characteristics are especially useful to cope with changes in the microenvironment and to support the healing of tissues (Fig. 2).
Fig. 2.
Schematic of how the changes in ischemic microenvironment can affect MSCs (red arrow), and in turned, be
modified by the presence of MSC administration (blue arrow). Changes in the environment such as oxygen and nutrition deprivation and host immune response can negatively affect the survival and proliferation of MSCs. On the other hand, the administration of MSCs during ischemic condition can help to reverse the damages caused by ischemic condition. Improvement of the condition is induced by the effect generated from MSCs characteristics of immunomodulation, microenvironment generation, homing and differentiation. MSCs generate secretome containing growth factors, cytokines and chemokines to modulate the host immune response, resolving cell death signal and balancing angiogenic and angiostatic pool in the ischemic site. MSCs can also home and guide the regeneration of nerve in the ischemic tissue to support tissue recovery
Homing capacity
MSCs are known for their ability to assist in the healing and recovery of damaged tissues by creating a microenvironment for regeneration. This is associated with the ability of MSCs to migrate from their site of origin to the site of injury. The ability of MSCs to travel and home to different tissue sites has been widely studied and discussed due to their expression of several molecules that assist with the process of cell movement [114]. MSCs can either be applied locally to the site of lesion or systemically in the circulation. Indeed, the transendothelial migration capacity of MSCs is an important characteristic which makes them advantageous in transplantation medicine [115]. Systemic transfer of MSCs involves steps that require the cells to attach to the endothelium and roll along the blood vessels to the injured tissues before the cells cross the endothelial membrane and successfully migrate across the tissue to reach the injured site [115, 116].
If administered systemically, the homing mechanism of MSCs to damaged tissues includes the attachment of MSCs to the endothelial cell (EC) walls via the interaction of CD44 on MSCs and selectins on the EC surface, which enable the MSCs to roll along the vessel wall [114]. Once in the vessel, MSCs are activated via the chemotactic effects of CXCR4, CXCR7 and other chemokines on the MSC surface with stromal derived factor-1 (SDF-1) on ECs, which also help MSCs to move towards the injury site [117]. Indeed, the expression of CXCR4 on MSCs has been shown to have controversial effects on the homing capacity of the cells. Some studies have suggested that the high expression of CXCR4 is associated with bone marrow homing but not transendothelial migration, while many studies have shown the role of CXCR4/SDF-1 chemotaxis in MSC migration to various tissues [101, 118–121]. Once reaching the destination, MSCs are arrested by the interaction of the very late antigen 4 (VLA-4), which is formed by the combination of integrin α4 and β1, with vascular cell adhesion molecule 1 (VCAM-1) [115, 116]. At the site of the arrest, transmigration or diapedesis occurs, allowing MSCs to cross the endothelial membrane into the interstitial space of the target tissue. To cross the endothelial membrane, MSCs are supported by the activity of matrix metalloproteinases (MMPs), which help to lyse the basement membrane of the endothelium, assisting MSCs to enter the interstitium [122]. Inside the interstitial space, MSCs and their secreted cytokines (such as CCR2, CCR3 and CXCR4) respond to several factors, including SDF-1, platelet derived growth factor (PDGF), IL-8 and insulin-like growth factor (IGF), to migrate to the target sites [123–125].
Differentiation capacity
MSCs are well-known for their differentiation potential. Several studies have been conducted to evaluate the differentiation of MSCs into certain cell types in vitro; many of them are of special interest, such as adipocytes, osteoblast and chondrocytes, while others are non-MSCs, such as hepatocytes, neurons and pancreatic islet cells [126–129]. Other cell types including smooth muscle cells, cardiomyocytes and keratocytes have also been successfully derived from different sources of MSCs [130, 131]. Depending on the origin of MSCs, the differentiation process can be optimized by selecting the MSC populations from certain sources; for example, BM-derived MSCs are the best source of cells for neural differentiation while MSCs from BM or adipose tissue may be more suitable for vascular muscle cell and adipocyte induction [129, 132].
MSCs with a certain expression profile may also be more favorable to commit to particular cell types. Thus, the selection of different MSC subpopulations in order to perform specific differentiation has also been a solution for optimization. For instance, MSCs with high expression of CD146 have been shown to be associated with vascular muscle cell differentiation. Therefore, the selection of the MSCs may be based on their advantage in angiogenesis [131]. In connection with homing, the above evidence raises a possibility that the selection of MSC subpopulations with high expression of both CXCR4 and CD146 may enhance the efficacy of MSCs in treating ischemic conditions. This is because MSCs with high expression of CXCR4 were shown to be efficient in homing to the injured sites, such as ischemic heart and brain [119, 133–135]. Other MSC subpopulations were also shown to be associated with different cell type commitments; for instance, CD200+ MSCs showed high osteogenic potential while CD140+ MSCs showed high adipogenic potential [136]. However, it must be noted that controlling the differentiation of MSCs in vivo is a challenging task that requires a broad knowledge of MSC behavior and function. Thus, further investigations of MSC differentiation in vitro and in vivo are warranted to understand the factors that can affect MSC differentiation in vivo.
Microenvironment generation
The ability to create a microenvironment for healing and homeostasis is another major characteristic of MSCs that is useful in the recovery of damaged tissues. Indeed, MSCs are the foundation for regenerative medicine. The purpose of using stem cells in regeneration not only relies on the ability of the transplanted cells to support the formation and healing of the injured tissues but also to prevent the damages that may cause irreversible tissue consequences, such as malfunction or dysfunction. MSCs with their varied secretion of protein molecules have been widely studied. Isolation of extracellular vesicles from MSCs has revealed several growth factors- such as hepatocyte growth factor (HGF), VEGF, transforming growth factor-β1 (TGFβ1), fibroblast growth factor 21 (FGF-21), and others- which enhance the ability of MSCs in endogenous repair and healing of tissues, including the promotion of vascularization to maintain tissue survival [137–140]. MSCs also secrete neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), to enhance neurogenesis in the case of the ischemic brain [141].
It was found that in the rat model of stroke, animals that were treated with activated MSCs had a significant increase in the number of cells of the oligodendrocytic lineage which express platelet-derived growth factor receptor α (PDGFRα), known to support the lipogenesis process [142]. The authors also identified several molecules in the secretome of activated MSCs that may have an important role in the healing and recovery of the damaged brain [142]. Furthermore, the presence of MSCs also helps to regulate autophagy to support nerve cell survival in cerebral ischemia via the activation of Akt/mTOR signaling pathway [143, 144]. This evidence suggests that MSCs have the potential to induce a healing environment in the injured tissues, which can be monitored by various mechanisms. In fact, several studies have put effort into enhancing the ability of MSCs to secrete exosomes that can be used for healing, but especially to promote angiogenesis as a part of the recovery process [138, 145, 146]. Stimulation and triggering factors can be applied when culturing MSCs to push the expression of angiogenic factors from MSCs and to push the secretion of these factors into the environment so that secretomes can be applied to the injured areas. Different types of triggers are exploited- including mechanical boundary culture conditions, hypoxic culture, and gene expression transfection- which promote angiogenesis and prevent apoptosis at the damaged tissue [145, 147, 148]. For example, exosomes from HIF-overexpressing MSCs showed an increased content of miRNA and proteins related to cell cycle, glycolysis and angiogenesis [147]. High levels of angiogenic factors, such as MMP-2, VEGF and TGF-β1, were found in the conditioned media of mechanically-stimulated MSCs [145]. It was found that even though the secretion of VEGF-A was not present in the conditioned medium from umbilical cord-derived MSCs (UC-MSCs), the effect on angiogenesis was maintained, suggesting that other factors were involved in angiogenic capacity [149]. Some signaling pathways have been identified as being responsible for the regulation of vascularization, such as the Notch signaling pathway and its downstream molecules Jagged-1 [147, 150]. Even though the identification of the triggering factors and activators in MSCs on angiogenesis may require further studies, the potential of using MSCs and their secretomes as a biomaterial in transplantation to treat ischemic diseases is, undoubtedly, promising.
Immunomodulation
The secretome from MSCs can modulate the immune response at the site of injury, preventing cell apoptosis and tissue necrosis mediated by the immune system. In vitro, when BM-MSCs and adipose-derived stem cells (ADSCs) were co-cultured with endothelial cells, high levels of IL-6 were detected in the media, which correlated with the reduction in neutrophil adhesion, suggesting that MSCs performed immunomodulation via the release of IL-6 into the environment [151]. Other secreted cytokines may include IL-1α, IL-1β, IL-10, and IL-12, which play important roles in the regulation of inflammation and immune modulation [152, 153].
While MSCs, in general, have been shown to perform immunomodulation at different levels, the presence of some subpopulations with specific origins has been shown to be more pronounced in immunomodulation function. For examples, BM-MSCs with high expression of CD146 were found to be more sensitive to immune stimulation, in which the exposure to inflammatory priming caused the cells to increase the expression of proteins related to immunomodulation, such as IL-10, IL-3, IL-4, and toll-like receptors, than cells with low or no expression of CD146 [153]. MSCs with low expression of CD105, from both human and mouse origins, have also been reported to have stronger immunomodulatory function compared to MSCs with high CD105 [154, 155]. Similarly, CD106 + MSCs were found to express more immunomodulatory factors than negative counterparts, which resulted in better Treg induction and inflammatory suppression [152]. Other types of MSC immunomodulation include the suppression of stimulated immune cell proliferation, T cell differentiation and macrophage activation, the induced polarization of macrophages into anti-inflammatory M2 phenotype, and the reduction in pro-inflammatory factors [153, 156, 157]. The ability of MSCs to inhibit INF-ϒ and induce IL-4 secretion has also been shown to shift the induction of TH1 cells to TH2 cells, which effectively reduces proinflammation and promotes anti-inflammation responses in graft-versus-host disease (GvHD) [158]. Immune activation of IFN-ϒ on MSCs can prolong the survival of MSCs by suppressing host T cell activation. Thus, the induced expression of IFN-ϒ on MSCs has been shown to be effective when co-transplanted with activated peripheral blood mononuclear cells [159]. These evidence suggest that MSCs can exert potent immunomodulation to assist in the control of tissue damage caused by immune responses while at the same time exerting potent healing effects. In conclusion, the use of MSCs can either facilitate recovery or suppression of immune responses; thus, the selection of the MSC subpopulation with high potency in tissue healing and protection can be optimized to enhance the use of MSCs in therapy.
Challenges of using MSCs in vivo and strategies to improve therapeutic efficacy of MSCs
The potential of MSCs in cell therapy has been proposed and investigated both in vitro and in vivo. However, several challenges directly affect the efficacy of MSCs in therapeutic applications, especially in the in vivo environment. These challenges include the low survival rate of MSCs in the in vivo environment, low growth rate, low ability to proliferate, and rejection from the host immune system. Using MSCs in cell therapy requires selected MSCs to be grown and expanded in vitro, after which the cells are transferred to the in vivo environment, either systemically or locally to the affected sites as a cell treatment. The changes in the environment provide the biggest challenge to the adaptation of MSCs and can effectively reduce the ability of MSCs to perform their function. Understanding the key challenges that MSCs may face in vivo will help to find solutions to improve the cell characteristics that support and sustain them in vivo, as well as solutions to increase the efficiency of cell therapy. Furthermore, it is critical to have good knowledge about the possible causes and mechanisms of these challenges so that they can be addressed and solved appropriately using different strategies (Table 2).
Table 2.
In vivo challenges that possibly affect MSCs efficacy in therapeutic application and strategies to improve MSCs adaptation
| In vivo challenges for MSCs | Improvement strategies |
|---|---|
| Survival |
Modulation of autophagy [143] Hypoxic preconditioning [160] Long-term starvation in culture [161] Selective transfer of CD34+ MSCs [162] Encapsulation of MSCs in microgel and lysophosphatidic acid (LPA) pretreatment [163] |
| Homing |
Selective transfer of CXCR4 MSCs or cell surface engineering with CXCR4 + [164, 165] Selective transfer of CD34+ MSCs [162] Ultrasound [166] Magnetic attraction using of superparamagnetic iron oxide (SPIO) nanoparticles [167] Enhancing the expression of CD44 by culturing MSCs on hyaluronic acid (HA) coated plated [168] Coculture with endothelial cells [169] |
| Proliferation | Preconditioning in hyperoxic condition and pan-caspase inhibitor treatment [170] |
| Host immune response |
IFN-ϒ loaded microparticle priming of MSC spheroids [159] 3D aggregates of MSCs Encapsulation of MSCs in microgel [163] Selection of CD106 + MSCs population [152] Selection of CD105- MSCs population [154, 155] Pretreatment of MSCs with IL-17A [171] Pretreatment of MSCs with IL-1β [172] Pro-inflammatory treatment of MSCs [173] |
| Trans-differentiation |
Combination with nitric oxide releasing hydrogel [174] Inhibition of cycloxygenase-2 [175] Increase of Akt and NFkB signals [176] Transfection with ETV2 [177] |
Survival and migration
The changes from in vitro to in vivo environment, especially in ischemic conditions, cause MSCs to face several microenvironmental restrictions, including nutrition deficiency, hypoxia, and oxidative stress (Fig. 2). For example, the injection of human MSCs into mouse ischemic heart showed that most of the injected cells were relocated into different organs, and only a small number of cells (~ 0.44%) were found in the heart at day 4 after injection [178]. In another study, when MSCs were transferred into patients via a coronary artery (intracoronary), only 1.3%-2.6% of cells were detected after 7 days, and only background detection was found when transferred to the patients via an antecubital vein (intravenously) [162]. Similarly, less than 1% MSCs were found in rat infarcted heart after 7 days of in vivo transfer [179]. These evidence suggest that in vivo, MSCs not only face survival problems but also face difficulty in migration, especially when administered systemically [180].
Problems with in vivo survival and migration in MSCs after transfer have led to an emerging interest in research on modifications of in vitro cell culture of MSCs to investigate ways to enhance the retention of MSCs in vivo and increase the migration of MSCs towards the target sites. Several studies have demonstrated different strategies to improve the survival and migration of MSCs. Stimulation that prolongs the survival of MSCs, including the modulation of autophagy and hypoxic pre-culturing, could effectively protect MSCs from apoptosis and hypoxic stress in animal models [143, 160]. It was found that long-term starvation of MSCs in culture, even though it changes the cell morphology and phenotype, did not change the cell stemness and genetic stability, which helped the cells reach quiescence and improve survival/adaptation in the in vivo environment [161]. The selection of certain MSC subpopulations has also shown advantages in enhancing cell survival in vivo. Particularly, selective transfer of CD34+ enriched MSCs showed a high cell retention rate in the heart compared to unselected MSCs [162]. Another strategy to improve MSC survival includes the encapsulation of MSCs in microgel before intravenous delivery and the induction of protection via treatment with lysophosphatidic acid (LPA), which showed that the survival time of MSCs increased fivefold over unencapsulated cells and the number of detected bare cells was doubled [163]. The enhancement of MSC migration has been tested with different strategies, including using mechanical and biological mechanisms. For instance, ultrasound of the CLI area was used to direct the homing of MSCs to the target site, and thereby to improve the efficacy of the treatment [166]. Even though more investigations on survival of MSCs in in vivo ischemic conditions are necessary, the improvements in cell survival and migration are, indeed, possible. This emphasizes the importance of enhancing survival rate and migration of MSCs when used as therapeutic applications.
Proliferation and differentiation
The exposure to hypoxia and nutrition restriction during ischemic conditions not only causes the reduction in cell survival but also influences the propagation of MSCs in the in vivo environment, which is followed by the risk of functional loss [181]. Several efforts have been made to improve the proliferation rate of MSCs in hypoxia, which includes the preconditioning of MSCs in hyperoxic conditions and pan-caspase inhibitor treatment [170]. However, whether these conditions provide similar effects in vivo requires further examination. Alternatively, the transfer of MSCs at the same time with Tregs has been shown to be effective at protecting MSCs and supporting the proliferation of MSCs in vivo in an ischemic heart model [182]. The evidence showed that the propagation capacity of MSCs in vivo could also be improved by appropriate preconditioning culture of MSCs. However, given the small number of studies, the efficiency of strategies to improve MSC proliferation are still limited. This could be due to the fact that the understanding of MSC behavior in vivo is not well-understood and the need to maintain MSC survival and migration in vivo are already major issues, making it more challenging to target the key factors that directly affect MSC proliferation. Further investigation is warranted to explore the mechanism that can be used to efficiently enhance MSC survival, migration and proliferation in therapeutic application.
Although transdifferentiation of MSCs into endothelial cells in both in vitro and in vivo conditions have been reported in some publications [183–187], the transdifferentiation process requires particular conditions, although the requirements are still unclear. In the commentary by Crisan (2013), the author discussed the results of the study by Corotchi and colleagues who evaluated the transdifferentiation of MSCs from Wharton’s jelly into endothelial cells [188] and who suggested the transdifferentiation potential of MSCs into endothelial cells, although the transdifferentiation remains controversial [185].
Host immune response
MSCs have low expression of the major histocompatibility complex (MHC) class I, no MHC class II, and low expression of costimulatory molecules (such as CD40, CD40 ligand, B7-1 and B7-2), which help MSCs to suppress the acceleration of T cells from the host immune system and inhibit NK cells from the host immune system during transplantation [158, 189, 190]. Even though MSCs possess great immunomodulation properties, such as being able to self-downregulate MHC-I after immune stimulation to maintain low immunogenicity [190], the transplantation of allogenic MSCs still face rejection by the host immune system. Notably, T cell activation and proliferation were only found following the intracranial injection of allogenic MSCs but not autologous MSCs [191, 192]. It has been widely discussed whether MSCs are as immune-privileged as thought and that mismatched MSCs are the major cause of host rejection in MSC transplantation [189, 193]. Studies in mice have shown that grafting of allogeneic MSCs mismatched in MHC-I and II were actively rejected by the host immune system, which was shown by the increase in T cells and NK cells in the host mice [194]. Different mechanisms leading to donor cell rejection have been well-described, including direct, indirect, or semi-direct allorecognition of MHC-mismatched MSCs [189, 195].
In direct allorecognition, MHC molecules on donor MSCs are recognized directly by host T cells, which activates T cells and accelerates immune responses to MSCs [189]. Indirect allorecognition of the host immune system works by the recognition of MHC peptides shed from the surface of MSCs by antigen-presenting cells (APCs) or dendritic cells (DCs), which then transfer the signals to activate T cells and trigger the immune system [189]. Semi-direct allorecognition includes the antigen-presenting properties of MHC molecules recognized by APCs or DCs, which will present them directly to T cells to activate the rejection response [195, 196]. Thus, suppressing MHC mismatch recognition appears to be the key to improving MSC survival during allogeneic graft. The immunosuppressive ability of MSCs is also helpful in GvHD [197–199]. Improving the immunomodulation ability of MSCs not only supports the cells to survive in the in vivo microenvironment but also enhances the cell capacity by improving the efficiency of MSCs in regulating the host immune response. Changes in culture condition and treatment have been shown to be effective in improving MSC ability to suppress the immune system [159, 200]. However, the mix of literature demonstrating MSC-mediated immune rejection and privilege might suggest that different MSC lines and individual host profiles may be correlating in performing either a strong or weak host immune response. Therefore, to predict the outcomes of a host immune response during MSC transplantation, a thorough examination is required to identify the correlation between host immune system reactions in different combinations of allogenic MSCs and host profiles.
How to overcome the challenges
In recent decades, the increase of aging populations has been met with the emerging incidence of vascular diseases, including ischemia, MI and stroke. The disease pathogenesis coming from blood flow restriction has become a serious health condition that results in decreased patient quality of life and increased burden on the health system. The restriction of blood supply critically changes the tissue function as the demands for nutrition and oxygen to maintain normal cell and tissue activity are not met, leading to complications including cell pathway alterations and immune response alterations, which may either support the tissue to adapt to the new conditions or may negatively accelerate apoptosis and necrosis as a consequence. Understanding the changes that occur within different cells at the ischemic sites will be beneficial in identifying the potential risk that may take place and addressing the promising targets that can be used to best support the treatment. The requirement to quickly regenerate the blood supply is critical to prevent severe and irreversible damage associated with ischemic events. Several therapies have been proposed and put into clinical trials, aiming to either prevent the damage of restricted blood flow or to enforce the recovery after the ischemic events. However, no current treatment has appeared to be effective in treating ischemic conditions, and most of them are temporary. Thus, there is a great need for new therapies that can safely improve and enhance the recovery after ischemic injury.
MSCs have recently been used in several applications due to their ability to self-renew and expand, differentiate into different cell types, home to target tissues, and exert immune modulation. MSCs have been effectively used as a cell therapy in different types of vascular diseases, including peripheral arterial diseases, ischemic heart, ischemic brain, and stroke [201, 202]. However, inconsistent clinical outcomes and limited data on post-treatment improvements have prevented large-scale trials of MSC transplantation and prevented MSCs to be further investigated as a potential cell therapy. These challenges not only come from the changes between in vitro and in vivo environments but are also derived from the alterations/modifications that occur at the ischemic sites, where nutrition and oxygen supply become short and host immune response is accelerated. Among the factors, nutrition and oxygen deprivation and host immune responses, as well as low adaptation, are the key factors that affect the survival of MSCs. The low homing potential of MSCs comes from the pre-treatment culture of the cells and the low adaptation in the in vivo environment, which causes the MSCs to express low chemotactic factors [115, 116]. Generally, there has not been much evidence in the proliferation capacity of MSCs in vivo after transplantation. Even though MSC proliferation may lead to concerns related to tumor formation, the ability to maintain MSC characteristics with controlled growth and proliferation may be a new aspect for future research of MSC-based therapy. Therefore, understanding of the challenges of MSCs in vivo is important to address where improvement can be made for future research.
In fact, several studies have proposed different modifications that can be applied to improve MSC characteristics to support the in vivo adaptation of MSCs, which have shown effective improvement in different models [12, 166, 203]. The improvement of MSC characteristics should be in coordination with other factors, such as the local environment conditions at the ischemic sites and the patient’s health profile. For example, the combination of MSC therapy with other cell types and treatments may be required in patients with different conditions, such as hypertension, obesity, diabetes or cancer, as the associated conditions may lower the efficacy of MSC treatment due to microenvironment changes. Thus, to achieve better outcomes with using MSC transplantation in ischemic conditions, further investigation of MSC-based therapy in various patients with different health problems is required to fully evaluate the efficacy of MSC transplantation for large-scale applications.
Conclusion
MSC transplantation provides a promising therapy for ischemic disease treatment. MSCs not only participate in angiogenesis but also promote the regeneration process of injured tissues by various mechanisms. However, the treatment efficacy of ischemic diseases by MSC transplantation has been met with challenges related to the proliferation as well as differentiation of MSCs at injured tissues. Some novel strategies to overcome these challenges have been suggested in recent studies that promote the treatment efficacy in animal models. Therefore, more preclinical trials and clinical trials using these new strategies are essential to evaluate and optimize this therapy for ischemic diseases.
Acknowledgements
All authors equally contributed in this work. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Calvert JW. Chapter 5 - Ischemic heart disease and its consequences. In: Willis MS, Homeister JW, Stone JR, editors. Cellular and molecular pathobiology of cardiovascular disease. San Diego: Academic Press; 2014. pp. 79–100. [Google Scholar]
- 2.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Wang X, Li J, Zhang J, Zhang F, Hu J, et al. Advances in the treatment of ischemic diseases by mesenchymal stem cells. Stem Cells Int. 2016;2016:5896061. doi: 10.1155/2016/5896061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong H, Yim HW, Park HJ, Cho Y, Hong H, Kim NJ, et al. Mesenchymal stem cell therapy for ischemic heart disease: systematic review and meta-analysis. Int J Stem Cells. 2018;11:1–12. doi: 10.15283/ijsc17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F, Tang H, Zhu J, Zhang JH. Transplanting mesenchymal stem cells for treatment of ischemic stroke. Cell Transplant. 2018;27:1825–1834. doi: 10.1177/0963689718795424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S, et al. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med. 2013;11:143. doi: 10.1186/1479-5876-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 8.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45:3618–3624. doi: 10.1161/STROKEAHA.114.007028. [DOI] [PubMed] [Google Scholar]
- 10.Silva LHA, Antunes MA, Dos Santos CC, Weiss DJ, Cruz FF, Rocco PRM. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res Ther. 2018;9:45. doi: 10.1186/s13287-018-0802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Chen X, Wang WE, Zeng C. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells Int. 2016;2016:9682757. doi: 10.1155/2016/9682757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo R, Lu Y, Liu J, Cheng J, Chen Y. Enhancement of the efficacy of mesenchymal stem cells in the treatment of ischemic diseases. Biomed Pharmacother. 2019;109:2022–2034. doi: 10.1016/j.biopha.2018.11.068. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Cho JH, Lee YH, Lee JH, Kim SS, Kim MY, et al. Improvement in left ventricular function with intracoronary mesenchymal stem cell therapy in a patient with anterior wall ST-segment elevation myocardial infarction. Cardiovasc Drugs Ther. 2018;32:329–338. doi: 10.1007/s10557-018-6804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penn MS, Ellis S, Gandhi S, Greenbaum A, Hodes Z, Mendelsohn FO, et al. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: phase I clinical study. Circ Res. 2012;110:304–311. doi: 10.1161/CIRCRESAHA.111.253427. [DOI] [PubMed] [Google Scholar]
- 15.Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial) Eur Heart J. 2015;36:1744–1753. doi: 10.1093/eurheartj/ehv136. [DOI] [PubMed] [Google Scholar]
- 16.Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Kofoed KF, Haack-Sørensen M, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. Eur J Heart Fail. 2020;22:884–892. doi: 10.1002/ejhf.1700. [DOI] [PubMed] [Google Scholar]
- 17.Ulus AT, Mungan C, Kurtoglu M, Celikkan FT, Akyol M, Sucu M, et al. Intramyocardial transplantation of umbilical cord mesenchymal stromal cells in chronic ischemic cardiomyopathy: a controlled, randomized clinical trial (HUC-HEART trial) Int J Stem Cells. 2020;13:364–376. doi: 10.15283/ijsc20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao W, Guo S, Gao C, Dai G, Gao Y, Li M, et al. A Randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. Int Heart J. 2017;58:238–244. doi: 10.1536/ihj.16-328. [DOI] [PubMed] [Google Scholar]
- 19.Qayyum AA, Mathiasen AB, Mygind ND, Vejlstrup NG, Kastrup J. Cardiac magnetic resonance imaging used for evaluation of adipose-derived stromal cell therapy in patients with chronic ischemic heart disease. Cell Transplant. 2019;28:1700–1708. doi: 10.1177/0963689719883592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Florea V, Rieger AC, DiFede DL, El-Khorazaty J, Natsumeda M, Banerjee MN, et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (The TRIDENT Study) Circ Res. 2017;121:1279–1290. doi: 10.1161/CIRCRESAHA.117.311827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gralla J, Brekenfeld C, Mordasini P, Schroth G. Mechanical thrombolysis and stenting in acute ischemic stroke. Stroke. 2012;43:280–285. doi: 10.1161/STROKEAHA.111.626903. [DOI] [PubMed] [Google Scholar]
- 23.Khazaei M, Davoodian A, Taheri M, Ghafouri-Fard S. Former antiplatelet drug administration and consequences of intravenous thrombolysis in acute ischemic stroke. Hum Antibodies. 2020;28:53–56. doi: 10.3233/HAB-190391. [DOI] [PubMed] [Google Scholar]
- 24.Su YH, Chen CH, Lin HJ, Chen YW, Tseng MC, Hsieh HC, et al. Safety and effectiveness of intravenous thrombolysis for acute ischemic stroke outside the coverage of national health insurance in Taiwan. Acta Neurol Taiwan. 2017;26:3–12. [PubMed] [Google Scholar]
- 25.Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain: J Neurol. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Kim AS, Johnson JN, et al. Two-year safety and clinical outcomes in chronic ischemic stroke patients after implantation of modified bone marrow-derived mesenchymal stem cells (SB623): a phase 1/2a study. J Neurosurg. 2018;23:1–11. doi: 10.3171/2017.10.JNS17971. [DOI] [PubMed] [Google Scholar]
- 27.Levy ML, Crawford JR, Dib N, Verkh L, Tankovich N, Cramer SC. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke. 2019;50:2835–2841. doi: 10.1161/STROKEAHA.119.026318. [DOI] [PubMed] [Google Scholar]
- 28.Jaillard A, Hommel M, Moisan A, Zeffiro TA, Favre-Wiki IM, Barbieux-Guillot M, et al. Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: a randomized clinical trial. Transl Stroke Res. 2020;11:910–923. doi: 10.1007/s12975-020-00787-z. [DOI] [PubMed] [Google Scholar]
- 29.Lu D, Jiang Y, Deng W, Zhang Y, Liang Z, Wu Q, et al. Long-Term outcomes of BMMSC compared with BMMNC for treatment of critical limb ischemia and foot ulcer in patients with diabetes. Cell Transplant. 2019;28:645–652. doi: 10.1177/0963689719835177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta PK, Krishna M, Chullikana A, Desai S, Murugesan R, Dutta S, et al. Administration of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells in critical limb ischemia due to Buerger's disease: phase II study report suggests clinical efficacy. Stem Cells Transl Med. 2017;6:689–699. doi: 10.5966/sctm.2016-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao WH, Gao HY, Li YT, Huang PP. Effectiveness of umbilical cord mesenchymal stem cells in patients with critical limb ischemia. Med Clin. 2019;153:341–346. doi: 10.1016/j.medcli.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 32.Huang P. Therapeutic outcomes of transplanting human umbilical cord mesenchymal stem cells in patients with critical limb ischemia. Eur J Vasc Endovasc Surg. 2019;58:e388–e389. doi: 10.1016/j.ejvs.2019.06.1024. [DOI] [Google Scholar]
- 33.Wang J, Zeng XX, Cai W, Han ZB, Zhu LY, Liu JY, et al. Safety and efficacy of placenta-derived mesenchymal stem cell treatment for diabetic patients with critical limb ischemia: a pilot study. Experim Clin Endocrinol Diabetes. 2019;1:1. doi: 10.1055/a-0978-4972. [DOI] [PubMed] [Google Scholar]
- 34.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HC, An SG, Lee HW, Park JS, Cha KS, Hong TJ, et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J. 2012;76:1750–1760. doi: 10.1253/circj.CJ-11-1135. [DOI] [PubMed] [Google Scholar]
- 36.Salter B, Sehmi R. The role of bone marrow-derived endothelial progenitor cells and angiogenic responses in chronic obstructive pulmonary disease. J Thor Dis. 2017;9:2168–2177. doi: 10.21037/jtd.2017.07.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bakondi B, Shimada IS, Perry A, Munoz JR, Ylostalo J, Howard AB, et al. CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol Ther. 2009;17:1938–1947. doi: 10.1038/mt.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasseri BA, Ebell W, Dandel M, Kukucka M, Gebker R, Doltra A, et al. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur Heart J. 2014;35:1263–1274. doi: 10.1093/eurheartj/ehu007. [DOI] [PubMed] [Google Scholar]
- 39.Forcillo J, Stevens LM, Mansour S, Prieto I, Salem R, Baron C, et al. Implantation of CD133+ stem cells in patients undergoing coronary bypass surgery: IMPACT-CABG pilot trial. Can J Cardiol. 2013;29:441–447. doi: 10.1016/j.cjca.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Stamm C, Kleine HD, Choi YH, Dunkelmann S, Lauffs JA, Lorenzen B, et al. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 41.Naseri MH, Madani H, Ahmadi Tafti SH, Moshkani Farahani M, Kazemi Saleh D, Hosseinnejad H, et al. COMPARE CPM-RMI trial: intramyocardial transplantation of autologous bone marrow-derived CD133+ cells and MNCs during CABG in patients with recent MI: a phase II/III, multicenter, placebo-controlled, randomized, Double-Blind Clinical Trial . Cell J. 2018;20:267–277. doi: 10.22074/cellj.2018.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet (London, England) 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mou Y, Yue Z, Zhang H, Shi X, Zhang M, Chang X, et al. High quality in vitro expansion of human endothelial progenitor cells of human umbilical vein origin. Int J Med Sci. 2017;14:294–301. doi: 10.7150/ijms.18137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Neill DW, Jiang Y, Leary E, Yavanian G, Eminli S, Marasco WA. A Lin-CD45-CD34+ population of extracellular vesicles in human blood that mimics very small embryonic-like stem cells (VSELs) by flow cytometry. Blood. 2012;120:4750. doi: 10.1182/blood.V120.21.4750.4750. [DOI] [Google Scholar]
- 46.Sovalat H, Scrofani M, Eidenschenk A, Pasquet S, Rimelen V, Hénon P. Identification and isolation from either adult human bone marrow or G-CSF−mobilized peripheral blood of CD34+/CD133+/CXCR4+/ Lin−CD45− cells, featuring morphological, molecular, and phenotypic characteristics of very small embryonic-like (VSEL) stem cells. Exp Hematol. 2011;39:495–505. doi: 10.1016/j.exphem.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Cui J, Peng W, Lu M. Intracoronary autologous CD34+ stem cell therapy for intractable angina. Cardiology. 2010;117:140–147. doi: 10.1159/000320217. [DOI] [PubMed] [Google Scholar]
- 48.Velagapudi P, Turagam M, Kolte D, Khera S, Hyder O, Gordon P, et al. Intramyocardial autologous CD34+ cell therapy for refractory angina: A meta-analysis of randomized controlled trials. Cardiovasc Revascular Med: Includ Mol Interv. 2019;20:215–219. doi: 10.1016/j.carrev.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 49.Park M, Seo JJ. Role of HLA in hematopoietic stem cell transplantation. Bone Marrow Res. 2012;2012:680841. doi: 10.1155/2012/680841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Latham K, Little AM, Madrigal JA. An overview of HLA typing for hematopoietic stem cell transplantation. Methods Mol Biol. 2014;1109:73–85. doi: 10.1007/978-1-4614-9437-9_5. [DOI] [PubMed] [Google Scholar]
- 51.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/S0301-472X(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Tian M, Wang F, Heng BC, Zhou J, Cai Z, et al. Understanding the immunological mechanisms of mesenchymal stem cells in allogeneic transplantation: from the aspect of major histocompatibility complex class I. Stem Cells Dev. 2019;28:1141–1150. doi: 10.1089/scd.2018.0256. [DOI] [PubMed] [Google Scholar]
- 53.Lee CW, Stabile E, Kinnaird T, Shou M, Devaney JM, Epstein SE, et al. Temporal patterns of gene expression after acute hindlimb ischemia in mice: insights into the genomic program for collateral vessel development. J Am Coll Cardiol. 2004;43:474–482. doi: 10.1016/j.jacc.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 54.Cave Alison C, Ingwall Joanne S, Friedrich J, Liao R, Saupe Kurt W, Apstein Carl S, et al. ATP synthesis during low-flow ischemia. Circulation. 2000;101:2090–2096. doi: 10.1161/01.CIR.101.17.2090. [DOI] [PubMed] [Google Scholar]
- 55.Weil MH, Afifi AA. Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock) Circulation. 1970;41:989–1001. doi: 10.1161/01.CIR.41.6.989. [DOI] [PubMed] [Google Scholar]
- 56.Chan TS, Cassim S, Raymond VA, Gottschalk S, Merlen G, Zwingmann C, et al. Upregulation of Krebs cycle and anaerobic glycolysis activity early after onset of liver ischemia. PLoS One. 2018;13:e0199177. doi: 10.1371/journal.pone.0199177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lundberg G, Wahlberg E, Swedenborg J, Sundberg CJ, Ungerstedt U, Olofsson P. Continuous assessment of local metabolism by microdialysis in critical limb ischaemia. Eur J Vascul Endovasc Surg. 2000;19:605–613. doi: 10.1053/ejvs.1999.1088. [DOI] [PubMed] [Google Scholar]
- 58.Bosco G, Yang ZJ, Nandi J, Wang J, Chen C, Camporesi EM. Effects of hyperbaric oxygen on glucose, lactate, glycerol and anti-oxidant enzymes in the skeletal muscle of rats during ischaemia and reperfusion. Clin Exp Pharmacol Physiol. 2007;34:70–76. doi: 10.1111/j.1440-1681.2007.04548.x. [DOI] [PubMed] [Google Scholar]
- 59.Muller M, Schmid R, Nieszpaur-Los M, Fassolt A, Lonnroth P, Fasching P, et al. Key metabolite kinetics in human skeletal muscle during ischaemia and reperfusion: measurement by microdialysis. Eur J Clin Invest. 1995;25:601–607. doi: 10.1111/j.1365-2362.1995.tb01752.x. [DOI] [PubMed] [Google Scholar]
- 60.Ryan TE, Yamaguchi DJ, Schmidt CA, Zeczycki TN, Shaikh SR, Brophy P, et al. Extensive skeletal muscle cell mitochondriopathy distinguishes critical limb ischemia patients from claudicants. JCI Insight. 2018;3:e123235. doi: 10.1172/jci.insight.123235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun D, Nguyen N, DeGrado TR, Schwaiger M, Brosius FC., 3rd Ischemia induces translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane of cardiac myocytes. Circulation. 1994;89:793–798. doi: 10.1161/01.CIR.89.2.793. [DOI] [PubMed] [Google Scholar]
- 62.Davey KA, Garlick PB, Warley A, Southworth R. Immunogold labeling study of the distribution of GLUT-1 and GLUT-4 in cardiac tissue following stimulation by insulin or ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H2009–H2019. doi: 10.1152/ajpheart.00663.2006. [DOI] [PubMed] [Google Scholar]
- 63.Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–E657. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- 64.Drake KJ, Shotwell MS, Wikswo JP, Sidorov VY. Glutamine and glutamate limit the shortening of action potential duration in anoxia-challenged rabbit hearts. Physiol Rep. 2015;3:1. doi: 10.14814/phy2.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bolotin G, Raman J, Williams U, Bacha E, Kocherginsky M, Jeevanandam V. Glutamine improves myocardial function following ischemia-reperfusion injury. Asian Cardiovasc Thorac Ann. 2007;15:463–467. doi: 10.1177/021849230701500603. [DOI] [PubMed] [Google Scholar]
- 66.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol. 2007;42:177–185. doi: 10.1016/j.yjmcc.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohiuddin M, Lee NH, Moon JY, Han WM, Anderson SE, Choi JJ, et al. Critical limb ischemia induces remodeling of skeletal muscle motor unit, myonuclear-, and mitochondrial-domains. Sci Rep. 2019;9:9551. doi: 10.1038/s41598-019-45923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tran TP, Tu H, Pipinos II, Muelleman RL, Albadawi H, Li YL. Tourniquet-induced acute ischemia-reperfusion injury in mouse skeletal muscles: Involvement of superoxide. Eur J Pharmacol. 2011;650:328–334. doi: 10.1016/j.ejphar.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ambrosio G, Becker LC, Hutchins GM, Weisman HF, Weisfeldt ML. Reduction in experimental infarct size by recombinant human superoxide dismutase: insights into the pathophysiology of reperfusion injury. Circulation. 1986;74:1424–1433. doi: 10.1161/01.CIR.74.6.1424. [DOI] [PubMed] [Google Scholar]
- 70.Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H566–H574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- 71.Forbes RA, Steenbergen C, Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ Res. 2001;88:802–809. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- 72.Kirmes I, Szczurek A, Prakash K, Charapitsa I, Heiser C, Musheev M, et al. A transient ischemic environment induces reversible compaction of chromatin. Genome Biol. 2015;16:246. doi: 10.1186/s13059-015-0802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Batie M, Del Peso L, Rocha S. Hypoxia and chromatin: a focus on transcriptional repression mechanisms. Biomedicines. 2018;6:1. doi: 10.3390/biomedicines6020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochem Biophys Acta. 2011;1813:1263–1268. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai Z, Luo W, Zhan H, Semenza GL. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci U S A. 2013;110:17462–17467. doi: 10.1073/pnas.1317158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kapitsinou PP, Sano H, Michael M, Kobayashi H, Davidoff O, Bian A, et al. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest. 2014;124:2396–2409. doi: 10.1172/JCI69073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarkar K, Fox-Talbot K, Steenbergen C, Bosch-Marce M, Semenza GL. Adenoviral transfer of HIF-1alpha enhances vascular responses to critical limb ischemia in diabetic mice. Proc Natl Acad Sci U S A. 2009;106:18769–18774. doi: 10.1073/pnas.0910561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pavo N, Lukovic D, Zlabinger K, Zimba A, Lorant D, Goliasch G, et al. Sequential activation of different pathway networks in ischemia-affected and non-affected myocardium, inducing intrinsic remote conditioning to prevent left ventricular remodeling. Sci Rep. 2017;7:43958. doi: 10.1038/srep43958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ganta VC, Choi M, Kutateladze A, Annex BH. VEGF165b modulates endothelial VEGFR1-STAT3 signaling pathway and angiogenesis in human and experimental peripheral arterial disease. Circ Res. 2017;120:282–295. doi: 10.1161/CIRCRESAHA.116.309516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu H, Xiao F, Wang G, Wei X, Jiang L, Chen Y, et al. STAT3 Regulates self-renewal of adult muscle satellite cells during injury-induced muscle regeneration. Cell Rep. 2016;16:2102–2115. doi: 10.1016/j.celrep.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 81.Choi EK, Yeo JS, Park CY, Na H, Lim J, Lee JE, et al. Inhibition of reactive oxygen species downregulates the MAPK pathway in rat spinal cord after limb ischemia reperfusion injury. Int J Surg. 2015;22:74–78. doi: 10.1016/j.ijsu.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 82.Yang J. The role of reactive oxygen species in angiogenesis and preventing tissue injury after brain ischemia. Microvasc Res. 2019;123:62–67. doi: 10.1016/j.mvr.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 83.Bentley K, Gerhardt H, Bates PA. Agent-based simulation of notch-mediated tip cell selection in angiogenic sprout initialisation. J Theor Biol. 2008;250:25–36. doi: 10.1016/j.jtbi.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 84.Moya IM, Umans L, Maas E, Pereira PN, Beets K, Francis A, et al. Stalk cell phenotype depends on integration of Notch and Smad1/5 signaling cascades. Dev Cell. 2012;22:501–514. doi: 10.1016/j.devcel.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tetzlaff F, Fischer A. Control of blood vessel formation by notch signaling. Adv Exp Med Biol. 2018;1066:319–338. doi: 10.1007/978-3-319-89512-3_16. [DOI] [PubMed] [Google Scholar]
- 86.Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harbor Perspect Med. 2013;3:a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang Q, Yan W, Li X, Hou L, Dong H, Wang Q, et al. Activation of canonical notch signaling pathway is involved in the ischemic tolerance induced by sevoflurane preconditioning in mice. Anesthesiology. 2012;117:996–1005. doi: 10.1097/ALN.0b013e31826cb469. [DOI] [PubMed] [Google Scholar]
- 88.Jin Z, Guo P, Li X, Ke J, Wang Y, Wu H. Neuroprotective effects of irisin against cerebral ischemia/ reperfusion injury via Notch signaling pathway. Biomed Pharmacother. 2019;120:109452. doi: 10.1016/j.biopha.2019.109452. [DOI] [PubMed] [Google Scholar]
- 89.Dimitrova E, Caromile LA, Laubenbacher R, Shapiro LH. The innate immune response to ischemic injury: a multiscale modeling perspective. BMC Syst Biol. 2018;12:50. doi: 10.1186/s12918-018-0580-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Santos-Zas I, Lemarie J, Tedgui A, Ait-Oufella H. Adaptive immune responses contribute to post-ischemic cardiac remodeling. Front Cardiovasc Med. 2018;5:198. doi: 10.3389/fcvm.2018.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rusinkevich V, Huang Y, Chen ZY, Qiang W, Wang YG, Shi YF, et al. Temporal dynamics of immune response following prolonged myocardial ischemia/reperfusion with and without cyclosporine A. Acta Pharmacol Sin. 2019;40:1168–1183. doi: 10.1038/s41401-018-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 93.Madaro L, Torcinaro A, De Bardi M, Contino FF, Pelizzola M, Diaferia GR, et al. Macrophages fine tune satellite cell fate in dystrophic skeletal muscle of mdx mice. PLoS Genet. 2019;15:e1008408. doi: 10.1371/journal.pgen.1008408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bless NM, Warner RL, Padgaonkar VA, Lentsch AB, Czermak BJ, Schmal H, et al. Roles for C-X-C chemokines and C5a in lung injury after hindlimb ischemia-reperfusion. Am J Physiol. 1999;276:L57–63. doi: 10.1152/ajplung.1999.276.1.L57. [DOI] [PubMed] [Google Scholar]
- 95.Jaworska K, Ratajczak J, Huang L, Whalen K, Yang M, Stevens BK, et al. Both PD-1 ligands protect the kidney from ischemia reperfusion injury. J Immunol. 2015;194:325–333. doi: 10.4049/jimmunol.1400497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nossent AY, Bastiaansen AJ, Peters EA, de Vries MR, Aref Z, Welten SM, et al. CCR7-CCL19/CCL21 axis is essential for effective arteriogenesis in a murine model of hindlimb ischemia. J Am Heart Assoc. 2017;6:1. doi: 10.1161/JAHA.116.005281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Latet SC, Hoymans VY, Van Herck PL, Vrints CJ. The cellular immune system in the post-myocardial infarction repair process. Int J Cardiol. 2015;179:240–247. doi: 10.1016/j.ijcard.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 98.Zuidema MY, Zhang C. Ischemia/reperfusion injury: The role of immune cells. World journal of cardiology. 2010;2:325–332. doi: 10.4330/wjc.v2.i10.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hofmann U, Frantz S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res. 2015;116:354–367. doi: 10.1161/CIRCRESAHA.116.304072. [DOI] [PubMed] [Google Scholar]
- 100.Stabile E, Kinnaird T, la Sala A, Hanson SK, Watkins C, Campia U, et al. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation. 2006;113:118–124. doi: 10.1161/CIRCULATIONAHA.105.576702. [DOI] [PubMed] [Google Scholar]
- 101.van Weel V, Toes RE, Seghers L, Deckers MM, de Vries MR, Eilers PH, et al. Natural killer cells and CD4+ T-cells modulate collateral artery development. Arterioscler Thromb Vasc Biol. 2007;27:2310–2318. doi: 10.1161/ATVBAHA.107.151407. [DOI] [PubMed] [Google Scholar]
- 102.Beneke A, Guentsch A, Hillemann A, Zieseniss A, Swain L, Katschinski DM. Loss of PHD3 in myeloid cells dampens the inflammatory response and fibrosis after hind-limb ischemia. Cell Death Dis. 2017;8:e2976. doi: 10.1038/cddis.2017.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oklu R, Albadawi H, Jones JE, Yoo HJ, Watkins MT. Reduced hind limb ischemia-reperfusion injury in Toll-like receptor-4 mutant mice is associated with decreased neutrophil extracellular traps. J Vasc Surg. 2013;58:1627–1636. doi: 10.1016/j.jvs.2013.02.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carbone F, Nencioni A, Mach F, Vuilleumier N, Montecucco F. Pathophysiological role of neutrophils in acute myocardial infarction. Thromb Haemost. 2013;110:501–514. doi: 10.1160/TH13-03-0211. [DOI] [PubMed] [Google Scholar]
- 105.Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, et al. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. 2003;108:205–210. doi: 10.1161/01.CIR.0000079225.50817.71. [DOI] [PubMed] [Google Scholar]
- 106.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–1663. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 107.Simons KH, Aref Z, Peters HAB, Welten SP, Nossent AY, Jukema JW, et al. The role of CD27-CD70-mediated T cell co-stimulation in vasculogenesis, arteriogenesis and angiogenesis. Int J Cardiol. 2018;260:184–190. doi: 10.1016/j.ijcard.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 108.Hellingman AA, Zwaginga JJ, van Beem RT, Hamming JF, Fibbe WE, Quax PH, et al. T-cell-pre-stimulated monocytes promote neovascularisation in a murine hind limb ischaemia model. Eur J Vasc Endovasc Surg. 2011;41:418–428. doi: 10.1016/j.ejvs.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 109.Niiyama H, Kai H, Yamamoto T, Shimada T, Sasaki K, Murohara T, et al. Roles of endogenous monocyte chemoattractant protein-1 in ischemia-induced neovascularization. J Am Coll Cardiol. 2004;44:661–666. doi: 10.1016/j.jacc.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 110.Choe MA, Kim KH, An GJ, Lee KS, Heitkemper M. Hindlimb muscle atrophy occurs from peripheral nerve damage in a rat neuropathic pain model. Biol Res Nurs. 2011;13:44–54. doi: 10.1177/1099800410382291. [DOI] [PubMed] [Google Scholar]
- 111.Tsuji N, Yamashita S, Sugawara Y, Kobayashi E. Effect of prolonged ischaemic time on muscular atrophy and regenerating nerve fibres in transplantation of the rat hind limb. J Plast Surg Hand Surg. 2012;46:217–221. doi: 10.3109/2000656X.2012.709726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ucuzian AA, Gassman AA, East AT, Greisler HP. Molecular mediators of angiogenesis. J Burn Care Res. 2010;31:158–175. doi: 10.1097/BCR.0b013e3181c7ed82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jung S, Panchalingam KM, Wuerth RD, Rosenberg L, Behie LA. Large-scale production of human mesenchymal stem cells for clinical applications. Biotechnol Appl Biochem. 2012;59:106–120. doi: 10.1002/bab.1006. [DOI] [PubMed] [Google Scholar]
- 114.Eseonu OI, De Bari C. Homing of mesenchymal stem cells: mechanistic or stochastic? Implications for targeted delivery in arthritis. Rheumatology (Oxford) 2015;54:210–218. doi: 10.1093/rheumatology/keu377. [DOI] [PubMed] [Google Scholar]
- 115.De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World journal of stem cells. 2016;8:73–87. doi: 10.4252/wjsc.v8.i3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ullah M, Liu DD, Thakor AS. Mesenchymal stromal cell homing: mechanisms and strategies for improvement. IScience. 2019;15:421–438. doi: 10.1016/j.isci.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zachar L, Bacenkova D, Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflam Res. 2016;9:231–240. doi: 10.2147/JIR.S121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wynn RF, Hart CA, Corradi-Perini C, O'Neill L, Evans CA, Wraith JE, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 119.Ryu CH, Park SA, Kim SM, Lim JY, Jeong CH, Jun JA, et al. Migration of human umbilical cord blood mesenchymal stem cells mediated by stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38 signal transduction pathways. Biochem Biophys Res Commun. 2010;398:105–110. doi: 10.1016/j.bbrc.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 120.Park SA, Ryu CH, Kim SM, Lim JY, Park SI, Jeong CH, et al. CXCR4-transfected human umbilical cord blood-derived mesenchymal stem cells exhibit enhanced migratory capacity toward gliomas. Int J Oncol. 2011;38:97–103. [PubMed] [Google Scholar]
- 121.Marquez-Curtis LA, Janowska-Wieczorek A. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. Biomed Res Int. 2013;2013:561098. doi: 10.1155/2013/561098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steingen C, Brenig F, Baumgartner L, Schmidt J, Schmidt A, Bloch W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008;44:1072–1084. doi: 10.1016/j.yjmcc.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 123.Bayo J, Real A, Fiore EJ, Malvicini M, Sganga L, Bolontrade M, et al. IL-8, GRO and MCP-1 produced by hepatocellular carcinoma microenvironment determine the migratory capacity of human bone marrow-derived mesenchymal stromal cells without affecting tumor aggressiveness. Oncotarget. 2017;8:80235–80248. doi: 10.18632/oncotarget.10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bi LK, Zhou N, Liu C, Lu FD, Lin TX, Xuan XJ, et al. Kidney cancer cells secrete IL-8 to activate Akt and promote migration of mesenchymal stem cells. Urol Oncol. 2014;32:607–612. doi: 10.1016/j.urolonc.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 125.Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 126.Pfützner A, Schipper D, Pansky A, Kleinfeld C, Roitzheim B, Tobiasch E. Mesenchymal stem cell differentiation into adipocytes is equally induced by insulin and proinsulin in vitro. Int J Stem Cells. 2017;10:154–159. doi: 10.15283/ijsc17033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Szaraz P, Gratch YS, Iqbal F, Librach CL. In vitro differentiation of human mesenchymal stem cells into functional cardiomyocyte-like cells. J Vis Experim JoVE. 2017;126:1. doi: 10.3791/55757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shivakumar SB, Lee HJ, Son YB, Bharti D, Ock SA, Lee SL, et al. In vitro differentiation of single donor derived human dental mesenchymal stem cells into pancreatic β cell-like cells. Bioscience Rep. 2019;39:1. doi: 10.1042/BSR20182051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Urrutia DN, Caviedes P, Mardones R, Minguell JJ, Vega-Letter AM, Jofre CM. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: An approach for their use in neural regeneration therapies. PLoS One. 2019;14:e0213032. doi: 10.1371/journal.pone.0213032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dos Santos A, Balayan A, Funderburgh ML, Ngo J, Funderburgh JL, Deng SX Differentiation capacity of human mesenchymal stem cells into keratocyte lineage. Invest Ophthalmol Vis Sci. 2019;60:3013–3023. doi: 10.1167/iovs.19-27008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Espagnolle N, Guilloton F, Deschaseaux F, Gadelorge M, Sensebe L, Bourin P. CD146 expression on mesenchymal stem cells is associated with their vascular smooth muscle commitment. J Cell Mol Med. 2014;18:104–114. doi: 10.1111/jcmm.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gu W, Hong X, Le Bras A, Nowak WN, Issa Bhaloo S, Deng J, et al. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J Biol Chem. 2018;293:8089–8102. doi: 10.1074/jbc.RA118.001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu Q, Liu L, Lin J, Wang Y, Xuan X, Guo Y, et al. SDF-1alpha/CXCR4 axis mediates the migration of mesenchymal stem cells to the hypoxic-ischemic brain lesion in a rat model. Cell J. 2015;16:440–447. doi: 10.22074/cellj.2015.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bang OY, Jin KS, Hwang MN, Kang HY, Kim BJ, Lee SJ, et al. The Effect of CXCR4 overexpression on mesenchymal stem cell transplantation in ischemic stroke. Cell Med. 2012;4:65–76. doi: 10.3727/215517912X647172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu SZ, Li YL, Huang W, Cai WF, Liang J, Paul C, et al. Paracrine effect of CXCR4-overexpressing mesenchymal stem cells on ischemic heart injury. Cell Biochem Funct. 2017;35:113–123. doi: 10.1002/cbf.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rostovskaya M, Anastassiadis K. Differential expression of surface markers in mouse bone marrow mesenchymal stromal cell subpopulations with distinct lineage commitment. PLoS One. 2012;7:e51221. doi: 10.1371/journal.pone.0051221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shahror RA, Ali AAA, Wu CC, Chiang YH, Chen KY. Enhanced Homing of Mesenchymal Stem Cells Overexpressing Fibroblast Growth Factor 21 to Injury Site in a Mouse Model of Traumatic Brain Injury. Int J Mol Sci. 2019;20:1. doi: 10.3390/ijms20112624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bian X, Ma K, Zhang C, Fu X. Therapeutic angiogenesis using stem cell-derived extracellular vesicles: an emerging approach for treatment of ischemic diseases. Stem Cell Res Ther. 2019;10:158. doi: 10.1186/s13287-019-1276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beegle JR, Magner NL, Kalomoiris S, Harding A, Zhou P, Nacey C, et al. Preclinical evaluation of mesenchymal stem cells overexpressing VEGF to treat critical limb ischemia. Mol Ther Methods Clin Dev. 2016;3:16053. doi: 10.1038/mtm.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arutyunyan I, Fatkhudinov T, Kananykhina E, Usman N, Elchaninov A, Makarov A, et al. Role of VEGF-A in angiogenesis promoted by umbilical cord-derived mesenchymal stromal/stem cells: in vitro study. Stem Cell Res Ther. 2016;7:46. doi: 10.1186/s13287-016-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jeong CH, Kim SM, Lim JY, Ryu CH, Jun JA, Jeun SS. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. Biomed Res Int. 2014;2014:129145. doi: 10.1155/2014/129145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tobin MK, Stephen TKL, Lopez KL, Pergande MR, Bartholomew AM, Cologna SM, et al. Activated mesenchymal stem cells induce recovery following stroke via regulation of inflammation and oligodendrogenesis. J Am Heart Assoc. 2020;9:e013583. doi: 10.1161/JAHA.119.013583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.He H, Zeng Q, Huang G, Lin Y, Lin H, Liu W, et al. Bone marrow mesenchymal stem cell transplantation exerts neuroprotective effects following cerebral ischemia/reperfusion injury by inhibiting autophagy via the PI3K/Akt pathway. Brain Res. 2019;1707:124–132. doi: 10.1016/j.brainres.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 144.Zheng Z, Zhang L, Qu Y, Xiao G, Li S, Bao S, et al. Mesenchymal stem cells protect against hypoxia-ischemia brain damage by enhancing autophagy through brain derived neurotrophic factor/mammalin target of rapamycin signaling pathway. Stem Cells. 2018;36:1109–1121. doi: 10.1002/stem.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kasper G, Dankert N, Tuischer J, Hoeft M, Gaber T, Glaeser JD, et al. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells. 2007;25:903–910. doi: 10.1634/stemcells.2006-0432. [DOI] [PubMed] [Google Scholar]
- 146.Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 147.Gonzalez-King H, Garcia NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepulveda P. Hypoxia inducible factor-1alpha potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem cells. 2017;35:1747–1759. doi: 10.1002/stem.2618. [DOI] [PubMed] [Google Scholar]
- 148.Serra J, Alves CPA, Brito L, Monteiro GA, Cabral JMS, Prazeres DMF, et al. Engineering of human mesenchymal stem/stromal cells with vascular endothelial growth factor-encoding minicircles for angiogenic ex vivo gene therapy. Hum Gene Ther. 2019;30:316–329. doi: 10.1089/hum.2018.154. [DOI] [PubMed] [Google Scholar]
- 149.Arutyunyan IV, Kananykhina EY, Fatkhudinov T, El'chaninov AV, Makarov AV, Raimova E, et al. Angiogenic potential of multipotent stromal cells from the umbilical cord: an in vitro study. Bull Exp Biol Med. 2016;161:141–149. doi: 10.1007/s10517-016-3365-7. [DOI] [PubMed] [Google Scholar]
- 150.Boareto M, Jolly MK, Ben-Jacob E, Onuchic JN. Jagged mediates differences in normal and tumor angiogenesis by affecting tip-stalk fate decision. Proc Natl Acad Sci USA. 2015;112:E3836–E3844. doi: 10.1073/pnas.1511814112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Munir H, Ward LSC, Sheriff L, Kemble S, Nayar S, Barone F, et al. Adipogenic differentiation of mesenchymal stem cells alters their immunomodulatory properties in a tissue-specific manner. Stem cells. 2017;35:1636–1646. doi: 10.1002/stem.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang ZX, Han ZB, Ji YR, Wang YW, Liang L, Chi Y, et al. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS One. 2013;8:e59354. doi: 10.1371/journal.pone.0059354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bowles AC, Kouroupis D, Willman MA, Orfei CP, Agarwal A, Correa D. CD146+CD107a+ mesenchymal stem/stromal cells with signature attributes correlate to therapeutic potency as “first responders” to injury and inflammation. bioRxiv. 2019;2019:787176. [Google Scholar]
- 154.Anderson P, Carrillo-Gálvez AB, García-Pérez A, Cobo M, Martín F. CD105 (endoglin)-negative murine mesenchymal stromal cells define a new multipotent subpopulation with distinct differentiation and immunomodulatory capacities. PLoS One. 2013;8:e76979. doi: 10.1371/journal.pone.0076979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pham LH, Vu NB, Van Pham P. The subpopulation of CD105 negative mesenchymal stem cells show strong immunomodulation capacity compared to CD105 positive mesenchymal stem cells. Biomed Res Ther. 2019;6:3131–3140. doi: 10.15419/bmrat.v6i4.538. [DOI] [Google Scholar]
- 156.Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, et al. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol. 2014;5:556. doi: 10.3389/fimmu.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Khare D, Or R, Resnick I, Barkatz C, Almogi-Hazan O, Avni B. Mesenchymal stromal cell-derived exosomes affect mRNA expression and function of B-lymphocytes. Front Immunol. 2018;9:3053. doi: 10.3389/fimmu.2018.03053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 159.Zimmermann JA, Hettiaratchi MH, McDevitt TC. Enhanced immunosuppression of T cells by sustained presentation of bioactive interferon-γ within three-dimensional mesenchymal stem cell constructs. Stem Cells Transl Med. 2017;6:223–237. doi: 10.5966/sctm.2016-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA, et al. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem cells. 2015;33:1818–1828. doi: 10.1002/stem.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ferro F, Spelat R, Shaw G, Duffy N, Islam MN, O'Shea PM, et al. Survival/adaptation of bone marrow-derived mesenchymal stem cells after long-term starvation through selective processes. Stem cells. 2019;37:813–827. doi: 10.1002/stem.2998. [DOI] [PubMed] [Google Scholar]
- 162.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 163.Mao AS, Özkale B, Shah NJ, Vining KH, Descombes T, Zhang L, et al. Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation. Proc Natl Acad Sci U S A. 2019;116:15392–15397. doi: 10.1073/pnas.1819415116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revascular Med: Includ Mol Interv. 2006;3:19–24. doi: 10.1016/j.carrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 165.Won YW, Patel AN, Bull DA. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials. 2014;35:5627–5635. doi: 10.1016/j.biomaterials.2014.03.070. [DOI] [PubMed] [Google Scholar]
- 166.Tebebi PA, Kim SJ, Williams RA, Milo B, Frenkel V, Burks SR, et al. Improving the therapeutic efficacy of mesenchymal stromal cells to restore perfusion in critical limb ischemia through pulsed focused ultrasound. Sci Rep. 2017;7:41550. doi: 10.1038/srep41550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yun WS, Choi JS, Ju HM, Kim MH, Choi SJ, Oh ES, et al. Enhanced homing technique of mesenchymal stem cells using iron oxide nanoparticles by magnetic attraction in olfactory-injured mouse models. Int J Mol Sci. 2018;19:1. doi: 10.3390/ijms19051376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Corradetti B, Taraballi F, Martinez JO, Minardi S, Basu N, Bauza G, et al. Hyaluronic acid coatings as a simple and efficient approach to improve MSC homing toward the site of inflammation. Sci Rep. 2017;7:7991. doi: 10.1038/s41598-017-08687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhidkova OV, Andreeva ER, Buravkova LB. Endothelial cells modulate differentiation potential and mobility of mesenchymal stromal cells. Bull Exp Biol Med. 2018;165:127–131. doi: 10.1007/s10517-018-4113-y. [DOI] [PubMed] [Google Scholar]
- 170.Saini U, Gumina RJ, Wolfe B, Kuppusamy ML, Kuppusamy P, Boudoulas KD. Preconditioning mesenchymal stem cells with caspase inhibition and hyperoxia prior to hypoxia exposure increases cell proliferation. J Cell Biochem. 2013;114:2612–2623. doi: 10.1002/jcb.24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sivanathan KN, Rojas-Canales D, Grey ST, Gronthos S, Coates PT. Transcriptome profiling of IL-17A preactivated mesenchymal stem cells: a comparative study to unmodified and IFN-γ modified mesenchymal stem cells. Stem Cells Int. 2017;2017:1025820. doi: 10.1155/2017/1025820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, et al. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473–481. doi: 10.1038/cmi.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pourgholaminejad A, Aghdami N, Baharvand H, Moazzeni SM. The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells. Cytokine. 2016;85:51–60. doi: 10.1016/j.cyto.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 174.Zhang K, Chen X, Li H, Feng G, Nie Y, Wei Y, et al. A nitric oxide-releasing hydrogel for enhancing the therapeutic effects of mesenchymal stem cell therapy for hindlimb ischemia. Acta Biomater. 2020;113:289–304. doi: 10.1016/j.actbio.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 175.Kaushik K, Das A. Cycloxygenase-2 inhibition potentiates trans-differentiation of Wharton's jelly-mesenchymal stromal cells into endothelial cells: transplantation enhances neovascularization-mediated wound repair. Cytotherapy. 2019;21:260–273. doi: 10.1016/j.jcyt.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 176.Liu C, Tsai AL, Li PC, Huang CW, Wu CC. Endothelial differentiation of bone marrow mesenchyme stem cells applicable to hypoxia and increased migration through Akt and NFκB signals. Stem Cell Res Ther. 2017;8:29. doi: 10.1186/s13287-017-0470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nguyen HT, Van Pham P. Conversion of human adipose derived stem cells into endothelial progenitor cells. Progress Stem Cell. 2017;4:229–240. [Google Scholar]
- 178.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 179.McGinley LM, McMahon J, Stocca A, Duffy A, Flynn A, O'Toole D, et al. Mesenchymal stem cell survival in the infarcted heart is enhanced by lentivirus vector-mediated heat shock protein 27 expression. Hum Gene Ther. 2013;24:840–851. doi: 10.1089/hum.2011.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dorland YL, Cornelissen AS, Kuijk C, Tol S, Hoogenboezem M, van Buul JD, et al. Nuclear shape, protrusive behaviour and in vivo retention of human bone marrow mesenchymal stromal cells is controlled by Lamin-A/C expression. Sci Rep. 2019;9:14401. doi: 10.1038/s41598-019-50955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Drela K, Stanaszek L, Nowakowski A, Kuczynska Z, Lukomska B. Experimental strategies of mesenchymal stem cell propagation: adverse events and potential risk of functional changes. Stem Cells Int. 2019;2019:7012692. doi: 10.1155/2019/7012692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhou Y, Singh AK, Hoyt RF, Jr, Wang S, Yu Z, Hunt T, et al. Regulatory T cells enhance mesenchymal stem cell survival and proliferation following autologous cotransplantation in ischemic myocardium. J Thorac Cardiovasc Surg. 2014;148:1131–1137. doi: 10.1016/j.jtcvs.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 184.Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 185.Crisan M. Transition of mesenchymal stem/stromal cells to endothelial cells. Stem Cell Res Ther. 2013;4:95. doi: 10.1186/scrt306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Alaminos M, Pérez-Köhler B, Garzón I, García-Honduvilla N, Romero B, Campos A, et al. Transdifferentiation potentiality of human Wharton's jelly stem cells towards vascular endothelial cells. J Cell Physiol. 2010;223:640–647. doi: 10.1002/jcp.22062. [DOI] [PubMed] [Google Scholar]
- 187.Dao TT-T, Vu NB, Phi LT, Le HT-N, Phan NK, Van Pham P. Human adipose-derived mesenchymal stem cell could participate in angiogenesis in a mouse model of acute hindlimb ischemia. Biomed Res Ther. 2016;3:770–779. doi: 10.7603/s40730-016-0037-1. [DOI] [Google Scholar]
- 188.Corotchi MC, Popa MA, Remes A, Sima LE, Gussi I, Lupu PM. Isolation method and xeno-free culture conditions influence multipotent differentiation capacity of human Wharton's jelly-derived mesenchymal stem cells. Stem Cell Research Ther. 2013;4:81. doi: 10.1186/scrt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Berglund AK, Fortier LA, Antczak DF, Schnabel LV. Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res Therapy. 2017;8:288. doi: 10.1186/s13287-017-0742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang Y, Huang J, Gong L, Yu D, An C, Bunpetch V, et al. The Plasticity of Mesenchymal Stem Cells in Regulating Surface HLA-I. iScience. 2019;15:66–78. doi: 10.1016/j.isci.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Isakova IA, Lanclos C, Bruhn J, Kuroda MJ, Baker KC, Krishnappa V, et al. Allo-reactivity of mesenchymal stem cells in rhesus macaques is dose and haplotype dependent and limits durable cell engraftment in vivo. PLoS One. 2014;9:e87238. doi: 10.1371/journal.pone.0087238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 195.Hoornaert CJ, Luyckx E, Reekmans K, Dhainaut M, Guglielmetti C, Le Blon D, et al. In vivo interleukin-13-primed macrophages contribute to reduced alloantigen-specific t cell activation and prolong immunological survival of allogeneic mesenchymal stem cell implants. Stem Cells. 2016;34:1971–1984. doi: 10.1002/stem.2360. [DOI] [PubMed] [Google Scholar]
- 196.Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, et al. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173:4828–4837. doi: 10.4049/jimmunol.173.8.4828. [DOI] [PubMed] [Google Scholar]
- 197.Amorin B, Alegretti AP, Valim V, Pezzi A, Laureano AM, da Silva MA, et al. Mesenchymal stem cell therapy and acute graft-versus-host disease: a review. Hum Cell. 2014;27:137–150. doi: 10.1007/s13577-014-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sato K, Ozaki K, Mori M, Muroi K, Ozawa K. Mesenchymal stromal cells for graft-versus-host disease: basic aspects and clinical outcomes. J Clin Exp Hematopathol. 2010;50:79–89. doi: 10.3960/jslrt.50.79. [DOI] [PubMed] [Google Scholar]
- 199.Godoy JAP, Paiva RMA, Souza AM, Kondo AT, Kutner JM, Okamoto OK. Clinical translation of mesenchymal stromal cell therapy for graft versus host disease. Front Cell Dev Biol. 2019;7:255. doi: 10.3389/fcell.2019.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Paronis E, Katsimpoulas M, Kadoglou NPE, Provost C, Stasinopoulou M, Spyropoulos C, et al. Cilostazol mediates immune responses and affects angiogenesis during the acute phase of hind limb ischemia in a mouse model. J Cardiovasc Pharmacol Ther. 2020;25:273–285. doi: 10.1177/1074248419897852. [DOI] [PubMed] [Google Scholar]
- 201.Yong KW, Choi JR, Mohammadi M, Mitha AP, Sanati-Nezhad A, Sen A. Mesenchymal stem cell therapy for ischemic tissues. Stem Cells Int. 2018;2018:8179075. doi: 10.1155/2018/8179075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yu H, Lu K, Zhu J, Wang J. Stem cell therapy for ischemic heart diseases. Br Med Bull. 2017;121:135–154. doi: 10.1093/bmb/ldw059. [DOI] [PubMed] [Google Scholar]
- 203.Haque N, Kasim NH, Rahman MT. Optimization of pre-transplantation conditions to enhance the efficacy of mesenchymal stem cells. Int J Biol Sci. 2015;11:324–334. doi: 10.7150/ijbs.10567. [DOI] [PMC free article] [PubMed] [Google Scholar]