Abstract
BACKGROUND:
Exosomes from mesenchymal stem cells (MSCs) show anti-inflammatory effect on osteoarthritis (OA); however, their biological effect and mechanism are not yet clearly understood. This study investigated the anti-inflammatory effect and mechanism of MSC-derived exosomes (MSC-Exo) primed with IL-1β in osteoarthritic SW982 cells.
METHODS:
SW982 cells were treated with interleukin (IL)-1β and tumor necrosis factor (TNF)-α to induce the OA phenotype. The effect of exosomes without priming (MSC-Exo) or with IL-1β priming (MSC-IL-Exo) was examined on the expression of pro- or anti-inflammatory factors, and the amount of IκBα was examined in SW982 cells. Exosomes were treated with RNase to remove RNA. The role of miR-147b was examined using a mimic and an inhibitor.
RESULTS:
MSC-IL-Exo showed stronger inhibitory effects on the expression of pro-inflammatory cytokines (IL-1β, IL-6, and monocyte chemoattractant protein-1) than MSC-Exo. The expression of anti-inflammatory factors (SOCS3 and SOCS6) was enhanced by MSCs-IL-Exo. Priming with IL-1β increased RNA content in MSC-IL-Exo, and pretreatment with RNase abolished anti-inflammatory effect in SW982 cells. miR-147b was found in much larger amounts in MSC-IL-Exo than in MSC-Exo. The miR-147b mimic significantly inhibited the expression of inflammatory cytokines, while the miR-147b inhibitor only partially blocked the anti-inflammatory effect of MSC-IL-Exo. MSC-IL-Exo and miR-147b mimic inhibited the reduction of IκBα, an nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) inhibitor, by IL-1β and TNF-α.
CONCLUSION:
This study showed that MSC exosomes with IL-1β priming exhibit significantly enhanced anti-inflammatory activity in osteoarthritic SW982 cells. The effect of IL-1β-primed MSC exosomes is mediated by miRNAs such as miR-147b and involves inhibition of the NF-κB pathway.
Electronic supplementary material
The online version of this article (10.1007/s13770-020-00324-x) contains supplementary material, which is available to authorized users.
Keywords: Exosome, MSCs, Priming, Anti-inflammation, Osteoarthritis, MicroRNA
Introduction
Mesenchymal stem cells (MSCs) possess anti-inflammatory and immunosuppressive functions and are regarded as a potential therapy for relevant diseases [1]. Many studies have shown that MSCs exert immunomodulatory activity by inducing functional changes in immune-related cells. They are known to directly inhibit the proliferation of T cells, B cells, natural killer cells and dendritic cells, modulate the cytokine-secretion profile of B cells, macrophages and dendritic cells, and inhibit the differentiation and maturation of antigen-presenting cells [2]. Moreover, MSCs can shift microenvironment at injury sites from a pro-inflammatory to an anti-inflammatory state through the secretion of trophic factors and anti-apoptotic molecules, which may provide a key mechanism responsible for their therapeutic effects. Interleukin 1 receptor antagonist secreted from MSCs can induce the polarization of macrophages toward type 2 phenotype [3]. In addition, anti-inflammatory monocytes are promoted by MSC-produced IL-6 and hepatocyte growth factor to secrete high levels of IL-10 [4]. Several studies have shown that the dynamic immunomodulatory profile of MSCs is based on their ability to sense injury or inflammation and then switch on a required response. Consequently, these properties of MSCs make them a potential cell-based therapy against many diseases such as type 1 diabetes mellitus, rheumatoid arthritis, graft versus host disease, acute renal failure, liver damage, cardiac injuries, and osteoarthritis (OA) [5–7].
It is being gradually accepted that the biological effects of MSCs are largely attributed to the secretion of trophic factors including exosomes. Exosomes are secretory vesicles of endocytic origin with a size range of 55–100 nm and are produced by a variety of cell types including MSCs [8]. Studies have shown that the amount and composition of the content of exosomes can vary depending on cell origin, and consequently affect their therapeutic potential [9]. It is thought that exosomes bear most of the biological activity of parent cells and can be a substitute for MSC-based therapy. In addition, recent studies are also underway to improve the therapeutic efficacy of exosomes using technologies such as exosome-mimetic Nanovesicles [10]. In that context, exosomes can offer a potential to overcome critical limitations of cell-based therapies in the handling and storage of live cells, potential immune response and adverse events, surgical operation, and cell viability in vivo. Further, previous studies have suggested that exosomes can mediate local and systemic cell-to-cell communication through the transfer of proteins, lipids, mRNAs, and microRNAs (miRNAs) [11]. Studies thus far have identified more than 150 miRNAs and 850 unique gene products that influence the therapeutic effect of exosomes. Among these, miRNAs such as miR-143 [12], miR-146a [13], miR-147 [14], and miR-155 [15] were found to play important roles in the regulation of immune and inflammatory responses by exosomes. MSC-derived exosomes can also be modulated to boost their anti-inflammatory actions through the inflammatory priming of MSCs via miRNA. For example, exosomes derived from lipopolysaccharide-primed MSCs showed improved macrophage polarization and resolution of chronic inflammation by shuttling let-7b [16]. Likewise, exosomes from IL-1β-primed MSCs effectively promoted macrophage polarization and immunomodulatory properties through the action of miR-146a [17].
OA is one of the most common joint diseases worldwide, affecting an estimated 10–13% of the >60-year-old population. OA has generally been regarded as a degenerative disease, and a recent study suggests that the disease employs immune reactions and local inflammatory responses to produce pro-inflammatory cytokines and metalloproteinases [18]. Pharmacological treatments for this joint disease are mainly analgesics and non-steroidal anti-inflammatory drugs, which reduce pain and inflammation, respectively. However, these treatments commonly cause high comorbidity in OA patients due to the side effects of drugs and inappropriate polypharmacy [19]. Intra-articular injection of hyaluronic acid is also commonly used; however, its therapeutic effect on OA has not yet been proven [20]. Accordingly, current OA treatments have many limitations and side effects, although minimal therapeutic effects have been demonstrated. MSC-based therapies have emerged as a novel opportunity to positively impact the outcome of OA [21, 22]. The therapeutic potential of MSCs is suggested to depend on its paracrine action to inhibit OA-related inflammation and protect degenerating chondrocytes [23]. Recent studies have shown that MSC-derived exosomes (MSC-Exo) exert a therapeutic effect on OA [24]. In these studies, MSC-Exo were injected into the knee joints and shown to reduce cartilage degeneration in OA models, possibly by regulating immune response, reducing cell apoptosis, and increasing chondrocyte proliferation.
Although many researchers have confirmed the inhibitory effect of primed MSC-Exo on OA progression, the mechanism underlying the anti-inflammatory effect of exosomes in OA synoviocytes has not yet been clearly elucidated. Moreover, whether the pretreatment of MSCs through priming could benefit the therapeutic efficacy of MSC-Exo against OA has not yet been investigated. Therefore, in this study, we aimed to investigate the anti-inflammatory effect and mechanism of exosomes derived from IL-1β-primed MSCs on the OA-like synovial sarcoma cell line SW982.
Materials and methods
MSCs culture and priming
Human bone marrow MSCs were purchased from CEFO (Seoul, Korea) and cultured in Eagle’s minimum essential medium alpha modification (α-MEM; HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; HyClone) and 1% penicillin/streptomycin at 37 °C under 5% CO2. Cells at passages 6–8 after purchase were used for all experiments. To obtain MSC-Exo, MSCs were cultured using FBS depleted of exosomes by centrifugation at 110,000 g for 18 h [25]. MSCs were unprimed or primed with 25 ng/mL IL-1β for 24 h to prepare naive (MSC-Exo) or primed exosomes (MSC-IL-Exo) as shown below. IL-1β is considered one of the crucial factors involved in the pathogenesis of OA [26], and MSC-IL-Exo showed better anti-inflammatory activity than exosomes with other priming factors such as poly I:C, interferon-γ, and tumor necrosis factor-α (TNF-α) in our preliminary study (Fig. S1 ). Conditioned medium of MSCs was harvested and centrifuged at 3000 g for 15 min to remove cells and cell debris (MSC-IL-CM). To obtain conditioned medium only with soluble fraction without exosomes (MSC-IL-SF), the medium was centrifuged at 110,000 g for 18 h, and pellets were resuspended in the culture medium.
Preparation of MSC-Exo
Exosomes were isolated from the culture medium of MSCs using ExoQuick-TC (Systems Biosciences, Palo Alto, CA, USA) according to the manufacturer’s instruction. Briefly, 5 mL of conditioned medium was mixed with 1 mL ExoQuick-TC solution and incubated overnight at 4 °C. Subsequently, the mixture was centrifuged at 1500 g for 30 min, and the supernatant was discarded. The exosome pellet was resuspended in phosphate buffered saline (PBS), and the amount of exosomes was quantified by protein concentration using Bradford assay (Bio-Rad, Hercules, CA, USA). To prepare exosomes without RNA content (RNase-IL-Exo), MSC-IL-Exo were incubated with 10 U/mL RNase (Ambion, Austin, TX, USA) for 3 h at 37 °C. The control samples were incubated in the same volume of PBS for 3 h at 37 °C. Then, the reaction was inactivated with SuperRase-in RNase inhibitor (Ambion) and harvested by centrifugation at 110,000 g for 60 min.
Characterization of MSC-Exo
Scanning electron microscopy (SEM) was performed to observe exosome morphology, as previously described [27]. Briefly, exosomes were fixed with 3.7% glutaraldehyde (Sigma–Aldrich, St. Louis, MO, USA) for 15 min and, after washing with PBS, were dehydrated sequentially with increasing concentrations of ethanol at 40%, 60%, 80%, and 98%. Samples were left at room temperature for 24 h on a slide glass to completely dry up and were analyzed using a SEM device (JEOL, Tokyo, Japan). To analyze size distribution, exosomes were diluted in distilled water at 100 μg/mL, and 3 mL of the exosome suspension was used for dynamic light scattering analysis using a particle size analyzer (ELSZ; Otsuka Electronics, Hirakata, Japan) according to the manufacturer’s instructions. Exosomes were treated with 500 μL TRIzol reagent (Invitrogen, Grand Island, NY, USA) for 10 min at 4 °C, and RNA content was measured using NanoDrop (JCBIO, Seoul, Korea) at 260 nm. Purified RNA was sent to Macrogen (Seoul, Korea) to analyze the profile of small RNAs using a Bioanalyzer (Agilent, CA, USA). To examine the cellular uptake of exosomes, 100 μg exosomes were labeled with 4 μL/mL PKH-26 for 4 min at 4 °C. The labeled exosomes were washed in an equal volume of 1% BSA and harvested by centrifugation at 110,000 g for 60 min. Subsequently, SW982 cells were treated with the PKH-26-labeled exosomes at 10 μg/mL for 24 h, and the uptake of exosomes in the cytoplasm of cells was observed under a fluorescence microscope (E600; Nikon, Minato, Tokyo, Japan).
Anti-inflammatory study in SW982 cells
The human synovial cell line SW982 was obtained from American Type Culture Cyollection (ATCC; Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium-high glucose (DMEM-HG; HyClone) supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C under 5% CO2. To induce an inflammatory response, SW982 cells in 12-well plates were treated with 10 ng/mL IL-1β and 25 ng/mL TNF-α for 24 h. MSC-IL-CM and MSC-IL-SF were treated with 1 mL each in a 12-well plate, and exosomes were treated with a concentration of 10 μg/mL. For the study using synthetic miRNAs, SW982 cells were transfected with miR-147b mimic or miR-147b inhibitor (all from Ambion) for 24 h using Lipofectamine 2000 (Invitrogen) before treatment with IL-1β and TNF-α.
Real time-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was performed according to a previously described protocol [28]. Briefly, RNA was extracted from SW982 cells using TRIzol (Invitrogen) and 1 μg total RNA was used for reverse transcription using a first-strand cDNA synthesis kit (Bio-Rad). RT-qPCR was performed to measure mRNA levels of pro-inflammatory cytokines (IL-1β, IL-6, and monocyte chemoattractant protein (MCP)-1) and anti-inflammatory genes (SOCS1 and SOCS3) using 1× SYBR Green Reaction Mix (Roche, Mannheim, Germany) and specific primers (Table 1). The mRNA level of each gene was calculated using the comparative CT method, and values normalized to glyceraldehyde-6-phosphate dehydrogenase (GAPDH) were presented. For the analysis of miRNAs, miRNAs were extracted from purified exosomes using miRCURY™ RNA isolation kit (Exiqon, Vedbaek, Denmark), and 1 μg of total RNA was used for reverse transcription using Universal RT cDNA synthesis kit (Exiqon). RT-qPCR was performed with ExiLENT SYBR Green master mix (Exiqon) using U6 control primer (#203907) and specific primers of hsa-miR-147b (#204368), hsa-miR-143 (#187544), hsa-miR-155 (#188755), and hsa-miR-146 (#187253), all purchased from Exiqon.
Table 1.
Primers used in RT-qPCR after the Reverse Transcription of mRNAs
| Primers | Sequences | Length (bp) | Annealing temp. (°C) |
|---|---|---|---|
| GAPDH |
F: 5′-TGCACCACCAACTGCTTAGC-3′ R: 5′-GGCATGGACTGTGGTCATGAG-3′ |
87 | 60 |
| IL-1β |
F: 5′-ACAAGGAGAAGAAAGTAATGAC-3′ R: 5′-GCTGTAGAGTGGGCTTAT-3′ |
104 | 60 |
| IL-6 |
F: 5′-CACCTCAGATTGTTGTTGTT-3′ R: 5′-AATAGTGTCCTAACGCTCAT-3′ |
119 | 60 |
| MCP-1 (CCL2) |
F: 5′-CCAGTCACCTGCTGTTAT-3′ R: 5′-GCTTCTTTGGGACACTTG-3′ |
98 | 60 |
| SOCS1 |
F: 5′-CACCTCCTACCTCTTCAT-3′ R: 5′-AATAAAGCCAGAGACCCT-3′ |
80 | 60 |
| SOCS3 |
F: 5′-AGACGGGACATCTTTCAC-3′ R: 5′-CGAATCTCTTAGCCAGACATA-3′ |
89 | 60 |
Western blot analysis
Total proteins were extracted from SW982 cells or purified exosomes using RIPA lysis buffer (Rockland, Gilbertsville, PA, USA). The protein concentration was quantified via Bradford assay (Bio-Rad). Samples were fractionated on a 4–20% sodium dodecyl sulfate-polyacrylamide gel (Bio-Rad) and transferred onto a polyvinylidene fluoride membrane (Bio-Rad). After blocking with 5% milk for 2 h, samples were incubated with primary antibodies against the following proteins: IL-1β (#ab156791, 1:1000), IL-6 (#ab6627, 1:500), MCP-1 (#ab9669, 1:500), SOCS1 (#ab62584, 1:1000), SOCS3 (#ab16030, 1:1000), IκBα (#ab12134, 1:500), and β-actin (#ab8227, 1:2000) for 24 h at 4 °C. Subsequently, samples were treated with horseradish peroxidase-conjugated anti-mouse secondary antibody (#ab6728, 1:3000) and anti-rabbit secondary antibody (#ab6721, 1:3000) for 2 h at room temperature (Abcam, Cambridge, UK). Signals were detected using an ECL kit (Thermo Fisher, MA, USA).
Statistical analysis
All quantitative data were expressed as mean ± standard deviation from three independent experiments (n = 3). Statistical significance was analyzed using t-test or one-way analysis of variance, followed by a Tukey-Kramer post-hoc test. A value of p < 0.05 was considered statistically significant (*p < 0.05, **p < 0.01, and ***p < 0.001).
Results
Exosomes derived from MSCs (MSC-Exo) and IL-1β-primed MSCs (MSC-IL-Exo) have similar characteristics
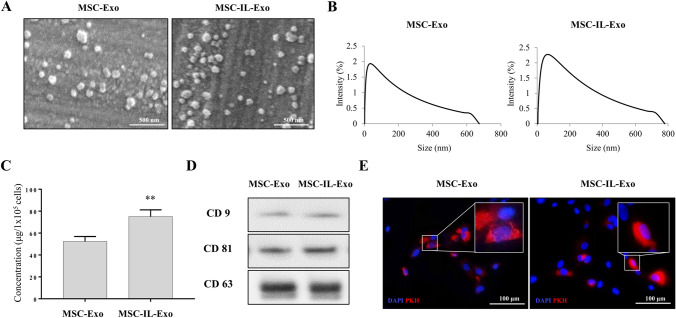
To determine the effect of IL-1β priming on the characteristics of exosomes, we first investigated the yield, morphology, size distribution, marker expression, and internalization potential of MSC-Exo and MSC-IL-Exo. Both categories of exosomes had a round shape in SEM images (Fig. 1A), and their mean diameters ranged from 50 to 200 nm (Fig. 1B), which was consistent with those of typical exosomes reported previously [27]. The protein concentration of exosomes from 1 × 105 cells was approximately 52.3 ± 4.5 μg for MSC-Exo and 75.0 ± 6.2 μg for MSC-IL-Exo, which showed approximately 38% increase in MSC-IL-Exo (Fig. 1C). Western blot analysis confirmed that both MSC-Exo and MSC-IL-Exo expressed common exosome markers CD9, CD63, and CD81 at similar levels (Fig. 1D). When these two groups of exosomes were PKH-26 labeled and used to treat SW982 cells, they were shown to enter the cells and reside in the cytoplasm as shown in the fluorescence images (Fig. 1E). These results revealed that both MSC-Exo and MSC-IL-Exo have typical exosome characteristics regardless of IL-1β priming of MSCs.
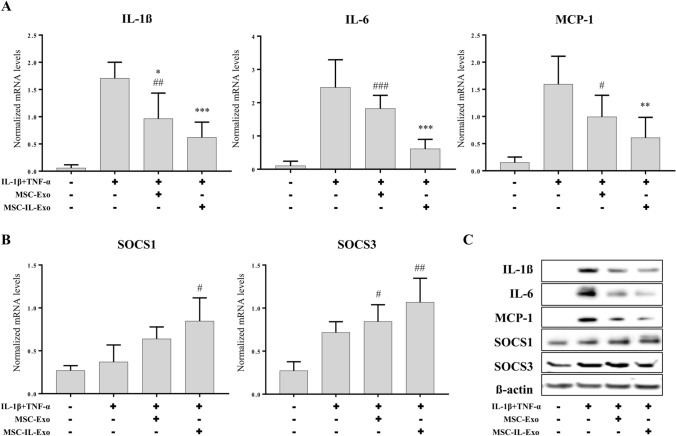
Fig. 2.
Anti-inflammatory activity of MSC-Exo and MSC-IL-Exo in SW982 cells. SW982 cells were treated with IL-1β (10 ng/mL) and TNF-α (25 ng/mL) for 24 h in the presence of 10 μg/mL MSC-Exo or MSC-IL-Exo. Expression of selected pro- or anti-inflammatory factors were analyzed via RT-qPCR and Western blot analyses. A mRNA levels of pro-inflammatory cytokines (IL-1β, IL-6, and MCP-1) normalized by that of GAPDH. B mRNA levels of anti-inflammatory proteins (SOCS1 and SOCS3) normalized by that of GAPDH. Data are presented as mean ± SD from three independent experiments (n = 3). #/*p < 0.05, ##/**p < 0.01, and ###/***p < 0.001 by one-way ANOVA for two exosome groups (MSC-Exo and MSC-IL-Exo) versus untreated control (#) or versus IL-1β and TFN-α groups (*). C Protein levels of the pro- or anti-inflammatory factors were examined via Western blot analysis. β-actin was used as an internal control
Fig. 1.
Characterization of exosomes derived from naive and IL-1β-primed MSCs. Exosomes were purified from the naive (MSC-Exo) or IL-1β-primed MSCs at 25 ng/mL for 24 h (MSC-IL-Exo) using ExoQuick-TC (Systems Biosciences). A Scanning electron microscope images of MSC-Exo and MSC-IL-Exo. Scale bar = 500 nm. B Size distribution of exosomes was analyzed by dynamic light scattering and presented as percent intensity in each group. C The amounts of MSC-Exo and MSC-IL-Exo were measured via Bradford assay. Values of 105 cells are presented as mean ± SD from three independent experiments (n = 3). **p < 0.01 by one-way ANOVA. D Protein levels of CD9, CD81, and CD63 in each exosome group were examined by Western blot analysis. E Exosomes labeled with PKH-26 were used to treat SW982 cells and visualized in red in the fluorescent images. DAPI staining for nucleus is shown in blue. Scale bar = 100 μm
MSC-IL-Exo showed higher ability to inhibit IL-1β-induced inflammatory events in SW982 cells than MSC-Exo
To compare the activity of MSC-Exo and MSC-IL-Exo, we examined their effect on the IL-1β-induced inflammatory response in SW982 cells. The RT-qPCR results showed that stimulation of SW982 cells with IL-1β (10 ng/mL) and TNF-α (25 ng/mL) significantly induced the expression of pro-inflammatory cytokines IL-1β, IL-6, and MCP-1, and slightly increased the anti-inflammatory genes SOCS1 and SOCS3 (Fig. 2). MSC-Exo inhibited IL-1β and TNF-α effects on the three pro-inflammatory cytokines; however, the inhibition was statistically significant for only IL-1β (Fig. 2A). In contrast, MSC-IL-Exo showed a stronger inhibitory effect with statistical significance for all three pro-inflammatory cytokines (Fig. 2A) compared to MSC-Exo. When compared with the untreated control group, the MSC-Exo group showed a statistically significant difference, while MSC-IL-Exo did not (Fig. 2A). The expression of anti-inflammatory cytokines was also slightly increased by MSC-Exo and increased at high levels by MSC-IL-Exo; however, both groups showed no statistically significant difference compared to the IL-1β and TNF-α group (Fig. 2B). Some exosome-treated groups showed statistically significant differences compared to the untreated control group, as indicated in Fig. 2B. Western blot analysis confirmed the effect of MSC-Exo and MSC-IL-Exo at the protein level (Fig. 2C). These results indicated that MSC-IL-Exo showed much higher anti-inflammatory effect than MSC-Exo on IL-1β- and TNF-α-induced inflammatory responses in SW982 cells.
Exosomes might play a key role in the anti-inflammatory paracrine action of MSCs
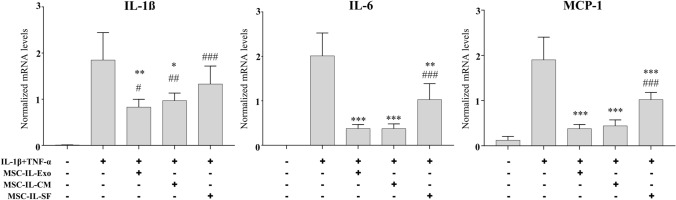
We investigated whether exosomes are responsible for the paracrine effect of MSCs. The anti-inflammatory effect of MSC-IL-Exo was compared with that of the conditioned medium or soluble fraction depleted of exosomes obtained from IL-1β-primed MSCs in the IL-1β- and TNF-α-induced inflammatory model of SW982 cells. Against the expression of anti-inflammatory cytokines IL-1β, IL-6, and MCP-1, MSC-Exo showed almost similar inhibitory effect to that of MSC-IL-CM, with clear statistically significant differences between the IL-1β and TNF-α groups (Fig. 3). In contrast, the soluble fraction (MSC-IL-SF) inhibited the expression of pro-inflammatory cytokines but showed much less inhibitory effect than MSC-IL-Exo and MSCs-IL-CM. When compared with the untreated control, only IL-6 and MCP-1 of both MSC-IL-Exo and MSC-IL-CM groups showed no statistically significant difference. These results suggested that exosomes play a key role in, but are not solely responsible for, the anti-inflammatory activity of MSC-IL-CM.
Fig. 3.
Role of exosomes in the anti-inflammatory paracrine action of IL-1β-primed MSCs. Conditioned medium (MSC-IL-CM) and the one depleted of exosome (MSC-IL-SF) were prepared from IL-β1-primed MSCs as described in Sect. 2. SW982 cells were treated with IL-1β (10 ng/mL) and TNF-α (25 ng/mL) for 24 h in the presence of 10 μg/mL of MSC-IL-Exo, 1 mL/well of MSC-IL-CM, or MSC-IL-SF. mRNA levels of selected pro-inflammatory cytokines (IL-1β, IL-6, and MCP-1) were analyzed via RT-qPCR. Values are normalized by that of GAPDH and presented as mean ± SD from three independent experiments (n = 3). #/*p < 0.05, ##/**p < 0.01, and ###/***p < 0.001 by one-way ANOVA for three treatment groups (MSC-IL-Exo, MSC-IL-CM, and MSC-IL-SF) versus untreated control (#), or versus IL-1β and TFN-α groups (*)
Anti-inflammatory action of MSC-IL-Exo could be mediated by exosome-shuttled RNAs
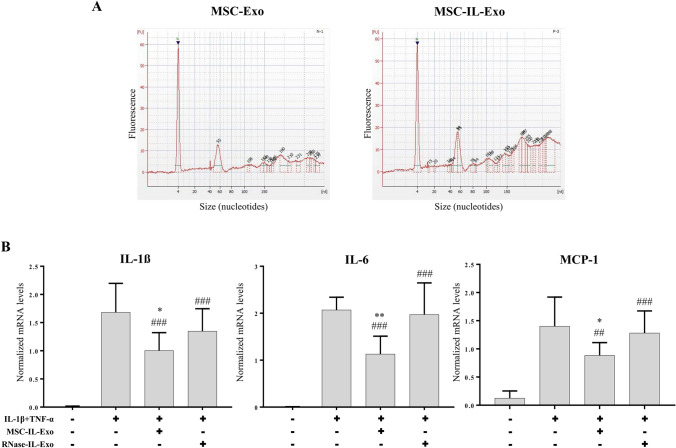
RNA profile was analyzed to determine whether priming of MSCs with IL-1β influences the content of small RNAs in secreted exosomes. In both MSC-Exo and MSC-IL-Exo, strong peaks at 4 nucleotide (nt) and at 55 nt were observed, of which the intensity of the 55 nt peak increased by IL-1β priming (Fig. 4A). Broad peaks of small RNAs observed in the range of 60–300 nt were also observed in both groups, and peaks larger than 150 nt showed a significant increase in the MSC-IL-Exo group (Fig. 4A) compared to the MSC-Exo group. To examine the role of the exosome-shuttled RNAs, IL-1β-primed exosomes depleted of RNAs (RNase-IL-Exo) were prepared using a high concentration of RNase. In contrast to MSC-IL-Exo, RNase-IL-Exo showed no inhibitory effect on the IL-1β- and TNF-α-induced expression of pro-inflammatory cytokines in SW982 cells (Fig. 4B). These results indicated that exosome-shuttled RNAs might have a crucial role in the anti-inflammatory action of MSC-IL-Exo.
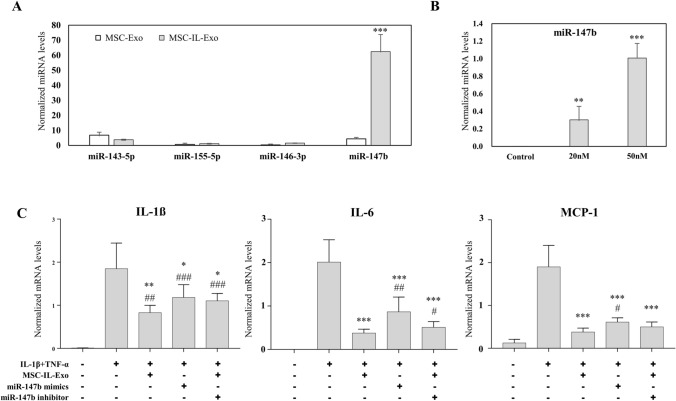
Fig. 5.
Role of miRNAs in the anti-inflammatory activity of MSC-IL-Exo. A Levels of miR-143, miR-146, miR-155, and miR-147b were examined in MSC-Exo and MSC-IL-Exo via RT-qPCR analysis. ***p < 0.001 between two groups by one-way ANOVA. B SW982 cells were transfected with synthetic miR-147b mimics at 0, 20, and 50 nM. The amount of miR-147b was examined via RT-qPCR analysis. **p < 0.01 and ***p < 0.001 versus the control group by one-way ANOVA. C SW982 cells were treated with IL-1β (10 ng/mL) and TNF-α (25 ng/mL) for 24 h in the presence of 10 μg/mL MSC-IL-Exo or after transfection of miR-147b mimics or miR-147b inhibitor. mRNA levels of selected pro-inflammatory cytokines (IL-1β, IL-6, and MCP-1) were analyzed by RT-qPCR. #/*p < 0.05, ##/**p < 0.01, and ###/***p < 0.001 for three treatment groups (MSC-IL-Exo, miR-147b mimics, and miR-147b inhibitor) versus untreated control (#) or versus IL-1β and TFN-α groups (*) by one-way ANOVA. All values are normalized by that of GAPDH and presented as mean ± SD from three independent experiments (n = 3)
Fig. 4.
Effect of RNase treatment on the anti-inflammatory activity of MSC-IL-Exo. A RNA profiles of MSC-Exo and MSC-IL-Exo are displayed up to 300 nt in the histograms. The peak at 4 nt is a low size marker (arrowhead). B MSC-IL-Exo was treated with 10 U/mL RNase (Ambion) for 3 h at 37 °C to prepare the sample without RNA content (RNase-IL-Exo). SW982 cells were treated with IL-1β (10 ng/mL) and TNF-α (25 ng/mL) for 24 h in the presence of 5 μg/mL of MSC-IL-Exo or RNase-IL-Exo. mRNA levels of selected pro-inflammatory cytokines (IL-1β, IL-6, and MCP-1) were analyzed via RT-qPCR. Values are normalized by that of GAPDH and presented as mean ± SD from three independent experiments (n = 3). *p < 0.05, ##/**p < 0.01, and ###p < 0.001 by one-way ANOVA for two exosome groups (MSC-Exo and MSC-IL-Exo) versus untreated control (#) or versus IL-1β and TFN-α groups (*)
MicroRNA 147b (miR-147b) contributed to the anti-inflammatory activity of MSC-IL-Exo in SW982 cells
miRNAs are known to be important in the biological function of exosomes. We selected miRNAs of miR-143 [12], miR-146 [13], miR-147b [14] and miR-155 [15] which are known to have immune or inflammation control activity. When their amounts were examined, a much larger amount of miR-147b was found in MSC-IL-Exo than those of the other miRNAs (Fig. 5A). To examine the role of miR-147b in the anti-inflammatory activity of MSC-IL-Exo, synthetic miR-147b mimics and miR-147b inhibitor were tested in the inflammatory model of SW982 cells. The miR-147b mimic was first tested at 20 and 50 nM to optimize the transfection condition. The expression level of miR-147b in SW982 cells was found to be 0.30 ± 0.12 at 20 nM and 0.98 ± 0.42 at 50 nM, depending on the amount of transfection (Fig. 5B). The miR-147b mimic or miR-147b inhibitor of 50 nM was used in the subsequent experiment. When tested on the IL-1β- and TNF-α-induced inflammatory responses in SW982 cells, the miR-147b mimic showed a significant inhibitory effect; however, its activity was somewhat lower than that of MSC-IL-Exo (Fig. 5C). The miR-147b inhibitor was co-treated with MSC-IL-Exo and found to partially block the anti-inflammatory effect of the primed exosomes with a statistically significant difference between IL-1β and IL-6. These results suggested that miR-147b can inhibit IL-1β- and TNF-α-induced inflammatory responses in SW982 cells and might contribute to the anti-inflammatory effect of MSC-IL-Exo to some extent.
Fig. 6.
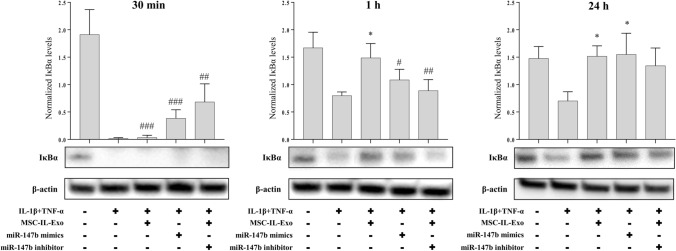
Effect of MSC-IL-Exo on the IκBα level in SW982 cells treated with IL-1β and TNF-α. SW982 cells were treated with IL-1β (10 ng/mL) and TNF-α (25 ng/mL) in the presence of 10 μg/mL MSC-IL-Exo or after transfection of miR-147b mimics or miR-147b inhibitor. Protein levels of IκBα was examined via Western blot analysis at 30 min, 1 h, and 24 h. β-actin was used as an internal control. Intensities of IκBα signal were quantified, and their normalized values by that of β-actin are presented in the graph as mean ± SD from three independent experiments (n = 3). #/*p < 0.05, ##p < 0.01, and ###p < 0.001 for three treatment groups (MSC-IL-Exo, miR-147b mimics, and miR-147b inhibitor) versus untreated control (#) or versus IL-1β and TNF-α groups (*) by one-way ANOVA
Anti-inflammatory effect of MSC-IL-Exo involved NF-κB activation
We investigated the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway as a target of the anti-inflammatory effect of MSC-IL-Exo and miR-147b. The amount of IκBα, an inhibitor or NF-κB pathway, was used as a surrogate marker and examined in the IL-1β- and TNF-α-induced inflammatory models of SW982 cells at 30 min, 1 h, and 24 h after treatment with MSC-IL-Exo and/or transfection of the miR-147b mimic and inhibitor (Fig. 6). Treatment with IL-1β and TNF-α rapidly decreased IκBα levels at 30 min and increased slightly at 1 and 24 h; however, it was still significantly lower than that in the untreated control. Co-treatment with MSC-IL-Exo showed a clear inhibitory effect against the IL-1β- and TNF-α-induced decrease in IκBα levels at 1 and 24 h but not at 30 min. Transfection of miR-147b mimic showed inhibitory effect against the activity of IL-1β and TNF-α at 30 min and 24 h but not at 1 h. Transfection of the miR-147b inhibitor together with MSC-IL-Exo showed unexpectedly increased IκBα levels at 30 min and blocked the action of MSC-IL-Exo only at 1 h. These results suggested that the anti-inflammatory effect of MSC-IL-Exo involves inhibition of NF-κB activation by IL-1β and TNF-α, and confirmed that exosome-shuttled miR-147b might have a partial role in the function of primed exosomes.
Discussion
In this study, we demonstrated that MSC-IL-Exo inhibited the IL-1β- and TNF-α-mediated increase in the expression of inflammatory mediators in SW982 human synovial cells. We also showed that exosomes play a crucial role in the paracrine action of MSCs, and priming with IL-1β enhances the anti-inflammatory effect of MSC-Exo on the employed model in SW982 cells. In addition, the effect of MSC-Exo depended on their RNA content, probably miRNAs, and miR-147b-mediated inhibition of the NF-κB pathway could be one of the key activities in its anti-inflammatory mechanism. Although it is premature to extrapolate from an in vitro study, our results suggest that MSC-IL-Exo could be an effective cell-free therapy to treat inflammatory diseases involving synoviocytes such as OA.
It is known that MSC-Exo can promote cell migration and proliferation and induce chondrogenic differentiation of stem cells [29]. These exosomes are known to diminish apoptosis of chondrocytes, promote cell proliferation, and delay OA progression by exerting an anti-inflammatory effect [24]. In addition, MSC-Exo are involved in the repair and regeneration of cartilage via chondroprotective and anti-inflammatory effects [30]. Tao et al. reported that exosomes secreted from human synovial MSCs overexpressing miR-140-5p inhibit cartilage degradation in a mouse OA model [31]. MSC-Exo overexpressing miR-92a-3p were also shown to inhibit cartilage degradation and promote cartilage formation in OA by targeting Wnt5a. In addition, various other miRNAs, such as miR-222, miR-199a-5p, and miR-140-5p, were found to exert an inhibitory effect on OA by reducing the amount of inflammatory cytokines in in vitro and in vivo models [32–34]. However, these studies have mostly reported phenomenal data without any information on their cellular or molecular mechanism. In this study, we confirmed the results of previous studies showing potential therapeutic effects of MSC-Exo on OA in vitro and suggested possible role of miR-147b in the anti-inflammatory activity of MSC-IL-Exo. IL-1β is commonly found in the synovial fluid of OA patients at high levels and is thought to play a causative role in OA progression. Therefore, our study design could mimic the clinical settings where endogenous or transplanted MSCs are placed in an OA environment in vivo, thereby providing meaningful implications for the therapeutic possibility of MSC-IL-Exo. Cell therapies using MSCs have many limitations in long-term storage, consistency in potency, dosing regime, and stability in vivo [35]. Exosome-based therapies can overcome these limitations and could be a practical alternative to MSC-based therapies in the future.
Paracrine effects of MSCs have since a long time been attributed to secreted soluble factors such as cytokines and growth factors. However, accumulating evidence has revealed the importance of exosomes and exosome-shuttled RNAs in these effects. Nakamura et al. showed that exosomes in MSC-IL-CM promote myogenesis and angiogenesis of MSCs more efficiently than their exosome-depleted counterparts, and this phenomenon is at least in part mediated by exosome-shuttled miRNAs such as miR-494 [36]. Furuta et al. showed that the injection of MSC-Exo promotes fracture healing in CD92/2 mice, whereas exosome-depleted medium showed no such effect. They also suggested that exosomal miRNAs are involved in the effect of these exosomes [37]. In this study, we have also shown that MSC-Exo exhibited a higher anti-inflammatory effect than exosome-depleted medium in SW982 cells and that this effect was abolished by RNase treatment. Exosomes can protect their RNA content from RNase attack [38]; however, high concentrations of RNase are known to inactivate exosomal RNAs [39]. Similarly, Reis et al. showed that pretreatment of MSC medium with RNase completely abolished its renal protective effect in a rat model of acute kidney injury [40]. Therefore, our results confirmed that exosomes and their RNA content play a key role in the paracrine action of MSCs in IL-1β and TNF-α-induced inflammatory models in SW982 cells.
In our study, the amount of miR-147b increased significantly in MSC-Exo by IL-1β priming, and transfection with the miR-147b mimic showed an inhibitory effect against inflammatory response in SW982 cells. However, transfection of the miR-147b inhibitor only partially blocked the anti-inflammatory action of MSC-IL-Exo in SW982 cells. Jones et al. reported that miR-147b is one of the upregulated miRNAs in bone from OA patients when compared to non-OA specimens among 157 human miRNAs identified [41]. However, the role of miR-147b in OA pathogenesis is not yet clear and is expected to exert an anti-inflammatory effect upon induction in an inflammatory environment by acting on the NF-κB pathway, as discussed below. miR-147b was reported as a negative regulator being induced in its expression by and inhibiting in return the toll-like receptor-induced inflammatory response in macrophages [14]. Interestingly, miR-147b has been reported to increase significantly in cardiac stem cells upon treatment with MSC-Exo [42]. Therefore, miR-147b may have other functions in addition to its anti-inflammatory activity. Considering the importance of RNA content, most likely of miRNAs, these results suggest that other miRNAs, in addition to miR-147b, also have a role in the anti-inflammatory mechanism of IL-1β-primed MSCs. For instance, Song et al. has shown that MSC-IL-Exo contained large amounts of miR-146a and exhibited improved therapeutic effects on sepsis in a mouse model [17]. miR-146a is also known to mediate anti-inflammatory effect of TNF-α-induced MSCs on urethral stricture [43] which suggests its possible role in OA suppression. In a separate experiment we have found that MSC-Exo with TNF-α priming also showed significant anti-inflammatory effect in SW982 cells, although it was inferior to IL-1β-primed exosomes (Supplementary data). However, the role of miR-146a controversial both in the anti-inflammatory activity of MSCs and OA progression [44] and needs further investigation for clear understanding.
We have shown in the present study that MSC-IL-Exo or miR-147b mimic inhibited IκBα degradation by IL-1β and TNF-α treatment in SW982 cells. The NF-κB pathway is known to play a central role in the cellular and molecular regulatory network of many inflammatory diseases and processes, including OA initiation and progression [45]. The phosphorylation and degradation of IκBα are essential steps in the activation of the NF-κB pathway. Van Buul et al. reported that secreted factors from MSCs stabilized IκBα and showed anti-inflammatory effects in human osteoarthritic synovium and cartilage explants in vitro [46]. Tang et al. reported similar findings that treatment of MSCs with IL-1β stabilized IκBα and decreased NF-κB levels both in IL-1β-treated chondrocytes and in a surgical OA model in mice [47]. In addition, Tofiño-Vian et al. showed that microvesicles from adipose MSCs decrease the amount of inflammatory cytokines and inhibit NF-κB activation in OA chondrocytes [48]. These results suggest that NF-κB is involved in OA and downregulated by the anti-inflammatory action of MSCs and their exosomes. The specific mechanism of NF-κB downregulation is not yet clear and is expected to vary depending on the functional molecules in MSCs and their exosomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Anti-inflammatory effect of MSC-derived exosomes primed with various factors. Exosomes isolated from MSCs were unprimed or primed with IL-1β, Poly I:C, IFN-γ, or TNF-α all at 10 μg/mL for 24 h. SW982 cells were treated with IL-1β (10 ng/mL) and TNF-α (25 ng/mL) for 24 h in the presence of each of the MSC-derived exosomes as indicated. mRNA levels of selected pro-inflammatory cytokines (IL-1b, IL-6, and MCP-1) were examined via RT-qPCR analysis. Values are normalized by that of GAPDH and presented as mean ± SD from three independent experiments (n = 3). #/*p < 0.05, ##/**p < 0.01, and ###/***p < 0.001 by one-way ANOVA for five treatment groups (Unprimed-Exo, IL-1β-Exo, poly I:C-Exo, IFN-γ-Exo, and TNF-α-Exo) versus untreated control (#) or versus IL-1β and TNF-α groups (*)
Acknowledgement
This study was supported by the National Research Foundation Grant (NRF-2019M3E5D1A02070861) of the Korea government.
Compliance with ethical standards
Conflict of Interest
The authors declare no conflict of interest.
Ethics statement
The study has no ethical issues to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Byung Hyune Choi, Email: bryan@inha.ac.kr
Byoung-Hyun Min, Email: bhmin@ajou.ac.kr
References
- 1.Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther. 2008;8:569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Q, Ren H, Han Z. Mesenchymal stem cells: immunomodulatory capability and clinical potential in immune diseases. J Cell Immunother. 2016;2:3–20. doi: 10.1016/j.jocit.2014.12.001. [DOI] [Google Scholar]
- 3.Luz-Crawford P, Djouad F, Toupet K, Bony C, Franquesa M, Hoogduijn MJ, et al. Mesenchymal stem cell-derived interleukin 1 receptor antagonist promotes macrophage polarization and inhibits B cell differentiation. Stem Cells. 2016;34:483–492. doi: 10.1002/stem.2254. [DOI] [PubMed] [Google Scholar]
- 4.Deng Y, Zhang Y, Ye L, Zhang T, Cheng J, Chen G, et al. Umbilical cord-derived mesenchymal stem cells instruct monocytes towards an IL10-producing phenotype by secreting IL6 and HGF. Sci Rep. 2016;6:37566. doi: 10.1038/srep37566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godoy JAP, Paiva RMA, Souza AM, Kondo AT, Kutner JM, Okamoto OK. Clinical translation of mesenchymal stromal cell therapy for graft versus host disease. Front Cell Dev Biol. 2019;7:255. doi: 10.3389/fcell.2019.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeedi P, Halabian R, Imani Fooladi AA. A revealing review of mesenchymal stem cells therapy, clinical perspectives and modification strategies. Stem Cell Investig. 2019;6:34. doi: 10.21037/sci.2019.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rostom DM, Attia N, Khalifa HM, Abou Nazel MW, El Sabaawy EA. The therapeutic potential of extracellular vesicles versus mesenchymal stem cells in liver damage. Tissue Eng Regen Med. 2020;17:537–552. doi: 10.1007/s13770-020-00267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko KW, Yoo YI, Kim JY, Choi B, Park SB, Park W, et al. Attenuation of tumor necrosis factor-alpha induced inflammation by umbilical cord-mesenchymal stem cell derived exosome-mimetic nanovesicles in endothelial cells. Tissue Eng Regen Med. 2020;17:155–163. doi: 10.1007/s13770-019-00234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 12.Che Y, Shi X, Shi Y, Jiang X, Ai Q, Shi Y, et al. Exosomes derived from miR-143-overexpressing MSCs inhibit cell migration and invasion in human prostate cancer by downregulating TFF3. Mol Ther Nucleic Acids. 2019;18:232–244. doi: 10.1016/j.omtn.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Nahid MA, Satoh M, Chan EK. Mechanistic role of microRNA-146a in endotoxin-induced differential cross-regulation of TLR signaling. J Immunol. 2011;186:1723–1734. doi: 10.4049/jimmunol.1002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A. 2009;106:15819–15824. doi: 10.1073/pnas.0901216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang P, Hou J, Lin L, Wang C, Liu X, Li D, et al. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J Immunol. 2010;185:6226–6233. doi: 10.4049/jimmunol.1000491. [DOI] [PubMed] [Google Scholar]
- 16.Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1beta-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35:1208–1221. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 18.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ. 2011;14:497–507. doi: 10.3111/13696998.2011.594347. [DOI] [PubMed] [Google Scholar]
- 20.Richards MM, Maxwell JS, Weng L, Angelos MG, Golzarian J. Intra-articular treatment of knee osteoarthritis: from anti-inflammatories to products of regenerative medicine. Phys Sportsmed. 2016;44:101–108. doi: 10.1080/00913847.2016.1168272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristjánsson B, Honsawek S. Current perspectives in mesenchymal stem cell therapies for osteoarthritis. Stem Cells Int. 2014;2014:194318. doi: 10.1155/2014/194318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Im GI. Perspective on intra-articular injection cell therapy for osteoarthritis treatment. Tissue Eng Regen Med. 2019;16:357–363. doi: 10.1007/s13770-018-00176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manferdini C, Maumus M, Gabusi E, Piacentini A, Filardo G, Peyrafitte JA, et al. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum. 2013;65:1271–1281. doi: 10.1002/art.37908. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 25.Liao Z, Muth DC, Eitan E, Travers M, Learman LN, Lehrmann E, et al. Serum extracellular vesicle depletion processes affect release and infectivity of HIV-1 in culture. Sci Rep. 2017;7:2558. doi: 10.1038/s41598-017-02908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan S, Vannberg FO, Dixon JB. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci Rep. 2016;6:24436. doi: 10.1038/srep24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim M, Kim J, Park SR, Park DY, Kim YJ, Choi BH, et al. Comparison of fetal cartilage-derived progenitor cells isolated at different developmental stages in a rat model. Dev Growth Differ. 2016;58:167–179. doi: 10.1111/dgd.12267. [DOI] [PubMed] [Google Scholar]
- 29.Toh WS, Lai RC, Hui JHP, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64. doi: 10.1016/j.semcdb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7:16214. doi: 10.1038/s41598-017-15376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180–195. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Guan SB, Lu Y, Wang F. MiR-140-5p inhibits synovial fibroblasts proliferation and inflammatory cytokines secretion through targeting TLR4. Biomed Pharmacother. 2017;96:208–214. doi: 10.1016/j.biopha.2017.09.079. [DOI] [PubMed] [Google Scholar]
- 33.Song J, Jin EH, Kim D, Kim KY, Chun CH, Jin EJ. MicroRNA-222 regulates MMP-13 via targeting HDAC-4 during osteoarthritis pathogenesis. BBA Clin. 2015;3:79–89. doi: 10.1016/j.bbacli.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu MH, Tsai CH, Huang YL, Fong YC, Tang CH. Visfatin promotes IL-6 and TNF-alpha production in human synovial fibroblasts by repressing miR-199a-5p through ERK, p38 and JNK signaling pathways. Int J Mol Sci. 2018;19:190. doi: 10.3390/ijms19010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim N, Cho SG. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells. 2015;8:54–68. doi: 10.15283/ijsc.2015.8.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589:1257–1265. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 37.Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N, et al. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl Med. 2016;5:1620–1630. doi: 10.5966/sctm.2015-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koga Y, Yasunaga M, Moriya Y, Akasu T, Fujita S, Yamamoto S, et al. Exosome can prevent RNase from degrading microRNA in feces. J Gastrointest Oncol. 2011;2:215–222. doi: 10.3978/j.issn.2078-6891.2011.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen WX, Liu XM, Lv MM, Chen L, Zhao JH, Zhong SL, et al. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS One. 2014;9:e95240. doi: 10.1371/journal.pone.0095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reis LA, Borges FT, Simões MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 2012;7:e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones SW, Watkins G, Le Good N, Roberts S, Murphy CL, Brockbank SM, et al. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage. 2009;17:464–472. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J Am Heart Assoc. 2016;5:e002856. doi: 10.1161/JAHA.115.002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang YC, Wu YP, Li XD, Chen SH, Ye XJ, Xue XY, et al. TNF-α-induced exosomal miR-146a mediates mesenchymal stem cell-dependent suppression of urethral stricture. J Cell Physiol. 2019;234:23243–23255. doi: 10.1002/jcp.28891. [DOI] [PubMed] [Google Scholar]
- 44.Pers YM, Ruiz M, Noël D, Jorgensen C. Mesenchymal stem cells for the management of inflammatory in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23:2027–2035. doi: 10.1016/j.joca.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 46.van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, et al. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis Cartilage. 2012;20:1186–1196. doi: 10.1016/j.joca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Tang J, Cui W, Song F, Zhai C, Hu H, Zuo Q, et al. Effects of mesenchymal stem cells on interleukin-1beta-treated chondrocytes and cartilage in a rat osteoarthritic model. Mol Med Rep. 2015;12:1753–1760. doi: 10.3892/mmr.2015.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tofiño-Vian M, Guillén MI, Pérez Del Caz MD, Silvestre A, Alcaraz MJ. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol Biochem. 2018;47:11–25. doi: 10.1159/000489739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anti-inflammatory effect of MSC-derived exosomes primed with various factors. Exosomes isolated from MSCs were unprimed or primed with IL-1β, Poly I:C, IFN-γ, or TNF-α all at 10 μg/mL for 24 h. SW982 cells were treated with IL-1β (10 ng/mL) and TNF-α (25 ng/mL) for 24 h in the presence of each of the MSC-derived exosomes as indicated. mRNA levels of selected pro-inflammatory cytokines (IL-1b, IL-6, and MCP-1) were examined via RT-qPCR analysis. Values are normalized by that of GAPDH and presented as mean ± SD from three independent experiments (n = 3). #/*p < 0.05, ##/**p < 0.01, and ###/***p < 0.001 by one-way ANOVA for five treatment groups (Unprimed-Exo, IL-1β-Exo, poly I:C-Exo, IFN-γ-Exo, and TNF-α-Exo) versus untreated control (#) or versus IL-1β and TNF-α groups (*)