Abstract
Concerns have recently grown about the health effects of secondhand smoke exposure and heated tobacco products. The analysis of tobacco smoke biomarkers is critical to assess the health effects of tobacco smoke exposure. For this purpose, the simultaneous determinations of exposure markers and health effect markers would provide a better evaluation of smoke exposure. In this study, nicotine metabolites (nicotine, cotinine, trans-3'-hydroxycotinine) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in urine were analyzed as exposure markers. The DNA damage markers, 7-methylguanine and 8-hydroxy-2'-deoxyguanosine, were simultaneously measured as health effect markers. The results revealed significant levels of urinary nicotine metabolites and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in the subjects exposed to secondhand smoke and heated tobacco products. In addition, the urinary levels of 7-methylguanine and 8-hydroxy-2'-deoxyguanosine tended to be high for secondhand smoke and heated tobacco products exposures, as compared to those of non-smokers. These biomarkers will be useful for evaluating tobacco smoke exposure.
Keywords: biomarker, urine, secondhand smoke (SHS), heated tobacco products (HTPs), DNA damage
Introduction
Tobacco smoke contains more than 7,000 chemicals, and at least 69 are potential carcinogens.(1) Smoking causes oxidative damage and DNA methylation, which can lead to a variety of cancers.(2) Although cigarette smoking has declined recently, secondhand smoke (SHS) is still an important issue. In addition to SHS exposure at home, the Centers for Disease Control and Prevention (CDC) in the United States reported that 19.9% of nonsmokers had some SHS exposure on the job.(3) The International Agency for Research on Cancer classified SHS as a human carcinogen (Group 1).(2) The National Institute for Occupational Safety and Health (NIOSH) has also concluded that SHS is an occupational carcinogen.(4) Several reports have evaluated the extent of SHS exposure with biomarkers in biological fluids, such as urine, saliva, and serum. Among them, cotinine, the main nicotine metabolite, has been extensively analyzed in urine. However, most of the cotinine is further metabolized into trans-3'-hydroxycotinine (3-HC).(5,6) In this study, the total nicotine equivalents (TNE; nicotine, cotinine, and 3-HC) were also determined. Nicotine and its metabolites can also be converted into glucuronides.(5,6) Therefore, the enzymatic hydrolysis of glucuronide conjugates was performed prior to the urine sample analysis. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) is a tobacco-specific nitrosamine and a metabolite of the carcinogenic 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).(7) The urinary NNAL levels have been investigated for smokers and secondhand smokers,(8,9) and are very useful in studies of human exposure to tobacco smoke. Urinary NNAL has a relatively longer half-life (10–40 days) than urinary cotinine (16 h) and can be detected even a few weeks after tobacco smoke exposure.(10) Therefore, the urinary NNAL levels were also determined in this study. A methanediazonium ion, a metabolite of NNK and NNAL, reacts with DNA to form methyl DNA base adducts, such as 7-methylguanine (m7Gua) and O6-methyl deoxyguanine.(11) An apurinic site in DNA is produced by the enzymatic elimination of m7Gua from the methylated DNA.(12) As a result, m7Gua is excreted into the urine. Apurinic sites frequently cause mutations in mammalian cells.(13,14) Higher levels of m7Gua have been detected in the lung DNA of current smokers.(15)
The levels of 8-hydroxy-2'-deoxyguanosine (8-OHdG), the most widely used biomarker for oxidative stress,(16–18) were previously determined in the urine,(19,20) blood,(21) and saliva(22,23) of cigarette smokers. Urinary m7Gua and 8-OHdG could be useful as early health effect markers for tobacco smoke exposure.
In 2015, Philip Morris International began selling heated tobacco products (HTPs) as a new type of tobacco in Japan. Since then, other tobacco companies have also started selling HTPs.(24) Hori et al.(25) reported that the number of HTPs users in Japan increased from 0.2 percent in 2015 to 11.3 percent in 2019. Although tobacco companies advertise the reduced risk of HTPs as compared to cigarettes, comparable levels of nicotine and other chemicals are present in the HTPs aerosol.(26,27) However, except for the tobacco company reports,(28,29) very few biological monitoring studies of these products have been published. Brief exposure to HTPs (IQOS) aerosols reportedly caused vascular endothelial dysfunction in rats, to the same extent as that caused by exposure to cigarette smoke.(30) Therefore, studies describing the true state of HTPs exposure to humans are needed.
The simultaneous analyses of cotinine and other tobacco biomarkers would provide a better evaluation of tobacco smoke exposure. In this study, we performed the simultaneous determinations of tobacco exposure markers (nicotine, cotinine, 3-HC, and NNAL) and DNA damage markers (m7Gua and 8-OHdG) in urine for never smokers, former smokers, SHS-exposed subjects, HTPs smokers and cigarette smokers.
Methods
Study participants
A total of 182 male volunteers (ages 18–64) from three Japanese companies participated in the study (Table 1). Urine samples (~10 ml) were collected in 15 ml polypropylene tubes between 8:00 am and 11:00 am, stored on ice in a cooler box during sampling and frozen at −30°C until analysis. A lifestyle questionnaire was administered at the time of urine collection. Information about smoking was obtained from the lifestyle questionnaire.
Table 1.
Participants in this study
| Smoking status |
|||||
|---|---|---|---|---|---|
| Never n = 34 |
Former n = 25 |
SHS n = 54 |
HTPs n = 22 |
Cigarettes n = 48 |
|
| Age (years) Median | 31.5 | 55 | 36 | 33.5 | 36.5 |
| (min–max) | (18–62) | (37–64) | (18–61) | (21–64) | (18–64) |
Categorization by smoking status
Participants were categorized as follows: Never smokers who had never smoked, former smokers who had smoked in the past, SHS-exposed persons, HTPs smokers, and cigarette smokers.
Analysis of tobacco exposure biomarkers
We determined the urinary nicotine, cotinine, 3-HC, and NNAL levels according to the method of Kawasaki et al.(31) with slight modifications. Briefly, 500 μl aliquots of the urine samples were thawed and mixed with 500 μl of 50 mmol acetate buffer (pH 4.0), followed by the addition of 2 μl of dl-cotinine (methyl-D3) and NNAL (1,2',3',4',5',6'-13C6) stock solutions as internal standards. β-Glucuronidase (1,000 U/500 μl of urine) was then added, and the solution was incubated at 37°C for 15 h. The mixture was loaded onto Isolute SLE+ column cartridges (Biotage Japan, Tokyo, Japan) and eluted with 6 ml of chloroform. The extract was evaporated to dryness under a continuous flow of nitrogen. The residue was dissolved in 200 μl of a 10% (v/v) acetonitrile solution containing 10 mM ammonium acetate. Analyses of urinary nicotine, cotinine, 3-HC, and NNAL were conducted using an HPLC (UltiMate 3000, Thermo Fisher Scientific, Yokohama, Japan) coupled to a hybrid quadrupole-Orbitrap mass spectrometer (Thermo Scientific Q Exactive Focus) with heated electrospray ionization (HESI-II). The sample separation was achieved on an L-column 3 C18 (2.1 mm × 100 mm, 3 μm, CERI, Tokyo, Japan) with a flow rate of 0.3 ml/min and a column temperature of 30°C. Mobile phase A was 10 mM ammonium formate and mobile phase B was acetonitrile. The following linear gradient program was used for the separation, with a total run time of 15 min. The percentage of B solvent changed as follows: 0–2 min, 3%; 6 min, 8%; 10 min, 30%; 10.1–12 min, 95%; 12.1–15 min, 3%. The injection volumes for the measurements were 2 μl for nicotine and cotinine, and 5 μl for NNAL. Other MS conditions were the same as previously reported.(31)
Analysis of DNA damage biomarkers
Urinary m7Gua and 8-OHdG concentrations were determined by the previously described method.(32) Briefly, a human urine sample was mixed with the same volume of a dilution solution containing the ribonucleoside marker, 8-hydroxyguanosine (8-OHG). A 20 μl aliquot of the diluted urine sample was injected into HPLC-1 (MCI GEL CA08F, 7 μm, 1.5 × 120 mm; elution, 2% acetonitrile in 0.54 mM sulfuric acid, 50 μl/min, 65°C), via the guard column (1.5 × 40 mm), and the chromatograms were recorded by a Gilson UV detector (UV/VIS-155 with 0.2 mm light path cell). Creatinine and m7Gua were detected at 235 and 305 nm, respectively. The 8-OHdG fraction was collected, depending on the relative elution position from the peak of the added marker, 8-OHG, and was automatically injected into the HPLC-2 column. The 8-OHdG fraction was fractionated by the HPLC-2 column [GL Sciences Inc., (Tokyo, Japan) Inertsil ODS-3, 3 μm, 4.6 × 250 mm; elution, 10 mM sodium phosphate buffer (pH 6.7) containing 5% methanol and the antiseptic reagent MB (100 μl/L), 0.7 ml/min, 45°C]. The 8-OHdG was detected by a Coulochem II EC detector (ESA Inc., Chemsford, MA) with a guard cell (5020) and an analytical cell (5011) (applied voltages: guard cell, 400 mV; E1, 150 mV; E2, 350 mV).
Statistical methods
The values of each biomarker were compared with the median, because the data did not follow a normal distribution. Only the 8-OHdG in urine was log-normally distributed, so a log-transformed parametric analysis was performed. Analysis of variance and multiple comparisons were performed using GraphPad Prism, ver. 7.04 (GraphPad Software, San Diego, CA). Multiple regression analysis was performed in JMP®, ver. 14.2.0 (SAS Institute Inc., Cary, NC) after log transformation for each marker. Two-sided p values less than 0.05 were considered significant.
Results
Urinary levels of tobacco exposure markers and smoking status
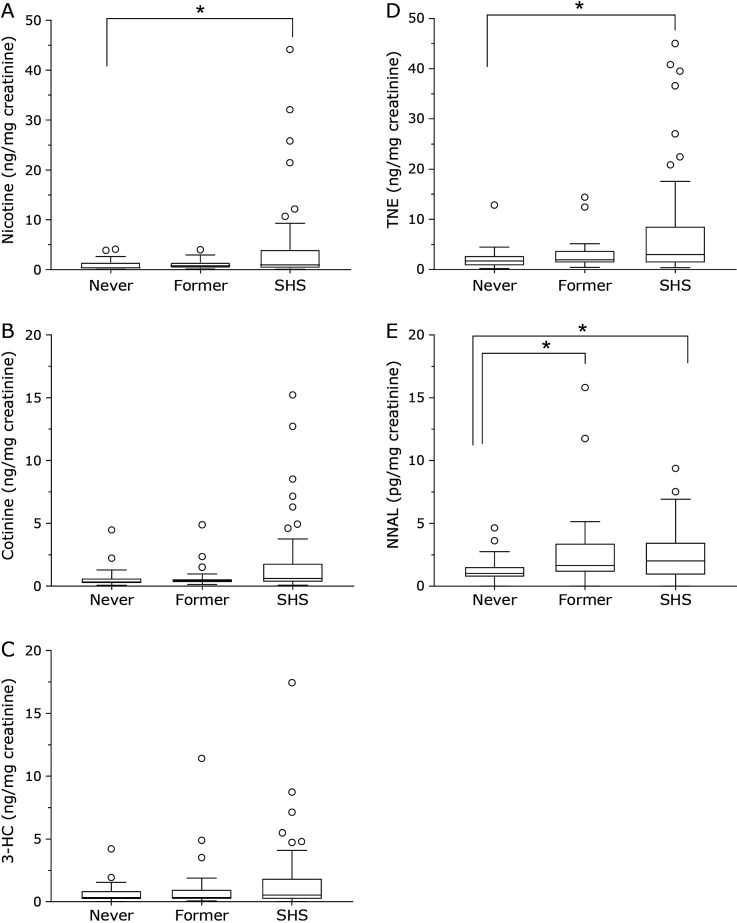
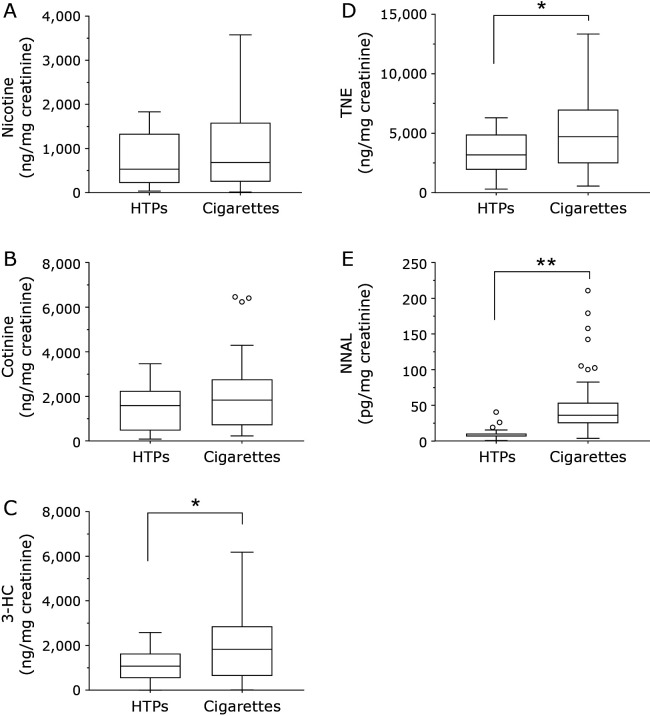
Because the urinary levels of each exposure marker differed greatly by the smoking status, the results are shown in two figures: non-smokers and passive smokers (Fig. 1) and smokers (Fig. 2). Non-smokers were further classified into two groups, as never smokers and former smokers. The urinary levels of nicotine, cotinine, and 3-HC as tobacco exposure markers were higher in passive smokers than in non-smokers (Fig. 1). Especially, the urinary nicotine and total nicotine equivalents (TNE) were significantly higher in passive smokers than in never smokers. There were no significant differences in the urinary levels of nicotine metabolites between never smokers and former smokers. The urinary NNAL levels were significantly higher in passive smokers than in never smokers. Among the non-smokers, the urinary NNAL levels were significantly higher in former smokers than in never smokers. Smokers were classified into two groups, as heated tobacco products (HTPs) smokers and cigarette smokers. (Fig. 2). The urinary nicotine and cotinine levels were not significantly different between HTPs smokers and cigarette smokers, but the 3-HC, TNE and NNAL levels were significantly higher in cigarette smokers. The urinary NNAL levels in HTPs smokers (6.91 pg/mg creatinine, 0.69–40.33: median, minimum–maximum) were significantly higher than those in never smokers (1.00 pg/mg creatinine, not detected (n.d.)–4.64), former smokers (1.67 pg/mg creatinine, n.d.–15.82) and SHS-exposed subjects (2.02 pg/mg creatinine, n.d.–9.37).
Fig. 1.
Tukey box plots of urinary tobacco exposure biomarker levels (A: nicotine, B: cotinine, C: 3-HC, D: TNE, E: NNAL) in nonsmokers, former smokers, and their respective passive smokers. The horizontal lines within boxes indicate median levels. P values are based on the Kruskal-Wallis test followed by the post hoc Dunn’s test. Multiple comparisons between the 3 groups. *p<0.05, **p<0.01.
Fig. 2.
Tukey box plots of urinary tobacco exposure biomarker levels (A: nicotine, B: cotinine, C: 3-HC, D: TNE, E: NNAL) in cigarette and HTPs smokers. The horizontal lines within boxes indicate median levels. P values are based on the Mann-Whitney U test. *p<0.05, **p<0.01.
Urinary levels of DNA damage markers and smoking status
The urinary levels of m7Gua were significantly higher in former smokers and cigarette smokers, as compared to never smokers (Fig. 3A). In addition, the urinary 8-OHdG levels were significantly higher in HTPs and cigarette smokers than in never smokers (Fig. 3B). Although the differences are not statistically significant, the urinary m7Gua levels of SHS-exposed subjects (6.88 μg/mg creatinine: median) and HTPs smokers (7.17 μg/mg creatinine) were higher than those of never smokers (5.47 μg/mg creatinine). The urinary 8-OHdG levels of SHS-exposed subjects (3.31 ng/mg creatinine) were also higher than those of never smokers (3.03 ng/mg creatinine).
Fig. 3.
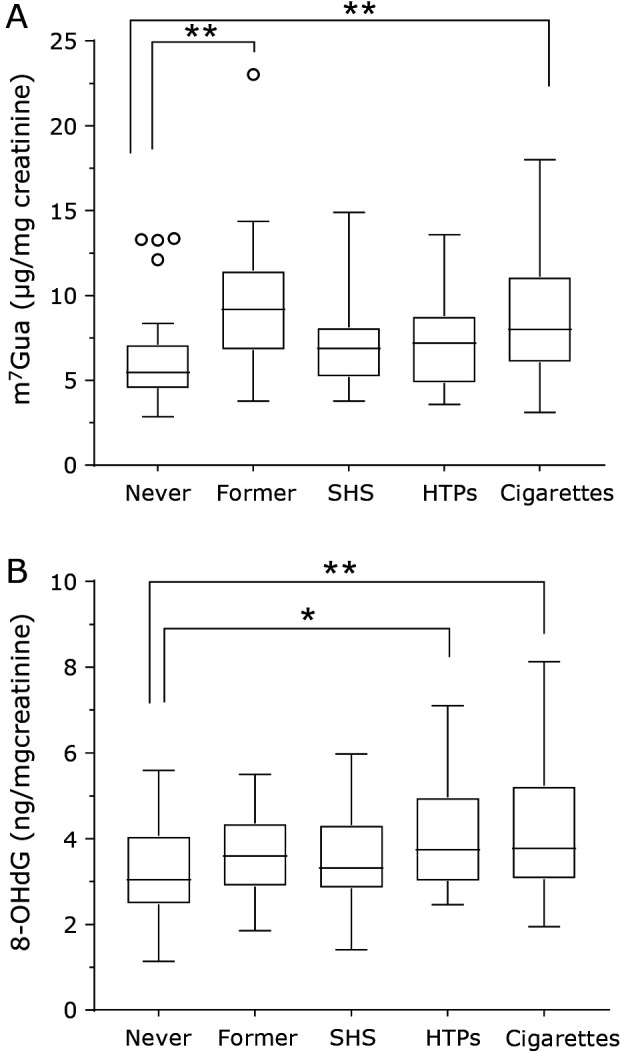
Tukey box plots of urinary DNA damage marker levels (A: m7Gua; B: 8-OHdG) for different smoking statuses. The horizontal lines within boxes indicate median levels. P values are based on the Kruskal-Wallis test followed by the post hoc Dunn’s test (m7Gua) or one-way ANOVA followed by Dunnett’s test (8-OHdG). Multiple comparisons of never smokers and each group. *p<0.05, **p<0.01.
Correlation between the urinary levels of each biomarker
Table 2 shows the Spearman's rank correlations of the continuous variables to the m7Gua and 8-OHdG levels. Urinary m7Gua and 8-OHdG were weakly but significantly correlated with the tobacco exposure markers nicotine, cotinine, 3-HC, and TNE. The age of the subjects was related to the m7Gua level, but not the 8-OHdG level.
Table 2.
Associations of urinary m7Gua and 8-OHdG with continuous variables
| Variables | m7Gua |
8-OHdG |
|||
|---|---|---|---|---|---|
| r | p | r | p | ||
| Nicotine | 0.21 | 0.005 | 0.21 | 0.004 | |
| Cotinine | 0.28 | <0.001 | 0.25 | <0.001 | |
| 3-HC | 0.26 | <0.001 | 0.23 | 0.002 | |
| TNE | 0.28 | <0.001 | 0.24 | 0.001 | |
| NNAL | 0.36 | <0.001 | 0.23 | 0.001 | |
| Age | 0.54 | <0.001 | 0.05 | 0.494 | |
Discussion
Nicotine metabolites have been widely measured as biomarkers of tobacco smoke exposure. In this study, nicotine, cotinine, and 3-HC were measured as tobacco-specific biomarkers. In humans, nicotine and its metabolites are transformed into glucuronides by uridine diphosphate-glucuronosyltransferase. Therefore, the glucuronide conjugates of urine samples were hydrolyzed by the enzyme prior to the sample analysis. Nicotine metabolites were also evaluated as TNE. The urinary levels of nicotine metabolites in SHS-exposed subjects were higher than those in non-smokers. Especially, the nicotine and TNE levels in the SHS exposure group were significantly higher than those in the never smokers. The urinary cotinine levels of the non-smoker and SHS exposure groups were consistent with the previous report.(33) Urinary NNAL, a carcinogenic tobacco-specific nitrosamine, and its glucuronide are valuable biomarkers for monitoring exposure to tobacco smoke. Several reports have evaluated SHS exposure. In those reports, the mean urinary NNAL concentration was 0.95–20.1 pg/ml for SHS exposure.(33) The urinary levels of NNAL for SHS exposure in this study were included in this range. As mentioned above, the usefulness of nicotine metabolites and NNAL in urine for evaluating SHS exposure was confirmed. Even in the current non-smokers, the NNAL levels of former smokers were significantly higher than those of never smokers. Former smokers may be more tolerant of SHS exposure. The smoking status grouping in this study was determined by self-reporting in the questionnaire. The separation of the non-smoking group into never smokers and former smokers is worthwhile to evaluate tobacco smoke exposure accurately. Although HTPs are becoming more popular, only a few biomonitoring studies for exposure and health effects have been reported. In this study, the exposure to HTPs was evaluated with urinary biomarkers. The nicotine metabolite levels in the urine of HTPs smokers tended to be slightly less than those of cigarette smokers. Even so, the HTPs smoker levels did not greatly differ from the cigarette smoker levels. With regard to the NNAL levels, the median value of the urinary levels in HTPs smokers was 5 times less than that in cigarette smokers. On the other hand, the 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK; precursor of NNAL) concentration in the HTPs mainstream smoke was about 10 times less than that in cigarette smoke.(26) The urinary NNAL levels did not directly reflect the NNK concentrations in the mainstream smoke of tobacco products. In HTPs, the mainstream smoke includes high amounts of glycerol and propylene glycol,(34) which might affect the absorption and metabolism of NNK. The results of this study showed that the urine of HTPs smokers has nicotine metabolite levels comparable to those of cigarette smokers, and meaningful amounts of NNAL.
Regarding the relationship between smoking status and urinary m7Gua levels, the m7Gua levels were significantly higher in cigarette smokers than in never smokers. These findings support the previously reported results.(35,36) In addition to the significantly higher levels of m7Gua in cigarette smokers, the m7Gua levels of former smokers were also higher than those of never smokers. Given that the urinary NNAL levels showed a similar tendency, tobacco exposure may contribute to increased urinary m7Gua levels. Although the difference is not statistically significant, the median m7Gua values of the SHS exposure and HTPs smoker groups were higher than that of the never smokers.
The urinary 8-OHdG levels of the HTPs and cigarette smoker groups were significantly higher than those of the never smokers. The higher levels of urinary 8-OHdG in cigarette smokers were previously reported.(19,20,37) However, at this time, no reliable study on the association between HTPs exposure and urinary 8-OHdG has been reported. The results of this study show that the use of HTPs may adversely affect the oxidative stress status. Although there was no statistically significant difference between the 8-OHdG levels in the never smoker, former smoker and SHS exposure groups, the median 8-OHdG levels of the former smoker and SHS exposure groups were higher than that of the never smokers. On the one hand, Howard et al.(38) reported significantly higher levels of plasma 8-OHdG in secondhand smokers, as compared to those of never smokers. On the other hand, some reports found that the 8-OHdG levels in plasma(39) or urine(40) of second-hand smokers were not different from those of never smokers. The discrepancy in those results might be attributable to variations in the passive smoking status between reports. For example, the median SHS exposure time per day in the present study was 20 min, whereas it was 6 h in the report by Howard et al.(38) In any case, SHS exposure presumably increases the 8-OHdG level in urine.
In this study, we evaluated the tobacco exposure markers (nicotine, cotinine, 3-HC, and NNAL) and the early health effect markers (m7Gua and 8-OHdG) in urine for the assessment of tobacco product use. The Spearman’s rank correlation test demonstrated that the tobacco exposure markers and DNA damage markers were all significantly correlated with each other. Although m7Gua and 8-OHdG are not specific markers for smoking, they could be useful biomarkers to evaluate the early health effects of smoking. Previous studies have probed the relationship between urinary m7Gua levels and age,(35,41,42) and the age of the subjects was positively correlated with the urinary m7Gua levels. An age-associated depression of the glutathione levels was proposed as one of the mechanisms, since glutathione may act as a scavenger for alkylating agents. Even so, the urinary m7Gua was positively correlated with the tobacco smoke exposure marker (NNAL) in the multiple regression analysis including age as one of the examination items.
In conclusion, significant levels of urinary nicotine metabolites and NNAL were detected in not only the cigarette smoker group, but also the SHS and HTPs exposure groups. The urinary levels of DNA damage markers (m7Gua and 8-OHdG) tended to be higher with SHS and HTPs exposure, as well as in cigarette smokers. Further use of these biomarkers for evaluating tobacco smoke exposure is expected.
Author Contributions
YK, SW and KK collected the samples and questionnaire survey. YK and KK analyzed nicotine, cotinine, 3-HC, and NNAL in urine. YSL and YO analyzed 8-OHdG and m7Gua in urine. YK statistically analyzed the data. KK and YK designed and critically discussed the study. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP17H01908. We thank Dr. Hiroshi Kasai for his helpful advice with the 8-OHdG measurement and Ms. Megumi Taketomi for her assistance with the urine collection and questionnaire survey.
Abbreviations
- 3-HC
trans-3'-hydroxycotinine
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- HTPs
heated tobacco products
- m7Gua
7-methylguanine
- NNAL
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- SHS
secondhand smoke
- TNE
total nicotine equivalents
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease, 2014; 139–584. [Google Scholar]
- 2.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs Vol. 83: Tobacco Smoke and Involuntary Smoking. Lyon: International Agency for Research on Cancer, 2004; 1–1438. [PMC free article] [PubMed] [Google Scholar]
- 3.Su CP, Syamlal G, Tamers S, Li J, Luckhaupt SE. Workplace secondhand tobacco smoke exposure among U.S. nonsmoking workers, 2015. MMWR Morb Mortal Wkly Rep 2019; 68: 604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The National Institute of Occupational Safety and Health. Current Intelligence Bulletin 54: Environmental Tobacco Smoke in the Workplace-Lung Cancer and Other Health Effects.Cincinnati, Ohio: US Department of Health and Human Services, Public Health Service, CDC, 1991; 91–108. [Google Scholar]
- 5.Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 2009; 192: 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakajima M, Yokoi T. Interindividual variability in nicotine metabolism: C-oxidation and glucuronidation. Drug Metab Pharmacokinet 2005; 20: 227–235. [DOI] [PubMed] [Google Scholar]
- 7.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs Vol. 89: Smokeless Tobacco and Some Tobacco-Specific N-Nitrosamines. Lyon: International Agency for Research on Cancer, 2007; 1–623. [PMC free article] [PubMed] [Google Scholar]
- 8.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis 2002; 23: 907–922. [DOI] [PubMed] [Google Scholar]
- 9.Hecht SS. Carcinogen derived biomarkers: applications in studies of human exposure to secondhand tobacco smoke. Tob Control 2004; 13 Suppl 1: i48–i56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob Res 2020; 22: 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson LA. Context matters: contribution of specific DNA adducts to the genotoxic properties of the tobacco-specific nitrosamine NNK. Chem Res Toxicol 2017; 30: 420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinne ML, He Y, Pachkowski BF, Nakamura J, Kelley MR. N-methylpurine DNA glycosylase overexpression increases alkylation sensitivity by rapidly removing non-toxic 7-methylguanine adducts. Nucleic Acids Res 2005; 33: 2859–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki T, Katayama Y, Komatsu Y, Kamiya H. Analysis of large deletion mutations induced by abasic site analog in human cells. Genes Environ 2018; 40: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rusyn I, Asakura S, Li Y, et al. Effects of ethylene oxide and ethylene inhalation on DNA adducts, apurinic/apyrimidinic sites and expression of base excision DNA repair genes in rat brain, spleen, and liver. DNA Repair (Amst) 2005; 4: 1099–1110. [DOI] [PubMed] [Google Scholar]
- 15.Crosbie PA, Harrison K, Shah R, et al. Topographical study of O(6)-alkylguanine DNA alkyltransferase repair activity and N7-methylguanine levels in resected lung tissue. Chem Biol Interact 2013; 204: 98–104. [DOI] [PubMed] [Google Scholar]
- 16.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2'-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res 1997; 387: 147–163. [DOI] [PubMed] [Google Scholar]
- 17.Kasai H, Kawai K. 8-Hydroxyguanine, an oxidative DNA and RNA modification. In: Jurga S, Erdmann VA, Barciszewski J, eds. Modified Nucleic Acids in Biology and Medicine. Cham: Springer International Publishing AG Switzerland, 2016; 147–185. [Google Scholar]
- 18.Kasai H. What causes human cancer? Approaches from the chemistry of DNA damage. Genes Environ 2016; 38: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loft S, Vistisen K, Ewertz M, Tjønneland A, Overvad K, Poulsen HE. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis 1992; 13: 2241–2247. [DOI] [PubMed] [Google Scholar]
- 20.Irie M, Tamae K, Iwamoto-Tanaka N, Kasai H. Occupational and lifestyle factors and urinary 8-hydroxydeoxyguanosine. Cancer Sci 2005; 96: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asami S, Hirano T, Yamaguchi R, Tomioka Y, Itoh H, Kasai H. Increase of a type of oxidative DNA damage, 8-hydroxyguanine, and its repair activity in human leukocytes by cigarette smoking. Cancer Res 1996; 56: 2546–2549. [PubMed] [Google Scholar]
- 22.Watanabe S, Kawasaki Y, Kawai K. Salivary 8-hydroxyguanine as a lifestyle-related oxidative stress biomarker in workers. J Clin Biochem Nutr 2020; 66: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawai K, Kasai H, Li YS, et al. Measurement of 8-hydroxyguanine as an oxidative stress biomarker in saliva by HPLC-ECD. Genes Environ 2018; 40: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heated tobacco products (HTPs) market monitoring information sheet. World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/273459/WHO-NMH-PND-18.7-eng.pdf?ua=1. Accessed 19 Nov 2020.
- 25.Hori A, Tabuchi T, Kunugita N. Rapid increase in heated tobacco product (HTP) use from 2015 to 2019: from the Japan ‘Society and New Tobacco’ Internet Survey (JASTIS). Tob Control 2020. DOI: 10.1136/tobaccocontrol-2020-055652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bekki K, Inaba Y, Uchiyama S, Kunugita N. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J UOEH 2017; 39: 201–207. [DOI] [PubMed] [Google Scholar]
- 27.Salman R, Talih S, El-Hage R, et al. Free-base and total nicotine, reactive oxygen species, and carbonyl emissions from IQOS, a heated tobacco product. Nicotine Tob Res 2019; 21: 1285–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lüdicke F, Ansari SM, Lama N, et al. Effects of switching to a heat-not-burn tobacco product on biologically relevant biomarkers to assess a candidate modified risk tobacco product: a randomized trial. Cancer Epidemiol Biomarkers Prev 2019; 28: 1934–1943. [DOI] [PubMed] [Google Scholar]
- 29.Bosilkovska M, Tran CT, de La Bourdonnaye G, Taranu B, Benzimra M, Haziza C. Exposure to harmful and potentially harmful constituents decreased in smokers switching to Carbon-Heated Tobacco Product. Toxicol Lett 2020; 330: 30–40. [DOI] [PubMed] [Google Scholar]
- 30.Nabavizadeh P, Liu J, Havel CM, et al. Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke. Tob Control 2018; 27: s13–s19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawasaki Y, Li YS, Ootsuyama Y, Nagata K, Yamato H, Kawai K. Effects of smoking cessation on biological monitoring markers in urine. Genes Environ 2020; 42: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasai H, Svoboda P, Yamasaki S, Kawai K. Simultaneous determination of 8-hydroxydeoxyguanosine, a marker of oxidative stress, and creatinine, a standardization compound, in urine. Ind Health 2005; 43: 333–336. [DOI] [PubMed] [Google Scholar]
- 33.Torres S, Merino C, Paton B, Correig X, Ramírez N. Biomarkers of exposure to secondhand and thirdhand tobacco smoke: recent advances and future perspectives. Int J Environ Res Public Health 2018; 15: 2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchiyama S, Noguchi M, Takagi N, et al. Simple determination of gaseous and particulate compounds generated from heated tobacco products. Chem Res Toxicol 2018; 31: 585–593. [DOI] [PubMed] [Google Scholar]
- 35.Tamae K, Kawai K, Yamasaki S, et al. Effect of age, smoking and other lifestyle factors on urinary 7-methylguanine and 8-hydroxydeoxyguanosine. Cancer Sci 2009; 100: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Narayanapillai S, Hu Q, Fujioka N, Xing C. Contribution of tobacco use and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone to three methyl DNA adducts in urine. Chem Res Toxicol 2018; 31: 836–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CY, Jhou YT, Lee HL, Lin YW. Simultaneous, rapid, and sensitive quantification of 8-hydroxy-2'-deoxyguanosine and cotinine in human urine by on-line solid-phase extraction LC-MS/MS: correlation with tobacco exposure biomarkers NNAL. Anal Bioanal Chem 2016; 408: 6295–6306. [DOI] [PubMed] [Google Scholar]
- 38.Howard DJ, Ota RB, Briggs LA, Hampton M, Pritsos CA. Environmental tobacco smoke in the workplace induces oxidative stress in employees, including increased production of 8-hydroxy-2'-deoxyguanosine. Cancer Epidemiol Biomarkers Prev 1998; 7: 141–146. [PubMed] [Google Scholar]
- 39.Mahrous MM, El-Barrany UM, Ismail MME-D, Gaballah IF, Rashed LA. Blood biomarkers of nicotine-induced toxicity in healthy males. Egypt J Forensic Sci 2019; 9: 28. [Google Scholar]
- 40.Pilger A, Germadnik D, Riedel K, Meger-Kossien I, Scherer G, Rüdiger HW. Longitudinal study of urinary 8-hydroxy-2'-deoxyguanosine excretion in healthy adults. Free Radic Res 2001; 35: 273–280. [DOI] [PubMed] [Google Scholar]
- 41.Gaubatz JW, Tan BH. Introduction, distribution, and removal of 7-methylguanine in different liver chromatin fractions of young and old mice. Mutat Res 1997; 375: 25–35. [DOI] [PubMed] [Google Scholar]
- 42.Park JW, Ames BN. 7-Methylguanine adducts in DNA are normally present at high levels and increase on aging: analysis by HPLC with electrochemical detection. Proc Natl Acad Sci U S A 1988; 85: 7467–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]