Abstract
Korean fermented kimchi is probiotic food preventing Helicobacter pylori (H. pylori)-associated atrophic gastritis in both animal and human trial. In order to reveal the effect of fermented kimchi against H. pylori infection, we performed clinical trial to document the changes of fecal microbiota in 32 volunteers (H. pylori (−) chronic superficial gastritis (CSG), H. pylori (+) CSG, and H. pylori (+) chronic atrophic gastritis (CAG) with 10 weeks kimchi. Each amplicon is sequenced on MiSeq of Illumina and the sequence reads were clustered into operational taxonomic units using VSEARCH and the Chao, Simpson, and Shannon Indices. Though significant difference in α- or β-diversity was not seen in three groups, kimchi intake led to significant diversity of fecal microbiome. As results, Klebsiella, Enterococcus, Ruminococcaceae, Streptococcus, Roseburia, and Clostirdiumsensu were significantly increased in H. pylori (+) CAG, while Akkermansia, Citrobacter, and Lactobacillus were significantly decreased in H. pylori (+) CAG. With 10 weeks of kimchi administration, Bifidobacterium, Lactobacillus, and Ruminococcus were significantly increased in H. pylori (+) CAG, whereas Bacteroides, Subdoligranulum, and Eubacterium coprostanolines were significantly decreased in H. pylori (−) CAG. 10 weeks of kimchi intake significantly improved pepsinogen I/II ratio (p<0.01) with significant decreases in interleukin-1β. Conclusively, fermented kimchi significantly changed fecal microbiota to mitigate H. pylori-associated atrophic gastritis.
Keywords: H. pylori, chronic atrophic gastritis, fermented kimchi, fecal microbiota, pepsinogen I/II ratio
Introduction
Chronic inflammation with Helicobacter pylori (H. pylori) is a major risk factor for gastric cancer, but only less than 3% of H. pylori-infected people develop gastric cancer.(1) As plausible explanations for these discrepancies, host changes in gut microbial composition are associated with intestinal metaplasia (IM), chronic atrophic gastritis (CAG), and gastric adenoma as precancerous lesions and gastric cancer, that is, the role of bacteria other than H. pylori is yet to be established.(2–4) A significant percentage of cancers arise from chronic microbial infections and consequent mutagenic inflammation, H. pylori infection and gastric cancer via gut microbiota changes is regarded as hallmarks of cancer-inducing microbe.(5) However, interventions using probiotics are still obscure implication, though the eradication is actively progressing in Japan in order to prevent H. pylori-associated CAG and cancer.(6–9)
Recent publications aimed to explore responsible gut or gastric microbiota in patients with different degree of gastritis including H. pylori and non-H. pylori bacteria were available;(10–12) profound alterations in human fecal microbiota of H. pylori infected individuals, the increased microbiota diversity associated with H. pylori infection, relative abundance of specific genera, all reflected the complex networking between H. pylori and its human host and gastric tumor microenvironment. For instances, H. pylori-infected individuals was shown to be increased of abundance of Candida glabrata and other unclassified fungi in addition to Succinivibrio, Coriobacteriaceae, Enterococcaceae, and Rikenellaceae,(13) linking possible role for H. pylori-associated changes in the gut microbiota in producing precancerous lesion as well as early stage gastric cancer, the knowledge and information may eventually lead to the development of novel biomarkers or therapeutic intervention.(14)
Put together of above backgrounds, we set hypothesis that fermented diet intervention might be ideal because food contains profuse and diverse kinds of probiotics, safety with long-term intake of food, and associated phytochemicals warranting rejuvenating actions of precancerous atrophic changes in food, ascertained antioxidative and anti-inflammatory actions of phytochemicals included in food,(15–21) considering the fact that accepted benefits of probiotic supplementation are relieving more gastrointestinal symptoms, some improvement in host immune responses, increasing compliance of eradication regimen, and increases of eradication rate.(17,22,23) Although consumption of over-the-counter probiotics to promote health has increased worldwide, there are conflicting clinical results that many probiotic strains and formulations can aggravate underlying diseases reported in high impact journal, so called possibility of “probiotics paradox”.(24–26)
Supported with facts that H. pylori as well as gut microbiota can be determining environmental factors influencing gastric pathologies including precancerous CAG as well as gastric cancer, in order to maintain the gut homeostasis and defensive condition against H. pylori infection, the modulation of gut microbiota with probiotics foods, fermented kimchi in the current study, can be basis for either ameliorating gastritis or rejuvenating atrophy, we have performed clinical trials to explore fecal microbiota changes relevant to probiotic foods in patients with gastritis and found significant contribution of probiotic kimchi in gastric homeostasis as well as the rejuvenation of CAG. In this investigation, deep understanding of the association between gut microbiota and H. pylori-related gastric lesion is very important for either evaluation of overall benefits of fermented kimchi or the optimization of H. pylori-associated gastric cancer prevention strategies.
Material and Methods
Bacteria DNA extraction from human stool samples
The human stool sample is filtered through a 40 μm-pore sized cell strainer after being diluted and incubated in 10 ml of PBS for 24 h. To separate the bacteria from human stool, bacteria in stool samples is isolated using centrifugation at 10,000 × g for 10 min at 4°C. After centrifugation, the pellet is comprised of bacteria. To extract the DNA out of the bacteria, bacteria is boiled for 40 min. DNA is extracted by using a DNeasy Power Soil Kit (QIAGEN, Hilden, Germany), for which standard protocol is followed as the kit guides and the DNA from bacteria in each sample is quantified by using QIAxpert system (QIAGEN).
Bacterial metagenomic analysis using DNA from human stool samples
Bacterial genomic DNA was amplified with 16S_V3_F (5'-TCG TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG CCT ACG GGN GGC WGC AG-3') and 16S_V4_R (5'-GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA GGA CTA CHV GGG TAT CTA ATC C-3') primers, which are specific for V3–V4 hypervariable regions of 16S rDNA gene. The libraries were prepared using PCR products according to MiSeq System guide (Illumina, Coyoacán, Mexico City, Mexico) and quantified using a QIAxpert. Each amplicon is then quantified, set equimolar ratio, pooled, and sequenced on MiSeq (Illumina) according to the manufacturer’s recommendations.
Analysis of bacterial composition in the microbiota
Paired-end reads that matched the adapter sequences were trimmed by cutadapt ver. 1.1.6.(27) The resulting FASTQ files containing paired-end reads were merged with CASPER and then quality filtered with Phred (Q) score based criteria described by Bokulich.(28,29) Any reads were shorter than 350 bp and longer than 550 bp after merging, were also discarded. To identify the chimeric sequences, a reference-based chimera detection step was conducted with VSEARCH against the SILVA gold database.(30,31) Next, the sequence reads were clustered into operational taxonomic units (OTUs) using VSEARCH with de novo clustering algorithm under a threshold of 97% sequence similarity. The representative sequences of the OTUs were finally classified using SILVA 132 database with UCLUST (parallel_assign_taxonomy_uclust.py script on QIIME ver. 1.9.1) under default parameters.(32) The Chao Indices, an estimator of richness of taxa per individual, were estimated to measure the diversity of each sample.
Volunteers recruitment and clinical trials
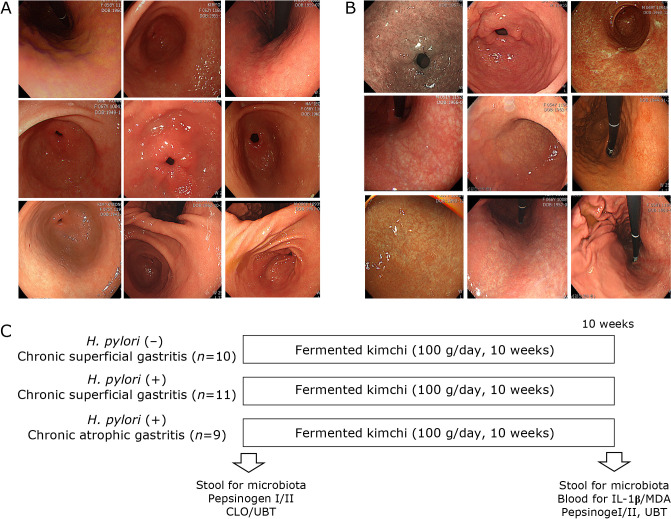
After Institutional Review Board, we have recruited 32 volunteers to accept the current clinical trial that 10 weeks of fermented kimchi intake can benefits against H. pylori infection, changes of pepsinogen I/II as marker for gastric atrophy, the urea breath test (UBT) as assessing H. pylori, measurement of serum IL-1β and malondialdehyde (MDA) as marker for inflammation as well as fecal microbiota changes. Volunteer subjects with H. pylori (−) chronic superficial gastritis (CSG), H. pylori (+) CSG, and H. pylori (+) chronic atrophic gastritis (CAG) were recruited at clinical trial center located at Digestive Disease Center, CHA University Bundang Hospital (Seongnam, Korea). The informed consents were obtained after explaining the aim and object of the current study. As shown in Fig. 1C, they were administered with fermented kimchi for 10 weeks after informed consent and stools were donated for microbiota analysis (MD Healthcare, Inc., Seoul, Korea) and blood samplings were saved for biochemical analysis including CBC, biochemistry, and levels of IL-1β, MDA, and pepsinogen I/II for lipid peroxidation as well as UBT for detecting the presence of H. pylori. Compliance for taking kimchi was checked above intake more than 95% and kimchi was prepared every 5 days to keep optimal fermentation state. All of these studies were approved with IRB (IRB #18-0202) of CHA University Bundang Hospital. There was no significant difference in mean ages, gender difference, and history of smoking and alcohol among groups on demographic analysis. As shown in Fig. 1A and B, representative gastroscopic pictures showing CSG, mild to moderate, in 10 patients, Male:Female = 5:5, mean ages, 44 ± 5, who was negative in H. pylori infection, CLO (−) and UBT (−), and Giemsa staining (−), the other group H. pylori (+) CSG, mild to moderate, in 11 patients, Male:Female = 6:5, mean ages, 48 ± 6, who was positive in H. pylori infection, CLO (+) and UBT (+), and Giemsa staining (+), and chronic atrophic gastritis (CAG), moderate to severe by Updated Sydney System, in 9 patients, Male:Female = 5:4, mean ages, 51 ± 3, who was positive in H. pylori infection, CLO (+) and UBT (+), Giemsa staining (+), and scored according to Updated Sydney System score. All the participating volunteers were sampled with blood to check the ratio of PG 1 and PG I), IL-1β, and MDA levels by ELISA. All volunteers were administered with fermented kimchi (CJ Food, Blossom Park, Suwon, Korea), 100 g/day for 10 weeks. Kimchi was prepared every 5 days to keep fermentation and was delivered to house of volunteers on exact day of delivery and keep at refrigerator. 100 g of kimchi was packed and supplied.
Fig. 1.
Clinical trial with fermented kimchi in patients with gastritis. (A) Representative gastroscopic pictures showing H. pylori-(−) chronic superficial gastritis (CSG), 10 patients, Male:Female = 5:5, mean ages, 44 ± 5, who was negative in H. pylori infection assessed with CLO (−), UBT (−), and Giemsa staining (−), H. pylori (+) CSG, mild to moderate, in 11 patients, Male:Female = 6:5, mean ages, 48 ± 6, who was positive in H. pylori infection assessed with CLO (+), UBT (+), and Giemsa staining (+), and H. pylori (+) chronic atrophic gastritis (CAG), moderate to severe by Updated Sydney System, in 9 patients, Male:Female = 5:4, mean ages, 51 ± 3, who was positive in H. pylori infection assessed with CLO (+), UBT (+), documentation of presence of H. pylori through Giemsa staining scored according to Updated Sydney System score. (B) Representative gastroscopic pictures showing H. pylori (+) CAG in 9 patients, Male:Female = 4:5, mean ages, 54 ± 4. (C) Schematic protocol for fecal microbiota measurement. All volunteers were administered with fermented kimchi (CJ Food, Suwon, Korea), 100 g/day for 10 weeks. All the volunteers were included after informed consent and their compliance for kimchi intake were more than 95%. Before starting, all were performed CLO/UBT/Giemsa staining and stool/blood collection for microbiota and serology analysis.
Biochemical tests, GastroPanel®, IL-1β
Serum levels of basal gastrin 17, pepsinogen (PG) I, PG II, and H. pylori antibody were measured by a chemiluminescent enzyme immunoassay using commercial kits (Biohit plc, Helsinki, Finland) and serum levels of IL-1β were measured with R/G human IL-1β ELISA kit (Mineapolis, MN). A 10 h fasting blood sample was obtained from all patients. Patients were not receiving anti-secretory treatment (including PPIs) 2 weeks before the extraction. EDTA tubes were centrifuged at 2,000 g for 15 min; 50 μl of G17 stabilizer was then added to plasma. Blood was stored at −20°C until the assay was performed.
Urea breath test (UBT)
The 13C urea breath test was performed two times, before clinical trial and after 10 weeks of kimchi intake using 75 mg urea (Urea 13C, Otsuka, Tokushima, Japan) according to the manufacturer’s instructions, briefly, as follows: a basal breath sample was obtained by asking the patient to take a deep breath then holding it for 10 s before blowing the exhale into a specific bag at zero time. After this, the patient was asked to drink the reagent that contains urea attached to a 13C carbon atom in 90 ml of water. Then, 30 min later, the patient was similarly asked to give a breath sample again, which was collected into a new specified bag. Samples were analyzed by infrared spectrophotometer.
Statistical analysis
Comparison of relative abundances (RA) of OTUs and α-diversity between groups was performed with the Mann-Whitney test. Statistical significance was considered if the p value was <0.05. The α-diversity of microbial composition was measured using the Observed, Chao1, Shannon, Simpson index and rarefied to compare species richness. Statistical analyses were performed with R software (ver. 3.6.0).
Results
α- and β-Diversity analysis according to gastric pathology and H. pylori status
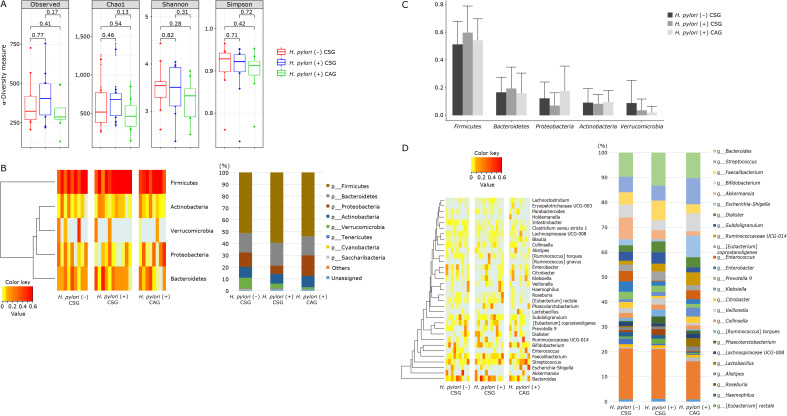
α-Diversity is the analysis of species diversity in samples, for which Chao1, Shannon, and Simpson indexes were explored in order to describe the diversity features of gut tract community according to gastric pathologic condition, H. pylori (−), mild to moderate degree of CSG, H. pylori (+), mild to moderate CSG, and H. pylori (+) CAG (Fig. 2A). In detail, Shannon, diversity indexes reflecting the diversity features of gut community, Chao indexes reflecting the species richness in the sample, and Simpson indexes reflecting community diversity including species richness and species evenness (33,34) were shown in Fig. 2 according to patient group. Generally, the larger the Shannon index and the smaller the Simpson index, the higher the species diversity in the sample were noted, regarding α-diversity according to gastric condition as shown in Fig. 2A, there was no significant difference in p value of these indexes between H. pylori (−) CSG and H. pylori (+) CSG (Chao1 index = 0.46, Shannon index = 0.82, and Simpson index = 0.71) and in p value of these indexes between H. pylori (+) CSG and H. pylori (+) CAG (Chao1 index = 0.13, Shannon index = 0.31, and Simpson index = 0.72). In order to further display differences in species diversity among samples, principal coordinate analysis was used to display differences among samples. In case that the two samples are close together, the species composition of the two samples is interpreted as similar. As result, no significant separation in bacterial community composition between H. pylori (−) CSG and H. pylori (−) CSG with 10 weeks of kimchi intake (Supplemental Fig. 1A*), between H. pylori (+) CSG and H. pylori (+) CSG with 10 weeks of kimchi intake (Supplemental Fig. 1B*, and between H. pylori (+) CAG and H. pylori (+) CAG with 10 weeks of kimchi intake (Supplemental Fig. 1C*). Relative abundance of gastritis associated microbiota including microbial composition of H. pylori (−) CSG, H. pylori (+) CSG, and H. pylori (+) CAG at the phylum level was shown in left and at genus level at right of Supplemental Fig. 1*.
Fig. 2.
Relative abundance of the phylum level (% similarity) in collected feces samples by pyrosequencing. (A) α-Diversity in H. pylori (−) CSG, H. pylori (+) CSG, mild to moderate, and H. pylori (+) CAG, moderate to severe degree. (B) Microbiota at phylum levels, Heatmap and bar display. (C) Microbiota at phylum levels. (D) Relative abundance of the genus level (% similarity) in collected feces samples by pyrosequencing. Significant changes in microbiota among H. pylori (−) CSG, H. pylori (+) CSG, and H. pylori (+) CAG, all before kimchi intake.
Phyla and genus result according to gastric pathology and H. pylori status
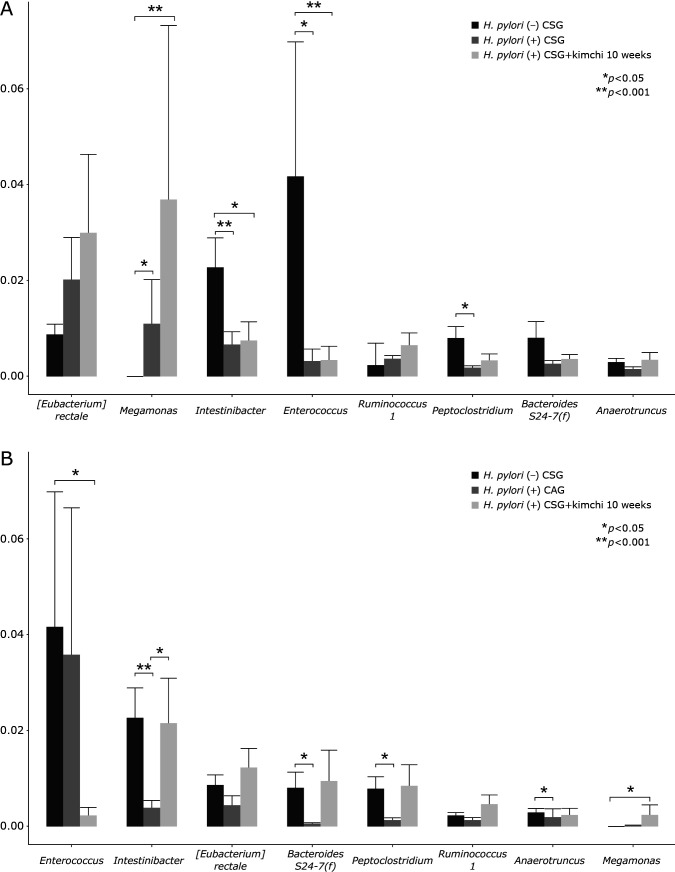
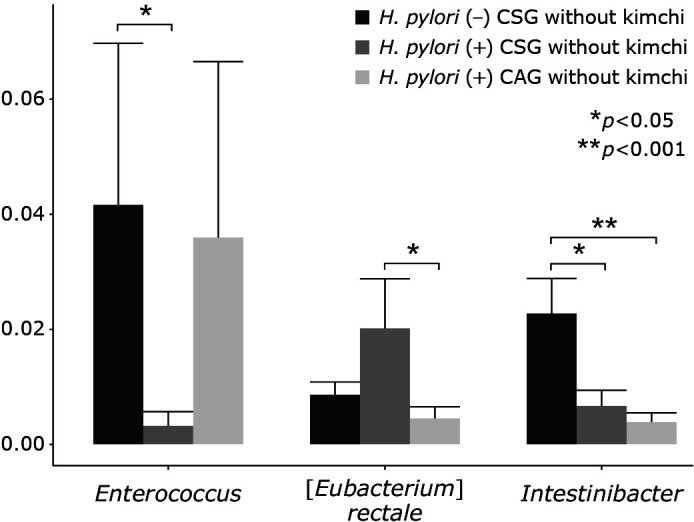
The most abundant phylum in total samples was Firmicutes, followed by followed by Bacteroidetes, Proteobacteria, Actinobacteria, and Verrucomicrobia (Fig. 2B and C). Conclusively, relative abundance of the phylum level in feces by pyrosequencing showed that there was no significant difference in RA of most abundant phyla among the three groups, although looks different according to gastric pathology and H. pylori status, but no statistical significance (p>0.05). RAs of gastritis-associated microbiota including microbial composition of H. pylori (−) CSG, H. pylori (+) CSG, and H. pylori (+) CAG at the genus level was shown in Fig. 2D and 3. RAs of microbiota among the three groups, H. pylori (−) CSG, H. pylori (+) CSG, and H. pylori (+) CAG in the genus level was shown in Fig. 2C depicting Klebsiella, Enterococcus, Ruminococcaceae, Streptococcus, Roseburia, and Clostirdiumsensu significantly increased in H. pylori-associated CAG, whereas Akkermansia, Citrobacter, and Lactobacillus significantly decreased in H. pylori-associated CAG (p<0.05). Individual microbiome was compared according to group, which was presented in Fig. 3 that Entercococcus and Intestinalis was significantly decreased in H. pylori-associated CSG compared to H. pylori negative CSG (p<0.05), whereas Eubacteriumrectalis was significantly increased in H. pylori (+) CSG compared to H. pylori (−) CSG (p<0.05). In cases of H. pylori (+) CAG, Eubacteriumrectalis was significantly decreased in H. pylori (+) CAG compared to H. pylori (+) CSG and Intestinalbacter was further decreased in H. pylori (+) CAG (p<0.001, Fig. 3).
Fig. 3.
Individual microbiota changes according to gastric pathology and H. pylori status.
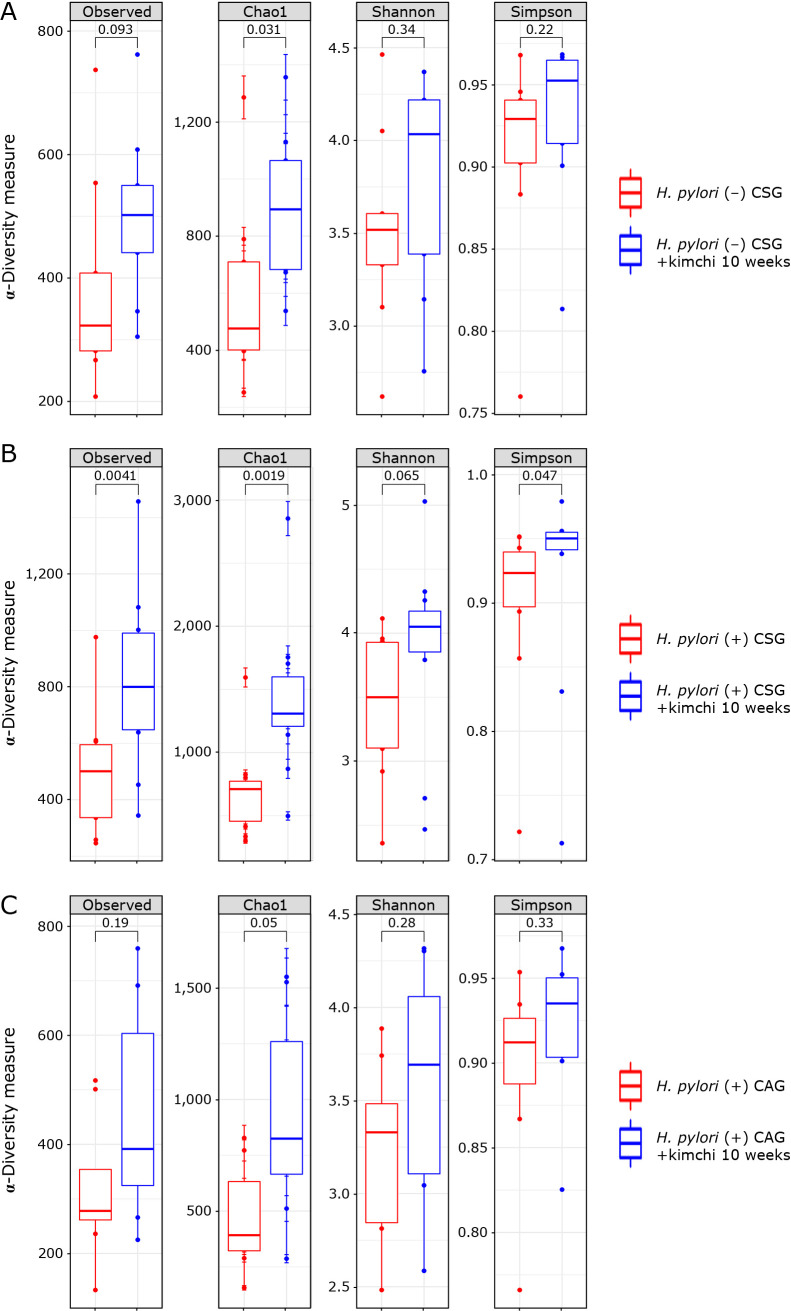
Evaluation of α- and β-diversity in the 3 groups after 10 weeks of kimchi intake
The Chao1 Richness index of the fecal microbiota was significant different between H. pylori (−) CSG and H. pylori (−) with 10 weeks of kimchi intake (p = 0.01, Fig. 4A), between H. pylori (+) CSG and H. pylori (+) CSG with 10 weeks of kimchi intake (p = 0.0019, Fig. 4B), between H. pylori (+) CAG and H. pylori (+) CAG with 10 weeks of kimchi intake (p = 0.05, Fig. 4C), while other indexes were only significant in Simpson index between H. pylori (+) CSG and H. pylori (+) CSG with 10 weeks of kimchi intake (p = 0.047, Fig. 4B). α-Diversity among H. pylori (−) CSG, H. pylori (+) CSG, and H. pylori (+) CAG was displayed in Fig. 5A. In order to further display differences in species diversity among samples including H. pylori (−) CSG, H. pylori (+) CSG, and H. pylori (+) CAG before and after 10 weeks of kimchi intake, principal coordinate analysis (PCoA) based on the unweighted UniFrac distance metrics is used to display differences among samples was presented in Supplemental Fig. 2A*: H. pylori (−) CSG, Supplemental Fig. 2B*: H. pylori (+) CSG, Supplemental Fig. 2C*: H. pylori (+) CAG, and all included group (Supplemental Fig. 2D*).
Fig. 4.
α-Diversity, phylum and genus level analysis after 10 weeks kimchi intake. (A) α-Diversity in H. pylori (−) CSG and H. pylori (−) CSG with 10 weeks of kimchi intake, (B) α-diversity in H. pylori (+) CSG and H. pylori (+) CSG with 10 weeks of kimchi intake, (C) α-diversity in H. pylori (+) CAG and H. pylori (+) CAG with 10 weeks of kimchi intake.
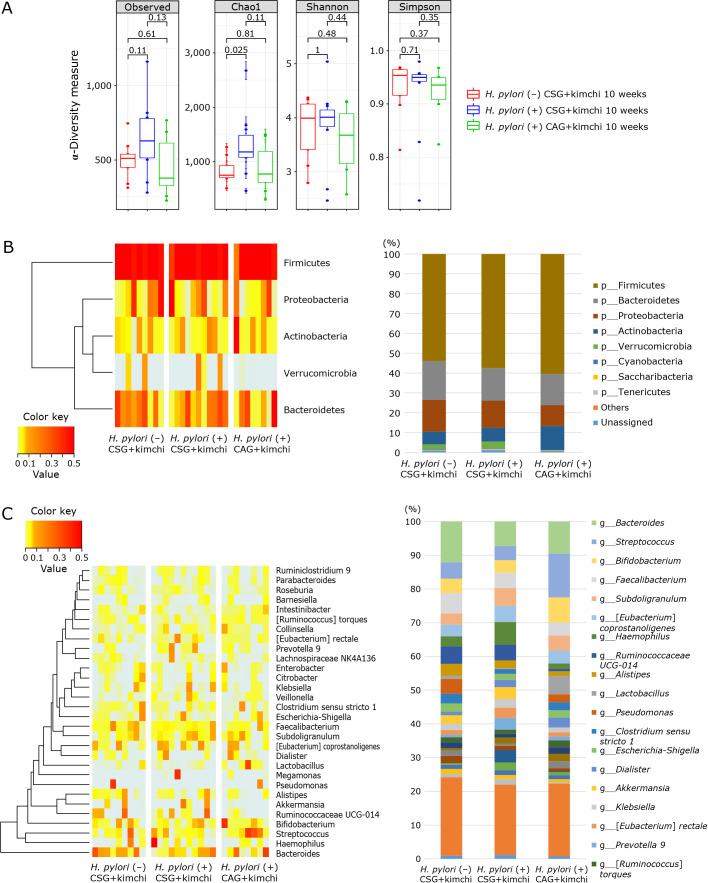
Fig. 5.
(A) α-Diversity in H. pylori (−) CSG, H. pylori (+) CSG, and H. pylori (+) CAG with 10 weeks of kimchi intake, respectively. (B, C) Significant changes in microbiota among H. pylori (−) CSG, H. pylori (+), and H. pylori (+) CAG with 10 weeks kimchi intake. (B) Phyllum level analysis; Phylum levels bar analysis (right). (C) Genus levels analysis; Heatmap analysis (left) and genus levels bar analysis (right).
Relative abundance of the three groups after 10 weeks of kimchi intake (the phylum level)
The most abundant phylum in fecal samples was Firmicutes, followed by followed by Bacteroidetes, Proteobacteria, Actinobacteria, and Verrucomicrobia (Fig. 5B), not significantly changes compared to before kimchi intake as shown in Fig. 2B. Conclusively, RA of the phylum level in feces by pyrosequencing showed that there was no significant difference in RA of most abundant phyla among the three groups, although looks different according to gastric pathology and H. pylori status, but no statistical significance (p>0.05). Individual separate Heatmap results compared between group before kimchi and group after kimchi at phylum levels were shown on Supplemental Fig. 1*.
Relative abundance of the three groups after 10 weeks of kimchi intake (genus level)
Relative abundance of microbiota among the three groups, H. pylori (−) CSG, H. pylori (+) CSG, and H. pylori (+) CAG in the genus level was shown in Fig. 5C depicting Bifidobacterium, Lactobacillus, and Ruminococcus were significantly increased in H. pylori (+) CAG with 10 weeks intake of kimchi intake, whereas Bacteroides, Subdoligranulum, and Eubacterium coprostanolines were significantly decreased in H. pylori (+) CAG with 10 weeks of kimchi intake (Fig. 5C). After assessing all the results from Heatmap and genus analysis (Fig. 6), we reached to findings that Megamonas was significantly increased in H. pylori (+) CSG compared to H. pylori (−) CSG (p<0.05), but its level was significantly increased with 10 weeks of kimchi intake compared to before (p<0.001), whereas Enterococcus was significantly decreased in H. pylori (+) CSG compared to H. pylori (−) CSG, its levels was slightly increased with kimchi intake (p<0.05), Peptoclostridium was significantly decreased with H. pylori infection, but increased with kimchi intake (p<0.05) (Fig. 6A). When analysis was extended to patients with H. pylori (+) CAG, as seen in Fig. 6B, Enterococcus was significantly decreased in patients with H. pylori (+) CAG with kimchi intake (p<0.05), whereas Intestinabacter was significantly decreased after H. pylori infection, but significantly restored with 10 weeks of fermented kimchi intake (p<0.05). Similar and significant findings were noted in Bacteroidates, Peptoclostridoium, Anaerotruncus, and Megamonas (Fig. 6B)
Fig. 6.
Significant microbiota changes according to group, (A) between H. pylori (+) CSG and H. pylori (+) CSG with 10 weeks of fermented kimchi, (B) between H. pylori (+) CAG and H. pylori (+) CAG with 10 weeks of fermented kimchi.
Changes of IL-1β and MDA after kimchi intake
Kimchi intake significantly decreased sera levels of IL-1β between H. pylori (−) CSG and H. pylori (+) CSG with 10 weeks of kimchi intake, though no significant changes were noted in MDA levels, reflecting antioxidative levels, in spite of kimchi intake and increases in PG I/II ratio. Interestingly, 10 weeks of kimchi intake led to unanticipated successful eradication with 10 weeks of fermented kimchi alone in whole 7 patients among 21 patients, by which we have high curiosity to analyze taxon between successful eradicated group and non-eradicated group (Supplemental Fig. 3C*).
Discussion
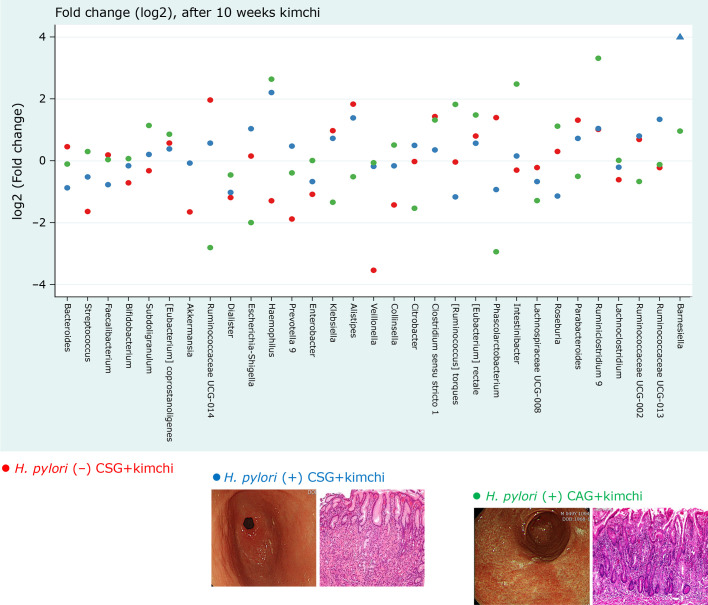
Though this study, we could document the real worlds evidence that fermented kimchi can be anticipatorily applied to either mitigate H. pylori-associated CSG or rejuvenate precancerous CAG. Though not assessed with follow up histology evaluation after kimchi intervention, GastroPanel® (Biohyt, Finland) dealing with the change of PG I/II ratio as marker for interpreting gastric atrophy and IL-1β levels as marker for gastric inflammation were significantly decreased with kimchi intake in addition to significant changes in fecal microbiota. In 7 out of 21 cases, kimchi intake alone led to eradication of bug. Furthermore, we could discover that Klebsiella, Enterococcus, Ruminococcaceae, Streptococcus, Roseburia, and Clostirdiumsensu were significantly increased in H. pylori (+) CAG, while Akkermansia, Citrobacter, and Lactobacillus were significantly decreased in H. pylori (+) CAG (p<0.05). With 10 weeks of kimchi administration, Bifidobacterium, Lactobacillus, and Ruminococcus were significantly increased in H. pylori (+) CAG, whereas Bacteroides, Subdoligranulum, and Eubacterium coprostanolines were significantly decreased in H. pylori (+) CAG (Fig. 7). Our study showed the role of the gut microbiome in H. pylori pathogenesis(35) and the possibility of prevention of H. pylori-associated gastric lesions through intake of probiotic foods.(36)
Fig. 7.
Schematic drawing explaining the significant contribution of fermented kimchi in either inhibition of H. pylori (+) CSG and blocking the advancement from H. pylori (+) CSG and H. pylori (+) CAG. Red dot; H. pylori (−) CSG, Blue dot; H. pylori (+) CSG, Green dot; H. pylori (+) CAG after 10 weeks of fermented kimchi intake. Fold changes (log2).
The human gastric lumen is one of the most hostile environments of the human body, featured with very acidic pH condition, to be sterile, and highly peristaltic condition until the discovery of H. pylori and the general advancement of next generation sequencing and bioinformatic analysis.(37,38) Now, functional genomics as well as the colonization of the gastric habitat can be documented by the 16S rRNA gene amplicon based bacterial microbiome using gastric biopsy or feces. For instance, the genera Actinomyces, Granulicatella, Veillonella, Fusobacterium, Neisseria, Helicobacter, Streptococcus, and Prevotella are significantly different between the H. pylori (+) and H. pylori (−) sample groups including gastric cancer.(39–41) In detail, according to Coker et al.(42) data, OTUs whose species identification corresponds to Parvimonas micra, Dialister pneumosintes, Slackia exigua, Peptostreptococcus stomatis, Prevotella intermedia, Fusobacterium nucleatum, Prevotella oris, and Catonella morbi were found to be significantly enriched in microbiome of gastric cancer compared with precancerous stages. Pathological gastric microbes including H. pylori and more bacteria especially in changed atrophic condition directly modulate its pathogenicity and carcinogenic potential.(43) Minor changes in diversity indices in gastritis, reduced microbial diversity, by decreased abundance of H. pylori, and by the enrichment of other bacterial genera was featured in the gastric carcinoma microbiota analysis,(44) concluding that dysbiosis can discriminate between gastritis and gastric cancer and the possible intervention of probiotics or probiotic foods as for preventive way.(45,46)
In the gut beyond stomach, the human gut microbiota is critical for maintenance of human health and plays an integral role in energy metabolism, absorption of nutrients, and defense against invading pathogens, but microbiota should exist within a delicate balance, homeostasis and hormesis, because in altered condition, dysbiosis contributed to aberrant pro-inflammatory, abnormal immune responses, susceptibility to invading pathogens, and initiation of disease processes including cancer.(47) In detail, H. pylori was present at relatively low abundance in patients with advanced premalignant lesion, but the microbiota of patients with gastric cancer were dominated by species of Lactobacillus, Streptococcus, Veillonella, and Prevotella(2) and a steady decrease in bacterial diversity of the gastric microbiota, with an increasing abundance of Lactobacillus and Lachnospiraceae in patients progressing to gastric carcinogenesis.(48–51)
Also, we could include detailed microbiota changes between successful eradicated subjects and non-eradicated subjects only with kimchi intake. Though we did not include gastric cancer patients, Instead, IM patients were included in our study, as per IM significance, as multiple risk factors associated with gastric carcinogenesis, IM interplays with H. pylori infection and bacterial genomics, host genetic factors, environmental factor such as hygiene, salt intake, diet, and gut microbiota.(52,53) Coker et al.(42) observed significant mucosa microbial dysbiosis in IM and gastric cancer subjects that enrichment of 21 and depletion of 10 bacterial taxa, Peptostreptococcus stomatis, S. anginosus, Parvimonas micra, Slackia exigua, and Dialister pneumosintes. Though a few in number, Sohn et al.(54) analyzed gastric biopsy samples from body and antrum according to H. pylori and non-Helicobacter infection in gastric cancer and found that the number of non-H. pylori urea-producing and non-H. pylori nitro-reducing bacteria was higher in H. pylori–(−) cancer groups than the others and these differences were more pronounced in the body, higher composition of Streptococcus pseudopneumoniae, S. parasanguinis, and S. oralis, emphasizing the importance of our study that long-term nutritional intervention like fermented food seems to be important in preventing H. pylori-associated gastric pathologies.(55)
Investigation correlating the changes of gut microbiota and parameter changes relevant to H. pylori infection is increasing, for instances, changes of IL-1β cytokine and PG I/II ratio in the current study adopting surrogate biomarker reflecting the improvement of atrophy with fermented kimchi via gut microbiota changes. Others published that changes in the gut microbiota such as the Bacteroidetes:Firmicutes (B:F) ratio after treatment with antimicrobials changed the plasma ghrelin level.(56) In a paper dealing with that H. pylori eradication has been found to be effective for gastric cancer prevention, but uncertainties remain about the possible adverse consequences such as microbial dysbiosis, the dominant phyla in fecal samples were Bacteroidetes, Firmicutes, and Proteobacteria with average relative and microbial diversity analysis showed that observed species and Shannon index were increased in subjects with past or current H. pylori infection compared with negative subjects,(57) signifying that the alterations of fecal microbiota, especially the dominant phyla of Bacteroidetes, Firmicutes, and Proteobacteria, may be involved in the process of H. pylori-related gastric lesion progression. After eradication of H. pylori, 1 year later, microbial co-occurrence was reduced and Acinetobacter lwoffii, Streptococcus anginosus, and Ralstonia were enriched, while Roseburia and Sphingomonas were depleted in patients with persistent inflammation.(58) Unexpectedly, kimchi intake alone for 10 weeks resulted in eradication in 7 patients, in which we analyzed genus level and found similar outcome (Supplemental Fig. 3C*).
Among 6 gene polymorphisms highly reported to be associated with H. pylori infection including MUC1, toll-like receptor 4 (TLR4), protein tyrosine phosphatase, non-receptor type 11 (PTPN11), IL-1β, and pepsinogens 3–5,(14) IL-1β 511 C/T SNP and levels reflect H. pylori-associated gastritis as well as cancer risk.(59–61) Though follow-up endoscopy for Updated Sydney System to evaluate atrophic gastritis in the current study is prerequisite, the analysis of serum biomarkers for the assessment of CAG using stomach-specific diagnostic performance of GastroPanel® was done.(62–64) As seen in Supplemental Fig. 3*, the detection of corpus CAG and severe CAG was significantly improved in group taken 10 weeks of fermented kimchi relevant to gut microbiota changes. In addition, though spontaneous eradication rate of H. pylori is regarded as very low, 1–3%,(65) 10 weeks of fermented kimchi intake led to 33% eradication.
As limitations, small in size, short-follow up interval, and analysis in phylum and genus level, we strictly excluded volunteers such as previous administration of proton pump inhibitor (PPI), other probiotics or nutraceuticals, case with autoimmune disease since prior PPI use or probiotics can influence the results.(66,67) Further with our in vitro and in vivo model study,(36) we dare to conclude long-term intake of fermented kimchi can be anticipating strategy either to mitigate H. pylori-associated gastric inflammation/carcinogenesis or to rejuvenate H. pylori-associated atrophic gastritis. Conclusively, though different ethnicity, different dietary style, and different cultural habits exist, our study should be extended more to reach to fermented kimchi as pharmanutrient fulfilling the efficacy of gut microbiota modulation against troublesome H. pylori infection, especially in Asian countries including Korea, Japan, and China.
Author Contributions
Study concept and design: JMP and KBH; acquisition of data: JMP, WHL, HS, and DYL; analysis and statistical analysis: JMP, WHL, and KBH; interpretation of data: HS, JYO, SJK, and KBH; drafting of manuscript: KBH.
Acknowledgments
This work was supported by Korean College of Helicobacter and Upper Gastrointestinal Research (to WJ Ko) and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (to KB Hahm, 116015-03-1-CG000). Prof. Sang Woon Choi provided valuable discussion for the study.
Abbreviations
- CAG
chronic atrophic gastritis
- CSG
chronic superficial gastritis
- IM
intestinal metaplasia
- MDA
malondialdehyde
- OTUs
operational taxonomic units
- PCoA
principal coordinate analysis
- PG I/II
pepsinogen I/II
- RA
relative abundance
- UBT
urea breath test
Conflict of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
References
- 1.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345: 784–789. [DOI] [PubMed] [Google Scholar]
- 2.Dicksved J, Lindberg M, Rosenquist M, Enroth H, Jansson JK, Engstrand L. Molecular characterization of the stomach microbiota in patients with gastric cancer and in controls. J Med Microbiol 2009; 58 (Pt 4): 509–516. [DOI] [PubMed] [Google Scholar]
- 3.Lee CW, Rickman B, Rogers AB, et al. Combination of sulindac and antimicrobial eradication of Helicobacter pylori prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer Res 2009; 69: 8166–8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 2013; 31: 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong H, Bording-Jorgensen M, Dijk S, Wine E. The complex interplay between chronic inflammation, the microbiome, and cancer: understanding disease progression and what we can do to prevent it. Cancers (Basel) 2018; 10: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asaka M, Kobayashi M, Kudo T, et al. Gastric cancer deaths by age group in Japan: outlook on preventive measures for elderly adults. Cancer Sci 2020; 111: 3845–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuda M, Asaka M, Kato M, et al. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter 2017; 22: e12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asaka M, Mabe K, Matsushima R, Tsuda M. Helicobacter pylori eradication to eliminate gastric cancer: the Japanese strategy. Gastroenterol Clin North Am 2015; 44: 639–648. [DOI] [PubMed] [Google Scholar]
- 9.Majima A, Handa O, Naito Y, et al. Early-stage gastric cancer can be found in improved atrophic mucosa over time from successful Helicobacter pylori eradication. Digestion 2017; 95: 194–200. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh YY, Tung SY, Pan HY, et al. Increased abundance of Clostridium and Fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Sci Rep 2018; 8: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frost F, Kacprowski T, Ruhlemann M, et al. Helicobacter pylori infection associates with fecal microbiota composition and diversity. Sci Rep 2019; 9: 20100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gantuya B, El-Serag HB, Matsumoto T, et al. Gastric microbiota in Helicobacter pylori-negative and -positive gastritis among high incidence of gastric cancer area. Cancers (Basel) 2019; 11: 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dash NR, Khoder G, Nada AM, Al Bataineh MT. Exploring the impact of Helicobacter pylori on gut microbiome composition. PLoS One 2019; 14: e0218274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Xu W, Lee A, et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: an open-label, randomized clinical trial. EBioMedicine 2018; 35: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saracino IM, Pavoni M, Saccomanno L, et al. Antimicrobial efficacy of five probiotic strains against Helicobacter pylori. Antibiotics (Basel) 2020; 9: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakiuchi T, Mizoe A, Yamamoto K, et al. Effect of probiotics during vonoprazan-containing triple therapy on gut microbiota in Helicobacter pylori infection: a randomized controlled trial. Helicobacter 2020; 25: e12690. [DOI] [PubMed] [Google Scholar]
- 17.Ji J, Yang H. Using probiotics as supplementation for Helicobacter pylori antibiotic therapy. Int J Mol Sci 2020; 21: 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han YM, Park JM, Jeong M, et al. Dietary, non-microbial intervention to prevent Helicobacter pylori-associated gastric diseases. Ann Transl Med 2015; 3: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SH, Kangwan N, Park JM, Kim EH, Hahm KB. Non-microbial approach for Helicobacter pylori as faster track to prevent gastric cancer than simple eradication. World J Gastroenterol 2013; 19: 8986–8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JS, Paek NS, Kwon OS, Hahm KB. Anti-inflammatory actions of probiotics through activating suppressor of cytokine signaling (SOCS) expression and signaling in Helicobacter pylori infection: a novel mechanism. J Gastroenterol Hepatol 2010; 25: 194–202. [DOI] [PubMed] [Google Scholar]
- 21.Dimidi E, Cox SR, Rossi M, Whelan K. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019; 11: 1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francavilla R, Lionetti E, Castellaneta SP, et al. Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: a pilot study. Helicobacter 2008; 13: 127–134. [DOI] [PubMed] [Google Scholar]
- 23.Homan M, Orel R. Are probiotics useful in Helicobacter pylori eradication? World J Gastroenterol 2015; 21: 10644–10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med 2019; 25: 716–729. [DOI] [PubMed] [Google Scholar]
- 25.Abid MB, Koh CJ. Probiotics in health and disease: fooling Mother Nature? Infection 2019; 47: 911–917. [DOI] [PubMed] [Google Scholar]
- 26.Freedman SB, Williamson-Urquhart S, Farion KJ, et al. Multicenter trial of a combination probiotic for children with gastroenteritis. N Engl J Med 2018; 379: 2015–2026. [DOI] [PubMed] [Google Scholar]
- 27.Kechin A, Boyarskikh U, Kel A, Filipenko M. cutPrimers: a new tool for accurate cutting of primers from reads of targeted next generation sequencing. J Comput Biol 2017; 24: 1138–1143. [DOI] [PubMed] [Google Scholar]
- 28.Kwon S, Lee B, Yoon S. CASPER: context-aware scheme for paired-end reads from high-throughput amplicon sequencing. BMC Bioinformatics 2014; 15 Suppl 9: S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bokulich NA, Subramanian S, Faith JJ, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 2013; 10: 57–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ 2016; 4: e2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 2013; 41 (Database issue): D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010; 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W, Zhang R, Shu R, et al. Study of the relationship between microbiome and colorectal cancer susceptibility using 16SrRNA sequencing. Biomed Res Int 2020; 2020: 7828392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J, Feng Q, Wong SH, et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 2017; 66: 70–78. [DOI] [PubMed] [Google Scholar]
- 35.Sheh A, Fox JG. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes 2013; 4: 505–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JM, Han YM, Oh JY, et al. Fermented kimchi rejuvenated precancerous atrophic gastritis via mitigating Helicobacter pylori-associated endoplasmic reticulum and oxidative stress. J Clin Biochem Nutr 2021. DOI: 10.3164/jcbn.20-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klymiuk I, Bilgilier C, Stadlmann A, et al. The human gastric microbiome is predicated upon infection with Helicobacter pylori. Front Microbiol 2017; 8: 2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engstrand L, Lindberg M. Helicobacter pylori and the gastric microbiota. Best Pract Res Clin Gastroenterol 2013; 27: 39–45. [DOI] [PubMed] [Google Scholar]
- 39.Delgado S, Cabrera-Rubio R, Mira A, Suárez A, Mayo B. Microbiological survey of the human gastric ecosystem using culturing and pyrosequencing methods. Microb Ecol 2013; 65: 763–772. [DOI] [PubMed] [Google Scholar]
- 40.Kienesberger S, Cox LM, Livanos A, et al. Gastric Helicobacter pylori infection affects local and distant microbial populations and host responses. Cell Rep 2016; 14: 1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulz C, Schütte K, Malfertheiner P. Helicobacter pylori and other gastric microbiota in gastroduodenal pathologies. Dig Dis 2016; 34: 210–216. [DOI] [PubMed] [Google Scholar]
- 42.Coker OO, Dai Z, Nie Y, et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018; 67: 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Espinoza JL, Matsumoto A, Tanaka H, Matsumura I. Gastric microbiota: an emerging player in Helicobacter pylori-induced gastric malignancies. Cancer Lett 2018; 414: 147–152. [DOI] [PubMed] [Google Scholar]
- 44.Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, et al. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018; 67: 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iizasa H, Ishihara S, Richardo T, Kanehiro Y, Yoshiyama H. Dysbiotic infection in the stomach. World J Gastroenterol 2015; 21: 11450–11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lam SY, Yu J, Wong SH, Peppelenbosch MP, Fuhler GM. The gastrointestinal microbiota and its role in oncogenesis. Best Pract Res Clin Gastroenterol 2017; 31: 607–618. [DOI] [PubMed] [Google Scholar]
- 47.Noto JM, Peek RM Jr. The gastric microbiome, its interaction with Helicobacter pylori, and its potential role in the progression to stomach cancer. PLoS Pathog 2017; 13: e1006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aviles-Jimenez F, Vazquez-Jimenez F, Medrano-Guzman R, Mantilla A, Torres J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Sci Rep 2014; 4: 4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Zhou J, Xin Y, et al. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur J Gastroenterol Hepatol 2016; 28: 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolig AS, Cech C, Ahler E, Carter JE, Ottemann KM. The degree of Helicobacter pylori-triggered inflammation is manipulated by preinfection host microbiota. Infect Immun 2013; 81: 1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eun CS, Kim BK, Han DS, et al. Differences in gastric mucosal microbiota profiling in patients with chronic gastritis, intestinal metaplasia, and gastric cancer using pyrosequencing methods. Helicobacter 2014; 19: 407–416. [DOI] [PubMed] [Google Scholar]
- 52.Jencks DS, Adam JD, Borum ML, Koh JM, Stephen S, Doman DB. Overview of current concepts in gastric intestinal metaplasia and gastric cancer. Gastroenterol Hepatol (N Y) 2018; 14: 92–101. [PMC free article] [PubMed] [Google Scholar]
- 53.Olmez S, Aslan M, Erten R, Sayar S, Bayram I. The prevalence of gastric intestinal metaplasia and distribution of Helicobacter pylori infection, atrophy, dysplasia, and cancer in its subtypes. Gastroenterol Res Pract 2015; 2015: 434039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sohn SH, Kim N, Jo HJ, et al. Analysis of gastric body microbiota by pyrosequencing: possible role of bacteria other than Helicobacter pylori in the gastric carcinogenesis. J Cancer Prev 2017; 22: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C, Powell SE, Betel D, Shah MA. The gastric microbiome and its influence on gastric carcinogenesis: current knowledge and ongoing research. Hematol Oncol Clin North Am 2017; 31: 389–408. [DOI] [PubMed] [Google Scholar]
- 56.Yanagi H, Tsuda A, Matsushima M, et al. Changes in the gut microbiota composition and the plasma ghrelin level in patients with Helicobacter pylori-infected patients with eradication therapy. BMJ Open Gastroenterol 2017; 4: e000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao JJ, Zhang Y, Gerhard M, et al. Association between gut microbiota and Helicobacter pylori-related gastric lesions in a high-risk population of gastric cancer. Front Cell Infect Microbiol 2018; 8: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sung JJY, Coker OO, Chu E, et al. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut 2020; 69: 1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang JM, Kim N, Shin CM, et al. Predictive factors for improvement of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication: a three-year follow-up study in Korea. Helicobacter 2012; 17: 86–95. [DOI] [PubMed] [Google Scholar]
- 60.Machado JC, Figueiredo C, Canedo P, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology 2003; 125: 364–371. [DOI] [PubMed] [Google Scholar]
- 61.Furuta T, El-Omar EM, Xiao F, Shirai N, Takashima M, Sugimura H. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology 2002; 123: 92–105. [DOI] [PubMed] [Google Scholar]
- 62.Chapelle N, Petryszyn P, Blin J, et al. A panel of stomach-specific biomarkers (GastroPanel®) for the diagnosis of atrophic gastritis: a prospective, multicenter study in a low gastric cancer incidence area. Helicobacter 2020; 25: e12727. [DOI] [PubMed] [Google Scholar]
- 63.Syrjänen K. A panel of serum biomarkers (GastroPanel®) in non-invasive diagnosis of atrophic gastritis. Systematic review and meta-analysis. Anticancer Res 2016; 36: 5133–5144. [DOI] [PubMed] [Google Scholar]
- 64.McNicholl AG, Forné M, Barrio J, et al. Accuracy of GastroPanel for the diagnosis of atrophic gastritis. Eur J Gastroenterol Hepatol 2014; 26: 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Y, Ye Z, Huang J, Huang Y, Yan W, Zhang Y. High prevalence and low spontaneous eradication rate of Helicobacter pylori infection among schoolchildren aged 7–12 years. Acta Paediatr 2018; 107: 1624–1628. [DOI] [PubMed] [Google Scholar]
- 66.Parsons BN, Ijaz UZ, D'Amore R, et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog 2017; 13: e1006653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruno G, Zaccari P, Rocco G, et al. Proton pump inhibitors and dysbiosis: current knowledge and aspects to be clarified. World J Gastroenterol 2019; 25: 2706–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.