Abstract
We examined the effects of a test food containing anthocyanin, astaxanthin, and lutein on the eye function in healthy Japanese adults with eye fatigue after operating visual display terminals. Forty-four subjects were randomly but equally assigned to the active or placebo group. Two active or placebo capsules were taken once daily for 6 weeks. Accommodative function, tear film break-up time, visual acuity, the value of Schirmer’s test, macular pigment optical density level, muscle hardness, and a questionnaire were evaluated before and after a 6-week intervention. Each group included 20 subjects in the efficacy analysis. The active group showed a significant improvement in the percentage of pupillary response of an average of both eyes and dominant eye pre- and post-visual display terminal operation at 6 weeks compared with the placebo group. Moreover, the active group showed a significant improvement in the scores of “A sensation of trouble in focusing the eyes” and “Difficulty in seeing objects in one’s hand and nearby, or fine print” compared with the placebo group between before and after ingestion. Therefore, 6-weeks consumption of the test food inhibited a decrease in the accommodative function caused by visual display terminal operation (UMIN000036989).
Keywords: anthocyanin, astaxanthin, lutein, visual display terminals, accommodative function
Introduction
In modern society, visual display terminals (VDTs), such as PCs and smartphones, are widely used and are indispensable in our daily lives. However, VDT operation is known to cause a decrease in the number of blinks,(1) dryness of the eye due to incomplete blinks,(2) decreased accommodative function, headache, and stiff shoulders.(3) Moreover, the percentage of people with subjective physical symptoms such as eye fatigue and stiff shoulders also increases as the duration of VDT operation increases.(4) Most of the light sources of VDTs are light emitting diodes (LEDs), and the time-dependent production of reactive oxygen species (ROS) in photoreceptor cells due to exposure to blue light generated by LEDs is thought to be a cause of eye strain and dry eye.(5,6) In addition, it has been reported that rhodopsin, which is involved in transmitting visual information to the brain, loses its function under oxidative stress in which ROS are abundant.(7) Therefore, the reduction of ocular cytotoxicity caused by ROS may be effective in preventing or reducing eye strain and dry eye. Many studies have been performed on the ocular function-improving effects of food components with antioxidant properties, such as anthocyanin, astaxanthin, and lutein.
Bilberry (Vaccinium myrtillus) is a perennial of the family Ericaceae, which widely grows throughout the northern and eastern parts of Europe.(8) Anthocyanins contained in bilberries have been shown to possess antioxidant properties.(9) Cyanidin-3-glucoside (C3G), a type of anthocyanin, has been reported to show high scavenging effects against some kinds of ROS.(10) Furthermore, C3G can bind to rhodopsin(11) and promotes the regeneration of rhodopsin after conformational change on light absorption.(12,13) According to previous clinical studies, anthocyanin is reported to help focusing on objects and relieve eye fatigue,(14,15) improve contrast sensitivity,(16) and improve tear fluid quality.(17)
Astaxanthin reduces the degradation of nitric oxide (NO), which is involved in the dilatation of blood vessels,(18) and showed blood flow improving effects in a clinical trial.(19) Regarding its effects on the eyes, astaxanthin is reported to significantly increase retinal capillary blood flow near the optic disc.(20) Therefore, astaxanthin is expected to restore the function of the ciliary body by improving blood circulation, leading to improved accommodative function and reduced eye fatigue and shoulder strain.
Lutein is distributed in the retina, where it is responsible for the visual center, and in the macula, the center of the retina, and plays an important role as an antioxidant in the body by eliminating the reactivity of radicals and other substances.(21) The previous study(22) on mice reported that lutein significantly inhibited the onset and progression of cataract via its antioxidant activity. In addition, lutein can absorb blue light and is thought to be capable of protecting tissues from damage caused by blue light. According to a previous study(23) on rhesus monkeys, the administration of lutein increased the macular pigment optical density and reduced the eye damage caused by blue light. Furthermore, the consumption of lutein by VDT operators is reported to improve visual acuity and contrast sensitivity.(24)
As aforementioned, individual substances of bilberry, astaxanthin, and lutein have been reported to contribute to the improvement of eye function. However, to the best of our knowledge, no previous studies have verified the effects of a combination of these three substances on the improvement of eye function. Therefore, this study aimed to verify the effect of the mixture of bilberry, astaxanthin, and lutein on eye function. In order to solve the problem of poor disintegration and solubility with soft capsules containing bilberry, in this study, an appropriate emulsifier was selected and a self-emulsifying formulation was designed. Since the self-emulsifying formulation designed for this study was expected to improve the absorption of not only bilberry but also other insoluble components, such as astaxanthin and lutein, the soft capsules containing both the three components and the self-emulsifying formulation were used to verify the effects on eye function.
Materials and Methods
Study design
This was a randomized, double-blind, placebo-controlled study. The allocation was based on a 1:1 ratio. The study protocol was approved by the independent ethical committee of the Medical Corporation Seishinkai, Takara Clinic, on May 27, 2019 (approval no. 1905-1904-BJ01-01-TC), and the protocol was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN000036989). This study was conducted in accordance with the Declaration of Helsinki (2013) and the Ethical Guidelines for Medical and Health Research involving human subjects of Japan and thoroughly considered medical ethics. The examinations were conducted at the Ario Nishiarai Eye Clinic (Tokyo, Japan).
Subjects
Inclusion criteria were defined as follows: (1) experiencing eye fatigue during VDT operation in healthy Japanese adult subjects; (2) corrected vision of both eyes with 1.0 or more and who do not use contact lenses, or who can switch to using eye glasses during the test period; (3) considered eligible to participate in the study by the principal physician; and (4) a relatively larger drop in the percentage of pupil constriction (average of both eyes) pre- and post-VDT operation at screening (examination before ingestion). Exclusion criteria were defined as follows: (a) a medical history of current treatment for malignancy, heart failure, or myocardial infarction; (b) using a pacemaker or an implantable cardioverter defibrillator; (c) current treatment for cardiac arrhythmia, hepatic, renal, or cerebrovascular disease, rheumatism, diabetes mellitus, hyperlipidemia, hypertension, or other chronic diseases; (d) diagnosed with or experiencing presbyopia; (e) the presence of ophthalmopathy, entropion, or trichiasis; (f) currently using eye drops for the treatment of an eye disease; (g) the presence of ametropia and no proper treatment of orthoptics; (h) underwent laser eye surgery (LASIK); (i) the presence of an irregular astigmatism; (j) eyestrain without accommodation function, including a neurological deficit; (k) daily consumption of “foods for specified health uses,” “foods with function claims,” or other functional foods/beverages; (l) regular use of medications, including herbal medicines and/or supplements; (m) allergic reaction to medications and/or products that contain the study components; (n) being pregnant, lactating, or planning to become pregnant; (o) enrollment in other clinical trials within the last 3 months before agreeing to participate in this study; and (p) ineligibility to participate in the study based on the evaluation of the principal physician.
Regularly, all subjects were enrolled through the website (https://www.go106.jp/) operated by ORTHOMEDICO Inc. (Tokyo, Japan). The study protocol was comprehensively explained to all the subjects. Written informed consent was obtained from all subjects before enrollment in the study at the ORTHOMEDICO Inc. office. It is worth noting that no subject was part of the sponsors or funding companies.
Sample size
There is no previous study that evaluates the change of percentage of pupillary response of the average of both eyes in pre- and post-VDT operation with continuous intake of a test food. Thus, the sample size was calculated with a minimum clinically meaningful difference of 4% between the groups in the change of percentage of pupillary response of the average of both eyes between pre- and post-VDT operation. In addition, the sample size was evaluated with an assumed SD of approximately 4.5, α value of 0.05, and a (1 − β) value of 0.80. Consequently, the sample size was finalized to be 20 subjects per group. Furthermore, we considered a dropout rate of 10% and added two extra subjects to each group (22 subjects per group).
Enrollment, randomization, and blinding
Of 61 subjects who signed informed consent forms, 44 eligible subjects who were considered appropriate for the study were selected by the physician. Subjects with a relatively larger negative percentage of pupil constriction (average of both eyes) between pre- and post-VDT operation at screening were selected as priority subjects for enrollment in this study. Allocation was performed according to a computer-generated randomization list by an allocation controller, who was not directly involved in this study. The allocation adjustment factors were the percentage of pupil constriction (average of both eyes) between pre- and post-VDT operation at screening, sex, and age at time of screening, and subjects were equally, but randomly, assigned to either the active group or the placebo group (n = 22 per group). Furthermore, subjects, the physician, the assessor of outcomes, and others who were associated with this study were not aware of group assignments and were not involved in the allocation. Moreover, the allocation controller locked the assignment sheet until the key-opening day.
Intervention
The active soft capsules included 36 mg of anthocyanin [100 mg/capsule; blueberry (bilberry) extract powder (BGG Japan Co., Ltd., Tokyo, Japan)], 3 mg of astaxanthin [60 mg/capsule; Haematococcus pluvialis-derived pigment (containing astaxanthin) (BGG Japan Co., Ltd.)], and 5 mg of lutein [12.5 mg/capsule; marigold (containing lutein) (OMNICA Co., Ltd., Tokyo, Japan)], and indigestible dextrin as placebo. Subjects were asked to consume either two active capsules (the active group) or two placebo capsules (the placebo group) daily with water after breakfast for six weeks. Both capsules were declared identical in color, odor, and flavor by the Ethics Committee.
Outcomes
Table 1 describes the schedule for this study. Subjects visited the clinic and underwent examinations before ingestion (Scr) and at six weeks after intake (6 weeks). The VDT operation at Scr and 6 weeks consisted of playing a video game with a handheld game console for 60 min. Each item was evaluated pre- and post-VDT operation at Scr and 6 weeks, and the change between pre- and post-VDT operation (post-VDT operation–pre-VDT operation) was calculated. The tear film break-up time (BUT) test, Schirmer’s test, and macular pigment optical density test were performed only pre-VDT operation. The assessments associated with the eyes were the average of both eyes, and dominant and non-dominant eyes.
Table 1.
Schedule of enrollment, intervention, and assessments
| Study period |
||||||||
|---|---|---|---|---|---|---|---|---|
| Enrollment | Screening |
Selection | Allocation | Start intake | Six weeks after the onset of test-food consumption (6 weeks) |
|||
| Pre-VDT operation | Post-VDT operation | Pre-VDT operation | Post-VDT operation | |||||
| ENROLLMENT: | ||||||||
| Eligibility screen | × | × | ||||||
| Informed consent | × | |||||||
| Dominant eye test (the hole-in-the card test) |
× | |||||||
| Other procedures | × | |||||||
| Allocation | × | |||||||
| INTERVENTIONS: | ||||||||
| Active group |  |
|||||||
| Placebo group |  |
|||||||
| ASSESSMENTS: | ||||||||
| Accommodative function test | × | × | × | × | ||||
| Tear film break-up time test | × | × | ||||||
| Visual acuity test | × | × | × | × | ||||
| Schirmer’s test | × | × | ||||||
| Macular pigment optical density test | × | × | ||||||
| Muscle hardness test | × | × | × | × | ||||
| Questionnaire | × | × | × | × | ||||
| Tonometry | × | × | ||||||
| Physical examination | × | × | × | |||||
| Urinalysis | × | × | ||||||
| Blood analysis | × | × | ||||||
| Medical questionnaire | × | × | ||||||
| Medical questionnaire |  |
|||||||
Primary outcome: change of percentage of pupillary response (average of both eyes) between pre- and post-VDT operation at Scr and 6 weeks
The percentage of pupillary responses were evaluated using TriIRIS C9000 (Hamamatsu Photonics K.K., Shizuoka, Japan), which is a near-point measuring device. The percentage of pupillary response obtained from TriIRIS C9000 corresponds to the moving distance of the accommodative target; therefore, the accommodative function of the pupil of healthy subjects could be quantified by the percentage of pupillary response at near-point measuring.(25,26) The percentage of pupillary response could be calculated from the following equation (1):(25,26)
| (1) |
The symptoms of eye fatigue caused by VDT operation include the reduced pupillary constriction and mydriasis function,(27) and increase of the percentage of pupillary response indicates enhancement of the accommodative function.
Secondary outcomes
All data obtained from the measurement of percentage of pupillary response except for the primary outcome was set as secondary outcomes. The percentage of pupillary response was evaluated by using TriIRIS C9000 and was calculated using the above equation (1).
BUT was measured after administering fluorescein into the eyes of subjects, and they were forbidden to blink. The time between the last blinking and the appearance of the first dry spot was observed and measured using a slit lamp.
Visual acuity was measured by using AutoREF/KERATOMETER ARK-700A (NIDEK CO., LTD., Aichi, Japan).
Schirmer’s value (the amount of lacrimal fluid) was measured by using Schirmer’s test strips.
Macular pigment optical density (MPOD) was measured by using MPS2 (M.E. TECHNICA CO., LTD., Tokyo, Japan).
Muscle hardness was measured by using Bio elasticity meter PEK-1 (Imoto Machinery Co., Ltd, Kyoto, Japan).
The subjective symptoms of eye fatigue were evaluated by the Likert scale method. Subjects expressed subjective sensations of the following symptoms: a tiredness sensation in the eyes; a sensation of dry eyes; objects appear to be blurred or hazy; watery eyes; a sensation of trouble in focusing the eyes; difficulty in seeing objects in one’s hand and nearby, or fine print; easy to feel dazzled by the light; stiffness in the neck and shoulders; a sensation of a heavy head; feeling fatigued in the back of one’s eyes; a sensation of a tired head (brain); difficulty in seeing objects in the dark; painful to look at the screen of a smartphone, cell phone or computer; do not feel like doing anything; cannot concentrate. All these questions were assessed on a scale from 1 (strongly disagree) to 6 (strongly agree).
Safety assessment
Safety evaluations were assessed in physical examination, urinalysis, blood analysis, and tonometry (Table 6-1–4). All subjects were asked to fill out a medical questionnaire for an understanding of their health conditions at each examination. In addition, subjects were asked to keep a daily record of consumption of the test food, health conditions, use of medications, and lifestyle.
Statistical analysis
Subjects visited the clinic twice, and the outcomes were assessed at Scr and 6 weeks. The data at Scr was set as baseline, and the data at 6 weeks was subtracted from the baseline and reported as the change in the value (Δ6 weeks). In addition, subjects’ background data were aggregated based on sex, age, and physical characteristics, and the active group was demographically compared with the placebo group using Student’s t test.
The primary outcome and secondary outcomes except for subjective symptoms, physical examination, urinalysis, blood analysis, and tonometry were presented as mean ± SD, which were analyzed using Student’s t test at baseline. Furthermore, we analyzed data at 6 weeks using the two-way analysis of covariance (ANCOVA). When ANCOVA was used for data analyses, we used the baseline values as covariates. Moreover, data on subjective symptoms were presented as median and interquartile range [first and third quartiles (Q1 and Q3, respectively)], which were analyzed using the Mann-Whitney U test. Furthermore, urinalysis and blood analysis data were assigned codes in which “1” was identified as within the normal range and “0” was identified as outside the normal range. The data were expressed as number of subjects and were analyzed using the chi-square test.
All statistical analyses in this study were two sided, and we set the significance level to 5% with no adjustment for multiple comparisons. Data analyses were performed using Windows SPSS ver. 23.0 (IBM Japan, Ltd., Tokyo, Japan).
Results
Subjects
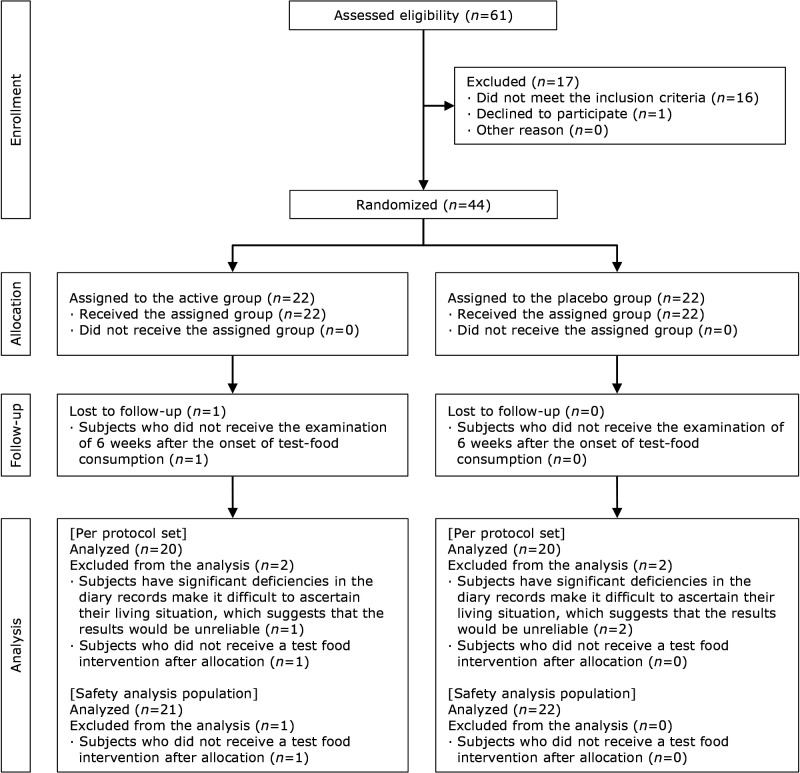
The study flowchart and subject disposition are shown in Fig. 1. This study was conducted from June 1, 2019, to December 13, 2019, and we recruited the subjects from June to August 2019. The target subjects were healthy Japanese adult subjects with eye fatigue sensations during VDT operation, and their corrected vision of both eyes with 1.0 or more and who did not use contact lenses, or who could switch to using eye glasses during the test period. Out of 61 subjects, 44 were eligible and were divided into either the active group or the placebo group (n = 22 each). At the case review meeting after the intervention, 3 subjects who violated the requirements for adherence, and 1 subject who did not receive a test food intervention after allocation were judged as ineligible for analysis and excluded from the analysis. Following key-opening, of the 3 subjects who violated the requirements for adherence, 1 was from the active group and 2 were from the placebo group. The subject who did not receive a test food intervention after allocation was in the active group. The number of subjects analyzed per protocol set was 20 subjects (8 men and 12 women) in the active group and 20 subjects (6 men and 14 women) in the placebo group. The number of subjects analyzed as the safety analysis set (analysis except for the subject who did not receive a test food intervention after allocation) was 21 subjects (8 men and 13 women) in the active group and 22 subjects (7 men and 15 women) in the placebo group. The background and age distribution of the study subjects are shown in Table 2-1–4. There was no significant difference between the background factors of both groups.
Fig. 1.
Flowchart of the study subjects.
Table 2-1.
Subjects’ background (per protocol set)
| Active group (n = 20) | Placebo group (n = 20) | p value | |
|---|---|---|---|
| Age (years) | 37.1 ± 8.2 | 36.1 ± 9.8 | 0.715 |
| Height (cm) | 165.7 ± 9.9 | 162.0 ± 7.2 | 0.183 |
| IgE (RIST) (IU/ml) | 179.4 ± 266.7 | 143.5 ± 159.1 | 0.608 |
| Dominant eye | |||
| Right eye | 16 (80.0%) | 11 (55.0%) | 0.176 |
| Left eye | 4 (20.0%) | 9 (45.0%) |
The data of age, height, and IgE are presented as the mean ± SD. The data of dominant eye are presented as the number of subjects and as a percentage of the each group.
Table 2-2.
Subjects’ background (safety analysis set)
| Active group (n = 21) | Placebo group (n = 22) | p value | |
|---|---|---|---|
| Age (years) | 36.7 ± 8.2 | 36.4 ± 9.4 | 0.925 |
| Height (cm) | 165.4 ± 9.8 | 162.2 ± 7.1 | 0.233 |
| IgE (RIST) (IU/ml) | 175.6 ± 260.5 | 185.9 ± 273.7 | 0.900 |
| Dominant eye | |||
| Right eye | 17 (81.0%) | 13 (59.1%) | 0.185 |
| Left eye | 4 (19.0%) | 9 (40.9%) |
The data of age, height, and IgE are presented as the mean ± SD. The data of dominant eye are presented as the number of subjects and as a percentage of the each group.
Table 2-3.
Subjects’ age distribution (per protocol set)
| Age (years) | Active group (n = 20) |
Placebo group (n = 20) |
|||
|---|---|---|---|---|---|
| Men (n) | Women (n) | Men (n) | Women (n) | ||
| 20–29 | 2 | 2 | 2 | 3 | |
| 30–39 | 3 | 4 | 2 | 5 | |
| 40–49 | 3 | 6 | 2 | 4 | |
| 50–59 | 0 | 0 | 0 | 2 | |
The data are presented as the number of subjects.
Table 2-4.
Subjects’ age distribution (safety analysis set)
| Age (years) | Active group (n = 21) |
Placebo group (n = 22) |
|||
|---|---|---|---|---|---|
| Men (n) | Women (n) | Men (n) | Women (n) | ||
| 20–29 | 2 | 3 | 2 | 3 | |
| 30–39 | 3 | 4 | 2 | 6 | |
| 40–49 | 3 | 6 | 3 | 4 | |
| 50–59 | 0 | 0 | 0 | 2 | |
The data are presented as the number of subjects.
Accommodative function
The results of the accommodative function are shown in Table 3. In the change between pre- and post-VDT operation, percentage of pupillary response of the average of both eyes at 6 weeks of the active group was significantly higher than that of the placebo group (active group, 0.0 ± 5.8%; placebo group, −4.4 ± 6.9%; p = 0.036). In addition, the percentage of pupillary response of the dominant eye at 6 weeks of the active group was significantly higher than that of the placebo group (active group, 0.1 ± 5.2%; placebo group, −5.8 ± 10.9%; p = 0.023).
Table 3.
The results of accommodative function
| Baseline |
6 weeks |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active group (n = 20) |
Placebo group (n = 20) |
p value | Active group (n = 20) |
Placebo group (n = 20) |
Mean difference |
95% Confidence interval |
p value | |||||
| Mean SD | Mean SD | Mean SD | Mean SD | Lower bound |
Upper bound |
|||||||
| Pre-VDT operation | ||||||||||||
| Percentage of pupillary response of average of both eyes (%) | 34.6 ± 12.1 | 32.7 ± 14.7 | 0.650 | 34.8 ± 15.3 | 37.2 ± 13.8 | −3.9 | −10.7 | 3.0 | 0.264 | |||
| Percentage of pupillary response of dominant eye (%) | 34.5 ± 10.9 | 33.0 ± 14.6 | 0.709 | 33.8 ± 14.0 | 37.1 ± 14.2 | −4.4 | −11.2 | 2.3 | 0.190 | |||
| Percentage of pupillary response of nondominant eye (%) | 34.8 ± 13.9 | 32.4 ± 15.7 | 0.617 | 35.8 ± 17.0 | 37.4 ± 14.7 | −3.2 | −10.9 | 4.6 | 0.410 | |||
| Post-VDT operation | ||||||||||||
| Percentage of pupillary response of average of both eyes (%) | 35.4 ± 14.9 | 33.6 ± 14.7 | 0.704 | 34.8 ± 13.0 | 32.8 ± 14.8 | 0.7 | −4.9 | 6.2 | 0.807 | |||
| Percentage of pupillary response of dominant eye (%) | 35.5 ± 15.1 | 33.7 ± 13.5 | 0.688 | 33.9 ± 12.0 | 31.3 ± 16.5 | 1.2 | −5.1 | 7.4 | 0.703 | |||
| Percentage of pupillary response of nondominant eye (%) | 35.2 ± 15.1 | 33.5 ± 16.4 | 0.726 | 35.7 ± 14.3 | 34.3 ± 15.3 | 0.2 | −6.3 | 6.8 | 0.942 | |||
| Change between pre- and post-VDT operation (post-VDT operation–pre-VDT operation) | ||||||||||||
| Percentage of pupillary response of average of both eyes (%) | 0.8 ± 5.3 | 0.9 ± 9.6 | 0.950 | 0.0 ± 5.8 | −4.4 ± 6.9 | 4.4 | 0.3 | 8.5 | 0.036* | |||
| Percentage of pupillary response of dominant eye (%) | 1.0 ± 7.3 | 0.7 ± 10.0 | 0.915 | 0.1 ± 5.2 | −5.8 ± 10.9 | 6.0 | 0.9 | 11.1 | 0.023* | |||
| Percentage of pupillary response of nondominant eye (%) | 0.5 ± 5.5 | 1.1 ± 10.8 | 0.824 | −0.1 ± 8.3 | −3.1 ± 6.3 | 3.1 | −1.6 | 7.7 | 0.187 | |||
The data are presented as the mean ± SD. Mean difference indicates the difference values between the mean value in two groups, and 95% confidence interval is for the mean difference. *p<0.05 vs the placebo group.
Visual acuity
The results of the visual acuity are shown in Table 4. In the change between pre- and post-VDT operation, visual acuity of the dominant eye at baseline in the active group were significantly lower than those in the placebo group (active group, −0.2 ± 0.3; placebo group, 0.0 ± 0.2; p = 0.022). Furthermore, the visual acuity of the dominant eye post-VDT operation at 6 weeks in the active group were significantly higher than those in the placebo group (active group, 0.7 ± 0.6; placebo group, 0.5 ± 0.4; p = 0.011).
Table 4.
The results of the visual acuity
| Baseline |
6 weeks |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active group (n = 20) |
Placebo group (n = 20) |
p value | Active group (n = 20) |
Placebo group (n = 20) |
Mean difference |
95% Confidence interval |
p value | |||||
| Mean SD | Mean SD | Mean SD | Mean SD | Lower bound |
Upper bound |
|||||||
| Pre-VDT operation | ||||||||||||
| Visual acuity of average of both eyes | 0.7 ± 0.6 | 0.5 ± 0.5 | 0.270 | 0.7 ± 0.6 | 0.5 ± 0.5 | 0.0 | −0.1 | 0.1 | 0.861 | |||
| Visual acuity of dominant eye | 0.7 ± 0.6 | 0.5 ± 0.5 | 0.203 | 0.7 ± 0.6 | 0.5 ± 0.5 | 0.0 | −0.2 | 0.1 | 0.581 | |||
| Visual acuity of nondominant eye | 0.6 ± 0.6 | 0.5 ± 0.5 | 0.395 | 0.7 ± 0.6 | 0.5 ± 0.5 | 0.1 | −0.1 | 0.2 | 0.285 | |||
| Post-VDT operation | ||||||||||||
| Visual acuity of average of both eyes | 0.6 ± 0.5 | 0.5 ± 0.4 | 0.529 | 0.6 ± 0.6 | 0.5 ± 0.4 | 0.1 | 0.0 | 0.2 | 0.157 | |||
| Visual acuity of dominant eye | 0.6 ± 0.5 | 0.5 ± 0.4 | 0.774 | 0.7 ± 0.6 | 0.5 ± 0.4 | 0.2 | 0.0 | 0.3 | 0.011* | |||
| Visual acuity of nondominant eye | 0.6 ± 0.6 | 0.5 ± 0.4 | 0.364 | 0.6 ± 0.5 | 0.5 ± 0.4 | 0.0 | −0.2 | 0.1 | 0.973 | |||
| Change between pre- and post-VDT operation (post-VDT operation–pre-VDT operation) | ||||||||||||
| Visual acuity of average of both eyes | −0.1 ± 0.2 | 0.0 ± 0.2 | 0.148 | 0.0 ± 0.2 | 0.0 ± 0.2 | 0.0 | −0.1 | 0.2 | 0.852 | |||
| Visual acuity of dominant eye | −0.2 ± 0.3 | 0.0 ± 0.2 | 0.022* | 0.0 ± 0.3 | 0.0 ± 0.3 | 0.1 | −0.1 | 0.3 | 0.251 | |||
| Visual acuity of nondominant eye | 0.0 ± 0.2 | 0.0 ± 0.2 | 0.940 | −0.1 ± 0.3 | 0.0 ± 0.3 | −0.1 | −0.2 | 0.1 | 0.336 | |||
The data are presented as the mean ± SD. Mean difference indicates the difference values between the mean value in two groups, and 95% confidence interval is for the mean difference. *p<0.05 vs the placebo group.
Subjective symptoms
The results of the subjective symptoms are shown in Table 5-1 (pre-VDT operation), Table 5-2 (post-VDT operation), and Table 5-3 (the change between pre- and post-VDT operation).
Table 5-1.
The results of questionnaire (pre-VDT operation)
| Baseline |
6 weeks |
Change values between baseline and 6 weeks (6 weeks–baseline; Δ6 weeks) |
p value |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active group (n = 20) |
Placebo group (n = 20) |
Active group (n = 20) |
Placebo group (n = 20) |
Active group (n = 20) |
Placebo group (n = 20) |
Baseline | 6 weeks | 6 weeks–basline | |||||||||||||||||||
| Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | ||||||||||
| A tiredness sensation in the eyes | 4.0 | 3.0 | 5.3 | 4.0 | 4.0 | 5.0 | 3.0 | 2.0 | 4.0 | 4.0 | 3.0 | 4.0 | −1.0 | −2.0 | 0.0 | −0.5 | −1.3 | 0.0 | 0.829 | 0.135 | 0.400 | ||||||
| Sensation of dry eyes | 2.5 | 1.8 | 3.0 | 3.0 | 2.0 | 3.0 | 3.0 | 2.0 | 3.0 | 3.0 | 2.0 | 3.3 | 0.0 | −0.3 | 1.0 | 0.0 | −1.0 | 1.0 | 0.125 | 0.243 | 0.950 | ||||||
| Objects appear to be blurred or hazy | 3.0 | 2.0 | 4.3 | 3.0 | 2.0 | 3.0 | 2.0 | 1.0 | 3.0 | 3.0 | 2.0 | 3.3 | −1.0 | −2.0 | 0.3 | 0.0 | −0.3 | 1.0 | 0.578 | 0.168 | 0.093 | ||||||
| Watery eyes | 3.0 | 2.0 | 4.0 | 3.0 | 3.0 | 4.0 | 2.0 | 1.0 | 3.0 | 3.0 | 2.0 | 4.0 | −0.5 | −2.0 | 0.3 | 0.0 | −1.0 | 1.0 | 0.359 | 0.061 | 0.461 | ||||||
| A sensation of trouble in focusing the eyes | 3.0 | 1.0 | 4.0 | 2.5 | 1.8 | 3.0 | 1.5 | 1.0 | 3.0 | 3.0 | 2.0 | 3.3 | 0.0 | −1.3 | 0.3 | 1.0 | 0.0 | 1.0 | 0.493 | 0.087 | 0.044* | ||||||
| Difficulty in seeing objects in one’s hand and nearby, or fine print | 1.5 | 1.0 | 3.0 | 2.0 | 1.0 | 3.0 | 1.0 | 1.0 | 2.3 | 2.5 | 1.0 | 3.0 | 0.0 | −1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.845 | 0.131 | 0.042* | ||||||
| Easy to feel dazzled by the light | 3.0 | 2.0 | 5.0 | 3.0 | 2.0 | 4.0 | 1.5 | 1.0 | 3.3 | 3.0 | 2.0 | 3.0 | −1.0 | −1.3 | 0.0 | 0.0 | −1.0 | 0.0 | 1.000 | 0.321 | 0.363 | ||||||
| Stiffness in the neck and shoulders | 4.5 | 3.0 | 6.0 | 4.0 | 2.8 | 6.0 | 3.0 | 2.0 | 5.0 | 4.0 | 3.0 | 5.0 | −1.0 | −2.0 | 0.0 | 0.0 | −1.0 | 1.0 | 0.861 | 0.101 | 0.098 | ||||||
| A sensation of a heavy head | 3.0 | 1.8 | 4.0 | 3.0 | 2.0 | 4.3 | 1.5 | 1.0 | 3.0 | 2.0 | 1.0 | 4.0 | −0.5 | −2.3 | 0.0 | −0.5 | −1.3 | 0.0 | 0.601 | 0.151 | 0.477 | ||||||
| Feeling fatigued in the back of one’s eyes | 3.0 | 1.8 | 5.0 | 4.0 | 2.8 | 5.0 | 3.0 | 1.0 | 3.3 | 3.0 | 2.8 | 4.0 | 0.0 | −1.3 | 0.0 | 0.0 | −1.3 | 1.0 | 0.321 | 0.144 | 0.686 | ||||||
| A sensation of a tired head (brain) | 3.0 | 2.0 | 3.5 | 2.5 | 2.0 | 4.0 | 2.0 | 1.0 | 3.0 | 3.0 | 1.8 | 3.0 | 0.0 | −1.3 | 0.0 | 0.0 | −1.0 | 1.0 | 0.924 | 0.388 | 0.305 | ||||||
| Difficulty in seeing objects in the dark | 3.0 | 2.0 | 4.0 | 2.5 | 2.0 | 3.3 | 2.5 | 1.0 | 3.0 | 2.0 | 1.8 | 3.0 | 0.0 | −1.3 | 0.0 | 0.0 | −1.0 | 0.0 | 0.446 | 0.926 | 0.786 | ||||||
| Painful to look at the screen of a smartphone, cell phone or computer | 2.0 | 1.0 | 4.0 | 2.0 | 2.0 | 3.0 | 2.0 | 1.0 | 3.0 | 2.0 | 1.8 | 3.0 | 0.0 | −1.0 | 0.0 | 0.0 | −1.0 | 0.3 | 0.868 | 0.457 | 0.757 | ||||||
| Do not feel like doing anything | 2.0 | 1.0 | 4.0 | 3.0 | 2.0 | 4.0 | 2.0 | 1.0 | 3.0 | 3.0 | 2.0 | 4.0 | 0.0 | −0.3 | 1.0 | 0.0 | −1.0 | 1.0 | 0.291 | 0.104 | 0.856 | ||||||
| Cannot concentrate | 2.0 | 1.0 | 4.0 | 2.0 | 2.0 | 4.0 | 2.0 | 1.0 | 3.0 | 2.5 | 2.0 | 3.0 | 0.0 | −1.3 | 0.3 | 0.0 | −1.0 | 0.3 | 0.530 | 0.133 | 0.752 | ||||||
The data are presented as median (Median), first quartile (Q1), and third quartile (Q3). 1, strongly disagree; 2, disagree; 3, slightly disagree; 4, slightly agree; 5, agree; 6, strongly agree. *p<0.05 vs the placebo group.
Table 5-2.
The results of questionnaire (post-VDT operation)
| Baseline |
6 weeks |
Change values between baseline and 6 weeks (6 weeks–baseline; Δ6 weeks) |
p value |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active group (n = 20) |
Placebo group (n = 20) |
Active group (n = 20) |
Placebo group (n = 20) |
Active group (n = 20) |
Placebo group (n = 20) |
Baseline | 6 weeks | 6 weeks–baseline | |||||||||||||||||||
| Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | ||||||||||
| A tiredness sensation in the eyes | 5.0 | 4.0 | 6.0 | 5.0 | 4.0 | 5.0 | 4.0 | 3.0 | 5.0 | 4.0 | 3.8 | 4.0 | −1.0 | −2.0 | 0.0 | −1.0 | −1.0 | 0.0 | 0.470 | 0.764 | 0.540 | ||||||
| Sensation of dry eyes | 4.0 | 3.0 | 5.0 | 4.0 | 4.0 | 4.0 | 3.0 | 2.0 | 4.3 | 4.0 | 3.0 | 4.0 | −1.0 | −1.0 | 0.0 | 0.0 | −1.0 | 0.0 | 0.570 | 0.333 | 0.255 | ||||||
| Objects appear to be blurred or hazy | 3.0 | 2.0 | 5.0 | 3.0 | 2.0 | 3.0 | 2.5 | 1.0 | 3.3 | 3.0 | 3.0 | 4.0 | 0.0 | −1.3 | 0.3 | 0.0 | 0.0 | 1.0 | 0.377 | 0.300 | 0.134 | ||||||
| Watery eyes | 4.0 | 3.8 | 5.0 | 4.0 | 3.0 | 4.0 | 3.0 | 1.0 | 5.0 | 3.0 | 3.0 | 4.0 | −1.0 | −3.0 | 0.0 | 0.0 | −1.0 | 0.0 | 0.108 | 0.465 | 0.243 | ||||||
| A sensation of trouble in focusing the eyes | 2.5 | 1.0 | 4.0 | 3.0 | 1.8 | 3.0 | 2.0 | 1.0 | 4.0 | 3.0 | 1.8 | 3.3 | 0.0 | −1.3 | 1.0 | 0.0 | 0.0 | 1.0 | 0.691 | 0.724 | 0.473 | ||||||
| Difficulty in seeing objects in one’s hand and nearby, or fine print | 2.5 | 1.0 | 4.0 | 2.0 | 1.0 | 3.0 | 2.0 | 1.0 | 2.3 | 2.0 | 1.0 | 3.0 | 0.0 | −2.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.370 | 0.319 | 0.059 | ||||||
| Easy to feel dazzled by the light | 3.5 | 1.8 | 4.0 | 3.0 | 2.0 | 3.3 | 2.0 | 1.0 | 3.3 | 2.5 | 2.0 | 3.0 | 0.0 | −1.3 | 0.0 | −0.5 | −1.0 | 0.3 | 0.546 | 0.373 | 0.546 | ||||||
| Stiffness in the neck and shoulders | 5.0 | 3.8 | 6.0 | 4.5 | 3.0 | 6.0 | 4.0 | 3.0 | 5.0 | 4.0 | 3.0 | 5.0 | 0.0 | −1.3 | 0.0 | 0.0 | −2.0 | 0.3 | 0.727 | 0.705 | 0.897 | ||||||
| A sensation of a heavy head | 4.0 | 1.8 | 5.0 | 3.0 | 2.0 | 5.0 | 3.0 | 1.8 | 3.3 | 2.5 | 2.0 | 4.0 | −0.5 | −1.3 | 0.0 | 0.0 | −1.0 | 0.3 | 0.928 | 0.838 | 0.401 | ||||||
| Feeling fatigued in the back of one’s eyes | 5.0 | 2.8 | 5.0 | 4.0 | 3.0 | 5.0 | 4.0 | 2.0 | 4.0 | 3.0 | 2.0 | 4.0 | −1.0 | −1.0 | 0.0 | −1.0 | −2.0 | 0.0 | 0.897 | 0.394 | 0.474 | ||||||
| A sensation of a tired head (brain) | 4.0 | 2.0 | 6.0 | 4.0 | 2.8 | 5.0 | 3.5 | 2.0 | 5.0 | 3.0 | 2.0 | 4.0 | −1.0 | −1.3 | 0.3 | −0.5 | −1.3 | 0.0 | 0.478 | 0.453 | 0.963 | ||||||
| Difficulty in seeing objects in the dark | 2.0 | 1.0 | 3.0 | 3.0 | 2.0 | 3.0 | 2.0 | 1.0 | 3.0 | 2.5 | 1.0 | 3.0 | 0.0 | −1.0 | 1.0 | 0.0 | −1.0 | 0.3 | 0.532 | 0.488 | 0.935 | ||||||
| Painful to look at the screen of a smartphone, cell phone or computer | 3.0 | 1.0 | 4.0 | 3.5 | 2.8 | 4.0 | 3.0 | 1.0 | 3.0 | 2.5 | 2.0 | 3.0 | 0.0 | −1.0 | 0.0 | −0.5 | −2.0 | 0.0 | 0.394 | 0.874 | 0.276 | ||||||
| Do not feel like doing anything | 3.0 | 1.8 | 4.0 | 3.0 | 2.0 | 3.3 | 2.5 | 1.0 | 3.0 | 2.5 | 1.8 | 3.0 | 0.0 | −1.0 | 0.3 | 0.0 | −1.0 | 0.0 | 0.961 | 0.979 | 0.484 | ||||||
| Cannot concentrate | 3.0 | 1.8 | 4.0 | 3.0 | 2.0 | 3.3 | 2.0 | 2.0 | 4.0 | 3.0 | 2.0 | 3.0 | 0.0 | −0.3 | 0.3 | 0.0 | −1.0 | 0.0 | 0.894 | 0.856 | 0.454 | ||||||
The data are presented as median (Median), first quartile (Q1), and third quartile (Q3). 1, strongly disagree; 2, disagree; 3, slightly disagree; 4, slightly agree; 5, agree; 6, strongly agree.
Table 5-3.
The results of questionnaire (the change between pre- and post-VDT operation)
| Baseline |
6 weeks |
Change values between baseline and 6 weeks (6 weeks–baseline; Δ6 weeks) |
p value |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active group (n = 20) |
Placebo group (n = 20) |
Active group (n = 20) |
Placebo group (n = 20) |
Active group (n = 20) |
Placebo group (n = 20) |
Baseline | 6 weeks | 6 weeks–baseline | |||||||||||||||||||
| Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | ||||||||||
| A tiredness sensation in the eyes | 0.0 | 0.0 | 2.0 | 0.5 | 0.0 | 1.0 | 1.0 | 0.0 | 2.0 | 0.0 | 0.0 | 1.0 | 0.0 | −1.0 | 1.3 | 0.0 | −1.0 | 0.3 | 0.742 | 0.164 | 0.877 | ||||||
| Sensation of dry eyes | 2.0 | 1.0 | 3.0 | 1.0 | 0.8 | 2.0 | 0.5 | 0.0 | 2.0 | 1.0 | 0.0 | 1.3 | −1.0 | −2.0 | 0.3 | 0.0 | −1.0 | 0.3 | 0.139 | 0.994 | 0.250 | ||||||
| Objects appear to be blurred or hazy | 0.0 | −1.0 | 1.0 | 0.0 | −1.0 | 1.0 | 0.0 | 0.0 | 1.3 | 0.0 | −0.3 | 1.0 | 0.5 | −1.0 | 1.0 | 0.0 | −1.0 | 1.0 | 0.842 | 0.539 | 0.639 | ||||||
| Watery eyes | 1.0 | 0.8 | 2.0 | 0.0 | −0.3 | 1.0 | 0.0 | 0.0 | 2.0 | 0.0 | 0.0 | 1.0 | 0.0 | −1.3 | 1.0 | 0.0 | −1.0 | 1.0 | 0.040* | 0.561 | 0.602 | ||||||
| A sensation of trouble in focusing the eyes | 0.0 | −0.3 | 1.0 | 0.0 | −0.3 | 1.0 | 0.0 | 0.0 | 1.0 | 0.0 | −0.3 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | −1.0 | 0.3 | 0.814 | 0.122 | 0.313 | ||||||
| Difficulty in seeing objects in one’s hand and nearby, or fine print | 0.0 | −1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | −1.0 | 0.0 | 0.0 | −1.0 | 1.0 | 0.0 | −0.3 | 0.0 | 0.888 | 0.321 | 0.672 | ||||||
| Easy to feel dazzled by the light | 0.0 | −1.0 | 1.0 | 0.0 | −1.0 | 1.0 | 0.0 | −1.0 | 0.3 | 0.0 | −0.3 | 1.0 | 0.5 | −1.3 | 1.0 | 0.0 | −0.3 | 1.0 | 0.635 | 0.840 | 0.994 | ||||||
| Stiffness in the neck and shoulders | 0.0 | −0.3 | 1.0 | 0.0 | −1.0 | 1.3 | 0.0 | 0.0 | 1.3 | 0.0 | −1.0 | 1.0 | 0.0 | −1.0 | 1.3 | −1.0 | −2.0 | 0.3 | 0.926 | 0.062 | 0.098 | ||||||
| A sensation of a heavy head | 0.0 | 0.0 | 1.0 | 0.0 | −1.0 | 1.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.0 | 0.0 | −1.0 | 1.0 | 0.433 | 0.496 | 0.647 | ||||||
| Feeling fatigued in the back of one’s eyes | 0.0 | 0.0 | 1.0 | 0.0 | −0.3 | 1.0 | 0.5 | 0.0 | 1.3 | 0.0 | −1.0 | 1.0 | 0.0 | −1.0 | 1.0 | −0.5 | −1.0 | 0.0 | 0.388 | 0.060 | 0.300 | ||||||
| A sensation of a tired head (brain) | 1.0 | 0.0 | 2.0 | 1.0 | 0.0 | 1.0 | 1.0 | 0.0 | 1.3 | 0.0 | 0.0 | 1.0 | 0.0 | −1.0 | 1.0 | −0.5 | −1.0 | 1.0 | 0.635 | 0.070 | 0.587 | ||||||
| Difficulty in seeing objects in the dark | 0.0 | −1.0 | 0.0 | 0.0 | −1.0 | 0.0 | 0.0 | −1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.0 | 0.175 | 0.246 | 0.731 | ||||||
| Painful to look at the screen of a smartphone, cell phone or computer | 0.0 | 0.0 | 1.0 | 1.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.3 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.0 | 0.0 | −1.0 | 0.0 | 0.139 | 0.405 | 0.047* | ||||||
| Do not feel like doing anything | 0.0 | 0.0 | 1.0 | 0.0 | −1.0 | 0.3 | 0.0 | 0.0 | 1.0 | 0.0 | −1.0 | 0.0 | 0.0 | −1.0 | 0.0 | 0.0 | −1.0 | 0.0 | 0.110 | 0.049* | 0.882 | ||||||
| Cannot concentrate | 0.0 | 0.0 | 1.0 | 0.0 | −0.3 | 1.0 | 0.5 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | −1.0 | 0.0 | 0.621 | 0.038* | 0.336 | ||||||
The data are presented as median (Median), first quartile (Q1), and third quartile (Q3). 1, strongly disagree; 2, disagree; 3, slightly disagree; 4, slightly agree; 5, agree; 6, strongly agree. *p<0.05 vs the placebo group.
Pre-VDT operation, the scale numbers of the question “A sensation of trouble in focusing the eyes” were median 0.0 (Q1–Q3, −1.3–0.3) in the active group and median 1.0 (Q1–Q3, 0.0–1.0) in the placebo group at Δ6 weeks, which were significantly lower in the active group than in the placebo group (p = 0.044). Also, the scale numbers of the question “Difficulty in seeing objects in one’s hand and nearby, or fine print” were median 0.0 (Q1–Q3, −1.0–0.0) in the active group and median 0.0 (Q1–Q3, 0.0–1.0) in the placebo group at Δ6 weeks, which were significantly lower in the active group than in the placebo group (p = 0.042).
In the change between pre- and post-VDT operation, the scale numbers of the question “Watery eyes” were median 1.0 (Q1–Q3, 0.8–2.0) in the active group and median 0.0 (Q1–Q3, −0.3–1.0) in the placebo group at baseline, which were significantly higher in the active group than in the placebo group (p = 0.040). Regarding the data at 6 weeks, the scale numbers of the question “Do not feel like doing anything” were median 0.0 (Q1–Q3, 0.0–1.0) in the active group and median 0.0 (Q1–Q3, −1.0–0.0) in the placebo group, which were significantly higher in the active group than in the placebo group (p = 0.049). Also, the scale numbers of the question “Cannot concentrate” were median 0.5 (Q1–Q3, 0.0–1.0) in the active group and median 0.0 (Q1–Q3, 0.0–0.0) in the placebo group, which were significantly higher in the active group than in the placebo group (p = 0.038). Furthermore, “Painful to look at the screen of a smartphone, cell phone or computer” were median 0.0 (Q1–Q3, 0.0–1.0) in the active group and median 0.0 (Q1–Q3, −1.0–0.0) in the placebo group at Δ6 weeks which were significantly higher in the active group than in the placebo group (p = 0.047).
BUT, Schirmer’s value, MPOD, and muscle hardness
There were no significant differences between the groups (data not shown).
Safety assessment
No medically problematic changes were observed with the continued ingestion of the test food (Table 6-1–4).
Table 6-1.
The results of the physical examination
| Baseline |
6 weeks |
p value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Active group (n = 20) |
Placebo group (n = 20) |
Active group (n = 20) |
Placebo group (n = 20) |
Baseline | 6 weeks | |||||
| Mean SD | Mean SD | Mean SD | Mean SD | |||||||
| Body weight (kg) | 61.4 ± 12.3 | 58.4 ± 12.5 | 61.2 ± 11.9 | 59.2 ± 13.2 | 0.443 | 0.126 | ||||
| BMI (kg/m2) | 22.3 ± 3.3 | 22.1 ± 4.4 | 22.3 ± 3.3 | 22.4 ± 4.6 | 0.877 | 0.150 | ||||
| Body fat percentage (%) | 24.2 ± 6.8 | 23.5 ± 9.9 | 25.1 ± 7.5 | 25.6 ± 9.1 | 0.766 | 0.174 | ||||
| Systolic blood pressure (mmHg) | 109.0 ± 13.1 | 109.9 ± 10.9 | 108.0 ± 10.6 | 112.5 ± 14.5 | 0.805 | 0.225 | ||||
| Diastolic blood pressure (mmHg) | 70.0 ± 11.0 | 71.4 ± 9.6 | 70.6 ± 8.7 | 74.7 ± 12.4 | 0.674 | 0.207 | ||||
| Pulse rate (bpm) | 74.0 ± 14.2 | 74.9 ± 9.3 | 74.5 ± 11.1 | 73.8 ± 9.6 | 0.799 | 0.611 | ||||
| Body temperature (ºC) | 36.5 ± 0.2 | 36.4 ± 0.4 | 36.5 ± 0.2 | 36.5 ± 0.4 | 0.266 | 0.320 | ||||
The data are presented as the mean ± SD.
Table 6-2.
The results of urinalysis
| Assessment point |
Active group (n = 20) |
Placebo group (n = 20) |
p value | ||||
|---|---|---|---|---|---|---|---|
| Within the reference range |
Outside the reference range |
Within the reference range |
Outside the reference range |
||||
| Protein | Baseline | 19 | 2 | 19 | 3 | 1.000 | |
| 6 weeks | 19 | 2 | 21 | 1 | 0.607 | ||
| Glucose | Baseline | 21 | 0 | 22 | 0 | N.A. | |
| 6 weeks | 21 | 0 | 21 | 1 | 1.000 | ||
| Urobilinogen | Baseline | 21 | 0 | 22 | 0 | N.A. | |
| 6 weeks | 21 | 0 | 22 | 0 | N.A. | ||
| Bilirubin | Baseline | 21 | 0 | 22 | 0 | N.A. | |
| 6 weeks | 21 | 0 | 22 | 0 | N.A. | ||
| pH | Baseline | 21 | 0 | 22 | 0 | N.A. | |
| 6 weeks | 20 | 1 | 22 | 0 | 0.488 | ||
| Occult blood | Baseline | 16 | 5 | 19 | 3 | 0.457 | |
| 6 weeks | 18 | 3 | 21 | 1 | 0.345 | ||
| Ketone bodies | Baseline | 20 | 1 | 20 | 2 | 1.000 | |
| 6 weeks | 20 | 1 | 20 | 2 | 1.000 | ||
The data are presented as the number of subjects. NA, not available.
Table 6-3.
The results of the blood analysis
| Reference range |
Baseline |
6 weeks |
p value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Active group (n = 20) |
Placebo group (n = 20) |
Active group (n = 20) |
Placebo group (n = 20) |
Baseline | 6 weeks | ||||||
| Mean SD | Mean SD | Mean SD | Mean SD | ||||||||
| Leukocyte count (/μl) | 3,300–9,000 | 5,509.5 ± 1,520.8 | 5,463.6 ± 1,212.5 | 5,547.6 ± 1,697.8 | 6,218.2 ± 1,890.2 | 0.913 | 0.157 | ||||
| Erythrocyte count (×104/μl) | Men: 430–570 Women: 380–500 |
456.9 ± 42.8 | 453.0 ± 51.4 | 457.8 ± 41.8 | 451.6 ± 44.7 | 0.791 | 0.567 | ||||
| Hemoglobin (g/dl) | Men: 13.5–17.5 Women: 11.5–15.0 |
13.4 ± 1.8 | 13.5 ± 1.2 | 13.6 ± 1.8 | 13.5 ± 1.0 | 0.855 | 0.554 | ||||
| Hematocrit value (%) | Men: 39.7–52.4 Women: 34.8–45.0 |
42.3 ± 4.6 | 42.3 ± 3.9 | 41.7 ± 4.2 | 41.7 ± 2.9 | 0.985 | 0.889 | ||||
| Platelet count (×104/μl) | 14.0–34.0 | 27.1 ± 5.8 | 28.4 ± 5.9 | 28.1 ± 5.9 | 30.4 ± 6.9 | 0.477 | 0.317 | ||||
| MCV (fl) | 85–102 | 92.5 ± 6.8 | 93.6 ± 5.2 | 91.1 ± 6.1 | 92.5 ± 4.6 | 0.548 | 0.425 | ||||
| MCH (pg) | 28.0–34.0 | 29.2 ± 2.7 | 29.8 ± 1.9 | 29.6 ± 2.6 | 30.1 ± 1.9 | 0.426 | 0.766 | ||||
| MCHC (%) | 30.2–35.1 | 31.5 ± 1.3 | 31.8 ± 0.8 | 32.4 ± 1.2 | 32.5 ± 0.9 | 0.385 | 0.497 | ||||
| Percentage of neutrophils (%) | 40.0–75.0 | 60.0 ± 9.7 | 59.1 ± 6.0 | 60.4 ± 8.5 | 59.8 ± 8.8 | 0.706 | 0.949 | ||||
| Percentage of lymphocytes (%) | 18.0–49.0 | 31.8 ± 9.0 | 33.0 ± 5.5 | 31.1 ± 7.7 | 31.9 ± 8.4 | 0.608 | 0.974 | ||||
| Percentage of monocytes (%) | 2.0–10.0 | 5.1 ± 1.5 | 5.3 ± 1.2 | 5.3 ± 1.4 | 5.8 ± 1.8 | 0.665 | 0.371 | ||||
| Percentages of eosinophils (%) | 0.0–8.0 | 2.4 ± 1.8 | 2.1 ± 1.6 | 2.6 ± 2.5 | 2.0 ± 1.4 | 0.546 | 0.554 | ||||
| Percentages of basophils (%) | 0.0–2.0 | 0.6 ± 0.3 | 0.5 ± 0.2 | 0.6 ± 0.4 | 0.6 ± 0.4 | 0.172 | 0.749 | ||||
| AST (U/L) | 10–40 | 18.0 ± 4.6 | 19.7 ± 5.9 | 19.9 ± 4.9 | 22.3 ± 5.8 | 0.288 | 0.316 | ||||
| ALT (U/L) | 5–45 | 15.9 ± 8.5 | 17.7 ± 12.4 | 16.9 ± 8.0 | 22.5 ± 13.4 | 0.579 | 0.014* | ||||
| γ-GT (U/L) | Men: ≤80 Women: ≤30 |
19.6 ± 10.8 | 22.4 ± 16.6 | 17.9 ± 9.0 | 26.5 ± 20.5 | 0.519 | 0.014* | ||||
| ALP (U/L) | 100–325 | 152.9 ± 47.8 | 163.8 ± 38.3 | 156.0 ± 40.0 | 170.8 ± 39.2 | 0.413 | 0.252 | ||||
| LD (U/L) | 120–240 | 170.9 ± 19.2 | 179.1 ± 37.3 | 163.5 ± 18.3 | 172.2 ± 30.7 | 0.371 | 0.512 | ||||
| LAP (U/L) | Men: 45–81 Women: 37–61 |
48.6 ± 7.6 | 50.7 ± 9.0 | 47.2 ± 8.4 | 50.3 ± 8.9 | 0.411 | 0.372 | ||||
| Total bilirubin (mg/dl) | 0.2–1.2 | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.3 | 0.8 ± 0.2 | 0.256 | 0.054 | ||||
| Direct bilirubin (mg/dl) | 0.0–0.2 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.184 | 0.986 | ||||
| Indirect bilirubin (mg/dl) | 0.2–1.0 | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.319 | 0.039* | ||||
| Cholinesterase (ChE) (U/L) | Men: 234–493 Women: 200–452 |
273.1 ± 58.7 | 305.5 ± 82.1 | 281.0 ± 55.9 | 315.2 ± 71.8 | 0.146 | 0.365 | ||||
| Total protein (g/dl) | 6.7–8.3 | 7.0 ± 0.2 | 7.1 ± 0.5 | 7.1 ± 0.4 | 7.2 ± 0.5 | 0.451 | 0.723 | ||||
| Urea nitrogen (mg/dl) | 8.0–20.0 | 10.5 ± 1.6 | 11.3 ± 3.5 | 12.4 ± 2.8 | 11.2 ± 2.5 | 0.381 | 0.087 | ||||
| Creatinine (mg/dl) | Men: 0.61–1.04 Women: 0.47–0.79 |
0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.991 | 0.008** | ||||
| Uric acid (mg/dl) | Men: 3.8–7.0 Women: 2.5–7.0 |
4.6 ± 1.2 | 4.8 ± 1.1 | 4.9 ± 1.3 | 4.9 ± 1.3 | 0.475 | 0.326 | ||||
| CK (U/L) | Men: 60–270 Women: 40–150 |
108.1 ± 48.5 | 119.6 ± 84.2 | 120.3 ± 72.1 | 95.8 ± 27.9 | 0.590 | 0.092 | ||||
| Sodium (mEq/L) | 137–147 | 139.9 ± 1.5 | 139.8 ± 1.8 | 138.8 ± 1.3 | 139.2 ± 1.9 | 0.939 | 0.333 | ||||
| Potassium (mEq/L) | 3.5–5.0 | 4.0 ± 0.2 | 4.0 ± 0.2 | 4.7 ± 0.4 | 4.8 ± 0.3 | 0.603 | 0.177 | ||||
| Chloride (mEq/L) | 98–108 | 101.4 ± 1.8 | 100.9 ± 1.6 | 101.2 ± 2.5 | 101.1 ± 1.9 | 0.327 | 0.801 | ||||
| Calcium (mEq/L) | 8.4–10.4 | 9.1 ± 0.2 | 9.2 ± 0.3 | 9.2 ± 0.4 | 9.4 ± 0.2 | 0.093 | 0.424 | ||||
| Inorganic phosphorus (mEq/L) | 2.5–4.5 | 3.5 ± 0.5 | 3.7 ± 0.7 | 3.3 ± 0.5 | 3.6 ± 0.5 | 0.237 | 0.264 | ||||
| Serum iron (μg/dl) | Men: 50–200 Women: 40–180 |
93.3 ± 36.7 | 100.2 ± 35.4 | 93.1 ± 42.7 | 94.5 ± 39.1 | 0.537 | 0.839 | ||||
| Serum amylase (U/L) | 40–122 | 80.7 ± 25.1 | 72.5 ± 22.3 | 83.0 ± 31.2 | 75.1 ± 24.9 | 0.268 | 0.898 | ||||
| Total cholesterol (mg/dl) | 120–219 | 183.5 ± 31.3 | 191.4 ± 30.9 | 190.6 ± 29.3 | 199.5 ± 38.9 | 0.410 | 0.772 | ||||
| HDL cholesterol (mg/dl) | Men: 40–85 Women: 40–95 |
61.8 ± 15.7 | 64.6 ± 18.4 | 64.9 ± 17.6 | 67.9 ± 20.8 | 0.585 | 0.985 | ||||
| LDL cholesterol (mg/dl) | 65–139 | 104.9 ± 24.7 | 107.5 ± 27.7 | 107.8 ± 20.8 | 113.0 ± 34.0 | 0.739 | 0.587 | ||||
| Triglyceride (mg/dl) | 30–149 | 79.0 ± 43.6 | 84.0 ± 57.4 | 78.9 ± 42.9 | 80.5 ± 75.7 | 0.755 | 0.743 | ||||
| Glucose (mg/dl) | 70–109 | 85.2 ± 7.4 | 78.5 ± 7.5 | 87.2 ± 9.4 | 81.4 ± 5.5 | 0.006** | 0.136 | ||||
| HbA1c (NGSP) (%) | 4.6–6.2 | 5.3 ± 0.2 | 5.4 ± 0.3 | 5.3 ± 0.2 | 5.4 ± 0.3 | 0.310 | 0.892 | ||||
| Glycoalbumin (%) | 12.3–16.5 | 13.7 ± 1.2 | 13.8 ± 1.3 | 14.1 ± 1.2 | 14.0 ± 1.2 | 0.801 | 0.247 | ||||
The data are presented as the mean ± SD. **p<0.01 and *p<0.05 vs the placebo group.
Table 6-4.
The results of the tonometry
| Baseline |
6 weeks |
p value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Active group (n = 20) |
Placebo group (n = 20) |
Active group (n = 20) |
Placebo group (n = 20) |
Baseline | 6 weeks | |||||
| Mean SD | Mean SD | Mean SD | Mean SD | |||||||
| Intraocular pressure of average of both eyes (mmHg) | 14.4 ± 3.1 | 15.0 ± 2.7 | 14.7 ± 2.7 | 15.1 ± 3.2 | 0.539 | 0.886 | ||||
| Intraocular pressure of dominant eye (mmHg) | 14.4 ± 3.1 | 15.2 ± 2.9 | 14.5 ± 2.7 | 14.9 ± 3.0 | 0.393 | 0.758 | ||||
| Intraocular pressure of nondominant eye (mmHg) | 14.4 ± 3.3 | 14.8 ± 2.8 | 14.9 ± 3.0 | 15.3 ± 3.6 | 0.737 | 0.906 | ||||
The data are presented as the mean ± SD.
Discussion
The percentage of pupillary response of the average of both eyes at 6 weeks of the active group were significantly higher than that of the placebo group, and the mean difference between the groups was 4.4%. In addition, the change of the percentage of pupillary response of the dominant eye between pre- and post-VDT operation at 6 weeks of the active group was significantly higher than that of the placebo group, and the mean difference between the groups was 6.0%. In a previous study which evaluated the accommodative function, a 4.3% change of the percentage of pupillary response was regarded as a clinically meaningful change.(28) Therefore, we judged a 4.4% change in the percentage of pupillary response observed in this study as a clinically meaningful change.
According to the reports by Ibi et al.,(29,30) the dominant eye is always mildly hypertonic compared to the non-dominant eye, and quickly responds to the far-to-near accommodation, thus the dominant eye is more likely to get accommodative malfunction caused by VDT operation. Therefore, the effect of VDT operation on the dominant eye was greater in this study, and it is assumed that there was a significant difference in the change of the percentage of pupillary response of the dominant eye between pre- and post-VDT operation after consumption of the test food. Moreover, the improvement of accommodative function by the consumption of the test food may have contributed to the change for the better of the scores of “A sensation of trouble in focusing the eyes (pre-VDT operation)” and “Difficulty in seeing objects in one’s hand and nearby, or fine print (pre-VDT operation)” between before and after ingestion.
Anthocyanins derived from bilberries are reported to have a vasorelaxing effect by increasing NO,(5) and astaxanthin has also been suggested to show blood flow improving effects by inhibiting the oxidative damage of erythrocyte membranes via the inhibition of lipid peroxidation on cell membranes and preserving the deformation ability of erythrocytes.(31) Improvement of blood circulation might contribute to the relaxation of the accommodative function related muscles, such as ciliary muscles, sphincter pupillae muscles, and dilator pupillae muscles. Therefore, ingestion of the test food is thought to improve the accommodative function by relaxing the tension of the accommodative function related muscles through the improvement of microcirculatory dynamics that transport nutrients to the ciliary muscles, sphincter pupillae muscles, and dilator pupillae muscles. In addition, the blood circulation improving effects of the test food may have contributed to the change for the better of the scores of “Stiffness in the neck and shoulders (pre-VDT operation)” between before and after ingestion.
As for the visual acuity, one of the secondary outcomes, the measured value and change of visual acuity of the dominant eye post-VDT operation at 6 weeks in the active group was significantly higher than that in the placebo group, and the mean difference between the groups was 0.2 in both the measured value and the change in values. The right eye is the dominant eye in most Japanese,(32) and the previous study reported that the visual acuity of the right eye of Japanese workers decreased from 0.09 to 0.12 one year after they started VDT operation.(33) Visual acuity is reported to decrease approximately by 0.1 with VDT operation; therefore, we judged a 0.2 increase in visual acuity observed in this study as a clinically meaningful change and the effect of the test food on visual acuity in the dominant eye was confirmed.
Decreased eye function is attributed to the hypofunction of rhodopsin and macular impairment, which is related to the production of ROS by blue light, the light source of VDTs.(34,35) C3G is suggested to promote the regeneration of rhodopsin(11,12) among the anthocyanins derived from bilberries.(10) In addition, a previous in vitro study using murine photoreceptor cells reported that an anthocyanin-rich bilberry extract suppressed the production of ROS in a concentration-dependent manner.(36) Regarding the macula, which is located in the center of the retina, its damage is known to reduce vision. Although the macula is usually thought to contribute to the prevention of age-related macular degeneration through blue light protection and its antioxidant capacity,(37) excessive exposure to blue light in the macula causes optical impairment. It has also been reported that the macula may improve visual performance by reducing chromatic aberration and increasing contrast sensitivity.(38) Since the macula contains lutein and zeaxanthin, several studies have evaluated the protective effects on the visual function of lutein and zeaxanthin intake, and they suggested the possibility of the prevention of age-related macular degeneration and the improvement of visual acuity.(39,40) Therefore, in the present study, the improvement of visual acuity was thought to be brought about by the fact that the intake of anthocyanin and lutein contained in the test food promoted the regeneration of rhodopsin, the reduction of ROS caused by blue light through the improvement of macular antioxidant capacity, and the improvement of contrast sensitivity through the reduction of chromatic aberration. Collectively, the reduction of eye function may have been attenuated by the inhibitory effect on rhodopsin regeneration by anthocyanin.
In this study, although the 6-week consumption of the test food improved accommodative and visual functions, there was no observation of the improving effects of the test food on the lacrimal fluid and the macula. The inclusion criteria of the previous study, which reported the improvement of the lacrimal fluid quality with consumption of anthocyanin, were subjects conscious of a dry eye.(17) However, the inclusion criteria of this study were subjects experiencing eye fatigue during VDT operation, and most of the subjects answered the questionnaire on subjective symptoms on eye dryness as “disagree” or “slightly disagree.” Contrary to the previous research, the lack of improvement in the lacrimal fluid quality in this study may have been due to the different inclusion criteria for the subjects. Macular pigment decreases with age, and most of the subjects included in this study were aged <50 years, which could be why it was difficult to evaluate the eye function with MPOD. One of the causes of age-related macular degeneration is the increase in photo-oxidative stress due to the decline in antioxidant capacity with aging.(41) However, prior research in Japanese elderly subjects reported intersex differences in the antioxidant property of astaxanthin in the eyes.(42) Estrogen, one of the female sex hormones, is known to have antioxidative effects, and oxidation reactions are prone to advance in postmenopausal women.(42) Indeed, according to that study, ROS scavenging activity of the aqueous humor, one of the watery fluids present in the eyes, was significantly improved only in women after the intake of astaxanthin.(42) In future studies, inclusion criteria different from that of this study may enable the evaluation of the effects of the test food on the other eye functions.
In addition, the antioxidant effects of anthocyanin, astaxanthin, and lutein are reported to contribute to the improvement of liver function.(43–45) In this study, ALT and γ-GT at 6 weeks of the active group were significantly lower than that of the placebo group, suggesting that the test food may improve liver function. Although the test food could improve liver function, this study aimed to verify the effects of the test food on the eye function, not to verify the effects of the test food on liver function. Further studies are required to evaluate the effects of the test food on the modulation of liver function.
The 6-week consumption of the test food containing anthocyanin, astaxanthin, and lutein on eye function in the healthy Japanese adult subjects with eye fatigue during VDT operation was investigated in this study. Through the test food consumption, the decline of accommodative function with VDT operation was suppressed, and the visual acuity of dominant eye and subjective symptoms related to VDT operation were improved. Furthermore, the test food consumption was found to be safe under the conditions of this study.
Author Contributions
YK, TS, and MK designed the study. TY conducted the study. YK, TS, HT, MK, and RK wrote the manuscript. TY had primary responsibility for the final content. All authors read and approved the final manuscript.
Acknowledgments
The authors thank all the subjects and staff who participated in this study.
Abbreviations
- BUT
tear film break-up time
- C3G
cyanidin-3-glucoside
- LED
light emitting diode
- MPOD
macular pigment optical density
- ROS
reactive oxygen species
- VDT
visual display terminal
Conflict of Interest
The sponsors of this study, BGG Japan Co., Ltd. and DHC Corporation, entrusted ORTHOMEDICO Inc. with conducting the study. KY and ST are employees of BGG Japan Co., Ltd., and MK, HT, and RK are members of DHC Corporation. TY (MD) is a staff of the Ario Nishiarai Eye Clinic. TY was the principal investigator and monitored all the conditions of the subjects.
References
- 1.Tsubota K, Nakamori K. Dry eyes and video display terminals. N Engl J Med 1993; 328: 584. [DOI] [PubMed] [Google Scholar]
- 2.Himebaugh NL, Begley CG, Bradley A, Wilkinson JA. Blinking and tear break-up during four visual tasks. Optom Vis Sci 2009; 86: E106–E114. [DOI] [PubMed] [Google Scholar]
- 3.Namba T, Fukahori A, Morikawa A, Yoneda T, Haruishi K, Tabuchi A. Reduction of natural vision control function by visual display terminal (VDT) work and recovery effect of circumocular thermotherapy. Kawasaki Med Welf J 2008; 17: 363–371. (in Japanese) [Google Scholar]
- 4.Ministry of Health, Labour and Welfare. Survey on technological innovation and labour 2008 (in Japanese). http://www.mhlw.go.jp/toukei/itiran/roudou/saigai/anzen/08/02.html. Accessed 11 Nov 2020.
- 5.Tosini G, Ferguson I, Tsubota K. Effects of blue light on the circadian system and eye physiology. Mol Vis 2016; 22: 61–72. [PMC free article] [PubMed] [Google Scholar]
- 6.Kuse Y, Ogawa K, Tsuruma K, Shimazawa M, Hara H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci Rep 2014; 4: 5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaiswal M, Haelterman NA, Sandoval H, et al. Impaired mitochondrial energy production causes light-induced photoreceptor degeneration independent of oxidative stress. PLoS Biol 2015; 13: e1002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uleberg E, Rohloff J, Jaakola L, et al. Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). J Agric Food Chem 2012; 60: 10406–10414. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima J, Tanaka I, Seo S, Yamazaki M, Saito K. LC/PDA/ESI-MS profiling and radical scavenging activity of anthocyanins in various berries. J Biomed Biotechnol 2004; 2004: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li CY, Xu HD, Zhao BT, Chang HI, Rhee HI. Gastroprotective effect of cyanidin 3-glucoside on ethanol-induced gastric lesions in rats. Alcohol 2008; 42: 683–687. [DOI] [PubMed] [Google Scholar]
- 11.Yanamala N, Tirupula KC, Balem F, Klein-Seetharaman J. pH-dependent interaction of rhodopsin with cyanidin-3-glucoside. 1. Structural aspects. Photochem Photobiol 2009; 85: 454–462. [DOI] [PubMed] [Google Scholar]
- 12.Tirupula KC, Balem F, Yanamala N, Klein-Seetharaman J. pH-dependent interaction of rhodopsin with cyanidin-3-glucoside. 2. Functional aspects. Photochem Photobiol 2009; 85: 463–470. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto H, Nakamura Y, Tachibanaki S, Kawamura S, Hirayama M. Stimulatory effect of cyanidin 3-glycosides on the regeneration of rhodopsin. J Agric Food Chem 2003; 51: 3560–3563. [DOI] [PubMed] [Google Scholar]
- 14.Kosehira M, Kitaichi N. Clinical effects of standard bilberry extract on eyestrain. Jpn Pharmacol Ther 2015; 43: 397–403. (in Japanese) [Google Scholar]
- 15.Kosehira M, Kageyama M, Kamohara S, Kitaichi N. Effect of standardized bilberry extract on eye strain—a double-blind randomized placebo controlled crossover study—. Jpn Pharmacol Ther 2015; 43: 1741–1749. (in Japanese) [Google Scholar]
- 16.Muth ER, Laurent JM, Jasper P. The effect of bilberry nutritional supplementation on night visual acuity and contrast sensitivity. Altern Med Rev 2000; 5: 164–173. [PubMed] [Google Scholar]
- 17.Sonoda K, Koikeda T, Masuda K, Saitoh M, Li Y. Verification of improve visual effect function by intake of bilberry extract powder supplement. Phannacometrics 2017; 92: 113–123. (in Japanese) [Google Scholar]
- 18.Hussein G, Nakamura M, Zhao Q, et al. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol Pharm Bull 2005; 28: 47–52. [DOI] [PubMed] [Google Scholar]
- 19.Miyawaki H, Takahashi J, Tsukahara H, Takehara I. Effects of astaxanthin on human blood rheology. J Clin Therap Med 2005; 21: 421–429. (in Japanese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagaki Y, Mihara M, Takahashi J, et al. The effect of astaxanthin on retinal capillary blood flow in normal volunteers. J Clin Therap Med 2005; 21: 537–542. (in Japanese) [Google Scholar]
- 21.Wooten BR, Hammond BR. Macular pigment: influences on visual acuity and visibility. Prog Retin Eye Res 2002; 21: 225–240. [DOI] [PubMed] [Google Scholar]
- 22.Kinoshita S, Sugawa H, Nanri T, et al. Trapa bispinosa Roxb. and lutein ameliorate cataract in type 1 diabetic rats. J Clin Biochem Nutr 2020; 66: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker FM, 2nd, Snodderly DM, Johnson EJ, et al. Nutritional manipulation of primate retinas, V: effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Investig Opthalmology Vis Sci 2011; 52: 3934–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma L, Lin XM, Zou ZY, Xu XR, Li Y, Xu R. A 12-week lutein supplementation improves visual function in Chinese people with long-term computer display light exposure. Br J Nutr 2009; 102: 186–190. [DOI] [PubMed] [Google Scholar]
- 25.Hiraoka M. Accommodative pupillography. Neuro-Ophthalmology Japan 2005; 22: 348–353. (in Japanese) [Google Scholar]
- 26.Hiraoka M, Moroda M, Touya Y, Hakamata N. Near triad meter—dynamic measurement of pupillometry with horizontal eye tracker by accommodative stimulation. J Jpn Ophthalmol Soc 2003; 107: 702–708. (in Japanese) [PubMed] [Google Scholar]
- 27.Hiraoka M. IT eye-strain. Ophthalmology 2005; 47: 63–70. (in Japanese) [Google Scholar]
- 28.Umigai N, Saito T, Yamashita S, Suzuki N, Yamada T. Effects of crocetin on the pupillary response during accommodation induced by visual display terminal work: a randomized, double-blind, placebo-controlled, crossover trial. Jpn J Compl Alternative Med 2017; 14: 9–16. (in Japanese) [Google Scholar]
- 29.Ibi K. Characteristics of dynamic accommodation responses: comparison between the dominant and non-dominant eyes. Ophthalmic Physiol Opt 1997; 17: 44–54. [PubMed] [Google Scholar]
- 30.Ibi K. Accommodation in technostress ophthalmopathy. JJOMT 2003; 51: 121–125. (in Japanese) [Google Scholar]
- 31.Tsukahara H, Koikeda T, Arai T, Hayashi H, Ohno S, Suzuki N. Supplementation effect of astaxanthin on blood flow and shoulder stiffness—a preliminary pilot study—. Jpn J Compl Alternative Med 2008; 5: 49–56. (in Japanese) [Google Scholar]
- 32.Masuda K, Saito A, Kanzaki J, Kunihiro T. Subjective visual vertical (SVV): effects of head position and visual conditions. Equilib Res 2003; 62: 181–189. (in Japanese) [Google Scholar]
- 33.Ibi K, Nakamura K, Iwasaki T, Higashi M, Kurimoto S, Saito S. Ophthalmological examination of VDT workers. The third report before and one year after the introduction of VDTs. Folia Opthalmol Jpn 1988; 39: 1214–1221. (in Japanese) [Google Scholar]
- 34.Widomska J, Subczynski WK. Mechanisms enhancing the protective functions of macular xanthophylls in the retina during oxidative stress. Exp Eye Res 2019; 178: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura M, Kuse Y, Tsuruma K, Shimazawa M, Hara H. The involvement of the oxidative stress in murine blue LED light-induced retinal damage model. Biol Pharm Bull 2017; 40: 1219–1225. [DOI] [PubMed] [Google Scholar]
- 36.Ooe E, Kuse Y, Yako T, et al. Bilberry extract and anthocyanins suppress unfolded protein response induced by exposure to blue LED light of cells in photoreceptor cell line. Mol Vis 2018; 24: 621–632. [PMC free article] [PubMed] [Google Scholar]
- 37.Jia YP, Sun L, Yu HS, et al. The pharmacological effects of lutein and zeaxanthin on visual disorders and cognition diseases. Molecules 2017; 22: 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renzi LM, Hammond BR. The effect of macular pigment on heterochromatic luminance contrast. Exp Eye Res 2010; 91: 896–900. [DOI] [PubMed] [Google Scholar]
- 39.Bernstein PS. The role of lutein and zeaxanthin in protection against age-related macular degeneration. Acta Hortic 2015; 1106: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renzi LM, Hammond BR Jr. The relation between the macular carotenoids, lutein and zeaxanthin, and temporal vision. Ophthalmic Physiol Opt 2010; 30: 351–357. [DOI] [PubMed] [Google Scholar]
- 41.Bellezza I. Oxidative stress in age-related macular degeneration: Nrf2 as therapeutic target. Front Pharmacol 2018; 9: 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashimoto H, Arai K, Takahashi J, Chikuda M. Effects of astaxanthin on VEGF level and antioxidation in human aqueous humor: difference by sex. J Clin Biochem Nutr 2019; 65: 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, Tan HY, Wang N, et al. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 2015; 16: 26087–26124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen JT, Kotani K. Astaxanthin as a potential protector of liver function: a review. J Clin Med Res 2016; 8: 701–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JE, Clark RM, Park Y, Lee J, Fernandez ML. Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet. Nutr Res Pract 2012; 6: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]