Abstract
Aims
This study aims to improve risk stratification for primary prevention implantable cardioverter defibrillator (ICD) implantation by developing a new mutation-specific prediction model for malignant ventricular arrhythmia (VA) in phospholamban (PLN) p.Arg14del mutation carriers. The proposed model is compared to an existing PLN risk model.
Methods and results
Data were collected from PLN p.Arg14del mutation carriers with no history of malignant VA at baseline, identified between 2009 and 2020. Malignant VA was defined as sustained VA, appropriate ICD intervention, or (aborted) sudden cardiac death. A prediction model was developed using Cox regression. The study cohort consisted of 679 PLN p.Arg14del mutation carriers, with a minority of index patients (17%) and male sex (43%), and a median age of 42 years [interquartile range (IQR) 27–55]. During a median follow-up of 4.3 years (IQR 1.7–7.4), 72 (10.6%) carriers experienced malignant VA. Significant predictors were left ventricular ejection fraction, premature ventricular contraction count/24 h, amount of negative T waves, and presence of low-voltage electrocardiogram. The multivariable model had an excellent discriminative ability {C-statistic 0.83 [95% confidence interval (CI) 0.78–0.88]}. Applying the existing PLN risk model to the complete cohort yielded a C-statistic of 0.68 (95% CI 0.61–0.75).
Conclusion
This new mutation-specific prediction model for individual VA risk in PLN p.Arg14del mutation carriers is superior to the existing PLN risk model, suggesting that risk prediction using mutation-specific phenotypic features can improve accuracy compared to a more generic approach.
Keywords: Phospholamban, Sudden cardiac death, Implantable cardioverter defibrillator, Risk stratification, Cardiomyopathy
Graphical Abstract
See page 2851 for the editorial comment on this article (doi:10.1093/eurheartj/ehab355)
Introduction
Hereditary cardiomyopathies are a leading cause of sudden cardiac death (SCD) in young adults.1 In the past years, substantial progress has been made in elucidating the genetic variants that contribute to the development of cardiomyopathy. Predominantly, alterations in genes coding for cytoskeletal, sarcomeric, desmosomal, and Z-disk proteins were found to be associated with the development of cardiomyopathy. Furthermore, variants in genes encoding calcium modulators are known to be associated with cardiomyopathies,2 including the gene encoding phospholamban (PLN), a protein essential for calcium homeostasis. Multiple pathogenic PLN variants are associated with the development of cardiomyopathy.3–8 The pathogenic variant in PLN, which has been studied most extensively, is c.40-42delAGA;p.Arg14del,9 which leads to a deletion of arginine 14 in the coding region of PLN and is associated with both dilated cardiomyopathy (DCM) and arrhythmogenic right ventricular cardiomyopathy (ARVC).10 This variant has been found in several European countries, the USA, Canada, and China11–13 and is one of the most prevalent single cardiomyopathy-related gene alterations in the world, in particular in the Netherlands.14 The p.Arg14del variant in PLN is characterized by a low-voltage electrocardiogram (ECG), repolarization abnormalities, development of cardiomyopathy, end-stage heart failure, ventricular arrhythmias (VA), and premature death. Affected individuals have a poor prognosis and high mortality from late adolescence onwards.4 , 10
Previously, two risk factors were identified that contribute to the prediction of malignant VA in p.Arg14del mutation carriers, i.e. left ventricular ejection fraction (LVEF <45%) and occurrence of sustained or non-sustained VA.10 These risk factors appeared to be more accurate than the general DCM criteria for primary prevention implantable cardioverter defibrillator (ICD) implantation.15 However, these risk factors were explored in a mixed cohort of carriers with and without sustained VA at baseline. Furthermore, the sensitivity and specificity of the identified risk factors remained limited and accurate quantification of individual risk is not possible. In addition to this PLN p.Arg14del specific risk model, an ARVC risk model for the prediction of malignant VA was recently published, designed to guide primary prevention ICD implantation in ARVC patients fulfilling the 2010 Task Force criteria.16 This model might also be valid in all p.Arg14del carriers as carriers can develop an ARVC phenotype.
This multicentre cohort study aims to improve risk stratification for primary prevention ICD implantation by developing a new prediction model for sustained VA in p.Arg14del mutation carriers without previous sustained VA. The proposed model will be compared to the existing PLN and ARVC models.
Methods
Study design
The prediction model was developed using data from a retrospective multicentre longitudinal cohort study and reported according to the TRIPOD statement.17 The study conformed to the principles of the Helsinki declaration. Design of the registry and collected variables were published earlier.18
Study population
The study cohort consists of index patients and relatives in whom the pathogenic p.Arg14del variant was identified for diagnostic purposes or cascade screening after genetic counselling in three Dutch University Hospitals and one Spanish University Hospital, between 2009 and May 2020. Genetic analysis of PLN was performed in index patients with clinical characteristics and family history or area of birth suggestive for a pathogenic PLN variant. Genetic analysis and counselling were also offered to relatives who were at risk of being p.Arg14del mutation carriers.
Data collection
All data were collected via chart review from first clinical contact to last follow-up with a cardiologist or clinical geneticist. All data were entered in electronic case report forms in compliance with good clinical practice. Follow-up was performed in both university and non-university hospitals. Baseline was defined as first-ever clinical contact and follow-up ended at the last available cardiology department visit. Data within 1 year of first clinical contact were considered baseline data.
Clinical outcomes
The primary outcome is malignant VA, defined as a composite endpoint of sustained ventricular tachycardia (VT), ventricular fibrillation (VF), appropriate ICD intervention, or (aborted) SCD. Sustained VT is defined as a VT which lasts ≥30 s, or <30 s when terminated electrically or pharmacologically. Appropriate ICD intervention is classified as ICD therapy (antitachycardia pacing or shock) for VF/VT. Aborted SCD is defined as basic life support for cardiac arrest with return of stable circulation. SCD is defined as death (with or without documented VF) within 1 h of acute symptoms or an unexplained death with no antecedent history of worsening symptoms. Carriers were censored if one of the endpoints occurred.
Predictor variables
Possible predictor variables were based on predictors for malignant VA in ARVC and DCM because p.Arg14del mutation carriers may display both ARVC or DCM phenotypes. Furthermore, possible predictors from the 2014 PLN p.Arg14del cohort study were included as possible predictors.10 All prespecified predictors and their definitions are displayed in Supplementary material online, Table S1. The final number of predictors chosen was based on the number of events, with a 10:1 ratio of events per considered predictor.
Missing data
As a result of the retrospective design, there were absence of diagnostic protocols and in some cases lack of clinical evaluation resulting in multiple variables with missing data. Missing data were considered missing at random and accounted for by multiple imputation. Imputation models for left and right ventricular ejection fraction included the qualitative assessment of ejection fraction. A total of 100 imputed datasets were generated.
Statistical analysis
SPSS Version 24.0.0.1 (IBM Corp., Armonk, NY, USA), R version 3.5.1, and RStudio Version 1.1.383 (RStudio, Boston, MA, USA) with packages rms, survminer, riskRegression, and mice were used for analysis. Continuous variables are expressed as mean ± standard deviation (SD) if normally distributed; median ± interquartile range (IQR) of 25–75% is used if the data were skewed. Categorical variables are reported as percentages (counts/total counts). Cox proportional hazards models were used to evaluate the effect of possible predictors while taking the time to event into account. To correct for family clustering, a frailty term for family id was used. Proportional-hazard assumptions were verified as well as linearity of the association for continuous predictors. The model was fitted using stepwise backward selection based on Akaike’s Information Criterion (AIC) where, in each step, candidate predictors that do not significantly lead to information loss of the model when deleted, e.g. significant difference in AIC value, were deleted. Stepwise selection was performed in each imputed dataset, where the final model consisted of variables selected in >50% of datasets. Results were pooled using Rubin’s rules. Discrimination of the model was described with Harrell’s C-statistic. Apparent C-statistic was estimated by fitting the final model on the original data. The model was internally validated using bootstrap sampling, 1000 bootstraps, to correct for overfitting. Predicted and observed outcomes were graphically evaluated with a calibration plot. Universal shrinkage factors were estimated using the calibration slope of the linear predictor. The ARVC model was applied to the complete PLN p.Arg14del cohort, regardless of phenotype, as carriers may display an ARVC (-like) phenotype and risk prediction with the ARVC model might be suitable for all PLN p.Arg14del carriers. Further details on model presentation, comparison to existing models and clinical utility methods can be found in the Supplementary material online.
Results
Study cohort
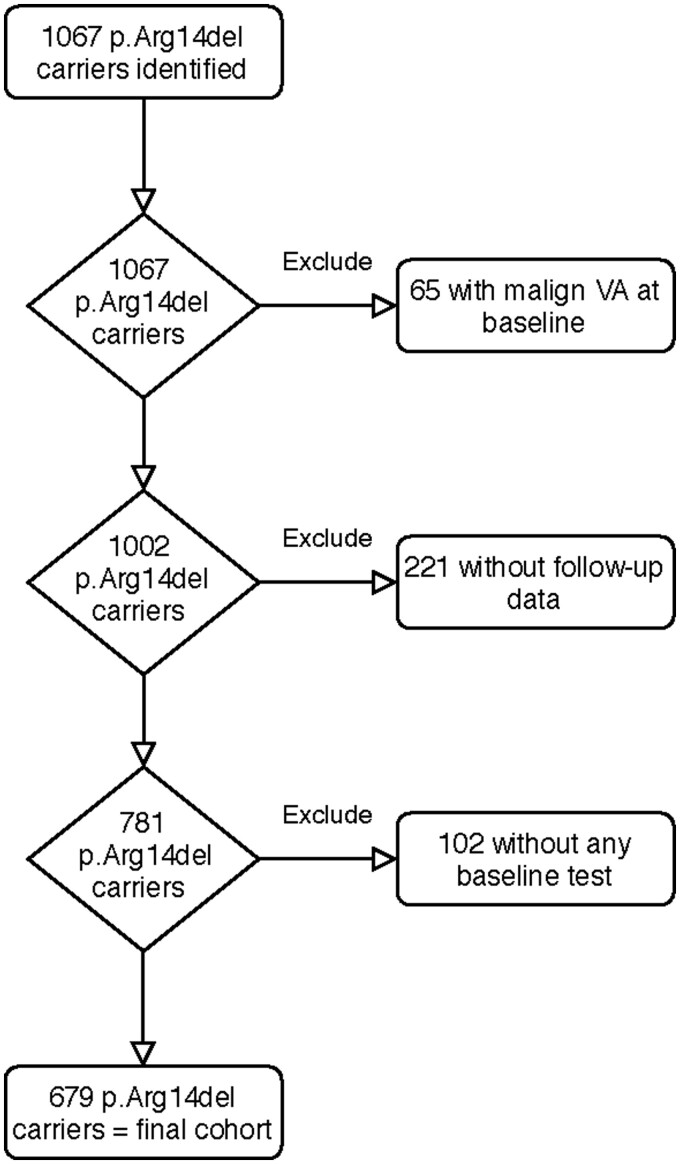
The final study cohort consists of 679 p.Arg14del mutation carriers (Figure 1). Most of the excluded carriers were only genetically counselled at participating centres without any cardiologic data available. Twenty (3%) of the 679 carriers were Spanish. The majority of the final cohort (83%) were family members of an index patient and most subjects were asymptomatic at baseline (85%). Almost half were male (43%), and the median age was 42 years (IQR 27–55). At baseline, only 10 (1%) already had an ICD, or received an ICD within 1 month. Finally, 28 (4%) p.Arg14del mutation carriers had an ARVC diagnosis by the Task Force criteria at baseline. Baseline characteristics are shown in Table 1.
Figure 1.
Flowchart of participant inclusion in the study. Diagram summarizing the inclusion and exclusion of study participants carrying the pathogenic PLN p.Arg14del variant. A baseline test should include at least an electrocardiogram, cardiac ultrasound, or magnetic resonance imaging. VA, ventricular arrhythmia.
Table 1.
Baseline characteristics
| p.Arg14del mutation carriers, n | 679 |
| Demographics | |
| Age at presentation (years) | 42 (27-55) |
| Male sex | 294 (43) |
| Caucasian ethnicity | 679 (100) |
| Proband | 113 (17) |
| History | |
| Cardiac syncope <6 months before presentation | 4 (0.6) |
| NYHA class ≥ II | 62 (9) |
| 1st degree family member with malignant VA | 91 (13) |
| Presentation reason: family screening vs. symptomatic or abnormal test result | 574 (85) |
| Fulfilment of 2010 ARVC TFC at baseline | 28 (4) |
| ECG/continuous rhythm monitoring | |
| Atrial fibrillation/flutter | 56 (8) |
| Low-voltage ECG (n = 618) | 95 (15) |
| TWI in ≥3 precordial leads (n = 559) | 77 (14) |
| TWI in ≥2 inferior leads (n = 559) | 49 (9) |
| NSVT | 67 (10) |
| 24 h PVC count >500 (n = 406) | 125 (31) |
| Imaging | |
| LVEF <45% (n = 627) | 105 (17) |
| Median LVEF (%) (n = 532) | 54 (48-58) |
| RV dysfunction (n = 608) | 62 (10) |
| Median RVEF (%) (n = 254) | 50 (45-55) |
| LGE on MRI (n = 262) | 77 (29) |
Values are given as n (%) or median (interquartile range).
ECG, electrocardiogram; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; NSVT, non-sustained ventricular tachycardia; NYHA, New York Heart Association; RV, right ventricular; RVEF, right ventricular ejection fraction; PVC, premature ventricular contraction; TFC, Task Force criteria; TWI, T-wave inversion; VA, ventricular arrhythmia.
Outcomes
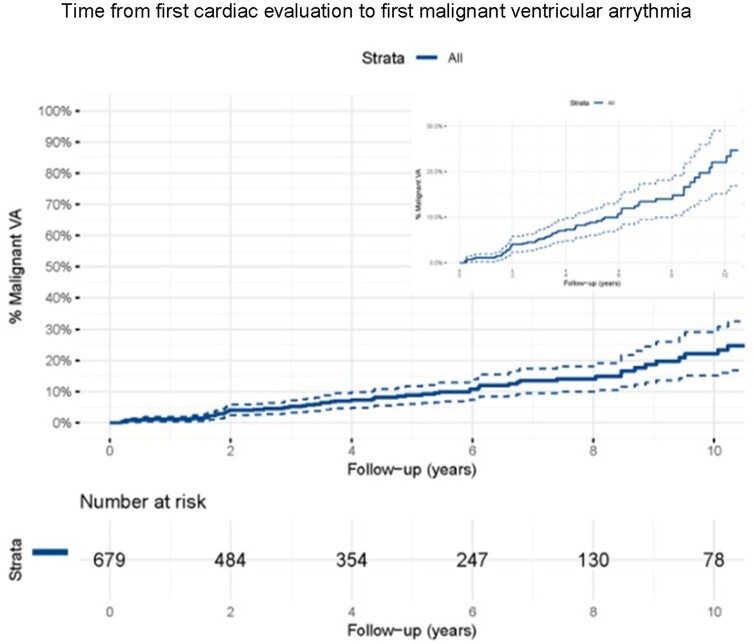
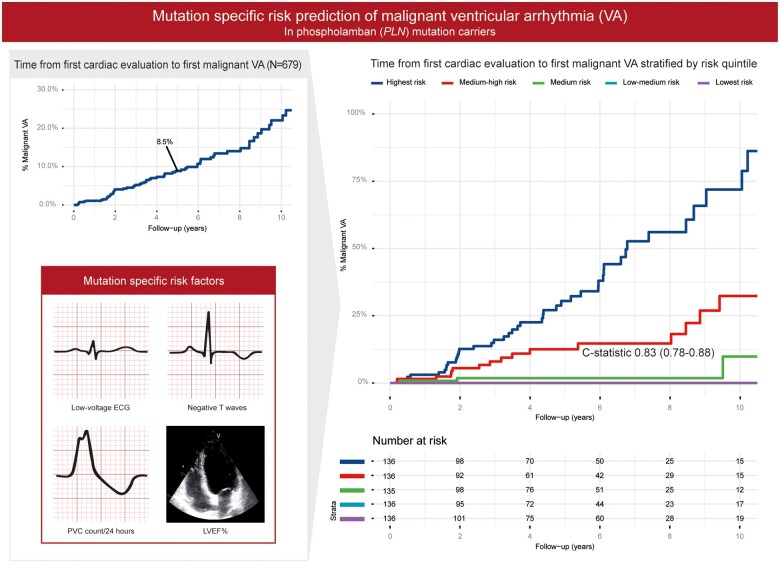
During a median follow-up of 4.3 years (IQR 1.7–7.4), 72 (10.6%) p.Arg14del mutation carriers experienced the composite endpoint of malignant VA, with a crude event rate of 2.0% per year. The Kaplan–Meier estimate of 5-year event rate was 8.5% [95% confidence interval (CI) 6–11%], with events occurring throughout follow-up (Figure 2).
Figure 2.
Survival analyses. Kaplan–Meier analysis with percentage of carriers with malignant ventricular arrhythmia (y-axis) and follow-up duration in years (x-axis). Upper right side is the Kaplan–Meier curve where the y-axis is zoomed in from 0-30%. VA, ventricular arrhythmia.
The primary composite outcome of malignant VA consisted of 37 appropriate ICD therapies, 25 sustained VT/VF, 6 SCD, 3 aborted SCD, and 1 VT storm. Arrhythmia characteristics were available in 51/72 (71%), of which 48/51 (94%) were sustained VT >190 b.p.m., VF, or (aborted) SCD. Arrhythmia characteristics are reported in Supplementary material online, Table S2. During follow-up, 148 patients received an ICD for primary or secondary prevention. At the end of follow-up, 44 (6%) p.Arg14del mutation carriers had died, 37 of a cardiac cause and 7 of a non-cardiac cause. Of the cardiac deaths, 25 were due to heart failure, 10 were due to SCD, and 2 were due to miscellaneous causes (after valve replacement procedure and left ventricular assist device failure). Characteristics of the patients with SCD are given in Supplementary material online, Table S3. Heart transplantation was performed in 17 carriers, and 12 received a left ventricular assist device.
Model development
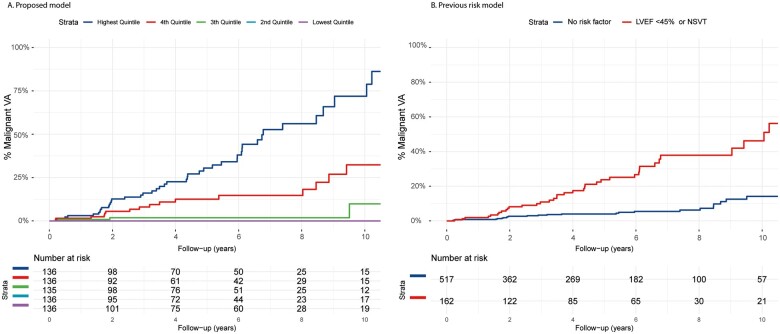
Each predictor was tested for linearity with the martingale residuals (diagnostic plots depicted in Supplementary material online, Figure S1). For 24-h premature ventricular contraction (PVC) count, the log linear conversion performed the best. T-wave inversion was used as a continuous variable, where the amount of precordial and inferior leads with T-wave inversion was counted. With 72 events, 7 candidate predictors were considered for the multivariable model, chosen from different diagnostic domains. Univariable hazard ratios of these pre-specified predictors and multivariable hazard ratios of the predictors selected in the final model are reported in Table 2. Univariable hazard ratios of all predictors are shown in Supplementary material online, Table S4. New York Heart Association class and right ventricular dysfunction are significant univariable predictors but are not selected for the multivariable model as their exclusion from the model does not significantly decrease the performance of the model. Final selected predictors are number of negative T waves, presence of low-voltage ECG, PVC count/24 h, and LVEF. The risk formula of the final model can be found in the Supplementary material online. With the final multivariable model, we calculated the predicted 5-year malignant VA risk of each carrier in the dataset. After dividing the population into quintiles of predicted risk, a clear distinction in risk between the risk groups is shown in Figure 3 with the lowest risk quintiles having a very low malignant VA rate.
Table 2.
Univariable hazard ratios of pre-specified predictors and multivariable hazard ratios of the predictors in the final model after backwards stepwise selection
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| NYHA class ≥II | 4.0 | 2.4-6.8 | <0.001 | – | – | – |
| Negative T waves inferior or precordial (per 1 increase) | 1.2 | 1-1.3 | 0.007 | 1.1 | 0.98-1.2 | 0.11 |
| Low-voltage ECG | 3.9 | 2.3-6.6 | <0.001 | 1.8 | 1.0-3.4 | 0.043 |
| Prior NSVT | 1.5 | 0.77-2.8 | 0.25 | – | – | |
| 24 h PVC count (per 1 log increase) | 1.5 | 1.3-1.7 | <0.001 | 1.4 | 1.2-1.6 | <0.001 |
| LVEF (per 1% decrease) | 1.08 | 1.05-1.09 | <0.001 | 1.04 | 1.01-1.06 | <0.001 |
| RV dysfunction | 4.9 | 2.6-9 | <0.001 | – | – | – |
Hazard ratios were corrected for family clustering using a frailty model.
CI, confidence interval; ECG, electrocardiogram; HR, hazard ratio; LVEF, left ventricular ejection fraction; NSVT, non-sustained ventricular tachycardia; NYHA, New York Heart Association; RV, right ventricular; PVC, premature ventricular contraction.
Figure 3.
Estimates for the cumulative risk of malignant ventricular arrhythmia in the current PLN p.Arg14del cohort (n = 679) stratified by predicted risk quintile compared to the existing phospholamban risk model. Cumulative incidence of malignant ventricular arrhythmia (y-axis) during follow-up (x-axis) stratified by predicted risk quintile with the proposed model (A) or by risk factor (B). LVEF, left ventricular ejection fraction; NSVT, non-sustained ventricular tachycardia.
Model validation
Internal validation with bootstrapping resulted in an optimism corrected C-statistic of the final model of 0.83 (95% CI 0.78–0.88), corresponding to a strong model. Visualization of calibration is represented in Supplementary material online, Figure S2. Agreement between predicted and observed 5-year malignant VA risk was excellent. With bootstrapping we calculated a calibration slope of 0.913.
Performance of existing risk models
Applying the previous PLN risk model in the current cohort yielded a C-statistic of 0.68 (95% CI 0.61–0.75). C-statistic of the proposed model was significantly higher (delta 0.11, P = 0.002). Applying the ARVC risk model to the complete current cohort of p.Arg14del mutation carriers regardless of ARVC diagnosis yielded a C-statistic of 0.74 (95% CI 0.68–0.80). C-statistic of the proposed model was significantly higher (delta 0.07, P = 0.006). Although more patients had right and left ventricular involvement, only a fraction of carriers had a Task Force-compatible ARVC diagnosis at baseline, and therefore, these results are probably not surprising; further results on the comparison with the ARVC model are provided in Supplementary material online, Figures S3 and S4. Calibration is shown in Supplementary material online, Figure S2, and high- and low-risk groups can be distinguished; however, calibration was not ideal with comparable observed outcomes in the medium-high- and high-risk quintiles.
Clinical utility
We explored scenarios where ICD implantation is based on the outcome of the prediction models. Figure 4 shows carriers with a predicted risk greater than the risk threshold that would be implanted with an ICD. As visualized, the proposed model leads to less false positives (ICD, no VA) or false negatives (No ICD, VA) compared to the existing PLN model. For example, by applying the proposed model to the study cohort (n = 679) with a risk threshold of >10% would result in 161 carriers receiving an ICD, with 42 having a malignant VA compared to 162 carriers with ICD, with 37 having a malignant VA with the existing model. Additionally, we compared diagnostic performance (e.g. sensitivity/specificity) of the risk models and potential cut-offs in Supplementary material online, Table S5. Not surprisingly, diagnostic performance was heavily dependent on the chosen risk threshold. Sensitivity and specificity of the previous PLN model was 66.1% and 80%, respectively. Sensitivity and specificity of the proposed model ranged from 58.9% to 98.2% and 53.9% to 88.1%, depending on the risk threshold.
Figure 4.
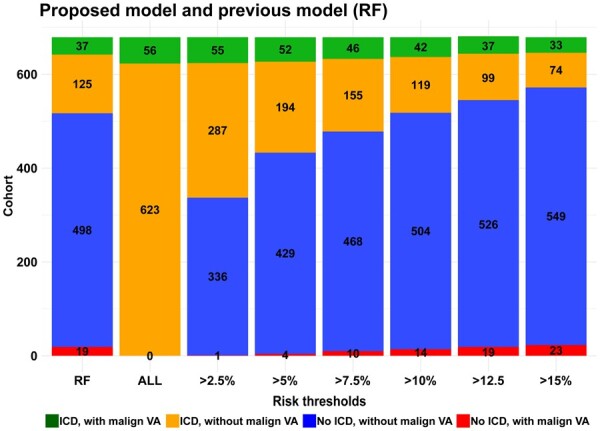
Risk threshold comparisons for the proposed model and the existing phospholamban risk model in 679 patients. The bars represent the clinical implication of using different 5-year malignant ventricular arrhythmia risk thresholds with the proposed model, where the risk threshold is on the x-axis. The existing phospholamban risk model based on two risk factors is named RF. Each bar represents the complete cohort, where the different colours represent the proportion of carriers experiencing malignant ventricular arrhythmia as well as the placement or non-placement of an implantable cardioverter defibrillator.
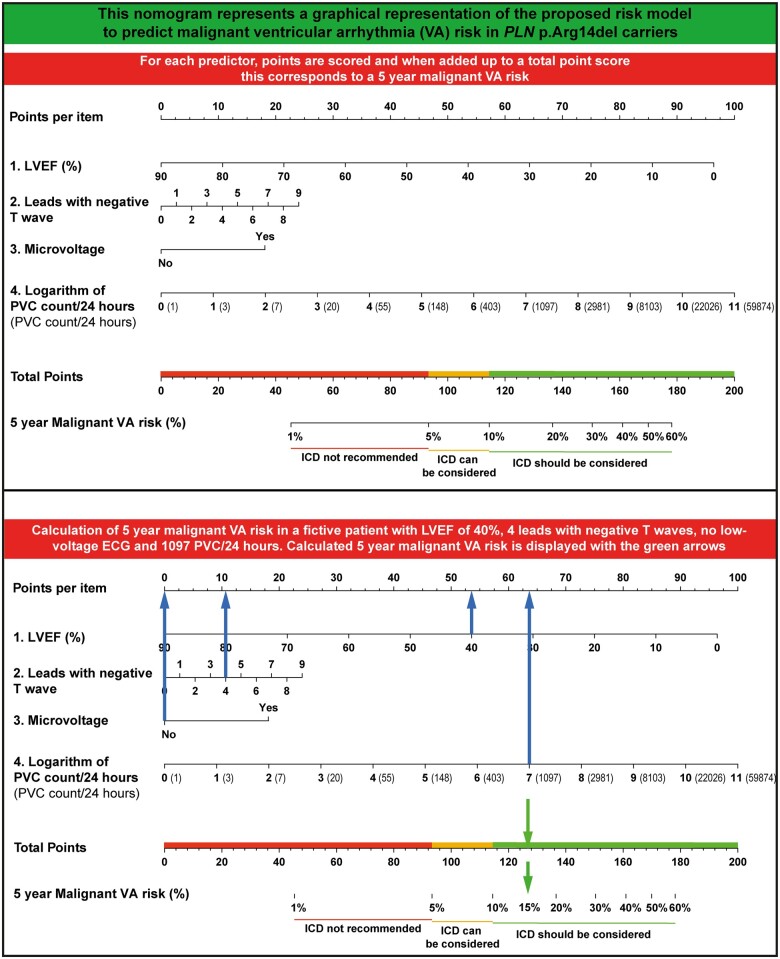
With the proposed risk model, several risk thresholds seem viable and factors other than malignant VA risk should be considered when deciding on a threshold for an individual patient. However, if one would adhere to the generally accepted risk threshold >5% in 5 years (i.e. 1%/year) to justify implantation of an ICD,19 the proposed model would result in 246 carriers being treated with an ICD, with 52 of them experiencing a malignant VA. Even more relevant, this risk threshold would result in 4 false negatives, while 433 ICD implantations are avoided compared to treating all carriers. Individual risk prediction can be made with an online calculator available at https://plnriskcalculator.shinyapps.io/final_shiny/ or nomogram (Figure 5).
Figure 5.
Nomogram to predict 5-year malignant ventricular arrhythmia risk.
Discussion
Main findings
While the event rate of incident malignant VA is significant in individuals with the PLN p.Arg14del variant, over 90% remain event-free at 5 years. Therefore, risk stratification is needed before ICD implantation to avoid unnecessary ICD implantations while protecting the majority of carriers from SCD (Graphical abstract).
Malignant VA risk prediction in PLN p.Arg14del carriers. With the mutation specific risk factors low and high risk groups can be identified. PLN, Phospholamban; VA, Ventricular arrhythmia.
In this study, we developed and internally validated a new prediction model for malignant VA in PLN p.Arg14del mutation carriers without prior malignant VA. Discriminative ability was excellent and calibration was adequate with agreement between predicted and observed risk (Supplementary material online, Figure S2). Using the proposed model is superior to using the existing models with a significantly higher discriminative ability (C-statistic) and less false positives or false negatives demonstrated in the scenario analyses. This suggests that a mutation-specific prediction model improves performance for this mixed phenotype population of PLN p.Arg14del mutation carriers, compared to applying phenotypic driven models for DCM and ARVC to all PLN p.Arg14del mutation carriers. For DCM, no direct comparison was made as the existing PLN p.Arg14del model has LVEF <45% as risk factor; thus, the comparison with the DCM model with LVEF <35% as risk factor was deemed redundant. The proposed model has more selected predictors than the previous PLN model. Adding PVC count, amount of negative T waves, and presence of low-voltage ECG as predictors can account for the increased model performance with the proposed model. The greatest clinical utility of this model lies in the individual estimations of 5-year risk derived with the nomogram or online calculator. While the acceptable risk threshold for an individual patient is also dependent on other factors, such as the country’s health system, shared decision-making, and non-SCD mortality risk, we suggest that a 5-year malignant VA risk of >5% should be the minimum to consider ICD therapy. Above this risk threshold, ICD implantation can be considered, with stronger recommendation with increasing predicted risks. Moreover, with a lower risk threshold, ICD harm probably outweighs ICD benefit given the fact that previous studies in inherited cardiomyopathies have shown a significant burden of ICD-related complications (27%) or inappropriate shocks (14%) in ICD-treated patients.20
Prior studies
Event rate of malignant VA was lower in this study compared to the 2014 p.Arg14del cohort of the previous model (crude annual rate 2% vs. 5.4%).10 We hypothesize ascertainment bias playing a role, with increasing inclusion of asymptomatic family members, 85% in this study vs. 58% in the previous report.21 In addition, we excluded carriers with malignant VA at baseline who are more likely to develop new malignant VA. Malignant VA rate was also lower than the ARVC risk model cohort (2.0% vs. 5.6% annual event rate).22 This was expected as included patients in the ARVC cohort were generally more severely affected because they had to fulfil the strict 2010 Task Force criteria.23 Risk stratification models have proven to be useful clinical tools for arrhythmic risk prediction in hypertrophic cardiomyopathy (HCM) and ARVC.16 , 24 Discriminative ability of the proposed model was at least similar to these tools, with a C-statistic of 0.83 vs. 0.70 and 0.77 of the HCM and ARVC models, respectively. Earlier research demonstrated gene and mutation-specific differences in disease manifestation and age-related penetrance.25 We hypothesize that gene- or maybe even mutation-specific risk stratification, whenever possible, is a potential superior approach.
Limitations and future directions
The prediction model is not suited for patients <16 years old because of the natural evolution of T waves during childhood. In addition, using our mutation-specific approach, it is uncertain that this risk model is suitable for other PLN variants as they might develop different phenotypes. Due to the retrospective design, not all patients were evaluated or treated based on a standardized protocol. In some patients, first contact with a cardiologist preceded the genetic diagnosis. Currently, all newly identified carriers will undergo extensive testing but in the early days, in some patients even before the discovery of PLN p.Arg14del as a pathogenic mutation, not all tests would have been considered as we do today, resulting in missing data. For instance, the amount of data on the presence of late gadolinium enhancement (LGE) was insufficient to consider it as a predictor for the main analysis. In a sub-analysis, presence of LGE was a univariable significant predictor of malignant VA (hazard ratio 9.6, 95% CI 3.1–29.0, P < 0.001). However, LGE was not selected for the multivariable model as leaving LGE out of the model did not decrease model performance significantly, likely because of significant overlap of predictive ability with other predictors such as low-voltage ECG and LVEF. In a future iteration of the prediction model, LGE can be further evaluated and new predictors might be included, such as strain imaging.26 We compared clinical characteristics of carriers with complete data vs. carriers with incomplete data to assess the impact of missing data on results. Clinical characteristics of the group with missing data were mostly similar to those of the group without missing data (Supplementary material online, Table S6). The only characteristic significantly different was the amount of negative T waves, which was higher in the group with incomplete data [1 (IQR 0–2) vs. 0 (IQR 0–1), P < 0.001].
True SCD risk is obscured by ICD implantation in the high-risk (deemed by the treating physician) group of the study cohort. However, removing ICD recipients from the cohort would not be a valid alternative. Firstly, this would lead to insufficient power, but more importantly would lead to new biases. Removing high-risk carriers from this cohort will lead to selection bias and underestimate true SCD risk. Similar issues pertain to other observational datasets where ICD implantation is performed, for example HCM or ARVC.16 , 24 For PLN mutations carriers a randomized controlled trial is not feasible. This is why we considered the composite endpoint of malignant VA as a surrogate marker for SCD. While being a serious event, not all carriers experiencing malignant VA would suffer from SCD. This should be kept in mind when choosing the risk threshold. In order to assess robustness of the combined endpoint, we analysed whether predictor effects of ICD therapy were similar to predictors for the other malignant VA endpoints (Supplementary material online, Table S7). Similarly, we analysed if predictor effects were different for life-threatening VA [defined as VT >240 b.p.m., VF, or (aborted) SCD] compared to the other VA (Supplementary material online, Table S8). Univariable hazard ratios of selected predictors were mostly similar for appropriate ICD therapy vs. other VA, and life-threatening VA vs. other VA. Thus, VA risk prediction with this model is valid but the predicted risk of the model reflects all malignant VA and not only life-threatening VA or SCD. Nearly all carriers who experienced SCD had a significant decrease in LVEF, which can be explained by it being a major risk factor for malignant VA. However, this may also be an indication that malignant VA are more lethal in carriers with a reduced ejection fraction.
We compared the proposed model to the existing PLN risk model for phenotypes of DCM (not directly) and ARVC. Importantly, these models were developed in patients with a DCM or ARVC phenotype and were not designed for patients with a DCM or ARVC causing genotype without phenotype. This is one of the reasons why this study cannot be seen as an external validation of the DCM and ARVC models, but rather tested if the model might work for all p.Arg14del mutation carriers, regardless of phenotype. Comparison of the models with only DCM or ARVC positive patients was not possible, as the power was insufficient for these analyses. Finally, external validation of the prediction model is desirable; however, it would currently be challenging to find a large enough cohort.
Conclusion
Based on the largest cohort of PLN p.Arg14del mutation carriers, we present a new mutation-specific model for the prediction of individual malignant VA risk. The proposed model is superior to the existing PLN risk model and will inform decision-making for primary prevention ICD implantation. Furthermore, this is a demonstration that risk prediction using mutation-specific phenotypic features can improve accuracy compared to a more generic approach.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We would like to thank Alba Garcia Garcia and David Fernández Vazquez for their contribution to this manuscript. We would like to thank Joris van Bentum for assistance with the figures. Folkert W. Asselbergs is supported by UCL Hospitals NIHR Biomedical Research Centre.
Funding
This work was supported by the PLN Foundation (www.plnheart.org) and the Netherlands Cardiovascular Research Initiative, an initiative with support of the Dutch Heart Foundation (2015-12 eDETECT; 2018-30 PREDICT2) and a Fondation Leducq Transatlantic Network of Excellence (Cure-PLaN).
Data availability
The data underlying this article cannot be shared due to the privacy of individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
Conflict of interest: J.P.v.T. reports grants from CVON-Netherlands Heart Foundation and grants from Leducq Foundation, during the conduct of the study. T.E.V. reports grants from PLN foundation and grants from CVON (Dutch Heart Foundation; Predict 2), during the conduct of the study. A.A.M.W. reports grants from CVON (Dutch Heart Foundation; Predict 2) and grants from PLN Foundation, during the conduct of the study. R.A.d.B. reports grants from Abbott, AstraZeneca, Bristol-Myers Squibb, NovoNordisk, and Roche and personal fees from Abbott, AstraZeneca, Bayer, Novartis, and Roche, outside the submitted work. The other authors have nothing to disclose.
Contributor Information
Tom E Verstraelen, Heart Center, Department of Cardiology, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, Netherlands.
Freyja H M van Lint, Department of Clinical Genetics, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, Netherlands; Department of Genetics, University Medical Center Utrecht, Utrecht University, Heidelberglaan 100, 3584 CX, Utrecht, Netherlands.
Laurens P Bosman, University Medical Center Utrecht, Division Heart and Lungs, Department of Cardiology, University of Utrecht, Heidelberglaan 100, 3584 CX, Utrecht, Netherlands.
Remco de Brouwer, University Medical Center Groningen, Department of Cardiology, University of Groningen, Hanzeplein 1, 9713 GZ, Groningen, Netherlands.
Virginnio M Proost, Heart Center, Department of Cardiology, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, Netherlands.
Bob G S Abeln, Heart Center, Department of Cardiology, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, Netherlands.
Karim Taha, University Medical Center Utrecht, Division Heart and Lungs, Department of Cardiology, University of Utrecht, Heidelberglaan 100, 3584 CX, Utrecht, Netherlands.
Aeilko H Zwinderman, Department of Clinical Epidemiology and Biostatistics, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, Netherlands.
Cathelijne Dickhoff, Department of Cardiology, Dijklander Ziekenhuis Hoorn, Maelsonstraat 3, 1624 NP, Hoorn, Netherlands.
Toon Oomen, Department of Cardiology, Antonius Ziekenhuis Sneek, Bolswarderbaan 1, 8601 ZK Sneek, Netherlands.
Bas A Schoonderwoerd, Medical Center Leeuwarden, Department of Cardiology, Henri Dunantweg 2, 8934 AD, Leeuwarden, Netherlands.
Gerardus P Kimman, Department of Cardiology, Noordwest Ziekenhuisgroep, Wilhelminalaan 12, 1815 JD, Alkmaar, Netherlands.
Arjan C Houweling, Department of Clinical Genetics, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, Netherlands.
Juan R Gimeno-Blanes, Department of Cardiology, Virgen de Arrixaca Hospital, Ctra,Murcia-Cartagena, s/n, 30120 El Palmar, Murcia, Spain; European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN GUARDHEART).
Folkert W Asselbergs, University Medical Center Utrecht, Division Heart and Lungs, Department of Cardiology, University of Utrecht, Heidelberglaan 100, 3584 CX, Utrecht, Netherlands; Institute of Cardiovascular Science and Institute of Health Informatics, Faculty of Population Health Sciences, University College London, Gower St, London WC1E 6BT, UK.
Paul A van der Zwaag, University Medical Center Groningen, Department of Clinical Genetics, University of Groningen, Hanzeplein 1, 9713 GZ, Groningen, Netherlands.
Rudolf A de Boer, University Medical Center Groningen, Department of Cardiology, University of Groningen, Hanzeplein 1, 9713 GZ, Groningen, Netherlands.
Maarten P van den Berg, University Medical Center Groningen, Department of Cardiology, University of Groningen, Hanzeplein 1, 9713 GZ, Groningen, Netherlands.
J Peter van Tintelen, Department of Clinical Genetics, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, Netherlands; Department of Genetics, University Medical Center Utrecht, Utrecht University, Heidelberglaan 100, 3584 CX, Utrecht, Netherlands.
Arthur A M Wilde, Heart Center, Department of Cardiology, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, Netherlands; European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN GUARDHEART).
References
- 1. Ackerman M, Atkins DL, Triedman JK. Sudden cardiac death in the young. Circulation 2016;133:1006–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Towbin JA. Inherited cardiomyopathies. Circ J 2014;78:2347–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, Fan GC, Tsiapras D, Hahn HS, Adamopoulos S, Liggett SB, Dorn GW 2nd, MacLennan DH, Kremastinos DT, Kranias EG. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest 2003;111:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RA, Fan GC, Tsiapras D, Parekh RR, Dorn GW 2nd, MacLennan DH, Kremastinos DT, Kranias EG. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci U S A 2006;103:1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medeiros A, Biagi DG, Sobreira TJ, de Oliveira PS, Negrao CE, Mansur AJ, Krieger JE, Brum PC, Pereira AC. Mutations in the human phospholamban gene in patients with heart failure. Am Heart J 2011;162:1088–1095.e1. [DOI] [PubMed] [Google Scholar]
- 6. Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 2003;299:1410–1413. [DOI] [PubMed] [Google Scholar]
- 7. Truszkowska GT, Bilinska ZT, Kosinska J, Sleszycka J, Rydzanicz M, Sobieszczanska-Malek M, Franaszczyk M, Bilinska M, Stawinski P, Michalak E, Malek LA, Chmielewski P, Foss-Nieradko B, Machnicki MM, Stoklosa T, Poninska J, Szumowski L, Grzybowski J, Piwonski J, Drygas W, Zielinski T, Ploski R. A study in Polish patients with cardiomyopathy emphasizes pathogenicity of phospholamban (PLN) mutations at amino acid position 9 and low penetrance of heterozygous null PLN mutations. BMC Med Genet 2015;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu GS, Morales A, Vafiadaki E, Lam CK, Cai WF, Haghighi K, Adly G, Hershberger RE, Kranias EG. A novel human R25C-phospholamban mutation is associated with super-inhibition of calcium cycling and ventricular arrhythmia. Cardiovasc Res 2015;107:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, Committee ALQA. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Rijsingen IA, van der Zwaag PA, Groeneweg JA, Nannenberg EA, Jongbloed JD, Zwinderman AH, Pinto YM, Dit Deprez RH, Post JG, Tan HL, de Boer RA, Hauer RN, Christiaans I, van den Berg MP, van Tintelen JP, Wilde AA. Outcome in phospholamban R14del carriers: results of a large multicentre cohort study. Circ Cardiovasc Genet 2014;7:455–465. [DOI] [PubMed] [Google Scholar]
- 11. Lopez-Ayala JM, Boven L, van den Wijngaard A, Penafiel-Verdu P, van Tintelen JP, Gimeno JR. Phospholamban p.arg14del mutation in a Spanish family with arrhythmogenic cardiomyopathy: evidence for a European founder mutation. Rev Esp Cardiol (Engl Ed) 2015;68:346–349. [DOI] [PubMed] [Google Scholar]
- 12. Posch MG, Perrot A, Geier C, Boldt LH, Schmidt G, Lehmkuhl HB, Hetzer R, Dietz R, Gutberlet M, Haverkamp W, Ozcelik C. Genetic deletion of arginine 14 in phospholamban causes dilated cardiomyopathy with attenuated electrocardiographic R amplitudes. Heart Rhythm 2009;6:480–486. [DOI] [PubMed] [Google Scholar]
- 13. Cheung CC, Healey JS, Hamilton R, Spears D, Gollob MH, Mellor G, Steinberg C, Sanatani S, Laksman ZW, Krahn AD. Phospholamban cardiomyopathy: a Canadian perspective on a unique population. Neth Heart J 2019;27:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Zwaag PA, van Rijsingen IA, Asimaki A, Jongbloed JD, van Veldhuisen DJ, Wiesfeld AC, Cox MG, van Lochem LT, de Boer RA, Hofstra RM, Christiaans I, van Spaendonck-Zwarts KY, Lekanne dit Deprez RH, Judge DP, Calkins H, Suurmeijer AJ, Hauer RN, Saffitz JE, Wilde AA, van den Berg MP, van Tintelen JP. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail 2012;14:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck K-H, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ. 2015 European Society of Cardiology Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death summarized by co-chairs. Eur Heart J 2015;36:2793– 2799. [DOI] [PubMed] [Google Scholar]
- 16. Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A, Bourfiss M, Fortier A, Lie OH, Saguner AM, Svensson A, Andorin A, Tichnell C, Murray B, Zeppenfeld K, van den Berg MP, Asselbergs FW, Wilde AAM, Krahn AD, Talajic M, Rivard L, Chelko S, Zimmerman SL, Kamel IR, Crosson JE, Judge DP, Yap SC, van der Heijden JF, Tandri H, Jongbloed JDH, Guertin MC, van Tintelen JP, Platonov PG, Duru F, Haugaa KH, Khairy P, Hauer RNW, Calkins H, Te Riele A, James CA. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2019;40:1850–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 2015;162:55–63. [DOI] [PubMed] [Google Scholar]
- 18. Bosman LP, Verstraelen TE, van Lint FHM, Cox M, Groeneweg JA, Mast TP, van der Zwaag PA, Volders PGA, Evertz R, Wong L, de Groot NMS, Zeppenfeld K, van der Heijden JF, van den Berg MP, Wilde AAM, Asselbergs FW, Hauer RNW, Te Riele A, van Tintelen JP; Netherlands ACM Registry. The Netherlands Arrhythmogenic Cardiomyopathy Registry: design and status update. Neth Heart J 2019;27:480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 20. Olde Nordkamp LR, Postema PG, Knops RE, van Dijk N, Limpens J, Wilde AA, de Groot JR. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm 2016;13:443–454. [DOI] [PubMed] [Google Scholar]
- 21. Nannenberg EA, van Rijsingen IAW, van der Zwaag PA, van den Berg MP, van Tintelen JP, Tanck MWT, Ackerman MJ, Wilde AAM, Christiaans I. Effect of ascertainment bias on estimates of patient mortality in inherited cardiac diseases. Circ Genom Precis Med 2018;11:e001797. [DOI] [PubMed] [Google Scholar]
- 22. Bosman LP, Sammani A, James CA, Cadrin-Tourigny J, Calkins H, van Tintelen JP, Hauer RNW, Asselbergs FW, Te Riele A. Predicting arrhythmic risk in arrhythmogenic right ventricular cardiomyopathy: a systematic review and meta-analysis. Heart Rhythm 2018;15:1097–1107. [DOI] [PubMed] [Google Scholar]
- 23. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J 2010;31:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, McKenna WJ, Omar RZ, Elliott PM; Hypertrophic Cardiomyopathy Outcomes I. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 2014;35:2010–2020. [DOI] [PubMed] [Google Scholar]
- 25. Nannenberg EA, Sijbrands EJ, Dijksman LM, Alders M, van Tintelen JP, Birnie M, van Langen IM, Wilde AA. Mortality of inherited arrhythmia syndromes: insight into their natural history. Circ Cardiovasc Genet 2012;5:183–189. [DOI] [PubMed] [Google Scholar]
- 26. Te Rijdt WP, Ten Sande JN, Gorter TM, van der Zwaag PA, van Rijsingen IA, Boekholdt SM, van Tintelen JP, van Haelst PL, Planken RN, de Boer RA, Suurmeijer AJH, van Veldhuisen DJ, Wilde AAM, Willems TP, van Dessel P, van den Berg MP. Myocardial fibrosis as an early feature in phospholamban p.Arg14del mutation carriers: phenotypic insights from cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 2019;20:92–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared due to the privacy of individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
Conflict of interest: J.P.v.T. reports grants from CVON-Netherlands Heart Foundation and grants from Leducq Foundation, during the conduct of the study. T.E.V. reports grants from PLN foundation and grants from CVON (Dutch Heart Foundation; Predict 2), during the conduct of the study. A.A.M.W. reports grants from CVON (Dutch Heart Foundation; Predict 2) and grants from PLN Foundation, during the conduct of the study. R.A.d.B. reports grants from Abbott, AstraZeneca, Bristol-Myers Squibb, NovoNordisk, and Roche and personal fees from Abbott, AstraZeneca, Bayer, Novartis, and Roche, outside the submitted work. The other authors have nothing to disclose.