Abstract
BACKGROUND:
PERTs are a new, multidisciplinary approach to PE care. They were conceived to efficiently identify and risk stratify PE patients and standardize care delivery. More research needs to be conducted to assess the effects that PERTs have had on PE care. This study sought to determine the effects of a PERT on quality and overall value of care.
METHODS:
This was a retrospective study of all patients 18 years of age or older who presented with a principal diagnosis of an acute PE based on available ICD codes from January 1, 2010 to December 31, 2018. Patients who did not have an imaging study, i.e., CTPA or ECHO, available were excluded. Patients were divided into Pre- (before October 2015) and Post-PERT eras (after October 2015) and stratified based on the presence of right heart strain/dysfunction on imaging. All quality outcomes were extracted from the EMR, and cost outcomes were provided by the financial department.
RESULTS:
530 individuals (226 Pre-PERT and 304 Post-PERT) were identified for analysis. Quality outcomes improved between the eras; most notably in-hospital mortality decreased (16.5 vs. 9.6) and hospital LOS decreased (7.7 vs. 4.4) (p<0.05). Total cost of care also decreased a statistically significant amount between the eras.
CONCLUSIONS:
The implementation of a PERT improved quality and cost of care, resulting in improved value. We hypothesize that this may be due to more timely identification and risk stratification leading to earlier interventions and streamlined decision making, but further research is required to validate these findings in larger cohorts.
Keywords: PE, PERT, Value, Risk Stratification, Cost Analysis
Introduction
Over 600,000 people in the United States experience a pulmonary embolism (PE) annually, with a 10–15% in-hospital mortality. It is most often the result of a thrombus in the deep veins of the legs (DVT) that embolizes to the pulmonary vasculature [1–3]. Patients experiencing a PE can present with a range of features that have variable risk of developing hemodynamic compromise or death. Historically, the management of these patients has been the responsibility of individual clinicians, aided by clinical guidelines and scoring systems [4]. The mainstay of treatment is anticoagulation, and systemic thrombolytic therapy or surgical embolectomy a consideration for high-risk patients. More recently, catheter-directed embolectomy and thrombolysis have emerged as potential treatment modalities for intermediate- and high-risk patients [5].
With the expansion of therapeutic options and lack of evidence from randomized clinical trials [6], it has become more difficult to select the most appropriate therapy. In 2008, the US Surgeon General issued a call to arms to more effectively prevent and treat DVTs and PEs [7]. In this context, the Pulmonary Embolism Response Teams (PERTs) were conceived to provide rapid multidisciplinary assessment and treatment of PE patients and first launched at the Massachusetts General Hospital (MGH) in 2012 [8]. As more institutions implemented PERTs, a national PERT Consortium emerged. [9] The creation of multidisciplinary PERTs has aided clinicians in quickly and efficiently risk stratifying patients presenting with the signs and symptoms of an acute PE and expedited the initiation of the most effective and specific treatment options [10].
In addition to the improved quality achieved from the rapid identification and risk stratification of PE patients and the focused initiation of appropriate therapies, the reduction in unnecessary physician practice variation, theoretically, should also decrease the cost of care delivery, as it has in other conditions [11]. In the era of value-based care, healthcare systems and providers are held to not only the quality of care delivered, but also to the level of resource utilization required to deliver that care. Improving outcomes or decreasing cost both improve value, but the impact of a PERT on value has yet to be experimentally assessed.
In this study, we assess the differences in major quality outcomes (i.e., length of stay, days on mechanical ventilation, in-hospital mortality, recurrent admissions, etc.) between the Pre-PERT and Post-PERT eras at the University of Kentucky. We also investigate whether there is a difference in the cost of care by instituting an evidence-based PERT protocol as well as employing rapid triage and potentially safer therapeutic strategies.
Methods
Patient Selection
This study was granted approval by the local institutional review board at the University of Kentucky through the Office of Research Integrity (protocol number: 49126). This was a retrospective study at a single, quaternary academic referral center of all patients 18 years of age or older who presented with a principal or primary diagnosis of an acute pulmonary embolism based on available ICD-9 and ICD-10 codes [12] within the electronic medical records system from January 1, 2010 to December 31, 2018. The PERT protocol was launched in October 2015 and included standardized reporting of right heart strain on CT pulmonary angiogram (CTPA) and a single-call activation of the PERT team for any patient with evidence of RV strain by imaging or cardiac biomarkers. For this analysis, patients were excluded if they did not have a CTPA or if they did not an have an evaluation of the right ventricle either by CT or echocardiogram. We chose to include only those patients with an assessment of the right heart as the PERT protocol was heavily influenced by right heart function. When a CTPA was available, the reported RV/LV ratio was used to make the determination of right heart strain. In the absence of a CTPA, the mild, moderate, or severe RV dilation on the echocardiogram was used. This designation on echocardiogram was used due to good correlation in previous studies between RV size on echocardiogram and RV/LV ratio on CT [13].
The patients were subdivided into two distinct eras: Pre-PERT, which spanned from January 2010 to September 2015, and Post-PERT, which spanned from October 2015 to December 2018). Within each group, sub-classification based on presence or absence of right heart strain was done.
Clinical variables
In addition to baseline demographics of age, gender, and reported ethnicity, patient co-morbidities as well as treatment modalities were documented. Pharmacy databases, catheterization laboratory records, billing data, and the extracorporeal life support organization (ELSO) database were accessed to identify therapeutic interventions administered. Individual charts were reviewed for all patients to ensure accuracy of these data sources.
Outcomes
Outcomes were divided broadly into quality and cost measures with both primary and secondary outcomes within each category. The primary quality outcome was in-hospital mortality. Secondary quality outcomes included hospital length of stay (LOS), intensive care unit (ICU) LOS, days on mechanical ventilation, and 30-day readmission rates. The quality outcomes were gathered via individual chart review and interrogation of the electronic data warehouse.
The primary cost outcome was total cost of care collected from payors, which included the sum of the index hospitalization and the 30 days after discharge. Secondary cost outcomes included the breakdown of the index hospitalization into professional and technical costs. All cost of care data was provided by UK Healthcare (UKHC) and the Kentucky Medical Services Foundation (KMSF).
Additionally, we investigated process measures to understand practice changes that may have been the result of the implementation of the PERT protocol. Process measures included documentation of right heart function on CTPA reports and the measurement of troponins. Given that the PERT protocol heavily emphasized activation for right heart strain/dysfunction, patients without right heart strain/dysfunction that underwent a PERT activation and patients with right heart strain/dysfunction that did not receive a PERT activation were assessed as deviations from the protocol.
Statistical Analysis
In order to ensure the confidentiality of UK Healthcare’s proprietary financial information, all cost values were divided by their subset specific Pre-PERT median value. Since dividing by the median is a linear transformation of the data, all relationships contained in the original data are preserved and all p-values are identical to their untransformed counterparts.
Participant characteristics and outcomes were compared by PERT group. For categorical variables, frequencies and column percentages (%) were reported and p-values were calculated using χ2 and Fisher’s exact tests, as appropriate. Continuous variables were tested for normality using the Shapiro-Wilk normality test along with histograms. Normally distributed continuous variables were reported using means and standard deviations (SD) and p-values were calculated using two-sample t-tests and one-way ANOVAs; otherwise, medians and first/third quartiles [Q1,Q3] were reported and p-values were calculated using Mann-Whitney U and Kruskal-Wallis tests.
Statistical significance was set at p≤0.05, and all tests were two-sided. Missing observations were reported and were excluded on an analysis-by-analysis basis. All analyses were done in R programming language, version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria). All graphics were produced using the R package ggplot2, version 3.2.1 (Hadley Wickham).
Results
Baseline demographics
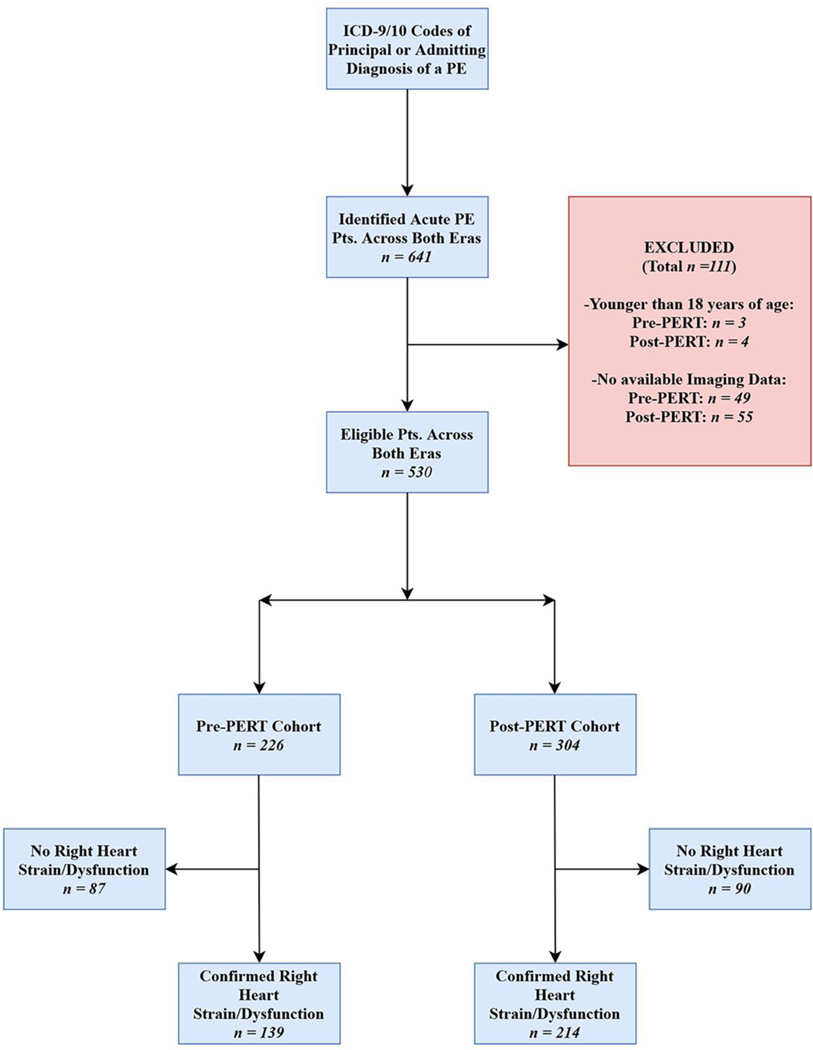
Initially, 634 patients aged 18 or older were identified who had a principal or admitting diagnosis of an acute PE based on ICD-9 and ICD-10 codes within the electronic data warehouse. 104 patients were excluded due to lack of a reported CTPA or echocardiogram in the UKHC system. The final cohort was comprised of a total of 530 patients. The patients were subdivided into two distinct eras: Pre-PERT, which spanned from January 2010 to September 2015, and Post-PERT, which spanned from October 2015 to December 2018, with 226 patients in the Pre-PERT, and 304 patients in the Post-PERT groups. The patients were further subdivided based upon the presence or absence of right heart/strain dysfunction on imaging (Figure 1).
Fig 1.
STROBE Flowchart of Study Cohorts
The baseline demographics in the Pre-PERT and post-PERT cohorts were similar (Table 1). Average age was 59.5 years in the Pre-PERT group and 58.1 years in the Post-PERT group (p=0.321). The majority of patients in both groups were female. Hypertension was the only condition that was significantly different between eras, 57.1% of Pre-PERT patients having documented hypertension versus 73% of Post-PERT patients (p<0.001). The presence of right heart strain or dysfunction was higher in the Post-PERT patients at 70.4% compared to only 61.5% Pre-PERT (p=0.04).
Table 1:
Baseline Demographics of the Pre-PERT and Post-PERT Eras
| Pre-PERT Era (n = 226) | Post-PERT Era (n = 304) | P-value | |
|---|---|---|---|
| Age, Years, Mean (SD) | 59.5 (14.9) | 58.1 (16.0) | 0.321 |
| Male, N (%) | 94 (41.6) | 143 (47.0) | 0.246 |
| BMI, Mean (SD) | 33.9 (13.6) | 35.0 (12.7) | 0.392 |
| Black/African American, N (%) | 23 (10.2%) | 40 (13.2%) | 0.371 |
| Presence of Right Heart Strain, N (%) | 139 (61.5) | 214 (70.4) | 0.040 |
| Comorbid Conditions | |||
| History of Cancer, N (%) | 63 (27.9) | 80 (26.3) | 0.763 |
| Active Cancer, N (%) | 54 (23.9) | 65 (21.4) | 0.562 |
| Congestive Heart Failure, N (%) | 58 (25.7) | 59 (19.4) | 0.107 |
| Chronic Kidney Disease, N (%) | 25 (11.1) | 32 (10.5) | 0.956 |
| Diabetes, N (%) | 61 (27.0) | 100 (32.9) | 0.172 |
| Hypertensive, N (%) | 129 (57.1) | 222 (73.0) | <0.001 |
| Coronary Artery Disease, N (%) | 42 (18.6) | 59 (19.4) | 0.899 |
| Therapeutic interventions | |||
| Systemic AC DOAC Use | 221 (97.8) 23 (10.4) | 298 (98.0) 145 (48.7) | 1.000 <0.001 |
| Catheter Directed Thrombolysis, N (%) | 46 (20.4) | 55 (18.1) | 0.587 |
| Systemic Thrombolysis, N (%) | 10 (4.4) | 7 (2.3) | 0.262 |
| Surgical or Mechanical Embolectomy, N (%) | 3 (1.3) | 8 (2.6) | 0.294 |
| On Inotropes, N (%) | 18 (8.0) | 23 (11.5) | 0.404 |
| ECMO, N (%) | 12 (5.3) | 12 (6.0) | 0.952 |
In regard to the utilization of various treatment modalities, i.e., systemic anticoagulation, systemic and catheter-directed (CDT) thrombolysis, surgical or mechanical embolectomy, and the use of extracorporeal membrane oxygenation (ECMO), there was no statistically significant difference between the two groups. However, in regard to type of anticoagulation used, there was a difference in rates of direct oral anticoagulants (DOACs) utilization between the Pre- and Post-PERT eras (i.e., 10.4% vs. 48.7%, respectively) (p<0.001).
In the subgroup of patients who had confirmed right heart strain or dysfunction, the baseline demographics were largely similar as well (Supplemental Table 1). The only significant difference between the Pre- and Post-PERT groups was the history of hypertension, with 58.3% of Pre-PERT patients having documented hypertension versus 73.4% of Post-PERT patients (p=0.005). Similar to the Pre- and Post-PERT groups in general, the right heart strain/dysfunction subgroup showed no significant difference in the utilization of the various therapeutic interventions, with the exception of DOAC use, which was higher in the Post-PERT group (48.8% vs. 13.2%; p<0.001).
Outcomes
Quality
The overall metrics of quality and utilization between the Pre-PERT and Post-PERT groups are presented in Table 2. In-hospital mortality was significantly higher at 16.5% in the Pre-PERT group as compared to 9.6% in the Post-PERT group (p=0.025). Median hospital LOS was approximately 3 days shorter in the Post-PERT group (p<0.001), and median ICU LOS was shorter by 1.5 days in the Post-PERT group (p=0.023). There was a 29% absolute risk reduction amongst patients who received any mechanical ventilation with the implementation of the PERT protocol, with median mechanical ventilation duration being over 2 days shorter in the Post-PERT group (p=0.025). There was no difference in 30-day readmission rates between the groups (p=0.910).
Table 2:
Quality and Utilization Outcomes Between the Pre-PERT and Post-PERT Eras
| Pre-PERT Era (n = 226) | Post-PERT Era (n = 304) | P-value | |
|---|---|---|---|
| In-Hospital Mortality, N (%) | 37 (16.5) | 29 (9.6) | 0.025 |
| 30-Day Readmission, N (%) | 34 (15.0) | 48 (15.8) | 0.910 |
| Hospital LOS, Days, Median [Q1,Q3] | 7.7 [3.9, 13.0] | 4.4 [2.4, 8.2] | <0.001 |
| Any ICU Time, N (%) | 155 (68.6) | 196 (64.5) | 0.370 |
| ICU LOS, Days, Median [Q1,Q3] | 4.4 [1.9, 10.5] | 2.9 [1.9, 6.0] | 0.023 |
| Any Time on Mechanical Vent, N (%) | 137 (60.6) | 96 (31.6) | <0.001 |
| Mechanical Vent Duration, Days, Median [Q1,Q3] | 7.6 [3.2, 14.0] | 5.0 [2.2, 10.9] | 0.025 |
The mortality benefit observed within the group was largely due to a reduction in mortality amongst those with RH strain/dysfunction (22.6% vs 9.0%, p=0.001). Overall hospital LOS was reduced amongst those with right heart strain/dysfunction (7.1 vs 4.0 days, p<0.001) and without right heart strain/dysfunction (7.8 vs 6.0 days, p=0.009). ICU LOS and mechanical ventilation time was numerically lower but did not reach statistical significance (Supplemental Table 2).
Cost
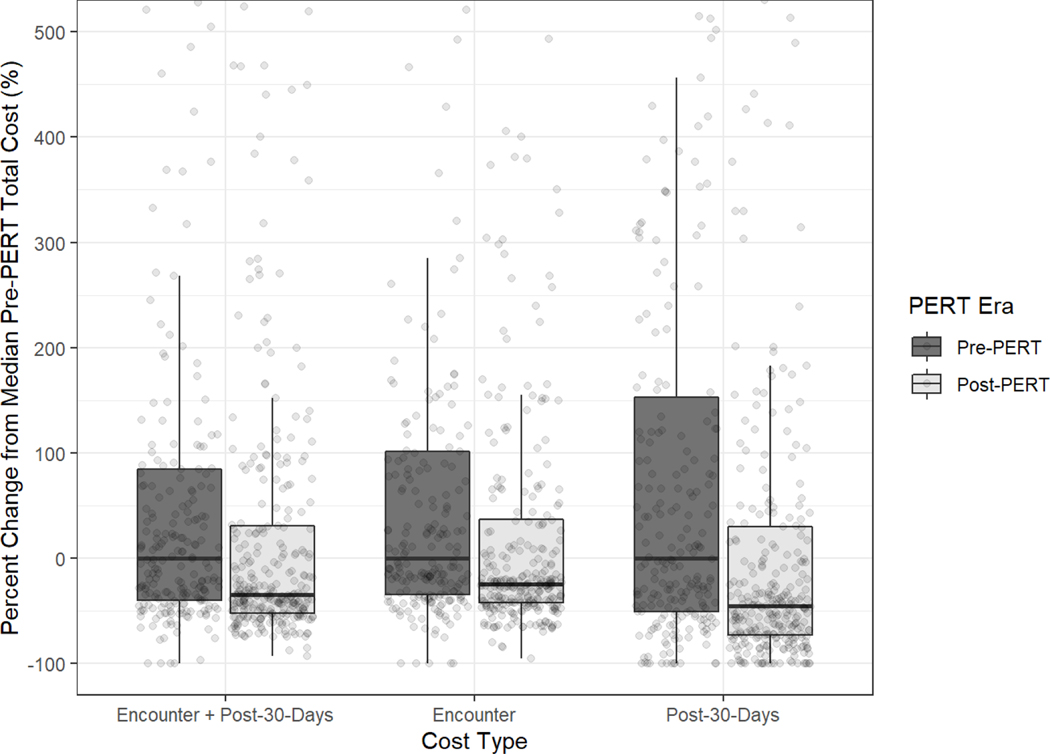
Implementing the PERT protocol also had a substantial reduction in the overall cost of care. Total cost of care for the index hospitalization and the 30 days post were reduced by ~34.3%. Greater than 80% of the costs incurred in both groups was during the index hospitalization. Total cost of care for the index hospitalization was reduced by 24.1%, with a 39.3% reduction in professional costs (p<0.001) and a 17.7% reduction in technical costs (p<0.001). Total costs of care in the 30 days after discharge was also significantly lower (p<0.001) (Figure 2) (Supplemental Table 3).
Fig 2.
Costs Stratified by PERT Era for the Entire Sample (n=530)
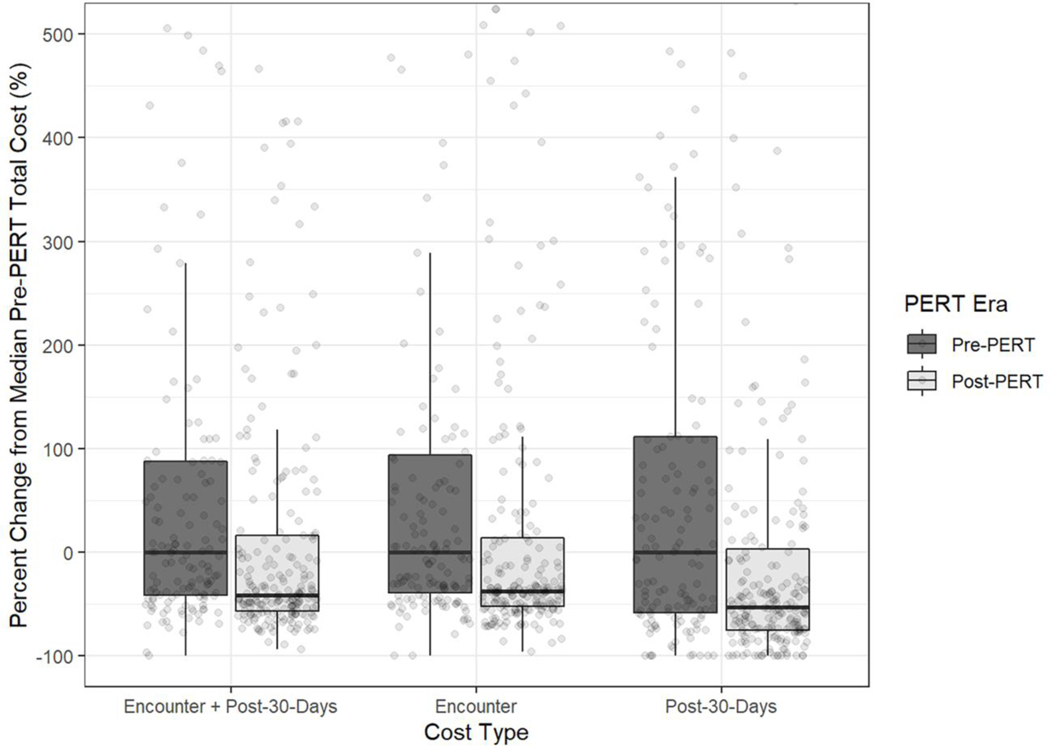
Similar to the quality measures, the majority of the cost reductions seen were in those with right heart strain/dysfunction, where costs were reduced by 41.1% (p<0.001) (Figure 3). While overall costs in those without right heart strain/dysfunction decreased with the PERT protocol by 25.1%, this did not reach statistical significance (p=0.178) (Supplemental Table 4).
Fig 3.
Costs Stratified by PERT Era Among Individuals with Right Heart Strain/Dysfunction (n=353)
Cost savings from implementing the PERT protocol exceeded $2.25 million at our institution over the three-year period of interest.
Process Measures
In the Post-PERT era, significantly more CTPA reports mentioned the presence or absence of right heart strain/dysfunction (98.4% vs. 91.1%; p=0.006). However, measurements of troponin were lower in the Post-PERT era at 55.6% as compared to 72.1% (p<0.001). Of note, 44 patients with right heart strain/dysfunction in the post-PERT era did not undergo a PERT activation. The PERT team was activated for an additional 30 patients did not have right heart strain/dysfunction. These 74 patients represent deviations from the PERT protocol, resulting in >75% fidelity with the care redesign.
Discussion
The implementation of optimal care pathways has been shown to decrease the time required for patients to receive appropriate medications and interventions in other disease states, most notably STEMI [14]. PE care, though, has only recently adopted such pathways in the form of PERTs, with the American Heart Association endorsing their role in 2019 [15]. However, the impact of a PERT protocol on value of care had not been studied prior to this investigation. This study highlights 2 primary findings: 1) quality of care improved after the initiation of a PERT protocol at our institution with a reduction in in-hospital mortality and 2) total overall cost of care decreased.
In regard to the baseline demographics of the Pre- and Post-PERT groups, there was virtually no difference between the two groups with the exception of the presence of right heart strain/dysfunction seen on imaging studies (61.5% vs. 70.4%, respectively), history of hypertension (57.1% vs. 73%, respectively), and utilization of DOACs for anticoagulation (10.4% vs. 48.7%, respectively). Given the correlation between right heart strain and clinical outcomes, this finding makes the observed gains more startling.
The most important finding from this investigation was the ~7% absolute risk reduction observed in in-hospital mortality after the initiation of the PERT protocol. This reduction in mortality is largely driven by those patients with confirmed right heart strain/dysfunction (22.6% vs. 9.0% between the eras, respectively) and was seen despite largely similar rates of different therapeutic interventions. This suggests that the reduction in mortality is likely the result of earlier recognition of higher-risk PE patients through more reliable reporting on imaging studies and a standardized approach to triaging these patients. Ultimately, this leads to the streamlined delivery of both clinical expertise and therapeutic interventions, especially to higher risk cohorts and can reduce mortality.
Another potential byproduct of expediting care to the highest risk cohorts is a reduction in overall LOS and ICU LOS. Some of this reduction may be due to the difference in rates of utilization of DOACs between the eras. DOACs do not require bridging therapy with heparin, which would save time. However, a large proportion of the decrease in overall hospital LOS was the result of a reduction in ICU LOS. The majority of the duration of the bridging therapy likely occurred outside of the ICU, so the reduction in ICU LOS was probably due to other underlying factors. Rapid identification of a decompensating patient and, therefore, the implementation of effective therapy, hopefully, reduces the risk of further decompensation, which would also decrease hospital and ICU LOS. While there was no difference in the utilization of rescue strategies, such as inotropes or ECMO, there was a reduction in mechanical ventilation. Whether mechanical ventilation itself is a risk of mortality (potentially due to positive pressure ventilation in a patient with RV dysfunction [16]) or whether it is a marker of a sicker patient is unclear.
Implementation of the PERT protocol at our institution reduced overall cost of care, which further improved value of care delivered. As previously mentioned, the implementation of a PERT protocol reduced overall resource utilization. The direct decrease in overall LOS, ICU LOS, and mechanical ventilation use correlates with substantially reduced overall cost of care during the index hospitalization. While both technical costs and professional costs decreased, the latter did so more. Given that most technical costs are DRG based, there is less expected variation within technical costs even with dramatic reduction in resource utilization. Professional fees are directly related to the complexity, intensity, and quantity of the interventions performed. Reducing physician variation, intervening earlier, and preventing further decompensation directly translate to earlier discharges and shorter ICU LOS. This, expectedly, will reduce professional costs more than technical costs. However, there are year-to-year variations in reimbursements and fee structure that have not been assessed with this analysis, but given the fact that costs correlate with more static measures of resource utilization (i.e., hospital LOS, ICU LOS, and days on mechanical ventilation), the implementation of a PERT protocol likely reduces true resource utilization. However, the net impact of this intervention on cost of care is tremendous. Potentially, anywhere from ~$1.7 to $7.3 billion could be saved across the health care system in this country annually [17].
With this reduction in index hospitalization cost of care, there was no increase in either 30-day readmission rates or cost of care at 30 days. This is important in the context of value-based purchasing, where outcomes and cost of care are attributed up to 30 days after the initial encounter.
Moreover, this analysis may not have captured those patients who passed away soon after discharge. However, the fact that there was no significant change in 30-day readmission rates between the Pre- and Post-PERT groups (15.0% vs. 15.8%, respectively) means that these patients were not being discharged prematurely, and the reduction in in-hospital mortality was likely due to other factors, i.e., the implementation of a PERT protocol.
In regard to the process outcomes that were expected to be improved as a result of the initiation of a PERT protocol, a statistically significant increase in the reporting of the presence or absence of right heart strain/dysfunction on CTPA was seen (91.1% vs. 98.4%, respectively). However, unexpectedly, a decrease in troponin collection was seen between the Pre- and Post-PERT groups (72.1% vs. 55.6%, respectively). The decrease in troponin collection seen in the Post-PERT group could possibly be due to a greater reporting of right heart strain on CTPA. However, the decrease in troponin ordering was far more than the increase in reporting of the right heart on CTPA and would not account for the decrease in isolation. Another potential explanation maybe the involvement of subspecialty expertise earlier, mitigating the need for more supportive data once a plan is in place. Either way, given the improvement in outcomes despite the decrease in troponin ordering, the role of cardiac biomarkers in the context of a PE response team needs to be revisited.
The deviations in the PERT protocol may have been the result of patients being transferred from other institutions who had seemingly been stable enough to endure the transit and, therefore, did not warrant a PERT activation. Furthermore, some patients may have been past the point of medical therapy, and their families may have discontinued further treatment, especially in the form of a PERT activation. For those patients who did not exhibit right heart strain/dysfunction on imaging studies but resulted in a PERT activation, their specific clinical context likely warranted an escalation of care, i.e., they were critically unstable in the setting of normal imaging findings.
Conclusions
This investigation showed that the implementation of a pulmonary embolism response team (PERT) significantly improved quality outcomes and decreased costs, resulting in an overall increase in the value of care provided to PE patients. Although these benefits were seen generally in the Post-PERT era, they were notably pronounced in the subset of patients who had confirmed right heart strain/dysfunction on imaging studies. We hypothesize that these findings are due to PERTs providing more timely and efficient recognition of higher-risk PE patients. As a result of this, clinical expertise and therapeutic interventions are likely implemented sooner, which could result in unnecessary resource utilization and variance in physician practice. This study provides a hypothesis that PERTs not only improve outcomes for high-risk PE patients but reduce costs across the spectrum of PE care. Future research should, therefore, aim to validate these findings and ascertain the system-wide and patient-level changes that prove to provide the most benefit is the next step. As more data becomes available, it will become easier to optimize these clinical teams to ensure that patients are receiving the best quality of care.
Limitations
The data in this study was largely gathered via an extraction directly from our electronic medical records system and heavily dependent on coded variables. As a result of this, the information extracted is only as good as the quality of the underlying documentation, especially in regard to the use of ICD-9 and ICD-10 codes for determining principal and admitting diagnoses. The small sample size of this study requires validation in larger studies. This study was not meant to identify any causal relationships between the implementation of a PERT and improvement in care but to simply report the findings that were seen as a result of said implementation.
Supplementary Material
Acknowledgments
Funding
Rahul Annabathula was supported by a 2019 AOA Carolyn L. Kuckein Student Research Fellowship. The project described was supported by the NIH National Center for Advancing Translational Sciences through grant number UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declarations
Disclosures
The authors have no conflicts to disclose.
Availability of Data and Material
Since the data collected is considered PHI, the authors alone have custody over it, per the agreement made with the IRB. However, deidentified data can made accessible upon request.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- [1].Rahimtoola A, & Bergin JD (2005). Acute pulmonary embolism: an update on diagnosis and management. Current problems in cardiology, 30(2), 61–114. doi: 10.1016/j.cpcardiol.2004.06.001 [DOI] [PubMed] [Google Scholar]
- [2].Beckman MG, Hooper WC, Critchley SE, & Ortel TL (2010). Venous thromboembolism: a public health concern. American journal of preventive medicine, 38(4), S495–S501. doi: 10.1016/j.amepre.2009.12.017 [DOI] [PubMed] [Google Scholar]
- [3].Goldhaber Samuel Z., “Deep Venous Thrombosis and Pulmonary Thromboembolism.” Harrison’s Principles of Internal Medicine , 19e Eds. Dennis Kasper, et al. New York, NY: McGraw-Hill, 2014, http://accessmedicine.mhmedical.com/content.aspx?bookid=1130§ionid=79744095. [Google Scholar]
- [4].Raja AS, Greenberg JO, Qaseem A, Denberg TD, Fitterman N, & Schuur JD (2015). Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Annals of internal medicine, 163(9), 701–711. doi: 10.7326/M14-1772 [DOI] [PubMed] [Google Scholar]
- [5].Xue X, & Sista AK (2018). Catheter-directed thrombolysis for pulmonary embolism: the state of practice. Techniques in vascular and interventional radiology, 21(2), 78–84. doi: 10.1053/j.tvir.2018.03.003 [DOI] [PubMed] [Google Scholar]
- [6].Rosovsky R, Zhao K, Sista A, Rivera‐Lebron B, & Kabrhel C (2019). Pulmonary embolism response teams: Purpose, evidence for efficacy, and future research directions. Research and practice in thrombosis and haemostasis, 3(3), 315–330. doi: 10.1002/rth2.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lavender Z, & MacNabb M (2019). Pulmonary embolism response team. Journal of the American Academy of PAs, 32(12), 52–53. doi: 10.1097/01.JAA.0000604872.64196.31 [DOI] [PubMed] [Google Scholar]
- [8].Provias T, Dudzinski DM, Jaff MR, Rosenfield K, Channick R, Baker J, Weinberg I, Donaldson C, Narayan R, Rassi AN, & Kabrhel C (2014). The Massachusetts General Hospital Pulmonary Embolism Response Team (MGH PERT): creation of a multidisciplinary program to improve care of patients with massive and submassive pulmonary embolism. Hospital Practice, 42(1), 31–37. doi: 10.3810/hp.2014.02.1089 [DOI] [PubMed] [Google Scholar]
- [9].Barnes GD, Kabrhel C, Courtney DM, Naydenov S, Wood T, Rosovsky R, Rosenfield K, Giri J, Balan P, Barnes G, & Courtney M (2016). Diversity in the pulmonary embolism response team model: an organizational survey of the national PERT consortium members. Chest, 150(6), 1414–1417. doi: 10.1016/j.chest.2016.09.034 [DOI] [PubMed] [Google Scholar]
- [10].Root CW, Dudzinski DM, Zakhary B, Friedman OA, Sista AK, & Horowitz JM (2018). Multidisciplinary approach to the management of pulmonary embolism patients: the pulmonary embolism response team (PERT). Journal of multidisciplinary healthcare, 11, 187. doi: 10.2147/JMDH.S151196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Butler TJ, Firestone KS, Grow JL, & Kantak AD (2014). Standardizing documentation and the clinical approach to apnea of prematurity reduces length of stay, improves staff satisfaction, and decreases hospital cost. The Joint Commission Journal on Quality and Patient Safety, 40(6), 263-AP1. doi: 10.1016/s1553-7250(14)40035-7 [DOI] [PubMed] [Google Scholar]
- [12].World Health Organization. (2014). International classification of diseases (ICD) information sheet. found at http://www.who.int/classifications/icd/factsheet/en.
- [13].Wake N, Kumamaru KK, George E, Bedayat A, Ghosh N, Quesada CG, Rybicki FJ, & Gerhard-Herman M (2014). Computed Tomography and Echocardiography in Patients With Acute Pulmonary Embolism: Part 1Correlation of Findings of Right Ventricular Enlargement. Journal of thoracic imaging, 29(1), W1–W6. doi: 10.1097/RTI.0000000000000047 [DOI] [PubMed] [Google Scholar]
- [14].Ryu DR, Choi JW, Lee BK, & Cho BR (2015). Effects of critical pathway on the management of patients with ST-elevation acute myocardial infarction in an emergency department. Critical pathways in cardiology, 14(1), 31–35. doi: 10.1097/HPC.0000000000000035 [DOI] [PubMed] [Google Scholar]
- [15].Giri J, Sista AK, Weinberg I, Kearon C, Kumbhani DJ, Desai ND, Piazza G, Gladwin MT, Chatterjee S, Kobayashi T, & Kabrhel C (2019). Interventional Therapies for Acute Pulmonary Embolism: Current status and principles for the development of novel evidence: a scientific statement From the American Heart Association. Circulation, 140(20), e774–e801. doi: 10.1161/CIR.0000000000000707 [DOI] [PubMed] [Google Scholar]
- [16].Fewell JE, Abendschein DR, Carlson CJ, Murray JF, & Rapaport ELLIOT (1980). Continuous positive-pressure ventilation decreases right and left ventricular end-diastolic volumes in the dog. Circulation research, 46(1), 125–132. doi: 10.1161/01.res.46.1.125 [DOI] [PubMed] [Google Scholar]
- [17].Turetz M, Sideris AT, Friedman OA, Triphathi N, & Horowitz JM (2018, June). Epidemiology, pathophysiology, and natural history of pulmonary embolism. In Seminars in interventional radiology (Vol. 35, No. 02, pp. 92–98). Thieme Medical Publishers. doi: 10.1055/s-0038-1642036 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.