Abstract
Objectives
To measure secondary attack rates (SARs) in prospectively followed household contacts of paediatric and adult cases of SARS-CoV-2 infection in England.
Methods
Self-taken nasal swabs from household contacts of PCR confirmed cases of COVID-19 and blood samples on day 35 were tested for evidence of infection with SARS-CoV-2 virus.
Results
The secondary attack rate (SAR) among 431 contacts of 172 symptomatic index cases was 33% (95% confidence intervals [CI] 25–40) and was lower from primary cases without respiratory symptoms, 6% (CI 0–14) vs 37% (CI 29–45), p = 0.030. The SAR from index cases <11 years was 25% (CI 12–38). SARs ranged from 16% (4–28) in contacts <11 years old to 36% (CI 28–45) in contacts aged 19–54 years (p = 0.119). The proportion infected who developed symptoms (78%) was similar by age (p = 0.44) though <19 year olds had fewer mean number of symptoms than adults (p = 0.001) and fewer reported loss of sense of taste or smell (p = 0.0001).
Conclusions
: There are high risks of transmission of SARS-CoV-2 virus in the home, including those where infection is introduced by a child. The risk of children acquiring infection was lower than that in adults and fewer developed typical symptoms of Covid-19 infection.
Keywords: SARS-CoV-2 transmission, Secondary attack rates by age, Households
Introduction
Following the emergence of the SARS-CoV-2 virus in early 2020, the World Health Organisation recommended that prospective studies should be conducted in the household setting to better understand the infectivity of the virus, the risk factors for transmission and the proportion of contacts infected without symptoms.1 As the pandemic progressed, a further key question to emerge in relation to the need for school closures was the extent to which children were able to transmit the virus to others and, if exposed, their likelihood of becoming infected.
A number of household transmission studies have now been published with a wide range of estimated secondary attack rates. A systematic review found significant heterogeneity in attack rates between the 54 included household studies, ranging from 7% to 45% with a weighted mean of 16.6%.2 This heterogeneity is perhaps unsurprising given the variation in study methods, such as the frequency and type of PCR testing, whether all contacts or only symptomatic contacts were tested, the definition used for categorising an index case and whether the studies were retrospective or prospective. The review, which excluded studies using serology to identify infected contacts, was unable to provide an estimate of the risk of transmission from children or from asymptomatically infected index cases.
At the end of March 2020, Public Health England (PHE) began a prospective follow up of households with a PCR confirmed index case in the community. As widespread community testing was not initially available in England,3 the sentinel surveillance network of the Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) the English primary care sentinel surveillance network was used to recruit index cases, the majority of whom were adults.4 Once widespread community testing was established, recruitment was focussed on index cases who were children. On identification of the index case, household members were followed up for clinical symptoms, and virological and serological evidence of SARS-CoV-2 infection. This paper reports the secondary infection rate in household contacts by age of both the index case and contact, the clinical presentation in the prospectively ascertained household cases and the accuracy of different symptoms in identifying those infected. Duration and quantity of viral shedding in relation to symptom onset and risk factors for transmission in the household setting are also reported.
Materials and methods
Recruitment and follow up
Cases of PCR confirmed SARS-CoV-2 infection reported to the PHE National Infection Service between 30th March and 17th November were telephoned by a study nurse to ask whether they and their household members would agree to take part in an enhanced surveillance of transmission in the household setting. Households with at least one other member not already known to be PCR positive were recruited. Symptoms in index cases and contacts were solicited through a standard questionnaire administered by the study nurses on the day of initial telephone contact (day 1) and again 14 days later. Packs containing virus transport medium and swabs for all household members to take their own nasal swab samples on receipt and again 7 days later, together with swabs for a self-taken oral fluid (OF) sample, were posted to households on day 1. Samples were posted back to the PHE Virus Reference Department (VRD), and from May 19th 2020 were couriered back. Diaries and thermometers were provided for daily recording of symptoms by each household member for 14 days starting on day 1 with diaries returned by post when completed. Solicited symptoms in the nurse questionnaires and diaries were: fever, cough, runny nose, sore throat, shortness of breath, loss of taste or smell, nausea, diarrhoea, fatigue, muscle/body pain, headache or other.
Approximately 5 weeks after day 1 venous blood samples were taken from consenting household members by the GP for an RCGP RSC recruited household or a trained phlebotomist who visited the home. A second OF swab was obtained at the same time.
Governance
The household surveillance protocol was approved by the PHE Research Ethics and Governance Group as part of the portfolio of PHE's enhanced surveillance activities in response to the pandemic and thus not requiring review by the NHS Health Research Authority (HRA) or the Confidentiality Advisory Group of the HRA. Oral informed consent for sampling and follow up was obtained by the nurses from household members who were free to decline to participate in the surveillance at any time. Consent for children was obtained by a parent or legal guardian. Test results were provided to GPs and to household members with interpretation and advice about compliance with national guidelines with respect to self -isolation if appropriate. The RCGP RSC's sentinel surveillance role is approved by the PHE Caldicott Guardian, under Regulation 3 of the Health Service (Control of Patient Information) Regulations 2002.
Laboratory testing
For index cases recruited from the RCGP sentinel surveillance the initial confirmatory swab was tested by the PHE VRD using a real-time reverse transcription (RT)-PCR with cycle threshold (Ct) values ≤39 considered positive.5 For index cases recruited via the network of community testing laboratories (termed Pillar 2 laboratories) a variety of RT-PCR assays were used.6 Nasal swabs taken from index cases and household members were tested at VRD by RT-PCR with virus culture conducted on PCR positive samples that were couriered in.
Day 35 blood samples were tested for IgG antibody to the nucleocapsid protein (NP) by a commercial NP assay and also by an in-house ELISA that used the receptor binding domain (RBD) as antigen (PHE,UK) as previously described.7 The cut-off for antibody positivity used in the analyses were ≥0.8 for the Abbott assay and ≥5.0 for the RBD ELISA. Both assays have shown high specificity at these cut-offs in serum samples taken before the emergence of SARS-CoV-2, 99.1% (96% confidence intervals [CIs] 98.4–99.6) for the Abbott and 98% (97.0–98.8) for the RBD ELISA (PHE unpublished). For participants with no day 35 blood sample but with a Day 35 OF sample, this was tested for antibodies to the spike and nucleocapsid proteins by an in-house IgG capture ELISA with a specificity of 99% for antibodies to both antigens and a sensitivity of 76% and 82%, respectively for the spike and NP assays (PHE unpublished). A household contact was defined as infected if PCR positive on either of the two self-taken nasal swabs and/or antibody positive at 35 days by either the Abbott or RBD assay, or in the day 35 OF antibody assay.
Statistical analysis
For estimation of secondary attack rates (SARs), the primary analysis excluded households in which the index case was not the primary case (i.e. with an infected household contact with symptom onset before or on the same day or day after the index case) and antibody positive PCR negative asymptomatic contacts whose timing of infection could not be established. In a sensitivity analysis SARs were estimated only with exclusion of household contacts who were prior or co-primary cases For both analyses only contacts with a day 35 blood or OF antibody result were included due to the low sensitivity of the self-administered nasal swabs in detecting infected contacts. Ages of index cases and contacts were grouped into ≤10, 11–18, 18–54, ≥55 years to broadly represent primary and secondary school-age children, young adults and parents with young children, and older adults
Because of the potential for false positive PCR test results on the index case's initial swab, particularly from June to end August8 when the incidence of SARS-CoV-2 infections in England was low,9 , 10 any household in which infection in the index case could not be confirmed by repeat PCR testing or development of antibodies to SARS-CoV-2, and with no infected household contacts, were excluded from the analysis.
A normal errors regression model was used to investigate the effect of age, presence of symptoms, symptom severity, gender and presence of a co-morbidity, after adjustment for interval from symptom onset, on factors associated with Ct value (as a continuous variable) in PCR positive contacts.
Results
Surveillance population
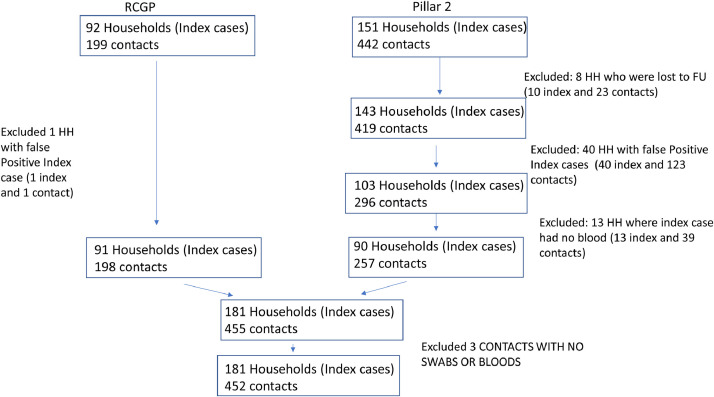
The numbers of index cases and contacts recruited and the numbers included in the analysis are shown in Fig. 1 . In 40 households, clustering among those recruited via Pillar 2 testing in June and July, infection in the index case could not be confirmed. All index cases were under 19 years old (as recruitment in this period was restricted to this age group) and all were antibody negative at 35 days and PCR negative in subsequent swabs as were all their household contacts. A further 13 households with a Pillar 2 index case recruited in June and July who did not provide a day 35 blood sample were also excluded based on negative PCR tests from the index case and all family members none of whom was antibody positive. Of the 92 index cases recruited via the RCGP sentinel surveillance prior to June only one was excluded as a false positive; the Ct value in the initial PCR surveillance swab from this index case was 39.
Fig. 1.
Flow diagram of study population and post-recruitment exclusions.
The demographic and clinical features of the remaining 181 index cases and the 452 contacts are shown in Table 1 . Health and social care workers comprised 47 (26%) index cases, with a further 11 (6%) in occupations such as bus drivers that required continuing public interaction despite the national lockdown in England from late March to June 2020; 91 index cases (50%) were pre-school or school children recruited from June onwards when recruitment was focussed on this age group. During this period nurseries and schools were closed except for children of key workers. One hundred and forty nine (82%) index cases said they were isolating from other household members after diagnosis and eight (4.4%) required hospital admission.
Table 1.
Demographic and clinical features of the 181 index cases and 452 household contacts.
| Feature | Level | INDEX CASES (N = 181) | CONTACTS (N = 452) |
| Household size (total number of participating individuals) | 2 | 43 (24%) | 43 (10%) |
| 3 | 42 (23%) | 84 (19%) | |
| 4 | 65 (36%) | 193 (43%) | |
| 5 | 25 (14%) | 99 (22%) | |
| 6 | 4 (2%) | 20 (4%) | |
| ≥7 | 2 (1%) | 13 (2%) | |
| Gender | Female | 103 (57%) | 226 (50%) |
| Male | 78 (43%) | 226 (50%) | |
| Age group (years) | 0 to 10 | 37 (20%) | 93 (21%) |
| 11 to 18 | 55 (30%) | 80 (18%) | |
| 19 to 54 | 66 (37%) | 233 (51%) | |
| >=55 | 23 (13%) | 46 (10%) | |
| Any Comorbidity | No | 149 (82%) | 380 (84%) |
| Yes | 32 (18%) | 72 (16%) | |
| Type of Comorbidity | Medicated asthma | 17 (9%) | 43 (10%) |
| (Subjects may have >1) | Diabetes | 7 (4%) | 11 (2%) |
| Respiratory disease | 1 (1%) | 5 (1%) | |
| Neurological condition | 5 (3%) | 4 (1%) | |
| Heart disease | 6 (3%) | 6 (1%) | |
| Liver/kidney disease | 2 (1%) | 6 (1%) | |
| Immune compromised | 3 (2%) | 6 (1%) | |
| Malignancy | 1 (<1%) | 7 (2%) |
Nine index cases with antibody at 35 days had no symptoms. None had PCR positive contacts and 7 of the 9 had OF samples taken at Day 1 of which five were antibody positive indicating infection from an earlier exposure. All 9 index cases were recruited during the low incidence summer period when there was a high false positive rate among the index cases. These nine index cases and their 21 contacts were therefore excluded, leaving a total of 431 contacts in 172 households in the final analysis.
Secondary attack rates
One or more swabs from 91 (21.1%) of the 431 contacts were PCR positive and antibodies to SARS-CoV-2 were found in 180 (46.9%) of the 383 contacts with a 35 day blood or OF sample. Overall, 194 of the 431 contacts (45.0%) had PCR and/or antibody evidence of infection. In contrast to the index children excluded as false positives, all 22 contacts aged under 19 years of age who were PCR positive and provided a 35 day blood or OF sample were antibody positive.
The SARs with exclusion of 55 households with prior or co-primary cases, and PCR negative asymptomatic contacts, is shown in Table 2 . In these prospectively followed households, 75/248 (33%) contacts were infected with an inverse relationship between household size and transmission rates. SARs in contacts under 11 years of age (16%) were lower than in older age groups though the trend with age was not significant (p = 0.119), neither did age of the index case significantly affect SARs (p = 0.346). Contacts in households in which the index case reported that they were isolating from other members did not experience a lower attack rate, nor was there evidence of a relationship between SAR and the minimum Ct value in the PCR positive swabs from the index case. SARs from index cases with respiratory or systemic symptoms were significantly higher than in those without such symptoms.
Table 2.
Secondary attack rates in contacts with a serological or oral fluid outcome. Analysis excludes 55 households with a prior or co-primary case and 22 asymptomatic antibody positive PCR negative contacts whose timing of infection could not be established.
| Factor | Level | n/N | % (95% CI) | P-value | |
| All | 75/248 | 33% (25–40) | |||
| Gender of contact | Female | 34/120 | 31% (22–39) | 0.516 | |
| Male | 41/128 | 34% (25–43) | |||
| Age of contact (years) | 0to10 | 7/40 | 16% (4–28) | 0.119 | |
| 11to18 | 11/40 | 33% (19–48) | |||
| 19to54 | 49/145 | 36% (28–45) | |||
| >=55 | 8/23 | 32% (14–51) | |||
| Gender of index case | Female | 42/139 | 33% (23–42) | 0.998 | |
| Male | 33/109 | 32% (22–43) | |||
| Age of index case | 0to10 | 14/61 | 25% (12–38) | 0.346 | |
| 11to18 | 26/94 | 30% (19–41) | |||
| 19to54 | 26/73 | 36% (23–49) | |||
| >=55 | 9/20 | 49% (24–73) | |||
| Index case self isolating | No | 8/29 | 30% (10–51) | 0.844 | |
| Yes | 66/217 | 33% (25–40) | |||
| Household size | 2 | 15/28 | 54% (35–72) | 0.009 | |
| 3 | 18/52 | 35% (20–50) | (trend) | ||
| 4 | 29/110 | 28% (18–38) | |||
| 5+ | 13/58 | 21% (8–35) | |||
| Minimum Ct value in index case | 35–40 | 12/38 | 31% (13–48) | 0.44 | |
| 30–34 | 13/40 | 33% (17–50) | (trend) | ||
| 25–29 | 13/36 | 38% (20–56) | |||
| 20–24 | 13/43 | 33% (16–51) | |||
| <20 | 6/14 | 45% (16–74) | |||
| Index case with loss taste/ smell | No | 20/77 | 28% (16–40) | 0.262 | |
| Yes | 53/153 | 37% (28–46) | |||
| 1Index case with respiratory symptoms | No | 2/38 | 6% (0–14) | 0.003 | |
| Yes | 73/210 | 37% (29–45) | |||
| 2Index case with gastro symptoms | No | 29/103 | 30% (19–40) | 0.526 | |
| Yes | 46/145 | 34% (25–44) | |||
| 3Index case with systemic symptoms | No | 2/22 | 10% (0–24) | 0.048 | |
| Yes | 73/226 | 35% (27–42) | |||
1. Cough
shortness of breath
sore throat
runny nose
2. Nausea and diarrhoea
3. Fever
headache
fatigue
muscle/body pain.
In the sensitivity analysis with just exclusion of any prior or co-primary cases results, 121/324 (39%) contacts were infected. Overall results were similar though unlike the primary analysis (Table 2) the inverse relationship between household size and SAR was not significant. (Table S1)
Symptoms in infected contacts
Of the 121 prospectively identified infected contacts, 94 (78%) reported at least one of the solicited symptoms. The proportion with symptomatic infection was similar across the age groups (Table 3 ). However, loss of sense of taste or smell was less commonly reported in under 19 year olds than in older age groups (p = 0.0001). Children also had fewer mean number of symptoms (p = 001). Among those with co-morbidities a similar proportion reported symptoms as in those without a co-morbidity.
Table 3.
Clinical features in 121 prospectively followed infected contacts (prior and co-primary infected contacts excluded).
| Feature | Level | n/N | % (95% CI) | P-value |
| Proportion reporting one or more solicited symptoms | 94/121 | 78% | ||
| Proportion with one or more solicited symptoms by age (years) | 0 to10 | 13/18 | 72% | 0.440 |
| 11to18 | 15/21 | 71% | ||
| 19to54 | 57/69 | 83% | ||
| >=55 | 9/13 | 69% | ||
| Proportion with symptoms by presence of co-morbidity | None | 73/96 | 76% | 0.590 |
| Any | 21/25 | 84% | ||
| Proportion symptomatic with respiratory symptoms | 0 to10 | 9/13 | 69% | |
| 11to18 | 15/15 | 100% | P = 0.034 | |
| 19to54 | 53/57 | 93% | ||
| >=55 | 9/9 | 100% | ||
| Proportion symptomatic with loss of taste/smell | 0 to10 | 3/12* | 25% | |
| 11to18 | 4/15 | 27% | P = 0.0001 | |
| 19to54 | 39/57 | 68% | ||
| >=55 | 6/9 | 67% | ||
| Proportion symptomatic with systemic symptoms | 0 to10 | 13/13 | 109 4 0% | |
| 11to18 | 14/15 | 93% | P = 0.635 | |
| 19to54 | 56/57 | 98% | ||
| >=55 | 9/9 | 100% | ||
| Mean number of symptoms | 0 to10 | 4.4 | ||
| 11to18 | 5.8 | P = 0.001 | ||
| 19to54 | 7.4 | |||
| >=55 | 7.4 | |||
*symptom not known for one child.
Serial intervals
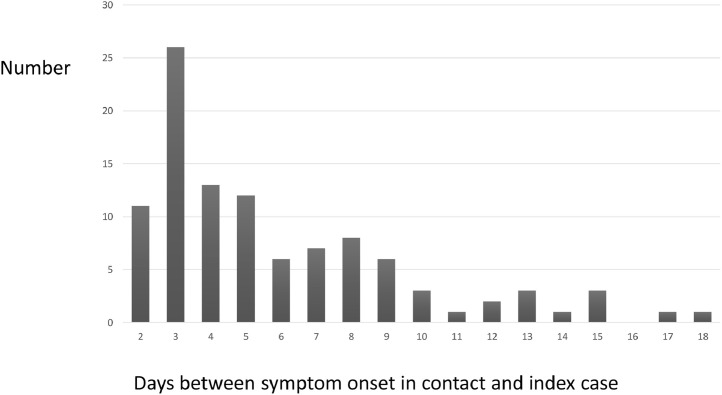
The serial intervals between symptom onset in index and secondary cases after exclusion of infected contacts with onsets before or within a day of the index case are shown in Fig. 2 . The median serial interval was 5 days with the interquartile range 3–8 days. The clustering around days 3, 7–9 and 13–15 suggests sequential transmission in the household.
Fig. 2.
Serial intervals within households: days between symptom onset in index and contact.
Factors affecting PCR positivity and CT values
Among the total of 180 contacts with antibody evidence of infection, 77 (43%) had one or more positive PCR swabs. The proportion PCR positive among infected contacts was similar in those with and without symptoms and by age (Table S2). PCR positivity in index cases and symptomatic infected contacts was highest when swabs were taken within 9 days of symptom onset, with the lowest Ct values found prior to symptom onset (Table 4 ). Persistent PCR positivity for more than 21 days after onset was found in 14.4% of swabs. Virus culture was carried out for 48 PCR positive swabs couriered to the laboratory of which 9 were culture positive (6/8 with a Ct value <25, 2/10 with a Ct value 25-<32 and 1/30 with a higher Ct value >32); no PCR positive swabs taken more than 7 days after symptom onset yielded viable virus. In the normal errors regression model after adjustment for interval from symptom onset, Ct values were lower in contacts than index cases (p = 0.015) and were lower in contacts with a shorter serial interval (p = 0.02).
Table 4.
Proportion of nasal swabs PCR positive, and mean Ct values in PCR positive swabs by time since onset of symptoms in infected contacts and index cases; first and second nasal swab combined.
| interval from symptom onset | PCR Positive | Total tested | % PCR Positive (95% CI) | mean Ct |
| −7 to −1 | 2 | 11 | 18.2% (2.3–51.8) | 23.22 |
| 0 to 2 | 11 | 21 | 52.4% (29.8–74.3) | 27.34 |
| 3 to 6 | 35 | 56 | 62.5% (48.5–75.1) | 27.33 |
| 7 to 9 | 45 | 85 | 52.9% (41.8–63.9) | 30.02 |
| 10 to 13 | 37 | 128 | 28.9% (21.2–37.6) | 32.95 |
| 14 to 20 | 27 | 197 | 13.7% (9.2–19.3) | 34.88 |
| 21 to 27 | 4 | 71 | 5.6% (1.6–13.8) | 36.35 |
| 28+ | 3 | 34 | 8.8% (1.9–23.7) | 36.51 |
| Total | 164 | 603 | 27.2% (23.7–3u0.9) | 30.94 |
*95% CI does not account for some individuals contributing two samples.
Sensitivity and positive predictive value of different symptoms
Among the 180 contacts with serological evidence of infection (i.e. including those with symptom onset prior to the index case) one or more of the solicited symptoms were reported by 153 (85%) compared with 107 (53%) of uninfected contacts (Table 5 ). Reporting at least one of the three symptoms that are triggers for community testing (fever, cough, loss of sense of taste or smell) had a sensitivity of 73% and captured 132/153 (86%) of all the symptomatic infected contacts. Among the 203 contacts without serological evidence of infection, 64 (32%) reported at least one of these three symptoms, though only 7% reported at least two and only 1% reported all three symptoms. The positive predictive value of different symptoms in this household study in which nearly half of the contacts were infected, was high with 67% of those reporting one or more of fever, cough or loss sense taste/smell being infected.
Table 5.
Sensitivity (% Infected with symptom) and positive predictive value of symptoms in contacts with a serological or oral fluid outcome, includes prior and co-primary cases.
| Reported symptoms | A: Infected (N = 180) with symptom | B: Uninfected (N = 203) with symptom | Positive predictive value A/A + B |
| Fever + cough + loss taste/smell | 42 (23%) | 3 (1%) | 93% |
| At least 2 of the above | 90 (50%) | 15 (7%) | 86% |
| At least one of the above | 132 (73%) | 64 (32%) | 67% |
| Other solicited symptom* | 21 (11.7%) | 43 (21%) | 33% |
| Any solicited symptom | 153 (85%) | 107 (53%) | 59% |
*Includes runny nose, sore throat, shortness of breath, nausea, diarrhoea, fatigue, muscle/body pain, headache.
Discussion
This prospective study of household transmission of SARS-CoV-2 shows high secondary attack rates when individuals are exposed in the home. Even after exclusion of asymptomatic antibody positive PCR negative contacts whose infection may have occurred in the past, evidence of transmission in the household setting was found in a third of contacts (Table 2). The risk of a primary case transmitting to other household contacts was lower in those without respiratory or systemic symptoms and was similar by age of the index case with a quarter of children under 11 years of age transmitting to another household member. When exposed in the home, children under 11 years of age had the lowest secondary attack rate though this was still substantial at 16%. Infected children were as likely as adults to report symptoms though they had fewer symptoms overall and were about two and a half times less likely than adults to report loss of taste or smell, a highly specific symptom for SARS-CoV-2 infection.
In this study self-isolation measures by the index cases to limit spread to other household members seemed ineffective. While the rigour with which self-isolation was followed could not be assessed, the lack of effect is consistent with the high infectivity of cases before symptom onset as shown by the Ct values in PCR positive swabs taken in this time period. Also with the short serial interval between onsets many contacts will already be incubating the infection by the time the PCR result in the index case is received and in some households (30% of those recruited in our study) there will be infected contacts with onset before that in the index case who were not tested. Use of routine testing data to assess household transmission rates, as recently reported,11 can therefore be misleading as the index case may not be the primary case in the household and many secondary cases may not be tested and remain undiagnosed. This is illustrated by the results of the HOSTED study in which routine Pillar 2 testing data was used to assess secondary attack rates in households and reported that only 5.5% of contacts were infected. 11
Our results show higher household transmission risks than reported in a meta-analysis of household transmission studies in which the weighted mean for the secondary attack rate was 16.6% (95% CI 14.0%−19.3%).2 However this analysis excluded studies that used antibody tests to diagnose infected contacts. In our study the secondary attack rate just based on PCR positive swabs from household contacts was similar at 16.9% whereas with the inclusion of serological testing the proportion infected more than doubled. It seems unlikely that the majority of the antibody positive PCR negative contacts had prior infection outside the home as most developed typical symptoms with onsets clustering around 3–5 days after the primary case. In our study the quality of the self-taken nasal swabs was likely to be variable and the timing of the day 1 and 7 swabs was not necessarily optimal in relation to symptom onset. In a smaller prospective household study in the United States in which contacts took daily nasal swabs for 14 days a secondary infection rate of 35% (95% CI 28%–43%) was found after exclusion of co-primary cases, similar to that in our study with inclusion of serology;12 the transmission risk from children who were the index cases was similar to that from adults and the risk of acquiring infection from an index case was similar for children and adults, mirroring the results of our study.
The overall proportion of prospectively ascertained infected contacts who did not develop symptoms (22%) was similar to that reported in a meta-analysis of outbreaks in closed or residential settings that found an overall rate of asymptomatic infection of 17% (95% CI 13%−20%) and evidence of a 42% lower risk of transmission.13 We were unable to assess transmission risk from asymptomatic cases as only nine were recruited and in none could a current infection be confirmed. Our study showed that some of the symptoms reported in infected contacts were likely to be non-specific background symptoms unrelated to SARS-CoV-2 infection as 53% of uninfected contacts reported at least one of the solicited symptoms before or during the 14 day follow up period (Table 5). When restricting symptoms to cough, fever and loss of sense of taste/smell the positive predictive value for infection was high in our household contact population in which 47% were infected but in a population with a substantially lower prevalence of infection, such as in the general population presenting for testing because of symptoms the positive predictive value of having cough, fever and loss of sense of taste/smell will be considerably lower, for example around 3% for a true overall prevalence of infection of 1%.
The sequential nasal swabs from index cases and contacts showed prolonged PCR positivity in some infected individuals though with high Ct values. As previously shown14 viable virus was rarely recovered at these high cycle numbers so such individuals are unlikely to be infectious. There was no relationship between Ct value and either age or illness severity, nor were secondary attack rates correlated with the minimum Ct value in the index case. Swabs were opportunistically taken at different times after onset and this was shown to be the major determinant of Ct value. Interestingly, after controlling for interval since onset, Ct values were lower in contacts than index cases, suggesting a greater viral load when infection is acquired under conditions of close exposure and more rapid development of symptoms as suggested by the shorter serial intervals in contacts with lower Ct values.
A high proportion of index cases recruited in the low incidence period between June and July 2020 were false positives. This is unsurprising as overall only 0.5% of Pillar 2 tests – the publicly bookable PCR tests for contacts or symptomatic members of the public - were positive during this period.9 Even with a highly specific PCR (e.g. 99.5%) with a true prevalence of 0.5%, about half of all positives detected will be false. The predominance of Pillar 2 tests reported as PCR positive based on a single gene target with a high Ct value during this low incidence period is consistent with false positives results.10 Two index cases had a positive PCR Pillar 2 test some months after their first, suggesting reinfection. However, neither of the two index cases nor their household members had any confirmatory PCR positive swabs nor any increase in antibody titres compared with the earlier time period, again consistent with a false positive second test. Our experience shows that it is important to confirm the status of the index case when studying household transmission and to fully investigate reported re-infections to confirm their validity.
This study was limited by the inability to study transmission from asymptomatic index cases and that it was largely conducted prior to the emergence of the Alpha (B.1.1.7) variant of concern first detected in Southern England in November 2020; sequencing of PCR positives from October onwards showed that only two families had the Alpha variant. The prospective household surveillance is being continued to compare the secondary attack rate in households infected with the Alpha and the more recent Delta variant, and from index cases who have received the AstraZeneca or Pfizer COVID-19 vaccines with that from unvaccinated individuals of similar age as an indication of the extent to which vaccination may provide additional protection against transmission from breakthrough infections in the household setting.15
Funding statement
This study was funded by Public Health England (an executive agency of the Department of Health) as part of the COVID-19 response. The authors had sole responsibility for the study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
Declaration of Competing Interest
The authors have declared no competing interest. SdeL is the Director of the Royal College of General Practitioners Research and Surveillance, and hold a grant from AstraZeneca both funded through his university.
Acknowledgments
We thank the members of the households who took in this enhanced surveillance, the PHE nurses who followed up the households, the administrative staff at PHE who sent out kits and arranged for the phlebotomy visits and Mary Matheson, Anna England, Debbie Powell, Jessica Fretwell, Lorraine Stapley and Rosie Watts at Porton PHE for receiving, spinning and storing the blood samples. We also thank staff of the Virus Reference Department of PHE Colindale who performed the molecular and serological testing and sequencing.
References
- 1.World Health organisation. Household transmission investigation protocol for 2019-novel coronavirus (COVID-19) infection. 28 February 2020 Available at https://www.who.int/publications-detail/household-transmission-investigation-protocol-for-2019-novel-coronavirus-(2019-ncov)-infection
- 2.Madewell Z.J., Yang Y., Longini I.M., Jr, Halloran M.E., Dean N.E. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.31756. Dec 1e2031756PMID: 33315116; PMCID: PMC7737089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boddington N.L., Charlett A., Elgohari S., Walker J.L., McDonald H.I., Byers C. COVID-19 in great Britain: epidemiological and clinical characteristics of the first few hundred (FF100) cases: a descriptive case series and case control analysis. Bull World Health Organ. 2020 [Preprint] E-pub: 22 May http://dx.doi.org/10.2471/BLT.20.265603. [Google Scholar]
- 4.de Lusignan S., Lopez Bernal J., Zambon M., Akinyemi O., Amirthalingam G., Andrews N. Emergence of a novel coronavirus (COVID-19): protocol for extending surveillance used by the royal college of general practitioners research and surveillance Centre and public health England. JMIR Public Health Surveill. 2020;6(2):e18606. doi: 10.2196/18606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guidance and standard operating procedure. COVID-19 virus testing in NHS laboratories https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/guidance-and-sop-covid-19-virus-testing-in-nhs-laboratories-v1.pdf
- 6.Department of Health and Social care. Scaling up our testing programme. 4th April 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/878121/coronavirus-covid-19-testing-strategy.pdf
- 7.Amirthalingam G. Vol. 27. 2021. Seroprevalence of SARS-CoV-2 among blood donors and changes after introduction of public health and social measures, London, UK; pp. 1795–1801. (Emerg Infect Dis). JulPMID: 34152947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coronavirus (COVID-19) in the UK. Cases in England. https://coronavirus.data.gov.uk/details/cases?areaType=nation&areaName=England. Accessed Monday 28 December 2020.
- 9.Surkova E., Nikolayevskyy N., Drobniewski F. False-positive COVID-19 results: hidden problems and costs. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30453-7. September 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker A.S., Pritchard E., House T., Robotham J.V., Birrel P.J., Bell J.I., The COVID-19 Infection Survey Team . doi. medRxiv; 2020. (Viral Load in Community SARS-CoV-2 Cases Varies Widely and Temporally). et al. .10.25.20219048. [DOI] [Google Scholar]
- 11.Hall J.A., Harris R.J., Zaidi A., Woodhall S.C., Dabrera G., Dunbar J.K. HOSTED-England's household transmission evaluation dataset: preliminary findings from a novel passive surveillance system of COVID-19 [published online ahead of print. Int J Epidemiol. 2021 doi: 10.1093/ije/dyab057. 2021 Apr 9] dyab057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grijalva C.G., Rolfes M.A., Zhu Y., McLean H.Q., Hanson K.E., Belongia E.A., Halasa N.B., Kim A., Reed C., Fry A.M., Talbot H.K. Vol. 69. 2020. Transmission of SARS-COV-2 infections in households - Tennessee and Wisconsin, April-September 2020; pp. 1631–1634. (MMWR Morb Mortal Wkly Rep). Nov 6PMID: 33151916; PMCID: PMC7643897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byambasuren O., Cardona M., Bell K., Clark J., McLaws M.-.L., Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Off J Assoc Med Microbiol Infect Dis Can. 2020;5(4) doi: 10.3138/jammi-2020-0030. December 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singanayagam A., Patel M., Charlett A., Lopez Bernal J., Saliba V., Ellis J., Ladhani S., Zambon M., Gopal R. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25(32) doi: 10.2807/1560-7917.ES.2020.25.32.2001483. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/951189/COVID-19_vaccine_surveillance_strategy.pdf AugPMID: 32794447; PMCID: PMC7427302. [DOI] [PMC free article] [PubMed] [Google Scholar]