ABSTRACT
Oocyte-specific knockdown of pericentrin (PCNT) in transgenic (Tg) mice disrupts acentriolar microtubule-organizing center (aMTOC) formation, leading to spindle instability and error-prone meiotic division. Here, we show that PCNT-depleted oocytes lack phosphorylated Aurora A (pAURKA) at spindle poles, while overall levels are unaltered. To test aMTOC-associated AURKA function, metaphase II (MII) control (WT) and Tg oocytes were briefly exposed to a specific AURKA inhibitor (MLN8237). Similar defects were observed in Tg and MLN8237-treated WT oocytes, including altered spindle structure, increased chromosome misalignment and impaired microtubule regrowth. Yet, AURKA inhibition had a limited effect on Tg oocytes, revealing a critical role for aMTOC-associated AURKA in regulating spindle stability. Notably, spindle instability was associated with disrupted γ-tubulin and lack of the liquid-like meiotic spindle domain (LISD) in Tg oocytes. Analysis of this Tg model provides the first evidence that LISD assembly depends expressly on aMTOC-associated AURKA, and that Ran-mediated spindle formation ensues without the LISD. These data support that loss of aMTOC-associated AURKA and failure of LISD assembly contribute to error-prone meiotic division in PCNT-depleted oocytes, underscoring the essential role of aMTOCs for spindle stability.
KEY WORDS: Oocyte, Spindle, Pericentrin, aMTOC, AURKA, TACC3, LISD
Summary: PCNT-depleted oocytes lack Aurora A at spindle pole aMTOCs and fail to assemble the liquid-like spindle domain, a critical reservoir of microtubule regulatory factors, leading to meiotic spindle instability.
INTRODUCTION
Chromosome segregation errors during female meiosis can lead to aneuploidy in oocytes and developing embryos upon fertilization, significantly contributing to congenital disorders and pregnancy loss in women (Jones and Lane, 2013; Nagaoka et al., 2012). Accurate chromosome segregation is contingent on the formation of a stable spindle microtubule (MT) apparatus and the establishment of correct bi-oriented (amphitelic) chromosome–MT attachments (Chmatal et al., 2015; Mihajlovic and FitzHarris, 2018). Notably, meiotic spindle assembly in mammalian oocytes differs from mitosis (Schuh and Ellenberg, 2007). Whereas somatic cells contain canonical centrosomes, composed of two centrioles surrounded by a pericentriolar matrix (PCM) that serve as the primary spindle microtubule-organizing centers (MTOCs) (Bettencourt-Dias and Glover, 2007), mammalian oocytes (mouse and human) lose centrioles early in development (Sathananthan et al., 2000; Szollosi et al., 1972). Despite the absence of centrioles, mouse oocytes express essential PCM proteins that are crucial for MT nucleation (Raynaud-Messina and Merdes, 2007), including γ-tubulin and associated γ-tubulin ring complex (γTuRC) proteins such as NEDD1 (Combelles and Albertini, 2001; Ma et al., 2010; Schuh and Ellenberg, 2007). The many structural and regulatory PCM proteins, including key kinases, form unique acentriolar microtubule-organizing centers (aMTOCs) with a distinctive ring-like structure at meiotic spindle poles (Schuh and Ellenberg, 2007; Baumann et al., 2017).
In previous studies we established that aMTOCs play a critical role in spindle stability and the accuracy of meiotic division in oocytes (Baumann et al., 2017). To test aMTOC function we developed a transgenic (Tg) mouse model with an oocyte-specific knockdown of pericentrin (PCNT), an essential scaffolding protein in somatic cell centrosomes (Chen et al., 2014; Delaval and Doxsey, 2010; Zimmerman et al., 2004) and oocyte aMTOCs (Ma and Viveiros, 2014). PCNT localizes specifically to aMTOCs throughout meiotic division (Luksza et al., 2013; Schuh and Ellenberg, 2007). Notably, oocyte-specific knockdown of PCNT in Tg mice disrupts the accumulation of PCM proteins and the formation of aMTOCs (Baumann et al., 2017). Although Ran GTPase activity (Dumont et al., 2007; Schuh and Ellenberg, 2007) promotes meiotic spindle assembly in PCNT-depleted oocytes, the loss of functional aMTOCs leads to meiotic spindle instability, highly error-prone meiotic division and female subfertility (Baumann et al., 2017). To determine the underlying basis of spindle instability in Tg oocytes, we assessed a key aMTOC-associated kinase, Aurora A (AURKA) and the newly identified ‘liquid-like meiotic spindle domain’ (LISD; So et al., 2019), which are critical for spindle assembly.
AURKA is a highly conserved serine/threonine kinase that localizes to the centrosome in somatic cells and regulates key events during mitosis, including centrosome maturation and spindle assembly (Barr and Gergely, 2007; Sardon et al., 2008; Joukov et al., 2014; Magnaghi-Jaulin et al., 2019). Its activation is dependent on phosphorylation of a conserved residue (threonine 288) in the activation loop of the catalytic domain. The MT-associated protein TPX2 has been identified as an important cofactor and activator of AURKA (Bayliss et al., 2003; Eyers and Maller, 2004; Walter et al., 2000). Disruption of AURKA in somatic cells leads to centriole separation failure, spindle defects and chromosome segregation errors (Joukov et al., 2014; Magnaghi-Jaulin et al., 2019; Marumoto et al., 2003). In oocytes, AURKA is phosphorylated upon the resumption of meiosis and localizes specifically to aMTOCs (Nguyen and Schindler, 2017; Saskova et al., 2008). Its activator, TPX2, is also expressed in oocytes and associates with meiotic spindle MTs (Brunet et al., 2008). Studies support a key role for AURKA in the regulation of spindle assembly upon the resumption of meiosis (Blengini et al., 2021; Bury et al., 2017; Saskova et al., 2008; Solc et al., 2012) as well as the maintenance of aMTOC organization and spindle structure in ovulated metaphase II (MII) oocytes (Wang et al., 2020). Notably, a recent study (So et al., 2019) demonstrated that AURKA activity and its target, TACC3 (transforming acidic coiled-coil-containing protein 3) (Burgess et al., 2018; Kinoshita et al., 2005), are essential to promote the formation of the unique and highly dynamic LISD in mammalian oocytes. The LISD is proposed to serve as an important reservoir for MT regulatory factors that are crucial for stable meiotic spindle assembly (So et al., 2019).
In this study we establish that PCNT-depleted oocytes from Tg mice lack phosphorylated AURKA (pAURKA) as well as the unique LISD at meiotic spindle poles. These data provide the first evidence that in vivo LISD assembly depends specifically on aMTOC-associated AURKA, rather than overall protein levels in the oocyte. Moreover, it demonstrates that Ran-mediated spindle formation in Tg oocytes occurs despite the absence of the LISD. Importantly, loss of aMTOC-associated AURKA and failure of LISD assembly contribute to error-prone meiotic division in PCNT-depleted oocytes, supporting the essential role of aMTOCs for meiotic spindle stability.
RESULTS
AURKA localization to oocyte aMTOCs is dependent on PCNT
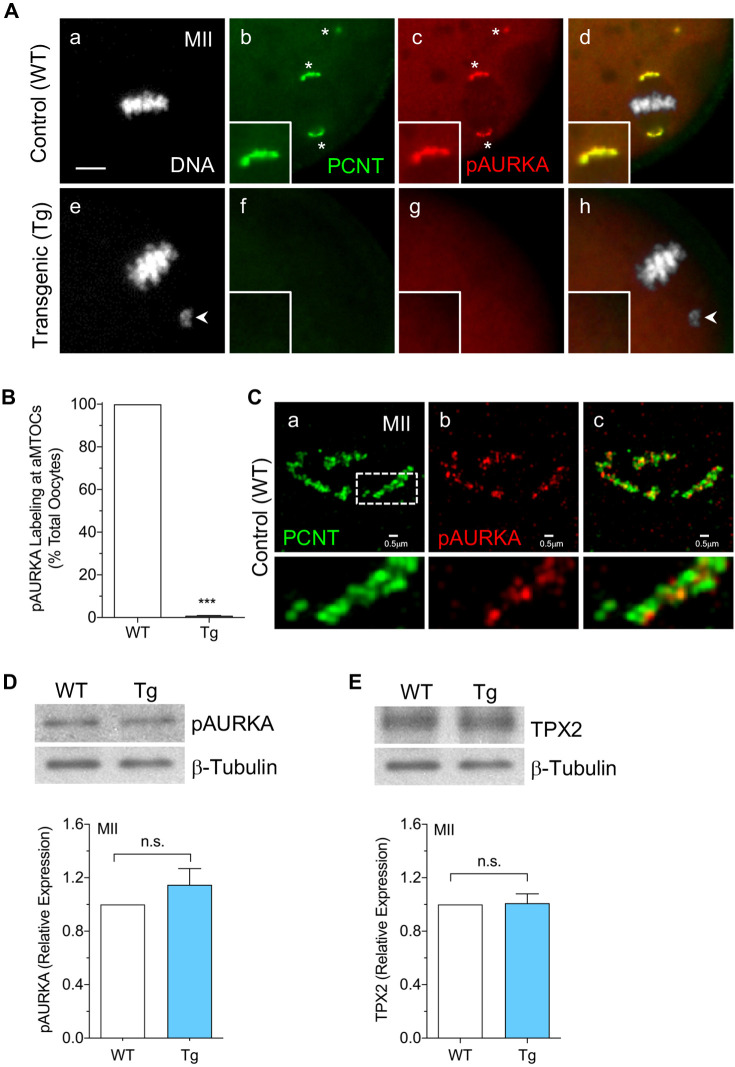
Phosphorylation of AURKA promotes a conformational change associated with kinase activation (Bayliss et al., 2003; Eyers and Maller, 2004). In oocytes, pAURKA localizes to aMTOCs upon the resumption of meiosis and throughout meiotic division (Balboula et al., 2016; Bury et al., 2017; So et al., 2019; Solc et al., 2012; Wang et al., 2020). To determine whether pAURKA associates with aMTOCs in a PCNT-dependent manner, we compared pAURKA labeling in wild-type (WT) control and PCNT-depleted oocytes from Tg mice (Baumann et al., 2017). Consistent with previous studies, all control MII oocytes showed bright pAURKA, colocalized with PCNT, at meiotic spindle pole aMTOCs as well as small distinct cytoplasmic aMTOCs (Fig. 1A panels a–d,B). We extended these findings using super-resolution structured illumination microscopy (SR-SIM) analysis of WT MII oocytes to reveal the spatial organization of pAURKA and PCNT at spindle pole aMTOCs. The two proteins were observed in an interlacing pattern within the partial or complete ring-like aMTOC structure (Fig. 1C). In sharp contrast, no pAURKA was detected at aMTOCs in PCNT-depleted metaphase I (MI)-stage (Fig. S1) or ovulated MII (Fig. 1A panels e–h,B) oocytes. Yet, western blots showed no difference in total pAURKA protein levels in lysates from WT control and Tg MII oocytes (Fig. 1D). Protein levels of TPX2, a key cofactor and activator of AURKA (Bayliss et al., 2003; Eyers and Maller, 2004), were also similar between WT and Tg MII-arrested oocytes (Fig. 1E). These data indicate that neither AURKA phosphorylation (activation) nor total pAURKA protein levels are disrupted in PCNT-depleted oocytes. In contrast, pAURKA localization to aMTOCs is dependent on PCNT and is absent from meiotic spindle pole aMTOCs in Tg (PCNT-depleted) oocytes.
Fig. 1.
pAURKA localization to oocyte aMTOCs is dependent on PCNT. (A) Representative images of ovulated MII control (n=98) and PCNT-depleted (n=70) oocytes collected from WT and Tg mice, respectively, double labeled using anti-PCNT (green) and anti-pAURKA (red) antibodies at aMTOCs (*). DAPI-labeled DNA is shown in gray. Merged images are shown in panels d and h. Arrowheads indicate misaligned chromosomes. Insets show 2× magnification of the spindle pole. Scale bar: 5 µm. (B) Percentage of total oocytes with pAURKA labeling at aMTOCs. Data are presented as mean±s.e.m. of three experiments. (C) SR-SIM of PCNT (green) and pAURKA (red) colocalization at spindle pole aMTOCs in ovulated MII oocytes (n=7) from WT mice. Merged image is shown in panel c. Lower panels show 3× magnification of the region indicated by a dashed box. (D,E) Western blot analysis of (D) pAURKA and (E) TPX2 protein levels in ovulated MII oocytes (50 oocytes/lane) from WT and Tg mice. β-tubulin was used as an internal control, and total protein levels in WT oocytes are normalized to 1.0 for comparisons. Data are presented as mean±s.e.m. of three experiments. ***P<0.001, n.s., not significant (two-tailed, unpaired, Student's t-test).
aMTOC-associated AURKA plays a critical role in regulating assembled spindle structure
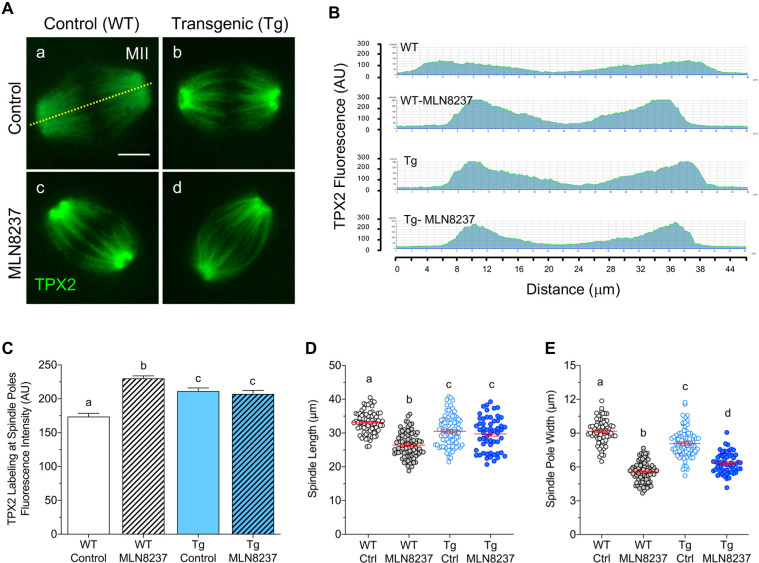
Next, we assessed whether pAURKA function is contingent on its aMTOC association and whether the absence of pAURKA from spindle pole aMTOCs contributes to the meiotic defects in PCNT-depleted oocytes. Our previous studies revealed altered spindle structure in PCNT-depleted oocytes (Baumann et al., 2017) and that AURKA activity affects assembled spindle organization in ovulated oocytes (Wang et al., 2020). Therefore, we compared meiotic spindle organization between WT and PCNT-depleted MII oocytes after a 4 h culture in either medium alone (control) or with a specific AURKA inhibitor (MLN8237). Spindle MTs were labeled with TPX2 in all groups (Fig. 2A–C); however, TPX2 labeling was significantly brighter towards the spindle poles in PCNT-depleted oocytes (Fig. 2A panel b). Significantly brighter TPX2 fluorescence towards the poles (Fig. 2B,C) was also observed after AURKA inhibition in both WT (Fig. 2A panel c) and Tg (Fig. 2A panel d) oocytes. Similar TPX2 labeling in Tg and MLN8237-treated oocytes indicates that aMTOC-associated AURKA influences TPX2 distribution along the spindle MTs.
Fig. 2.
Loss of PCNT and AURKA inhibition alter TPX2 distribution and assembled spindle structure. (A) Representative images of MT-associated TPX2 (green) labeling along the meiotic spindle in MII oocytes from WT and Tg mice, following a 4 h culture in either medium alone (control) or with 500 nM MLN8237. Dashed line represents the spindle length transect used to measure TPX2 fluorescence. Scale bar: 10 µm. (B) Representative scans of TPX2 fluorescence along the spindle length in oocytes from each group. (C) Mean±s.e.m. fluorescence intensity of TPX2 at spindle pole minus end MTs in control and MLN8237-treated WT (n=58 and n=54, respectively) and Tg (n=65 and n=44, respectively) MII oocytes. (D,E) Quantitative analysis (mean±s.e.m.) of the meiotic (D) spindle length and (E) average spindle pole width in control (Ctrl) and MLN8237-treated WT (n=73 and n=81, respectively), and Tg (n=86 and n=56, respectively) MII oocytes. Different letters denote significant differences (P<0.05) between groups (one-way ANOVA with Tukey's multiple comparisons test). AU, arbitrary units.
Analysis of meiotic spindle length and pole width (Fig. 2D,E) also points to AURKA and aMTOC regulation of assembled spindle structure. Significantly shorter spindles were observed in WT oocytes following AURKA inhibition (Fig. 2D) (Wang et al., 2020). Loss of PCNT and aMTOCs in Tg oocytes also leads to shorter spindles (Baumann et al., 2017), but to a lesser extent than AURKA inhibition in WT oocytes, as demonstrated by direct comparison (Fig. 2D). Notably, exposure of Tg oocytes to the AURKA inhibitor failed to further decrease the spindle length (Fig. 2D), supporting that aMTOC-associated AURKA, rather than overall activity in the oocyte, plays a key role in the regulation of spindle length. Inhibition of AURKA also leads to highly focused spindle poles (Fig. 2E) (Wang et al., 2020). Direct comparisons showed reduced pole width in Tg (PCNT-depleted) oocytes, albeit to a lesser extent than AURKA inhibition (Fig. 2E). MLN8237 exposure further reduced the spindle pole width in Tg oocytes (Fig. 2E). These data support that spindle structure alterations in PCNT-depleted oocytes are linked to the loss of pAURKA from the spindle pole aMTOCs.
Loss of PCNT and aMTOC-associated AURKA disrupts spindle microtubule regrowth and chromosome alignment
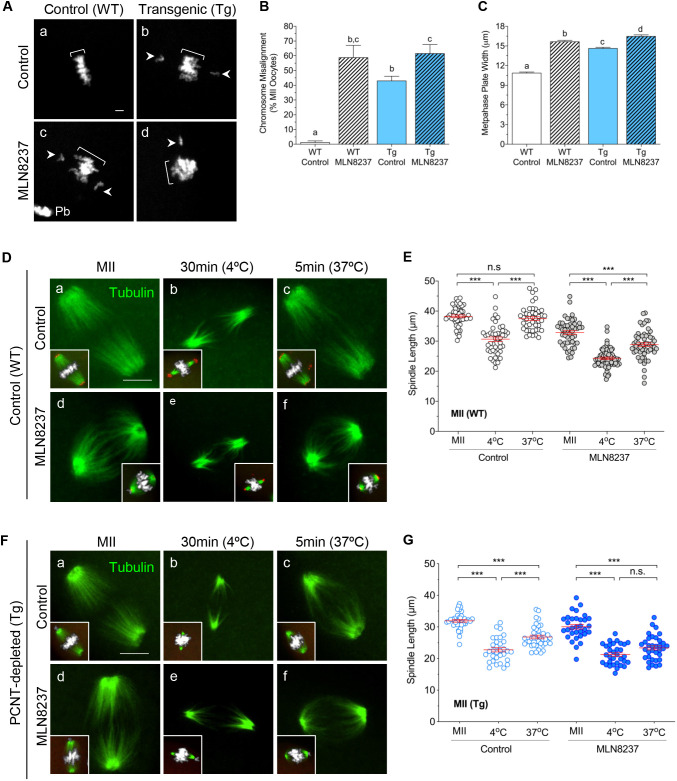
Next, we assessed chromosome alignment and MT regrowth in ovulated WT and PCNT-depleted Tg MII oocytes, following a brief (4 h) culture in medium alone or with the AURKA inhibitor (Fig. 3A–C). Over 95% of WT control oocytes maintained tightly congressed chromosomes that fully aligned at the metaphase plate (Fig. 3A panel a). Similar to our previous studies (Baumann et al., 2017), we observed that PCNT-depleted oocytes in medium alone exhibited high rates of chromosomes misalignment and disrupted chromosome congression (Fig. 3A panel b). Inhibition of AURKA activity also increased chromosome misalignment (Fig. 3B) and disrupted chromosome congression (Fig. 3C) in both WT and Tg oocytes (Fig. 3A panels c,d). Interestingly, chromosome misalignment rates were comparable between WT and Tg MLN8237-treated oocytes. These findings support that aMTOC-associated AURKA plays an important role in maintaining chromosome alignment, likely through its regulation of spindle stability. Thus, the high incidence of chromosome errors observed in PCNT-depleted oocytes may be attributed to the loss of aMTOC-associated pAURKA.
Fig. 3.
Loss of PCNT as well as AURKA inhibition promote increased chromosome errors and impaired MT regrowth. (A) Representative images of chromosome misalignment (arrowheads) in control and MLN8237-treated WT oocytes (n=132 and n=156, respectively), compared to Tg PCNT-depleted oocytes (n=152 and n=119, respectively). Chromosomes are labeled with DAPI. Brackets indicate metaphase plate width; Pb, polar body. Scale bar: 10 µm. (B) Mean±s.e.m. percentage of total oocytes with chromosome misalignment in each group. n=4 experiments. (C) Mean±s.e.m. width of the metaphase plate, indicative of chromosome congression. In B and C, different letters denote significance (P<0.05) between groups (one-way ANOVA with Tukey's multiple comparisons test). (D–G) Representative images of control and MLN8237-treated oocytes from (D) WT and (F) Tg mice. Ovulated oocytes were fixed either after 4 h culture (MII), 30 min after cold treatment at 4°C to depolymerize MTs, or 5 min post re-warming at 37°C to assess MT regrowth. Insets show spindle MTs and chromosome configurations. Oocytes were double labeled with anti-acetylated tubulin (green) and anti-PCNT (red) antibodies to detect MTs and aMTOCs, respectively. DAPI-labeled DNA is shown in gray. Scale bars: 10 µm. Mean±s.e.m. spindle length measured in (E) WT and (G) Tg oocytes from each group: control MII (WT, n=50; Tg, n=36), post 4°C cold treatment (WT, n=45; Tg, n=32) and post 37°C re-warming (WT, n=43; Tg, n=39), compared to MLN8237-treated oocytes at MII (WT, n=49; Tg, n=32), 4°C cold treatment (WT, n=63; Tg, n=32) and 37°C re-warming (WT, n=56; Tg, n=34). ***P<0.001; n.s., not significant (one-way ANOVA with Tukey's multiple comparisons test).
Because AURKA plays an important role in regulating MT stability (Sardon et al., 2008), we evaluated the persistence of cold-stable MTs as well as MT regrowth following cold treatment (Fig. 3D–G) in control and MLN8237-treated MII oocytes from both WT (Fig. 3D,E) and Tg (Fig. 3F,G) mice. Following a 30 min. incubation at 4°C, cold-stable MTs were primarily detected towards the spindle poles (Fig. 3D,F), and the mean spindle length (distance from pole to pole) was significantly reduced in all groups (Fig. 3E,G). Next, we assessed MT regrowth, as denoted by brighter interpolar MTs and the spindle length, following a brief (5 min) incubation at 37°C in pre-warmed medium. Interestingly, the ‘recovered’ spindle length differed between the control and inhibitor-treated groups in both WT and PCNT-depleted (Tg) oocytes. Upon re-warming, the ‘increased/recovered’ spindle length in WT controls was comparable to oocytes prior to cold treatment (Fig. 3D panels a–c,E). However, WT oocytes exposed to the AURKA inhibitor showed limited MT regrowth and contained significantly shorter spindles following re-warming (Fig. 3D panels d–f,E). Notably, quantitative analysis of Tg oocytes in medium alone also revealed significantly shorter lengths post re-warming (Fig. 3F panels a–c,G), indicative of impaired MT regrowth. In turn, Tg oocytes exposed to the AURKA inhibitor showed no significant increase in spindle length post re-warming (Fig. 3F panels d–f,G). These data indicate that aMTOC-associated pAURKA plays a critical, albeit not exclusive, role in regulating MT regrowth that can influence meiotic spindle stability in oocytes.
Inhibition of AURKA disrupts aMTOC organization and γ-tubulin targeting to spindle MTs
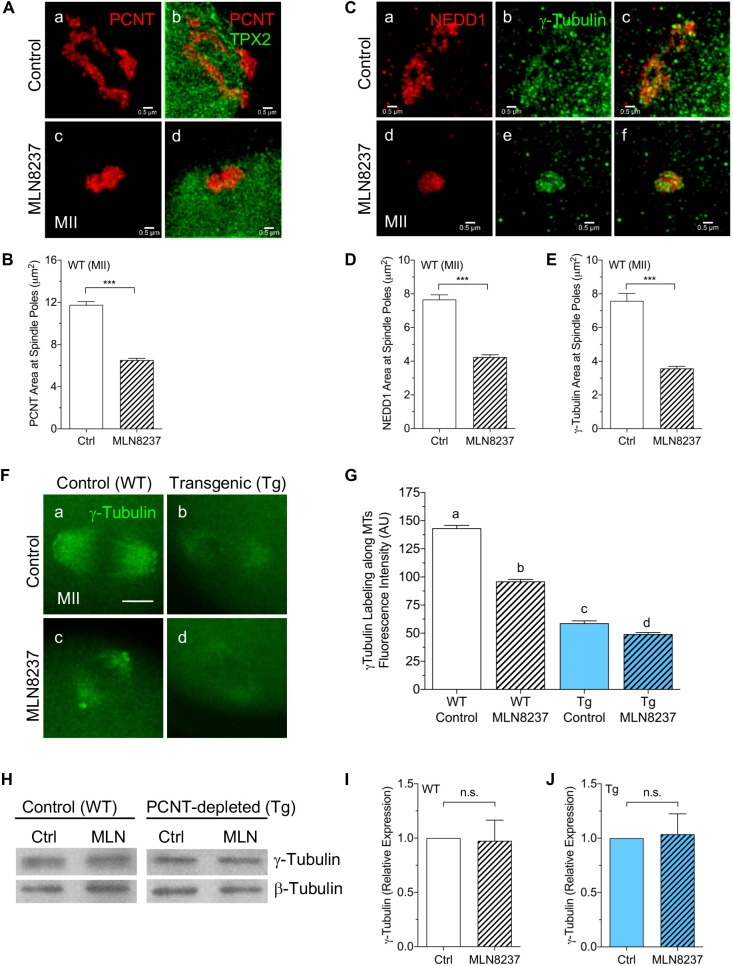
To gain insight into the underlying mechanisms that contribute to limited MT regrowth in response to PCNT loss and AURKA inhibition, we assessed the distribution and expression of key aMTOC-associated factors that promote MT nucleation: γ-tubulin and its anchoring protein in the γTuRC, NEDD1 (Ma et al., 2010; Raynaud-Messina and Merdes, 2007). Consistent with previous observations (Bury et al., 2017; Wang et al., 2020), additional SR-SIM analysis revealed that brief exposure to MLN8237 leads to a striking disruption of the ring-like aMTOC organization, with marked clustering of PCNT into compact foci at the spindle poles (Fig. 4A,B). Pronounced clustering of both NEDD1 and γ-tubulin into small foci was also observed in response to treatment with the AURKA inhibitor (Fig. 4C–E). Super-resolution imaging of control oocytes (Fig. 4C panels a–c) showed γ-tubulin colocalized with NEDD1 in an interweaving pattern at aMTOCs. Whereas NEDD1 labeling was restricted to aMTOCs, γ-tubulin was also detected along the spindle MTs (Fig. 4C panels b,c). Interestingly, AURKA inhibition diminished MT-associated γ-tubulin (Fig. 4C panels e,f).
Fig. 4.
AURKA activity regulates aMTOC organization and γ-tubulin targeting to spindle MTs. (A) SR-SIM imaging of PCNT (red) and TPX2 (green) at spindle poles in WT control (n=9) and MLN8237-treated (n=11) MII oocytes. (B) Mean±s.e.m. PCNT area at the spindle poles of control (Ctrl; n=103) and MLN8237-treated (n=114) WT oocytes. (C) SR-SIM images showing NEDD1 (red) and γ-tubulin (green) at spindle pole aMTOCs in control (n=5) and MLN8237-treated (n=7) WT oocytes. Merged images are shown in panels c and f. (D,E) Mean±s.e.m. area of (D) NEDD1 and (E) γ-tubulin at spindle poles of control (n=56, NEDD1; n=54, γ-tubulin) and MLN8237-treated (n=71, NEDD1; n=81, γ-tubulin) WT oocytes. ***P<0.001 (two-tailed, unpaired Student's t-test). (F) Fluorescent γ-tubulin labeling along spindle MTs in control and MLN8237-treated WT (n=67 and n=80, respectively) and Tg (n=63 and n=67, respectively) MII oocytes. (G) Fluorescence intensity of γ-tubulin along spindle MTs. Different letters denote significance (P<0.05) between groups (one-way ANOVA with Tukey's multiple comparisons test). (H–J) Western blot analysis of γ-tubulin levels in oocytes (n=50/lane) from WT and Tg mice, following a 4 h culture in either medium alone or with 500 nM MLN8237 (MLN). β-tubulin was used as an internal control. Mean±s.e.m. γ-tubulin protein levels are shown for (I) WT and (J) Tg oocytes, with control levels normalized to 1.0 for comparisons (n=3 experiments). n.s., not significant (two-tailed, unpaired Student's t-test). Scale bars: 0.5 µm (A,C), 10 µm (F).
Therefore, we assessed the fluorescence intensity of γ-tubulin along the spindle MTs in both WT and Tg MII oocytes, following a brief (4 h) culture with or without the AURKA inhibitor (Fig. 4F,G). In the WT control group, γ-tubulin was detected at spindle pole aMTOCs and diffusely along the spindle MTs (Fig. 4F panel a). However, after MLN8237 exposure, γ-tubulin labeling became dimmer and restricted to MTs towards the spindle poles as well as clustered aMTOCs (Fig. 4F panel c). Notably, Tg oocytes exhibited only dim γ-tubulin labeling along the spindle MTs (Fig. 4F panel b), which was further reduced by AURKA inhibition (Fig. 4F panel d). Quantitative analysis (Fig. 4G) confirmed significantly lower γ-tubulin fluorescence intensity along spindle MTs in Tg oocytes and following MLN8237 treatment in both groups. Nonetheless, western blot analysis showed no significant difference in total protein levels between control and MLN8237-treated oocytes from either the WT or Tg groups (Fig. 4H–J), confirming that loss of PCNT and/or AURKA inhibition specifically disrupts the localization of γ-tubulin to meiotic spindle MTs. Taken together, these data support that AURKA activity plays an important role in maintaining aMTOC organization as well as γ-tubulin targeting to the spindle MTs.
LISD assembly is dependent on PCNT and aMTOC-associated AURKA
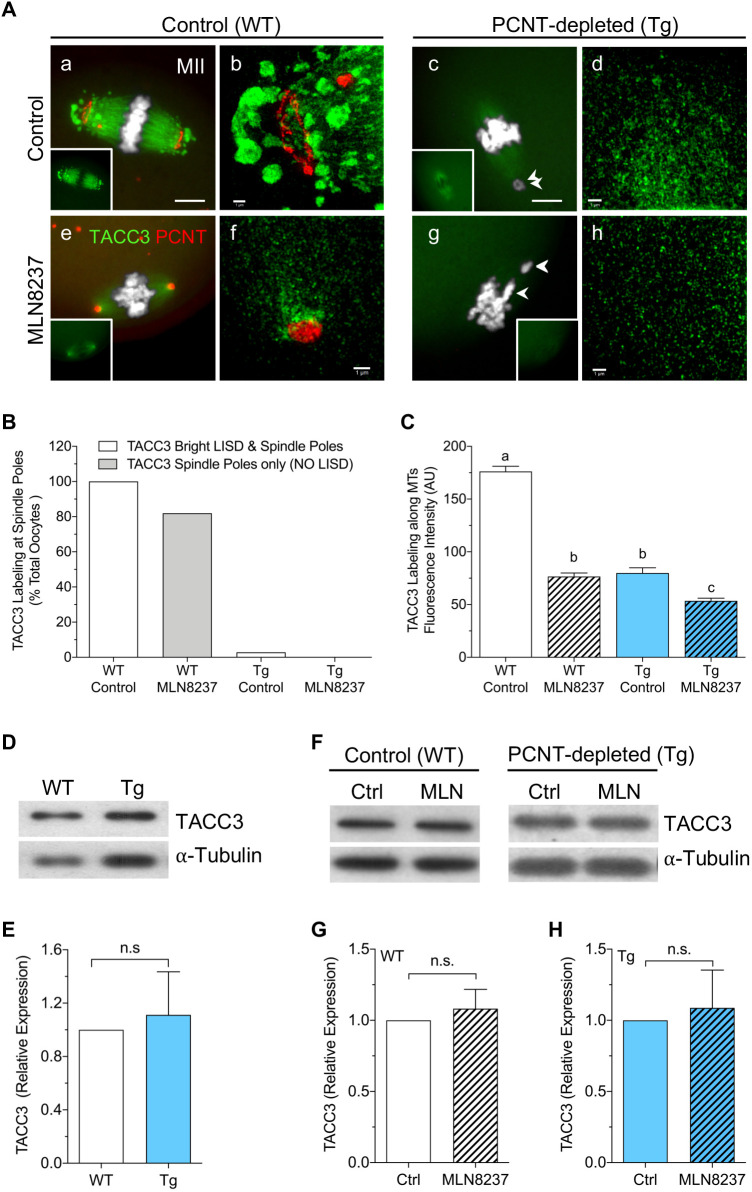
Recent studies have identified a unique LISD in mammalian oocytes, which serves as an important reservoir for MT regulatory factors that are critical for stable meiotic spindle assembly (So et al., 2019). AURKA and its downstream target, TACC3, play an essential role in LISD formation (So et al., 2019). Hence, we assessed whether LISD assembly occurs in PCNT-depleted oocytes from Tg mice. A key LISD component protein, TACC3, was used as a specific LISD marker in WT and Tg MII oocytes, with and without AURKA inhibition (Fig. 5A–C). In WT control oocytes, bright TACC3 was detected along the MTs as well as at prominent LISD protrusions at both spindle poles (Fig. 5A panel a; Movies 1 and 2). SR-SIM analysis revealed the spatial distribution of the LISD protrusions, which extend beyond the spindle poles (Fig. 5A panel b). The aMTOCs were brightly labeled with PCNT, revealing their distinct ring-like organization, but no TACC3 labeling was detected at the aMTOCs (Fig. 5A panel b; Movie 3). Brief (4 h) exposure of WT oocytes to the AURKA inhibitor caused complete loss of the LISD protrusions as well as a significant decrease in TACC3 labeling along the spindle MTs (Fig. 5A panel e,B,C; Movies 4 and 5). SR-SIM analysis of the spindle pole area also showed loss of the ring-like aMTOC organization and pronounced clustering of PCNT at highly focused poles with no LISD (Fig. 5A panel f; Movie 6). Similar to previous studies in MI oocytes (So et al., 2019), these data support an essential role for AURKA activity in maintaining the LISD at assembled meiotic spindles in ovulated MII oocytes.
Fig. 5.
PCNT-depleted oocytes lack the LISD at spindle poles. (A) Fluorescent (panels a,c,e,g) and SR-SIM (panels b,d,f,h) imaging of PCNT (red) and TACC3 (green) labeling of aMTOCs and the LISD, respectively, in WT and Tg (PCNT-depleted) MII oocytes, following a 4 h culture in either medium alone or MLN8237 (500 nM). DNA is labeled using DAPI (gray). Insets show TACC3 staining. Arrowheads indicate misaligned chromosomes. Scale bars:10 µm (a,c,e,g), 0.5 µm (b,d,f,h). (B) Percentage of oocytes with TACC3 labeling at meiotic spindle poles in control or MLN8237-treated WT (n=83 and n=94, respectively) and PCNT-depleted Tg (n=70 and n=72, respectively) groups. (C) Mean±s.e.m. TACC3 fluorescence intensity along MTs in all groups (AU, arbitrary units). Different letters denote significance (P<0.05) between groups (one-way ANOVA with Tukey's multiple comparisons test). (D) Western blot analysis of TACC3 protein levels in WT and Tg ovulated MII oocytes (n=50/lane). α-tubulin was used as an internal control. (E) Quantification shows mean±s.e.m. TACC3 protein levels (n=4), with Wt levels normalized to 1.0 for comparisons. (F) Western blot analysis of TACC3 levels in ovulated oocytes (n=50/lane) from WT and Tg mice, following a 4 h culture in either medium alone (Ctrl) or with 500 nM MLN8237 (MLN). α-tubulin was used as an internal control. (G,H) Quantification of mean±s.e.m. TACC3 protein levels (n=5) in (G) WT and (H) Tg oocytes, with control levels normalized to 1.0 for comparisons. n.s., not significant (two-tailed, unpaired, Student's t-test).
Tg mice provided a unique in vivo model to test whether LISD assembly is dependent on oocyte PCNT and aMTOCs. Notably, fluorescence analysis of TACC3 labeling revealed a striking lack of the LISD in ovulated MII oocytes collected from Tg mice (Fig. 5A panel c; Movies 1 and 2). No LISD protrusions were detected in any PCNT-depleted oocytes (Fig. 5B), and SR-SIM revealed only faint TACC3 staining along MTs (Fig. 5A panel d; Movie 3). Similarly, analysis of TACC3 distribution at MI showed TACC3 labeling along the spindle MTs, but no LISD protrusions in Tg (PCNT-depleted) MI oocytes (Fig. S2, Movie 7). Brief exposure to the AURKA inhibitor (Fig. 5A panels g,h; Movies 4 and 5) further decreased TACC3 fluorescence intensity along the MTs in ovulated MII Tg oocytes (Fig. 5C). Yet, western blot analysis showed no significant difference in total TACC3 levels between ovulated WT and Tg (PCNT-depleted) oocytes (Fig. 5D,E). TACC3 levels were also similar in control and MLN8237-treated oocytes from both the WT and Tg groups (Fig. 5F–H), pointing to the redistribution of these proteins into the cytoplasm. In sum, these findings support that AURKA activity is crucial for maintaining the LISD at assembled spindle poles during MII arrest. Notably, analysis of Tg oocytes establishes that LISD assembly depends specifically on aMTOC-associated AURKA.
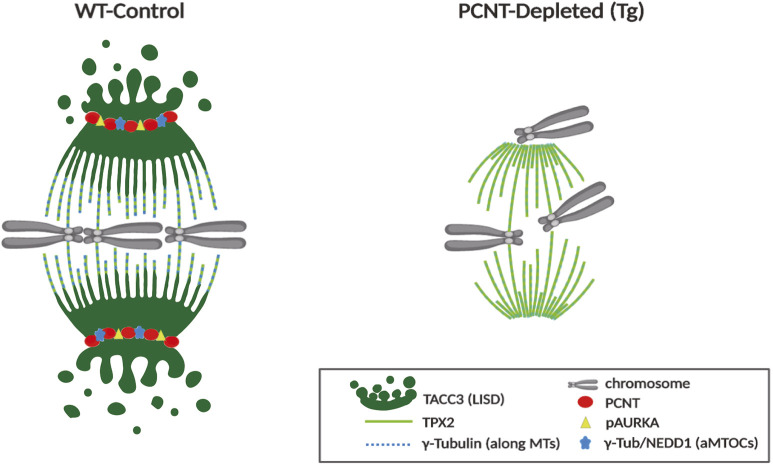
Taken together, our findings identify key underlying mechanisms that contribute to spindle instability in PCNT-depleted oocytes that lack functional aMTOCs. A schematic summary illustrates crucial differences between WT and Tg (PCNT-depleted) oocytes, including spindle structure, LISD assembly and aMTOC-associated factors (Fig. 6).
Fig. 6.
Loss of aMTOCs disrupts meiotic spindle organization and stability. Schematic illustrating crucial differences in meiotic spindle organization, key aMTOC-associated factors and the LISD at spindle poles between WT control and PCNT-depleted (Tg) ovulated oocytes that lack functional aMTOCs (γ-Tub, γ-tubulin).
DISCUSSION
We previously demonstrated that oocyte-specific knockdown of PCNT in Tg mice disrupts the formation of aMTOCs and, thus, the meiotic spindle assembles in a Ran-GTPase-dependent manner (Baumann et al., 2017). Notably, Tg oocytes show marked spindle instability, leading to error-prone meiotic division, aneuploidy and female subfertility (Baumann et al., 2017). However, the underlying mechanisms by which aMTOCs promote meiotic spindle stability are not fully understood. Here, we report that PCNT-depleted oocytes lack pAURKA at spindle pole aMTOCs and fail to assemble the unique LISD. Analyses of ovulated oocytes from Tg mice establish that LISD assembly depends specifically on aMTOC-associated AURKA activity rather than overall protein levels, and that Ran-mediated meiotic spindle formation in PCNT-depleted oocytes occurs without the LISD. Our findings identify key underlying mechanisms that contribute to error-prone meiotic division in PCNT-depleted oocytes (Fig. 6) and further supports the essential role of aMTOCs in safeguarding meiotic spindle stability.
Immunofluorescence analysis of ovulated MII oocytes from Tg mice revealed the absence of AURKA at spindle pole aMTOCs, despite unaltered total protein levels for both AURKA and a key activator, TPX2. PCNT-depleted oocytes also lacked AURKA at aMTOCs during MI, supporting that AURKA localization at aMTOCs is dependent on PCNT. This is consistent with studies in somatic cells, which demonstrate that AURKA binds CEP192 and is targeted to the centrosome in a PCNT-dependent manner (Joukov et al., 2014). Super-resolution analysis using SR-SIM confirmed colocalization of AURKA and PCNT at aMTOCs and revealed a distinct ‘interweaving’ pattern of the proteins. This is suggestive of higher-order organization and is similar to our observations for PCNT and CEP215 (CDK5RAP2) in mouse oocytes (Wang et al., 2020). Therefore, PCNT-depleted oocytes provide a unique in vivo model to test whether AURKA regulation of meiotic spindle organization and/or stability is contingent on its localization to aMTOCs at the spindle poles.
It is well established that AURKA plays an essential role in initial meiotic spindle assembly upon the resumption of meiosis (Bury et al., 2017; Solc et al., 2012), as well as the maintenance of assembled spindle and aMTOC organization in ovulated MII oocytes (Wang et al., 2020). Moreover, while this article was under review, a new study reported the development of an oocyte-conditional Aurka-knockout mouse model. Their analysis revealed that knockout of Aurka in oocytes disrupts formation of the first meiotic spindle, leading to meiotic arrest at MI and female infertility (Blengini et al., 2021). To test aMTOC-associated AURKA function, we compared assembled meiotic spindle organization between ovulated WT and Tg oocytes, following a brief (4 h) incubation in medium alone or with MLN8237 to inhibit overall kinase activity. Control Tg oocytes contained significantly shorter spindles with reduced pole width compared to those of WT controls, as we previously reported (Baumann et al., 2017). Similar alterations were observed in WT oocytes following AURKA inhibition, confirming that AURKA is a key regulator of meiotic spindle organization and size. Notably, AURKA inhibition failed to decrease the spindle length in Tg oocytes and had a more limited effect on pole focusing compared to the effects in WT oocytes. These data support that aMTOC-associated AURKA, rather than overall protein levels in the oocyte, plays a critical role in regulating meiotic spindle length. It also suggests that regulation of pole focusing may involve AURKA on both spindle pole aMTOCs and spindle MTs. Although further studies are needed to determine the precise mechanisms by which AURKA regulates assembled MII meiotic spindle organization and size, similarities to mitotic spindle regulation are plausible. In somatic cells, several known AURKA substrates play a role in pole focusing, including ASPM and CEP215 (Sardon et al., 2008; Tungadi et al., 2017). Moreover, AURKA has been shown to maintain spindle length by negatively regulating the activity of KIF2A, a MT minus end-binding depolymerase (Jang et al., 2009; Nehlig et al., 2021). Studies also demonstrate that disruption of AURKA and TPX2 interaction leads to shorter spindles (Bird and Hyman, 2008). Interestingly, our analysis revealed altered TPX2 distribution along the meiotic spindle in Tg oocytes and in WT oocytes exposed to MLN8237, with significantly brighter TPX2 labeling observed towards the spindle poles. Besides activating AURKA, TPX2 can influence spindle length by regulating MT flux (Fu et al., 2015) and promotes branching MT nucleation from existing MTs for spindle stability (Petry et al., 2013). Moreover, TPX2 is important for Ran-mediated spindle MT formation in mitotic cells (Gruss et al., 2002) as well as mouse oocytes, where it regulates spindle pole integrity (Brunet et al., 2008).
Alterations in spindle size and pole organization observed in PCNT-depleted (Tg) oocytes and following AURKA inhibition were associated with chromosome errors as well as impaired MT growth. Our previous studies revealed high rates of chromosome misalignment and disruption of chromosome congression in Tg oocytes, during both MI and ovulated MII oocyte stages, together with increased rates of chromosome–MT attachment errors and aneuploidy at MII (Baumann et al., 2017). Interestingly, the highest rates of chromosome errors were observed following AURKA inhibition in both WT and Tg oocytes. This is consistent with the recognized role for AURKA in regulating kinetochore–MT interactions at the centromere (DeLuca et al., 2018; Katayama et al., 2008) as well as promoting MT assembly in mitotic (Sardon et al., 2008) and meiotic (Bury et al., 2017) spindles. Analysis of MT regrowth following cold treatment in oocytes revealed impaired MT dynamics in Tg oocytes and following inhibition of AURKA activity. Notably, Tg oocytes exposed to the AURKA inhibitor showed no recovery of the spindle length, pointing to disrupted MT polymerization and/or depolymerization dynamics. MT regrowth is likely hindered by the loss of γ-tubulin along the spindle MTs as well as disruption of the ring-like aMTOC organization in WT oocytes, denoted by pronounced clustering of γ-tubulin and its γTuRC anchoring protein (NEDD1) (Ma et al., 2010; Raynaud-Messina and Merdes, 2007). How this striking disruption of aMTOC organization might also impact spindle organization and/or stability remains to be determined. In Tg oocytes, γ-tubulin is absent from the spindle poles but persists along the spindle MTs (Baumann et al., 2017). Interestingly, AURKA inhibition decreased MT-associated γ-tubulin in Tg oocytes, which correlated with higher rates of chromosome misalignment. This disruption of key MT-nucleating factors likely contributes to meiotic spindle instability. Further studies are warranted to assess the role of additional aMTOC-associated factors and to test whether there are potential differences during meiosis I and meiosis II. Nevertheless, these data support that loss of aMTOC-associated AURKA is a key contributor to meiotic spindle instability, leading to error-prone meiotic division in Tg (PCNT-depleted) oocytes.
Aurora A activity has recently been shown to regulate assembly of the newly identified LISD, which is unique to mammalian oocytes. The LISD serves as an important reservoir for MT regulatory factors that are essential for stable meiotic spindle formation (So et al., 2019). This highly dynamic domain is comprised of multiple centrosomal and spindle-associated proteins, including the known AURKA target TACC3, which promotes spindle stability in mitosis and meiosis (Peset and Vernos, 2008). TACC3 localizes to the dynamic LISD protrusions that extend outward from the poles (So et al., 2019). Previous studies report that TACC3 binds to spindle MTs and is phosphorylated by TPX2 in somatic cells and oocytes (Brunet et al., 2008; Burgess et al., 2018). Inhibition and/or depletion of AURKA or TACC3 disrupts LISD formation, with dispersal of the MT regulatory factors into the cytoplasm, leading to severe spindle defects with significant loss of k-fibers and interpolar MTs as well as reduced spindle volume (So et al., 2019). Oocyte-specific knockout of Aurka also leads to loss of TACC3 labeling, disrupted spindle formation and arrest at MI (Blengini et al., 2021). Here, we demonstrate that PCNT-depleted oocytes from Tg mice lack the LISD domain at both MI and ovulated MII stages. Analysis of this unique mouse model has established that LISD assembly in vivo depends specifically on oocyte aMTOC-associated AURKA, not just overall levels of this key kinase. In addition, since meiotic spindle assembly occurs in a Ran-dependent manner in PCNT-depleted oocytes (Baumann et al., 2017), the current study also reveals that Ran-mediated spindle assembly can proceed without the LISD in Tg oocytes. Further studies are needed to determine whether additional aMTOC-associated factors contribute to LISD formation. Nevertheless, this study provides novel mechanistic insight into the underlying basis of meiotic spindle instability and error-prone meiotic division in Tg oocytes that lack PCNT and aMTOCs. Moreover, it identifies a key limitation in Ran-mediated meiotic spindle formation.
In summary, this study demonstrates that the localization of AURKA to meiotic spindle poles is dependent on PCNT and aMTOCs in mouse oocytes. Additionally, we provide evidence that aMTOC-associated AURKA, rather than overall activity in the oocyte, plays a critical role in the regulation of meiotic spindle size, organization and stability. Importantly, we establish that in vivo LISD assembly in oocytes is dependent on aMTOCs. Loss of aMTOC-associated AURKA and failure of LISD assembly contribute to error-prone meiotic division in PCNT-depleted oocytes, underscoring the essential role of aMTOCs for meiotic spindle stability.
MATERIALS AND METHODS
Animals
All mice were housed at a constant temperature (24–26°C) and a controlled light cycle (12 h light/dark), with food and water provided ad libitum. Animal-use protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Georgia (Athens, GA, USA), and all experiments were conducted in accordance with the specified guidelines.
Ovulated oocyte collection and culture
Oocytes from control wild-type (WT) and transgenic (Tg) mice with an oocyte-specific knockdown of PCNT (Baumann et al., 2017) were used for analysis. All experiments utilized in vivo matured MII oocytes, with a fully assembled spindle, collected from super-ovulated females. The mice were treated with 5 IU pregnant mare serum gonadotrophin (EMD Biosciences, La Jolla, CA) followed by 5 IU human chorionic gonadotrophin (hCG; EMD Biosciences) 44–48 h later, to promote follicle development and ovulation. The oocytes were recovered from the oviducts ∼16 h after hCG treatment and transferred to minimal essential medium (MEM) supplemented with 3 mg/ml bovine serum albumin (MEM/BSA; Sigma-Aldrich, St Louis, MO). Surrounding cumulus cells were removed by gentle pipetting in MEM/BSA supplemented with hyaluronidase (0.3 mg/ml; Sigma-Aldrich) followed by several rinses in fresh medium alone. When indicated, oocytes were cultured at 37°C in MEM/BSA for specified times. All cultures were maintained at 37°C in MEM/BSA under 5% CO2, 5% O2 and 90% N2.
Immunofluorescence
Oocytes were fixed and immunolabeled with specific antibodies as previously described (Baumann and Viveiros, 2015) to detect pAURKA (1:1000; NB100-2371; Novus Biologicals, Centennial, CO), PCNT (1:1000; 611815; BD Biosciences, San Jose, CA; and PRB432C; Covance, Princeton, NJ), TPX2 (1:500; NB500-179; Novus Biologicals, Centennial, CO), NEDD1 (1:500; H00121441-M05; Abnova), γ-tubulin (1:500; T3559; Sigma-Aldrich), acetylated α-tubulin (1:1000; T6793; Sigma-Aldrich) and TACC3 (1:400; ab134154; Abcam, Cambridge, MA). In brief, fixed oocytes were incubated with the primary antibodies overnight at 4°C, then washed and incubated (1 h) at 37°C with specific Alexa Fluor 488- or Alexa Fluor 555-conjugated secondary antibodies (1:1000; A11070, A21425, A11017 and A21430; Life Technologies, Eugene, OR). After a final wash, the oocytes were transferred onto glass slides and overlaid with mounting medium (Vectashield, Vector Laboratories, Burlingame, CA) containing DAPI (4′,6-diamidino-2-phenylindole) to counterstain the DNA. Fluorescence was assessed using a Leica DMRE upright fluorescence microscope with imaging software (Leica Microsystems). Where indicated, confocal imaging and three-dimensional (3D) reconstruction of meiotic spindles was performed using a Nikon Eclipse Ti-U/D-Eclipse C1 laser scanning confocal microscope equipped with a 40× objective lens following sequential (frame lambda) excitation of GFP and RFP fusion proteins with a 488 nm and 561 nm Coherent Sapphire laser, respectively. Image acquisition was conducted using EZ-C1 software (Nikon) with a step size of 0.5 µm and a z-stack range of 40 µm. Imaging data were subsequently analyzed by maximum intensity and 3D reconstructions using NIH Elements software (Nikon). In some experiments, the oocytes were assessed by super-resolution structured illumination microscopy (SR-SIM) using a Zeiss Elyra S1 system equipped with a 100× oil immersion lens and ZEN 2011 software with a SIM analysis module for image acquisition at the Biomedical Microscopy Core (BMC) facility, University of Georgia.
Western blot analysis
Oocyte (n=50 per group) samples were frozen in RIPA buffer supplemented with protease and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA) as previously described (Wang et al., 2020). In preparation for analysis, the samples were thawed on ice, mixed with the 4× loading buffer (161-0747; BioRad), and heated at 98°C for 7 min. The proteins were separated on 8% acrylamide gels containing SDS and then transferred onto hydrophobic PVDF membranes (Millipore, Burlington, MA). Following transfer, the membranes were blocked in Tris-buffered saline supplemented with 2% Tween 20 (TBST) containing 5% nonfat milk powder for 1 h at room temperature, then incubated at 4°C overnight, with specific primary antibodies (1:1000 dilution) for the detection of either phosphorylated pT288 AURKA (3079; Cell Signaling Technology), TPX2 (NB500-179; Novus Biologicals), γ-tubulin (T3559; Sigma-Aldrich) or TACC3 (ab134154; Abcam). Incubation with primary antibodies for detection of either α- or β-tubulin (1:2000; T6793, clone 6-11B-1; T4026, clone TUB 2.1; Sigma-Aldrich), under similar conditions, was used as an internal control. After several washes in TBST, the membranes were incubated with peroxidase-conjugated secondary antibodies (115-036-062 and 111-036-144; Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature. ECL (Millipore) was used for chemiluminescent detection. Individual band intensity was quantified using ImageJ software (NIH, Bethesda, MD), and the relative total protein values in each group were compared to the control, which was normalized to 1.0.
AURKA inhibition
Aurora kinase A (AURKA) plays an essential role in meiotic spindle assembly (Bury et al., 2017; Solc et al., 2012), as well as the maintenance of assembled spindle and aMTOC organization in ovulated oocytes (Wang et al., 2020). This key kinase is phosphorylated (activated) upon the resumption of meiosis and localizes specifically to aMTOCs. To determine whether AURKA function in MII oocytes is dependent on its association with the aMTOCs, we compared meiotic spindle organization between WT control and PCNT-depleted oocytes following AURKA inhibition. In brief, ovulated MII control and PCNT-depleted oocytes were collected from WT and Tg mice, respectively, then incubated for 4 h in MEM/BSA supplemented with 500 nM MLN8237 (Thermo Scientific), a selective inhibitor for AURKA (Sloane et al., 2010) previously used in mouse oocytes at this concentration (Bury et al., 2017). Control group oocytes from WT and Tg mice were incubated in medium alone under similar conditions. All cultures were maintained at 37°C in MEM/BSA under 5% CO2, 5% O2 and 90% N2. The oocytes were immediately frozen for western blotting or fixed for immunofluorescence analysis to assess the meiotic spindle and chromosome configurations.
Spindle MT stability and regrowth analysis
Spindle MT stability following cold treatment was assessed in response to AURKA inhibition in both WT control and PCNT-depleted oocytes. In brief, ovulated MII oocytes were cultured for 4 h in MEM/BSA medium supplemented with 500 nM MLN8237 (Thermo Fisher Scientific), while control oocytes were incubated in medium alone. Following the 4 h culture, the oocytes were transferred to cold (4°C) M2 medium (M7167-50ML; Sigma-Aldrich), either alone (controls) or supplemented with 500 nM MLN8237, for 30 min to depolymerize the spindle MTs. After cold treatment, half of the oocytes from each group were immediately fixed for analysis, while the other half were transferred to pre-warmed MEM, either alone (controls) or supplemented with 500 nM MLN8237, at 37°C for MT regrowth, then were fixed at 5 min post re-warming. The oocytes were immunolabeled with anti-acetylated α-tubulin to assess spindle MT organization. Fluorescence was evaluated using a Leica Microsystem fluorescence microscope with imaging software.
Statistical analysis
All data are presented as the mean±s.e.m. from a minimum of three independent experimental replicates. GraphPad Prism 8 software was used for data analysis and preparation of all graphs. The data were analyzed by either two-tailed, unpaired Student's t-test for comparison among two groups, or one-way ANOVA followed by Tukey's multiple comparison test between three or more groups. Differences were considered to be significant when P<0.05.
Supplementary Material
Acknowledgements
The authors thank Dr M. K. Kandasamy for advice with super-resolution (SR-SIM) microscopy at the Biomedical Microscopy Core facility, University of Georgia. Moreover, we thank Ms Luhan Yang for helpful discussions during manuscript preparation.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: R.D.L.F., M.M.V.; Methodology: C.B.; Formal analysis: X.W., C.B., M.M.V.; Investigation: X.W., C.B.; Resources: R.D.L.F.; Writing - original draft: X.W., M.M.V.; Writing - review & editing: X.W., C.B., R.D.L.F., M.M.V.; Visualization: X.W., M.M.V.; Funding acquisition: R.D.L.F., M.M.V.
Funding
This work was funded by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD092857 to M.M.V.) and a graduate fellowship from the Department of Physiology and Pharmacology, University of Georgia (to X.W.). R.D.L.F. and C.B. are supported by the National Institutes of Health (NIH; HD093383) and the National Science Foundation Center for Cell Manufacturing (CMaT). Deposited in PMC for release after 12 months.
References
- Balboula, A. Z., Nguyen, A. L., Gentillo, A. S., Quartuccio, S. M., Drutovic, D., Solc, P. and Schindler, K. (2016). Haspin kinase regulates microtubule-organizing center clustering and stability through Aurora kinase C in mouse oocytes. J. Cell Sci. 129, 3648-3660. 10.1242/jcs.189340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr, A. R. and Gergely, F. (2007). Aurora-A: the maker and breaker of spindle poles. J. Cell Sci. 120, 2987-2996. 10.1242/jcs.013136 [DOI] [PubMed] [Google Scholar]
- Baumann, C. and Viveiros, M. M. (2015). Meiotic spindle assessment in mouse oocytes by siRNA-mediated Silencing. J. Vis. Exp. 104, e53586. 10.3791/53586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann, C., Wang, X., Yang, L. and Viveiros, M. M. (2017). Error-prone meiotic division and subfertility in mice with oocyte-conditional knockdown of pericentrin. J. Cell Sci. 130, 1251-1262. 10.1242/jcs.196188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss, R., Sardon, T., Vernos, I. and Conti, E. (2003). Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell 12, 851-862. 10.1016/S1097-2765(03)00392-7 [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias, M. and Glover, D. M. (2007). Centrosome biogenesis and function: centrosomics brings new understanding. Nat. Rev. Mol. Cell Biol. 8, 451-463. 10.1038/nrm2180 [DOI] [PubMed] [Google Scholar]
- Bird, A. W. and Hyman, A. A. (2008). Building a spindle of the correct length in human cells requires the interaction between TPX2 and Aurora A. J. Cell Biol. 182, 289-300. 10.1083/jcb.200802005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blengini, C. S., Ibrahimian, P., Vaskovicova, M., Dutrovic, D., Solc, P. and Schindler, K. (2021). Aurora kinase A is essential for meiosis in mouse oocytes. PLoS Genet. 17, e1009327. 10.1371/journal.pgen.1009327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet, S., Dumont, J., Lee, K. W., Kinoshita, K., Hikal, P., Gruss, O. J., Maro, B. and Verlhac, M.-H. (2008). Meiotic regulation of TPX2 protein levels governs cell cycle progression in mouse oocytes. PLoS ONE 3, e3338. 10.1371/journal.pone.0003338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, S. G., Mukherjee, M., Sabir, S., Joseph, N., Gutiérrez-Caballero, C., Richards, M. W., Huguenin-Dezot, N., Chin, J. W., Kennedy, E. J., Pfuhl, M.et al. (2018). Mitotic spindle association of TACC3 requires Aurora-A-dependent stabilization of a cryptic α-helix. EMBO J. 37, e97902. 10.15252/embj.201797902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury, L., Coelho, P. A., Simeone, A., Ferries, S., Eyers, C. E., Eyers, P. A., Zernicka-Goetz, M. and Glover, D. M. (2017). Plk4 and Aurora A cooperate in the initiation of acentriolar spindle assembly in mammalian oocytes. J. Cell Biol. 216, 3571-3590. 10.1083/jcb.201606077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.-T., Hehnly, H., Yu, Q., Farkas, D., Zheng, G., Redick, S. D., Hung, H.-F., Samtani, R., Jurczyk, A., Akbarian, S.et al. (2014). A unique set of centrosome proteins requires pericentrin for spindle-pole localization and spindle orientation. Curr. Biol. 24, 2327-2334. 10.1016/j.cub.2014.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmátal, L., Yang, K., Schultz, R. M. and Lampson, M. A. (2015). Spatial regulation of kinetochore microtubule attachments by destabilization at spindle poles in meiosis I. Curr. Biol. 25, 1835-1841. 10.1016/j.cub.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combelles, C. M. H. and Albertini, D. F. (2001). Microtubule patterning during meiotic maturation in mouse oocytes is determined by cell cycle-specific sorting and redistribution of γ-tubulin. Dev. Biol. 239, 281-294. 10.1006/dbio.2001.0444 [DOI] [PubMed] [Google Scholar]
- Delaval, B. and Doxsey, S. J. (2010). Pericentrin in cellular function and disease. J. Cell Biol. 188, 181-190. 10.1083/jcb.200908114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca, K. F., Meppelink, A., Broad, A. J., Mick, J. E., Peersen, O. B., Pektas, S., Lens, S. M. A. and DeLuca, J. G. (2018). Aurora A kinase phosphorylates Hec1 to regulate metaphase kinetochore-microtubule dynamics. J. Cell Biol. 217, 163-177. 10.1083/jcb.201707160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont, J., Petri, S., Pellegrin, F., Terret, M.-E., Bohnsack, M. T., Rassinier, P., Georget, V., Kalab, P., Gruss, O. J. and Verlhac, M.-H. (2007). A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J. Cell Biol. 176, 295-305. 10.1083/jcb.200605199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyers, P. A. and Maller, J. L. (2004). Regulation of Xenopus Aurora A activation by TPX2. J. Biol. Chem. 279, 9008-9015. 10.1074/jbc.M312424200 [DOI] [PubMed] [Google Scholar]
- Fu, J., Bian, M., Xin, G., Deng, Z., Luo, J., Guo, X., Chen, H., Wang, Y., Jiang, Q. and Zhang, C. (2015). TPX2 phosphorylation maintains metaphase spindle length by regulating microtubule flux. J. Cell Biol. 210, 373-383. 10.1083/jcb.201412109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss, O. J., Wittmann, M., Yokoyama, H., Pepperkok, R., Kufer, T., Silljé, H., Karsenti, E., Mattaj, I. W. and Vernos, I. (2002). Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat. Cell Biol. 4, 871-879. 10.1038/ncb870 [DOI] [PubMed] [Google Scholar]
- Jang, C.-Y., Coppinger, J. A., Seki, A., Yates, J. R., III and Fang, G. (2009). Plk1 and Aurora A regulate the depolymerase activity and the cellular localization of Kif2a. J. Cell Sci. 122, 1334-1341. 10.1242/jcs.044321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, K. T. and Lane, S. I. R. (2013). Molecular causes of aneuploidy in mammalian eggs. Development 140, 3719-3730. 10.1242/dev.090589 [DOI] [PubMed] [Google Scholar]
- Joukov, V., Walter, J. C. and De Nicolo, A. (2014). The Cep192-organized aurora A-Plk1 cascade is essential for centrosome cycle and bipolar spindle assembly. Mol. Cell 55, 578-591. 10.1016/j.molcel.2014.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama, H., Sasai, K., Kloc, M., Brinkley, B. R. and Sen, S. (2008). Aurora kinase-A regulates kinetochore/chromatin associated microtubule assembly in human cells. Cell Cycle 7, 2691-2704. 10.4161/cc.7.17.6460 [DOI] [PubMed] [Google Scholar]
- Kinoshita, K., Noetzel, T. L., Pelletier, L., Mechtler, K., Drechsel, D. N., Schwager, A., Lee, M., Raff, J. W. and Hyman, A. A. (2005). Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis. J. Cell Biol. 170, 1047-1055. 10.1083/jcb.200503023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łuksza, M., Queguigner, I., Verlhac, M.-H. and Brunet, S. (2013). Rebuilding MTOCs upon centriole loss during mouse oogenesis. Dev. Biol. 382, 48-56. 10.1016/j.ydbio.2013.07.029 [DOI] [PubMed] [Google Scholar]
- Ma, W. and Viveiros, M. M. (2014). Depletion of pericentrin in mouse oocytes disrupts microtubule organizing center function and meiotic spindle organization. Mol. Reprod. Dev. 81, 1019-1029. 10.1002/mrd.22422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, W., Baumann, C. and Viveiros, M. M. (2010). NEDD1 is crucial for meiotic spindle stability and accurate chromosome segregation in mammalian oocytes. Dev. Biol. 339, 439-450. 10.1016/j.ydbio.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Magnaghi-Jaulin, L., Eot-Houllier, G., Gallaud, E. and Giet, R. (2019). Aurora a protein kinase: to the centrosome and beyond. Biomolecules 9, 28. 10.3390/biom9010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumoto, T., Honda, S., Hara, T., Nitta, M., Hirota, T., Kohmura, E. and Saya, H. (2003). Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J. Biol. Chem. 278, 51786-51795. 10.1074/jbc.M306275200 [DOI] [PubMed] [Google Scholar]
- Mihajlović, A. I. and FitzHarris, G. (2018). Segregating chromosomes in the mammalian oocyte. Curr. Biol. 28, R895-R907. 10.1016/j.cub.2018.06.057 [DOI] [PubMed] [Google Scholar]
- Nagaoka, S. I., Hassold, T. J. and Hunt, P. A. (2012). Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13, 493-504. 10.1038/nrg3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig, A., Seiler, C., Steblyanko, Y., Dingli, F., Arras, G., Loew, D., Welburn, J., Prigent, C., Barisic, M. and Nahmias, C. (2021). Reciprocal regulation of Aurora kinase A and ATIP3 in the control of metaphase spindle length. Cell. Mol. Life. Sci. 78, 1765-1779. 10.1007/s00018-020-03614-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, A. L. and Schindler, K. (2017). Specialize and Divide (Twice): functions of three aurora kinase homologs in mammalian oocyte meiotic maturation. Trends Genet. 33, 349-363. 10.1016/j.tig.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peset, I. and Vernos, I. (2008). The TACC proteins: TACC-ling microtubule dynamics and centrosome function. Trends Cell Biol. 18, 379-388. 10.1016/j.tcb.2008.06.005 [DOI] [PubMed] [Google Scholar]
- Petry, S., Groen, A. C., Ishihara, K., Mitchison, T. J. and Vale, R. D. (2013). Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell 152, 768-777. 10.1016/j.cell.2012.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud-Messina, B. and Merdes, A. (2007). γ-tubulin complexes and microtubule organization. Curr. Opin. Cell Biol. 19, 24-30. 10.1016/j.ceb.2006.12.008 [DOI] [PubMed] [Google Scholar]
- Sardon, T., Peset, I., Petrova, B. and Vernos, I. (2008). Dissecting the role of Aurora A during spindle assembly. EMBO J. 27, 2567-2579. 10.1038/emboj.2008.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saskova, A., Solc, P., Baran, V., Kubelka, M., Schultz, R. M. and Motlik, J. (2008). Aurora kinase A controls meiosis I progression in mouse oocytes. Cell Cycle 7, 2368-2376. 10.4161/cc.6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathananthan, A. H., Selvaraj, K. and Trounson, A. (2000). Fine structure of human oogonia in the foetal ovary. Mol. Cell. Endocrinol. 161, 3-8. 10.1016/S0303-7207(99)00216-6 [DOI] [PubMed] [Google Scholar]
- Schuh, M. and Ellenberg, J. (2007). Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 130, 484-498. 10.1016/j.cell.2007.06.025 [DOI] [PubMed] [Google Scholar]
- Sloane, D. A., Trikic, M. Z., Chu, M. L. H., Lamers, M. B. A. C., Mason, C. S., Mueller, I., Savory, W. J., Williams, D. H. and Eyers, P. A. (2010). Drug-resistant aurora A mutants for cellular target validation of the small molecule kinase inhibitors MLN8054 and MLN8237. ACS Chem. Biol. 5, 563-576. 10.1021/cb100053q [DOI] [PubMed] [Google Scholar]
- So, C., Seres, K. B., Steyer, A. M., Mönnich, E., Clift, D., Pejkovska, A., Möbius, W. and Schuh, M. (2019). A liquid-like spindle domain promotes acentrosomal spindle assembly in mammalian oocytes. Science 364, eaat9557. 10.1126/science.aat9557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solc, P., Baran, V., Mayer, A., Bohmova, T., Panenkova-Havlova, G., Saskova, A., Schultz, R. M. and Motlik, J. (2012). Aurora kinase A drives MTOC biogenesis but does not trigger resumption of meiosis in mouse oocytes matured in vivo. Biol. Reprod. 87, 85. 10.1095/biolreprod.112.101014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szollosi, D., Calarco, P. and Donahue, R. P. (1972). Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J. Cell Sci. 11, 521-541. 10.1242/jcs.11.2.521 [DOI] [PubMed] [Google Scholar]
- Tungadi, E. A., Ito, A., Kiyomitsu, T. and Goshima, G. (2017). Human microcephaly ASPM protein is a spindle pole-focusing factor that functions redundantly with CDK5RAP2. J. Cell Sci. 130, 3676-3684. 10.1242/jcs.203703 [DOI] [PubMed] [Google Scholar]
- Walter, A. O., Seghezzi, W., Korver, W., Sheung, J. and Lees, E. (2000). The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation. Oncogene 19, 4906-4916. 10.1038/sj.onc.1203847 [DOI] [PubMed] [Google Scholar]
- Wang, X., Baumann, C., De La Fuente, R. and Viveiros, M. M. (2020). CEP215 and AURKA regulate spindle pole focusing and aMTOC organization in mouse oocytes. Reproduction 159, 261-274. 10.1530/REP-19-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, W. C., Sillibourne, J., Rosa, J. and Doxsey, S. J. (2004). Mitosis-specific anchoring of γ tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell 15, 3642-3657. 10.1091/mbc.e03-11-0796 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.