ABSTRACT
Background
Dietary sources of metals are not well established among pregnant women in the United States.
Objective
We aimed to perform a diet-wide association study (DWAS) of metals during the first trimester of pregnancy.
Methods
In early pregnancy (11.3 ± 2.8 weeks of gestation), 1196 women from Project Viva (recruited 1999–2002 in eastern Massachusetts) completed a validated FFQ (135 food items) and underwent measurements of erythrocyte metals [arsenic (As), barium, cadmium, cesium (Cs), copper, mercury (Hg), magnesium, manganese, lead (Pb), selenium (Se), zinc]. The DWAS involved a systematic evaluation and visualization of all bivariate relations for each food–metal combination. For dietary items with strong associations with erythrocyte metals, we applied targeted maximum likelihood estimations and substitution models to evaluate how hypothetical dietary interventions would influence metals’ concentrations.
Results
Participants’ mean ± SD age was 32.5 ± 4.5 y and prepregnancy BMI was 24.8 ± 5.4 kg/m2; they were mostly white (75.9%), college graduates (72.4%), married or cohabitating (94.6%), had a household income >$70,000/y (63.5%), and had never smoked (67.1%). Compared with other US-based cohorts, the overall diet quality of participants was above average, and concentrations of erythrocyte metals were lower. The DWAS identified significant associations of several food items with As, Hg, Pb, Cs, and Se; for example, As was higher for each SD increment in fresh fruit (11.5%; 95% CI: 4.9%, 18.4%), white rice (17.9%; 95% CI: 9.4%, 26.9%), and seafood (50.9%; 95% CI: 42.8%, 59.3%). Following the guidelines for pregnant women to consume ≤3 servings/wk of seafood was associated with lower As (−0.55 ng/g; 95% CI: −0.82, −0.28 ng/g) and lower Hg (−2.67 ng/g; 95% CI: −3.55, −1.80 ng/g). Substituting white rice with bread, pasta, tortilla, and potato was also associated with lower As (35%–50%) and Hg (35%–70%).
Conclusions
Our DWAS provides a systematic evaluation of diet–metals relations. Prenatal diet may be an important source of exposures to metals.
Keywords: metals, environmental exposure, diet, seafood, rice, arsenic, mercury, cesium, pregnancy
See corresponding editorial on page 414.
Introduction
Metals are natural elements that can contaminate a particular environment from anthropogenic activities, such as mining, industrial manufacturing, and agricultural pesticide application. Metals can enter the food web and may bioaccumulate. For example, arsenic (As), a metalloid, is naturally found in rocks and sediments and can dissolve into groundwater in its highly toxic inorganic form (1). Crops, such as rice, grown in As-contaminated water and soil have been shown to contain elevated amounts of inorganic As (iAs) (2). Man-made causes such as the application of organic arsenical pesticides and herbicides, use of As-based animal drugs and antibiotics, As compounds added in animal feeds, and the historical use of iAs on fruits and potato fields have also led to As contamination in food sources (3). Fish, shellfish, meat, poultry, dairy products, and cereals have been reported as dietary sources of exposure to As, although in concentrations much lower than those detected in groundwater, and seafood mainly harbors the less toxic organic form of As (4). Other examples of dietary sources of exposure to environmental metals include mercury (Hg) in seafood (5) and cadmium (Cd) in cereal products and vegetables (6).
Exposure to metals during pregnancy, both essential and nonessential, may have short- and long-term health consequences to both the mother and the fetus. Because maternal dietary intake directly influences the nutritional needs for fetal development and the fetus's exposure to metals, identifying food choices that balance good nutrition and low exposure to toxic metals may have lifelong consequences. Literature examining dietary exposure to metals in pregnant women in the United States is limited, and most studies examined only a few food items or metals at a time. A diet-wide association study (DWAS) is a systematic and comprehensive evaluation of multiple dietary components (e.g., food items, food groups, and diet scores/patterns) and their associations with biomarkers of environmental chemicals. A DWAS can help identify potential sources of exposure and assess the relative contribution of exposure from diet (7).
The goals of this study are to apply a DWAS using data from a New-England-based pregnancy cohort, Project Viva, to evaluate the associations of food intake and dietary patterns with blood metal profiles. We hypothesized the DWAS would identify food items that had previously been reported to be sources of metal exposures, such as rice and seafood for As and Hg, and uncover new dietary characteristics associated with variations in metal concentrations. Based on the findings from the DWAS, we applied a causal inference method to estimate how different hypothetical dietary interventions could reduce blood metal concentrations.
Methods
Study population
Project Viva is a longitudinal research study that examines how diet and other factors during and after pregnancy affect maternal and child health outcomes. The study team recruited pregnant women at their first-trimester visit from Atrius Harvard Vanguard Medical Associates in eastern Massachusetts during 1999–2002. Trained research staff obtained written informed consent immediately after the women's initial clinical prenatal visit; administered a brief interview; provided self-administered questionnaires to inquire about demographics, lifestyle, and dietary habits; and collected blood samples for biomarker analyses. Details of the study protocol and recruitment and retention procedures are available elsewhere (8). This analysis included 1196 pregnant women with data on erythrocyte metals (n = 1181 for Hg), dietary assessment, and all covariates used in this analysis (see the study flowchart in Supplemental Figure 1). All variables reflect participants’ characteristics in the first trimester of pregnancy at enrollment (mean ± SD: 11.3 ± 2.9 weeks of gestation); Supplemental Table 1 presents the complete list of variables considered in the DWAS. The study protocols were approved by the Institutional Review Board at Harvard Pilgrim Health Care.
Dietary assessment
Project Viva used a semiquantitative FFQ (9) to collect self-reported dietary habits in early pregnancy, from the last menstrual period until the date of FFQ completion (mean ± SD: 11.3 ± 2.9 weeks of gestation). The FFQ was developed based on an extensively validated Willett FFQ used in the Nurses’ Health Study and another large prospective cohort of adults (10) and included 166 questions on the average consumption frequency of specified foods. Nutrient intakes estimated from the FFQ were comparable with biomarker concentrations of several nutrients measured in the blood (9). We derived diet scores to reflect dietary patterns and diet quality, including the Alternate Healthy Eating Index for Pregnancy (AHEI-P), Mediterranean diet score (MDS), Western diet score, and Prudent diet score. The AHEI-P (90-point) is a measure of diet quality based on modified recommendations from the USDA for pregnant women (11). The MDS (9-point) was calculated based on consumption of food groups associated with a traditional Mediterranean diet (12), including fish, dairy products, fruits, legumes, nuts, vegetables, whole grains products, red and processed meat, and unsaturated-to-saturated fat ratio; we did not include alcohol consumption because it is not recommended for pregnant women and few pregnant women drank alcohol in our study cohort (13). The Prudent diet score was made up of green leafy vegetables, cabbages, fruits, tomatoes, poultry and fish, whereas the Western diet score was made up of red meat, processed meat, refined grains, snacks, sweets, desserts, French fries, and pizza; they were derived previously using principal factor component analysis (13). We also grouped individual food items into 18 categories (11). Supplemental Table 1 shows the complete list of dietary variables used in this analysis.
Erythrocyte metal concentrations
The primary outcome we assessed in the study was erythrocyte metal concentrations and there was no secondary outcome. Blood samples were collected from all participants at the first trimester (the same time as the dietary assessment), centrifuged at 492 g force for 10 min at 4°C to separate RBCs from plasma, and stored at −70°C. We retained only RBCs but not whole blood, which is the predominate matrix used in clinical tests for blood metal profiles. Although metals in RBCs cannot be directly compared with concentrations measured in whole blood, RBCs are better biomarkers for some metals; for example, erythrocyte copper (Cu) and cadmium (Cd) concentrations are more reflective of long-term status (14, 15) and erythrocyte magnesium (Mg) and manganese (Mn) are deemed more diagnostic for clinical status (16, 17). For As, however, blood is not as good a biomarker as other biomarkers such as urine or nails because it can only reflect short-term exposure to total As (speciation analysis cannot be performed to differentiate between the more toxic inorganic form and the less toxic organic form); however, a high correlation has been found between blood As and urinary As (18). Erythrocyte Hg reflects total Hg and cannot differentiate between elemental, inorganic Hg and organic Hg [methylmercury (MeHg), the more toxic form], but has good correlation with total Hg in whole blood (19). We performed a short review comparing RBC and whole blood as biomarkers of metals and present it in Supplemental Method 1.
We measured concentrations of As, barium (Ba), Cd, cesium (Cs), Cu, Mg, Mn, lead (Pb), selenium (Se), and zinc (Zn) using triple quadrupole inductively coupled plasma mass spectrometry (ICP-MS; Agilent 8800 ICP-QQQ) in MS/MS mode, which provides higher sensitivity and lower background interference than traditional single quadrupole ICP-MS. Briefly, 0.5 mL stored packed RBCs is weighed and digested in 2 mL ultra-pure concentrated HNO3 acid for 48 h and then further digested with 1 mL 30% ultra-pure hydrogen peroxide for another 48 h after dilution with 10 mL deionized water. Sample handling was performed in an International Organization for Standardization (ISO) class 6 clean room with an ISO class 5 laminar flow clean hood. Hg concentration was measured in an RBC sample separately divided into aliquots using the Direct Mercury Analyzer 80 (Milestone Inc.). We ran initial and continuous calibrations, included procedural blanks, and repeated analysis of 2% of the sample to ensure quality control (QC). In addition, we analyzed Seronorm-Blood L3 once daily to monitor accuracy and included high- and low-level blind QC samples in each batch of the analysis. Supplemental Method 2 presents more detailed analytical methods and QCs. The limit of detection (LOD) was 0.153 ng/g for As, 0.3 ng/g for Hg, and 0.587 ng/g for Cs (see Supplemental Table 2 for the LODs of other metals). The analysis included duplicated blinded samples for QC and calculated relative percentage difference (RPD) and intraclass correlations (ICCs) to evaluate precision and reliability, respectively, between duplicated measurements (see Supplemental Table 2 for the QC results). All metals included in the analysis had ICCs > 0.5 and percentages detected above the LOD of ≥85%. We imputed values below the LOD as LOD/square root of 2 (20).
Covariates
We included in our regression models a priori covariates likely to be important predictors of metal exposure and which may be correlated with dietary characteristics using a directed acyclic graph (21). We next included all potential confounders in the linear regression model to assess them for multicollinearity and removed covariates with tolerance <0.1 (variance inflation factor >10). The final list of covariates included were age at enrollment, self-reported race/ethnicity (white, black, Asian, Hispanic, others), education (college graduate, yes or no), household income (≤$70,000/y, >$70,000/y), marital status (married or cohabitating, yes or no), smoking status (yes or no), nulliparity (yes or no), prepregnancy height and weight (which we used to calculate BMI), hemoglobin concentration, and total calorie intake. Participants self-reported their race/ethnicity at enrollment as Hispanic or Latina, white or Caucasian, black or African American, Asian or Pacific Islander, American Indian or Alaskan Native, or other. Owing to low sample sizes, we grouped American Indian or Alaskan Native and other in the same category (“others”). We modeled age, prepregnancy BMI, hemoglobin concentration, and total calorie intake as continuous variables and the rest as categorical variables.
Statistical analysis
We described participants’ characteristics as means ± SDs for quantitative traits and percentages for categorical variables. We summarized dietary variables using z scores to reflect the number of SDs from the mean and reported medians [IQRs] for all erythrocyte metal concentrations. Normality testing showed metal concentrations were right skewed and thus we ln transformed them for linear regression analyses. We calculated Spearman rank correlation coefficients to examine the strength of correlations between dietary variables and metal concentrations.
The DWAS included an evaluation of all bivariate relations between each pair of dietary variables (135 food items, 18 food groups, and 4 diet scores) and metal concentrations (11 metals) using both univariate and multivariable ordinary least squares regression models (referred to as linear regressions). We adjusted for multiple testing using the false discovery rate (FDR) method (22) to account for type I errors and declared statistical significance if the FDR-adjusted P value, or q value (23), was <0.01. To improve interpretation and comparability, we reported the magnitude of associations as relative change (%) in median metal concentrations per SD change in the dietary variable (24), and used similar visualization methods as in previous DWASs (7, 25, 26) to summarize results. We examined the robustness of associations by systematically deleting visually apparent outliers.
To estimate the effects of seafood consumption on As and Hg concentrations, we performed targeted maximum likelihood estimation (TMLE) analysis (27) to estimate the marginal geometric mean (GM) difference in As and Hg concentrations if all pregnant women followed a hypothetical intervention of eating ≤3 servings of seafood per week compared with a scenario in which none of the pregnant women followed the intervention, i.e., all ate >3 servings of seafood per week. We dichotomized at 3 servings/wk to be consistent with the international and US dietary recommendations (4, 28). We used the Super Learner algorithm, an ensemble machine learning algorithm that builds the optimal weighted combination of predictions which minimize the cross-validated mean square error to estimate the outcome regression. TMLE provides double-robust estimators with valid statistical inference, whereas the machine learning algorithm minimizes the risk of model misspecification (29).
We also evaluated how substituting white rice with other grains would influence erythrocyte As and Hg concentrations by using an isoenergetic substitution model (30). Specifically, we assumed the consumption of total grain remained constant and calories from white rice would be replaced only by calories from other grain items. We fitted a multivariable linear regression model with ln-transformed erythrocyte As or Hg as the dependent variable and included all grain items, except white rice, as the independent variables. The model controlled for total grain intake and adjusted for potential confounders, including age, parity, education, household income, marital status, race/ethnicity, prepregnancy BMI, smoking, hemoglobin concentration, and seafood intake. The parameter estimate was transformed (24) and interpreted as the relative change (%) in median As or Hg concentrations by replacing each serving of white rice per week with that grain item.
We performed bivariate analyses using SAS version 9.4 (SAS Institute Inc.) and used R version 3.5 (R Foundation for Statistical Computing) for visualization and building prediction models. We used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for cohort studies to ensure adequate reporting of study methods and findings.
Results
Population characteristics
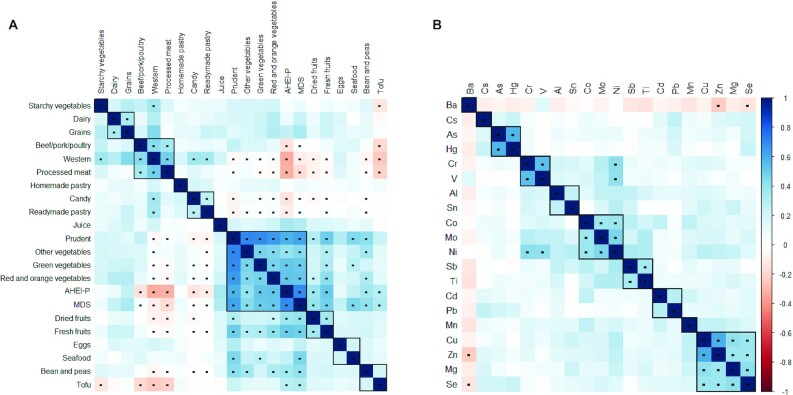
The study participants had a mean ± SD age of 32.5 ± 4.5 y and prepregnancy BMI of 24.8 ± 5.4 kg/m2; and were predominately white (75.9%), college graduates (72.4%), married or cohabitating (94.6%), with household income >$70,000/y (63.5%), and had never smoked (67.1%) (see Table 1 for a summary of characteristics). Mean ± SD AHEI-P was 60.5 ± 10.2 based on a 90-point scale and mean MDS was 4.5 based on a 9-point scale (Table 1). Figure 1A shows the Spearman rank correlation coefficients of the food groups and diet scores. Erythrocyte concentrations of toxic metals among the study population were generally low: median [IQR] concentrations were 0.8 [0.4–1.6] ng/g for As, 0.4 [0.3–0.6] ng/g for Cd, 3.3 [1.6–6.6] ng/g for Hg, and 17.6 [13.5–23.9] ng/g for Pb (Table 1). Some erythrocyte metal concentrations were moderately correlated with each other, e.g., As and Hg (r = 0.58, P < 0.01) and Cu and Zn (r = 0.60, P < 0.01); see Figure 1B for a heat map of the Spearman correlations among metals.
TABLE 1.
Characteristics of 1196 pregnant women in Project Viva included in this analysis
| Participants’ characteristics | Mean ± SD, n (%), or median [IQR] |
|---|---|
| Age, y | 32.5 ± 4.5 |
| Prepregnancy BMI, kg/m2 | 24.8 ± 5.4 |
| Race/ethnicity | |
| White | 908 (75.9) |
| Black | 122 (10.2) |
| Asian | 56 (4.7) |
| Hispanic | 72 (6.0) |
| Others | 38 (3.2) |
| College graduate | |
| No | 330 (27.6) |
| Yes | 866 (72.4) |
| Married or cohabitating | |
| No | 64 (5.4) |
| Yes | 1132 (94.6) |
| Household income, $/y | |
| ≤70,000 | 436 (36.5) |
| >70,000 | 760 (63.5) |
| Smoking | |
| Former | 254 (21.2) |
| Smoked during pregnancy | 139 (11.6) |
| Never | 803 (67.1) |
| Nulliparity | |
| No | 605 (50.6) |
| Yes | 591 (49.4) |
| Hemoglobin concentration, g/dL | 12.6 ± 0.9 |
| Dietary characteristics, unit | |
| AHEI-P, 90-point scale | 60.5 ± 10.2 |
| Mediterranean diet, 9-point scale | 4.5 ± 1.9 |
| Western diet, z score | 0.02 ± 1.0 |
| Prudent diet, z score | −0.01 ± 0.9 |
| Erythrocyte metal(loid) concentration | |
| As, ng/g | 0.8 [0.4–1.6] |
| Ba, ng/g | 3.2 [2.0–5.9] |
| Cd, ng/g | 0.4 [0.3–0.6] |
| Cs, ng/g | 2.6 [2.1–3.2] |
| Cu, ng/g | 564.0 [516.5–617.0] |
| Hg,1 ng/g | 3.3 [1.6–6.6] |
| Mg, μg/g | 41.2 [37.2–46.3] |
| Mn, ng/g | 16.2 [13.2–20.5] |
| Pb, ng/g | 17.6 [13.5–23.9] |
| Se, ng/g | 248.0 [221.0–282.0] |
| Zn, μg/g | 10.5 [9.3–11.7] |
n = 1181 with measurement for erythrocyte Hg.
FIGURE 1.
Spearman rank correlations of dietary variables (food groups and diet scores) (A) and erythrocyte metal concentrations (B) for 1196 pregnant women in Project Viva (n = 1181 for Hg). Dots indicate Spearman rank correlations with P value < 0.05. AHEI-P, Alternate Healthy Eating Index for Pregnancy; MDS, Mediterranean Diet Score.
DWAS: cross-sectional associations between dietary variables and erythrocyte metal concentrations
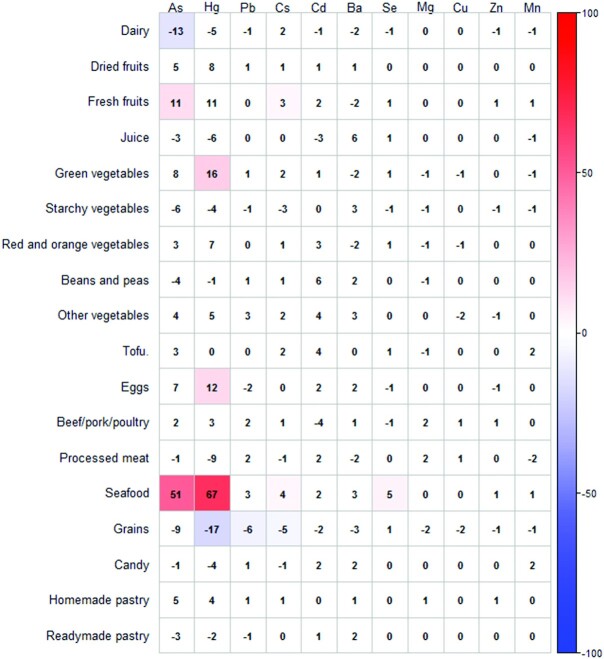
Out of the 135 food items, the DWAS identified 13, 12, 7, 4, and 1 food items with significant associations with Hg, As, Cs, Se, and Pb, respectively, after accounting for multiple comparisons (FDR < 0.01). The DWAS did not show any significant diet–metal associations for Ba, Cd, Cu, Mg, Mn, and Zn (Supplemental Figure 2A–F). Findings remained consistent in the sensitivity analysis after removing visible outliers. For ease of interpretation, we summarized intakes of the 135 food items into 18 food groups and present the results in Figure 2, with numbers indicating the relative change (%) in median erythrocyte metal concentration per SD increase in intake. The positive associations included fresh fruits with As (11.5%; 95% CI: 4.9%, 18.4%) and Cs (3.2%; 95% CI: 1.1%, 5.4%); green vegetables with Hg (16.2%; 95% CI: 7.6%, 25.5%); egg with Hg (12.0%; 95% CI: 3.8%, 20.9%); and seafood with As (50.9%; 95% CI: 42.8%, 59.3%), Hg (66.9%; 95% CI: 55.8%, 78.7%), and Se (4.8%; 95% CI: 3.5%, 6.0%). We also observed some inverse associations, including dairy (−12.5%; 95% CI: −18.2%, −6.5%) with As and grains with Hg (−17.3%; 95% CI: −24.7%, −9.3%), Pb (−6.3%; 95% CI: −9.2%, −3.3%), and Cs (−4.7%; 95% CI: −7.1%, −2.3%). Within the grains food group, white rice had positive associations with As (17.9%; 95% CI: 9.4%, 26.9%) and Hg (13.8%; 95% CI: 3.5%, 25.1%), whereas other grain items (white bread, French fries, potato, and other crackers) had inverse associations with As and Hg (Supplemental Figure 2E).
FIGURE 2.
Relation between dietary intakes (food groups) and erythrocyte metal concentrations for 1196 pregnant women in Project Viva (n = 1181 for Hg). Numbers indicate the relative change (%) in median erythrocyte metal concentrations at early pregnancy per SD increase in intake, adjusting for age, race, education, income, smoking status, prepregnancy BMI, hemoglobin concentration, and total calorie intake. Parameter estimates with false discovery rate–adjusted P values < 0.01 are shaded with colored backgrounds that correspond to the magnitude of the effect sizes.
Table 2 summarizes the associations by diet scores. Higher AHEI-P was associated with higher Cs (3.4%; 95% CI: 1.4%, 5.5%, per SD increase), Se (1.9%; 95% CI: 0.7%, 3.2%), and Hg (12.7%; 95% CI: 4.7%, 21.3%); MDS was positively associated with As (24.3%; 95% CI: 16.9%, 32.3%), Cs (6.2%; 95% CI: 4.0%, 8.6%), Se (2.2%; 95% CI: 0.9%, 3.5%), and Hg (32.6%; 95% CI: 22.7%, 43.3%). Participants with a more Western diet had lower Cs (−6.5%; 95% CI: −8.9%, −4.1%) and Hg (−17.3%; 95% CI: −24.9%, −9.1%), whereas participants with a more Prudent diet had higher As (21.2%; 95% CI: 13.9%, 29.1%), Cs (4.0%; 95% CI: 1.7%, 6.3%), and Hg (31.1%; 95% CI: 21.2%, 41.7%).
TABLE 2.
Estimated relative change (%) in median erythrocyte metal concentrations associated with each SD increase in different diet scores for 1196 pregnant women in Project Viva1
| Metal | AHEI-P, % (95% CI) | Mediterranean diet,% (95% CI) | Western diet, % (95% CI) | Prudent diet, % (95% CI) |
|---|---|---|---|---|
| As | 5.8 (−0.2, 12.2) | 24.3 (16.9, 32.3)2 | −9.4 (−16.0, −2.2) | 21.2 (13.9, 29.1)2 |
| Ba | 0.2 (−4.8, 5.5) | 1.4 (−4.0, 7.2) | 5.0 (−1.8, 12.3) | 1.9 (−3.6, 7.7) |
| Cd | 0.9 (−2.3, 4.2) | 0.9 (−2.7, 4.5) | 1.8 (−2.3, 6.2) | 4.2 (0.7, 7.9) |
| Cs | 3.4 (1.4, 5.5)2 | 6.2 (4.0, 8.6)2 | −6.5 (−8.9, −4.1)2 | 4.0 (1.7, 6.3)2 |
| Cu | −0.3 (−1.3, 0.7) | −0.5 (−1.6, 0.6) | 0.5 (−0.9, 1.8) | −1.3 (−2.3, −0.2) |
| Hg3 | 12.7 (4.7, 21.3)2 | 32.6 (22.7, 43.3)2 | −17.3 (−24.9, −9.1)2 | 31.1 (21.2, 41.7)2 |
| Mg | −1.4 (−2.5, −0.3) | −0.6 (−1.8, 0.6) | 1.0 (−0.5, 2.5) | −0.1 (−1.3, 1.1) |
| Mn | 1.4 (−0.6, 3.5) | 2.0 (−0.1, 4.3) | −1.7 (−4.2, 1.0) | 0.6 (−1.5, 2.9) |
| Pb | −1.7 (−4.1, 0.8) | −0.1 (−2.8, 2.6) | 3.5 (0.2, 7.0) | 4.0 (1.2, 6.8) |
| Se | 1.9 (0.7, 3.2)2 | 2.2 (0.9, 3.5)2 | −1.1 (−2.6, 0.5) | 1.5 (0.2, 2.8) |
| Zn | 0.1 (−1.0, 1.1) | −0.2 (−1.4, 1.0) | −0.1 (−1.4, 1.4) | −0.6 (−1.8, 0.6) |
We used multivariable linear regression to estimate all effect estimates and 95% CIs adjusting for age, race/ethnicity, education, household income, marital status, smoking status, parity, prepregnancy BMI, hemoglobin concentration, and total calorie intake. AHEI-P, Alternate Healthy Eating Index for Pregnancy; SD: standard deviation.
False discovery rate–adjusted P value < 0.01.
n = 1181.
Fish and shellfish consumption
From the DWAS result, we observed strong positive associations of fish and shellfish intake with erythrocyte As and Hg (Figure 3, Supplemental Figure 2D). We further explored the causal relation using TMLE and found that under the hypothetical intervention in which all women in the population followed the dietary recommendation of eating ≤3 servings of fish and shellfish per week, the marginal GM of erythrocyte As was 0.55 ng/g lower (95% CI: 0.28, 0.82 ng/g) and erythrocyte Hg was 2.67 ng/g lower (95% CI: 1.80, 3.55 ng/g), than had all women consumed >3 servings of fish and shellfish per week, given intakes of all other food groups remaining constant (Table 3). These differences were comparable with the median concentrations in this population (As: 0.8 ng/g [IQR: 0.4–1.6 ng/g]; Hg: 3.3 ng/g [IQR: 1.6–6.6 ng/g]) (Table 1).
FIGURE 3.
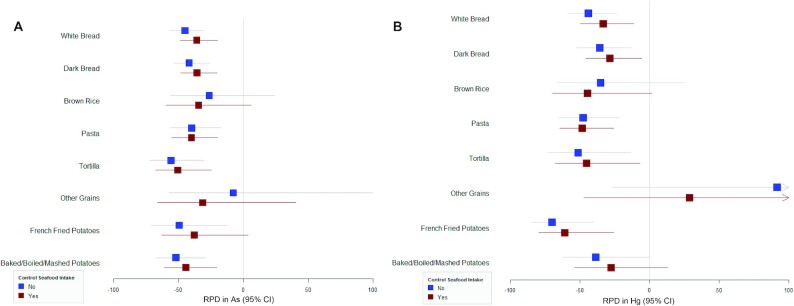
Relative change (%) in median erythrocyte As (n = 1196) (A) and Hg (n = 1181) (B) concentrations by substituting white rice with other grains (per serving/d) for pregnant women in Project Viva.
TABLE 3.
Marginal GM difference in erythrocyte As and Hg concentrations under a hypothetical intervention scenario of consumption (≤3 servings/wk compared with >3 servings/wk) for pregnant women in Project Viva1
| Marginal GM difference,2 ng/g | ||||
|---|---|---|---|---|
| Metals | Exposed/total, n/n | Model 1,3 ψ (95% CI) | Model 2,4 ψ (95% CI) | Model 3,5 ψ (95% CI) |
| As | 1026/1196 | −0.49 (−0.75, −0.24) | −0.58 (−0.81, −0.35) | −0.55 (−0.82, −0.28) |
| Hg | 1014/1181 | −3.10 (−4.21, −1.99) | −3.33 (−4.43, −2.22) | −2.67 (−3.55, −1.80) |
GM, geometric mean.
Marginal GM difference in erythrocyte metal concentrations estimated using targeted maximum likelihood estimation under the hypothetical intervention scenario that all women followed the dietary guideline of ≤3 servings of fish and shellfish consumption per week (A = 1) compared with the scenario of exceeding the dietary recommendation for weekly fish and shellfish consumption (A = 0).
Model 1: adjusted for baseline covariates, including age, parity, education, household income, marital status, race, prepregnancy BMI, smoking, and hemoglobin concentration.
Model 2: Model 1 + total calorie intake (isocaloric comparison).
Model 3: Model 1 + all other food groups (maintaining the same intake amounts for other food groups).
White rice consumption
We observed strong positive associations of erythrocyte As and Hg with white rice intake, but not with other grains (Supplemental Figure 2E). We estimated the effect of replacing white rice with other grains using substitution models and found that, after accounting for covariates including seafood intake, substituting white rice with white bread, dark bread, pasta, tortilla, and potato was associated with lower As and Hg (Figure 3). We observed the strongest effect for reducing erythrocyte As by replacing white rice with tortilla (−51.9%; 95% CI: −63.3%, −37.0% per serving). Replacement with brown rice and “other grains” did not have a significant effect on erythrocyte As and Hg (Figure 3).
Discussion
There is a growing interest in uncovering dietary sources of exposures to environmental chemicals, especially for pregnant women (31, 32). We applied a DWAS to systematically evaluate the relations between self-reported food intakes and blood concentrations of 11 metals using a population of pregnant women living in the New England area. We identified several previously known diet–metal associations, including Hg with seafood and As with white rice, in this population. We also uncovered new potential sources of metal exposures to As and Hg, such as fresh fruits, green vegetables, and eggs.
To our knowledge, this is the first study to use diet scores to characterize the association between diet patterns and blood metal concentrations among pregnant women. Pregnant women who had healthier dietary patterns, as characterized by higher AHEI-P, Mediterranean, and Prudent diet scores, also had higher erythrocyte As and Hg, whereas those with the unhealthier Western diet pattern had lower erythrocyte Hg. Although this finding was expected from the DWAS results, because fruits, vegetables, and seafood were the main components of the healthy eating diet scores, this finding highlighted the fact that food can be a source of nutrients and exposure to environmental contaminants at the same time, which can lead to confounding in health outcome studies that examine food intake as an exposure. There is a fair amount of literature investigating the negative confounding in the relation between the risks (MeHg) and benefits (PUFAs and other nutrients) associated with eating fish and seafood (33); however, because more studies have showed links between diet and chemical exposures (25, 26, 32), we should extend this assessment of confounding beyond just fish and seafood. Statistical methods for analyzing correlated complex mixtures, such as quantile g-computation (34) and Bayesian weight-quantile sum regressions (35), are available to concurrently analyze the effect of a mixture (e.g., diet) which has both beneficial and harmful effects on a health outcome; Bayesian kernel machine regression (36) has the functionality to assess interactions within the mixture and can determine risk from contaminates given a range of nutritional backgrounds.
Overall, our findings were consistent with studies from other countries, specifically, self-reported fish and shellfish consumption were associated with higher biomarker concentrations of As and Hg (25, 31, 32, 37–39) and white rice intake was associated with higher As biomarker concentrations (37, 40). We note that the biomarker we used measured total As and total Hg, which only included fractions of the more toxic species (iAs and MeHg); although, previous reports had indicated As found in erythrocytes was mostly iAs (41) and 70%–95% of the total Hg in whole blood was MeHg and bonded to hemoglobin (19).
Fish, shellfish, meat, poultry, dairy products, rice, cereals, and apple juices had been shown to contain As, and our study provided supporting evidence for seafood, white rice, and fruits. The strong association we observed between fish intake and erythrocyte As was expected because seafood has been identified as the major source of As. The proportion of iAs in fish is very low and the major constituent of As in fish is organic As, such as arsenosugars and arsenolipids. Although organic As is assumed to be less harmful owing to having a shorter half-life than iAs (42), actual studies on its toxicity are rare; studies on the long-term health effect of As exposure from seafood intake are still warranted. The source of As in fruits and rice likely was arsenical pesticides and herbicides, or iAs naturally occurring or contaminated in the environment. Rice can absorb more iAs than other food crops (10 times higher than wheat) (43). From our data, substituting white rice with other grains during pregnancy could lower the body burden of total As. Currently, there is no dietary guideline on rice consumption for pregnant women in the United States, but the US FDA is monitoring the iAs concentration in rice and rice products and is currently finalizing an action level of 100 µg/kg for iAs in infant rice cereal (44). Notably, the US Geological Survey detected moderate to high concentrations of iAs in groundwater sampled from the New England area where this study was based (45); however, we did not detect a significant association between plain water intake and erythrocyte total As concentration in this population (Supplemental Figure 2F).
The 2015–2020 Dietary Guidelines for Americans recommended eating ≥8 and ≤12 ounces of seafood low in Hg per week. Our data provided evidence that following the guideline of consuming <3 servings of fish per week can lower erythrocyte Hg concentration. Despite the risk of having higher Hg exposure, we previously showed that pregnant women with higher fish intake also had offspring with better cognitive outcomes (46, 47), again highlighting the importance of accounting for both the risk and the benefit when providing dietary advice to pregnant women, as well as providing support for guidance on choosing seafood species known to have lower concentrations of environmental contaminants such as Hg.
Not hypothesized, we observed positive associations of Hg with white rice, egg, and some fruit and vegetable items (blueberries, spinach, and roman lettuce) and Cs with fresh fruits, which warrant more investigations. Contaminated environment (soil, air, water), fishmeal in poultry feeds, or industrialized poultry and agricultural production systems are possible routes for Hg to enter the food system. NHANES data showed links between rice and Hg (7), especially among non–seafood consumers (48) and those who self-identified as Asian (49). Hg contamination in egg and vegetables had been reported but most studies were done in contaminated areas (50). Cs naturally occurs in the environment and concentrations can elevate because of mining and milling of certain ores. Cs is only mildly toxic in its stable form, but its radio isotopes present a high health risk. Nuclear power plants release radioactive Cs during normal operations and surface water and different types of food have been found to contain detectable concentrations of radioactive Cs (51).
Our study had several strengths. We used observational data and objective biomarkers to identify potential exposure sources for metals. It is difficult and costly to perform randomized controlled trials to intervene on dietary intakes, therefore this type of observational evidence is highly valuable. We applied stringent statistical approaches that reduced the chance of false discoveries. We confirmed the internal validity of our data by conducting multiple sensitivity tests. We performed dietary characterization using multiple approaches to provide a comprehensive evaluation and utilized a causal inference method with a double-robust estimator that reduced bias from misclassification to provide relevant estimates based on dietary recommendations.
Some of the limitations in our study included potential recall bias from intake self-reported using an FFQ, although a previous validation study with biomarkers showed that the FFQ had good power in discriminating high from low intake (9). The list of foods available in the FFQ was not designed specifically to capture potential sources of metal exposures, such as cooking, processing, and packaging methods. Future studies on diet–metal relations can incorporate other methods such as the NOVA Food Classification System scores (https://world.openfoodfacts.org) or questionnaires on food preparation methods to account for other potential sources of exposure. We measured total As and total Hg only and did not quantify iAs and MeHg, which are more relevant to adverse health effects. Some metals have different half-lives in RBCs and might not accurately reflect long-term exposure. Future studies should incorporate speciation analyses on As and Hg. Our findings, owing to their cross-sectional nature, can only suggest association. Our study participants were not aware of their erythrocyte metal concentrations at the time of interview, thus, reverse causation is unlikely because we did not expect erythrocyte concentrations of metals to influence the reporting of dietary behavior, nor do we expect there to be any biological mechanism that could change dietary reporting based on erythrocyte concentrations of metals. Finally, our participants were mostly white, college graduates with high socioeconomic status, residing in the New England region of the northeastern United States; the external validity of our study is limited by these characteristics and findings may not be generalizable to populations with different dietary habits and levels of metal exposure.
In conclusion, in a population of pregnant women recruited from eastern Massachusetts, we conducted a DWAS on metals. The DWAS confirmed many previously known dietary sources of exposure, including As and Hg from rice and seafood, and uncovered some previously unknown associations, for example, Hg from eggs and Cs from fresh fruits. In this population, we found positive associations of intakes of fruit, green vegetables, white rice, and seafood with erythrocyte As and Hg. Prenatal diet may be an important source of metal exposures, especially As and Hg.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the research team at Project Viva for collecting and managing data; Inbar Brenner, Karen Ruderman, Marleny Ortega, and Chelsea Jenter at the Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute providing administrative support; and the Children's Health Exposure Analysis Resource (CHEAR) Data Center of the Department of Environmental Medicine and Public Health at Icahn School of Medicine at Mount Sinai for performing the quantification of metal concentrations in RBCs.
The authors’ responsibilities were as follows—P-iDL and EO: were in charge of the design of the research, including project conception, development of the overall research plan, and study oversight; P-iDL: performed the statistical analyses, drafted the manuscript, and had primary responsibility for the final content; ROW and CA: were responsible for the erythrocyte metal measurements; SLR-S, M-FH, and EO: were involved with data collection; and all authors: read and approved the final manuscript and provided significant contributions to the revision of the manuscript. The authors report no conflicts of interest.
Notes
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development grant R01 HD034568 (to EO), NIH Office of the Director grant UG3 OD023286 (to EO), and National Institute of Environmental Health Sciences (NIEHS) grant R01ES031259 (to AC). The Children's Health Exposure Analysis Resource (CHEAR) funded the measurement of metals (CHEAR award #2017-1740; grant U2CES026561) which was carried out at the Mount Sinai CHEAR Network Laboratory. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIH, NIEHS, or the Environmental influences on Child Health Outcomes (ECHO) program.
Supplemental Tables 1 and 2, Supplemental Figures 1 and 2, and Supplemental Methods 1 and 2 and are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AHEI-P, Alternate Healthy Eating Index for Pregnancy; DWAS, diet-wide association study; FDR, false discovery rate; GM, geometric mean; iAs, inorganic As; ICC, intraclass correlation; ICP-MS, inductively coupled plasma mass spectrometry; ISO, International Organization for Standardization; LOD, limit of detection; MDS, Mediterranean Diet Score; MeHg, methylmercury; QC, quality control; TMLE, targeted maximum likelihood estimation.
Contributor Information
Pi-i D Lin, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA.
Andres Cardenas, Division of Environmental Health Sciences, School of Public Health, University of California, Berkeley, Berkeley, CA, USA.
Sheryl L Rifas-Shiman, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA.
Marie-France Hivert, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA; Diabetes Unit, Massachusetts General Hospital, Boston, MA, USA.
Tamarra James-Todd, Department of Environmental Health, Harvard TH Chan School of Public Health, Boston, MA, USA.
Chitra Amarasiriwardena, Department of Environmental Medicine, Icahn School of Medicine at Mount Sinai, New York City, NY, USA; Institute for Exposomic Research, Icahn School of Medicine at Mount Sinai, New York City, NY, USA.
Robert O Wright, Department of Environmental Medicine, Icahn School of Medicine at Mount Sinai, New York City, NY, USA; Institute for Exposomic Research, Icahn School of Medicine at Mount Sinai, New York City, NY, USA.
Mohammad L Rahman, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA.
Emily Oken, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA.
Data availability
Data described in the article, code book, and analytic code will be made available upon request pending approval. Metals’ concentrations are available on the CHEAR Data Center Repository (https://cheardatacenter.mssm.edu/) at doi:10.36043/1740_225.
References
- 1. National Research Council . Critical aspects of EPA's IRIS assessment of inorganic arsenic: interim report. Washington (DC): National Academies Press; 2013. [Google Scholar]
- 2. Imamul Huq SM, Joardar JC, Parvin S, Correll R, Naidu R. Arsenic contamination in food-chain: transfer of arsenic into food materials through groundwater irrigation. J Health Popul Nutr. 2006;24(3):305–16. [PMC free article] [PubMed] [Google Scholar]
- 3. Adamse P, Van der Fels-Klerx HJI, de Jong J. Cadmium, lead, mercury and arsenic in animal feed and feed materials – trend analysis of monitoring results. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2017;34(8):1298–311. [DOI] [PubMed] [Google Scholar]
- 4. WHO . Food and nutrition policy for schools: a tool for the development of school nutrition programmes in the WHO European Regions. Copenhagen (Denmark): WHO, Programme for Nutrition and Food Security; 2006. [Google Scholar]
- 5. Sunderland EM, Li M, Bullard K. Decadal changes in the edible supply of seafood and methylmercury exposure in the United States. Environ Health Perspect. 2018;126(1):017006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim K, Melough MM, Vance TM, Noh H, Koo SI, Chun OK. Dietary cadmium intake and sources in the US. Nutrients. 2019;11(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis MA, Gilbert-Diamond D, Karagas MR, Li Z, Moore JH, Williams SM, Frost HR. A dietary-wide association study (DWAS) of environmental metal exposure in US children and adults. PLoS One. 2014;9(9):e104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, Rich-Edwards JW, Rifas-Shiman SL, Sagiv S, Taveras EMet al. Cohort profile: Project Viva. Int J Epidemiol. 2015;44(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol. 2004;14(10):754–62. [DOI] [PubMed] [Google Scholar]
- 10. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26.; discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 11. Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc. 2009;109(6):1004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–608. [DOI] [PubMed] [Google Scholar]
- 13. Lange NE, Rifas-Shiman SL, Camargo CA Jr, Gold DR, Gillman MW, Litonjua AA. Maternal dietary pattern during pregnancy is not associated with recurrent wheeze in children. J Allergy Clin Immunol. 2010;126(2):250–5.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walk-in-Lab . Copper blood test, RBC. [Internet]. Slidell (LA): Walk-in-Lab; 2020; [cited 2020 May 9]. Available from: https://www.walkinlab.com/products/view/copper-rbc-plasma-blood-test. [Google Scholar]
- 15. Li YV, Zhang JH. Metal ion in stroke. New York: Springer-Verlag; 2012. [Google Scholar]
- 16. Mayo Clinic Laboratories . Manganese, blood. [Internet]. Rochester (MN): Mayo Clinic Laboratories; 2020; [cited 2020 May 9]. Available from: https://neurology.testcatalog.org/show/MNB. [Google Scholar]
- 17. Vormann J. Magnesium: nutrition and metabolism. Mol Aspects Med. 2003;24(1–3):27–37. [DOI] [PubMed] [Google Scholar]
- 18. Hall M, Chen Y, Ahsan H, Slavkovich V, van Geen A, Parvez F, Graziano J. Blood arsenic as a biomarker of arsenic exposure: results from a prospective study. Toxicology. 2006;225(2–3):225–33. [DOI] [PubMed] [Google Scholar]
- 19. Mortensen ME, Caudill SP, Caldwell KL, Ward CD, Jones RL. Total and methyl mercury in whole blood measured for the first time in the U.S. population: NHANES 2011–2012. Environ Res. 2014;134:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112(17):1691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hernán MA, Robins JM. Graphical representation of causal effects. In: Causal inference: what if. Boca Raton (FL): CRC Press; 2020. p. 69–82. [Google Scholar]
- 22. Boca SM, Leek JT. A direct approach to estimating false discovery rates conditional on covariates. PeerJ. 2018;6:e6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lai Y. A statistical method for the conservative adjustment of false discovery rate (q-value). BMC Bioinformatics. 2017;18(S3):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barrera-Gómez J, Basagaña X. Models with transformed variables: interpretation and software. Epidemiology. 2015;26(2):e16–7. [DOI] [PubMed] [Google Scholar]
- 25. Lin P-ID, Bromage S, Mostofa MG, Allen J, Oken E, Kile ML, Christiani DC. Associations between diet and toenail arsenic concentration among pregnant women in Bangladesh: a prospective study. Nutrients. 2017;9(4):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin P-ID, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert M-F, Fleisch AF, Calafat AM, Sanchez-Guerra M, Osorio-Yáñez Cet al. Dietary characteristics associated with plasma concentrations of per- and polyfluoroalkyl substances among adults with pre-diabetes: cross-sectional results from the Diabetes Prevention Program Trial. Environ Int. 2020;137:105217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schuler MS, Rose S. Targeted maximum likelihood estimation for causal inference in observational studies. Am J Epidemiol. 2017;185(1):65–73. [DOI] [PubMed] [Google Scholar]
- 28. Environmental Protection Agency (EPA)/FDA . Advice about eating fish: for women who are or might become pregnant, breastfeeding mothers, and young children. [Internet]. Silver Spring (MD): FDA; 2019; [cited 2020 Feb 10]. Available from: https://www.fda.gov/media/102331/download. [Google Scholar]
- 29. Van der Laan MJ, Rose S. Targeted learning: causal inference for observational and experimental data. New York: Springer; 2011. [Google Scholar]
- 30. Færch K, Lau C, Tetens I, Pedersen OB, Jørgensen T, Borch-Johnsen K, Glümer C. A statistical approach based on substitution of macronutrients provides additional information to models analyzing single dietary factors in relation to type 2 diabetes in Danish adults: the Inter99 study. J Nutr. 2005;135(5):1177–82. [DOI] [PubMed] [Google Scholar]
- 31. Caspersen IH, Thomsen C, Haug LS, Knutsen HK, Brantsæter AL, Papadopoulou E, Erlund I, Lundh T, Alexander J, Meltzer HM. Patterns and dietary determinants of essential and toxic elements in blood measured in mid-pregnancy: the Norwegian Environmental Biobank. Sci Total Environ. 2019;671:299–308. [DOI] [PubMed] [Google Scholar]
- 32. Papadopoulou E, Haug LS, Sakhi AK, Andrusaityte S, Basagaña X, Brantsaeter AL, Casas M, Fernández-Barrés S, Grazuleviciene R, Knutsen HKet al. Diet as a source of exposure to environmental contaminants for pregnant women and children from six European countries. Environ Health Perspect. 2019;127(10):107005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi AL, Cordier S, Weihe P, Grandjean P. Negative confounding in the evaluation of toxicity: the case of methylmercury in fish and seafood. Crit Rev Toxicol. 2008;38(10):877–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keil AP, Buckley JP, O'Brien KM, Ferguson KK, Zhao S, White AJ. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect. 2020;128(4):047004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Colicino E, Pedretti NF, Busgang SA, Gennings C. Per- and poly-fluoroalkyl substances and bone mineral density: results from the Bayesian weighted quantile sum regression. Environ Epidemiol. 2020;4(3):e092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, Godleski JJ, Coull BA. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ashrap P, Watkins DJ, Mukherjee B, Boss J, Richards MJ, Rosario Z, Vélez-Vega CM, Alshawabkeh A, Cordero JF, Meeker JD. Predictors of urinary and blood metal(loid) concentrations among pregnant women in Northern Puerto Rico. Environ Res. 2020;183:109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Golding J, Steer CD, Hibbeln JR, Emmett PM, Lowery T, Jones R. Dietary predictors of maternal prenatal blood mercury levels in the ALSPAC birth cohort study. Environ Health Perspect. 2013;121(10):1214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hinwood AL, Callan AC, Ramalingam M, Boyce M, Heyworth J, McCafferty P, Odland JO. Cadmium, lead and mercury exposure in non smoking pregnant women. Environ Res. 2013;126:118–24. [DOI] [PubMed] [Google Scholar]
- 40. Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, Baker ER, Jackson BP, Folt CL, Karagas MR. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci U S A. 2011;108(51):20656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guo M, Wang B, Liu S, Wang W, Gao C, Hu S, Fan S, Hai X, Zhou J. Time course of arsenic species in red blood cells of acute promyelocytic leukemia (APL) patients treated with single agent arsenic trioxide. Exp Rev Clin Pharmacol. 2019;12(4):371–8. [DOI] [PubMed] [Google Scholar]
- 42. Molin M, Ulven SM, Meltzer HM, Alexander J. Arsenic in the human food chain, biotransformation and toxicology – review focusing on seafood arsenic. J Trace Elem Med Biol. 2015;31:249–59. [DOI] [PubMed] [Google Scholar]
- 43. Davis MA, Signes-Pastor AJ, Argos M, Slaughter F, Pendergrast C, Punshon T, Gossai A, Ahsan H, Karagas MR. Assessment of human dietary exposure to arsenic through rice. Sci Total Environ. 2017;586:1237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. US FDA . For consumers: seven things pregnant women and parents need to know about arsenic in rice and rice cereal. [Internet]. Silver Spring (MD): FDA; 2020; [cited 2020 Nov 3]. Available from: https://www.fda.gov/consumers/consumer-updates/consumers-seven-things-pregnant-women-and-parents-need-know-about-arsenic-rice-and-rice-cereal. [Google Scholar]
- 45. Welch AH, Ryker SJ, Helsel DR, Hamilton PA. Arsenic in ground water of the United States: an overview. Water Well J. 2001;2(3):30–3. [Google Scholar]
- 46. Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, Hu H, Gillman MW. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol. 2008;167(10):1171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW. Maternal fish consumption, hair mercury, and infant cognition in a U.S. cohort. Environ Health Perspect. 2005;113(10):1376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wells EM, Kopylev L, Nachman R, Radke EG, Segal D. Seafood, wine, rice, vegetables, and other food items associated with mercury biomarkers among seafood and non-seafood consumers: NHANES 2011–2012. J Exposure Sci Environ Epidemiol. 2020;30(3):504–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Awata H, Linder S, Mitchell LE, Delclos GL. Association of dietary intake and biomarker levels of arsenic, cadmium, lead, and mercury among Asian populations in the United States: NHANES 2011–2012. Environ Health Perspect. 2017;125(3):314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Overmeire I, Pussemier L, Hanot V, De Temmerman L, Hoenig M, Goeyens L. Chemical contamination of free-range eggs from Belgium. Food Addit Contam. 2006;23(11):1109–22. [DOI] [PubMed] [Google Scholar]
- 51. Agency for Toxic Substances and Disease Registry (ATSDR) . Public health statement for cesium. Atlanta (GA): ATSDR; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will be made available upon request pending approval. Metals’ concentrations are available on the CHEAR Data Center Repository (https://cheardatacenter.mssm.edu/) at doi:10.36043/1740_225.