ABSTRACT
Background
Plant-rich diets are associated with lower cardiometabolic risks and longer survival in the general population, but their association with mortality in cancer survivors is still unclear.
Objectives
We aimed to examine the associations of 3 postdiagnostic plant-based diet indices with all-cause mortality in omnivorous long-term colorectal cancer (CRC) survivors.
Methods
Diet was assessed with FFQs at a median of 6 years after diagnosis in 1404 CRC survivors (56% male; median age, 69 years) in a Northern German prospective cohort study. An overall, a healthful plant-based, and an unhealthful plant-based diet index were derived by scoring intakes of animal foods reversely and intakes of healthy (whole grains, vegetables, fruits, legumes, nuts, oils, tea/coffee) and less healthy plant foods (refined grains, fruit juices, sugar-sweetened beverages, potatoes, sweets/desserts) positively or reversely, depending on the index. Vital status follow-up was conducted via population registries. Cox proportional hazards regression was applied to estimate HRs for all-cause mortality according to plant-based diet adherence.
Results
Within 7 years (median) after diet assessment, 204 deaths occurred. The overall plant-based diet index displayed a significant, inverse association with all-cause mortality (HR per 10-point increase in diet index, 0.72; 95% CI, 0.57–0.91). Although not statistically significant, higher healthful plant-based diet scores showed a strong tendency towards lower mortality (HR, 0.82; 95% CI, 0.67–1.01). The unhealthful plant-based diet index was associated with higher mortality, but lost statistical significance after multivariable adjustment (HR, 1.19; 95% CI, 0.96–1.48). A subgroup analysis revealed that the tendency towards a positive association of the unhealthful plant-based diet with mortality was restricted to less physically active individuals (<95 metabolic equivalent of task hours/week).
Conclusions
An overall plant-based diet was inversely associated with all-cause mortality in long-term CRC survivors. However, more research is needed to further disentangle the impacts of different qualities of plant-based diets on cancer survivors’ health.
Keywords: plant-based diet, colorectal cancer, long-term survivors, survival, mortality
Introduction
Colorectal cancer (CRC) is among the most common types of cancer worldwide, and thus causes a substantial burden for patients, their families, and the health-care system (1, 2). Earlier and enhanced diagnostics, as well as progress and innovation in treatment strategies, has reduced the mortality associated with CRC, leading to a growing population of long-term survivors (2–4). To further improve their general health, quality of life, and survival after cancer, many CRC survivors are seeking advice, particularly regarding beneficial lifestyle modifications (5, 6). Several dietary factors, as well as adherence to defined dietary patterns, have previously been shown to be associated with survival after a diagnosis of CRC (7–9). For example, in a prior analysis of the present CRC survivor cohort, greater adherence to the Mediterranean diet or to the healthy Nordic diet was associated with lower all-cause mortality (10).
Over the past decades, potential health effects of plant-based diets have been increasingly recognized (11, 12). To assess the degree of adherence to plant foods within an omnivorous diet, Satija et al. (13, 14) developed 3 different plant-based diet indices that allow a plant-based diet to be quantified on a continuous scale instead of being a binary trait (e.g., vegetarian compared with nonvegetarian), similar to the provegetarian food pattern developed by Martínez-González et al. (15). The application of these dietary indices also enables the appraisal of the quality of a plant-based diet by providing an overall plant-based diet index (PDI), a healthful plant-based diet index (hPDI), and an unhealthful plant-based diet index (uPDI). Whereas the PDI represents a general plant-based, less animal-based diet, the hPDI emphasizes healthy plant foods, such as whole grains, vegetables, fruits, nuts, and legumes, and the uPDI is defined by high intake of rather unhealthy plant foods, like refined grains, sugar-sweetened beverages, fruit juices, and sweets and desserts (14).
In prior publications, these plant-based diet indices revealed statistically significant associations with diabetes mellitus, cardiovascular diseases, weight change, obesity, visceral adipose tissue, and mortality in general population-based cohorts (13, 14, 16–19). However, their relevance for survival in individuals after a cancer diagnosis is still unknown.
Therefore, we aimed to investigate the associations of the degree of adherence to the 3 different plant-based diet indices (PDI, hPDI, uPDI) with all-cause mortality in a cohort of omnivorous long-term CRC survivors.
Methods
Study design and study population
A cohort of 2733 patients with a histologically confirmed CRC diagnosis was recruited by the biobank popgen between 2004 and 2007 and was followed up prospectively. The study design has been previously reported in more detail (20–22). Briefly, patients with a diagnosis of CRC between 2002 and 2005 were identified through medical records of surgical departments from 23 hospitals in Northern Germany and via the cancer registry of the state of Schleswig-Holstein. From the University Hospital Kiel, patients who were diagnosed between 1993 and 2005 were included. Patients were invited to participate in the study by their treating physicians and were asked to fill in a questionnaire about clinical and sociodemographic characteristics and selected lifestyle factors. The study protocol was approved by the institutional ethics committee of the Medical Faculty of Kiel University, and written informed consent was provided by all study participants.
A follow-up of study participants was conducted between 2009 and 2011, in which a total of 2263 patients who initially agreed to be recontacted were asked to complete another questionnaire on clinical and sociodemographic characteristics, as well as an FFQ (23) with a set of additional questions on usual physical activity attached. Of the 2263 individuals contacted for this follow-up, 354 participants were deceased and 31 were lost to follow-up (due to an unknown residential address). Of the remaining 1878 participants, 1685 individuals completed the general questionnaire; of these, 1452 filled in the FFQ. Of the 1452 study participants with dietary data, we excluded individuals with missing information on the year of CRC diagnosis (n = 21) and those who had a diagnosis of small intestinal cancer instead of CRC (n = 3). Furthermore, participants with implausible information on the length of follow-up (n = 3) and participants with missing information on vital status (n = 21) were excluded from analyses, resulting in a final study sample of 1404 CRC survivors. A participant flow chart is depicted in Supplementary Figure 1.
Ascertainment of vital status
The ascertainment of vital status has previously been described in more detail (10). Vital status of all study participants was updated from March to June 2016 via population registries, and the date of death was recorded if participants were deceased. In total, 204 deaths had occurred since diet assessment, and the date of death could be verified for all cases. For the present study, the start of survival follow-up was the date of diet assessment. The follow-up ended with the verified date of death or the date of the last vital status assessment, whichever came first.
Assessment of diet and calculation of diet indices
Usual dietary intake was assessed with a validated, semi-quantitative, web-based FFQ that was developed by the Department of Epidemiology at the German Institute of Human Nutrition in Potsdam-Rehbrücke (23). A paper-based version was available on request and was used by 84% of participants. The 112-item FFQ evaluates the consumption frequencies of predefined foods and beverages during the previous 12 months. Frequency categories consisted of 4 to 11 options, ranging from “never” and “once a day” to “eleven times a day or more frequently.” Quantities were provided as portions, grams, milliliters, slices, pieces, or spoons. Total daily energy intake and intakes of each inquired food or food group in grams per day were calculated for each participant based on the FFQ data using the German Food Code and Nutrient Database (version II.3) (24). According to gram-per-day intake data of meat and fish products, none of the study participants followed a vegetarian (exclusion of meat and fish) or vegan (exclusion of all animal-derived foods) diet.
Plant-based diet indices were calculated based on the available dietary data using the approach developed and published by Satija et al. (13, 14). Specifically, a total of 18 food groups were derived, representing 3 food categories: 1) healthy plant foods (whole grains, vegetables, fruits, legumes, nuts, vegetable oils, and tea and coffee); 2) less healthy plant foods (refined grains, potatoes, fruit juices, sugar-sweetened beverages, and sweets and desserts); and 3) animal foods (meat, animal fat, dairy, fish/seafood, eggs, and miscellaneous animal-based foods).
The categorization of healthy and less healthy plant foods was based on evidence concerning associations between different foods and chronic disease outcomes (13). Alcoholic beverages, which could not be clearly rated as healthy or less healthy, were not included in the indices (13) but were adjusted for in the multivariable regression analyses. Subsequently, based on the intake values, each of the 18 food groups was ranked according to sex-specific quintiles, and scores from 1 to 5 were assigned positively (Q1 = 1, Q2 = 2, Q3 = 3, Q4 = 4, Q5 = 5) or reversely (Q5 = 1, Q4 = 2, Q3 = 3, Q2 = 4, Q1 = 5), depending on the respective diet index. For the overall PDI, food groups of both healthy and less healthy plant-food categories were assigned positive scores and food groups of the animal food category were assigned reverse scores. To derive the hPDI, food groups of the healthy plant food category were given positive scores and food groups of the less healthy plant food category and foods of the animal food category were given reverse scores. For the uPDI, food groups of the less healthy plant food category were scored positively, and food groups of the healthy plant food category and the animal food category were scored reversely. Finally, to obtain the 3 plant-based diet indices, the scores of the food groups were summed for each index, resulting in a total score ranging from 18 to 90, with a higher index indicating a more plant-based (overall, healthful, or unhealthful) and less animal-based diet.
Clinical and sociodemographic data
Clinical factors, including information on tumor location, the occurrence of metastases or other types of cancer (both reported at baseline and follow-up), and use of neoadjuvant and adjuvant cancer therapies in addition to surgery, were obtained from each participant by self-administered questionnaires. In a subset of 181 patients, physician information was available and was used to validate self-reported information on tumor location, type of therapy, and metastases against medical records, which revealed an overall good agreement (87% concordance).
Using questionnaires from study participants, we also assessed information on sociodemographic factors, including sex, age at diagnosis, age at diet assessment (follow-up), smoking status at follow-up, and post-diagnostic body weight and height at follow-up. BMI was defined as kg/m². The FFQ included additional validated questions on physical activity (walking, cycling, sports, gardening, housework, home repair, stair climbing) during the past 12 months (25). To obtain intensity levels that could be summed to a value of total physical activity, we assigned corresponding metabolic equivalent of task (MET) values, according to the 2000 Compendium of Physical Activity (26), to each activity (27).
Statistical analyses
Main participant characteristics were compared across quartiles of the PDI, and tests for statistical differences included the Kruskal-Wallis test for continuous variables and the chi-square test for categorical variables.
Associations of each of the 3 plant-based diet indices (PDI, hPDI, uPDI) with all-cause mortality were assessed using Cox proportional hazard regression models, calculating HRs and 95% CIs for all-cause mortality according to diet index quartile (considering Quartile 1 as the reference) and per 10-point increases in the diet index (continuously). The time interval from age at diet assessment to age at death or last follow-up was the underlying time variable for this survival analysis. To control for confounding factors, a first regression model was adjusted for sex and age at diet assessment. A second model was additionally adjusted for BMI at diet assessment (continuous in kg/m²), physical activity (continuous in METs/week), total energy intake (continuous in kcal/day), alcohol intake (continuous in g/day), smoking status (never, former, current, unknown), survival time from CRC diagnosis until diet assessment (continuous in years), tumor location (colon, rectum, both, unknown), occurrence of metastases (yes, no, unknown), occurrence of other cancers (yes, no, unknown), and type of neo-/adjuvant therapy (none, chemotherapy, radiation, both, unknown). The assumption of proportional hazards was tested by the Schoenfeld residuals method and by including time-dependent variables in the models. Because 3 variables (age, BMI, and metastases) did not meet the assumption of proportional hazards, respective multiplicative time-covariate interaction terms (time × age, time × BMI, time × metastases) were included in the models. We tested for a linear trend across quartiles by modeling the median value of the diet score quartiles as a continuous variable and included it as exposure in the respective regression models.
We additionally tested for differences in slopes of hPDI and uPDI in their relation to all-cause mortality in the Cox proportional hazards regression models.
The presence of nonlinear associations between the 3 diet indices and all-cause mortality was analyzed with restricted cubic spline regression, fitted with 4 knots [5th, 35th, 65th, and 95th percentiles (28)] and the median of the reference category (Quartile 1) as a reference point. These regression analyses were adjusted for the same covariates as the main multivariable models (as described above).
We tested for statistical interactions of PDI, hPDI, and uPDI with BMI, physical activity, and occurrence of metastases, by including respective interaction terms (cross product of diet index and covariate) in the multivariable-adjusted models. In cases of significant statistical interactions, we performed subgroup analyses stratifying the multivariable Cox proportional hazards regression model, with the continuous (per 10-point increase) diet score as exposure, by categories of the respective covariate.
To examine the effects of the individual food groups that are components of the plant-based diet indices on survival, we additionally conducted analyses relating each food group separately to all-cause mortality and calculating HRs for the food group quintiles, using Quintile 1 as the reference category and adjusting for the same confounding variables as in the main survival analysis (see above). Also for the food group analysis, we calculated P values for a linear trend (procedure explained above).
Furthermore, we conducted 3 sensitivity analyses. First, we repeated the Cox regression analysis with the plant-based diet indices as exposures, excluding BMI as a covariate to examine the impact of the BMI adjustment on the association between dietary indices and survival. Second, we investigated the association of the post-diagnostic diet indices with all-cause mortality after excluding CRC survivors who died within 12 months after diet assessment, to exclude those patients whose dietary behavior might have been influenced by malaise, suggesting reverse causation. Third, we excluded individuals who had a diagnosis of metastases related to their CRC, to preclude individuals with an overall worse health status where the effect of dietary factors on survival might be less strong.
All statistical analyses were conducted using SAS version 9.4 software (SAS Institute, Inc.), and 2-sided P values < 0.05 were considered statistically significant.
Results
Participant characteristics of the overall study sample and stratified by quartiles of the PDI are presented in Table 1. In total, 56% of study participants were males, and the median ages at diagnosis and at diet assessment were 62 and 69 years, respectively. On average (median), there were 6 years between CRC diagnosis and the assessment of dietary behavior. Of all the CRC survivors, 17% reported a diagnosis of metastases and 21% had another cancer disease (before, during, or after CRC). About half of the study participants had no indication for adjuvant or neoadjuvant therapy and were only treated surgically. The other half of the study participants underwent chemotherapy or a combination of chemotherapy and radiation in addition to surgery (Table 1). Comparing characteristics across quartiles of PDI adherence, participants in a higher PDI quartile were slightly younger, reported more physical activity, and had a higher total energy intake when compared to participants in a lower PDI quartile (Table 1).
TABLE 1.
Characteristics of the total sample of CRC survivors (n = 1404) and stratified by quartiles of the overall PDI
| PDI quartiles | ||||||
|---|---|---|---|---|---|---|
| Participant characteristics | Total sample | Q1 | Q2 | Q3 | Q4 | P 1 |
| Total number of individuals, n (%) | 1404 (100) | 331 (24) | 332 (24) | 406 (29) | 335 (24) | |
| Number of deaths during follow-up, n (%) | 204 (15) | 59 (29) | 50 (25) | 65 (32) | 30 (15) | 0.01 |
| Plant-based diet indices, score | ||||||
| PDI | 54 (50–58) | 47 (44–48) | 51 (51–52) | 56 (54–57) | 61 (60–63) | |
| hPDI | 54 (49–59) | 50 (47–55) | 52 (48–57) | 56 (50–60) | 58 (54–63) | <0.0001 |
| uPDI | 54 (49–59) | 56 (51–61) | 55 (51–60) | 54 (48–58) | 52 (48–57) | <0.0001 |
| Sex, n (%) | ||||||
| Male | 788 (56) | 181 (55) | 183 (55) | 236 (58) | 188 (56) | |
| Female | 616 (44) | 150 (45) | 149 (45) | 170 (42) | 147 (44) | 0.78 |
| Age at diagnosis, y | 62 (57–66) | 62 (57–67) | 63 (58–67) | 61 (56–66) | 61 (56–65) | 0.004 |
| Age at diet assessment, y | 69 (64–73) | 70 (65–75) | 69 (65–74) | 68 (63–73) | 69 (64–73) | 0.03 |
| BMI, kg/m² | 26.2 (23.9–29.3) | 26.5 (23.9–29.8) | 26.4 (23.9–29.4) | 26.3 (24.1–29.2) | 25.8 (23.5–28.3) | 0.08 |
| BMI categories, n (%) | ||||||
| <20 kg/m² | 47 (3) | 10 (3) | 10 (3) | 9 (2) | 18 (5) | |
| 20 to <25 kg/m² | 489 (35) | 112 (34) | 114 (34) | 144 (36) | 119 (36) | |
| 25 to <30 kg/m² | 608 (43) | 134 (40) | 144 (44) | 184 (45) | 146 (44) | |
| ≥30 kg/m² | 260 (19) | 75 (23) | 64 (19) | 69 (17) | 52 (15) | 0.19 |
| Smoking status, n (%) | ||||||
| Never | 565 (40) | 130 (39) | 134 (40) | 159 (39) | 142 (42) | |
| Former | 692 (49) | 160 (48) | 163 (49) | 207 (51) | 162 (48 | |
| Current | 126 (9) | 35 (11) | 28 (8) | 37 (9) | 26 (8) | |
| Unknown | 21 (2) | 6 (2) | 7 (2) | 3 (1) | 5 (1) | 0.82 |
| Physical activity, METs/wk | 95 (63–132) | 80 (51–117) | 95 (65–130) | 94 (61–131) | 110 (73–150) | <0.0001 |
| Alcohol (ethanol) intake, g/day | 7.0 (1.9–19.6) | 6.3 (1.8–20.8) | 7.4 (1.8–19.2) | 6.7 (2.0–20.4) | 6.7 (1.9–17.7) | 0.94 |
| Energy intake, kcal/d | 2183 (1782–2605) | 1847 (1590–2234) | 2143 (1697–2539) | 2253 (1853–2696) | 2379 (2072–2952) | <0.0001 |
| Time between CRC diagnosis and diet assessment, y | 6 (5–8) | 6 (5–8) | 6 (5–8) | 7 (5–8) | 6 (5–8) | 0.16 |
| Tumor location, n (%) | ||||||
| Colon | 666 (47) | 146 (44) | 151 (45) | 200 (49) | 169 (50) | |
| Rectum | 594 (42) | 150 (45) | 143 (43) | 169 (42) | 132 (39) | |
| Both | 63 (5) | 11 (3) | 17 (5) | 22 (5) | 13 (4) | |
| Unknown | 81 (6) | 24 (7) | 21 (6) | 15 (4) | 21 (6) | 0.32 |
| Metastases, n (%) | ||||||
| Yes | 238 (17) | 60 (18) | 54 (16) | 67 (17) | 57 (17) | |
| No | 928 (66) | 216 (65) | 216 (65) | 168 (66) | 228 (68) | |
| Unknown | 238 (17) | 55 (17) | 62 (19) | 71 (17) | 50 (15) | 0.90 |
| Other cancer, n (%) | ||||||
| Yes | 297 (21) | 71 (21) | 64 (19) | 89 (22) | 73 (22) | |
| No | 1077 (77) | 256 (77) | 264 (80) | 305 (75) | 252 (75) | |
| Unknown | 30 (2) | 4 (1) | 4 (1) | 12 (3) | 10 (3) | 0.38 |
| Therapy, n (%) | ||||||
| None | 734 (52) | 164 (50) | 177 (53) | 215 (53) | 178 (53) | |
| Chemotherapy | 313 (22) | 74 (22) | 68 (20) | 94 (23) | 77 (23) | |
| Radiation | 45 (3) | 16 (5) | 9 (3) | 13 (3) | 7 (2) | |
| Chemotherapy and radiation | 289 (21) | 73 (22) | 71 (21) | 80 (20) | 65 (19) | |
| Unknown | 23 (2) | 4 (1) | 7 (2) | 4 (1) | 8 (2) | 0.66 |
Values are n (%) or median (IQR).
Based on chi-squared test for categorical variables and Kruskal-Wallis test for continuous variables.
Abbreviations: CRC, colorectal cancer; hPDI, healthful plant-based diet index; MET, metabolic equivalent of task; PDI, plant-based diet index; Q, quartile; uPDI, unhealthful plant-based diet index.
During a median follow-up time of 7 years, 204 of the 1404 study participants died. In multivariable-adjusted Cox proportional hazards regression analyses, higher adherence to the PDI (fourth compared with first quartile and per 10-point increase in index) was statistically significantly associated with lower all-cause mortality in CRC survivors (Table 2). Specifically, a 10-point increment in PDI decreased the risk of dying from all causes by 28% (95% CI, 9%–43%). The hPDI also showed a strong tendency towards an inverse association with all-cause mortality, but was not statistically significant (HR per 10-point increase in hPDI, 0.82; 95% CI, 0.67–1.01; P = 0.06). Additionally, when a linear trend was forced across the hPDI quartiles, the association with mortality was not statistically significant (P = 0.20; Table 2). Furthermore, a 10-point increase in uPDI was related to higher all-cause mortality in age- and sex-adjusted models, but this association lost statistical significance after multivariable adjustment (Table 2). Nevertheless, it is possible that the mortality associations with healthful and unhealthful plant-based diets are significantly different, as the test for differences in slopes revealed a P value of 0.04.
TABLE 2.
Associations between adherence to plant-based diets and all-cause mortality in 1404 CRC survivors
| Diet index quartiles | Continuous (per 10-point increase in diet index) | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P trend1 | ||
| Plant-based diet index | ||||||
| Total number of individuals (deaths), n | 331 (59) | 332 (50) | 406 (65) | 335 (30) | — | |
| Score, median (IQR) | 47 (44–48) | 51 (51–52) | 56 (54–57) | 61 (60–63) | — | |
| Age- and sex-adjusted, HR (95% CI) | 1 (Ref.) | 0.88 (0.60–1.28) | 0.96 (0.67–1.28) | 0.52 (0.33–0.81) | 0.01 | 0.76 (0.61–0.94) |
| Multivariable-adjusted,2 HR (95% CI) | 1 (Ref.) | 0.86 (0.58–1.27) | 0.90 (0.62–1.31) | 0.46 (0.29–0.75) | 0.01 | 0.72 (0.57–0.91) |
| Healthful plant-based diet index | ||||||
| Total number of individuals (deaths), n | 373 (58) | 354 (52) | 314 (46) | 363 (48) | — | |
| Score, median (IQR) | 46 (43–48) | 52 (51–53) | 56 (56–57) | 62 (60–65) | — | |
| Age- and sex-adjusted, HR (95% CI) | 1 (Ref.) | 0.88 (0.61–1.28) | 0.92 (0.63–1.36) | 0.83 (0.57–1.21) | 0.38 | 0.86 (0.70–1.04) |
| Multivariable-adjusted,2 HR (95% CI) | 1 (Ref.) | 0.87 (0.60–1.28) | 0.87 (0.58–1.28) | 0.76 (0.51–1.14) | 0.20 | 0.82 (0.67–1.01) |
| Unhealthful plant-based diet index | ||||||
| Total number of individuals (deaths), n | 325 (41) | 392 (44) | 312 (53) | 375 (66) | — | |
| Score, median (IQR) | 45 (43–47) | 52 (50–53) | 56 (56–57) | 62 (60–65) | — | |
| Age- and sex-adjusted, HR (95% CI) | 1 (Ref.) | 0.79 (0.52–1.21) | 1.36 (0.91–2.05) | 1.33 (0.90–1.97) | 0.04 | 1.22 (1.00–1.48) |
| Multivariable-adjusted,2 HR (95% CI) | 1 (Ref.) | 0.77 (0.50–1.19) | 1.37 (0.90–2.10) | 1.29 (0.84–1.98) | 0.07 | 1.19 (0.96–1.48) |
Associations are estimated with Cox proportional hazard regression.
Calculated by modeling the median value of diet score quartiles as a continuous variable.
Adjusted for sex, age at diet assessment, BMI, physical activity, survival time from CRC diagnosis until diet assessment, tumor location, metastases, other cancer, type of therapy, smoking status, alcohol intake, total energy intake, time × age, time × BMI, and time × metastases.
Abbreviation: CRC, colorectal cancer
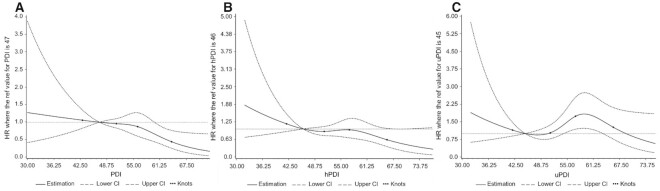
The test for nonlinearity resulted in a significant nonlinear relationship between the uPDI and all-cause mortality (P = 0.02) depicting a slightly overhead-N-shaped form (Figure 1). In contrast, the PDI and the hPDI did not provide any evidence for significant nonlinear associations with all-cause mortality (P values > 0.05; Figure 1).
FIGURE 1.
Multivariable-adjusted HRs for all-cause mortality according to adherence to the (A) PDI, (B) hPDI, and (C) uPDI, calculated with restricted cubic spline regression (all n = 1404). The solid line depicts HRs and the dashed lines are the 95% CIs. The points indicate the knots on the 5th, 35th, 65th, and 95th percentiles. The reference value is the median of the first quartile of the respective plant-based diet index. The models were adjusted for sex, age at diet assessment, BMI, physical activity, survival time from CRC diagnosis until diet assessment, tumor location, metastases, other cancer, type of therapy, smoking status, total energy intake, and alcohol intake. The P values for nonlinearity are (A) 0.25, (B) 0.41, and (C) 0.02 (Wald chi-square test). Abbreviations: CRC, colorectal cancer; hPDI, healthful plant-based diet index; PDI, plant-based diet index; uPDI, unhealthful plant-based diet index.
The tests for statistical interaction only revealed a significant interaction between the uPDI and physical activity in the association with mortality (P = 0.046). All other tested statistical interactions resulted in a P value > 0.10 (data not shown). In the subgroup analysis relating the uPDI to all-cause mortality, stratified by the median level of physical activity (<95 MET-hours/week compared with ≥95 MET-hours/week), we observed a strong tendency towards an association between a higher uPDI and greater mortality (HR per 10-point increment in uPDI, 1.31; 95% CI, 0.99–1.73; P = 0.06) in participants who were less physically active (<95 MET-hours/week; n = 698). In contrast, in the group of participants with higher physical activity (≥95 MET-hours/week; n = 706), the uPDI and mortality showed no association (HR, 0.98; 95% CI, 0.67–1.42).
When examining the individual food groups that are components of the plant-based diet indices with respect to their association with survival, some food groups revealed statistically significant associations with mortality, even though in most cases the effect strengths varied across food group quintiles (Table 3). Of the healthy plant food groups, whole grains, nuts, legumes, and vegetable oils provided some evidence of an inverse association with all-cause mortality. Among the less healthy plant food groups, a high intake of sugar-sweetened beverages was associated with a higher mortality risk, whereas moderate fruit juice consumption (Quintiles 2 and 3) was significantly related to lower all-cause mortality when compared to the lowest category of fruit juice intake (Quintile 1). Regarding the animal food groups, only greater intake of animal fat (Quintile 3 compared with Quintile 1) was suggested to be associated with a higher mortality risk. A significant linear trend across quintiles was only found for nuts and sugar-sweetened beverages (both P values = 0.004; Table 3).
TABLE 3.
Multivariable-adjusted associations between the intakes of the individual food groups and all-cause mortality in 1404 CRC survivors
| Food group quintiles | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P trend1 | |
| Healthy plant food groups | ||||||
| Whole grains, intake2 | 0.75 (0.63–0.81) | 1.07 (0.95–1.17) | 1.39 (1.35–1.42) | 1.57 (1.50–1.71) | 3.08 (2.42–3.67) | |
| HR (95% CI) | 1 (Ref.) | 0.59 (0.39–0.90) | 0.67 (0.44–1.02) | 0.54 (0.35–0.83) | 0.68 (0.44–1.06) | 0.36 |
| Fruits, intake3 | 82 (58–102) | 157 (137–169) | 200 (187–231) | 305 (236–338) | 481 (423–556) | |
| HR (95% CI) | 1 (Ref.) | 1.05 (0.66–1.66) | 1.03 (0.65–1.63) | 1.04 (0.66–1.66) | 1.12 (0.70–1.81) | 0.70 |
| Vegetables, intake3 | 119 (106–129) | 154 (148–162) | 183 (175–193) | 230 (216–245) | 362 (300–454) | |
| HR (95% CI) | 1 (Ref.) | 0.64 (0.41–1.00) | 0.79 (0.51–1.22) | 0.65 (0.41–1.04) | 0.78 (0.49–1.24) | 0.62 |
| Nuts, intake3 | 0.43 (0.43–0.44) | 0.92 (0.77–0.92) | 1.62 (1.07–2.87) | 2.93 (2.36–4.91) | 9.25 (6.77–16.85) | |
| HR (95% CI) | 1 (Ref.) | 0.71 (0.48–1.06) | 0.88 (0.59–1.31) | 0.68 (0.41–1.13) | 0.48 (0.31–0.75) | 0.004 |
| Legumes, intake3 | 0.58 (0.58–0.58) | 1.82 (1.82–1.82) | 1.20 (1.20–1.20) | 3.69 (3.69–3.69) | 6.81 (3.69–6.81) | |
| HR (95% CI) | 1 (Ref.) | 0.88 (0.54–1.43) | 0.51 (0.28–0.93) | 0.92 (0.46–1.81) | 0.88 (0.53–1.48) | 0.91 |
| Vegetable oils, intake3 | 3.7 (2.7–4.5) | 6.5 (5.8–7.0) | 8.9 (8.1–9.6) | 12.0 (11.1–13.1) | 20.1 (16.3–25.3) | |
| HR (95% CI) | 1 (Ref.) | 1.07 (0.71–1.59) | 0.93 (0.61–1.42) | 0.50 (0.30–0.82) | 0.78 (0.50–1.22) | 0.08 |
| Tea and coffee, intake3 | 299 (233–247) | 402 (388–418) | 528 (484–593) | 744 (670–839) | 1134 (982–1421) | |
| HR (95% CI) | 1 (Ref.) | 0.83 (0.55–1.25) | 0.79 (0.52–1.20) | 0.91 (0.60–1.37) | 0.64 (0.39–1.06) | 0.16 |
| Less healthy plant food groups | ||||||
| Fruit juices, intake3 | 15 (14–18) | 28 (24–33) | 60 (46–75) | 149 (121–193) | 414 (322–630) | |
| HR (95% CI) | 1 (Ref.) | 0.61 (0.39–0.94) | 0.64 (0.42–0.98) | 0.66 (0.43–1.02) | 0.74 (0.49–1.13) | 0.73 |
| Sugar-sweetened beverages, intake3 | 5.5 (5.5–5.5) | 14.3 (13.6–14.3) | 49.4 (49.4–49.4) | 157.7 (157.7–157.7) | 806.5 (806.5–1734.5) | |
| HR (95% CI) | 1 (Ref.) | 1.28 (0.89–1.85) | 1.01 (0.60–1.72) | 1.70 (0.97–2.94) | 2.34 (1.32–4.16) | 0.004 |
| Refined grains, intake2 | 0.10 (0.08–0.15) | 0.23 (0.21–0.29) | 0.38 (0.35–0.43) | 0.58 (0.53–0.64) | 0.95 (0.81–1.29) | |
| HR (95% CI) | 1 (Ref.) | 1.10 (0.71–1.71) | 1.18 (0.76–1.83) | 1.00 (0.64–1.55) | 1.24 (0.78–1.97) | 0.50 |
| Potatoes, intake3 | 36 (23–45) | 69 (61–93) | 102 (85–105) | 138 (116–148) | 168 (142–183) | |
| HR (95% CI) | 1 (Ref.) | 1.08 (0.70–1.68) | 0.83 (0.53–1.32) | 0.79 (0.49–1.26) | 0.99 (0.63–1.55) | 0.61 |
| Sweets and desserts, intake3 | 44 (34–50) | 71 (64–77) | 93 (87–103) | 122 (111–137) | 182 (157–215) | |
| HR (95% CI) | 1 (Ref.) | 0.77 (0.49–1.19) | 0.87 (0.56–1.34) | 0.73 (0.46–1.15) | 0.64 (0.38–1.06) | 0.07 |
| Animal food groups | ||||||
| Animal fat, intake3 | 1.5 (1.1–2.1) | 4.3 (3.5–5.4) | 9.8 (8.6–11.9) | 16.2 (14.9–19.9) | 24.8 (19.2–37.2) | |
| HR (95% CI) | 1 (Ref.) | 1.39 (0.85–2.27) | 1.45 (0.92–2.27) | 1.69 (1.07–2.65) | 1.33 (0.82–2.14) | 0.21 |
| Dairy, intake3 | 88 (71–106) | 159 (142–173) | 223 (209–237) | 309 (282–342) | 465 (418–538) | |
| HR (95% CI) | 1 (Ref.) | 1.09 (0.69–1.71) | 1.10 (0.69–1.75) | 1.42 (0.91–2.20) | 1.30 (0.82–2.05) | 0.18 |
| Egg, intake3 | 4.1 (2.9–7.9) | 9.3 (8.8–10.0) | 18.5 (17.8–19.8) | 20.3 (19.4–20.8) | 23.8 (22.0–43.3) | |
| HR (95% CI) | 1 (Ref.) | 1.43 (0.92–2.20) | 1.25 (0.81–1.94) | 1.11 (0.70–1.77) | 1.24 (0.76–2.01) | 0.61 |
| Fish and seafood, intake3 | 5.3 (3.6–7.3) | 19.6 (14.0–19.6) | 32.2 (22.2–32.2) | 44.4 (35.2–44.7) | 57.1 (56.0–81.9) | |
| HR (95% CI) | 1 (Ref.) | 1.04 (0.68–1.59) | 1.06 (0.70–1.60) | 0.99 (0.63–1.55) | 0.79 (0.50–1.25) | 0.33 |
| Meat, intake3 | 41 (34–62) | 84 (56–98) | 116 (75–132) | 151 (98–173) | 205 (139–253) | |
| HR (95% CI) | 1 (Ref.) | 1.15 (0.75–1.76) | 0.99 (0.63–1.54) | 1.10 (0.69–1.74) | 1.05 (0.64–1.73) | 0.93 |
| Miscellaneous animal-based foods, intake2 | 0.03 (0.02–0.08) | 0.22 (0.18–0.25) | 0.32 (0.30–0.35) | 0.42 (0.38–0.43) | 0.56 (0.50–0.70) | |
| HR (95% CI) | 1 (Ref.) | 0.72 (0.46–1.12) | 0.95 (0.63–1.43) | 0.89 (0.58–1.36) | 0.68 (0.44–1.05) | 0.20 |
Associations were estimated with Cox proportional hazards regression, adjusted for sex, age at diet assessment, BMI, physical activity, survival time from CRC diagnosis until diet assessment, tumor location, metastases, other cancer, type of therapy, smoking status, alcohol intake, total energy intake, time × age, time × BMI, and time × metastases.
Calculated by modeling the median value of food group quintiles as a continuous variable.
Intake in portions per day, values are medians (IQRs).
Intake in grams per day, values are medians (IQRs).
Abbreviations: CRC, colorectal cancer; Q, quintiles.
In the sensitivity analysis of the Cox model excluding BMI as a covariate, effect estimates were only very marginally different (Supplementary Table 1) from the main results. Likewise, in the 2 other sensitivity analyses, we observed slightly weaker but similar associations of the plant-based diet indices with mortality, excluding individuals who died within 12 months of the diet assessment (n = 1,385,185 deaths; Supplementary Table 2) or excluding individuals who had a diagnosis of metastases (n = 1,166,149 deaths; Supplementary Table 3).
Discussion
We observed the following key results: first, a higher overall PDI was associated with statistically significantly lower all-cause mortality over 7 years (median) of survival follow-up. The hPDI also showed a strong tendency towards an inverse association with mortality, but was not statistically significant. By contrast, higher adherence to the uPDI revealed a (nonsignificant) association with higher mortality and displayed a statistically significant nonlinear association with survival in restricted cubic spline regression. Second, the combination of uPDI and physical activity had a statistically significant interaction in the association with mortality. A subgroup analysis revealed a strong tendency towards a positive association between the uPDI and all-cause mortality in individuals that were less physically active (<95 MET-hours/week), whereas no association between the uPDI and mortality was observable in individuals with higher physical activity (≥95 MET-hours/week). Third, some of the individual food groups (e.g., whole grains, nuts, legumes, sugar-sweetened beverages, animal fat) revealed associations with mortality when examined individually, although a clear linear trend across quintiles was not evident in most cases.
The majority of studies investigating plant-based or vegetarian/vegan diets in relation to cancer and/or mortality considered the cancer incidence as an endpoint or analyzed disease-specific and overall mortality in general population samples. A meta-analysis including 10 prospective population-based cohort studies reported a significantly reduced incidence of total cancer in those who did not eat meat (vegans and vegetarians) but no association with all-cause or cancer mortality (29). In a community-based cohort of 12168 middle-aged adults, stronger adherence to a healthy plant-based diet was associated with lower risks of cardiovascular and all-cause mortality, while the less healthy plant-based diet revealed no association with mortality (30). In 2 large US cohorts, 12-year changes in PDI, hPDI, and uPDI were associated with total and cardiovascular mortality. However, only the PDI was associated with cancer mortality (18). In our study, we examined survival in cancer survivors but considered all-cause mortality as the endpoint.
While many analyses were conducted in general population cohorts, there is initial evidence from cancer survivors. In breast cancer survivors (mean survival time, 80 months), higher consumption of fiber and vegetables was significantly associated with better overall survival (31). However, dietary information was assessed for the year before diagnosis, not after diagnosis like in our study. A Newfoundland study of 529 CRC patients did not observe any significant association between a posteriori-derived prudent vegetable (similar to our hPDI) or high sugar patterns (similar to our uPDI) and survival. Only a high processed meat pattern was detrimentally associated with survival after CRC diagnosis (32). To our knowledge, no prior study has examined the 3 indices of PDI, hPDI, and uPDI in relation to mortality in CRC survivors.
The potential beneficial effects of plant diets on cancer survival are multifaceted. Plant-based diets are usually rich in dietary fiber, plant proteins, micronutrients, and phytochemicals, which have anti-inflammatory and anti-oxidative properties (33–36) and may prevent CRC progression and recurrence, lower blood glucose, blood lipid concentrations, and body weight, and improve bowel function (7, 37–42). Polyphenols, for example, exert chemopreventive effects and may induce tumor cell apoptosis (43). Plant-based diets are additionally healthy by limiting detrimental animal products and constituents like saturated fats, dietary cholesterol, antibiotics from meat, heme iron, and chemical contaminants from high-temperature cooking of animal products (44, 45). Particularly for the incidence of CRC, processed meat is among the most evident nutritional risk factors (45).
The reasons for the less distinct associations of the hPDI (inverse association) and of the uPDI (positive association) with mortality are not entirely clear. It is conceivable, though, that not all foods included in the plant food groups labeled as “less healthy” (counting inversely in the hPDI and positively in the uPDI) are as detrimental as assumed (e.g., potatoes, fruit juices). This concept is supported by our analysis relating individual food groups to mortality, where fruit juices, for example, provided some evidence for an inverse association with mortality. However, it is also possible that the FFQ used in our study does not assess food intake in enough detail to clearly differentiate between healthy and less healthy plant foods (e.g., refined and whole grains), and therefore does not enable a reliable and biologically plausible categorization.
We observed that the association between greater adherence to an unhealthful plant-based diet and a higher mortality risk was stronger in CRC survivors who were less physically active (<95 MET-hours per week) than in those more physically active. Thus, in this risk factor constellation, a healthy diet might be more influential in increasing health and survival.
Strengths of our study are the relatively large sample size, use of a standardized dietary assessment, long follow-up time, use of a validated vital status assessment, and application of predefined dietary indices that model plant-based diets on a continuous (rather than on a binary) scale. Limitations include that the majority of our data were self-reported, bearing the potential of information bias. However, a validation of self-reported clinical information against medical records in a subset of 181 patients revealed a concordance of 87%. The study sample is restricted to long-term cancer survivors who, at baseline, had survived a median of 6 years after CRC diagnosis, which might introduce survivor bias. The generalizability of our results to all CRC patients is therefore unknown. The relatively small number of deaths in our cohort is also an indication for long-term survival. As some observations only revealed tendencies but were not statistically significant, we assume that a larger study sample with more statistical power might have provided more statistically significant associations. Unfortunately, no information on tumor stage and comorbidities was available in our cohort, but data on metastases and other cancers were used. Furthermore, we only had information on all-cause mortality, but not on disease-specific mortality. Thus, future studies examining the effects of plant-based diets on cause-specific mortality are warranted.
In conclusion, our study results suggest that greater adherence to overall plant-based diets may be associated with lower all-cause mortality in CRC long-term survivors. While we found some evidence that plant-based diet patterns may be differentially associated with survival in long-term CRC survivors, further studies with well-characterized study samples are needed to strengthen the evidence and to clearly define the influence of different qualities of plant-based dietary patterns on survival. Lifestyle modifications, like transitioning to a more plant-based diet and reducing or eliminating animal-based foods, might be especially relevant in clinical practice because cancer survivors would be able to actively improve their health and may even be able to increase survival.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows – IR, JE, MK, WL: designed the research; JH, GB, UN, IR conducted the research; IR: analyzed the data or performed the statistical analysis and wrote the paper; IR, WL: had primary responsibility for the final content; and all authors: made significant intellectual contributions and read and approved the final manuscript. WL acknowledges funding from the German Research Foundation within the Clusters of Excellence “Precision Medicine in Chronic Inflammation” (EXC 2167). MK receives funding from the National Institute on Aging (grant # K01 AG 066817). All the other authors report no conflicts of interest.
Notes
The authors acknowledge the following funding: WL from the German Research Foundation within the Clusters of Excellence “Precision Medicine in Chronic Inflammation” (EXC 2167); MK from the National Institute on Aging (grant # K01 AG 066817).
Abbreviations used: CRC, colorectal cancer; hPDI, healthful plant-based diet index; MET, metabolic equivalent of task; PDI, plant-based diet index; uPDI, unhealthful plant-based diet index.
Contributor Information
Ilka Ratjen, Institute of Epidemiology, University Hospital Schleswig-Holstein, University of Kiel, Kiel, Germany.
Janna Enderle, Institute of Epidemiology, University Hospital Schleswig-Holstein, University of Kiel, Kiel, Germany.
Greta Burmeister, Department of General, Visceral, Vascular, and Transplantation Surgery, University Hospital Rostock, Rostock, Germany.
Manja Koch, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Ute Nöthlings, Nutritional Epidemiology, Department of Nutrition and Food Science, Rheinische Friedrich-Wilhelms-University Bonn, Bonn, Germany.
Jochen Hampe, Medical Department 1, University Hospital Dresden, Technical University Dresden, Dresden, Germany.
Wolfgang Lieb, Institute of Epidemiology, University Hospital Schleswig-Holstein, University of Kiel, Kiel, Germany.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
References
- 1. Sharma R. An examination of colorectal cancer burden by socioeconomic status: Evidence from GLOBOCAN 2018. EPMA J. 2020;11(1):95–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ann Transl Med. 2019;7(21):609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Cancer Institute . SEER stat fact sheets: Colon and rectum cancer. [Internet]. Available from: http://seer.cancer.gov/statfacts/html/colorect.html. [Google Scholar]
- 4. Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, Capocaccia R, Baili P, Rachet B, Gatta G, Hakulinen Tet al. . Cancer survival in five continents: A worldwide population-based study (CONCORD). Lancet Oncol. 2008;9(8):730–56. [DOI] [PubMed] [Google Scholar]
- 5. Young JM, Durcinoska I, DeLoyde K, Solomon MJ. Patterns of follow up and survivorship care for people with colorectal cancer in new South Wales, Australia: A population-based survey. BMC Cancer. 2018;18(1):339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson AS, Steele R, Coyle J. Lifestyle issues for colorectal cancer survivors–Perceived needs, beliefs and opportunities. Support Care Cancer. 2013;21(1):35–42. [DOI] [PubMed] [Google Scholar]
- 7. Song M, Wu K, Meyerhardt JA, Ogino S, Wang M, Fuchs CS, Giovannucci EL, Chan AT. Fiber intake and survival after colorectal cancer diagnosis. JAMA Oncol. 2018;4(1):71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang B, McCullough ML, Gapstur SM, Jacobs EJ, Bostick RM, Fedirko V, Flanders WD, Campbell PT. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: The Cancer Prevention Study–II Nutrition Cohort. J Clin Oncol. 2014;32(22):2335–43. [DOI] [PubMed] [Google Scholar]
- 9. Fung TT, Kashambwa R, Sato K, Chiuve SE, Fuchs CS, Wu K, Giovannucci E, Ogino S, Hu FB, Meyerhardt JA. Post diagnosis diet quality and colorectal cancer survival in women. PLOS One. 2014;9(12):e115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ratjen I, Schafmayer C, di Giuseppe R, Waniek S, Plachta-Danielzik S, Koch M, Nothlings U, Hampe J, Schlesinger S, Lieb W. Postdiagnostic Mediterranean and healthy Nordic dietary patterns are inversely associated with all-cause mortality in long-term colorectal cancer survivors. J Nutr. 2017;147(4):636–44. [DOI] [PubMed] [Google Scholar]
- 11. Magkos F, Tetens I, Bugel SG, Felby C, Schacht SR, Hill JO, Ravussin E, Astrup A. A perspective on the transition to plant-based diets: A diet change may attenuate climate change, but can it also attenuate obesity and chronic disease risk?. Adv Nutr. 2020;11(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Springmann M, Wiebe K, Mason-D'Croz D, Sulser TB, Rayner M, Scarborough P. Health and nutritional aspects of sustainable diet strategies and their association with environmental impacts: A global modelling analysis with country-level detail. Lancet Planet Health. 2018;2(10):e451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: Results from three prospective cohort studies. PLOS Med. 2016;13(6):e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, Rexrode KM, Rimm EB, Hu FB. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in US adults. J Am Coll Cardiol. 2017;70(4):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martínez-González MA, Sánchez-Tainta A, Corella D, Salas-Salvadó J, Ros E, Arós F, Gómez-Gracia E, Fiol M, Lamuela-Raventós RM, Schröder Het al. . A provegetarian food pattern and reduction in total mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr. 2014;100(Suppl 1):320S–8S. [DOI] [PubMed] [Google Scholar]
- 16. Satija A, Malik V, Rimm EB, Sacks F, Willett W, Hu FB. Changes in intake of plant-based diets and weight change: Results from 3 prospective cohort studies. Am J Clin Nutr. 2019;110(3):574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baden MY, Satija A, Hu FB, Huang T. Change in plant-based diet quality is associated with changes in plasma adiposity-associated biomarker concentrations in women. J Nutr. 2019;149(4):676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baden MY, Liu G, Satija A, Li Y, Sun Q, Fung TT, Rimm EB, Willett WC, Hu FB, Bhupathiraju SN. Changes in plant-based diet quality and total and cause-specific mortality. Circulation. 2019;140(12):979–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ratjen I, Morze J, Enderle J, Both M, Borggrefe J, Muller HP, Kassubek J, Koch M, Lieb W. Adherence to a plant-based diet in relation to adipose tissue volumes and liver fat content. Am J Clin Nutr. 2020;112(2):354–63. [DOI] [PubMed] [Google Scholar]
- 20. Schafmayer C, Buch S, Volzke H, von Schonfels W, Egberts JH, Schniewind B, Brosch M, Ruether A, Franke A, Mathiak Met al. . Investigation of the colorectal cancer susceptibility region on chromosome 8q24.21 in a large German case-control sample. Int J Cancer. 2009;124(1):75–80. [DOI] [PubMed] [Google Scholar]
- 21. Castro FA, Forsti A, Buch S, Kalthoff H, Krauss C, Bauer M, Egberts J, Schniewind B, Broering DC, Schreiber Set al. . TLR-3 polymorphism is an independent prognostic marker for stage II colorectal cancer. Eur J Cancer. 2011;47(8):1203–10. [DOI] [PubMed] [Google Scholar]
- 22. Schlesinger S, Walter J, Hampe J, von Schonfels W, Hinz S, Kuchler T, Jacobs G, Schafmayer C, Nöthlings U. Lifestyle factors and health-related quality of life in colorectal cancer survivors. Cancer Causes Control. 2014;25(1):99–110. [DOI] [PubMed] [Google Scholar]
- 23. Nöthlings U, Hoffmann K, Bergmann MM, Boeing H. Fitting portion sizes in a self-administered food frequency questionnaire. J Nutr. 2007;137(12):2781–6. [DOI] [PubMed] [Google Scholar]
- 24. Dehne LI, Klemm C, Henseler G, Hermann-Kunz E. The German food code and nutrient database (BLS II.2). Eur J Epidemiol. 1999;15(4):355–9. [DOI] [PubMed] [Google Scholar]
- 25. Haftenberger M, Schuit AJ, Tormo MJ, Boeing H, Wareham N, Bueno-de-Mesquita HB, Kumle M, Hjartaker A, Chirlaque MD, Ardanaz Eet al. . Physical activity of subjects aged 50-64 years involved in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2002;5(6B):1163–76. [DOI] [PubMed] [Google Scholar]
- 26. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR Jr., Schmitz KH, Emplaincourt POet al. . Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(Suppl 9):S498–504. [DOI] [PubMed] [Google Scholar]
- 27. Friedenreich C, Norat T, Steindorf K, Boutron-Ruault MC, Pischon T, Mazuir M, Clavel-Chapelon F, Linseisen J, Boeing H, Bergman Met al. . Physical activity and risk of colon and rectal cancers: The European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2398–407. [DOI] [PubMed] [Google Scholar]
- 28. Harrell FE. Regression modeling strategies. With applications to linear models, logistic regression, and survival analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 29. Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57(17):3640–9. [DOI] [PubMed] [Google Scholar]
- 30. Kim H, Caulfield LE, Garcia-Larsen V, Steffen LM, Coresh J, Rebholz CM. Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J Am Heart Assoc. 2019;8(16):e012865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McEligot AJ, Largent J, Ziogas A, Peel D, Anton-Culver H. Dietary fat, fiber, vegetable, and micronutrients are associated with overall survival in postmenopausal women diagnosed with breast cancer. Nutr Cancer. 2006;55(2):132–40. [DOI] [PubMed] [Google Scholar]
- 32. Zhu Y, Wu H, Wang PP, Savas S, Woodrow J, Wish T, Jin R, Green R, Woods M, Roebothan Bet al. . Dietary patterns and colorectal cancer recurrence and survival: A cohort study. BMJ Open. 2013;3(2):e002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bellik Y, Boukraa L, Alzahrani HA, Bakhotmah BA, Abdellah F, Hammoudi SM, Iguer-Ouada M. Molecular mechanism underlying anti-inflammatory and anti-allergic activities of phytochemicals: An update. Molecules. 2012;18(1):322–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bolori P, Setaysh L, Rasaei N, Jarrahi F, Yekaninejad MS, Mirzaei K. Adherence to a healthy plant diet may reduce inflammatory factors in obese and overweight women–A cross-sectional study. Diabetes Metab Syndr. 2019;13(4):2795–802. [DOI] [PubMed] [Google Scholar]
- 35. Cassidy A, Rogers G, Peterson JJ, Dwyer JT, Lin H, Jacques PF. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am J Clin Nutr. 2015;102(1):172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Medawar E, Huhn S, Villringer A, Veronica Witte A. The effects of plant-based diets on the body and the brain: A systematic review. Transl Psychiatry. 2019;9(1):226–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82(1):163–73. [DOI] [PubMed] [Google Scholar]
- 38. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steffen LM, Jacobs DR Jr., Murtaugh MA, Moran A, Steinberger J, Hong CP, Sinaiko AR. Whole grain intake is associated with lower body mass and greater insulin sensitivity among adolescents. Am J Epidemiol. 2003;158(3):243–50. [DOI] [PubMed] [Google Scholar]
- 40. Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kahleova H, Levin S, Barnard N. Cardio-metabolic benefits of plant-based diets. Nutrients. 2017;9(8):848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chiu YF, Hsu CC, Chiu TH, Lee CY, Liu TT, Tsao CK, Chuang SC, Hsiung CA. Cross-sectional and longitudinal comparisons of metabolic profiles between vegetarian and non-vegetarian subjects: A matched cohort study. Br J Nutr. 2015;114(8):1313–20. [DOI] [PubMed] [Google Scholar]
- 43. Alam MN, Almoyad M, Huq F. Polyphenols in colorectal cancer: Current state of knowledge including clinical trials and molecular mechanism of action. Biomed Res Int. 2018;2018:4154185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hever J. Plant-based diets: A physician's guide. Perm J. 2016;20(3):15–082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johnson IT. The cancer risk related to meat and meat products. Br Med Bull. 2017;121(1):73–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.