ABSTRACT
Background
Epidemiologic studies examining the relations between dairy product and calcium intakes and breast cancer have been inconclusive, especially for tumor subtypes.
Objective
To evaluate the associations between intakes of specific dairy products and calcium and risk of breast cancer overall and for subtypes defined by estrogen receptor (ER) status.
Method
We pooled the individual-level data of over 1 million women who were followed for a maximum of 8–20 years across studies. Associations were evaluated for dairy product and calcium intakes and risk of incident invasive breast cancer overall (n = 37,861 cases) and by subtypes defined by ER status. Study-specific multivariable hazard ratios (HRs) were estimated and then combined using random-effects models.
Results
Overall, no clear association was observed between the consumption of specific dairy foods, dietary (from foods only) calcium, and total (from foods and supplements) calcium, and risk of overall breast cancer. Although each dairy product showed a null or very weak inverse association with risk of overall breast cancer (P, test for trend >0.05 for all), differences by ER status were suggested for yogurt and cottage/ricotta cheese with associations observed for ER-negative tumors only (pooled HR = 0.90, 95% CI: 0.83, 0.98 comparing ≥60 g/d with <1 g/d of yogurt and 0.85, 95% CI: 0.76, 0.95 comparing ≥25 g/d with <1 g/d of cottage/ricotta cheese). Dietary calcium intake was only weakly associated with breast cancer risk (pooled HR = 0.98, 95% CI: 0.97, 0.99 per 350 mg/d).
Conclusion
Our study shows that adult dairy or calcium consumption is unlikely to associate with a higher risk of breast cancer and that higher yogurt and cottage/ricotta cheese intakes were inversely associated with the risk of ER-negative breast cancer, a less hormonally dependent subtype with poor prognosis. Future studies on fermented dairy products, earlier life exposures, ER-negative breast cancer, and different racial/ethnic populations may further elucidate the relation.
Keywords: breast cancer, calcium, cheese, dairy products, diet, milk, pooled analysis, yogurt
Introduction
Worldwide, breast cancer is the most commonly diagnosed malignancy and the leading cause of cancer death in women, accounting for 2.1 million cases each year and 15% of all cancer deaths (1). Breast cancer is a heterogeneous disease with subtypes based on expression of hormone receptors, indicating different etiologies, clinical characteristics, and survival rates. Hormone-receptor-negative tumors have poorer prognosis and fewer treatment options (2–4). Since these subtypes are less hormonally dependent, any association between dietary exposures and breast cancer risk would be more likely to show up. One challenge in studying hormone-receptor-negative tumors is that they account for <20% of all breast cancers (5), so many studies have inadequate statistical power to analyze them separately.
Dairy products have been hypothesized to influence breast carcinogenesis in conflicting ways. They are the main dietary sources of conjugated linoleic acid, calcium, and vitamin D (in fortified fluid milk and yogurt), all of which have been suggested to have anticarcinogenic properties by regulating cell proliferation, differentiation, and apoptosis (6–9). Dairy products also contain branched chain amino acids and potentially increase circulating insulin-like growth factor 1 (IGF-1) concentrations (10), which may promote cell growth, elevate mitotic activity, and increase DNA replication errors (11, 12). Bovine sex hormones and hormone drugs used in dairy management practices (e.g. trenbolone acetate, zeranol, melengestrol acetate) might increase breast cancer risk as well (11, 13). A meta-analysis of 22 prospective cohort studies and 5 case-control studies reported that high total dairy consumption was associated with a modestly lower risk of breast cancer (risk ratio = 0.90, 95% CI: 0.83, 0.98, comparing >600 g/d with <200 g/d) (14). The number of studies reporting on specific dairy products was limited, and results were not reported separately for breast cancer subtypes in that meta-analysis.
To evaluate the associations between intakes of specific dairy products and calcium and risk of female breast cancer overall and for subtypes defined by estrogen receptor (ER) status, we conducted a pooled analysis within 21 cohorts in the Pooling Project of Prospective Studies of Diet and Cancer (Diet and Cancer Pooling Project, DCPP).
Methods
Study population
The DCPP is an international consortium of prospective cohort studies (15). In this study, we analyzed 21 (16–36) cohorts (Table 1, Supplemental Table 1) that met the following inclusion criteria: 1) ≥1 publication on any diet and cancer association; 2) assessed dairy product and calcium intake with a comprehensive long-term (e.g. the past 6 mo or 1 y) dietary assessment tool; 3) validated the dietary assessment tool or a closely related instrument; and 4) included ≥25 incident ER-negative breast cancer cases.
TABLE 1.
Characteristics by cohort study1 in the breast cancer analyses in the Pooling Project of Prospective Studies of Diet and Cancer
| Baseline cohort size3 | Year(s) of recruitment | Median follow-up (y) | No. of breast cancer cases | ||||
|---|---|---|---|---|---|---|---|
| Cohort2 | Country | Total | ER-positive | ER-negative | |||
| BCDDP | USA | 42,061 | 1987–1989 | 8.7 | 1305 | 793 | 166 |
| BWHS | USA | 52,576 | 1995 | 13.0 | 670 | 416 | 254 |
| CARET | USA | 6000 | 1985–1994 | 12.3 | 367 | 193 | 31 |
| CLUE II | USA | 8279 | 1989 | 16.5 | 288 | 198 | 50 |
| CNBSS | Canada | 45,185 | 1980–1985 | 16.5 | 1240 | 367 | 125 |
| CPS II | USA | 74,137 | 1992–1993 | 9.8 | 2999 | 1835 | 323 |
| CTS | USA | 100,067 | 1995–1999 | 8.1 | 2696 | 1930 | 343 |
| IWHS | USA | 34,584 | 1986 | 18.9 | 1849 | 1329 | 238 |
| JPHC I | Japan | 21,609 | 1990–1992 | 14.5 | 289 | 111 | 69 |
| MCCS | Australia | 22,456 | 1990–1994 | 13.4 | 799 | 493 | 171 |
| MEC | USA | 92,435 | 1993–1997 | 10.7 | 3308 | 2169 | 543 |
| NHS a | USA | 88,618 | 1980–1982 | 6.5 | 1122 | 528 | 255 |
| NHS b4 | USA | 68,394 | 1986 | 19.9 | 4467 | 3075 | 757 |
| NHS II | USA | 93,778 | 1991–1993 | 12.0 | 1331 | 846 | 303 |
| NIH-AARP | USA | 200,049 | 1995–1997 | 7.5 | 5972 | 2322 | 464 |
| NLCS | Netherlands | 62,573 | 1986 | 6.6 | 2013 | 700 | 183 |
| NYUWHS | USA | 13,257 | 1985 | 17.1 | 919 | 392 | 121 |
| ORDET | Italy | 9044 | 1987–1992 | 12.5 | 283 | 206 | 67 |
| PLCO | USA | 28,292 | 1993–2002 | 9.1 | 1090 | 858 | 137 |
| SMC | Sweden | 60,950 | 1987–1990 | 16.9 | 2605 | 1605 | 384 |
| WHS | USA | 38,385 | 1992–1995 | 10.1 | 1177 | 937 | 187 |
| WLHS | Sweden | 47,514 | 1991–1992 | 15.3 | 1072 | 737 | 196 |
| Total | 1,141,849 | 37,861 | 22,040 | 5367 | |||
For each study, information on the study population, dietary assessment method, and the validation study of the dietary assessment method, as well as the reference(s), are provided in Supplemental Table 1.
BCDDP, Breast Cancer Detection Demonstration Project Follow-up Study; BWHS, The Black Women's Health Study; CARET, β-Carotene and Retinol Efficacy Trial; CLUE II, CLUE II: Campaign Against Cancer and Heart Disease; CNBSS, Canadian National Breast Screening Study; CPS II, Cancer Prevention Study II Nutrition Cohort; CTS, California Teachers Study; ER, estrogen receptor; IWHS, Iowa Women's Health Study; JPHC I, Japan Public Health Center-Based Study Cohort I; MCCS, Melbourne Collaborative Cohort Study; MEC, Multiethnic Cohort; NHS a, Nurses’ Health Study (part a); NHS b, Nurses’ Health Study (part b); NHS II, Nurses’ Health Study II; NIH-AARP, NIH-AARP Diet and Health Study; NLCS, Netherlands Cohort Study; NYUWHS, New York University Women's Health Study; ORDET, Hormones and Diet in the Etiology of Breast Cancer Study; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; SMC, Swedish Mammography Cohort; WHS, Women's Health Study; WLHS, Women's Lifestyle and Health Study.
Cohort size reflects the size after application of study-specific exclusion criteria and further exclusion of participants with energy intakes beyond 3 SDs of their loge-transformed study-specific mean energy intake and history of cancer diagnosis at baseline (except for nonmelanoma skin cancer); the Netherlands Cohort Study was analyzed as a case-cohort study, and the above exclusions were not applied to its baseline cohort size.
The Nurses’ Health Study (NHS) was analyzed as 2 separate cohorts [1980–86, NHS (a); 1986–2006, NHS (b)] to utilize the comprehensive dietary assessment administered in 1986. As a result, the participants in the Nurses’ Health Study (b) were not added into the total baseline cohort size because the participants in this study were included in the Nurses’ Health Study (a).
Assessment of dietary and nondietary factors
Dietary intake was assessed at baseline by a validated study-specific FFQ, generally covering the past year. Total milk, hard cheese, cottage/ricotta cheese, yogurt, and ice cream were examined (see Supplemental Table 2 for the items in each group and the daily intake in each participating cohort). All studies estimated dietary calcium intake (from foods) and 12 studies estimated total calcium intake that also included calcium from multivitamins and other supplements. Dietary and total calcium intakes were energy adjusted using the residual method (37). The Pearson correlation coefficients comparing intakes from the FFQ used in these studies or closely related FFQs with either multiple 24-h recalls or dietary records generally ranged from 0.5 to 0.9 for intake of dairy products (25, 38–44) and 0.5–0.8 for dietary calcium (41, 42, 45–48) (Supplemental Table 1).
Each study collected age, height, and body weight at baseline. Most studies also assessed family history of breast cancer, educational attainment, physical activity, smoking habits, and several reproductive factors (covariate availability for each cohort is summarized in Supplemental Methods).
Case ascertainment
Breast cancer was defined by International Classification of Diseases 9 (ICD-9) code 174.0 or ICD-10 code C50. Incident invasive breast cancer cases were identified by follow-up questionnaires and subsequent review of medical records, through linkage to cancer registries, or by both methods. Some cases were also identified using linkage to mortality registries. Follow-up generally exceeded 90% for the studies (15). Estrogen and progesterone receptor status (ER status obtained for 85% and PR status obtained for 80% of all cases) was identified through cancer registries, pathology reports, medical records, or laboratory determinations. Cases with borderline hormone receptor status (<1% among those with ER data) were considered as positive for that hormone receptor.
Ethics
Each included study and the consortium were approved by their respective Institutional Review Board.
Statistics
The primary aims for the study were to assess the associations of dairy product and calcium intake and risk of female breast cancer overall and of breast cancer subtypes defined by ER status; as secondary analyses we examined breast cancer subtypes defined jointly by ER and PR status. We analyzed the primary participant-level data in each cohort. The Netherlands Cohort Study was analyzed as a case-cohort study (49), as required by its study design. The Nurses’ Health Study was separated into 2 cohorts (1980–1986 Nurses’ Health Study a; 1986–2006 Nurses’ Health Study b) because of the more detailed dietary assessment after 1986 compared with 1980.
We excluded women who reported total energy intake outside of 3 SDs from the mean loge-transformed energy intake in that study and who had been diagnosed with any cancer other than nonmelanoma skin cancer prior to baseline. The 10 studies that did not measure supplemental calcium intake were excluded from analyses of total calcium intake.
The associations for dairy products, dietary calcium, and total calcium intake and risk of breast cancer overall and for subtypes defined by ER status and by ER/PR status jointly were evaluated for each study using Cox proportional hazards regression. Dairy product and calcium intakes were modeled using categories defined by common absolute intake cut points. Calcium intake was modeled using study-specific quintiles as well. Most of the dairy products evaluated were comprised of a limited number of items and had relatively discrete intake distributions, thus we did not model them using quantiles. For each participant, we calculated person-years of follow-up from the age at baseline questionnaire return to the age of diagnosis of incident breast cancer, death, loss to follow-up, or end of follow-up, whichever occurred first. We used age at baseline and year of baseline questionnaire return as stratification factors to account for age, calendar time, and time since study entry. In multivariable analyses, we adjusted for established and suspected breast cancer risk factors (see Table 2 for specific variables and categorization) directly in the model for studies with >200 cases of the outcome of interest (i.e. overall breast cancer, ER-positive tumors, and ER-negative tumors); otherwise we included propensity scores (50, 51). We created missing indicator variables for confounders with missing values (the proportion of missing data was generally <10% for all covariates). We evaluated the main exposures for divergence from the proportional hazards assumption by examining figures of Schoenfeld residuals (52) and did not find evidence of significant violation.
TABLE 2.
Pooled multivariable1 adjusted HRs and 95% CIs for intake of dairy products and risk of breast cancer overall and for subtypes defined by estrogen receptor (ER)2 status
| Categories of intake (g/d) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pooled HR (95% CI) | ||||||||
| Dairy group3 | Reference | Category 1 | Category 2 | Category 3 | P trend 4 | P het 5 | I 2 6 | P com 7 |
| Total milk8 | ||||||||
| No. cases | 4774 | 12,235 | 9150 | 10,740 | ||||
| (ER+/–) | (2835/740) | (7039/1784) | (5366/1234) | (6204/1412) | ||||
| Total | 1.00 (Ref) | 0.97 (0.94, 1.01) | 0.99 (0.95, 1.03) | 0.95 (0.91, 0.98) | 0.02 | 0.72 | <1% | |
| ER+ | 1.00 (Ref) | 0.97 (0.92, 1.02) | 1.00 (0.94, 1.06) | 0.95 (0.90, 1.00) | 0.13 | 0.40 | 5% | 0.91 |
| ER– | 1.00 (Ref) | 1.01 (0.92, 1.11) | 0.99 (0.89, 1.09) | 0.95 (0.85, 1.05) | 0.25 | 0.37 | 7% | |
| Hard cheese9 | ||||||||
| No. cases | 7369 | 24,602 | 3934 | 1675 | ||||
| (ER+/–) | (3792/946) | (14,833/3577) | (2252/560) | (974/241) | ||||
| Total | 1.00 (Ref) | 1.02 (0.98, 1.06) | 0.99 (0.93, 1.06) | 1.01 (0.93, 1.10) | 0.76 | 0.29 | 13% | |
| ER+ | 1.00 (Ref) | 1.05 (1.00, 1.10) | 1.00 (0.92, 1.10) | 1.03 (0.93, 1.15) | 0.82 | 0.36 | 8% | 0.84 |
| ER– | 1.00 (Ref) | 0.96 (0.88, 1.04) | 0.94 (0.81, 1.09) | 1.00 (0.78, 1.29) | 0.56 | 0.18 | 23% | |
| Cottage/ricotta cheese10 | ||||||||
| No. cases | 13,217 | 10,373 | 3277 | 3596 | ||||
| (ER+/–) | (7271/1883) | (6202/1377) | (1945/448) | (2073/474) | ||||
| Total | 1.00 (Ref) | 0.99 (0.96, 1.02) | 0.96 (0.92, 1.00) | 0.97 (0.93, 1.01) | 0.19 | 0.53 | <1% | |
| ER+ | 1.00 (Ref) | 1.00 (0.96, 1.04) | 0.96 (0.90, 1.03) | 0.96 (0.90, 1.02) | 0.10 | 0.35 | 9% | 0.07 |
| ER– | 1.00 (Ref) | 0.91 (0.85, 0.99) | 0.93 (0.79, 1.10) | 0.85 (0.76, 0.95) | 0.07 | 0.47 | <1% | |
| Yogurt11 | ||||||||
| No. cases | 14,616 | 10,146 | 3675 | 6997 | ||||
| (ER+/–) | (8534/2189) | (5846/1344) | (2049/513) | (4163/961) | ||||
| Total | 1.00 (Ref) | 0.98 (0.96, 1.01) | 0.97 (0.93, 1.01) | 0.96 (0.92, 0.99) | 0.01 | 0.25 | 17% | |
| ER+ | 1.00 (Ref) | 0.99 (0.96, 1.03) | 0.96 (0.91, 1.02) | 0.98 (0.94, 1.03) | 0.20 | 0.94 | <1% | 0.07 |
| ER– | 1.00 (Ref) | 0.92 (0.86, 0.99) | 0.93 (0.84, 1.03) | 0.90 (0.83, 0.98) | 0.02 | 0.62 | <1% | |
| Ice cream12 | ||||||||
| No. cases | 11,359 | 17,381 | 3749 | 2406 | ||||
| (ER+/–) | (6757/1494) | (10,294/2568) | (2264/582) | (1449/345) | ||||
| Total | 1.00 (Ref) | 1.01 (0.98, 1.04) | 1.03 (0.99, 1.07) | 1.00 (0.95, 1.05) | 0.96 | 0.71 | <1% | |
| ER+ | 1.00 (Ref) | 1.00 (0.96, 1.04) | 1.02 (0.97, 1.08) | 0.99 (0.92, 1.06) | 0.53 | 0.32 | 12% | 0.53 |
| ER– | 1.00 (Ref) | 1.03 (0.96, 1.11) | 1.12 (0.99, 1.26) | 1.04 (0.90, 1.21) | 0.42 | 0.25 | 17% | |
Multivariable model includes race/ethnicity (white, African American, Hispanic, Asian), education (<high school, high school, >high school), BMI (<23, 23 to <25, 25 to <30, ≥30 kg/m2), height (<1.60, 1.60 to <1.65, 1.65 to <1.70, 1.70 to <1.75, ≥1.75 m except Japan Public Health Center-Based Study Cohort I: <1.50, 1.50 to <1.55, 1.55 to <1.60, 1.60 to <1.65, ≥1.65 m), alcohol consumption (0, >0 to <5, 5 to <15, 15 to <30, ≥30 g/d), energy intake (kcal/d, continuous), smoking status (never, past, current), physical activity (low, medium, high), age at menarche (<11, 11/12, 13/14, ≥15 y except Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: <10, 10/11, 12/13, ≥14 y), the combination of menopausal status and hormone replacement therapy use [premenopausal women and among postmenopausal women separate categories for never, past, and current users of hormone replacement therapy, except in the California Teachers Study, Canadian National Breast Screening Study, Multiethnic Cohort, New York University Women's Health Study (NYUWHS), Hormones and Diet in the Etiology of Breast Cancer Study, Swedish Mammography Cohort, and Women's Lifestyle and Health Study where the combination was modeled as premenopausal women and among postmenopausal women separate categories for never and ever users of hormone replacement therapy], oral contraceptive use (ever, never), parity (0, 1 to 2, ≥3, except for NYUWHS 0, ≥1), age at first birth (≤25, >25 y), history of benign breast disease (yes, no), family history of breast cancer (yes, no). Age in years and year of questionnaire return were included as stratification variables.
ER: estrogen receptor.
Total milk group included skim milk, 1% milk, 2% milk, whole milk, buttermilk, evaporated milk; hard cheese contained hard cheese, high-fat cheese, low-fat cheese, cheddar cheese, feta cheese, and unspecified cheese; cottage/ricotta cheese contained cottage and ricotta cheese; yogurt included high-fat and low-fat yogurt but not frozen yogurt.
P trend: the P value, test for trend was calculated using the Wald test statistic.
P het: the P value, test for between-studies heterogeneity for the highest category was calculated using the Q statistic.
I 2 for the highest category.
Pcom: the P value, test for common effects for different subtypes defined by estrogen receptor status for the highest category was calculated using the Wald test statistic.
The intake of total milk for reference, category 1, category 2, category 3 is 0, 1–124.9, 125–249.9, ≥250 g/d, respectively.
The intake of hard cheese for reference, category 1, category 2, category 3 is 0, 1–24.9, 25–49.9, ≥50 g/d, respectively.
The intake of cottage/ricotta cheese for reference, category 1, category 2, category 3 is 0, 1–12.4, 12.5–24.9, ≥25 g/d, respectively.
The intake of yogurt for reference, category 1, category 2, category 3 is 0, 1–29.9, 30–59.9, ≥60 g/d, respectively.
The intake of ice cream for reference, category 1, category 2, category 3 is 0, 1–16.9, 17–33.9, ≥34 g/d, respectively.
We pooled the study-specific HRs using random-effects models (53). Between-studies heterogeneity was evaluated using the Q (53) and I2 statistics (54).
To test for a linear trend across categories of intake for each participant, we assigned the study-specific median value of their exposure category, modeled that variable as a continuous variable, and tested the coefficient using the Wald test. We compared nonparametric regression curves using restricted cubic splines with the linear model using the likelihood ratio test (55) to test for nonlinearity in the associations for dairy products, dietary calcium, and total calcium. As the P value test for nonlinearity exceeded 0.05 for all associations, we also conducted analyses in which we modeled dairy food and calcium intakes as continuous variables.
In prespecified stratification analyses, we investigated whether the associations of interest varied by menopausal status at diagnosis using a previously described algorithm (56) (premenopausal, postmenopausal), age at diagnosis (<64, ≥64 y), BMI (<25, ≥25 kg/m2), region (North America, other), and follow-up years (<5, ≥5 y) using a mixed-effects metaregression model (57). We used a contrast test to obtain the P value, test for common effects of breast cancer subtypes defined by ER status and ER/PR status (58) (details in Supplemental Methods).
For each study that evaluated calcium intake in their validation study, we corrected for the bias in the estimated HRs due to measurement error in calcium intake (16–19, 21, 23–25, 27–33), using a regression calibration method (59, 60).
For all tests of statistical hypotheses, 2-sided Wald 95% CIs were calculated, and 2-sided P < 0.05 were considered statistically significant. All analyses were conducted using SAS software versions 9.2–9.4 (SAS Institute).
Results
After applying the inclusion and exclusion criteria (Supplemental Figure 1), across the 21 prospective studies with maximum follow-up ranging from 8 to 20 y, 37,861 incident cases of invasive breast cancer (22,040 ER-positive and 5,367 ER-negative breast cancer cases) were diagnosed among 1,141,849 women (Table 1, Supplemental Table 1).
Dairy product and calcium consumption varied substantially across studies. Median dietary calcium intake ranged from 490 to 853 mg/d. Median total calcium intakes ranged from 675 to 1173 mg/d (Supplemental Table 2). Dietary calcium intake was highly correlated with total milk intake (median Pearson correlation coefficient across studies = 0.74) and reduced-fat milk intake (median Pearson correlation = 0.73); correlations for total calcium intake with these 2 food items were weaker. Very weak correlations were observed between calcium and other dairy products (Supplemental Table 3).
Only multivariable results are presented because results from age-adjusted models were similar. For all dairy products evaluated, null or very weak associations were observed for risk of breast cancer overall with pooled multivariable HRs comparing the highest with the lowest category ranging from 0.95 to 1.01 across the dairy products (Table 2, Supplemental Table 4). When we expanded the highest category of total milk to ≥500 g/d, the pooled HR did not decrease further (data not shown).
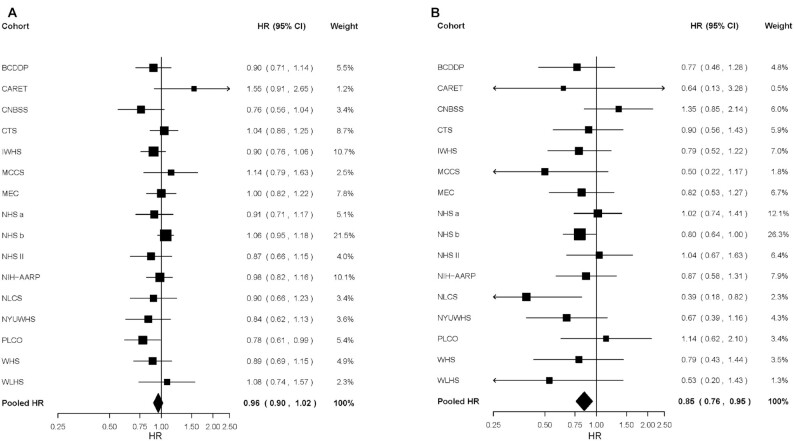
When we estimated associations for subtypes of breast cancer defined by ER status (Table 2), differences between ER-positive and ER-negative tumors were suggested only for yogurt and cottage/ricotta cheese consumption, with statistically significant inverse associations being observed for ER-negative tumors only. The pooled HRs comparing ≥60 g/d with <1 g/d yogurt intake were 0.90 (95% CI: 0.83, 0.98) for ER-negative tumors and 0.98 (95% CI: 0.94, 1.03) for ER-positive tumors (P, test for common effects by ER status = 0.07) (Table 2, Figure 1). Similarly, higher cottage/ricotta cheese consumption was associated with a 15% lower risk of ER-negative (pooled HR comparing ≥25 g/d to <1 g/d = 0.85, 95% CI: 0.76, 0.95) but not ER-positive breast cancer (pooled HR = 0.96, 95% CI: 0.90, 1.02; P, test for common effects by ER status = 0.07) (Table 2, Figure 2). When intakes were modeled as continuous variables, we did not observe significant associations for any dairy product with risk of breast cancer overall (Figure 3) or for subtypes defined by ER (Supplemental Table 5) or joint ER/PR status (Supplemental Table 6).
FIGURE 1.
Study-specific and pooled multivariable HRs comparing ≥60 versus 0 g/d of yogurt intake and risk of (A) estrogen-receptor-positive breast cancer and (B) estrogen-receptor-negative breast cancer. The squares and horizontal lines correspond to the study-specific HRs and 95% CIs for the comparison of ≥60 g/d to 0 g/d of yogurt intake. The BCDDP (Breast Cancer Detection Demonstration Project Follow-up Study), CLUE II (CLUE II: Campaign Against Cancer and Heart Disease), and JPHC I (Japan Public Health Center-Based Study Cohort I) were excluded from the figure because these studies did not measure yogurt intake. The size of the squares reflects the study-specific weight (inverse of the variance). The diamond represents the pooled multivariable HR and 95% CI. BWHS, The Black Women's Health Study; CARET, β-Carotene and Retinol Efficacy Trial; CNBSS, Canadian National Breast Screening Study; CPS II, Cancer Prevention Study II Nutrition Cohort; CTS, California Teachers Study; IWHS, Iowa Women's Health Study; MCCS, Melbourne Collaborative Cohort Study; MEC, Multiethnic Cohort; NHS a, Nurses’ Health Study (part a); NHS b, Nurses’ Health Study (part b); NHS II, Nurses’ Health Study II; NIH-AARP, NIH-AARP Diet and Health Study; NLCS, Netherlands Cohort Study; NYUWHS, New York University Women's Health Study; ORDET, Hormones and Diet in the Etiology of Breast Cancer Study; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; SMC, Swedish Mammography Cohort; WHS, Women's Health Study; WLHS, Women's Lifestyle and Health Study.
FIGURE 2.
Study-specific and pooled multivariable HRs comparing ≥25 versus 0 g/d of cottage/ricotta cheese intake and risk of (A) estrogen-receptor-positive breast cancer and (B) estrogen-receptor-negative breast cancer. The squares and horizontal lines correspond to the study-specific HRs and 95% CIs for the comparison of ≥25 g/d to 0 g/d of cottage/ricotta cheese intake. The BWHS (The Black Women's Health Study), CLUE II (CLUE II: Campaign Against Cancer and Heart Disease), CPS II (Cancer Prevention Study II Nutrition Cohort), JPHC I (Japan Public Health Center-Based Study Cohort I), ORDET (Hormones and Diet in the Etiology of Breast Cancer Study), and SMC (Swedish Mammography Cohort) were excluded from the figure because these studies did not measure cottage/ricotta cheese intake. The size of the squares reflects the study-specific weight (inverse of the variance). The diamond represents the pooled multivariable HR and 95% CI. BCDDP, Breast Cancer Detection Demonstration Project Follow-up Study; CARET, β-Carotene and Retinol Efficacy Trial; CNBSS, Canadian National Breast Screening Study; CTS, California Teachers Study; IWHS, Iowa Women's Health Study; MCCS, Melbourne Collaborative Cohort Study; MEC, Multiethnic Cohort; NHS a, Nurses’ Health Study (part a); NHS b, Nurses’ Health Study (part b); NHS II, Nurses’ Health Study II; NIH-AARP, NIH-AARP Diet and Health Study; NLCS, Netherlands Cohort Study; NYUWHS, New York University Women's Health Study; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; WHS, Women's Health Study; WLHS, Women's Lifestyle and Health Study.
FIGURE 3.
Pooled multivariable HRs and 95% CIs for intake of dairy products and risk of breast cancer stratified by participant characteristics, region, and follow-up time. Pooled multivariable HRs (solid circles) of breast cancer and 95% CIs (horizontal lines) are plotted on a log scale for intake of (A) total milk (per 250 g/d increment), (B) total hard cheese (per 14 g/d increment), (C) cottage/ricotta cheese (per 10 g/d increment), and (D) total yogurt (per 25 g/d increment) stratified by participant characteristics, region, and follow-up time. The P value, test for between-studies heterogeneity (P-het) was calculated using the Q statistic; the P value, test for interaction (P-inter) was calculated using metaregression.
Dietary calcium intake showed a significant inverse trend with risk of breast cancer overall (P, test for trend = 0.004), although the result for the highest intake category (≥1400 mg/d) was not statistically significant (Table 3). Weak inverse associations were also observed when dietary calcium intake was modeled using study-specific quintiles (pooled HR comparing quintile 5 with 1 = 0.95, 95% CI: 0.91, 0.98, P, test for trend = 0.001, Supplemental Table 7) or as a continuous variable (pooled HR for a 350 mg/d increment = 0.98, 95% CI: 0.97, 0.99, Figure 4). After correcting for measurement error, the pooled age- and energy-adjusted HR for a 350 mg/d increment of dietary calcium intake changed from 0.98 (95% CI: 0.96, 0.99) to 0.95 (95% CI: 0.91, 0.98) for overall breast cancer. Results for dietary calcium intake were similar in magnitude when we limited the analyses to only those studies included in the total calcium analyses or when limited to individuals with no supplemental calcium intake (results not shown). The associations between total calcium intake and risk of breast cancer were all weak and statistically nonsignificant when intake was modeled as a categorical variable (Table 3), as quintiles (Supplemental Table 7), or continuously (Figure 4). Results for dietary and total calcium intake did not differ by ER (Table 3) or joint ER/PR subtypes (P, test for common effects >0.4, Supplemental Table 8).
TABLE 3.
Pooled multivariable1 adjusted HRs and 95% CIs for calcium intake and risk of breast cancer overall and for subtypes defined by estrogen receptor (ER)2 status
| Categories of intake | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pooled multivariable HR (95% CI) | ||||||||||
| Nutrients | Reference | Category 1 | Category 2 | Category 3 | Category 4 | Category 5 | P trend 3 | P het 4 | I 2 5 | Pcom 6 |
| Dietary calcium7 | ||||||||||
| No. cases | 6976 | 11,699 | 9394 | 5268 | 3194 | 1327 | ||||
| (ER+/–) | (3999/1061) | (6899/1708) | (5449/1281) | (3057/717) | (1861/442) | (774/158) | ||||
| Total | 1.00 (Ref) | 0.99 (0.96, 1.02) | 0.98 (0.95, 1.02) | 0.95 (0.91, 1.00) | 0.93 (0.88, 0.99) | 0.99 (0.91, 1.07) | 0.004 | 0.19 | 22% | |
| ER+ | 1.00 (Ref) | 1.00 (0.96, 1.04) | 0.98 (0.93, 1.04) | 0.96 (0.91, 1.02) | 0.93 (0.86, 1.01) | 1.00 (0.91, 1.11) | 0.08 | 0.21 | 21% | 0.71 |
| ER– | 1.00 (Ref) | 0.99 (0.91, 1.08) | 0.97 (0.88, 1.07) | 0.96 (0.85, 1.08) | 0.97 (0.86, 1.02) | 1.04 (0.86, 1.26) | 0.18 | 0.32 | 11% | |
| Total calcium8 | ||||||||||
| No. cases | 2749 | 5129 | 4698 | 3759 | 3915 | 5574 | ||||
| (ER+/–) | (1553/395) | (3006/778) | (2754/666) | (2209/511) | (2351/510) | (3408/684) | ||||
| Total | 1.00 (Ref) | 0.99 (0.95, 1.04) | 0.97 (0.93, 1.02) | 0.96 (0.92, 1.02) | 0.97 (0.91, 1.04) | 0.97 (0.91, 1.04) | 0.25 | 0.19 | 28% | |
| ER+ | 1.00 (Ref) | 1.02 (0.96, 1.08) | 1.00 (0.93, 1.06) | 0.98 (0.92, 1.05) | 1.01 (0.94, 1.10) | 1.01 (0.93, 1.09) | 0.80 | 0.20 | 26% | 0.92 |
| ER– | 1.00 (Ref) | 1.08 (0.95, 1.22) | 1.04 (0.89, 1.22) | 1.03 (0.86, 1.23) | 1.02 (0.88, 1.18) | 1.02 (0.88, 1.17) | 0.57 | 0.82 | <1% | |
Multivariable model includes race/ethnicity (white, African American, Hispanic, Asian), education (<high school, high school, >high school), BMI (<23, 23 to <25, 25 to <30, ≥30 kg/m2), height (<1.60, 1.60 to <1.65, 1.65 to <1.70, 1.70 to <1.75, ≥1.75 m except Japan Public Health Center-Based Study Cohort I: <1.50, 1.50 to <1.55, 1.55 to <1.60, 1.60 to <1.65, ≥1.65 m), alcohol consumption (0, >0 to <5, 5 to <15, 15 to <30, ≥30 g/d), energy intake (kcal/d, continuous), smoking status (never, past, current), physical activity (low, medium, high), age at menarche (<11, 11/12, 13/14, ≥15 y except Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: <10, 10/11, 12/13, ≥14 y), the combination of menopausal status and hormone replacement therapy use [premenopausal women and among postmenopausal women separate categories for never, past, and current users of hormone replacement therapy, except in the California Teachers Study, Canadian National Breast Screening Study, Multiethnic Cohort, New York University Women's Health Study (NYUWHS), Hormones and Diet in the Etiology of Breast Cancer Study, Swedish Mammography Cohort, and Women's Lifestyle and Health Study where the combination was modeled as premenopausal women and among postmenopausal women separate categories for never and ever users of hormone replacement therapy], oral contraceptive use (ever, never), parity (0, 1 to 2, ≥3, except for NYUWHS 0, ≥1), age at first birth (≤25, >25 y), history of benign breast disease (yes, no), family history of breast cancer (yes, no). Age in years and year of questionnaire return were included as stratification variables.
ER: estrogen receptor.
P trend: the P value, test for trend was calculated using the Wald test statistic.
P het: the P value, test for between-studies heterogeneity for the highest category was calculated using the Q statistic.
I 2 for the highest category.
P com: the P value, test for common effects for different subtypes defined by estrogen receptor status for the highest category was calculated using the Wald test statistic.
The intake of dietary calcium for reference, category 1, category 2, category 3, category 4, and category 5 is <500, 500 to <700, 700 to <900, 900 to <1100, 1100 to <1400, ≥1400 mg/d, respectively.
The intake of total calcium for reference, category 1, category 2, category 3, category 4, and category 5 is <500, 500 to <700, 700 to <900, 900 to <1100, 1100 to <1400, ≥1400 mg/d, respectively.
FIGURE 4.
Pooled multivariable HRs and 95% CIs for calcium intake and risk of breast cancer stratified by participant characteristics, region, and follow-up time. Pooled multivariable HRs (solid circles) of breast cancer and 95% CIs (horizontal lines) are plotted on a log scale for intake of (A) dietary calcium (per 350 mg/d) and (B) total (from foods and supplements) calcium (per 350 mg/d) stratified by participant characteristics, region, and follow-up time. The P value, test for between-studies heterogeneity (P-het) was calculated using the Q statistic; the P value, test for interaction (P-inter) was calculated using metaregression.
The associations between total milk, hard cheese, cottage/ricotta cheese, yogurt, dietary calcium, and total calcium intake and risk of overall, ER-positive, and ER-negative breast cancer generally did not vary significantly by BMI, menopausal status, or age at diagnosis. Some differences by region and follow-up time were noted (Figures 3 and 4, Supplemental Tables 5 and 9). For example, the following associations were modified by region: hard cheese intake and risk of ER-positive breast cancer (P, test for interaction = 0.03), cottage/ricotta cheese and risk of ER-negative breast cancer (P, test for interaction = 0.001), and dietary calcium and risk of ER-negative breast cancer (P, test for interaction = 0.02) where stronger associations were observed among cohorts outside of North America. A suggestively stronger association was also observed for total milk intake and risk of ER-negative breast cancer among cohorts outside of North America (P, test for interaction = 0.06). The associations for dietary and total calcium did not vary by total vitamin D intake (P, test for interaction >0.19, results not shown). When we examined supplemental calcium intake stratified by tertiles of dietary calcium intake, no evidence of an interaction was found for the association between supplemental calcium intake and risk of breast cancer (P, test for interaction = 0.86, results not shown).
Discussion
In this pooled analysis, we found null or very weak inverse associations for the consumption of total milk, yogurt, hard cheese, cottage/ricotta cheese, ice cream, dietary calcium, and total calcium with risk of overall and ER-positive breast cancer. For ER-negative breast cancer, modest inverse associations were observed for yogurt and cottage/ricotta cheese consumption when modeled as categorical variables. Results were generally consistent across studies and population subgroups defined by menopausal status at diagnosis, age at diagnosis, and BMI.
A previous meta-analysis of cohort and case-control studies showed a modest inverse relation between dairy consumption and overall breast cancer risk and stronger inverse associations for yogurt and low-fat dairy products (14). Of the 17 cohorts in that meta-analysis examining diet during mid to later adulthood, 7 were included in our pooled analysis but had 1–7 y longer follow-up. The meta-analysis included 10 studies that did not compute total energy intake, did not measure long-term usual diet covering the past 6 mo or 1 y, or did not validate the dietary assessment tool, thus these cohorts were not included in our study as they did not meet our inclusion criteria. Our study included another 14 cohorts that had not previously examined dairy products and breast cancer, minimizing the influence of publication bias, a common limitation of meta-analyses of the published literature (61, 62). The meta-analysis did not examine breast cancer subtypes, whereas our study showed stronger associations for yogurt and cottage/ricotta cheese intake with risk of ER-negative than ER-positive breast cancer.
There are a few explanations for the inverse association observed for yogurt intake. Yogurt consumption does not increase circulating IGF-1 as has been shown for other dairy products (63). Probiotics and fermented dairy products have been shown to boost intestinal microbiome richness, which might increase urinary estrogen excretion (64), induce apoptosis of breast cancer cell lines (65), and counteract dietary and genetic predisposition to mammary cancer in mice (66). Moreover, probiotics have been shown to be enriched in controls compared with breast cancer cases (67).
A lower risk of ER-negative breast cancer was also associated with a higher intake of cottage/ricotta cheese but not hard cheese. This may be attributable to differences in viable bacterial counts between cottage/ricotta cheese and hard cheese that occur during manufacturing and storage (68–70); for example, evidence has shown that the abundance of probiotic strains in low-fat hard cheese decreased over the aging process (71). When we stratified by region, the inverse association for cottage/ricotta cheese intake and ER-negative breast cancer was shown only among studies outside of North America. The regional differences might potentially be due to differences in food regulations, food processing, farming practices, or nutrient content.
Our findings on calcium intake and overall breast cancer risk are consistent with a meta-analysis of 11 prospective cohort studies (of which 6 were included in our study) (9). Studies suggest that higher dietary calcium can markedly suppress Western-diet induced hyperproliferation of epithelial cells in mice (72, 73), exert a prodifferentiation effect on mammary gland cells (74), and reduce the incidence of mammary tumors in rats (75). Yet, in the Women's Health Initiative, calcium and vitamin D supplementation was not associated with breast cancer risk (76), nor with mammographic density [higher breast density is associated with higher breast cancer risk (77)] between the intervention and placebo groups (78). However, the low intervention dose, study duration, population studied (>60% were ≥60 y old at baseline), and nonadherence may have contributed to the null findings. Our study and the meta-analysis (9) both found slightly stronger, although still weak, associations for dietary calcium than for calcium from supplements, suggesting a synergistic effect of calcium and other nutrients in dairy foods and/or effects of nutrients in dairy foods that are highly correlated with calcium. It is also possible that supplemental calcium reduces breast cancer risk only among women who are calcium deficient, resulting in supplementation having minimal benefit above and beyond adequate calcium intake from food. Although we do not have a measure of whether women were calcium deficient, the association for supplemental calcium intake with risk of overall breast cancer did not vary across tertiles of dietary calcium intake in our study.
The main strength of our study is that we analyzed the primary participant-level data from 21 prospective cohort studies, which made it possible to harmonize the definitions of the outcomes, exposures, and confounding variables. Harmonization, in turn, reduced potential sources of heterogeneity across studies due to different exposure and covariate definitions and use of different analytic approaches. Analyzing the participant-level data also permitted more flexibility in the analyses and estimation of finer dose-response relations than possible in meta-analyses of published studies. In addition, the large sample size provided adequate statistical power to examine breast cancer subtypes defined by hormone receptor status, particularly the less common subtypes. Lastly, the adjustments for known breast cancer risk factors minimized the likelihood of residual confounding strongly influencing our results. In fact, despite the differences in the assessment methods used across studies for diet and confounding variables, there was no significant between-study heterogeneity in any of our main analyses.
Our study has limitations. Dietary intakes were inevitably measured with error. However, moderate to high correlations between the measurements by FFQ and by dietary record or similar instruments have been reported in validation studies (25, 38–48). After correcting for measurement error, the association between calcium intake and risk of breast cancer changed only slightly. Misclassification of the exposure and the covariates might exist given that only baseline measurements were available, however, the results stratified by follow-up time indicated little difference across strata. We were not able to estimate consumption during earlier life periods, which could be biologically more relevant (79). A recent study found that adolescent consumption of high-fat dairy products was positively associated with ER-negative-PR-negative breast cancer (80). We also could not further characterize other subtypes including luminal A, B, and basal-like subtypes. Although the amount of missing data was generally low for most of the covariates measured in each cohort study, some covariates were not available in a number of cohorts such as use of menopausal hormone therapy and history of benign breast disease (Supplemental Methods). However, we do not expect that the unmeasured covariates had a strong influence on the observed associations as the age-adjusted estimates were very similar to the multivariable estimates. Lastly, our study population consisted predominantly of white women. The results might not be applicable to populations of other racial or ethnic compositions.
In summary, although concerns were raised that dairy products might contribute to a higher risk of breast cancer, this international study of over 1 million women suggested that adult dairy consumption is unlikely to be associated with higher risk of this common cancer and that higher fermented dairy product intake could potentially decrease the risk of the ER-negative subtype, if the aforementioned associations were causal. Evaluation of these associations in more racially/ethnically diverse populations and in those with higher fermented dairy product consumption may help elucidate further any relation between dairy foods and breast cancer risk.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Shiaw-Shyuan Yaun and Tao Hou for their contributions to data management and statistical support. The author's contributions were as follows—all authors of this research manuscript: have participated in the planning, execution, and/or analysis of the study, and have read and approved the final manuscript. For each cohort, acknowledgments, and disclaimers are listed in Supplemental Table 10. The authors report no conflicts of interest.
Notes
The centralization, checking, harmonization, and statistical analyses of the participant-level data from each of the cohorts were supported by NIH (grant CA55075 to WCW) and the Breast Cancer Research Foundation (to WCW). YW was supported by the China Scholarship Council, the Muriel K. and David R. Pokross and Joan P. and Ronald C. Curhan Doctoral Student Support Fund in Nutrition, and the Mayer Fund. For each cohort, funding, acknowledgements, and disclaimers are listed in Supplemental Table 10. The funders did not have any role in the design, implementation, analysis, or interpretation of the data. The Pooling Project of Prospective Studies of Diet and Cancer is a project within the National Cancer Institute Cohort Consortium.
Supplemental Figure 1 and Supplemental Tables 1–10 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
RH and YW are joint first authors.
Abbreviations used: DCPP, Diet and Cancer Pooling Project; ER, estrogen receptor; ICD, International Classification of Diseases; IGF-1, insulin-like growth factor 1; PR, progesterone receptor.
Contributor Information
You Wu, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Ruyi Huang, Department of Medical Education, E-DA Hospital and School of Medicine for International Students, School of Medicine, I-SHOU University, Kaohsiung City, Taiwan; Department of Family Medicine, National Taiwan University Hospital, Taipei, Taiwan; Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Molin Wang, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Leslie Bernstein, Department of Population Sciences, Beckman Research Institute, City of Hope, Duarte, CA, USA.
Traci N Bethea, Slone Epidemiology Center at Boston University, Boston, MA, USA; Department of Medicine, Boston University School of Medicine, Boston, MA, USA.
Chu Chen, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Yu Chen, Division of Epidemiology, Department of Population Health and Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA.
A Heather Eliassen, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Neal D Freedman, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD, USA.
Mia M Gaudet, Department of Population Science, American Cancer Society, Atlanta, GA, USA.
Gretchen L Gierach, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD, USA.
Graham G Giles, Cancer Epidemiology Division, Cancer Council Victoria, Melbourne, Victoria, Australia; Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, The University of Melbourne, Melbourne, Victoria, Australia; Precision Medicine, School of Clinical Sciences at Monash Health, Monash University, Clayton, Victoria, Australia.
Vittorio Krogh, Epidemiology and Prevention Unit Department of Research, IRCCS National Cancer Institute Foundation, Milan, Italy.
Susanna C Larsson, Department of Surgical Sciences, Uppsala University, Uppsala, Sweden; Unit of Nutritional Epidemiology, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
Linda M Liao, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD, USA.
Marjorie L McCullough, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; Department of Population Science, American Cancer Society, Atlanta, GA, USA.
Anthony B Miller, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada.
Roger L Milne, Cancer Epidemiology Division, Cancer Council Victoria, Melbourne, Victoria, Australia; Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, The University of Melbourne, Melbourne, Victoria, Australia; Precision Medicine, School of Clinical Sciences at Monash Health, Monash University, Clayton, Victoria, Australia.
Kristine R Monroe, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Marian L Neuhouser, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Julie R Palmer, Slone Epidemiology Center at Boston University, Boston, MA, USA; Department of Medicine, Boston University School of Medicine, Boston, MA, USA.
Anna Prizment, Division of Hematology, Oncology and Transplantation, University of Minnesota Medical School, Minneapolis, MN, USA; Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Peggy Reynolds, Department of Epidemiology and Biostatistics, University of California San Francisco, Berkeley, CA, USA.
Kim Robien, Department of Exercise and Nutrition Sciences, Milken Institute School of Public Health, George Washington University, WA, USA.
Thomas E Rohan, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Sven Sandin, Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden; Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Seaver Autism Center for Research and Treatment at Mount Sinai, New York, NY, USA.
Norie Sawada, Epidemiology and Prevention Group, Center for Public Health Sciences, National Cancer Center, Tokyo, Japan.
Sabina Sieri, Epidemiology and Prevention Unit Department of Research, IRCCS National Cancer Institute Foundation, Milan, Italy.
Rashmi Sinha, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD, USA.
Rachael Z Stolzenberg-Solomon, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD, USA.
Shoichiro Tsugane, Epidemiology and Prevention Group, Center for Public Health Sciences, National Cancer Center, Tokyo, Japan.
Piet A van den Brandt, Department of Epidemiology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, The Netherlands; Department of Epidemiology, Care and Public Health Institute (CAPHRI), Maastricht University, Maastricht, The Netherlands.
Kala Visvanathan, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Elisabete Weiderpass, International Agency for Research on Cancer, WHO, Lyon, France.
Lynne R Wilkens, University of Hawaii Cancer Center, Honolulu, HI, USA.
Walter C Willett, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Alicja Wolk, Department of Surgical Sciences, Uppsala University, Uppsala, Sweden; Unit of Nutritional Epidemiology, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
Anne Zeleniuch-Jacquotte, Division of Epidemiology, Department of Population Health and Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA.
Regina G Ziegler, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD, USA.
Stephanie A Smith-Warner, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Data Availability
The data described in the manuscript will not be made available because we do not have permission to disclose or release the data from the participating cohorts as specified in their executed data use agreements.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13:1558–68. [PubMed] [Google Scholar]
- 3. Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, Gaudet M, Schmidt MK, Broeks A, Cox Aet al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103:250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. JNCI Journal of the National Cancer Institute. 2004;96:218–28. [DOI] [PubMed] [Google Scholar]
- 5. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K. SEER Cancer Statistics Review1975–2016. Bethesda (MD): National Cancer Institute; 2019. [Google Scholar]
- 6. Zhang S, Zeng X, Ren M, Mao X, Qiao S. Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol. 2017;8:10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Białek A, Tokarz A. Conjugated linoleic acid as a potential protective factor in prevention of breast cancer. Postepy Hig Med Dosw (Online). 2013;67:6–14. [DOI] [PubMed] [Google Scholar]
- 8. Davoodi H, Esmaeili S, Mortazavian AM. Effects of milk and milk products consumption on cancer: a review. Comprehensive Reviews in Food Science and Food Safety. 2013;12:249–64. [Google Scholar]
- 9. Hidayat K, Chen G-C, Zhang R, Du X, Zou S-Y, Shi B-M, Qin L-Q. Calcium intake and breast cancer risk: meta-analysis of prospective cohort studies. Br J Nutr. 2016;116:158–66. [DOI] [PubMed] [Google Scholar]
- 10. Harrison S, Lennon R, Holly J, Higgins JPT, Gardner M, Perks C, Gaunt T, Tan V, Borwick C, Emmet Pet al. Does milk intake promote prostate cancer initiation or progression via effects on insulin-like growth factors (IGFs)? A systematic review and meta-analysis. Cancer Causes Control. 2017;28:497–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nachman KE, Smith TJS. Hormone use in food animal production: assessing potential dietary exposures and breast cancer risk. Current Environmental Health Reports. 2015;2:1–14. [DOI] [PubMed] [Google Scholar]
- 12. Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer. 2015;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farlow DW, Xu X, Veenstra TD. Quantitative measurement of endogenous estrogen metabolites, risk-factors for development of breast cancer, in commercial milk products by LC-MS/MS. J Chromatogr B. 2009;877:1327–34. [DOI] [PubMed] [Google Scholar]
- 14. Zang J, Shen M, Du S, Chen T, Zou S. The association between dairy intake and breast cancer in Western and Asian populations: a systematic review and meta-analysis. Journal of Breast Cancer. 2015;18:313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith-Warner SA, Spiegelman D, Ritz J, Albanes D, Beeson WL, Bernstein L, Berrino F, van den Brandt PA, Buring JE, Cho Eet al. Methods for pooling results of epidemiologic studies. Am J Epidemiol. 2006;163:1053–64. [DOI] [PubMed] [Google Scholar]
- 16. McCullough ML, Rodriguez C, Diver WR, Feigelson HS, Stevens VL, Thun MJ, Calle EE. Dairy, calcium, and vitamin D intake and postmenopausal breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2898–904. [DOI] [PubMed] [Google Scholar]
- 17. Shin MH, Holmes MD, the SHJO . Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–11. [DOI] [PubMed] [Google Scholar]
- 18. Lin J, Manson JE, Lee I-M, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;167:1050–9. [DOI] [PubMed] [Google Scholar]
- 19. Omenn GS, Goodman G, Thornquist M, Grizzle J, Rosenstock L, Barnhart S, Balmes J, Cherniack MG, Cullen MR, Glass A. The β-Carotene and Retinol Efficacy Trial (CARET) for chemoprevention of lung cancer in high risk populations: smokers and asbestos-exposed workers. Cancer Res. 1994;54(7 Suppl):2038s–43s. [PubMed] [Google Scholar]
- 20. Toniolo P, Riboli E, Shore RE, Pasternack BS. Consumption of meat, animal products, protein, and fat and risk of breast cancer: a prospective cohort study in New York. Epidemiology. 1994;5:391–7. [DOI] [PubMed] [Google Scholar]
- 21. Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, Fogel R, Gelmann EP, Gilbert F, Hasson MAet al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21:273S–309S. [DOI] [PubMed] [Google Scholar]
- 22. Giles GG, English DR. The Melbourne Collaborative Cohort Study. IARC Sci Publ. 2002;156:69–70. [PubMed] [Google Scholar]
- 23. Kumle M, Weiderpass E, Braaten T, Persson I, Adami H-O, Lund E. Use of oral contraceptives and breast cancer risk: the Norwegian-Swedish Women's Lifestyle and Health Cohort Study. Cancer Epidemiol Biomarkers Prev. 2002;11:1375–81. [PubMed] [Google Scholar]
- 24. Terry P, Jain M, Miller AB, Howe GR, Rohan TE. Dietary carotenoids and risk of breast cancer. Am J Clin Nutr. 2002;76:883–8. [DOI] [PubMed] [Google Scholar]
- 25. Genkinger JM, Makambi KH, Palmer JR, Rosenberg L, Adams-Campbell LL. Consumption of dairy and meat in relation to breast cancer risk in the Black Women's Health Study. Cancer Causes Control. 2013;24:675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Le Marchand L, Haiman CA, Wilkens LR, Kolonel LN, Henderson BE. MTHFR polymorphisms, diet, HRT, and breast cancer risk: the Multiethnic Cohort Study. Cancer Epidemiol Biomarkers Prev. 2004;13:2071–7. [PubMed] [Google Scholar]
- 27. Sieri S, Krogh V, Pala V, Muti P, Micheli A, Evangelista A, Tagliabue G, Berrino F. Dietary patterns and risk of breast cancer in the ORDET cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:567–72. [PubMed] [Google Scholar]
- 28. Velie EM, Schairer C, Flood A, He J-P, Khattree R, Schatzkin A. Empirically derived dietary patterns and risk of postmenopausal breast cancer in a large prospective cohort study. Am J Clin Nutr. 2005;82:1308–19. [DOI] [PubMed] [Google Scholar]
- 29. Sellers TA, Vierkant RA, Djeu J, Celis E, Wang AH, Kumar N, Cerhan JR. Unpasteurized milk consumption and subsequent risk of cancer. Cancer Causes Control. 2008;19:805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Umesawa M, Iso H, Ishihara J, Saito I, Kokubo Y, Inoue M, Tsugane S. Dietary calcium intake and risks of stroke, its subtypes, and coronary heart disease in Japanese. Stroke. 2008;39:2449–56. [DOI] [PubMed] [Google Scholar]
- 31. Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Premenopausal dietary fat in relation to pre- and post-menopausal breast cancer. Breast Cancer Res Treat. 2014;145:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larsson SC, Andersson S-O, Johansson J-E, Wolk A. Cultured milk, yogurt, and dairy intake in relation to bladder cancer risk in a prospective study of Swedish women and men. Am J Clin Nutr. 2008;88:1083–7. [DOI] [PubMed] [Google Scholar]
- 33. Goldbohm RA, Chorus AMJ, Galindo Garre F, Schouten LJ, van den Brandt PA. Dairy consumption and 10-y total and cardiovascular mortality: a prospective cohort study in the Netherlands. Am J Clin Nutr. 2011;93:615–27. [DOI] [PubMed] [Google Scholar]
- 34. Link LB, Canchola AJ, Bernstein L, Clarke CA, Stram DO, Ursin G, Horn-Ross PL. Dietary patterns and breast cancer risk in the California Teachers Study cohort. Am J Clin Nutr. 2013;98:1524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol. 2004;160:1223–33. [DOI] [PubMed] [Google Scholar]
- 36. Nyante SJ, Dallal CM, Gierach GL, Park Y, Hollenbeck AR, Brinton LA. Risk factors for specific histopathological types of postmenopausal breast cancer in the NIH-AARP Diet and Health Study. Am J Epidemiol. 2013;178(3):359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Willett W. Nutritional epidemiology. Oxford: Oxford University Press; 2013. p. 274–5. [Google Scholar]
- 38. Sasaki S, Takahashi T, Iitoi Y, Iwase Y, Kobayashi M, Ishihara J, Akabane M, Tsugane S. Food and nutrient intakes assessed with dietary records for the validation study of a self-administered food frequency questionnaire in JPHC Study Cohort I. J Epidemiol. 2003;13:23–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Romieu I, Stampfer MJ, Stryker WS, Hernandez M, Kaplan L, Sober A, Rosner B, Willett WC. Food predictors of plasma beta-carotene and alpha-tocopherol: validation of a food frequency questionnaire. Am J Epidemiol. 1990;131:864–76. [DOI] [PubMed] [Google Scholar]
- 40. Koralek DO, Bertone-Johnson ER, Leitzmann MF, Sturgeon SR, Lacey JV, Schairer C, Schatzkin A. Relationship between calcium, lactose, vitamin D, and dairy products and ovarian cancer. Nutr Cancer. 2006;56:22–30. [DOI] [PubMed] [Google Scholar]
- 41. Goldbohm RA, van den Brandt PA, Brants HA, van't Veer P, Sturmans F, Al M, Hermus RJ. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur J Clin Nutr. 1994;48:253–65. [PubMed] [Google Scholar]
- 42. Flagg EW, Coates RJ, Calle EE, Potischman N, Thun MJ. Validation of the American Cancer Society Cancer Prevention Study II Nutrition Survey Cohort Food Frequency Questionnaire. Epidemiology. 2000;11:462–8. [DOI] [PubMed] [Google Scholar]
- 43. Kaaks R. Pilot phase studies on the accuracy of dietary intake measurements in the EPIC project: overall evaluation of results. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26:26S–36. [DOI] [PubMed] [Google Scholar]
- 44. Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 45. Munger RG, Folsom AR, Kushi LH, Kaye SA, Sellers TA. Dietary assessment of older Iowa women with a food frequency questionnaire: nutrient intake, reproducibility, and comparison with 24-hour dietary recall interviews. Am J Epidemiol. 1992;136:192–200. [DOI] [PubMed] [Google Scholar]
- 46. Bassett JK, English DR, Fahey MT, Forbes AB, Gurrin LC, Simpson JA, Brinkman MT, Giles GG, Hodge AM. Validity and calibration of the FFQ used in the Melbourne Collaborative Cohort Study. Public Health Nutr. 2016;19:2357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women's Health Study. Am J Epidemiol. 1993;137:1302–17. [DOI] [PubMed] [Google Scholar]
- 48. Kumanyika S. Relative validity of food frequency questionnaire nutrient estimates in the Black Women's Health Study. Ann Epidemiol. 2003;13:111–8. [DOI] [PubMed] [Google Scholar]
- 49. Prentice R. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 50. Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150:327–33. [DOI] [PubMed] [Google Scholar]
- 51. Cepeda MS. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. 2003;158:280–7. [DOI] [PubMed] [Google Scholar]
- 52. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 53. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 54. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 56. Smith-Warner SA, Spiegelman D, Yaun S-S, van den Brandt PA, Folsom AR, Goldbohm RA, Graham S, Holmberg L, Howe GR, Marshall JRet al. Alcohol and breast cancer in women. JAMA. 1998;279:535. [DOI] [PubMed] [Google Scholar]
- 57. Stram DO. Meta-analysis of published data using a linear mixed-effects model. Biometrics. 1996;52:536–44. [PubMed] [Google Scholar]
- 58. Wang M, Spiegelman D, Kuchiba A, Lochhead P, Kim S, Chan AT, Poole EM, Tamimi R, Tworoger SS, Giovannucci Eet al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35:782–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for measurement error: the case of multiple covariates measured with error. Am J Epidemiol. 1990;132:734–45. [DOI] [PubMed] [Google Scholar]
- 60. Rosner B, Willett WC, Spiegelman D. Correction of logistic regression relative risk estimates and confidence intervals for systematic within-person measurement error. Stat Med. 1989;8:1051–69.-discussion1071–3. [DOI] [PubMed] [Google Scholar]
- 61. Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sterne JAC, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–29. [DOI] [PubMed] [Google Scholar]
- 63. Romo Ventura E, Konigorski S, Rohrmann S, Schneider H, Stalla GK, Pischon T, Linseisen J, Nimptsch K. Association of dietary intake of milk and dairy products with blood concentrations of insulin-like growth factor 1 (IGF-1) in Bavarian adults. Eur J Nutr. 2020;59(4):1413–20. [DOI] [PubMed] [Google Scholar]
- 64. Flores R, Shi J, Fuhrman B, Xu X, Veenstra TD, Gail MH, Gajer P, Ravel J, Goedert JJ. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hassan Z, Mustafa S, Rahim RA, Isa NM. Anti-breast cancer effects of live, heat-killed and cytoplasmic fractions of Enterococcus faecalis and Staphylococcus hominis isolated from human breast milk. In Vitro Cell Dev Biol Anim. 2016;52:337–48. [DOI] [PubMed] [Google Scholar]
- 66. Lakritz JR, Poutahidis T, Levkovich T, Varian BJ, Ibrahim YM, Chatzigiagkos A, Mirabal S, Alm EJ, Erdman SE. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int J Cancer. 2014;135:529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Goedert JJ, Jones G, Hua X, Xu X, Yu G, Flores R, Falk RT, Gail MH, Shi J, Ravel Jet al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J Natl Cancer Inst. 2015;107:995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Baruzzi F, Morea M, Matarante A, Cocconcelli PS. Changes in the Lactobacillus community during ricotta forte cheese natural fermentation. J Appl Microbiol. 2000;89:807–14. [DOI] [PubMed] [Google Scholar]
- 69. Lovayová V, Dudriková E, Rimárová K, Siegfried L. Quantity of selected probiotic cultures in semi-hard cheese with low-cooking curd during the maturation process. J Food Sci Technol. 2015;52:4697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kok CR, Hutkins R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr Rev. 2018;76:4–15. [DOI] [PubMed] [Google Scholar]
- 71. Ganesan B, Weimer BC, Pinzon J, Dao Kong N, Rompato G, Brothersen C, McMahon DJ. Probiotic bacteria survive in Cheddar cheese and modify populations of other lactic acid bacteria. J Appl Microbiol. 2014;116:1642–56. [DOI] [PubMed] [Google Scholar]
- 72. Xue L, Lipkin M, Newmark H, Wang J. Influence of dietary calcium and vitamin D on diet-induced epithelial cell hyperproliferation in mice. JNCI Journal of the National Cancer Institute. 1999;91:176–81. [DOI] [PubMed] [Google Scholar]
- 73. McGrath CM, Soule HD. Calcium regulation of normal human mammary epithelial cell growth in culture. In Vitro. 1984;20:652–62. [DOI] [PubMed] [Google Scholar]
- 74. Jacobson EA, James KA, Newmark HL, Carroll KK. Effects of dietary fat, calcium, and vitamin D on growth and mammary tumorigenesis induced by 7,12-dimethylbenz(a)anthracene in female Sprague-Dawley rats. Cancer Res. 1989;49:6300–3. [PubMed] [Google Scholar]
- 75. Abou-Issa H, Moeschberger M, el-Masry W, Tejwani S, Curley RW, Webb TE. Relative efficacy of glucarate on the initiation and promotion phases of rat mammary carcinogenesis. Anticancer Res. 1995;15:805–10. [PubMed] [Google Scholar]
- 76. Prentice RL, Pettinger MB, Jackson RD, Wactawski-Wende J, Lacroix AZ, Anderson GL, Chlebowski RT, Manson JE, Van Horn L, Vitolins MZet al. Health risks and benefits from calcium and vitamin D supplementation: Women's Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:1133–44. [PubMed] [Google Scholar]
- 78. Bertone-Johnson ER, McTiernan A, Thomson CA, Wactawski-Wende J, Aragaki AK, Rohan TE, Vitolins MZ, Tamimi RM, Johnson KC, Lane Det al. Vitamin D and calcium supplementation and one-year change in mammographic density in the Women's Health Initiative calcium and vitamin D trial. Cancer Epidemiol Biomarkers Prev. 2012;21:462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hjartåker A, Laake P, Lund E. Childhood and adult milk consumption and risk of premenopausal breast cancer in a cohort of 48,844 women – the Norwegian women and cancer study. Int J Cancer. 2001;93:888–93. [DOI] [PubMed] [Google Scholar]
- 80. Farvid MS, Eliassen AH, Cho E, Chen WY, Willett WC. Dairy consumption in adolescence and early adulthood and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2018;27:575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in the manuscript will not be made available because we do not have permission to disclose or release the data from the participating cohorts as specified in their executed data use agreements.