Abstract
Background:
Coronavirus Disease 2019 (COVID-19) pandemic raised global attention especially due to the severe acute respiratory symptoms associated to it. However, almost one third of patients also develop neurological symptoms. The aim of the present study is to describe the case of a previously health adult that evolved cerebral ventricular empyema in the IV ventricle during COVID-19 infection treatment.
Case Description:
A 49-year-old man with COVID-19 developed pneumonia caused by multidrug-resistant Acinetobacter baumannii. After treating adequate treatment, sedation was switched off without showing appropriate awakening. Brain CT was performed with evidence of communicating hydrocephalus. External ventricular shunt (EVD) was implant with intraoperative cerebrospinal fluid suggestive of meningitis with a positive culture for oxacillin-sensitive Staphylococcus hominis. Twenty days after EVD, meningitis treatment was finished and with 2 negative cultures, conversion to ventriculoperitoneal shunt was performed. In the following week, during the evaluation of the patient in intensive care, quadriplegia and absence of spontaneous respiratory movement were evidenced, just maintaining head movement. Brain MRI was performed with a diagnosis of ventriculitis associated with pus collections on the IV ventricle. The patient underwent microsurgical drainage removal of the shunt, with a positive intraventricular collection culture for Klebsiella pneumoniae carbapenemase and multidrug-resistant Pseudomonas aeruginosa, without improvement in the neurological condition. After 14 weeks of hospitalization, the patient died.
Conclusion:
It is well known that COVID-19 has potential to directly attack and cause severe damage to the central nervous system; however, ventricular empyema is an extremely rare life-threatening complication.
Keywords: Coronavirus disease 2019, External ventricular shunt, Hydrocephalus, Motor Evoked Potentials, Ventricular empyema

INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in Wuhan, China, and has become a global health threat.[14,20] Although most patients with COVID-19 manifest fever, fatigue, and cough, more severe cases of the disease can include respiratory distress complicating with renal and cardiac failure and eventually death.[10,12,23] Extra-respiratory manifestations may also involve other organs/systems, including gastrointestinal, hepatic, cutaneous, hematological, ocular, cardiac, renal, and neurological symptoms.[1,5,11,18] It was recently documented that 36.4% of patients with COVID-19 developed neurological symptoms, such as headache, disturbed consciousness, and paresthesia.[2,6,13,15] Severely affected individuals are more likely to develop neurological disorders than patients who have mild or moderate disease.[2,6,13] However, abscess or empyema affecting the central nervous system (CNS) is an extremely rare event in COVID-19 patients with few cases reports to date.[19]
The aim of the present study is to describe the case of a previously health adult that evolved a voluminous ventricular empyema in the IV ventricle during COVID-19 infection treatment.
CASE DESCRIPTION
A 49-year-old, previously healthy patient, diagnosed with COVID-19 in December/2020 was hospitalized in an intensive care unit with respiratory symptoms and no neurological deficits, but confusion and lethargy. He developed pneumonia caused by multidrug-resistant Acinetobacter baumannii and sinusitis. After treating such infections with antibiotic therapy, the patient’s sedation was switched off without showing adequate awakening, then a brain CT was performed with evidence of communicating hydrocephalus with signs of cerebrospinal fluid transudation. The patient underwent an external ventricular shunt (EVD) implant with intraoperative cerebrospinal fluid suggestive of meningitis with a positive culture for oxacillin-sensitive Staphylococcus hominis. Twenty days after EVD, meningitis treatment was finished and with 2 negative cultures, conversion to ventriculoperitoneal shunt was performed. In the following week, during the evaluation of the patient in intensive care, quadriplegia and absence of spontaneous respiratory movement were evidenced, just maintaining head movement, characterizing lock-in syndrome. A brain MRI was performed with a diagnosis of ventriculitis associated with an empyema on the 4th ventricle [Figures 1 and 2]. The patient underwent microsurgical drainage of the 4th ventricle pus collection through large suboccipital telovelar approach and removal of the shunt, with a positive intraventricular collection culture for Klebsiella pneumoniae carbapenemase and multidrug-resistant Pseudomonas aeruginosa, without improvement in the neurological condition. Prolonged intraventricular infusion of polymyxin was performed. A neurophysiologist performed the intraoperative neurophysiological monitoring with the NIM-Eclipse System (Medtronic, FL). As previously described,[9] the setup included transcranial electrical stimulation (TES) motor evoked potentials with recording from upper and lower extremities and from muscles innervated by VII, IX, X, XI, and XII cranial pairs (TES-CoBMEP). Furthermore, it included somatosensory evoked potentials with stimulation of median nerves at wrist and posterior tibial nerves at ankle and recording in the scalp and continuous and trigged electromyography with recording from muscles innervated by VII, IX, X, XI, and XII cranial pairs. Throughout the surgical procedure, recordable responses were observed only in TES-CoBMEP of the Orbicularis Oris muscle, innervated by the VII cranial pair [Figure 3]. Control brain CT with evidence of hydrocephalus was achieved, and an EVD was again performed. After 14 weeks of hospitalization, the patient died.
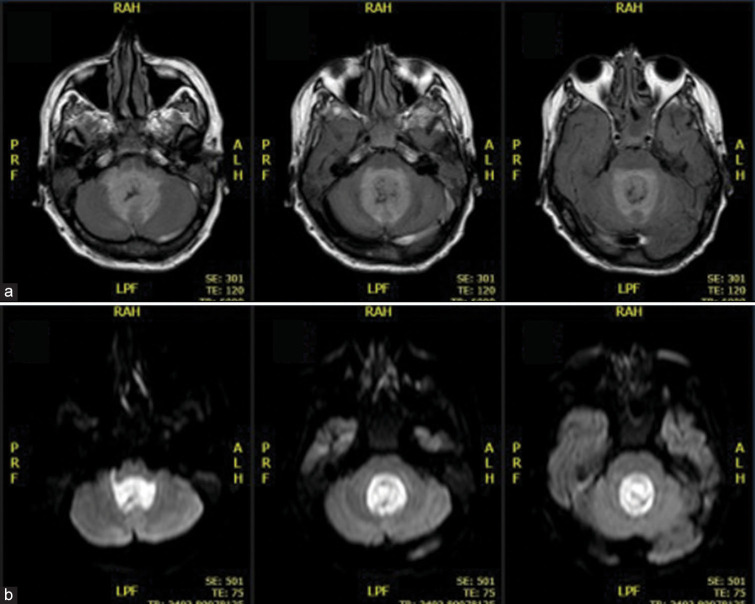
Figure 1:
Brain MRI showing ventricular empyema (a) axial T2-weighted fluid-attenuated inversion recovery imaging; (b) axial diffusion-weighted Imaging imaging).
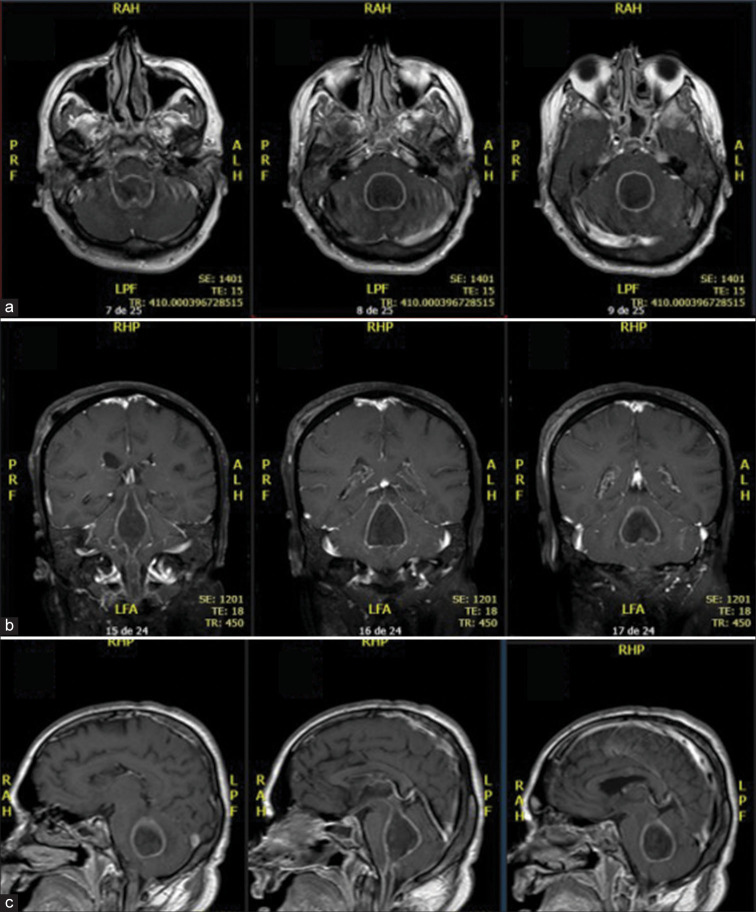
Figure 2:
Brain MRI showing ventricular empyema in Gadolinium-enhanced T1-weighted images (a) Axial; (b) Coronal; (c) Sagittal.
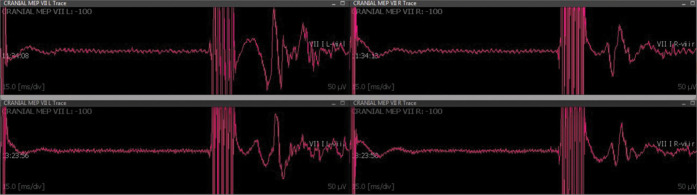
Figure 3:
Transcranial electrical stimulation-CoBMEP recorded from the left and right orbicularis oris muscle, showing the stability responses throughout the surgical procedure. Upper and lower panels depict, respectively, recorded responses at the beginning and the end of the surgery.
DISCUSSION
COVID-19 is a novel respiratory disease caused by SARSCoV-2 and has become a global health threat.[8,17] The lungs are the most affected organs because the virus accesses host cells through angiotensin-converting enzyme 2 (ACE2), which is most abundant on type II alveolar cells.[7,16] However, extra-respiratory manifestations of SARS-CoV-2 infection have recently been observed in the rapidly increasing number of COVID-19 cases.[1,5,11,18] About one-third of infected patient’s present neurological symptoms during the course of the disease and, even in some cases, neurologic symptoms may be the initial or only presentation of the COVID-19.[2,6,13,15]
The mechanisms involved in the neurological affections resulting from SARS-CoV-2 infection are not completely understood. However, it has been proposed that the virus gains access to the CNS by systemic vascular dissemination or, more locally, across the cribriform plate of the ethmoid bone.[3] According to Baig et al., once in the systemic circulation, the virus invades neural tissue due to its properties of neurotropism. Here, it binds to and interacts with ACE2 receptors in the capillary endothelium.[3] After accessing the CNS, there is an increasing body of evidence suggesting that the virus may damage neuronal cells through direct infection, hypoxic injury, or immune offence.[3,4]
COVID-19 patients may present central (CNS) and peripheric nervous system (PNS)-associated signs and symptoms. Several CNS-related manifestations have been reported and include headache, dizziness, impaired consciousness, acute cerebrovascular disease, epilepsy, ataxia, acute disseminated encephalomyelitis, and viral encephalitis.[3,4,21,22] Older individuals and patients with preexisting chronic medical conditions are at an elevated risk of worst CNS associated manifestations.[3,4,21,22] PNS signs and symptoms of COVID-19 are usually less severe and include hyposmia/anosmia, hypogeusia/ageusia, muscle pain, and Guillain-Barre syndrome.[3,4,21,22] However, abscess or empyema affecting the CNS is an extremely rare event in COVID-19 patients with only few cases reported to date.[19]
Talamonti et al. described six consecutive COVID-19 patients with primary spinal epidural abscess (SEA) that was managed surgically.[19] The authors hypothesize SEA may develop because an asymptomatic bacterial colonization co-exists with damage to the vascular endothelium induced by COVID-19.[19] Additionally, immunocompromised state or nosocomial superinfection may all play a role for pus collection around the CNS.[19] In the present case, we describe a neurological complication in a previously health man admitted with COVID-19 that evolved during the course of viral infection with ventricular empyema associated with severe pyogenic meningitis. We believe that prolonged external ventricular drainage predisposed to infection of the CNS. In our understanding, the immunocompromised state and nosocomial superinfection may have played a significant role for pus collection. Although, appropriated neurosurgical drainage and prolonged intravenous/ intrathecal antibiotic therapy were performed, the patient presented no neurological improvement postoperatively and died. Although preoperative lock-in syndrome was evident, intraoperative neurophysiological monitoring was used for clinical documentation of preoperative neurological status and better understands whether after appropriate brainstem decompression any function would recovery. To the best of our knowledge, this is the first case reported of ventricular empyema in a COVID-19 patient. Further scientific investigation may clarify if SARS-CoV-2 infection may increase the risk of bacterial meningitis and ventricular empyema.
CONCLUSION
It is well known that COVID-19 has potential to directly attack and cause severe damage to the CNS; however, intraventricular pus collection is an extremely rare life-threatening complication in COVID-19 patient. Further scientific investigations are necessary to understand whether the viral infection increases the risk of ventricular empyema.
Footnotes
How to cite this article: Meguins LC, Rocha AS, Laurenti MR, de Morais DF. Ventricular empyema associated with severe pyogenic meningitis in COVID-19 adult patient: Case report. Surg Neurol Int 2021;12:346.
Contributor Information
Lucas Crociati Meguins, Email: lucascmeguins@gmail.com.
Andre Salotto Rocha, Email: salotto.andre@gmail.com.
Matheus Rodrigo Laurenti, Email: mrlaurenti@yahoo.com.br.
Dionei Freitas de Morais, Email: dionei@cerebroecoluna.com.br.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Abobaker A, Raba AA, Alzwi A. Extrapulmonary and atypical clinical presentations of COVID-19. J Med Virol. 2020;92:2458–64. doi: 10.1002/jmv.26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: A literature review. J Clin Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–8. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 4.Baig AM. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther. 2020;26:499–501. doi: 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behzad S, Aghaghazvini L, Radmard AR, Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: Radiologic and clinical overview. Clin Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger JR. COVID-19 and the nervous system. J Neurovirol. 2020;26:143–8. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of Coronavirus disease 2019 (COVID-19) J Pathol. 2020;251:228–48. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chau CH, Strope JD, Figg WD. COVID-19 clinical diagnostics and testing technology. Pharmacotherapy. 2020;40:857–68. doi: 10.1002/phar.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deletis V, Fernández-Conejero I. Intraoperative monitoring and mapping of the functional integrity of the brainstem. J Clin Neurol. 2016;12:262–73. doi: 10.3988/jcn.2016.12.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge H, Wang X, Yuan X, Xiao G, Wang C, Deng T, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020;39:1011–9. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–32. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 12.Huang X, Wei F, Hu L, Wen L, Chen K. Epidemiology and clinical characteristics of COVID-19. Arch Iran Med. 2020;23:268–71. doi: 10.34172/aim.2020.09. [DOI] [PubMed] [Google Scholar]
- 13.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16–27e1. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Liu C, Liu G, Luo W, Xia N. COVID-19: Progress in diagnostics, therapy and vaccination. Theranostics. 2020;10:7821–35. doi: 10.7150/thno.47987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niazkar HR, Zibaee B, Nasimi A, Bahri N. The neurological manifestations of COVID-19: A review article. Neurol Sci. 2020;41:1667–71. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pathangey G, Fadadu PP, Hospodar AR, Abbas AE. Angiotensin-converting enzyme 2 and COVID-19: Patients, comorbidities, and therapies. Am J Physiol Lung Cell Mol Physiol. 2021;320:L301–30. doi: 10.1152/ajplung.00259.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samudrala PK, Kumar P, Choudhary K, Thakur N, Wadekar GS, Dayaramani R, et al. Virology, pathogenesis, diagnosis and in-line treatment of COVID-19. Eur J Pharmacol. 2020;883:173375. doi: 10.1016/j.ejphar.2020.173375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkesh A, Daei Sorkhabi A, Sheykhsaran E, Alinezhad F, Mohammadzadeh N, Hemmat N, et al. Extrapulmonary clinical manifestations in COVID-19 patients. Am J Trop Med Hyg. 2020;103:1783–96. doi: 10.4269/ajtmh.20-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talamonti G, Colistra D, Crisà F, Cenzato M, Giorgi P, D’Aliberti G. Spinal epidural abscess in COVID-19 patients. J Neurol. 2021;268:2320–6. doi: 10.1007/s00415-020-10211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan DY, Luo XY, Dong W, Zhang ZW. Current practice and potential strategy in diagnosing COVID-19. Eur Rev Med Pharmacol Sci. 2020;24:4548–53. doi: 10.26355/eurrev_202004_21039. [DOI] [PubMed] [Google Scholar]
- 21.Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: A systematic review and current update. Acta Neurol Scand. 2020;142:14–22. doi: 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou M, Zhang X, Qu J. Coronavirus disease 2019 (COVID-19): A clinical update. Front Med. 2020;14:126–35. doi: 10.1007/s11684-020-0767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]