Abstract
Background:
Cough is one of the most common symptoms of coronavirus disease 2019 (COVID-19) infection. This relatively benign symptom may lead to serious sequelae, especially in postoperative neurosurgical patients.
Case Description:
Here, we report a case of bone flap displacement, pseudomeningocele formation, and consequent cerebrospinal fluid leak in a patient with COVID-19 infection who recently underwent craniotomy for excision of cerebral metastasis. We highlight the pathophysiologic mechanisms of cough that may cause increased intracranial pressure (ICP), leading to the postoperative morbidity.
Conclusion:
Aside from additional risks to the patient’s health and increased treatment costs, these complications also lead to subsequent delays in the management of the underlying disease. Symptomatic treatment of cough is advised to prevent complications resulting from increased ICP.
Keywords: Cancer, Complication, Coronavirus disease 2019, Cough, Pseudomeningocele

INTRODUCTION
Coronavirus disease 2019 (COVID-19) is a global pandemic affecting 132 million people worldwide as of this writing.[5] Cough is one of its most common symptoms, occurring in about 75% of patients.[2] Coughing causes a Valsalva effect, which increases intracranial pressure (ICP).[4] Increased ICP is detrimental to neurosurgical patients, as this can lead to adverse outcomes.[1] Here, we report a case of COVID-19-induced severe persistent cough resulting in bone flap displacement, pseudomeningocele formation, and consequent cerebrospinal fluid (CSF) leak in a postoperative neurosurgical patient.
CASE DESCRIPTION
The patient is a 40-year-old female diagnosed with breast cancer in 2019. She underwent modified radical mastectomy in July 2019 and was on trastuzumab chemotherapy. She was referred to the neurosurgical service for a right parietal tumor on metastatic workup [Figure 1] and was scheduled for craniotomy for tumor resection. Before surgery, she underwent two COVID-19 polymerase chain reaction (PCR) tests in accordance with our preoperative protocol, and both were negative. During surgery, gross total tumor resection was achieved and the dura was closed in a watertight fashion using absorbable 4–0 sutures. The bone flap was anchored using silk 2–0 sutures. The postoperative course was uneventful and she was discharged home on the 3rd postoperative day. Histopathology confirmed the diagnosis of metastatic breast cancer.
Figure 1:
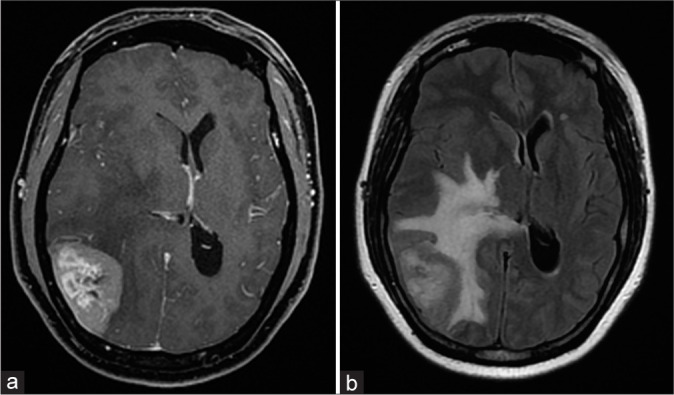
(a) Preoperative contrast cranial magnetic resonance imaging, T1 sequence, showing the contrast-enhancing right parietal tumor; (b) FLAIR sequence showing the marked perilesional vasogenic edema surrounding the tumor and midline shift.
One week after discharge, the patient experienced severe and persistent cough that kept her awake at night. She self-medicated with antitussives with minimal relief. Three days later, she noted a gradually bulging fluid-filled mass at her postoperative site, as well as the sensation that there was something moving underneath the fluid collection. After a 2 more days, fluid leaked from the surgical incision, prompting the patient to consult at the emergency department.
On examination, the patient was awake, oriented, and able to follow commands. She did not have fever or nuchal rigidity. She had a pseudomeningocele over the postoperative site, with dehiscence of a portion of the inferior limb of the surgical incision and watery fluid draining from it. On palpation, the bone flap was found to be mobile and displaced inferiorly. As part of the hospital protocol for admission, the patient underwent a COVID-19 PCR test, which was positive. She was thus transferred to the COVID-19 isolation unit.
A contrast cranial computed tomography (CT) scan showed postoperative changes at the right parietal area and no evidence of enhancing tumor. There was also no evidence of hydrocephalus, subdural empyema, or brain abscess. The bone flap was displaced inferiorly [Figure 2], and there was a fluid-filled collection at the right parietal area, consistent with a pseudomeningocele. Microbiological studies of the lumbar CSF and subgaleal fluid showed that there was no infection.
Figure 2:
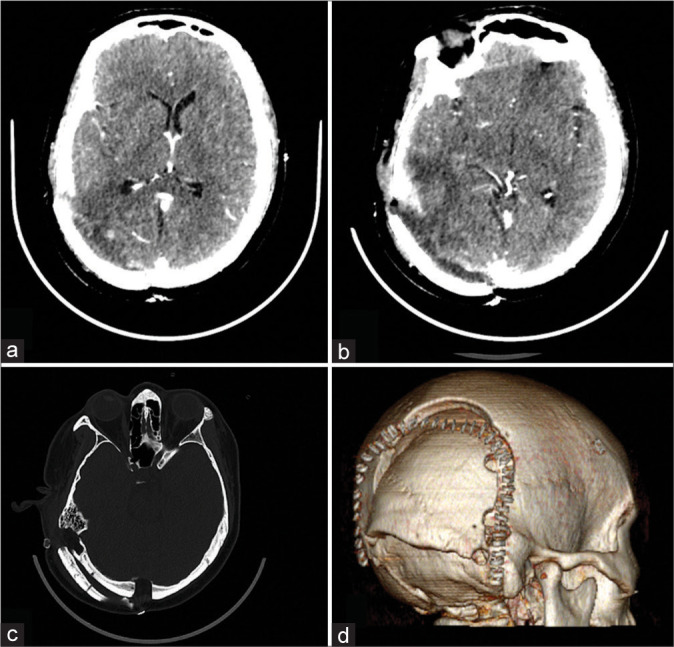
(a) Postoperative contrast cranial computed tomography (CT) showing postoperative changes at the right parietal area, no evidence of enhancing tumor, and no evidence of hydrocephalus; (b) same CT showing pseudomeningocele formation and outward displacement of the bone flap; (c) cranial CT, bone window, showing dislodged bone flap; (d) 3D reconstruction of the cranial CT highlighting the dislodged bone flap.
She was started on acetazolamide and mannitol to decrease CSF production and ICP. The leak site was sutured, and a lumbar drain was inserted to divert CSF and keep the postoperative site dry. The opening pressure at the time of lumbar drain insertion was normal at 7 cm H2O, likely due to the administration of mannitol, the CSF leak at the postoperative site, and the pseudomeningocele formation. Antibiotics were also given. The patient was quarantined in the COVID unit for 2 weeks and was only transferred to the regular ward after a negative COVID-19 PCR test.
The pseudomeningocele recurred after the lumbar drain was clamped, indicating failure of treatment. Thus, she underwent debridement, craniectomy, and duraplasty using fascia lata graft. Intraoperatively, the bone flap was found to be unsecured and displaced inferiorly, and the sutures securing the bone flap had become unraveled. A 3 cm × 4 cm dural defect was found along the margins of the previous dural repair, and a fascia lata graft was used to repair this. The bone flap was not reimplanted since the brain was swollen and herniating slightly past the craniectomy defect, likely due to cerebral edema from the infection as a consequence of the CSF leak. A new lumbar drain was inserted, then removed after a week. The patient’s postoperative site remained dry and flat, and she was discharged home.
On follow-up after 1 month, the patient was well, with no recurrence of the pseudomeningocele or CSF leak. She had no neurologic deficits and her cough had resolved completely.
DISCUSSION
COVID-19 infection has been shown to result in adverse outcomes in surgical patients, especially in terms of respiratory complications.[2] In addition, its symptoms may also cause complications in postoperative patients.[3] In this paper, we have reported the impact of one of its main respiratory symptoms, cough, on a postoperative neurosurgical patient. The cough seen in COVID-19 patients has been described as persistent, occurring 3–4 times in a 24 h period.[2]
Coughing is the sudden forceful expulsion of air through the large airways to clear them of irritants, particles, and fluids.[4] It consists of three phases: inhalation, forced exhalation through a closed glottis, and vigorous release of air once the glottis opens, producing the characteristic sound of coughing.[4] The pathophysiology of increased ICP during coughing is related to these phases.[1] During the phases of coughing, intra-abdominal and intrathoracic pressures increase, causing blood to pool in the valveless veins around the vertebrae and epidural veins and compressing the spinal dura, resulting in an upward pressure wave that increases ICP.[1] The increase in intrathoracic and intra-abdominal pressures also prevents cranial venous drainage, which increases intracranial blood volume and consequently, ICP, according to the Monro-Kellie doctrine.[1,4] In laboratory studies by Williams, coughing has been shown to increase ICP to as much as 100 mmHg during bursts of coughing, well above the normal ICP limit of 15–20 mmHg.[4]
In our case, we postulate that the repetitive increase in ICP caused by the severe persistent COVID-19-induced coughing led to a breakdown in the initial dural repair, causing CSF to leak out of the dura and accumulate under the scalp to form a pseudomeningocele. The severe and persistent coughing may have exerted pressure on the dural repair and unraveled the sutures. The continuous coughing further increased the size of the dural tear until it reached the dimensions seen during the surgery. This led to CSF leakage into the epidural space and accumulation beneath the bone flap. Subsequently, the increased pressure of the CSF probably exerted pressure on the overlying bone flap, unraveling the sutures securing the flap, and dislodging it inferiorly. In normal tissue, the pressures brought about by coughing would be transmitted evenly along the brain tissue and skull, but in a postoperative patient, pressures would intuitively be transmitted disproportionately toward the weakest point in the skull – that is, the postoperative site – resulting in the sequelae we have observed.[3] The normal ICP noted during lumbar tap was not surprising, since mannitol had already been started to decrease the ICP, and some of the CSF had already exited the intracranial compartment and formed a pseudomeningocele.
Our case is important because it illustrates that COVID-19 infection and the cough associated with it may still cause problems even after the surgical procedure. Coughing may not be a benign symptom and may lead to similar types of adverse events. Symptomatic treatment of cough, especially those associated with COVID-19, is advised in postoperative neurosurgical patients to prevent adverse sequelae stemming from increased ICP.
CONCLUSION
Although cough may be thought of as a benign symptom of COVID-19, it may have adverse sequelae related to increase ICP. Symptomatic treatment of cough is advised in the postoperative neurosurgical patient to prevent complications resulting from increased ICP.
Acknowledgments
None.
Footnotes
How to cite this article: Pascual JS, Chan KI, Khu KJ. Severe and persistent coronavirus disease 2019 cough resulting in bone flap displacement and pseudomeningocele. Surg Neurol Int 2021;12: 348.
Contributor Information
Juan Silvestre Grecia Pascual, Email: jgpascual@up.edu.ph.
Kevin Ivan Peñaverde Chan, Email: kpchan@up.edu.ph.
Kathleen Joy Ong-Lopez Khu, Email: kathleen.khu@neurosurgery.ph.
Ethics approval
Not applicable.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Donnelly J, Czosnyka M, Harland S, Varsos GV, Cardim D, Robba C, et al. Increased ICP and its cerebral haemodynamic sequelae. Acta Neurochir Suppl. 2018;126:47–50. doi: 10.1007/978-3-319-65798-1_10. [DOI] [PubMed] [Google Scholar]
- 2.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of Coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35:1545–9. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knisely A, Zhou ZN, Wu J, Huang Y, Holcomb K, Melamed A, et al. Perioperative morbidity and mortality of patients with COVID-19 who undergo urgent and emergent surgical procedures. Ann Surg. 2021;273:34–40. doi: 10.1097/SLA.0000000000004420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams B. Cerebrospinal fluid pressure: Changes in response to coughing. Brain. 1976;96:331–46. doi: 10.1093/brain/99.2.331. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Geneva: World Health Organization; 2020. Weekly Operational Update on COVID-19; pp. 1–10. Available from: http://www.who.int/publications/m/item/weekly-update-on-covid-19 [Last assessed on 2020 Oct 16] [Google Scholar]


