Abstract
Multiple myeloma (MM) is the second most common type of hematological disease with its incidence rising in the elderly. In MM, the extent of the bone disease increases both morbidity and mortality. The detection of lytic bone lesions on imaging, especially computerized tomography (CT) and magnetic resonance imaging (MRI) is crucial to separate asymptomatic from symptomatic MM patients even when no clinical symptoms are present. Although radiology is essential in the staging and management of patients with MM there is still high variability in the choice between MRI and CT. In addition, there is still suboptimal agreement among readers. The potential of medical imaging in MM is largely under-evaluated: artificial intelligence, radiomics and new quantitative methods to report CT and MRI will improve imaging usage.
Keywords: Multiple myeloma, Imaging, Magnetic resonance imaging, Computed tomography, Quantitative imaging
Core Tip: Introduction of new quantitative scores and biomarkers to predict multiple myeloma (MM) prognosis, possibly outperforming current staging methods to create new reliable standards for disease prediction and monitoring is an opportunity for further research in MM imaging.
INTRODUCTION
Multiple myeloma belongs to the so-called plasma cell dyscrasias which are pathological conditions including monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM), and full-blown multiple myeloma (MM)[1]. Epidemiological studies show that, on the one hand, around 5% population over 70 is MGUS carriers and around 1% of them will turn into MM every year. On the other hand, around 10% SMM population evolves into full-blown MM[1]. Finally, the early MM mortality, i.e. the number of MM patients that dye within the first year after diagnosis, is nowadays around 28%, with a peak of 35% among older patients[1]. The single or, more frequently, multiple bone lesions are biologically determined by the proliferation of abnormal cells from a single clone and the excessive and unbalanced activation of osteoclasts eroding the bone starting from the medulla and then reaching the cortical bone and even the extra-osseous soft-tissues. However, MM has a heterogenous genetic architecture which is evident among different patients with the same disease. Genetic heterogenicity is evident also in the same patient where different focal bone lesions may have different genetic patterns[2-4]. MM patients are classically described and defined by the CRAB-criteria (Calcium elevation, Renal insufficiency, Anemia, Bone lesion), indeed symptoms of MM patients vary from bone pain or pathological fractures over renal failure and anemia to calcium elevation and even immune deficiency. It is not known why up to 20% of patients with SMM become symptomatic within 2 years, while one third does not progress to MM within a decade[5], therefore there are several unmet research questions that need to be addressed. In MM patients, having a single focal lesion > 5 mm in diameter identified by mean of computed tomography (CT) or magnetic resonance imaging (MRI) is currently used to identify high-risk SMM patients to upstage them to MM according to the International myeloma working group updated criteria for the diagnosis of multiple myeloma[6]. Therefore, detection of lytic bone lesions on imaging has been recognized crucial since 2003 when the international myeloma working group replaced the classical Durie-Salmon staging system with a more complex and complete revised version called Durie-Salmon plus system. This latter system replaced radiography for identifying bone involvement with the increased sensitivity of MRI, CT or Positron emission tomography (PET)[7]. Therefore, the detection of lytic bone lesions on imaging, especially CT and MRI, is becoming crucial from the clinical viewpoint to separate asymptomatic from symptomatic MM patients. According to Rajkumar et al[8] bone imaging in MM is relevant for diagnosis because osteolytic lesion detection justifies the beginning of a treatment. Medical imaging is required for several reasons: (1) Localization of bone pain; (2) Prevention of complications such as pathologic fractures on long bones (i.e. femur) and vertebral pathological fractures; (3) Identification of focal lesions with high risk of progression; (4) To identify sites of extra-medullary disease; and (5) Identification of sites at potential risk of neurologic complications (Figure 1). In spite of the pivotal role of medical imaging in MM patient care, there is still considerable heterogeneity in clinical practice regarding imaging usage in MM, essentially due to the high variability in the choice between various imaging methods and the high variability in image interpretation[9,10]. In this editorial, the unmet research questions in the usage of imaging in MM are reported and possible future directions are discussed.
Figure 1.
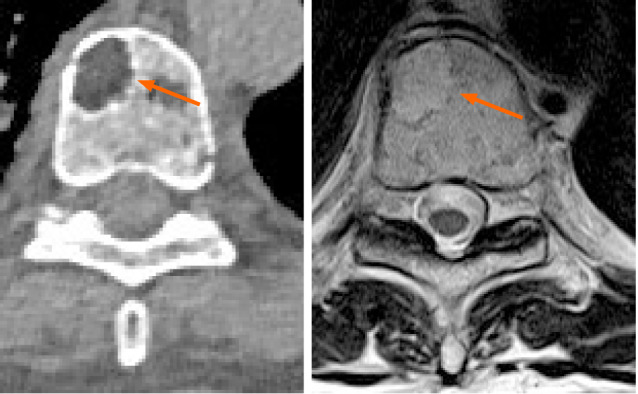
Computed tomography and magnetic resonance imaging of the same lytic lesion located into the vertebral body acquired in the same week for different reasons. No major differences in detection capabilities are evident.
POTENTIAL OF MEDICAL IMAGING IN MM
Firstly, it must be underlined that the detection of lytic bone lesions with a diameter > 5 mm can be done with both CT and MRI and no study directly compared the two modalities regarding patients’ outcomes after CT or MRI. At least in theory, MRI could have some advantages, such as the possibility to introduce functional sequences such as diffusion weighted sequences, but, no clear advantage of one technique over another has been found, even when a systematic review approach was adopted[11,12]. Regelink et al[12] found that there was only few additional lesions detected by both PET and MRI if CT was used as reference test (detection rate 1.00 and 1.00-1.25 respectively). In addition, the review by Regelink et al[12] review was limited by the suboptimal methodological quality of the involved studies due to lack of a technical details. It could be suggested that both MRI and CT have equal diagnostic value and there is no clear advantage to prefer one of the two techniques (Table 1). The scientific community is waiting for thorough comparative future studies, possibly focusing on prognostic value and follow-up. Furthermore, an analysis of multiple bone lesions detected on CT and MRI could be performed using artificial intelligence and radiomics[13]. Up-to-date, radiomics[14] is a quantitative radiological promising technique, with the ultimate goal to improve cancer treatment by improving prognostic capabilities of medical imaging. Radiomics is a complex, quantitative feature-based tool for image analysis described as the conversion of images to higher dimensional data and the subsequent mining of these data for improved decision support[14]. In MM, a recent application of radiomics improved the radiological evaluation of focal and diffuse pattern on CT by increasing the area under the curve of radiologists[15]. Accuracy of radiologists compared to the reference standard was lower (64%) than the accuracy using a radiomics approach (79%)[15]. In addition, machine learning-based classifiers resulted a satisfactory in differentiating MM lesions from those of tumor metastasis of the spine evaluated on MRI[16]. Radiomics was also on PET/CT in MM to elaborate a prognosis model predicting outcome in transplant-eligible newly diagnosed patients[17]. Finally, radiomics has been used with MRI to correlate features with the clinical and hematological response in multiple myeloma patients undergoing systemic treatment. In detail, one textural feature (GLSZM large area low gray level emphasis), in the study by Ekert et al[18] resulted to be correlated also with the bioptic degree of bone marrow infiltration.
Table 1.
Specific advantages and disadvantages or computed tomography and magnetic resonance imaging in multiple myeloma
|
|
Availability
|
Reader expertise
|
Radiation dose
|
Repeatability among different readers
|
Repeatability among different scanners
|
Availability of reporting guidelines
|
Ability to detect > 5 mm focal lesions
|
Exam duration
|
| CT | High | Medium | Similar to total body CT | High | Medium | Low | High | Less than 10 min |
| MRI | Medium | Low | None | Medium | Medium | Low | High | More than 30 min |
CT: Computed tomography; MRI: Magnetic resonance imaging.
CONCLUSION
Introduction of new quantitative scores and biomarkers to refine diagnosis, to predict MM prognosis, possibly outperforming current staging methods to create new reliable standards for disease prediction and monitoring is an opportunity for further research in MM imaging.
Footnotes
Conflict-of-interest statement: Dr. Tagliafico has no conflicts of interest to disclose.
Manuscript source: Invited manuscript
Peer-review started: March 15, 2021
First decision: April 6, 2021
Article in press: June 18, 2021
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Karavaş E, Xiao B S-Editor: Zhang H L-Editor: Filipodia P-Editor: Yuan YY
References
- 1.Jameson J, Fauci A, Kasper D, Hauser S, Longo D. Harrison’s Principles of Internal Medicine. 20th ed. McGraw Hill, 2018. [Google Scholar]
- 2.Rasche L, Chavan SS, Stephens OW, Patel PH, Tytarenko R, Ashby C, Bauer M, Stein C, Deshpande S, Wardell C, Buzder T, Molnar G, Zangari M, van Rhee F, Thanendrarajan S, Schinke C, Epstein J, Davies FE, Walker BA, Meissner T, Barlogie B, Morgan GJ, Weinhold N. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat Commun. 2017;8:268. doi: 10.1038/s41467-017-00296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neben K, Jauch A, Hielscher T, Hillengass J, Lehners N, Seckinger A, Granzow M, Raab MS, Ho AD, Goldschmidt H, Hose D. Progression in smoldering myeloma is independently determined by the chromosomal abnormalities del(17p), t(4;14), gain 1q, hyperdiploidy, and tumor load. J Clin Oncol. 2013;31:4325–4332. doi: 10.1200/JCO.2012.48.4923. [DOI] [PubMed] [Google Scholar]
- 4.Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, Morgan G, Van Ness B, Chesi M, Minvielle S, Neri A, Barlogie B, Kuehl WM, Liebisch P, Davies F, Chen-Kiang S, Durie BG, Carrasco R, Sezer O, Reiman T, Pilarski L, Avet-Loiseau H International Myeloma Working Group. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23:2210–2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, Larson DR, Plevak MF, Jelinek DF, Fonseca R, Melton LJ 3rd, Rajkumar SV. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582–2590. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BG, Miguel JF. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar SV. Evolving diagnostic criteria for multiple myeloma. Hematology Am Soc Hematol Educ Program. 2015;2015:272–278. doi: 10.1182/asheducation-2015.1.272. [DOI] [PubMed] [Google Scholar]
- 8.Hillengass J. Evolving Concepts in the Diagnosis and Staging of Multiple Myeloma. Natl Compr Cancer Netw. 2020;18:1770–1772. [Google Scholar]
- 9.Tagliafico AS, Belgioia L, Bonsignore A, Rossi F, Succio G, Bignotti B, Dominietto A. Subspecialty Second-Opinion in Multiple Myeloma CT: Emphasis on Clinically Significant Lytic Lesions. Medicina (Kaunas) 2020;56 doi: 10.3390/medicina56040195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tagliafico AS, Dominietto A, Belgioia L, Campi C, Schenone D, Piana M. Quantitative Imaging and Radiomics in Multiple Myeloma: A Potential Opportunity? Medicina (Kaunas) 2021;57 doi: 10.3390/medicina57020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caers J, Withofs N, Hillengass J, Simoni P, Zamagni E, Hustinx R, Beguin Y. The role of positron emission tomography-computed tomography and magnetic resonance imaging in diagnosis and follow up of multiple myeloma. Haematologica. 2014;99:629–637. doi: 10.3324/haematol.2013.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regelink JC, Minnema MC, Terpos E, Kamphuis MH, Raijmakers PG, Pieters-van den Bos IC, Heggelman BG, Nievelstein RJ, Otten RH, van Lammeren-Venema D, Zijlstra JM, Arens AI, de Rooy JW, Hoekstra OS, Raymakers R, Sonneveld P, Ostelo RW, Zweegman S. Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: a systematic review. Br J Haematol. 2013;162:50–61. doi: 10.1111/bjh.12346. [DOI] [PubMed] [Google Scholar]
- 13.Fiz F, Marini C, Campi C, Massone AM, Podestà M, Bottoni G, Piva R, Bongioanni F, Bacigalupo A, Piana M, Sambuceti G, Frassoni F. Allogeneic cell transplant expands bone marrow distribution by colonizing previously abandoned areas: an FDG PET/CT analysis. Blood. 2015;125:4095–4102. doi: 10.1182/blood-2015-01-618215. [DOI] [PubMed] [Google Scholar]
- 14.Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tagliafico AS, Cea M, Rossi F, Valdora F, Bignotti B, Succio G, Gualco S, Conte A, Dominietto A. Differentiating diffuse from focal pattern on Computed Tomography in multiple myeloma: Added value of a Radiomics approach. Eur J Radiol. 2019;121:108739. doi: 10.1016/j.ejrad.2019.108739. [DOI] [PubMed] [Google Scholar]
- 16.Xiong X, Wang J, Hu S, Dai Y, Zhang Y, Hu C. Differentiating Between Multiple Myeloma and Metastasis Subtypes of Lumbar Vertebra Lesions Using Machine Learning-Based Radiomics. Front Oncol. 2021;11:601699. doi: 10.3389/fonc.2021.601699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamet B, Morvan L, Nanni C, Michaud AV, Bailly C, Chauvie S, Moreau P, Touzeau C, Zamagni E, Bodet-Milin C, Kraeber-Bodéré F, Mateus D, Carlier T. Random survival forest to predict transplant-eligible newly diagnosed multiple myeloma outcome including FDG-PET radiomics: a combined analysis of two independent prospective European trials. Eur J Nucl Med Mol Imaging. 2021;48:1005–1015. doi: 10.1007/s00259-020-05049-6. [DOI] [PubMed] [Google Scholar]
- 18.Ekert K, Hinterleitner C, Baumgartner K, Fritz J, Horger M. Extended Texture Analysis of Non-Enhanced Whole-Body MRI Image Data for Response Assessment in Multiple Myeloma Patients Undergoing Systemic Therapy. Cancers (Basel) 2020;12 doi: 10.3390/cancers12030761. [DOI] [PMC free article] [PubMed] [Google Scholar]


