Abstract
BACKGROUND
Fractional flow reserve (FFR) measurement is commonly used in the cardiac catheterization laboratory to assess the functional significance of coronary arterial plaques. Robust real-world data on complications and modes of failure of FFR guidewires are limited.
AIM
To characterize these outcomes by analyzing the post-marketing surveillance data from the United States Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database for commonly used FFR guidewires.
METHODS
The MAUDE database was queried from January 2010 through April 2020 for 3 FFR guidewires [PressureWireTM X (Abbott), CometTM (Boston Scientific), and VerrataTM (Philips)] by searching for the following events: “Injury”, “malfunction”, “death”, and “other”. This yielded 544 reports. After excluding incomplete reports, 486 reports were analyzed.
RESULTS
Guidewire tip fracture was the most commonly reported mode of failure, in 174 (35.8%) cases followed by guidewire kinking (n = 152, 31.3%), communication failure (n = 141, 29.0%), and shaft fracture (n = 67, 13.8%). In total, 133 (27.4%) device failures resulted in patient adverse events. The most common adverse event was retained guidewire tip, in 71 (53.4%) cases, followed by freshly deployed stent dislodgment (n = 26, 19.6%) and coronary artery dissection (n = 23, 17.3%). Seven deaths were reported.
CONCLUSION
FFR guidewire failures can occur because of various mechanisms and cause patient adverse events. The MAUDE database serves as an important platform for improved collaboration among clinicians, device manufacturers, and regulators to improve device performance and optimize patient outcomes. Our analysis provides mechanistic insights of FFR guidewire failure and associated adverse events but cannot verify causality or provide a comparison among different guidewires.
Keywords: Fractional flow reserve, Coronary guidewire, Adverse events, Modes of failure, Food and Drug Administration, Manufacturer and user facility device experience
Core Tip: We analyzed post-marketing surveillance data from the Food and Drug Administration Manufacturer and User Facility Device Experience database to outline the most common adverse events and modes of failure encountered with Fractional Flow Reserve (FFR) coronary guidewires. Guidewire tip fracture was the most commonly reported mode of failure, in 35.8% of cases; retained guidewire tip was the most common patient complication (53.4% of cases). FFR is an important frontline measurement in the cardiac catheterization laboratory to assess intracoronary physiology. Our analysis demonstrates that in real-world practice, FFR guidewire failures can occur because of myriad mechanisms and result in patient complications.
INTRODUCTION
Fractional flow reserve (FFR) is an essential measurement in the cardiac catheterization laboratory to assess intracoronary physiology. It is obtained by using a pressure sensing guidewire to calculate flow in the epicardial coronary arteries and determine the functional significance of stenosis. The benefits of an FFR-based revascularization strategy in coronary artery disease are well-established. Landmark clinical trials[1-5] have demonstrated that an FFR-guided decision to perform percutaneous coronary intervention reduces major adverse cardiovascular events and decreases the rate of urgent interventions. Likewise, physicians can safely defer revascularization for FFR-negative lesions[6], sparing patients the risk of invasive procedures and long-term antiplatelet therapy. FFR has considerable clinical advantages and is widely utilized.
Performing FFR requires the insertion of an additional guidewire into the patient’s arterial system, which increases the risk of procedural complications. However, robust real-world data on the complications and modes of failure of commonly used FFR guidewires are limited. We aim to characterize these outcomes by analyzing the post-marketing surveillance data from the United States Food and Drug Administration (FDA) Manufacturer and User Facility Device Experience (MAUDE) database for commonly used FFR guidewires.
MATERIALS AND METHODS
The MAUDE database is an electronic repository created by the FDA to capture major adverse events involving medical devices[7]. Reporting is either mandatory (manufacturers and device-user facilities) or voluntary (medical personnel, patients, and consumers). Developed in the 1990s, the database is updated monthly, with each report containing information on the device, event date, and event description by the provider and the manufacturer. The MAUDE database was queried from January 1, 2010, through April 1, 2020, for three commonly utilized FFR guidewires [PressurewireTM X (Abbott), CometTM (Boston Scientific), and VerrataTM (Philips)] by searching for the following events: “Injury”, “malfunction”, “death”, and “other”. This yielded 544 reports. Each report included a narrative description of the failure event and the results of a standardized inspection of the device if it was returned to the manufacturer. After excluding incomplete reports, duplicate reports, and older devices not in current use, 486 reports were included in the final analysis. This study was conducted from a publicly available database; therefore, an approval from the institutional review board was not required. Patients were not required to give informed consent for the study because the analysis used anonymous clinical data that were obtained from a freely accessible database. Although the MAUDE database is a passive monitoring framework, it can provide important insight into the most commonly reported complications associated with interventional devices. Reports on safety and monitoring of approved interventional devices based on this database have been previously reported[8].
RESULTS
Tables 1 and 2 show a complete list of reported modes of failure and adverse patient events, respectively, categorized by each FFR coronary guidewire. Percentages represent the proportion of total submitted MAUDE reports and do not reflect the incidence rates. Guidewire tip fracture occurred in 174 (35.8%) cases and was the most commonly reported mode of failure. Guidewire kinking was reported in 152 (31.3%) cases, communication failure in 141 (29.0%) cases, and shaft fracture in 67 (13.8%) cases. In total, 133 (27.4%) device failures resulted in adverse patient events. The most common adverse patient events were a retained guidewire tip, in 71 (53.4%) cases, followed by freshly deployed stent dislodgment in 26 (19.6%) cases and coronary artery dissection in 23 (17.3%) cases. Seven patient deaths were reported. The device-related adverse events were most commonly reported during physiologic evaluation of the left anterior descending (LAD) artery, accounting for 54.8% of the reported adverse events. The relative involvement of other target coronary arteries is demonstrated in Figure 1.
Table 1.
Summary of device modes of failure for fractional flow reserve coronary guidewires
|
Device mode of failure
|
VerrataTM (Philips), n = 199
|
CometTM (Boston Scientific), n = 180
|
PressureWireTM X (Abbott), n = 107
|
Total, n = 486
|
| Guidewire distal tip fracture | 55 (27.6) | 68 (37.8) | 51 (47.7) | 174 (35.8) |
| Guidewire kinking | 37 (18.6) | 103 (57.2) | 12 (11.2) | 152 (31.3) |
| Communication failure | 64 (32.2) | 59 (32.8) | 18 (16.8) | 141 (29.0) |
| Failure to advance guidewire | 62 (31.2) | 36 (20.0) | 14 (13.1) | 112 (23.0) |
| Peeled guidewire coating | 6 (3.0) | 83 (46.1) | 0 (0) | 89 (18.3) |
| Guidewire shaft fracture | 25 (12.6) | 22 (12.2) | 10 (9.3) | 67 (13.8) |
Percentages represent proportion of reported events and do not reflect the incidence rates. Results reported as n (%).
Table 2.
Summary of patient adverse events for fractional flow reserve coronary guidewires
|
Adverse patient events
|
VerrataTM (Philips), n = 58
|
CometTM (Boston Scientific), n = 29
|
PressureWireTM X (Abbott), n = 46
|
Total, n = 133
|
| Retained guidewire tip | 27 (46.6) | 22 (75.9) | 22 (47.8) | 71 (53.4) |
| Stent dislodgement | 18 (31.0) | 0 (0) | 8 (17.4) | 26 (19.6) |
| Vessel dissection | 8 (13.0) | 4 (13.8) | 11 (23.9) | 23 (17.3) |
| Death | 2 (3.4) | 2 (6.9) | 3 (6.5) | 7 (5.3) |
| Vessel perforation | 3 (5.2) | 1 (3.5) | 2 (4.4) | 6 (4.5) |
Percentages represent proportion of reported events and do not reflect the incidence rates. Results reported as n (%).
Figure 1.
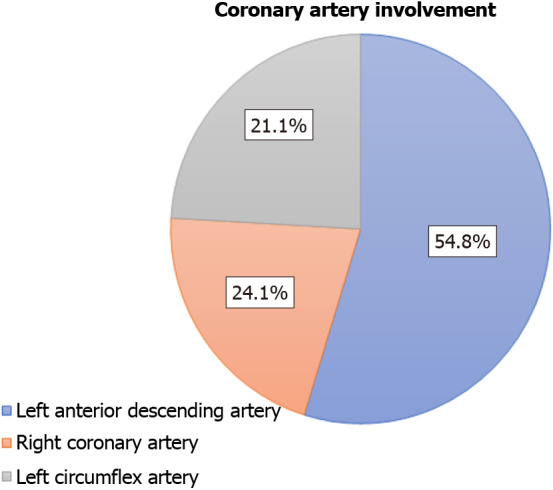
Adverse events stratified by target vessels for fractional flow reserve coronary guidewires.
DISCUSSION
This study demonstrates the modes of failure of commonly used FFR guidewires and highlights the potential for adverse patient outcomes. Two general categories of guidewire failure can occur: structural failure and errors in signal communication. The most common structural failures reported in this study were distal tip fractures (35.8%), kinking (31.3%), peeled coating (18.3%), and shaft fractures (13.8%). A majority of devices in this study failed from more than one of the above mechanisms. Most structural failures were attributed to operator handling issues, defined as having occurred at any point after the device was removed from packaging. Structural failures add complexity for the operator, as demonstrated by 23% of reports noting the inability to advance the guidewire. Structural failures are also directly associated with patient harm: 53% of adverse patient events were related to fractured guidewire tips that remained in the coronary arteries. Retained guidewire fragments increase the risk of dissection, embolization, and thrombus formation and necessitate further interventions[9]. All options, including percutaneous retrieval with a snare, surgical retrieval combined with coronary bypass, or stenting with antiplatelet therapy, increase the risk of further procedural complications and morbidity. Given the high incidence of guidewire fractures, operators should assess the integrity of the device upon removal and ensure that there are no retained pieces in the patient.
The other category of guidewire failure is an error in signal communication, which contributed to 29% of guidewire failures in this study. Pressure sensors at the tip of FFR guidewires transmit data to an external hub that processes information for clinician interpretation. In all three FFR guidewires assessed in this study, communication between the tip and hub occurs via internal cables threaded through the shaft of the guidewire. Errors in signal communication can manifest as the inability to zero the sensor, significant drift, or no signal detected by the hub. The currently available pressure wire sensors are either piezo-electric or optical; these pressure wires differ from routine workhorse wire, as they require integration of thin wires or optical fibers that transmit the pressure signals[10]. While the exact etiologies of communication errors of FFR guidewires were not captured in our data, they can occur after any structural failure that causes sensor or cable damage, or from manufacturing defects. Communication errors are important to detect, as they can result in inaccurate measurements, prolonged procedures, and the need for additional instrumentation.
FFR guidewires can cause patient adverse events. This study demonstrates that vessel dissection, vessel perforation, and stent dislodgement can occur with FFR guidewire use. These are clinically significant complications that must be recognized early and managed carefully to avoid further patient harm. The age of dislodged stents was not documented in the reports; however, operators should exercise caution when advancing or withdrawing an FFR guidewire across any freshly deployed stents. Seven deaths were reported among the cases reviewed. While this is noteworthy, there is not enough information to link the use of FFR guidewires with these deaths.
A majority of all reported events (54.8%) occurred in the LAD, followed by 24.1% in the right coronary artery and 21.1% in the left circumflex artery. A higher rate of reported failures in the LAD may be attributed to generally higher rates of FFR procedures performed in this vessel; however, this information was not captured in our data. Additionally, coronary characteristics such as tortuosity, calcification, and disease severity can also impact individual coronary outcomes. Without this information, the clinical significance of higher reported failures in the LAD is uncertain. Further studies that include data on coronary characteristics and operator technique would be useful to better determine whether FFR guidewires have higher failure rates in any one coronary artery.
Limitations
The MAUDE database has several inherent limitations that impact the interpretation of our study. Notably, only cases with adverse events are reported in the database, and reporting is partially voluntary. Successful cases are not reported, and there is no information on the overall frequency of the device use. Without this information, we cannot derive the incidence of failure rates associated with the use of these guidewires or compare outcomes among different devices. Adverse events may be reported both by users and manufacturers, leading to duplicate reports and difficulty in discriminating. Another notable limitation of the database is that data are provided in a non-standardized narrative form. Not all failed devices were returned to the manufacturer for standard inspections. Without standardized information on the clinical context of failure events, these data cannot be used to imply causality or comparison between procedures or devices.
CONCLUSION
The landmark clinical trials that support the routine frontline use of FFR did not specifically address adverse events related to the use of FFR guidewires. Consequently, the present study is important in understanding the common pitfalls of this widely used procedure. Our analysis demonstrates that real-world use of FFR is associated with complications and adverse patient outcomes. Knowledge of the methods of FFR guidewire failure is critical for operators to develop situational awareness, anticipate difficult situations, assess for common complications, and mitigate adverse patient events. Interpretation of MAUDE data also provides critical feedback to manufacturers and allows for collaboration among clinicians, device manufacturers, and regulators to improve devices and patient outcomes.
ARTICLE HIGHLIGHTS
Research background
Fractional flow reserve (FFR) measurement is an essential tool in the cardiac catheterization laboratory to assess the functional significance of coronary artery lesions. Robust real-world data on the commonly reported complications and modes of failure associated with the FFR guidewires are scarce.
Research motivation
The landmark clinical trials that support routine physiologic lesion assessment with FFR did not specifically address adverse events associated with the use of FFR guidewires. Accordingly, this provided us the impetus to explore common shortcomings with one of the most common applied technology in the cardiac catheterization laboratory. With broadened global utilization of the FFR and newer iterations, standard reporting of adverse events and failure modes may improve patient selection, operator expertise and device technology.
Research objectives
The objective of our study was to investigate the most commonly reported adverse events and failure modes associated with commonly used FFR guidewires by analyzing the post-marketing surveillance data from the United States Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database.
Research methods
We queried the MAUDE database from January 2010 through April 2020 for 3 FFR guidewires [PressureWireTM X (Abbott), CometTM (Boston Scientific), and VerrataTM (Philips)] by searching for the following events: “Injury”, “malfunction”, “death”, and “other”. The search yielded 544 reports. After excluding incomplete and duplicate reports, 486 reports were included in the final analysis.
Research results
The most commonly reported mode of failure was guidewire tip fracture described in 174 (35.8%) cases followed by guidewire kinking (n = 152, 31.3%), communication failure (n = 141, 29.0%), and shaft fracture (n = 67, 13.8%). One hundred thirty-three (27.4%) device failures caused patient adverse events. The most commonly reported adverse event was retained guidewire tip described in 71 (53.4%) cases, followed by freshly deployed stent dislodgment (n = 26, 19.6%) and coronary artery dissection (n = 23, 17.3%). Seven deaths were reported.
Research conclusions
FFR guidewire failures can occur because of myriad mechanisms and cause patient adverse events. Understanding the methods of FFR guidewire failure is critical for interventionalists to develop operational awareness, forebode challenging situations, evaluate common complications, and assuage adverse patient events. The MAUDE database serves as an important pulpit for improved collaboration among physicians, device manufacturers, and regulators to improve device performance and optimize patient outcomes.
Research perspectives
Intermediate coronary lesions are commonly encountered during cardiac catheterization and present a diagnostic dilemma. Physiologic testing using a pressure wire-based system is appropriate for these lesions. The introduction of newer nonhyperemic pressure-based indices of stenosis severity such as instant wave-Free Ratio (iFR) and coregistered iFR pressure mapping may augur a paradigm shift for functional lesion assessment. It is pivotal for interventionalists to familiarize themselves with the common pitfalls associated not only with the standard FFR system but also with the newer iterations.
Footnotes
Institutional review board statement: This study was conducted from a publicly available database, therefore, an approval from the institutional review board was not required.
Informed consent statement: Patients were not required to give informed consent for the study because the analysis used anonymous clinical data that were obtained from a freely accessible database.
Conflict-of-interest statement: We have no financial relationships to disclose.
Manuscript source: Invited manuscript
Peer-review started: January 17, 2021
First decision: February 14, 2021
Article in press: July 5, 2021
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leowattana W S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY
Contributor Information
Nauman Khalid, Department of Interventional Cardiology, St. Francis Medical Center, Monroe, LA 71201, United States.
Yagya Pandey, Department of Internal Medicine, Baylor College of Medicine, Houston, TX 77030, United States.
Umair Khalid, Department of Interventional Cardiology, Michael E. DeBakey VA Medical Center, Houston, TX 77030, United States.
Hassan Kamran, Department of Interventional Cardiology, Baylor College of Medicine, Houston, TX 77030, United States.
Jason P Wermers, Health Sciences, University of Maryland Graduate School, Baltimore, MD 21201, United States.
Lovely Chhabra, Department of Cardiology, Westchester Medical Center Network Advanced Physician Services, New York, NY 12601, United States.
Mahboob Alam, Department of Interventional Cardiology, Baylor College of Medicine, Houston, TX 77030, United States.
Hani Jneid, Department of Interventional Cardiology, Michael E. DeBakey VA Medical Center, Houston, TX 77030, United States.
Waleed Tallat Kayani, Department of Interventional Cardiology, Baylor College of Medicine, Houston, TX 77030, United States. waleed83@gmail.com.
Data sharing statement
No additional data are available.
References
- 1.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van' t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 2.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Elrashidi MY, Flammer AJ, Lennon RJ, Bell MR, Holmes DR, Bresnahan JF, Rihal CS, Lerman LO, Lerman A. Long-term outcomes of fractional flow reserve-guided vs. angiography-guided percutaneous coronary intervention in contemporary practice. Eur Heart J. 2013;34:1375–1383. doi: 10.1093/eurheartj/eht005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smits PC, Abdel-Wahab M, Neumann FJ, Boxma-de Klerk BM, Lunde K, Schotborgh CE, Piroth Z, Horak D, Wlodarczak A, Ong PJ, Hambrecht R, Angerås O, Richardt G, Omerovic E Compare-Acute Investigators. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376:1234–1244. doi: 10.1056/NEJMoa1701067. [DOI] [PubMed] [Google Scholar]
- 5.Ahn JM, Park DW, Shin ES, Koo BK, Nam CW, Doh JH, Kim JH, Chae IH, Yoon JH, Her SH, Seung KB, Chung WY, Yoo SY, Lee JB, Choi SW, Park K, Hong TJ, Lee SY, Han M, Lee PH, Kang SJ, Lee SW, Kim YH, Lee CW, Park SW, Park SJ IRIS-FFR Investigators†. Fractional Flow Reserve and Cardiac Events in Coronary Artery Disease: Data From a Prospective IRIS-FFR Registry (Interventional Cardiology Research Incooperation Society Fractional Flow Reserve) Circulation. 2017;135:2241–2251. doi: 10.1161/CIRCULATIONAHA.116.024433. [DOI] [PubMed] [Google Scholar]
- 6.Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, Stella PR, Boersma E, Bartunek J, Koolen JJ, Wijns W. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103:2928–2934. doi: 10.1161/01.cir.103.24.2928. [DOI] [PubMed] [Google Scholar]
- 7.FDA MAUDE database. [cited 10 January 2021]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm .
- 8.Khalid N, Javed H, Rogers T, Hashim H, Shlofmitz E, Wermers JP, Chen Y, Musallam A, Khan JM, Torguson R, Bernardo NL, Waksman R. Adverse events with orbital atherectomy: an analytic review of the MAUDE database. EuroIntervention. 2020;16:e325–e327. doi: 10.4244/EIJ-D-19-00295. [DOI] [PubMed] [Google Scholar]
- 9.Al-Moghairi AM, Al-Amri HS. Management of retained intervention guide-wire: a literature review. Curr Cardiol Rev. 2013;9:260–266. doi: 10.2174/1573403X11309030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kern MJ. Comparing FFR tools: New wires and a pressure microcatheter. Cath Lab Digest. 2016;24:5. [cited 10 January 2021]. Available from: https://www.cathlabdigest.com/article/Comparing-FFR-Tools-New-Wires-Pressure-Microcatheter . [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.


