Abstract
Sitosterolemia is an inherited metabolic disorder characterized by increased levels of plant sterols, such as sitosterol. This disease is caused by loss-of-function genetic mutations in ATP-binding cassette (ABC) subfamily G member 5 or member 8 ( ABCG5 or ABCG8 , respectively), both of which play important roles in selective excretion of plant sterols from the liver and intestine, leading to failure to prevent absorption of food plant sterols. This disorder has been considered to be extremely rare. However, accumulated clinical data as well as genetics suggest the possibility of a much higher prevalence. Its clinical manifestations resemble those observed in patients with familial hypercholesterolemia (FH), including tendon xanthomas, hyper LDL-cholesterolemia, and premature coronary atherosclerosis. We provide an overview of this recessive genetic disease, diagnostic as well as therapeutic tips, and the latest diagnostic criteria in Japan.
Keywords: Sitosterolemia, ABCG5, ABCG8, Familial hypercholesterolemia
Introduction
Sitosterolemia (OMIM #210250, and #618666) is an autosomal recessive disorder of lipid metabolism characterized by increased absorption and decreased biliary excretion of plant sterols and cholesterol, resulting in prominently elevated serum concentrations of plant sterols, such as sitosterol, campesterol, and stigmasterol (Fig.1) 1, 2) . This condition was first described by Bhattacharyya and Connor in 1974 3) . Patients with sitosterolemia primarily exhibit tendinous and tuberous xanthomas and premature coronary atherosclerosis, resembling these characteristics in patients with familial hypercholesterolemia (FH) 4-9) . Severity of the phenotypes of sitosterolemia appears to be more variable than for FH possibly due to its greater dependency on dietary sterol intake 10, 11) . In addition, they have a wider variety, which includes hemolysis, splenomegaly, platelet abnormalities, and arthralgia/arthritis 12) . This disease is caused by biallelic (homozygous/compound heterozygous) loss-of-function (LOF) mutations in either ATP-binding cassette (ABC) subfamily G member 5 or member 8 ( ABCG5 and ABCG8 , respectively) that play an important role in excreting sterols from the liver and intestine (Fig.1) 13, 14) . Therefore, increased absorption of plant sterols from the intestine and their decreased secretion from the liver are the primary cause of sitosterolemia 15, 16) . Several (adaptive) secondary changes in lipid metabolism have been found to be associated with the accelerated sterol absorption; for example, altered solubilization of sterols in intestinal micelles, increased activity of acyl CoA: cholesterol acyltransferase (ACAT), and changes in intracellular transport processes of sterols.
Fig.1. Schema of sterol metabolism focusing on ABCG5/8 and NPC1L1.
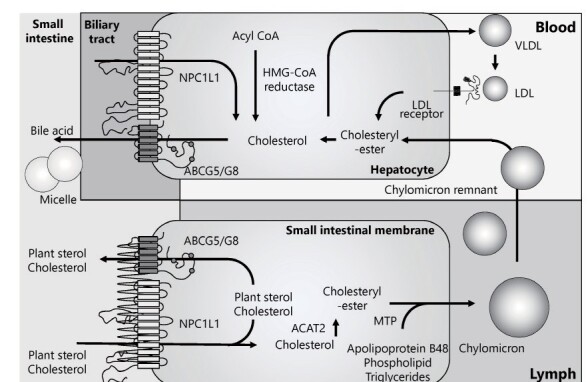
In the intestine, plant sterols and cholesterol are absorbed via NPC1L1, while they are excreted via ABCG5/8. The same pattern is observed in hepatocytes.
Sitosterolemia used to be considered an extremely rare disorder but recent studies indicate the possibility of a much higher prevalence in the general population 6-10) .
Based on the pathophysiology of this disease, ezetimibe, an inhibitor of Niemann-Pick C1 Like 1 (NPC1L1) that mediates absorption of dietary cholesterol in the intestine 17) , has been shown to be effective in reducing serum sitosterol as well as cholesterol in sitosterolemic patients, together with dietary management to restrict intake of these sterols 18 , 19) . In this review article, we discuss the current understanding of sitosterolemia, its diagnostic criteria, and future perspectives.
Plant Sterols
Plant sterols (sitosterol, campesterol, and stigmasterol) are sterol molecules naturally contained at low levels in plant foods such as fruits, vegetables, nuts and cereals 20) . Sitosterol is usually the most abundant plant sterol in the diet 2) and the average Japanese diet and Western diets contain similar amounts of cholesterol and plant sterols. Although approximately 50% of dietary cholesterol is absorbed, less than 5% of plant sterols is absorbed in normal individuals 21-24) , resulting in lower levels of plant sterols than cholesterol in the body. In a recent investigation of plasma concentrations of plant sterols in 667,718 subjects, they seemed to be dependent on age, gender, and apolipoprotein E genotype 25) .
Accumulation of plant sterols in patients with sitosterolemia would contribute to atherogenesis. However, dietary intake of plant sterols is generally considered beneficial for normal individuals as they competitively inhibit cholesterol absorption, which is then selectively excreted resulting in lower cholesterol levels 26) . The European Atherosclerosis Society Consensus Panel currently recommends taking plant sterols for patients with a relatively high risk for cardiovascular disease and/or statin intolerance 20) . In addition, the proinflammatory properties of sitosterol appear to be much weaker than those of cholesterol 27) .
Epidemiology
Sitosterolemia has long been considered an extremely rare disorder. Indeed, only 45 sitosterolemic subjects were reported in a review article published in 2003 28) . Its autosomal recessive inheritance may have caused us to think it is a rare disease. However, the Exome Aggregation Consortium (ExAC) Exome Browser, a public genetic database, has suggested that 1 in ~220 general individuals have LOF mutations in the ABCG5 or ABCG8 gene 29) . Therefore, a rough estimate of the number of homozygous/compound heterozygous patients with sitosterolemia is 1 in ~200,000 general individuals. Moreover, a recent study has shown that a certain proportion of patients clinically diagnosed as FH could in fact have sitosterolemia 30) . Accordingly, this disorder appears to be much more prevalent than previously thought.
Genetic Backgrounds and Pathophysiology
In 2001, the cause of this disease was identified as double LOF mutations in the ABCG5 or ABCG8 gene 13, 14) . So far, most patients with recognized sitosterolemia have come from consanguineous marriages, which has lead to homozygous mutations in the ABCG5 or ABCG8 gene. However, recent advances in genetic analysis have revealed that there are also a number of cases with compound heterozygous mutations in the ABCG5 and ABCG8 genes. Relatively common pathogenic mutations are c.1166G>A/p.Arg389His, and c.1256G>A/p.Arg419His in ABCG5 gene 31-33) .
The ABCG5 and ABCG8 proteins form heterodimers and act as a complex, which functions as a transporter of sterols in the bile and intestine. Accordingly, patients with sitosterolemia exhibit either homozygous or compound heterozygous mutations in the ABCG5 or ABCG8 gene. Besides the above mutations, Tada et al. previously reported a unique case of sitosterolemia caused by double heterozygous mutations in the ABCG5 and ABCG8 genes, suggesting that specific combinations of mutations and/or quite deleterious heterozygous mutations may cause sitosterolemia 34) .
Recent genome-wide association studies (GWAS) indicated that the ABCG5 and ABCG8 genes are significantly associated with LDL cholesterol levels and increased prevalence of coronary artery disease (CAD) 35, 36) , suggesting that these genes contribute to high LDL cholesterol and high plant sterol levels in plasma and risk for CAD among the general population as well. In addition, Tada et al. have recently shown that deleterious mutations of the ABCG5 or ABCG8 gene contribute substantially to mimicking and exacerbation of the FH phenotype 30) .
Clinical Manifestations
Individuals suffering from sitosterolemia primarily present with tendinous and tuberous xanthomas and premature coronary atherosclerosis, resembling those in FH. Therefore, a certain proportion of patients with sitosterolemia could be misdiagnosed as FH due to tendon xanthomas and elevated LDL cholesterol 30) . However, the severity of LDL cholesterol elevation and xanthomas appears to be more variable in sitosterolemia than in FH. In a case of myocardial infarction in a 25-year-old woman previously described by Kawamura et al. 9) , Achilles tendon xanthomas, as well as significantly elevated LDL cholesterol levels and sitosterol levels were found, and she was initially misdiagnosed as FH. However, consideration of the recessive pattern of inheritance, great responsiveness to dietary counseling together with statin plus ezetimibe (LDL cholesterol was reduced from 220 mg/dl to 55 mg/dl) lead to the accurate diagnosis of sitosterolemia.
Typical cases in infancy have also been described. LDL cholesterol levels in FH tend to be constantly high, whereas those in sitosterolemia may vary with the latest dietary intake of sterols. The most extreme cases have been infants who are breastfeeding. They have been found to have cutaneous xanthomas associated with significant elevation in LDL cholesterol levels, resembling those in homozygous FH, 6, 37, 38) . It has been noted that weaning alone can reduce their LDL cholesterol levels, causing the cutaneous xanthomas to regress, despite sitosterol levels that remain significantly elevated. These observations suggest that they are quite vulnerable to a sterol-rich diet, and that dietary management is very important in infants with sitosterolemia. However, we have experienced several independent infantile cases of transient hypercholesterolemia associated with breastfeeding without any signs of cutaneous xanthomas, where the patients turned out to be carriers of heterozygous mutations of the ABCG5 gene (data not shown). Thus, it appears that some infantile cases of “breastfed hypercholesterolemia” can be explained by heterozygous mutations of the ABCG5 gene.
In addition, a variety of other phenotypes, such as hemolysis, splenomegaly, platelet abnormalities, arthralgia/arthritis have been documented among patients with sitosterolemia, and some of them have been shown to be associated with accumulation of sitosterol in an animal model 39) . The underlying mechanism responsible for the hematologic abnormalities observed in some patients with sitosterolemia appears to be accumulation of circulating sterols in blood cell membranes, leading to abnormal morphology and function 40) . Regarding arthralgia/arthritis, the case of a sitosterolemic patient who also had a history of recurrent arthritis has been described. Whole exome sequencing analysis revealed that this patient had another concomitant genetic disorder that had caused familial Mediterranean fever where arthritis is documented as one of the major manifestations 7) .
Since sitosterolemia is a recessive disorder, in many cases there is a consanguineous marriage in the background. This could lead to the coincidence of other recessive genetic disorders, although there is no clear evidence suggesting an association between their genotypes or inheritance patterns and the severity or variety of sitosterolemia phenotypes. Comprehensive genetic analyses in such patients could shed light on the causal (genetic) backgrounds of their phenotypes.
Sitosterol or Cholesterol?
Sitosterolemia was named for the significant elevation in serum sitosterol level in this disease. As sitosterol and other plant sterols have been shown to accumulate in atherosclerotic lesions of patients with sitosterolemia 4, 41) , lowering serum sitosterol has long been considered to be a target for therapy. However, a causative relationship between marked elevation of sitosterol in serum and its tissue deposition and development of atherosclerotic cardiovascular diseases remains to be demonstrated. The results of studies regarding an association between serum sitosterol levels and atherosclerosis have been controversial 42- 45) .
Currently available data as well as the fact that sitosterolemic patients with premature atherosclerotic cardiovascular diseases tend to exhibit hyper-LDL cholesterolemia suggest that LDL cholesterol, rather than sitosterol is the main causal factor for atherogenicity. Therefore, further studies assessing the role of sitosterol in the development of atherosclerosis are needed
Diagnostic Criteria
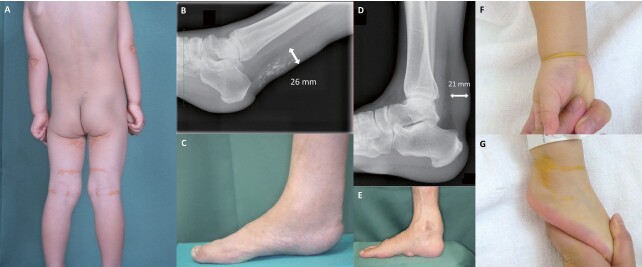
Diagnostic criteria for sitosterolemia in Japan are described in Table 1 . Serum sitosterol levels could be measured using high-sensitive gas chromatography. Their reference ranges in Japanese individuals have been determined as 0.99 - 3.88 µg/mL in males, and 1.03 - 4.45 µg/mL in females 46) . It is vitally important to perform differential diagnosis to distinguish it from FH ( Fig.2A ) , autosomal recessive hypercholesterolemia (ARH) ( Fig.2B, 2C ) and cerebrotendinous xanthomatosis (CTX) ( Fig.2D, 2E ) . It is not easy to make a differential clinical diagnosis of sitosterolemia ( Fig.2F, 2G ) just based on physical manifestations 47-50) .
Table 1. Diagnostic criteria.
|
A.Clinical manifestations 1.Cutaneous or tendon xanthomas 2.Premature coronary artery disease (male <45 yr, female <55 yr) B.Laboratory testing 1.Serum sitosterol ≥ 1 mg/dL (10 µg/mL) C.Differential diagnosis Exclude familial hypercholesterolemia and cerebrotendinous xanthomatosis D.Genetic analysis Pathogenic mutations in ABCG5 or ABCG8 gene |
Definite: fulfills A-1, B-1, C, and D
Probable: fulfills A-1, B-1, and C
Possible: fulfills A-1, A-2, and B-1
Fig.2. Xanthomas in patients with dyslipidemias.
(A) Systemic xanthomas in a patient with homozygous FH (3-year-old boy)
(B) X-ray of Achilles’ tendon in a patient with ARH (67-year-male)
(C) Achilles’ tendon xanthomas in a patient with ARH (67-year-male)
(D) X-ray of Achilles’ tendon in a patient with CTX (63-year-male)
(E) Achilles’ tendon xanthomas in a patient with CTX (63-year-male)
(F) Xanthomas at the ankle in a patient with sitosterolemia (1-year-old girl)
(G) Xanthomas at the wrist in a patient with sitosterolemia (1-year-old girl)
Diagnostic Tips for Sitosterolemia
As stated above, patients with sitosterolemia typically exhibit tendinous and tuberous xanthomas and premature coronary atherosclerosis, resembling the manifestations of FH. Therefore, patients with premature coronary atherosclerosis should be examined to see whether they have a special genetic background including that for sitosterolemia. Absence of a family history of hypercholesterolemia as well as premature CAD is likely to indicate sitosterolemia rather than FH. However, it is of note that some patients with sitosterolemia have a family history of hypercholesterolemia and tendon xanthomas despite its recessive pattern of inheritance. The tendon xanthomas of sitosterolemia tend to be more severe than those of heterozygous FH, despite lower levels of LDL cholesterol. Thus, sitosterolemia should be considered in differential diagnosis for heterozygous FH, which is now considered a relatively frequent genetic metabolic disease. In addition, LDL cholesterol levels of sitosterolemic patients tend to vary dramatically depending on their latest dietary intake of plant sterols, and this would be useful information in making a clinical diagnosis of this disease. ARH and CTX are extremely rare autosomal recessive diseases, and almost all patients with these diseases are from consanguineous marriages. It is sometimes quite difficult to differentiate sitosterolemia from ARH based on a single assessment; however, responsiveness to dietary counseling differs between sitosterolemia and ARH. On the other hand, patients with CTX can be differentiated from those with sitosterolemia based on several factors, such as absence of hypercholesterolemia, chronic diarrhea during childhood, juvenile cataracts, and neurological symptoms 49) .
Management of Sitosterolemia
Restriction of plant sterols as well as cholesterol should be the first line strategy. Sitosterolemic patients should avoid plant sterol-rich foods, such as corn oil, sesame seeds, peanuts, soybeans, rapeseed oil, sesame oil, rice oil, margarine, avocado, chocolate, and shellfish, whereas, other vegetables and fruits, such as potato, carrot, and apple contain less plant sterols 51) . In addition to plant sterols, they also need to avoid cholesterol-rich foods, including animal liver and eggs. Regarding medication, ezetimibe and bile-acid sequestrant resins have been established as standard therapies because the primary cause of this disease is increased absorption of plant sterols from the intestine and their decreased secretion from the liver 15) . Both could reduce sitosterol (~ 20% by ezetimibe, and ~ 30% by resins) 18, 52) and LDL cholesterol in sitosterolemia. Ezetimibe has also been shown to favorably increase platelet count 19) .
Patients with sitosterolemia usually do not respond to statins because HMG-CoA reductase activity is already maximally inhibited 52) . However, statins are effective in reducing LDL cholesterol, at least in some sitosterolemic patients 9, 45) , although they may increase sitosterol levels in others 53) . Considering the lack of a clear association between sitosterol levels and frequency of atherosclerotic cardiovascular disease 44) , as well as the fact that some patients with sitosterolemia are treated with statins due to being misdiagnosed with FH 30) , statins could at least be used for patients in a secondary prevention setting. For patients with advanced atherosclerotic lesions and resistance to the standard treatments mentioned above, LDL apheresis could be considered if applicable, although it is not officially covered by the Japanese national health insurance 8) .
Liver transplantation was performed in a case of sitosterolemia with liver cirrhosis and reportedly resulted in a dramatic reduction in serum plant sterol levels 54) . Regarding target levels of LDL cholesterol, there is plenty of clinical evidence suggesting that lowering LDL cholesterol is associated with reduced risk for atherosclerotic cardiovascular diseases. In addition, sitosterolemia has been considered as a phenocopy of FH and therefore, the target LDL cholesterol level should be the same as that of FH. However, there has been no definite evidence for an association of sitosterol lowering and prevention of atherosclerotic cardiovascular diseases so far. Accordingly, LDL cholesterol, rather than sitosterol, should be the main biomarker when treating patients with sitosterolemia. Dietary restriction of plant sterols, ezetimibe, and bile-acid sequestrant resins have been shown to reduce both LDL cholesterol and sitosterol levels and thus these strategies should be considered as standard treatment for patients with sitosterolemia. The plant sterol content of foods and food ingredients varies widely from 7 mg/100 g in potatoes and tomatoes to 686-952 mg/100 g in corn oil 55) . It is therefore rational to recommend patients with sitosterolemia and reduced function of ABCG5 or ABCG8 to avoid vegetables rich in plant sterols.
Conclusions and Perspectives
Sitosterolemia is a monogenic disorder that has been considered rather rare. However, its prevalence may currently be substantially underestimated 56) , so we should be more careful to identify this disease among hypercholesterolemic patients with xanthomas. In particular, to raise awareness of sitosterolemia among pediatricians and dermatologists, education for them focusing on its typical manifestations is important. Measurement of serum sitosterol is not covered by Japanese national health insurance but we firmly believe that it is reasonable for it to be covered now that we have reference data for serum sitosterol levels among Japanese healthy individuals as well as patients with sitosterolemia. Ideally, prospective randomized controlled trials investigating if specific lowering of serum sitosterol leads to reduced risk for atherosclerotic cardiovascular diseases should be performed. More large-scale observational studies attempting to demonstrate an independent association between sitosterol levels and atherosclerotic cardiovascular diseases are also needed.
To establish the clinical importance of this disease for public health, more accurate prevalence and clinical manifestation data should be accumulated, supported by the health insurance system and comprehensive genetic analyses. Diagnostic criteria of sitosterolemia proposed by the Japanese Ministry of Health, Labor and Welfare scientific research team for hyperlipidemia would facilitate the accumulation of such data on this unique disorder.
Acknowledgments and Notice of Grant Support
This work has been supported by Health, Labour and Welfare Sciences Research Grant for Research on Rare and Intractable Diseases (H30-nanji-ippan-003).
Conflicts of Interest
Atsushi Nohara has nothing to disclose. Hayato Tada has nothing to disclose. Masatsune Ogura has received honoraria from Amgen Inc., Astellas Pharma Inc. Sachiko Okazaki has received scholarship grants from Minophagen Pharmaceutical Co., Ltd., Kowa Company, Ltd. Koh Ono has nothing to disclose. Hitoshi Shimano has nothing to disclose. Hiroyuki Daida has received honoraria from Amgen Inc., Daiichi-Sankyo Co., Ltd., Kowa Co., Ltd., and MSD K.K., Novartis Pharma K.K., Bayer Yakuhin, Ltd. and received clinical research funding from Canon Medical Systems Corporation, Philips Japan, Ltd., Toho Holdings Co., Ltd., Asahi Kasei Corporation, and Inter Reha Co., Ltd. HD has also received scholarship grants from Nippon Boehringer Ingelheim Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sanofi K.K., MSD K.K., Daiichi-Sankyo Co., Ltd., Pfizer Co., Ltd., Mitsubishi Tanabe Pharma Corp., Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Teijin Pharma, Ltd., Shionogi & Co., Ltd., Actelion Pharmaceuticals, Ltd., Actelion Ltd., Kowa Co., Ltd., Bayer Yakuhin, Ltd. HD has also courses endowed by companies, including Philips Japan, Ltd., ResMed, Fukuda Denshi Co., Ltd., and Paramount Bed Co., Ltd. Kazushige Dobashi has nothing to disclose. Toshio Hayashi has nothing to disclose. Mika Hori has nothing to disclose. Kota Matsuki has nothing to disclose. Tetsuo Minamino has nothing to disclose. Shinji Yokoyama has nothing to disclose. Mariko Harada-Shiba has received stock holdings or options from Liid Pharma, honoraria from Amgen Inc., Astellas Pharma Inc., Sanofi, and scholarship grants from Aegerion Pharmaceuticals, Inc., Recordati Rare Diseases Japan, and Kaneka Corporation. Katsunori Ikewaki has nothing to disclose. Yasushi Ishigaki has nothing to disclose. Shun Ishibashi has received honoraria from Kowa Co., Ltd., and a scholarship grant from Ono Pharmaceutical Co., Ltd. Kyoko Inagaki has nothing to disclose. Hirotoshi Ohmura has nothing to disclose. Hiroaki Okazaki has received scholarship grants from Minophagen Pharmaceutical Co., Ltd., Kowa Company, Ltd. Masa-aki Kawashiri has nothing to disclose. Masayuki Kuroda has nothing to disclose. Masahiro Koseki has received clinical research funding from Kowa Company, Ltd., Rohto Pharmaceutical Co., Ltd. Takanari Gotoda has nothing to disclose. Shingo Koyama has nothing to disclose. Yoshiki Sekijima has nothing to disclose. Manabu Takahashi has nothing to disclose. Yasuo Takeuchi has nothing to disclose. Misa Takegami has nothing to disclose. Kazuhisa Tsukamoto has received honoraria from Bayer Yakuhin, Ltd., MSD Ltd., Takeda Pharmaceutical Company Ltd., and scholarship grants from Mitsubishi Tanabe Pharma Corporation., Bayer Yakuhin, Ltd., Sanofi K.K. Atsuko Nakatsuka has nothing to disclose. Kimitoshi Nakamura has nothing to disclose. Satoshi Hirayama has nothing to disclose. Hideaki Bujo has nothing to disclose. Daisaku Masuda has received clinical research funding from MSD K.K., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Kowa Co., Ltd. Takashi Miida has nothing to disclose. Yoshihiro Miyamoto has nothing to disclose. Takeyoshi Murano has nothing to disclose. Takashi Yamaguchi has nothing to disclose. Shizuya Yamashita has received honoraria from Kowa Company, Ltd., MSD K.K. Masashi Yamamoto has nothing to disclose. Koutaro Yokote has received honoraria from Kowa Company, Ltd., MSD K.K., Astellas Pharma Inc., Mitsubishi Tanabe Pharma Corp., Amgen K.K., Takeda Pharmaceutical Company Limited, Sanofi K.K., Ono Pharmaceutical Co., Ltd., AstraZeneca K.K., Daiichi-Sankyo Co., Ltd., Novartis Pharma K.K., Sumitomo Dainippon Pharma Co., Ltd., Kyowa Kirin Co., Ltd., Pfizer Japan Inc., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K., Taisho Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., and received clinical research funding from Taisho Pharmaceutical Co., Ltd. KY has also received scholarship grants from Mitsubishi Tanabe Pharma Corp., Takeda Pharmaceutical Co., Ltd., MSD K.K., Pfizer Japan Inc., Novo Nordisk Pharma Ltd., Taisho Pharmaceutical Co., Ltd., Kao Corporation, Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Daiichi-Sankyo Co., Ltd., Teijin Pharma, Ltd., Shionogi Co., Ltd., Bayer Yakuhin, Ltd. Jun Wada has nothing to disclose.
References
- 1).Escolà-Gil JC, Quesada H, Julve J, Martín-Campos JM, Cedó L, Blanco-Vaca F. Sitosterolemia: diagnosis, investigation, and management. Curr Atheroscler Rep, 2014; 16: 424 [DOI] [PubMed] [Google Scholar]
- 2).Salen G, Shefer S, Nguyen L, Ness GC, Tint GS, Shore V. Sitosterolemia. J Lipid Res, 1992; 33: 945-955 [PubMed] [Google Scholar]
- 3).Bhattacharyya AK, Connor WE. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest, 1974; 53: 1033-1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Salen G, Horak I, Rothkopf M, Cohen JL, Speck J, Tint GS, Shore V, Dayal B, Chen T, Shefer S. Lethal atherosclerosis associated with abnormal plasma and tissue sterol composition in sitosterolemia with xanthomatosis. J Lipid Res, 1985; 26: 1126-1133 [PubMed] [Google Scholar]
- 5).Kolovou G, Voudris V, Drogari E, Palatianos G, Cokkinos DV. Coronary bypass grafts in a young girl with sitosterolemia. Eur Heart J, 1996; 17: 965-966 [DOI] [PubMed] [Google Scholar]
- 6).Tada H, Kawashiri MA, Takata M, Matsunami K, Imamura A, Matsuyama M, Sawada H, Nunoi H, Konno T, Hayashi K, Nohara A, Inazu A, Kobayashi J, Mabuchi H, Yamagishi M. Infantile cases of sitosterolaemia with novel mutations in the ABCG5 Gene: Extreme hypercholesterolaemia is exacerbated by breastfeeding. JIMD Rep, 2015; 21: 115-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Tada H, Kawashiri MA, Okada H, Endo S, Toyoshima Y, Konno T, Nohara A, Inazu A, Takao A, Mabuchi H, Yamagishi M, Hayashi K. A Rare Coincidence of Sitosterolemia and Familial Mediterranean Fever Identified by Whole Exome Sequencing. J Atheroscler Thromb, 2016; 23: 884-890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Sakuma N, Tada H, Mabuchi H, Hibino T, Kasuga H. Lipoprotein apheresis for sitosterolemia. Ann Intern Med, 2017; 167: 896-899 [DOI] [PubMed] [Google Scholar]
- 9).Kawamura R, Saiki H, Tada H, Hara A. Acute myocardial infarction in a 25-year-old woman with sitosterolemia. J Clin Lipidol, 2018; 12: 246-249 [DOI] [PubMed] [Google Scholar]
- 10).Hansel B, Carrié A, Brun-Druc N, Leclert G, Chantepie S, Coiffard AS, Kahn JF, Chapman MJ, Bruckert E. Premature atherosclerosis is not systematic in phytosterolemic patients: severe hypercholesterolemia as a confounding factor in five subjects. Atherosclerosis, 2014; 234: 162-168 [DOI] [PubMed] [Google Scholar]
- 11).Wang J, Joy T, Mymin D, Frohlich J, Hegele RA. Phenotypic heterogeneity of sitosterolemia. J Lipid Res, 2004; 45: 2361-2367 [DOI] [PubMed] [Google Scholar]
- 12).Bastida JM, Benito R, Janusz K, Díez-Campelo M, Hernández-Sánchez JM, Marcellini S, Girós M, Rivera J, Lozano ML, Hortal A, Hernández-Rivas JM, González-Porras JR. Two novel variants of the ABCG5 gene cause xanthelasmas and macrothrombocytopenia: a brief review of hematologic abnormalities of sitosterolemia. J Thromb Haemost, 2017; 15: 1859-1866 [DOI] [PubMed] [Google Scholar]
- 13).Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R, Srivastava AK, Salen G, Dean M, Patel SB. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet, 2001; 27: 79-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Lu K, Lee MH, Hazard S, Brooks-Wilson A, Hidaka H, Kojima H, Ose L, Stalenhoef AF, Mietinnen T, Bjorkhem I, Bruckert E, Pandya A, Brewer HB Jr, Salen G, Dean M, Srivastava A, Patel SB. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am J Hum Genet, 2001; 69: 278-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Salen G, Shore V, Tint GS, Forte T, Shefer S, Horak I, Horak E, Dayal B, Nguyen L, Batta AK, Lindgren FT, Kwiterovich PO Jr. Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J Lipid Res, 1989; 30: 1319-1330 [PubMed] [Google Scholar]
- 16).Lütjohann D, Björkhem I, Beil UF, von Bergmann K. Sterol absorption and sterol balance in phytosterolemia evaluated by deuterium-labeled sterols: effect of sitostanol treatment. J Lipid Res, 1995; 36: 1763-1773 [PubMed] [Google Scholar]
- 17).Yu L. The structure and function of Niemann-Pick C1-like 1 protein. Curr Opin Lipidol, 2008; 19: 263-269 [DOI] [PubMed] [Google Scholar]
- 18).Salen G, von Bergmann K, Lütjohann D, Kwiterovich P, Kane J, Patel SB, Musliner T , Stein P , Musser B ; Multicenter Sitosterolemia Study Group. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation, 2004; 109: 966-971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Othman RA, Myrie SB, Mymin D, Merkens LS, Roullet JB, Steiner RD, Jones PJ. Ezetimibe reduces plant sterol accumulation and favorably increases platelet count in sitosterolemia. J Pediatr, 2015; 166: 125-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Gylling H, Plat J, Turley S, Ginsberg HN, Ellegård L, Jessup W, Jones PJ, Lütjohann D, Maerz W, Masana L, Silbernagel G, Staels B, Borén J, Catapano AL, De Backer G, Deanfield J, Descamps OS, Kovanen PT, Riccardi G, Tokgözoglu L, Chapman MJ; European Atherosclerosis Society Consensus Panel on Phytosterols. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis, 2014; 232: 346-360 [DOI] [PubMed] [Google Scholar]
- 21).Othman RA, Myrie SB, Jones PJ. Non-cholesterol sterols and cholesterol metabolism in sitosterolemia. Atherosclerosis, 2013; 231: 291-299 [DOI] [PubMed] [Google Scholar]
- 22).Bastida JM, Girós ML, Benito R, Janusz K, Hernández-Rivas JM, González-Porras JR. Sitosterolemia: Diagnosis, Metabolic and Hematological Abnormalities, Cardiovascular Disease and Management. Curr Med Chem, 2019; 26: 6766-6775 [DOI] [PubMed] [Google Scholar]
- 23).Hirai K, Shimazu C, Takezoe R, Ozeki Y. Cholesterol, phytosterol and polyunsaturated fatty acid levels in 1982 and 1957 Japanese diets. J Nutr Sci Vitaminol (Tokyo), 1986; 32: 363-372 [DOI] [PubMed] [Google Scholar]
- 24).Ostlund RE Jr, McGill JB, Zeng CM, Covey DF, Stearns J, Stenson WF, Spilburg CA. Gastrointestinal absorption and plasma kinetics of soy Delta(5)-phytosterols and phytostanols in humans. Am J Physiol Endocrinol Metab, 2002; 282: E911-916 [DOI] [PubMed] [Google Scholar]
- 25).Dayspring TD, Varvel SA, Ghaedi L, Thiselton DL, Bruton J, McConnell JP. Biomarkers of cholesterol homeostasis in a clinical laboratory database sample comprising 667,718 patients. J Clin Lipidol, 2015; 9: 807-881 [DOI] [PubMed] [Google Scholar]
- 26).Izar MC, Tegani DM, Kasmas SH, Fonseca FA. Phytosterols and phytosterolemia: gene-diet interactions. Genes Nutr, 2011; 6: 17-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Kurano M, Iso -O N, Hara M, Noiri E, Koike K, Kadowaki T, Tsukamoto K. Plant sterols increased IL-6 and TNF-α secretion from macrophages, but to a lesser extent than cholesterol. J Atheroscler Thromb, 2011; 18: 373-383 [DOI] [PubMed] [Google Scholar]
- 28).Pullinger CR, Kane JP, Malloy MJ. Primary hypercholesterolemia: genetic causes and treatment of five monogenic disorders. Expert Rev Cardiovasc Ther, 2003; 1: 107-119 [DOI] [PubMed] [Google Scholar]
- 29).Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG; Exome Aggregation Consortium. Analysis of protein-coding genetic variation in 60,706 humans. Nature, 2016; 536: 285-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Tada H, Okada H, Nomura A, Yashiro S, Nohara A, Ishigaki Y, Takamura M, Kawashiri MA. Rare and deleterious mutations in ABCG5/ABCG8 genes contribute to mimicking and worsening of familial hypercholesterolemia phenotype. Circ J, 2019; 83: 1917-1924 [DOI] [PubMed] [Google Scholar]
- 31).Niu DM, Chong KW, Hsu JH, Wu TJ, Yu HC, Huang CH, Lo MY, Kwok CF, Kratz LE, Ho LT. Clinical observations, molecular genetic analysis, and treatment of sitosterolemia in infants and children. J Inherit Metab Dis, 2010; 33: 437-443 [DOI] [PubMed] [Google Scholar]
- 32).Yagasaki H, Nakane T, Toda T, Kobayashi K, Aoyama K, Ichikawa T, Sugita K. Carotid intima media thickness in a girl with sitosterolemia carrying a homozygous mutation in the ABCG5 gene. J Pediatr Endocrinol Metab, 2017; 30: 1007-1011 [DOI] [PubMed] [Google Scholar]
- 33).Su X, Shao Y, Lin Y, Zhao X, Zhang W, Jiang M, Huang Y, Zeng C, Liu L, Li X. Clinical features, molecular characteristics, and treatments of a Chinese girl with sitosterolemia: A case report and literature review. J Clin Lipidol, 2019; 13: 246-250 [DOI] [PubMed] [Google Scholar]
- 34).Tada H, Nomura A, Yamagishi M, Kawashiri MA. First case of sitosterolemia caused by double heterozygous mutations in ABCG5 and ABCG8 genes. J Clin Lipidol, 2018; 12: 1164- 1168 [DOI] [PubMed] [Google Scholar]
- 35).Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Do R, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkila K, Hypponen E, Isaacs A, Jackson AU, Johansson A, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikainen LP, Magnusson PKE, Mangino M, Mihailov E, Montasser ME, Muller-Nurasyid M, Nolte IM, O’Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney ASF, Doring A, Elliott P, Epstein SE, Ingi Eyjolfsson G, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJP, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimaki T, Lin SY, Lindstrom J, Loos RJF, Mach F, McArdle WL, Meisinger C, Mitchell BD, Muller G, Nagaraja R, Narisu N, Nieminen TVM, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stan.akova A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YI, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrieres J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Jarvelin MR, Jula A, Kahonen M, Kaprio J, Kesaniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, Marz W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njolstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PEH, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BHR, Ordovas JM, Boerwinkle E, Palmer CNA, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Kathiresan S, Mohlke KL, Ingelsson E, Abecasis GR; Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat Genet, 2013; 45: 1274-1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Lu X, Peloso GM, Liu DJ, Wu Y, Zhang H, Zhou W, Li J, Tang CS, Dorajoo R, Li H, Long J, Guo X, Xu M, Spracklen CN, Chen Y, Liu X, Zhang Y, Khor CC, Liu J, Sun L, Wang L, Gao YT, Hu Y, Yu K, Wang Y, Cheung CYY, Wang F, Huang J, Fan Q, Cai Q, Chen S, Shi J, Yang X, Zhao W, Sheu WH, Cherny SS, He M, Feranil AB, Adair LS, Gordon-Larsen P, Du S, Varma R, Chen YI, Shu XO, Lam KSL, Wong TY, Ganesh SK, Mo Z, Hveem K, Fritsche LG, Nielsen JB, Tse HF, Huo Y, Cheng CY, Chen YE, Zheng W, Tai ES, Gao W, Lin X, Huang W, Abecasis G; GLGC Consortium, Kathiresan S, Mohlke KL, Wu T, Sham PC, Gu D, Willer CJ. Exome chip meta-analysis identifies novel loci and East Asian-specific coding variants that contribute to lipid levels and coronary artery disease. Nat Genet, 2017; 49: 1722-1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Park JH, Chung IH, Kim DH, Choi MH, Garg A, Yoo EG. Sitosterolemia presenting with severe hypercholesterolemia and intertriginous xanthomas in a breastfed infant: case report and brief review. J Clin Endocrinol Metab, 2014; 99: 1512-1518 [DOI] [PubMed] [Google Scholar]
- 38).Wang W, Jiang L, Chen PP, Wu Y, Su PY, Wang LY. A case of sitosterolemia misdiagnosed as familial hypercholesterolemia: A 4-year follow-up. J Clin Lipidol, 2018; 12: 236-239 [DOI] [PubMed] [Google Scholar]
- 39).Kanaji T, Kanaji S, Montgomery RR, Patel SB, Newman PJ. Platelet hyperreactivity explains the bleeding abnormality and macrothrombocytopenia in a murine model of sitosterolemia. Blood, 2013; 122: 2732-2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Su Y, Wang Z, Yang H, Cao L, Liu F, Bai X, Ruan C. Clinical and molecular genetic analysis of a family with sitosterolemia and co‐existing erythrocyte and platelet abnormalities. Haematologica, 2006; 91: 1392-1395 [PubMed] [Google Scholar]
- 41).Helske S, Miettinen T, Gylling H, Mäyränpää M, Lommi J, Turto H, Werkkala K, Kupari M, Kovanen PT. Accumulation of cholesterol precursors and plant sterols in human stenotic aortic valves. J Lipid Res, 2008; 49: 1511-1518 [DOI] [PubMed] [Google Scholar]
- 42).Wilund KR, Yu L, Xu F, Vega GL, Grundy SM, Cohen JC, Hobbs HH. No association between plasma levels of plant sterols and atherosclerosis in mice and men. Arterioscler Thromb Vasc Biol, 2004; 24: 2326-2332 [DOI] [PubMed] [Google Scholar]
- 43).Assmann G, Cullen P, Erbey J, Ramey DR, Kannenberg , Schulte H. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case-control analysis of the Prospective Cardiovascular Münster (PROCAM) study. Nutr Metab Cardiovasc Dis, 2006; 16: 13-21 [DOI] [PubMed] [Google Scholar]
- 44).Genser B, Silbernagel G, De Backer G, Bruckert E, Carmena R, Chapman MJ, Deanfield J, Descamps OS, Rietzschel ER, Dias KC, März W. Plant sterols and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J, 2012; 33: 444-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Hansel B, Carrié A, Brun-Druc N, Leclert G, Chantepie S, Coiffard AS, Kahn JF, Chapman MJ, Bruckert E. Premature atherosclerosis is not systematic in phytosterolemic patients: severe hypercholesterolemia as a confounding factor in five subjects. Atherosclerosis, 2014; 234: 162-168 [DOI] [PubMed] [Google Scholar]
- 46).Yoshida H, Tada H, Ito K, Kishimoto Y, Yanai H, Okamura T, Ikewaki K, Inagaki K, Shoji T, Bujo H, Miida T, Yoshida M, Kuzuya M, Yamashita S. Reference intervals of serum non-cholesterol sterols by gender in healthy Japanese individuals. J Atheroscler Thromb, 2020; 27: 409-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Mabuchi H. Half a Century Tales of Familial Hypercholesterolemia (FH) in Japan. J Atheroscler Thromb, 2017; 24: 189-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Tada H, Inaba S, Pozharitckaia D, Kawashiri MA. Prominent tendon xanthomas and abdominal aortic aneurysm associated with cerebrotendinous xanthomatosis identified using whole exome sequencing. Intern Med, 2018; 57: 1119-1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Sekijima Y, Koyama S, Yoshinaga T, Koinuma M, Inaba Y. Nationwide survey on cerebrotendinous xanthomatosis in Japan. J Hum Genet, 2018; 63: 271-280 [DOI] [PubMed] [Google Scholar]
- 50).Tada H, Kawashiri MA, Ikewaki K, Terao Y, Noguchi T, Nakanishi C, Tsuchida M, Takata M, Miwa K, Konno T, Hayashi K, Nohara A, Inazu A, Kobayashi J, Mabuchi H, Yamagishi M. Altered metabolism of low-density lipoprotein and very-low-density lipoprotein remnant in autosomal recessive hypercholesterolemia: results from stable isotope kinetic study in vivo. Circ Cardiovasc Genet, 2012; 5: 35-41 [DOI] [PubMed] [Google Scholar]
- 51).Moreau RA. Composition of Plant Sterols and Stanols in Supplemented Food Products. J AOAC Int, 2015; 98: 685-690 [DOI] [PubMed] [Google Scholar]
- 52).Nguyen LB, Cobb M, Shefer S, Salen G, Ness GC, Tint GS. Regulation of cholesterol biosynthesis in sitosterolemia: effects of lovastatin, cholestyramine, and dietary sterol restriction. J Lipid Res, 1991; 32: 1941-1948 [PubMed] [Google Scholar]
- 53).Uusitupa MI, Miettinen TA, Happonen P, Ebeling T, Turtola H, Voutilainen E, Pyörälä K. Lathosterol and other noncholesterol sterols during treatment of hypercholesterolemia with lovastatin alone and with cholestyramine or guar gum. Arterioscler Thromb, 1992; 12: 807-813 [DOI] [PubMed] [Google Scholar]
- 54).Miettinen TA, Klett EL, Gylling H, Isoniemi H, Patel SB. Liver transplantation in a patient with sitosterolemia and cirrhosis. Gastroenterology, 2006; 130: 542-547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Piironrn V, Lampi AM. Occurrence and levels of phytosterols in foods. In: Dutta PC, editor. Phytosterols as functional food components and nutraceuticals. New York: Marcel Dekker, Inc; 2004. Pp. 1-32 [Google Scholar]
- 56).Tada H, Nohara A, Inazu A, Sakuma N, Mabuchi H, Kawashiri MA. Sitosterolemia, Hypercholesterolemia, and Coronary Artery Disease. J Atheroscler Thromb, 2018; 25: 783-789 [DOI] [PMC free article] [PubMed] [Google Scholar]