Abstract
Aim: Pneumococcal and influenza infections can cause serious morbidity and mortality in patients with cardiovascular diseases. The purpose of this study was to investigate the safety and efficacy of simultaneous inoculations of 23-valent pneumococcal polysaccharide vaccine (PPSV23) and trivalent influenza vaccine (TIV) in patients with coronary artery disease (CAD).
Methods: This was a prospective, randomized, single-blind, placebo-controlled study. A total of 40 patients with CAD were randomly assigned to the TIV+PPSV23 (simultaneous inoculations of TIV and PPSV23) and TIV+Placebo (inoculations of TIV and placebo) groups. Primary outcomes were the safety of simultaneous vaccinations and the changing of circulating cardiovascular biomarkers before, at 4-, and at 12-weeks after vaccinations.
Results: The baseline characteristics between the two groups were identical. The prevalence of injection-site pain, swelling, and reddening were 47%, 37%, and 37% in the TIV+PPSV23 group, and 10%, 5%, and 0% in the TIV+Placebo group, respectively. All reactions were self-limited. Body temperature >37.0℃ or serious injection-related reaction was not observed. The levels of white blood cells, high-sensitivity C-reactive protein, N-terminal pro-B-type natriuretic peptide, pentraxin-3, and malondialdehide-modified low-density lipoprotein (LDL), were not significantly different between the two groups before and after vaccinations. The levels of anti-oxidized LDL were significantly and step-wisely decreased from baseline, to 4-, and 12-weeks vaccinations in the both groups. No significant changes of other markers were observed in both groups at each time point.
Conclusion: Simultaneous inoculations of TIV and PPSV23 were safety in patients with CAD, suggesting that dual vaccinations can be considered even in patients with CAD.
Keywords: 23-valent pneumococcal polysaccharide vaccine, Influenza vaccine, Inflammation, Oxidized low-density lipoprotein, Coronary artery disease
See editorial vol. 28: 813-815
Introduction
Systemic inflammation is linked to an increased risk of cardiovascular events 1- 3) . Indeed, previous studies demonstrated that acute infections are associated with a transient increase in the risk of myocardial infarction and stroke 4, 5) . Streptococcus pneumoniae and influenza viruses are common pathogens in community-acquired pneumonia, which can cause serious morbidity and mortality in patients with cardiovascular diseases 6, 7) .
It has been reported that inoculation of influenza vaccine is associated with reduced risk of major cardiovascular events 8, 9) . In addition, previous studies, including a meta-analysis, have also demonstrated that inoculation of 23-valent pneumococcal polysaccharide vaccine (PPSV23) is also associated with decreased risk of cardiovascular events and mortality 10- 12) . Several studies have reported that dual inoculations of PPSV23 and influenza vaccine reduced the risk of respiratory infection comparing with inoculation of I influenza vaccine alone 13, 14) . In addition, Hung et al. demonstrated that dual inoculations of PPSV23 and influenza vaccine are effective in preventing of myocardial infarction and stroke in elderly patients with chronic illness 15) . Although the Ministry of Health, Labor and Welfare of Japan has approved of the simultaneous inoculation of PPSV23 and influenza vaccine at the physician’s discretion, simultaneous inoculation is not necessarily a popular clinical practice, especially in elderly patients.
The mechanisms, by which inoculations of PPSV23 and influenza vaccine reduce the risk of cardiovascular events, have been investigated. Acute infections in respiratory system, which can elicit both a systemic and local inflammation, may induce vascular inflammation, promote thrombogenesis, and stimulate biomechanical stress 5, 15- 17) . These pathophysiological events may also induce disruption and trigger thrombosis in a pre-existing vulnerable coronary lesion 16, 17) . A basic study showed that pneumococcal immunization induced high levels of oxidized low-density lipoprotein (oxLDL)-specific IgM antibody, which was cross-reactive with pneumococcal determinants and decreased the extent of atherosclerosis in LDL receptor deficient mice 18) , suggesting that pneumococcal immunization may ameliorate the levels of oxLDL and/or oxLDL-specific antibodies.
However, the safety and efficacy for inflammatory and oxidative responses of simultaneous inoculations of PPSV23 and influenza vaccine in high risk patients for cardiovascular events have not been investigated. Therefore, we conducted a prospective randomized study, which investigate the safety and the changing of inflammatory and oxidative markers after simultaneous inoculations of PPSV23 and influenza vaccine or inoculation of influenza vaccine alone in patients with coronary artery disease (CAD).
Methods
Subjects
We recruited 41 patients with stable CAD, who requested influenza vaccination at regular check-up in the outpatient clinic at Juntendo University Hospital, to participate this study from November 2013 to December 2013. Inclusion criteria were age ≥ 50 years, patients with documented CAD defined as ≥ 50% diameter stenosis by coronary angiography, or history of myocardial infarction, prior percutaneous coronary revascularization, or prior coronary artery bypass surgery. Exclusion criteria were patients treated with PPSV23 previously, patients treated with another vaccination within 3 months, patients with allergy against vaccine component, egg, chicken, and chicken-derived ingredients, patients with acute and severe diseases, patients with liver disorder (aspartate aminotransferase and/or alanine aminotransferase ≥ 2.5 times or larger than upper normal limits), patients with renal disorder (creatinine level ≥ 2.0 mg/dL), history of myocardial infarction within 6 months, history of coronary revascularization within 3 months, patients with congestive heart failure (New York Heart Association Class III or IV), patients with poor control diabetes (HbA1c ≥ 10%), patients with poor control hypertension (systolic blood pressure ≥ 200 mmHg or DBP ≥ 110 mmHg), and patients who judged as ineligible by clinical investigators. No subjects changed either their internal medications or daily dietary habits during the study period. This study was approved by the Ethical Committee of Juntendo University. Subjects received full verbal and written explanations of the nature and purpose of this study, and gave their written informed consent. This study has been registered in the UMIN Clinical Trials Registry System http://www.umin.ac.jp/ctr/ as the trial ID UMIN000012017.
Study Design
This was a prospective, randomized, single-blind, placebo-controlled study. The patients were randomly assigned to the simultaneous inoculations of trivalent influenza vaccine (TIV) (The Chemo-Sero-Therapeutic Research Institute, Kumamoto, Japan) and PPSV23 (Pneumovax NP, MSD, K.K., Tokyo, Japan) (TIV+PPSV23) group and inoculations of TIV and placebo (TIV+Placebo) group using a computer-assisted random numbers table system. A single staff member performed the randomization without any knowledge of the clinical information. In the TIV+PPSV23 group, each patient was simultaneously and subcutaneously immunized with 0.5 ml of TIV and 0.5 ml of PPSV23 in the extensor of right upper arm and the extensor of left upper arm, respectively. In the TIV+Placebo group, each patient was simultaneously and subcutaneously immunized with 0.5 ml of TIV and 0.5 ml of saline in the extensor of right upper arm and the extensor of left upper arm, respectively. The patients were blinded after assignment to vaccinations until 12-week visit. Primary outcomes were related to the safety of simultaneous vaccinations (including changes in body temperature after 14 days and injection-site responses, such as pain, swelling, and reddening, after 5 days) and changes in circulating cardiovascular biomarkers before vaccinations and 4 and 12 weeks thereafter.
Measurements of Body Temperature and Injection-site Responses
We requested all subjects to measure body temperature every morning for 14 days after vaccinations. We also requested to check and record the pain, swelling, and reddening in each injection-site for 5 days after vaccinations. The pain scores 0,1,2, and 3 were defined as none, mild, moderate, and severe, respectively. The reaction sizes of swelling and reddening were measured by a simple measurement ruler.
Blood Sampling and Measurements
Blood sampling was performed before, at 4-, and at 12-weeks after vaccinations. Complete blood count, aspartate aminotransferase, alanine aminotransferase, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), lactate dehydrogenase, creatinine, uric acid, blood glucose, hemoglobin A1c, and high-sensitivity C-reactive protein (hs-CRP), were analyzed by standardized measurement methods. The level of low-density lipoprotein cholesterol (LDL-C) was calculated by Friedewald’s formula. The plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels were measured using a commercially available immunoassay kit (Elecsys proBNP, Roche Diagnostics). For the measurement of the following parameters, we utilized commercially available enzyme immunoassay kits: pentraxin (PTX)-3 was measured by a monoclonal antibody (Perseus Proteomics Inc., Tokyo, Japan); malondialdehide-modified low-density lipoprotein (MDA-LDL) by a monoclonal antibody (Sekisui Medical Co., Ltd., Tokyo, Japan); anti-oxLDL by a monoclonal antibody (Immundiagnostik AG, Bensheim, Germany) 19) .
Statistical Analysis
The results are presented in the mean values±SD. Statistical intergroup differences at the baseline were analyzed by the chi-square test and unpaired Student’s t -test. The differences at the follow-up were analyzed by the two-way ANOVA to detect any significant differences, which were later evaluated by post hoc analysis (i.e., Tukey’s test). All statistical analyses were performed with SPSS version 20 (SPSS, Inc). A P -value of less than 0.05 was considered significant.
Results
Clinical Characteristics of the TIV+PPSV23 and the TIV+Placebo Groups
We randomised 41 patients with stable CAD in this study. One patient withdrew the consent just before inoculations of TIV and PPSV23. Finally, 40 patients (mean age 66 years, 16 males) were enrolled in this study ( Fig.1 ) . The clinical characteristics at enrollment are shown in Table 1 . There were no significant differences in age, gender, body mass index, the prevalence of hypertension, diabetes mellitus, dyslipidemia, smoking history or other clinical profiles between the two groups. The concomitant use of each medication was also identical between the two groups.
Fig.1. Flow Diagram Describing the Study Patients Included in This Study.
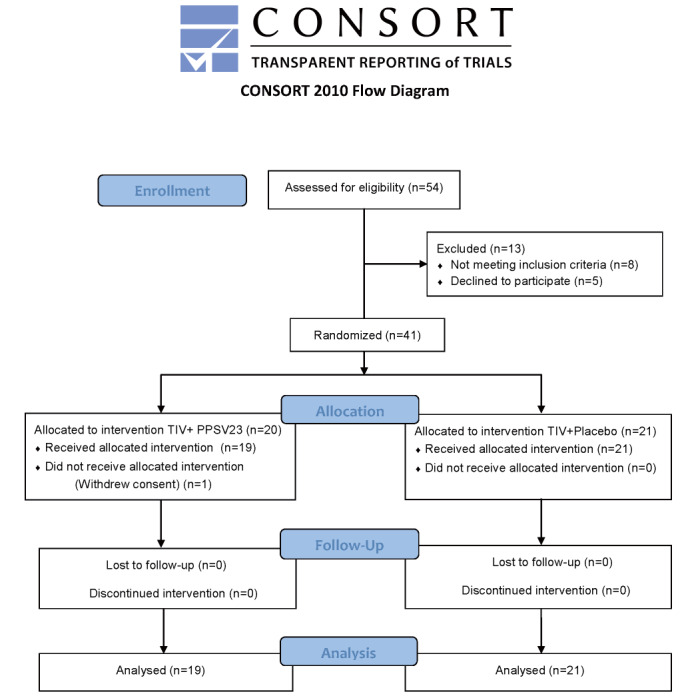
TIV; trivalent influenza vaccine, PPSV23; 23-valent pneumococcal polysaccharide vaccine.
Table 1. Clinical characteristics in the TIV+PPSV23 and the TIV+Placebo groups.
| TIV+PPSV23 ( n = 19) | TIV+Placebo ( n = 21) | P Value | |
|---|---|---|---|
| Age, years | 66±8 | 67±8 | 0.663 |
| Male, (%) | 17 (89) | 18 (86) | 0.720 |
| Body mass index, kg/m 2 | 24.5±4.3 | 24.5±3.4 | 0.992 |
| Hypertension, (%) | 14 (74) | 17 (81) | 0.583 |
| Diabetes mellitus, (%) | 10 (53) | 11 (52) | 0.987 |
| Dyslipidemia, (%) | 18 (95) | 20 (95) | 0.942 |
| Current smoker, (%) | 3 (16) | 6 (29) | 0.334 |
| Family history of premature CAD, | 4 (21) | 6 (29) | 0.583 |
| Previous myocardial infarction, (%) | 8 (42) | 9 (43) | 0.962 |
| Prior PCI, (%) | 10 (53) | 13 (62) | 0.554 |
| Prior CABG, (%) | 4 (21) | 1 (5) | 0.120 |
| No. of diseased vessels | |||
| One, (%) | 5 (26) | 10 (48) | 0.165 |
| Two, (%) | 8 (42) | 8 (38) | 0.796 |
| Three, (%) | 6 (32) | 3 (14) | 0.191 |
| Ejection fraction, % | 64±9 | 64±10 | 0.791 |
| Medications | |||
| Aspirin, (%) | 16 (84) | 19 (90) | 0.550 |
| Thienopyridine, (%) | 7 (37) | 8 (38) | 0.935 |
| Calcium channel blockers, (%) | 11 (58) | 8 (38) | 0.210 |
| Beta-blockers, (%) | 7 (37) | 9 (43) | 0.698 |
| ACE inhibitors or ARB, (%) | 11 (58) | 16 (76) | 0.217 |
| Diuretics, (%) | 3 (16) | 7 (33) | 0.201 |
| Statins, (%) | 17 (89) | 17 (81) | 0.451 |
| Sulfonylurea, (%) | 1 (6) | 4 (19) | 0.188 |
| Thiazolidine, (%) | 1 (6) | 0 (0) | 0.287 |
| Biguanide, (%) | 1 (6) | 3 (14) | 0.342 |
| DPP-IV inhibitors, (%) | 4 (21) | 7 (33) | 0.385 |
| Insuline, (%) | 2 (11) | 0 (0) | 0.127 |
Values are mean±SD. INF = influenza; PC = pneumococcal; CAD = coronary artery disease; PCI = percutaneous coronary intervention; CABG = coronary artery bypass grafting; ACE = angiotensin-converting enzyme; ARB = angiotensin II type 1 receptor blockers; DPP = Dipeptidyl peptidase.
Comparison of Body Temperature between the TIV+PPSV23 and TIV+Placebo Groups
The changing of body temperature in each group is shown in Fig.2 . There were no significant differences in body temperature during 14 days after vaccinations between the two groups.
Fig.2. Comparison of Body Temperature between the TIV+PPSV23 and TIV+Placebo Groups.
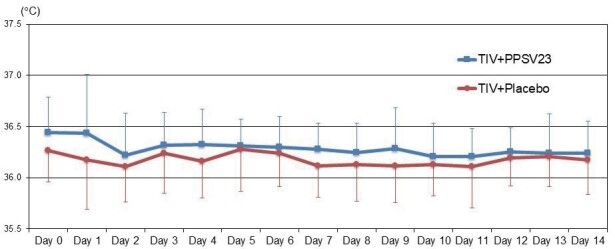
Each error bar (1SD) was demonstrated by one-side because of retativly large values of SD. TIV; trivalent influenza vaccine, PPSV23; 23-valent pneumococcal polysaccharide vaccine.
Changing in Injection-Site Swelling, Reddening, and Pain in the TIV+PPSV23 and TIV+Placebo Groups
The prevalence of injection-site swelling, reddening, and pain in the left upper arm were 37%, 37%, and 47%, in the TIV+PPSV23 group, and 5%, 0%, and 10%, in the TIV+Placebo group, respectively (all P <0.01). The changing of injection-site swelling, reddening, and pain in each group are shown in Fig.3 (right upper arm) and Fig.4 (left upper arm). In the right upper arm, there were no significant differences in swelling, reddening, and pain during 5 days after vaccinations between the two groups. In the left upper arm, the sizes of swelling at Day 1 (1.1±1.6 vs. 0.0±0.0 inch, P <0.01), Day 2 (0.9±1.3 vs. 0.0±0.0 inch, P <0.01), and Day 3 (0.5±0.9 vs. 0.0±0.0 inch, P <0.01), were significantly larger in the TIV+PPSV23 group than those in the TIV+Placebo group. The sizes of reddening at Day 1 (1.1±1.5 vs. 0.1±0.3 inch, P <0.01), Day 2 (0.9±1.4vs. 0.0±0.0 inch, P <0.01), and Day 3 (0.7±1.0 vs. 0.1±0.3 inch, P <0.05), were significantly larger in the TIV+PPSV23 group than those in the TIV+Placebo group. The pain grading at Day 1 (0.8±1.0 vs. 0.0±0.0, P <0.01), Day 2 (0.6±0.7 vs. 0.0±0.2, P <0.01), Day 3 (0.4±0.6 vs. 0.0±0.2, P <0.01), and Day 4 (0.3±0.6 vs. 0.0±0.0, P <0.05), were significantly higher in the TIV+PPSV23 group than those in the TIV+Placebo group. All swelling, reddening, and pain were self-limited.
Fig.3. Changes in Injection-site Swelling, Reddening, and Pain in the TIV+PPSV23 and TIV+Placebo Groups (Right Upper Arm).
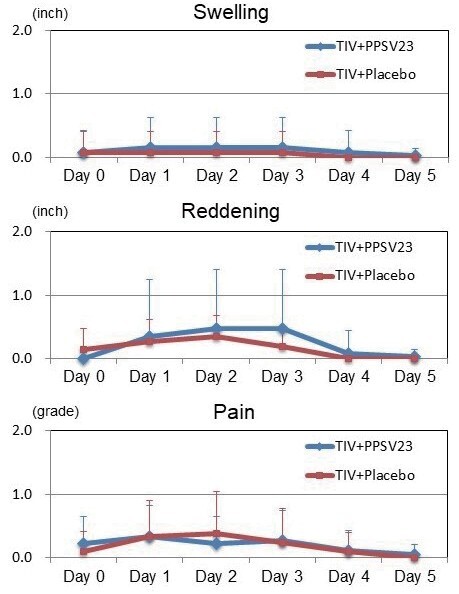
In the right upper arm, no significant differences in swelling, reddening, and pain 5 days after vaccinations was observed between both groups.
Each error bar (1SD) was demonstrated by one-side because of retativly large values of SD. TIV; trivalent influenza vaccine, PPSV23; 23-valent pneumococcal polysaccharide vaccine.
Fig.4. Changes in Injection-site Swelling, Reddening, and Pain in the TIV+PPSV23 and TIV+Placebo Groups (Left Upper Arm).
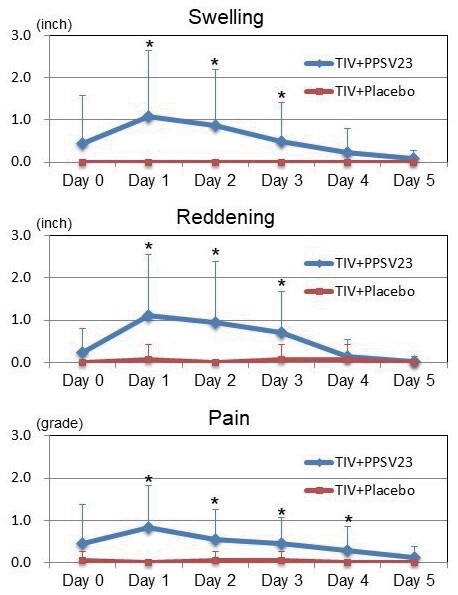
In the left upper arm, the TIV+PPSV23 group had significantly more swelling at Day 1 (1.1±1.6 vs. 0.0±0.0 inches; P <0.01), Day 2 (0.9±1.3 vs. 0.0±0.0 inch; P <0.01), and Day 3 (0.5±0.9 vs. 0.0±0.0 inch; P <0.01), significantly more reddening at Day 1 (1.1±1.5 vs. 0.1±0.3 inches; P <0.01), Day 2 (0.9±1.4 vs. 0.0±0.0 inches; P <0.01), and Day 3 (0.7±1.0 vs. 0.1±0.3 inches; P <0.05), and significantly higher pain grading at Day 1 (0.8±1.0 vs. 0.0±0.0; P <0.01), Day 2 (0.6±0.7 vs. 0.0±0.2; P <0.01), Day 3 (0.4±0.6 vs. 0.0±0.2; P <0.01), and Day 4 (0.3±0.6 vs. 0.0±0.0; P <0.05) compared to the TIV+Placebo group.
Each error bar (1SD) was demonstrated by one-side because of retativly large values of SD. TIV; trivalent influenza vaccine, PPSV23; 23-valent pneumococcal polysaccharide vaccine.
* P <0.05 compared with the TIV+Placebo group.
Changes in Blood Count, Blood Chemistry Parameters, and Inflammatory and Oxidative Markers between the TIV+PPSV23 and TIV+Placebo Groups
The changing of blood count, blood chemistry parameters, and inflammatory and oxidative markers, in each group is shown in Table 2 . All these values except anti-oxLDL levels were not significantly different between the two groups before and after vaccinations. No significant changes of these markers except anti-oxLDL levels were also observed in both groups at each time point. The levels of anti-oxLDL were significantly and step-wisely decreased from baseline, to 4-, and 12-weeks vaccinations in the TIV+PPSV23 group (baseline: 100704936, 4 weeks: 55411773, vs. baseline P <0.05, 12 weeks: 38392014, vs. baseline P <0.01). Same trends of anti-oxLDL levels were observed in the TIV+Placebo group (baseline: 94107377, 4 weeks: 57041186, vs. baseline P <0.05, 12 weeks: 3540864, vs. baseline P <0.01). However, there were no significant differences of anti-oxLDL levels between the two groups in any time-points.
Table 2. Changes of blood count, blood chemistry parameteres, and inflammatory and oxidative markers between the TIV+ PPSV23 and the TIV+Placebo groups.
| TIV+PPSV23 ( n = 19) | TIV+Placebo ( n = 21) | |||||
|---|---|---|---|---|---|---|
| Baseline | 4 weeks | 12 weeks | Baseline | 4 weeks | 12 weeks | |
| Complete blood count | ||||||
| White blood cell count, 10 3 /mL | 6.0±1.9 | 6.3±2.0 | 5.9±1.5 | 6.0±1.1 | 5.9±1.4 | 5.9±1.2 |
| Hemoglobin, g/dL | 14.0±2.1 | 14.1±2.1 | 14.4±1.9 | 14.2±1.3 | 14.2±1.3 | 14.4±1.4 |
| Platelet, 10 3 /mL | 20.1±4.7 | 21.4±4.5 | 22.0±5.1 | 19.3±4.2 | 18.2±5.0 | 20.5±4.1 |
| Blood chemistry parameteres | ||||||
| Aspartate aminotransferase, U/L | 26±12 | 27±13 | 23±8 | 26±13 | 26±11 | 29±17 |
| Alanine aminotransferase, U/L | 25±15 | 26±16 | 22±11 | 29±18 | 29±17 | 29±17 |
| Lactate dehydrogenase, U/L | 197±36 | 193±32 | 191±31 | 187±34 | 184±29 | 184±29 |
| Blood urea nitrogen, g/dL | 16.8±5.0 | 18.2±5.8 | 16.3±5.7 | 16.2±4.2 | 16.7±3.2 | 16.4±3.7 |
| Creatinine, g/dL | 0.83±0.24 | 0.84±0.26 | 0.84±0.32 | 0.80±0.23 | 0.80±0.25 | 0.80±0.22 |
| Uric acid, mg/dL | 5.9±0.9 | 6.1±1.0 | 6.0±0.9 | 5.4±1.3 | 5.4±1.4 | 5.4±1.3 |
| Total cholesterol, mg/dL | 164±25 | 167±20 | 165±21 | 162±29 | 167±32 | 162±23 |
| Triglyceride, mg/dL | 151±78 | 145±52 | 153±62 | 131±85 | 131±62 | 131±56 |
| LDL-cholesterol, mg/dL | 83±21 | 83±21 | 86±19 | 91±25 | 83±21 | 88±24 |
| HDL-cholesterol, mg/dL | 50±15 | 50±12 | 49±12 | 48±11 | 49±13 | 48±10 |
| Fasting blood glucose, mg/dL | 125±35 | 129±64 | 117±36 | 127±30 | 128±39 | 123±35 |
| HbA1c, % | 6.3±0.8 | 6.4±0.8 | 6.5±0.8 | 6.2±0.7 | 6.3±0.8 | 6.4±0.7 |
| NT-proBNP, pg/mL | 431±638 | 404±559 | 373±663 | 236±537 | 187±286 | 164±269 |
| Inflammatory and oxidative markers | ||||||
| High-sensitivity CRP, mg/L | 1.21±1.45 | 1.65±2.33 | 2.97±5.63 | 0.90±1.53 | 1.00±1.32 | 1.46±0.95 |
| PTX-3, ng/mL | 1.88±0.98 | 1.91±0.78 | 1.86±1.17 | 1.94±0.91 | 1.88±0.71 | 1.88±0.95 |
| MDA-LDL, U/L | 110±27 | 113±41 | 124±48 | 103±34 | 103±35 | 109±36 |
| Anti-oxLDL, U/mL | 10070±4936 | 5541±1773 * | 3879±2014 **# | 9410±7377 | 5704±1186 * | 3540±864 **# |
Values are mean±SD. INF = influenza; PC = pneumococcal; CAD = coronary artery disease; LDL = low-density lipoprotein; HDL = high-density lipoprotein; CRP = C-reactive protein; PTX = pentraxin; NT-pro BNP = N-terminal pro brain natriuretic peptide; MDA = malondialdehyde; oxLDL = oxidized LDL
* P <0.05 compared with at baseline
** P <0.005 compared with at baseline
# P <0.05 compared with after 4 weeks
Prevalence of Respiratory Tract Infections and Other Serious Events during the Study Period
During 12 weeks follow up, upper tract infection was observed 3 and 5 subjects in the TIV+PPSV23 and TIV+Placebo groups, respectively. No influenza infection, other vaccine related-serious adverse reactions, cardiovascular events, or other inflammatory diseases were observed during 12 weeks.
Discussion
This study showed that the prevalence of PPSV23 injection-site pain, swelling, and reddening were higher in the TIV+PPSV23 group comparing with the TIV+Placebo group. However, all reactions were mild and self-limited. No severe injection-related reactions or other inflammatory disorders were observed during the study period. To the best of our knowledge, this is the first report, which demonstrated the safety of simultaneous inoculations of PPSV23 and TIV in patients with CAD conducted by a prospective, randomized, single blinded placebo-controlled design.
It has been reported that inoculation of pneumococcal vaccine as well as influenza vaccine reduces the risk of major cardiovascular events 8-12) . In addition, dual inoculations of pneumococcal and influenza vaccines are supposed to additionally decrease the risk of myocardial infarction and stroke as well as respiratory infection comparing with inoculation of influenza vaccine alone 13-15) . However, there are few evidences, which showed the clinical usefulness of simultaneous inoculations of pneumococcal and influenza vaccines in patients at high risk for cardiovascular events. In the present study, we clearly demonstrated the low risk of injection-related reactions even in patients with CAD. Although the vaccination rate of TIV is relatively high, the percentage of PPSV23 inoculation is still low even in Japan 20) . Indeed, the mean vaccination rate of PPSV23 in 2017 was 35.0% in elderly Japanese persons 21) . Therefore, clinical physicians must encourage inoculating pneumococcal vaccine as well as influenza vaccine, especially in patients with cardiovascular disease, who are at high risk for morbidity and mortality of pneumonia and/or cardiovascular events. In these cases, the present study provided evidence that simultaneous inoculations of pneumococcal and influenza vaccines prior to the beginning of the influenza season would be considerably useful and helpful.
In this study period, body temperature >37.0℃ or serious injection-related reaction was not observed. The prevalence of PPSV23 injection-site swelling, reddening, and pain, was significantly higher in the TIV+PPSV23 group than the TIV+Placebo group. The maximum levels of each reaction were also significantly higher in the TIV+PPSV23 group than the TIV+Placebo group. However, all these swelling, reddening, and pain were mild and self-limited. A previous study reported that adverse reactions to immunization in 541 individuals receiving simultaneous inoculations of PPSV23 and TIV, and in 320 recipients of PPSV23 alone 22) . The prevalence of reddening, swelling, and soreness, was 20.7%, 16.2%, and 34.9%, in the PPSV23 and TIV group, and 15.0%, 13.1%, and 30.6%, in the PPSV23 alone group 22) . In the interview form of Pneumovax ® NP, the prevalence of reddening, swelling, and soreness, was reported as 26.2%, 23.1%, and 72.3% 23) . Therefore, we believe that local adverse reaction of simultaneous inoculations of TIV and PPSV23 is limited and acceptable.
The levels of white blood cell, hsCRP, NT-proBNP, PTX-3, and MDA-LDL, were not significantly different between the two groups before and after vaccinations. In addition, no significant changes of these markers were observed in both groups at each time point. These findings suggest that simultaneous inoculations of TIV and PPSV23 as well as TIV administration were safe even in patients with CAD. Meanwhile, the levels of anti-oxLDL IgG antibody were significantly and step-wisely decreased from baseline, to 4-, and 12-weeks vaccinations in both groups. The precise mechanism, by which the levels of anti-oxLDL IgG antibody were decreased after vaccinations, is uncertain. A previous basic study showed that pneumococcal immunization induced oxLDL-specific IgM antibody, which was cross-reactive with pneumococcal determinants 18) . The presence of anti-oxLDL IgG antibody was associated with anti-pneumococcal IgG titer in a clinical study 24) . However, it was reported that pneumococcal vaccination did not increase circulating levels of IgM antibodies to oxLDL 25) . Another study reported that influenza vaccination caused elevations in OxLDL antibody levels at 2 days and 14 days 26) . These discrepancies may be attributed to differences between mice and humans, differences of study subjects, a booster effect of complete Freund adjuvant for mice immunization, differences in anti-oxLDL IgG antibodies, differences in blood sampling time, and seasonal variations in study period. Further study must be needed to clarify these concerns.
Limitation
There are several potential limitations in the present study. First, this was a small sample-sized and single center study. Second, this is a preliminary and pilot study. Therefore, a power calculation was not performed to determine the number of subjects. Third, this study consisted of only subjects who requested influenza vaccination in patients with CAD. Therefore, there may be some selection bias and the study group may not be representative of all patients with CAD. Finally, only IgG antibody, but not IgM, against oxLDL was measured in the present study. Further studies are needed to confirm the findings in this study.
Conclusions
Simultaneous inoculations of PPSV23 and TIV were safety in patients with CAD. These data suggested that simultaneous inoculations of PPSV23 and TIV can be considered even in patients with CAD.
Acknowledgement
This work was supported by a research grant from MSD K.K., a subsidiary of Merck Sharp & Dohme Corp., in accordance with the Investigator Initiated Studies Program of Merck Sharp & Dohme Corp. The opinions expressed in this paper are those of the authors and do not necessarily represent those of MSD K.K. nor Merck Sharp & Dohme Corp. The authors wish to thank each of the participants for their data collection.
Conflict of Interest
K Shimada received lecture fees from MSD K.K. (Tokyo, Japan) and Astellas Pharma Inc. (Tokyo, Japan). K Miyauchi received lecture fees from Amgen Astellas (Tokyo, Japan), Astellas Pharma Inc. (Tokyo, Japan), MSD K.K. (Tokyo, Japan), Bayer Health Care (Tokyo, Japan), Sanofi (Tokyo, Japan), Daiichi-Sankyo (Tokyo, Japan), Boehringer-Ingelheim (Tokyo, Japan), and Bristol-Myers Squibb (Tokyo, Japan). H Daida received lecture fees from Amgen inc (Tokyo, Japan), Sanofi K.K. (Tokyo, Japan), KOWA PHARMACEUTICAL COMPANY LTD (Tokyo, Japan), manuscript fees from Daiichi-Sankyo Company, Ltd. (Tokyo, Japan), research funding from MSD K.K. (Tokyo, Japan), Astellas Pharma Inc. (Tokyo, Japan), TEIJIN PHARMA LTD. (Tokyo, Japan), Nippon Boehringer-Ingelheim Co., Ltd. (Tokyo, Japan), Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan), Bayer Yakuhin Ltd, (Tokyo, Japan), Shionogi & Co., Ltd. (Tokyo, Japan), Sanofi K.K. (Tokyo, Japan), Pfizer Co., Ltd. (Tokyo, Japan), Daiichi-Sankyo Company, Ltd. (Tokyo, Japan), Novartis Pharma K.K. (Tokyo, Japan), Takeda Pharmaceutical Co., Ltd. (Tokyo, Japan), Actelion Pharmaceutical Co., Ltd. (Tokyo, Japan), HeartFlow Japan G.K. (Tokyo, Japan), Bristol-Myers Squibb (Tokyo, Japan), and Nihon Medi-Physics Co., Ltd. (Tokyo, Japan), and scholarship grants from MSD K.K. (Tokyo, Japan), Philips Japan, Ltd. (Tokyo, Japan), TOHO HOLDINGS CO., LTD. (Tokyo, Japan), CANON MEDICAL SYSTEMS CORPORATION (Tokyo, Japan), Philips Respironics GK (Tokyo, Japan), Fukuda Denshi Co., Ltd. (Tokyo, Japan), ResMed, Kowa Company Ltd. (Tokyo, Japan), Nihon Medi-Physics Co., Ltd. (Tokyo, Japan), Daiichi-Sankyo Company, Ltd. (Tokyo, Japan), and Inter Reha Co., Ltd. (Tokyo, Japan). Other authors claimed no conflict of interest.
References
- 1).Shimada K: Immune system and atherosclerotic disease: heterogeneity of leukocyte subsets participating in the pathogenesis of atherosclerosis. Circ J, 2009; 73: 994-1001 [DOI] [PubMed] [Google Scholar]
- 2).Yagita Y: Cholesterol and Inflammation in Stroke Recurrence. J Atheroscler Thromb, 2019; 26: 406-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Kitagawa K, Hosomi N, Nagai Y, Kagimura T, Ohtsuki T, Maruyama H, Origasa H, Minematsu K, Uchiyama S, Nakamura M, Matsumoto M; J-STARS collaborators : Cumulative effects of LDL cholesterol and CRP levels on recurrent stroke and TIA. J Atheroscler Thromb, 2019; 26: 432-441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Spodick DH, Flessas AP, Johnson MM: Association of acute respiratory symptoms with onset of acute myocardial infarction: prospective investigation of 150 consecutive patients and matched control patients. Am J Cardiol, 1984; 53: 481-482 [DOI] [PubMed] [Google Scholar]
- 5).Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P: Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med, 2004; 351: 2611-2618 [DOI] [PubMed] [Google Scholar]
- 6).Musher DM, Rueda AM, Kaka AS, Mapara SM: The association between pneumococcal pneumonia and acute cardiac events. Clin Infect Dis, 2007; 45: 158-165 [DOI] [PubMed] [Google Scholar]
- 7).Corrales-Medina VF, Suh KN, Rose G, Chirinos JA, Doucette S, Cameron DW, Fergusson DA: Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med, 2011; 8: e1001048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Johnstone J, Loeb M, Teo KK, Gao P, Dyal L, Liu L, Avezum A, Cardona-Munoz E, Sleight P, Fagard R, Yusuf S; Ongoing Telmisartan Alone and in Combination With Ramipril Global EndPoint Trial (ONTARGET); Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) Investigators: Influenza vaccination and major adverse vascular events in high-risk patients. Circulation, 2012; 126: 278-286 [DOI] [PubMed] [Google Scholar]
- 9).Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, Ciszewski A, Vakili H, Hoffman EB, Farkouh ME, Cannon CP: Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA, 2013; 310: 1711-1720 [DOI] [PubMed] [Google Scholar]
- 10).Lamontagne F, Garant MP, Carvalho JC, Lanthier L, Smieja M, Pilon D: Pneumococcal vaccination and risk of myocardial infarction. CMAJ, 2008; 179: 773-777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Eurich DT, Johnstone JJ, Minhas-Sandhu JK, Marrie TJ, Majumdar SR: Pneumococcal vaccination and risk of acute coronary syndromes in patients with pneumonia: population-based cohort study. Heart, 2012; 98: 1072-1077 [DOI] [PubMed] [Google Scholar]
- 12).Vlachopoulos CV, Terentes-Printzios DG, Aznaouridis KA, Pietri PG, Stefanadis CI: Association between pneumococcal vaccination and cardiovascular outcomes: a systematic review and meta-analysis of cohort studies. Eur J Prev Cardiol, 2015; 22: 1185-1199 [DOI] [PubMed] [Google Scholar]
- 13).Furumoto A, Ohkusa Y, Chen M, Kawakami K, Masaki H, Sueyasu Y, Iwanaga T, Aizawa H, Nagatake T, Oishi K : Additive effect of pneumococcal vaccine and influenza vaccine on acute exacerbation in patients with chronic lung disease. Vaccine, 2008; 26: 4284-4289 [DOI] [PubMed] [Google Scholar]
- 14).Kawakami K, Ohkusa Y, Kuroki R, Tanaka T, Koyama K, Harada Y, Iwanaga K, Yamaryo T, Oishi K: Effectiveness of pneumococcal polysaccharide vaccine against pneumonia and cost analysis for the elderly who receive seasonal influenza vaccine in Japan. Vaccine, 2010; 28: 7063-7069 [DOI] [PubMed] [Google Scholar]
- 15).Hung IF, Leung AY, Chu DW, Leung D, Cheung T, Chan CK, Lam CL, Liu SH, Chu CM, Ho PL, Chan S, Lam TH, Liang R, Yuen KY: Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clin Infect Dis, 2010; 51: 1007-1016 [DOI] [PubMed] [Google Scholar]
- 16).Corrales-Medina VF, Madjid M, Musher DM: Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis, 2010; 10: 83-92 [DOI] [PubMed] [Google Scholar]
- 17).Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, Siadaty MS, Sanati S, Casscells W: Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein e-deficient mice. Circulation, 2003; 107: 762-768 [DOI] [PubMed] [Google Scholar]
- 18).Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ: Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between streptococcus pneumoniae and oxidized LDL. Nat Med, 2003; 9: 736-743 [DOI] [PubMed] [Google Scholar]
- 19).Moohebati M, Kabirirad V, Ghayour-Mobarhan M, Esmaily H, Tavallaie S, Akhavan Rezayat A, Pourghadamyari H, Sahebkar A: Investigation of serum oxidized low-density lipoprotein IgG levels in patients with angiographically defined coronary artery disease. Int J Vasc Med, 2014; 2014: 845960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Naito T, Matsuda N, Tanei M, Watanabe Y, Watanabe A: Relationship between public subsidies and vaccination rates with the 23-valent pneumococcal vaccine in elderly persons, including the influence of the free vaccination campaign after the Great East Japan Earthquake. J Infect Chemother, 2014; 20: 450-453 [DOI] [PubMed] [Google Scholar]
- 21).https: //www.mhlw.go.jp/topics/bcg/other/5.html (in Japanese, May 30, 2020 accessed) [Google Scholar]
- 22).Socan M, Frelih T, Janet E, Petras T, Peternelj B: Reactions after pneumococcal vaccine alone or in combination with influenza vaccine. Vaccine, 2004; 22: 3087-3091 [DOI] [PubMed] [Google Scholar]
- 23).https: //www.msdconnect.jp/static/mcijapan/images/if_pneumovaxnp.pdf (in Japanese, May 30, 2020 accessed) [Google Scholar]
- 24).Suthers B, Hansbro P, Thambar S, McEvoy M, Peel R, Attia J: Pneumococcal vaccination may induce anti-oxidized low-density lipoprotein antibodies that have potentially protective effects against cardiovascular disease. Vaccine, 2012; 30: 3983-3985 [DOI] [PubMed] [Google Scholar]
- 25).Damoiseaux J, Rijkers G, Tervaert JW: Pneumococcal vaccination does not increase circulating levels of IgM antibodies to oxidized LDL in humans and therefore precludes an anti-atherogenic effect. Atherosclerosis, 2007; 190: 10-11 [DOI] [PubMed] [Google Scholar]
- 26).Liuba P, Aburawi E, Pesonen E, Andersson S, Truedsson L, Ylä-Herttuala S, Holmberg: Residual adverse changes in arterial endothelial function and LDL oxidation after a mild systemic inflammation induced by influenza vaccination. Ann Med, 2007; 39: 392-399 [DOI] [PubMed] [Google Scholar]


