Abstract
Aims: We aimed to develop and validate risk prediction models to estimate the absolute 10-year risk of death from coronary heart disease (CHD), stroke, and cardiovascular disease (CVD).
Methods: We evaluated a total of 44,869 individuals aged 40–79 years from eight Japanese prospective cohorts to derive coefficients of risk equations using cohort-stratified Cox proportional hazard regression models. Discrimination (C-index) of the equation was examined in each cohort and summarised using random-effect meta-analyses. Calibration of the equation was assessed using Hosmer-Lemeshow chi-squared statistic.
Results: Within a median follow-up of 12.7 years, we observed 765 deaths due to CVD (276 CHDs and 489 strokes). After backward selection, age, sex, current smoking, systolic blood pressure (SBP), proteinuria, prevalent diabetes mellitus, the ratio of total cholesterol to high-density lipoprotein cholesterol (TC/HDLC), interaction terms of age by SBP, and age by current smoking were retained as predictors for CHD. Sex was excluded in the stroke equation. We did not consider TC/HDLC as a risk factor for the stroke and CVD equations. The pooled C-indices for CHD, stroke, and CVD were 0.83, 0.80, and 0.81, respectively, and the corresponding p -values of the Hosmer-Lemeshow tests were 0.18, 0.003, and 0.25, respectively.
Conclusions: Risk equations in the present study can adequately estimate the absolute 10-year risk of death from CHD, stroke, and CVD. Future work will evaluate the system as an education and risk communication tool for primary prevention of CHD and stroke.
Keywords: Coronary heart disease, Stroke, Cardiovascular disease, Mortality, Prediction model
See editorial vol. 28: 811-812
Introduction
Cardiovascular disease (CVD) is one of the leading causes of death, accounting for 31.8% of total global deaths in 2017, which has increased by 21.1% over the past decade along with population aging 1 , 2) . There is a pressing need for early CVD prevention to reduce increasing disease burden. A risk prediction model based on routinely collected medical data is a common tool for identifying high-risk individuals and planning interventions 3 - 5) . However, existing risk equations, which were derived mainly from white or black descendants in the USA or European countries, reportedly perform poorly in other populations of different risk levels 6 - 8) . For example, Japan experienced a rapid decline in haemorrhagic stroke occurrence 9) until around 1990 and has since attained the lowest incidence and mortality rate of CVD among the Organisation for Economic Cooperation and Development countries. In addition, the case mix of CVD in Japan is also substantially different from that of Western populations 10 , 11) , with stroke and lacunar stroke being more prevalent than coronary heart disease (CHD) 12 , 13) and large-artery occlusion 14 , 15) , respectively. Therefore, it would be relevant to construct ethnicity-specific prediction models of CVD outcomes based on more recent epidemiological data.
Although mortality is influenced by advances in medical technology and accessibility and quality of health care under any given incidence, CVD death rates are reportedly well proportioned to occurrences in specific populations 16) . Risk equations using death as the outcome can be easily recalibrated and updated for different populations because death statistics are usually more readily available and reliable than disease incidence. In addition, differences in surveillance methods may introduce systematic bias when associating risk factors with these endpoints, as well as absolute risks. However, it is often difficult to employ or find studies using common incidence surveillance methods between different countries and even within a country.
To date, there are only three risk equations available in the primary medical setting to predict absolute risk of CVD death: the NIPPON DATA 80 risk chart, SCORE 11) , and Globorisk 17) . The latter two were derived from individual meta-analysis of multiple cohorts in Western countries with a wide span in baseline year (1972–1991 and 1948–1993, respectively), and are the most widely used risk charts. The NIPPON DATA 80 risk chart for Japan was also developed early with a baseline year of 1980 18) .
Aim
In this pooled analysis of individual-level data for 44,869 Japanese, encompassing residential (rural and urban) and occupational cohorts with baseline year 1988–2002, we aimed to develop and validate risk prediction equations to estimate the absolute 10-year risk of death from CHD, stroke, and CVD combined. In addition to conventional predictors employed in earlier studies, we considered proteinuria 19) and interaction terms for all variables by sex and age 20) .
Methods
Study Population
Of the 15 cohorts participating in the Evidence for Cardiovascular Prevention from Observational Cohorts in Japan research group (EPOCH-JAPAN), we excluded one cohort with a baseline year earlier than 1985, one cohort with a median follow-up of less than 10 years, and five cohorts without baseline information on high-density lipoprotein cholesterol (HDLC), proteinuria, and prevalent diabetes mellitus (DM). The remaining cohorts consisted of two urban community-based cohorts (Suita, Osaka), three rural community-based cohorts (Hisayama, JMS, Osaki), one nation-wide general population sample cohort (NIPPON DATA 90), and two occupational cohorts (YKK, Aichi), with a total of 34,552 women and 31,214 men. We further excluded those aged <40 years or ≥ 80 years ( N =10,579), those with missing data for any risk factor ( N =8,392), and those with reported history of CVD at baseline ( N =1,926), leaving 23,378 women and 21,491 men for the final analysis.
All participants provided informed consent. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethical Review Committee of Keio University (20110192), and Shiga University of Medical Science (23-125-1).
Follow-Up
Subjects in each cohort were followed up prospectively from baseline. For some cohorts, causes of death were determined by reviewing the death certificate or referring to the National Vital Statistics with further review of medical records or autopsy reports. The underlying cause of death was coded according to the International Classification of Disease (ICD) for National Vital Statistics based on the criteria proposed by the World Health Organization. These classifications were based on the ICD-9 until the end of 1994 and on the ICD-10 from the beginning of 1995. In the present study, death from CHD was defined as ICD-9:410-414, ICD-10: I20-I25; death from stroke was defined as ICD-9: 430-438, ICD-10: I60-I69; and death from CVD was defined as a combination of CHD and stroke.
Statistical Analysis
Age, sex, systolic blood pressure (SBP), current smoking, proteinuria, and prevalent DM were considered as potential predictors for all end-points, whereas blood lipid indices including total cholesterol (TC), HDLC, non-HDLC, and ratio of TC to HDLC (TC/HDLC) were considered only for CHD, i.e., not for stroke or CVD, due to the inverse relationship observed for intracerebral haemorrhagic and ischemic strokes 4) . Non-normally distributed continuous variables, namely age, SBP, and blood lipid indices, were logarithmically transformed to obtain a better fit. Prevalent diabetes mellitus was defined by the World Health Organization diagnostic criteria as any one of the following essential conditions: fasting blood glucose ≥ 126 mg/dl, non-fasting blood glucose ≥ 200 mg/dl, HbA1c ≥ 6.5%, or using medication for diabetes. Female sex, non-current smoking status, negative or trace proteinuria, and non-prevalent DM per cohort was set as the reference group. Data were analysed collectively in men and women, but the model also included sex. All interaction terms of all variables listed above by sex and age were considered as potential predictors in the risk prediction model.
Cox proportional hazards regression models stratified by cohort were used to estimate variables coefficients, assuming variable effects were constant across cohorts. Since study area and cohort are highly correlated in the present study, we did not treat area as an additional stratification variable or an adjusted confounder. The statistical procedure used to determine the final predictors for each end point was similar to that used in the Framingham Study multivariable statistical models to estimate CHD risk 21) . All significant ( p <0.10) risk factors and interaction terms identified in a series of univariable models were entered into a multivariate model with backward selection procedure (retention criteria: p <0.10). The mean baseline survival rate S 0 (t) at the 10-year follow-up from each cohort was derived and then averaged by weighting the corresponding number of deaths from each cohort to obtain the pooled baseline survival function.
The discrimination of the equation was estimated using Harrell’s overall C-index for survival analysis 22) and standard error was calculated following the method introduced by Pencina et al. 23) . A C-index using 100-sample bootstrap sampling was calculated within each individual cohort, which was then pooled using a random-effect meta-analysis to obtain the pooled C-index. The model was calibrated by ranking subjects into deciles of predicted risk, then comparing observed and predicted event percentages within the 10-year follow-up according to a modified Hosmer-Lemeshow chi-squared statistic introduced by D’Agostino and Nam 24) with eight degrees of freedom.
Since absolute risk is often difficult to interpret, we present sex- and five-year age group-specific reference values defined as the average of the estimated risks of all individuals in that category in the present study.
All analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
The baseline mean (standard deviation) values for age, SBP, TC, HDLC, and TC/HDLC were 57.0 (10.1) years, 131.1 (19.8) mmHg, 204.8 (36.7) mg/dl, 53.4 (14.1) mg/dl, and 4.1 (3.2), respectively. The proportion (%) of men, current smokers, those with proteinuria, and those with prevalent DM were 47.9, 26.7, 2.8, and 4.6, respectively ( Table 1 ) .
Table 1. Baseline characteristics of the participating cohorts (means (standard deviation) or percentage), EPOCH-JAPAN Study.
| Cohort | N | Baseline year | Age (years) | Men (%) | Current smoking |
SBP (mmHg) |
TC (mg/dl) |
HDLC (mg/dl) |
TC/ HDLC |
Proteinuria + |
Prevalent DM + |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hisayama | 2,475 | 1988 | 57.5 (10.4) | 42.5 | 25.2 | 132.4 (20.4) | 207.0 (42.0) | 50.4 (11.7) | 4.3 (1.2) | 5.7 | 8.8 |
| NIPPON DATA 90 | 5,367 | 1990 | 56.1 (10.5) | 42.8 | 28.1 | 138.3 (20.3) | 206.6 (37.9) | 53.8 (15.4) | 4.1 (1.4) | 2.8 | 4.3 |
| YKK | 2,797 | 1990 | 47.1 (5.3) | 67.4 | 39.2 | 119.2 (15.7) | 202.6 (35.0) | 52.9 (13.2) | 4.1 (1.2) | 4.0 | 2.5 |
| Suita | 5,016 | 1991 | 58.6 (10.7) | 47.5 | 29.3 | 129.8 (22.0) | 210.8 (37.1) | 52.7 (13.8) | 4.3 (1.3) | 6.1 | 4.6 |
| JMS | 9,535 | 1994 | 57.3 (9.1) | 38.7 | 21.9 | 130.3 (20.7) | 194.2 (34.7) | 51.2 (12.9) | 4.0 (1.2) | 1.8 | 3.0 |
| Osaki | 10,898 | 1995 | 61.5 (9.4) | 47.6 | 26.0 | 131.2 (17.5) | 204.0 (35.2) | 51.7 (12.8) | 4.2 (1.2) | 1.7 | 5.5 |
| Osaka | 4,767 | 1996 | 57.3 (9.6) | 36.3 | 23.7 | 135.4 (20.5) | 213.4 (36.7) | 59.4 (14.8) | 4.3 (9.3) | 2.0 | 3.5 |
| Aichi | 4,014 | 2002 | 50.1 (5.6) | 81.2 | 31.1 | 127.4 (15.7) | 211.7 (34.8) | 58.7 (15.8) | 3.8 (1.1) | 2.3 | 6.7 |
| Overall | 44,869 | — | 57.0 (10.1) | 47.9 | 26.7 | 131.1 (19.8) | 204.8 (36.7) | 53.4 (14.1) | 4.1 (3.2) | 2.8 | 4.6 |
SBP indicates systolic blood pressure; TC, total cholesterol, HDLC, high-density lipoprotein cholesterol; DM, diabetes mellitus
During a median follow-up of 12.7 years, 765 (325 women, 440 men) died from CVD (276 from CHD (91 women, 185 men) and 489 from stroke (234 women, 255 men). Supplementary Table 1 shows the crude hazard ratios of each risk factor for each end point. Neither TC nor non-HDLC were significant predictors of CHD death in the crude model ( p >0.10). After backward variable selection, the final model for CHD included age, sex, SBP, current smoking, proteinuria, prevalent DM, TC/HDLC, and interactions of age by SBP and age by current smoking. HDLC did not remain a significant predictor of CHD death in the multivariable model with or without TC/HDLC. The final model for stroke included age, SBP, current smoking, proteinuria, prevalent DM, and interactions of age by SBP and age by current smoking. Further, the same predictors, as well as sex, were retained in the final model for CVD. All interaction terms of sex by variables were removed from the final models. The coefficients and standard errors of all predictors for each end point are presented in Table 2 .
Supplementary Table 1. Univariable hazard ratios (HRs) and 95% confidence intervals (95% CIs) for death from coronary heart disease, stroke and cardiovascular disease, EPOCH-JAPAN Study.
| Coronary heart disease | Stroke | Cardiovascular disease | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Ln-age (year) | 1,469 (533-4,050) | <0.0001 | 2,486 (1,130-5,468) | <0.0001 | 2,047 (1,098-3,815) | <0.0001 |
| Men vs. women | 2.65 (2.06-57.06) | <0.0001 | 1.43 (1.19-15.25) | <0.0001 | 1.77 (1.53-60.09) | <0.0001 |
| Current- vs. non-smoking | 2.07 (1.63-35.26) | <0.0001 | 1.40 (1.16-11.93) | 0.0006 | 1.62 (1.40-40.57) | <0.0001 |
| Prevalent DM (+) vs. (-) | 2.69 (1.82-25.09) | <0.0001 | 2.26 (1.67-27.42) | <0.0001 | 2.41 (1.90-51.64) | <0.0001 |
| Proteinuria vs. (trace/-) | 3.04 (1.95-24.38) | <0.0001 | 2.72 (1.90-30.11) | <0.0001 | 2.83 (2.15-54.25) | <0.0001 |
| Ln-SBP (mmHg) | 34.86 (16.16-75.20) | <0.0001 | 28.40 (15.77-51.15) | <0.0001 | 30.63 (19.20-48.87) | <0.0001 |
| Ln-TC/HDLC | 1.75 (1.20-2.42) | 0.003 | — | — | — | — |
Ln indicates natural logarithm; DM, diabetes mellitus; SBP, systolic blood pressure; TC, total cholesterol; HDLC, high density lipoprotein cholesterol
Table 2. Coefficients and standard errors of variables included in the final model from multivariable Cox hazard regression analysis, EPOCH-JAPAN Study.
| Coronary heart disease | Stroke | Cardiovascular disease | ||||
|---|---|---|---|---|---|---|
| β (standard error) | p value | β (standard error) | p value | β (standard error) | p value | |
| Ln-age (years) | 61.19918 (15.75274) | 0.0001 | 37.40606 (12.30594) | 0.002 | 45.54988 (9.75022) | <0.0001 |
| Men vs. women | 0.65869 (0.14501) | <0.0001 | — | — | 0.30093 (0.08433) | 0.0004 |
| Current- vs. non-smoking | 15.36389 (4.34143) | 0.0004 | 8.24292 (3.27491) | 0.01 | 10.64932 (2.59050) | <0.0001 |
| Prevalent DM (+) vs. (-) | 0.56252 (0.19977) | 0.005 | 0.45679 (0.15697) | 0.004 | 0.49413 (0.12339) | <0.0001 |
| Proteinuria vs. (trace/-) | 0.58243 (0.22974) | 0.01 | 0.63621 (0.18467) | 0.0006 | 0.62120 (0.14385) | <0.0001 |
| Ln-SBP (mmHg) | 46.36999 (13.31927) | 0.0005 | 26.39953 (10.44811) | 0.01 | 33.30729 (8.26619) | <0.0001 |
| Ln-TC/HDLC | 0.35931 (0.18845) | 0.057 | — | — | — | — |
| Ln-age×Ln-SBP | –10.61448 (3.17158) | 0.0008 | –5.92290 (2.48755) | 0.02 | –7.54461 (1.96807) | 0.0001 |
| Ln-age×Current smoking | –3.51974 (1.03357) | 0.0007 | –1.84505 (0.78233) | 0.02 | –2.41998 (0.61808) | <0.0001 |
| S 0 (t) | 0.9981 | 0.9961 | 0.9942 | |||
Ln indicates natural logarithm; DM, diabetes mellitus; SBP, systolic blood pressure; TC, total cholesterol; HDLC, high-density lipoprotein cholesterol
Women aged 70–74 years on average had a 1.04%, 2.55%, and 3.25% risk of death from CHD, stroke, and CVD within 10 years, respectively ( Table 3 ) . Men aged 65–69 years had a 1.38%, 1.78%, and 3.03% risk of death from CHD, stroke, and CVD, respectively.
Table 3. Sex-specific mean 10-year risk (%) of death from coronary heart disease, stroke, and cardiovascular disease according to 5-year age groups, EPOCH-JAPAN Study.
| Age range (years) | Coronary heart disease | Stroke | Cardiovascular disease | |||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| 40–44 | 0.008 | 0.06 | 0.02 | 0.06 | 0.03 | 0.11 |
| 45–49 | 0.02 | 0.12 | 0.07 | 0.13 | 0.08 | 0.24 |
| 50–54 | 0.06 | 0.26 | 0.17 | 0.28 | 0.21 | 0.51 |
| 55–59 | 0.13 | 0.50 | 0.36 | 0.58 | 0.45 | 1.03 |
| 60–64 | 0.28 | 0.89 | 0.74 | 1.09 | 0.94 | 1.89 |
| 65–69 | 0.55 | 1.38 | 1.39 | 1.78 | 1.76 | 3.03 |
| 70–74 | 1.04 | 2.29 | 2.55 | 3.01 | 3.25 | 5.05 |
| 75–80 | 1.84 | 3.63 | 4.38 | 4.83 | 5.60 | 8.00 |
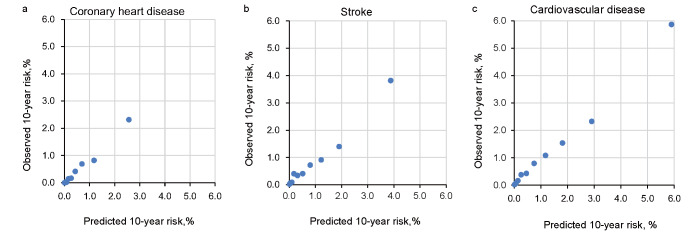
The pooled C-index at 10-year follow-up for CHD, stroke, and CVD were 0.83 (0.66–1.05), 0.80 (0.67–0.95) and 0.81 (0.70–0.93), respectively. There was no heterogeneity in discrimination across individual cohorts in all three end points. The p -values of Hosmer-Lemeshow tests indicating model calibration for CHD, stroke, and CVD were 0.18, 0.003, and 0.25, respectively ( Fig.1a–c ) . When model performance was measured according to sex, the corresponding C-indices were 0.86 (0.56–1.32), 0.79 (0.61–1.02), and 0.81 (0.65–1.00) for women, and 0.78 (0.58–1.06), 0.79 (0.61–1.03), and 0.79 (0.65–0.95) for men, respectively. The corresponding p -values of the Hosmer-Lemeshow test were 0.27, 0.002, and 0.04 for women, and 0.51, 0.49, and 0.25 for men, respectively (data not shown).
Fig.1.
Calibration plots for death from coronary heart disease (a), stroke (b), and cardiovascular disease (c), showing predicted and observed 10-year risk according to deciles of predicted 10-year risk. Solid points denote each decile.
(a). Coefficient of the regression=0.8725, constant of the regression= –0.0238, R 2 =0.9866. Calibration for x 2 =11.39, p =0.18.
(b). Coefficient of the regression=0.9289, constant of the regression=–0.0172, R 2 =0.9745. Calibration for x 2 =23.69, p =0.003.
(c). Coefficient of the regression=0.9573, constant of the regression=–0.0249, R 2 =0.9889. Calibration for x 2 =10.20, p =0.25.
Discussion
We developed risk equations to estimate the absolute 10-year risk of death from CHD, stroke, and CVD using relatively contemporary healthy Japanese population cohorts. The models performed well in men and women for discrimination across all end points. Deciles of predicted 10-year risk generally matched well with observed risk for all end points in men, but are likely suboptimal for stroke and CVD in women.
Prediction models for CHD, stroke, and CVD incidence have been reported in Japan 25 - 29) . The primary advantage when using mortality as the endpoint is that mortality statistics are readily available domestically in Japan as well as internationally. Further, they can be standardized across different populations, which facilitates model recalibration to different times and populations 30) . We recognize that it would be straightforward to develop an incidence model that aids primary prevention effort. However, mortality models can be more easily updated or recalibrated for specific places at different times.
Regardless of the types of endpoints, these risk-estimation systems, including ours, can aid decision-making for clinicians to deliver appropriate care for patients according to their absolute risks and also allow them to better communicate disease risks with patients to help modify behaviour, including receiving pharmacological interventions. However, the benefits of implementing such systems to better control risk factors have not yet been entirely elucidated. One approach is to present risk as years of life lost for the individual’s actual age 31 , 32) . As a previous study from Japan proposed the use of vascular age 33) , we have presented average risks of all individuals in five-year age groups in each sex as reference risk values to better communicate a patient’s risks.
The present study included common risk factors for CVD and observed positive associations of age, SBP, current smoking, and prevalent DM with CHD and stroke death. The finding is consistent with those of previous studies using incidence or mortality of CHD, stroke, or CVD as end-points 34) . The stronger associations for SBP, current smoking, and prevalent DM with CHD death than stroke death were consistent with findings from another larger scale contemporary Japanese study 35) . Further prospective studies supported interaction effect of age by SBP on CHD or stroke 20 , 36) , as well as age by current smoking on CHD 37) or stroke 38) .
While proteinuria has been increasingly recognised as an important risk factor for CHD 19 , 39) , stroke 19) , and CVD 40) across diverse populations, it has never been incorporated into existing risk prediction models for long-term CVD. Because proteinuria can be easily and inexpensively detected using a urine dipstick test, our predictive model that included proteinuria can be applied to primary care settings.
TC is the most widely used variable related to lipid metabolism in prediction models for incident CVD 34) or CVD death, including SCORE, Globorisk, and NIPPON DATA 80 11 , 17 , 18) . Indeed, the US latest lipid guidelines 41) recommended lipid screening for primary prevention of CVD with TC and HDLC. We examined several combinations of lipid markers in the present study and found that only TC/HDLC but not TC, HDLC, or non-HDLC, was a significant predictor of CHD death. This finding is consistent with the SCORE model that included TC/HDLC. Although the latest Japanese guidelines recommend using LDLC for screening 4) , we did not examine LDLC as it could not be calculated using the Friedewald equation 42) in cohorts without fasting blood sampling. The reasons for not observing positive associations of TC or non-HDLC alone with CHD are unclear; however, we found that low TC was a poor prognostic factor in CHD patients 43 , 44) , most likely because we used CHD death as the outcome. Another possibility may be the broader use of statins for primary and secondary CHD prevention, as the present study consisted of more recent cohorts compared to previous SCORE 11) and NIPPON DATA 80 cohorts 18) . However, limited data on cholesterol-lowering therapy during follow-up in the present study inhibited further interpretation.
Our stroke model included similar predictors to those in previous Japanese studies of stroke incidence 33) and showed good discrimination performance. However, our stroke prediction model may lack accuracy when predicting stroke death in women. Specifically, it generally overestimated risk across risk deciles. Whether further data such as antihypertensive or cholesterol-lowering medication, or female specific risk factors such as menopause, would serve to generate better goodness of fit for stroke death when building the prediction model warrants further investigation.
The C-index of the model for CVD death in the current study (0.81) was superior to those reported from SCORE’s TC/HDLC risk chart 11) for low-risk region (0.75), SCORE’s TC risk chart (0.74), and Globorisk 17) for fatal CVD (0.76). Yet we could not compare our results with those in the NIPPON DATA 80 18) as it did not report internal validation results. The C-indices of CHD and the stroke model in the present study were 0.85 and 0.80, which were comparable to prediction models in a recent large-scale Japanese cohort: incident CHD 29) , 0.81 and incident stroke 33) , 0.78.
The present study is subject to some limitations. First, developing the risk equation relied solely on baseline measurements of risk factors, which may have attenuated effect size estimates. Second, our model did not include medication for dyslipidemia due to unavailable variables in many of our cohorts, which may have led to less accurate predictions.
Our study integrated individual participant data from multiple high-quality prospective cohorts using a relatively recent baseline year. The large sample size enabled us to build separate prediction models for CHD and stroke. Including a diverse population encompassing residential (urban and rural) and occupational cohorts ensured a representative sample of the general population, which increased model applicability to the overall Japanese population. In addition, we constructed models that included proteinuria, an approach that may expand the use of proteinuria in primary care settings and allow more accurate risk stratification.
In conclusion, the EPOCH-JAPAN risk prediction models created in the present study can adequately estimate the absolute 10-year risk of death from CHD, stroke, CVD. Therefore, they can be used as education and risk communication tools for primary prevention of CHD and stroke. Further risk factors in addition to those used in the present study may be needed for more accurate predictions for stroke in women.
Acknowledgements
The Evidence for Cardiovascular Prevention from Observational Cohorts in Japan (EPOCH–JAPAN) Research Group is composed of the following investigators. Co-Chairperson: Hirotsugu Ueshima (Shiga University of Medical Science); Co–Chairperson: Tomonori Okamura (Keio University School of Medicine); Co–Chairperson: Yoshitaka Murakami (Toho University).
Executive committee: Hirotsugu Ueshima (Shiga University of Medical Science), Yutaka Imai (Tohoku Institute for Management of Blood Pressure), Takayoshi Ohkubo (Teikyo University School of Medicine), Fujiko Irie (Ibaraki Prefecture), Hiroyasu Iso (Osaka University Graduate School of Medicine), Akihiko Kitamura (Tokyo Metropolitan Institute of Gerontology), Toshiharu Ninomiya (Kyushu University Graduate School of Medicine), Yutaka Kiyohara (Hisayama Research Institute for Lifestyle Diseases), Katsuyuki Miura (Shiga University of Medical Science), Sachiko Tanaka (Shiga University of Medical Science), Yoshitaka Murakami (Toho University), Hideaki Nakagawa (Kanazawa Medical University), Masaru Sakurai (Kanazawa Medical University), Takeo Nakayama (Kyoto University School of Public Health), Akira Okayama (Research Institute of Strategy for Prevention), Toshimi Sairenchi (Dokkyo Medical University), Shigeyuki Saitoh (Sapporo Medical University), Hirofumi Ohnishi (Sapporo Medical University), Kiyomi Sakata (Iwate Medical University), Akiko Tamakoshi (Hokkaido University Faculty of Medicine), Ichiro Tsuji (Tohoku University Graduate School of Medicine), Michiko Yamada (Radiation Effects Research Foundation), Masahiko Kiyama (Osaka Center for Cancer and Cardiovascular Disease Prevention), Yoshihiro Miyamoto (National Cerebral and Cardiovascular Center), Shizukiyo Ishikawa (Jichi Medical University), Hiroshi Yatsuya (Nagoya University), and Tomonori Okamura (Keio University School of Medicine).
Funding
This research was supported by a grant–in–aid from the Ministry of Health, Labor and Welfare, Health and Labor Sciences research grants, Japan (Research on Health Services: H17–Kenkou–007; Comprehensive Research on Cardiovascular Disease and Life–Related Disease: H18–Junkankitou [Seishuu]–Ippan–012; H19–Junkankitou [Seishuu]–Ippan–012; H20–Junkankitou [Seishuu]–Ippan–013; H23–Junkankitou [Seishuu]–Ippan–005; H26-Junkankitou [Seisaku]-Ippan-001 and H29–Junkankitou–Ippan–003.
COI
The authors have no COI to disclose.
References
- 1).O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, Lopez-Jaramillo P, Damasceno A, Langhorne P, McQueen MJ, Rosengren A, Dehghan M, Hankey GJ, Dans AL, Elsayed A, Avezum A, Mondo C, Diener HC, Ryglewicz D, Czlonkowska A, Pogosova N, Weimar C, Iqbal R, Diaz R, Yusoff K, Yusufali A, Oguz A, Wang X, Penaherrera E, Lanas F, Ogah OS, Ogunniyi A, Iversen HK, Malaga G, Rumboldt Z, Oveisgharan S, Al Hussain F, Magazi D, Nilanont Y, Ferguson J, Pare G, Yusuf S and INTERSTROKE Investigators: Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet, 2016; 388: 761-775 [DOI] [PubMed] [Google Scholar]
- 2).McGill HC, Jr., McMahan CA and Gidding SS: Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation, 2008; 117: 1216-1227 [DOI] [PubMed] [Google Scholar]
- 3).Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC, Jr., Virani SS, Williams KA, Sr., Yeboah J and Ziaeian B: 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol, 2019; 74: 1376-1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S, Committee for E and Clinical Management of A: Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I and Verschuren WMM: [2016 European guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts. Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation]. G Ital Cardiol (Rome), 2017; 18: 547-612 [DOI] [PubMed] [Google Scholar]
- 6).D’Agostino RB, Sr., Grundy S, Sullivan LM, Wilson P and CHD Risk Prediction Group: Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA, 2001; 286: 180-187 [DOI] [PubMed] [Google Scholar]
- 7).Sawano M, Kohsaka S, Okamura T, Inohara T, Sugiyama D, Watanabe M, Nakamura Y, Higashiyama A, Kadota A, Okud N, Murakami Y, Ohkubo T, Fujiyoshi A, Miura K, Okayama A, Ueshima H, National Integrated Project for Prospective Observation of Non-Communicable Disease and its Trends in the Aged (NIPPON DATA 80) Research Group: Validation of the european SCORE risk chart in the healthy middle-aged Japanese. Atherosclerosis, 2016; 252: 116-121 [DOI] [PubMed] [Google Scholar]
- 8).DeFilippis AP, Young R, McEvoy JW, Michos ED, Sandfort V, Kronmal RA, McClelland RL and Blaha MJ: Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J, 2017; 38: 598-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Statistics and Information Department, Ministry of Health, Labour and Welfare. Vital Statistics of Japan, 1951-2013. Tokyo: MHLW [Google Scholar]
- 10).Goff DC, Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr., Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr., Sorlie P, Stone NJ and Wilson PWF: 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 2014; 63: 2935-2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetiere P, Jousilahti P, Keil U, Njolstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM and SCORE project group: Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J, 2003; 24: 987-1003 [DOI] [PubMed] [Google Scholar]
- 12).Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y, Watanabe M, Kadota A, Okuda N, Kadowaki T, Nakamura Y and Okamura T: Cardiovascular disease and risk factors in Asia: a selected review. Circulation, 2008; 118: 2702-2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, Gotoh S, Fukuhara M, Ikeda F, Shikata K, Yoshida D, Yonemoto K, Kamouchi M, Kitazono T and Kiyohara Y: Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the Hisayama Study (1961-2009). Circulation, 2013; 128: 1198-1205 [DOI] [PubMed] [Google Scholar]
- 14).Yatsuya H, Yamagishi K, North KE, Brancati FL, Stevens J, Folsom AR and Atherosclerosis Risk in Communities Study Investigators: Associations of obesity measures with subtypes of ischemic stroke in the ARIC Study. J Epidemiol, 2010; 20: 347-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Li Y, Yatsuya H, Iso H, Yamagishi K, Saito I, Kokubo Y, Sawada N and Tsugane S: Body Mass Index and Risks of Incident Ischemic Stroke Subtypes: The Japan Public Health Center-Based Prospective (JPHC) Study. J Epidemiol, 2019; 29: 325-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Saito I, Yamagishi K, Kokubo Y, Yatsuya H, Iso H, Sawada N, Inoue M and Tsugane S: Association between mortality and incidence rates of coronary heart disease and stroke: The Japan Public Health Center-based prospective (JPHC) study. Int J Cardiol, 2016; 222: 281-286 [DOI] [PubMed] [Google Scholar]
- 17).Hajifathalian K, Ueda P, Lu Y, Woodward M, Ahmadvand A, Aguilar-Salinas CA, Azizi F, Cifkova R, Di Cesare M, Eriksen L, Farzadfar F, Ikeda N, Khalili D, Khang YH, Lanska V, Leon-Munoz L, Magliano D, Msyamboza KP, Oh K, Rodriguez-Artalejo F, Rojas-Martinez R, Shaw JE, Stevens GA, Tolstrup J, Zhou B, Salomon JA, Ezzati M and Danaei G: A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): a pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol, 2015; 3: 339-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).NIPPON DATA80 Research Group: Risk assessment chart for death from cardiovascular disease based on a 19-year follow-up study of a Japanese representative population. Circ J, 2006; 70: 1249-1255 [DOI] [PubMed] [Google Scholar]
- 19).Irie F, Iso H, Sairenchi T, Fukasawa N, Yamagishi K, Ikehara S, Kanashiki M, Saito Y, Ota H and Nose T: The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int, 2006; 69: 1264-1271 [DOI] [PubMed] [Google Scholar]
- 20).Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, Kaptoge S, Whitlock G, Qiao Q, Lewington S, Di Angelantonio E, Vander Hoorn S, Lawes CM, Ali MK, Mozaffarian D, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group, Asia-Pacific Cohort Studies Collaboration (APCSC), Diabetes Epidemiology: Collaborative analysis of Diagnostic criteria in Europe (DECODE), Emerging Risk Factor Collaboration (ERFC) and Prospective Studies Collaboration (PSC): The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One, 2013; 8: e65174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Sullivan LM, Massaro JM and D’Agostino RB, Sr.: Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med, 2004; 23: 1631-1660 [DOI] [PubMed] [Google Scholar]
- 22).Harrell FE, Jr., Lee KL and Mark DB: Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med, 1996; 15: 361-387 [DOI] [PubMed] [Google Scholar]
- 23).Pencina MJ and D’Agostino RB: Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med, 2004; 23: 2109-2123 [DOI] [PubMed] [Google Scholar]
- 24).D’Agostino R and Nam B: Evaluation of the Performance of Survival Analysis Models: Discrimination and Calibration Measures. Handbook of Statistics, 2003; 23: 1-25 [Google Scholar]
- 25).Nakai M, Watanabe M, Kokubo Y, Nishimura K, Higashiyama A, Takegami M, Nakao YM, Okamura T and Miyamoto Y: Development of a Cardiovascular Disease Risk Prediction Model Using the Suita Study, a Population-Based Prospective Cohort Study in Japan. J Atheroscler Thromb, 2020; 27: 1160-1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Nishimura K, Okamura T, Watanabe M, Nakai M, Takegami M, Higashiyama A, Kokubo Y, Okayama A and Miyamoto Y: Predicting coronary heart disease using risk factor categories for a Japanese urban population, and comparison with the framingham risk score: the suita study. J Atheroscler Thromb, 2014; 21: 784-798 [DOI] [PubMed] [Google Scholar]
- 27).Harada A, Ueshima H, Kinoshita Y, Miura K, Ohkubo T, Asayama K, Ohashi Y and Japan Arteriosclerosis Longitudinal Study Group: Absolute risk score for stroke, myocardial infarction, and all cardiovascular disease: Japan Arteriosclerosis Longitudinal Study. Hypertens Res, 2019; 42: 567-579 [DOI] [PubMed] [Google Scholar]
- 28).Tanabe N, Iso H, Okada K, Nakamura Y, Harada A, Ohashi Y, Ando T, Ueshima H and Japan Arteriosclerosis Longitudinal Study Group: Serum total and non-high-density lipoprotein cholesterol and the risk prediction of cardiovascular events - the JALS-ECC. Circ J, 2010; 74: 1346-1356 [DOI] [PubMed] [Google Scholar]
- 29).Yatsuya H, Iso H, Li Y, Yamagishi K, Kokubo Y, Saito I, Sawada N, Inoue M and Tsugane S: Development of a Risk Equation for the Incidence of Coronary Artery Disease and Ischemic Stroke for Middle-Aged Japanese- Japan Public Health Center-Based Prospective Study. Circ J, 2016; 80: 1386-1395 [DOI] [PubMed] [Google Scholar]
- 30).Cooney MT, Dudina A, D’Agostino R and Graham IM: Cardiovascular risk-estimation systems in primary prevention: do they differ? Do they make a difference? Can we see the future? Circulation, 2010; 122: 300-310 [DOI] [PubMed] [Google Scholar]
- 31).Soureti A, Hurling R, Murray P, van Mechelen W and Cobain M: Evaluation of a cardiovascular disease risk assessment tool for the promotion of healthier lifestyles. Eur J Cardiovasc Prev Rehabil, 2010; 17: 519-523 [DOI] [PubMed] [Google Scholar]
- 32).D’Agostino RB, Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM and Kannel WB: General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation, 2008; 117: 743-753 [DOI] [PubMed] [Google Scholar]
- 33).Yatsuya H, Iso H, Yamagishi K, Kokubo Y, Saito I, Suzuki K, Sawada N, Inoue M and Tsugane S: Development of a point-based prediction model for the incidence of total stroke: Japan public health center study. Stroke, 2013; 44: 1295-1302 [DOI] [PubMed] [Google Scholar]
- 34).Damen JA, Hooft L, Schuit E, Debray TP, Collins GS, Tzoulaki I, Lassale CM, Siontis GC, Chiocchia V, Roberts C, Schlussel MM, Gerry S, Black JA, Heus P, van der Schouw YT, Peelen LM and Moons KG: Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ, 2016; 353: i2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Matsunaga M, Yatsuya H, Iso H, Yamashita K, Li Y, Yamagishi K, Tanabe N, Wada Y, Wang C, Ota A, Tamakoshi K, Tamakoshi A and JACC Study Group: Similarities and differences between coronary heart disease and stroke in the associations with cardiovascular risk factors: The Japan Collaborative Cohort Study. Atherosclerosis, 2017; 261: 124-130 [DOI] [PubMed] [Google Scholar]
- 36).Lawes CM, Rodgers A, Bennett DA, Parag V, Suh I, Ueshima H, MacMahon S and Asia Pacific Cohort Studies Collaboration: Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens, 2003; 21: 707-716 [DOI] [PubMed] [Google Scholar]
- 37).Tolstrup JS, Hvidtfeldt UA, Flachs EM, Spiegelman D, Heitmann BL, Balter K, Goldbourt U, Hallmans G, Knekt P, Liu S, Pereira M, Stevens J, Virtamo J and Feskanich D: Smoking and risk of coronary heart disease in younger, middle-aged, and older adults. Am J Public Health, 2014; 104: 96-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Shinton R and Beevers G: Meta-analysis of relation between cigarette smoking and stroke. BMJ, 1989; 298: 789-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M and Alberta Kidney Disease Network: Relation between kidney function, proteinuria, and adverse outcomes. JAMA, 2010; 303: 423-429 [DOI] [PubMed] [Google Scholar]
- 40).Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J and Gansevoort RT: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet, 2010; 375: 2073-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Jr., Sperling L, Virani SS and Yeboah J: 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2019; 139: e1046-e1081 [DOI] [PubMed] [Google Scholar]
- 42).Miida T, Nishimura K, Okamura T, Hirayama S, Ohmura H, Yoshida H, Miyashita Y, Ai M, Tanaka A, Sumino H, Murakami M, Inoue I, Kayamori Y, Nakamura M, Nobori T, Miyazawa Y, Teramoto T and Yokoyama S: A multicenter study on the precision and accuracy of homogeneous assays for LDL-cholesterol: comparison with a beta-quantification method using fresh serum obtained from non-diseased and diseased subjects. Atherosclerosis, 2012; 225: 208-215 [DOI] [PubMed] [Google Scholar]
- 43).Reddy VS, Bui QT, Jacobs JR, Begelman SM, Miller DP, French WJ and Investigators of National Registry of Myocardial Infarction (NRMI) 4b–5: Relationship between serum low-density lipoprotein cholesterol and in-hospital mortality following acute myocardial infarction (the lipid paradox). Am J Cardiol, 2015; 115: 557-562 [DOI] [PubMed] [Google Scholar]
- 44).Sacks FM, Tonkin AM, Craven T, Pfeffer MA, Shepherd J, Keech A, Furberg CD and Braunwald E: Coronary heart disease in patients with low LDL-cholesterol: benefit of pravastatin in diabetics and enhanced role for HDL-cholesterol and triglycerides as risk factors. Circulation, 2002; 105: 1424-1428 [DOI] [PubMed] [Google Scholar]