Abstract
Purpose
To describe two cases of medium-sized uveal melanoma presenting with hemorrhagic choroidal detachments.
Observations
The first case is a 39-year-old man who presented with choroidal hemorrhage and angle closure glaucoma. The second case is a 42-year-old man who presented with choroidal hemorrhage and posterior scleritis. Vitrectomy with transvitreous fine needle aspiration biopsy was ultimately required to diagnose malignant uveal melanoma in each case.
Conclusions and importance
Intraocular hemorrhage is a rare presenting sign of uveal melanoma. When it does occur, it is typically associated with large tumors. Hemorrhagic choroidal detachments are particularly rare in uveal melanoma, and can limit the diagnostic utility of clinical exam, B-scan ultrasonography, and magnetic resonance imaging. Although it is uncommon, it is important to maintain a high index of suspicion for choroidal melanoma in any patient with unexplained choroidal hemorrhage.
Keywords: Adult ocular oncology, Choroidal melanoma, Choroidal hemorrhage, Retinal and vitreous surgery
1. Introduction
Intraocular hemorrhage is a rare presenting sign of uveal melanoma, present in less than 3 % of new cases.1 When it does occur, the hemorrhage usually within the retina or the vitreous and is typically associated with large uveal melanomas.2, 3, 4, 5 The presence of suprachoroidal hemorrhage at the time of presentation can make melanoma detection challenging due to limitations on clinical exam and imaging modalities such as B-scan ultrasonography and magnetic resonance imaging (MRI), which may not reliably differentiate between choroidal hemorrhage and a choroidal mass.4 Here we describe two cases of medium-sized uveal melanoma, stage IIA by American Joint Commission on Cancer 8th edition,6 that presented with acute choroidal hemorrhage: one with choroidal hemorrhage and angle closure glaucoma, and the other with choroidal hemorrhage and posterior scleritis. After initial conservative management, vitrectomy was ultimately needed to remove associated non-clearing vitreous hemorrhage, visualize the underlying pathology, and sample the choroidal mass with a fine needle aspiration biopsy (FNAB). In each case, the FNAB obtained at the time of vitrectomy demonstrated malignant melanoma.
2. Findings
Case 1: A 39-year-old man presented with sudden vision loss and pain in the right eye. Visual acuity (VA) on presentation was light perception and the intraocular pressure (IOP) was elevated to 38 mmHg in the affected eye. Slit lamp exam was notable for a shallow anterior chamber, and a dilated fundus exam demonstrated large hemorrhagic choroidal detachments obscuring view to the posterior pole. A B-scan ultrasound was performed, which demonstrated large hemorrhagic choroidal detachments as well as an indistinct, dome-shaped mass in the temporal periphery that measured 7.7mm in height and 14.2mm in largest base diameter with heterogenous internal reflectivity (Fig. 1A). No intrinsic vascularity was noted on ultrasound of the lesion. MRI of the orbits demonstrated choroidal hemorrhage with an underlying mass. The mass was hyperintense on pre-gadolinium T1-weighted images (Fig. 1C) and did not show significant enhancement on post-contrast images (Fig. 1D). There was enhancement of the structures adjacent to the lesion in the lateral orbit. Computed tomography scans of the abdomen and chest were obtained to rule out choroidal metastasis from an unknown primary and were unremarkable.
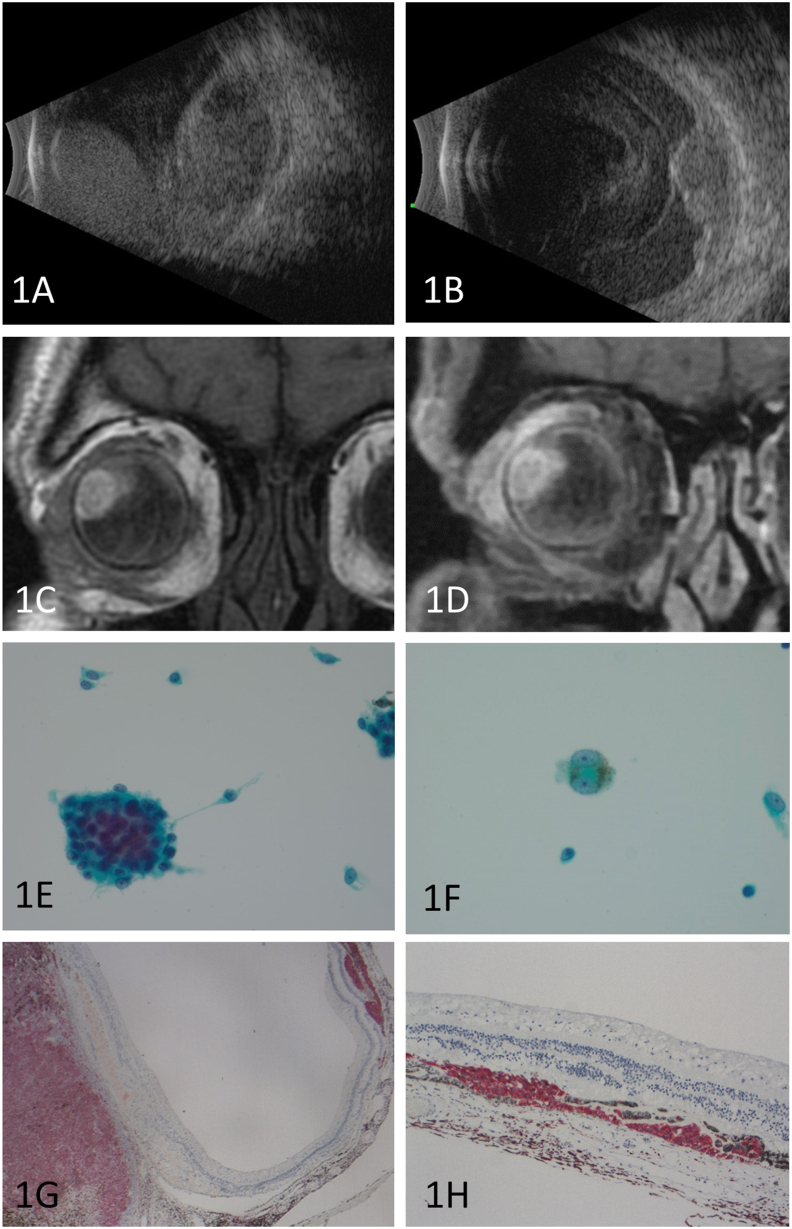
Fig. 1.
1A: B scan ultrasound demonstrating large hemorrhagic choroidal detachments with a heterogeneous mass.
1B: B scan ultrasound 2 months after presentation showing marked reduction in mass size.
1C: Pre-gadolinium T1 coronal Magnetic resonance image.
1D: Post-gadolinium T1 coronal Magnetic resonance image.
1E: Fine needle aspiration biopsy showing clump of atypical, pigmented melanocytes with both epithelioid and spindled characteristics.
1F: High magnification of a binucleate atypical cell with distinct nucleoli and dusty brown intracytoplasmic pigment consistent with melanin.
1G: Melan A immunohistochemistry showing the choroidal melanoma with remote seeding.
1H: High magnification of Melan A immunohistochemistry showing extensive subretinal pagetoid spread away from the main mass. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Initial management included topical and oral IOP-reducing agents. Serial exams demonstrated improvement in the choroidal hemorrhage and progressive decrease in the size of the choroidal mass. At two months after presentation, his VA remained light perception, and IOP was 15 mmHg on topical IOP-lowering drops. The choroidal hemorrhage persisted, but had decreased in size. The choroidal mass height had decreased to 4mm (Fig. 1B). However, recurrent vitreous hemorrhage precluded adequate examination of the lesion so the decision was made to proceed with pars plana vitrectomy and transvitreous fine needle aspiration biopsy of the choroidal lesion. Intraoperative examination of the retina demonstrated widespread retinal atrophy, pigment migration, and increased pigmentation throughout the fundus. Needle biopsy was obtained with a 25-gauge needle through pars plana valved cannulas. Cytology demonstrated highly atypical melanocytic cell proliferation, consistent with a choroidal melanoma (Fig. 1E and F).
The patient elected to pursue enucleation. Surgical pathology demonstrated a choroidal melanoma with a basal diameter of 13mm and a maximum height of 4mm (pT2a, stage IIA). The neoplasm breached Bruch's membrane and showed patchy but extensive growth in the suprachoroidal plane, resembling pagetoid spread (Fig. 1G). Bruch's membrane was otherwise well preserved outside of the focal disruption overlying the tumor. The remaining globe contained a broad region of chronic suprachoroidal hemorrhage with rare suprachoroidal nests of melanoma cells seen at the periphery of the hemorrhage (Fig. 1H). No definite intravascular melanoma or thrombus formation was identified, though many choroidal vessels appeared congested and dilated. The iridocorneal angle was uninvolved by neoplasm, and there was no evidence of significant peripheral anterior synechiae formation.
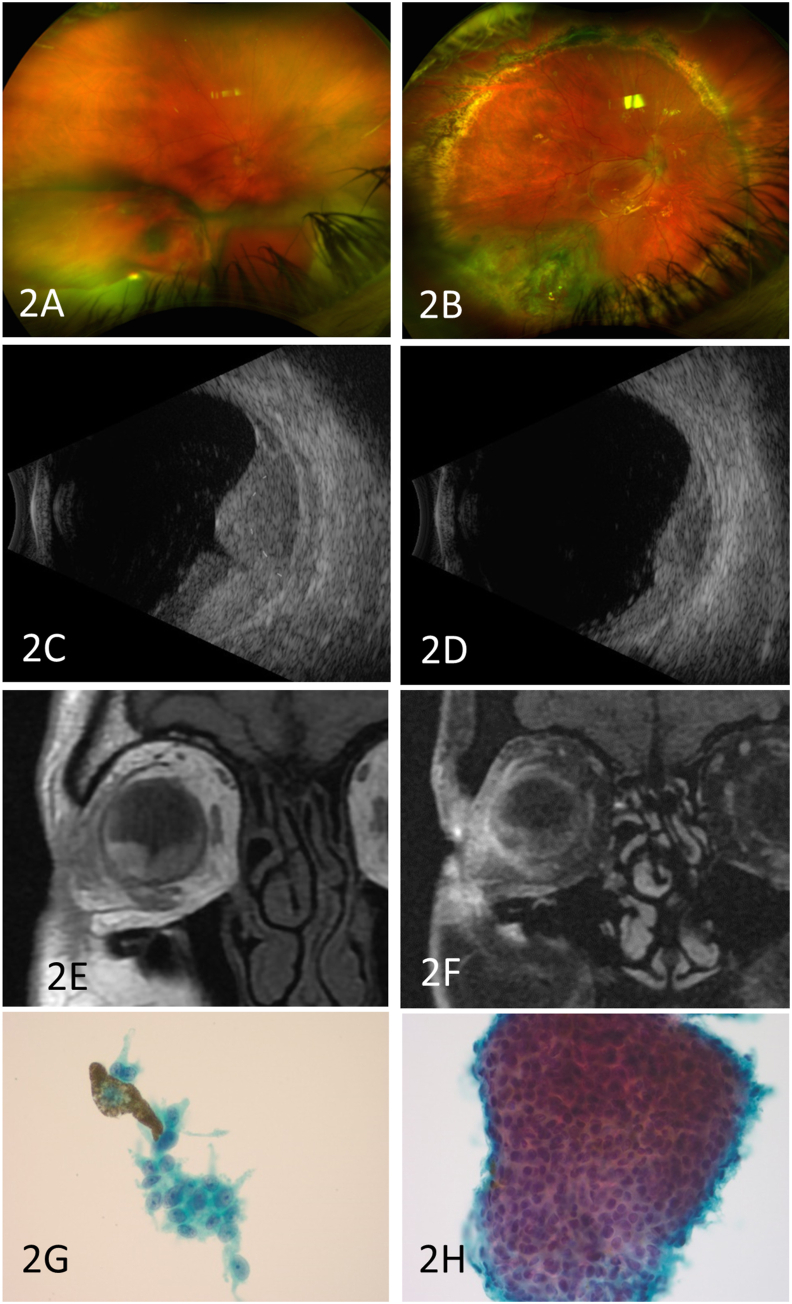
Case 2: A 42-year-old man presented with three days of vision loss in his superior visual field and severe pain in the right eye. Best corrected visual acuity on presentation was 20/70 and IOP was 11 mmHg. His exam was notable for mild eyelid edema with reactive ptosis, conjunctival chemosis, diffuse non-blanching scleral injection, mild vitreous hemorrhage, and inferior subretinal and choroidal hemorrhage (Fig. 2A). B-scan ultrasound showed massive choroidal thickening with a faintly visible dome-shaped mass underlying the irregularly shaped choroidal hemorrhage. The mass showed a homogenous hypoechoic signal and measured 5.1mm in height and 11mm in largest base, and no intrinsic vascularity was detected (Fig. 2C). An MRI of the orbits was obtained. Pre-gadolinium T1-weighted imaging demonstrated hyperintensity of the mass and choroidal hemorrhage (Fig. 2E). Post-gadolinium T1-weighted images demonstrated enhancement of the lateral orbit, lacrimal gland, and lateral sclera. There was questionable heterogeneous enhancement of the choroidal lesion (Fig. 2F). The patient was managed with multiple interventions including oral steroids, non-steroidal anti-inflammatory drugs, and antibiotics for a diagnosis of posterior scleritis of unknown etiology. His pain improved, and serial B-scan ultrasound exams demonstrated progressive decrease in size of both the choroidal hemorrhage and the choroidal lesion, which measured 3.1mm in height and 9.0mm in largest base three weeks after his initial presentation (cT1a, stage IIA, Fig. 2D). Initial CT scans of his abdomen and chest were unremarkable. One month after presentation he developed worsening vitreous hemorrhage. A B-scan ultrasound at that time showed recurrent choroidal hemorrhages and a new total retinal detachment with subretinal hemorrhage. He underwent external drainage of choroidal hemorrhage from the superonasal quadrant, vitrectomy, transvitreous biopsy of the inferotemporal choroidal lesion with a 25-gauge needle, membrane peel, fluid air exchange, endolaser, and gas tamponade. Cytology showed highly atypical melanocytic cell proliferation, consistent with malignant melanoma (Fig. 2G and H). The patient wished to pursue proton beam irradiation therapy. However, prior to radiation and one month after his initial surgery, he developed a recurrent total open-funnel retinal detachment. He underwent cataract extraction with intraocular lens placement, vitrectomy, membrane peel, 360-degree retinectomy, and fluid air exchange with silicone oil tamponade. Tantalum ring markers were placed at the time of his repeat retinal detachment repair to guide his radiation therapy. Three months post operatively, his tumor had regressed after proton beam irradiation, and his vision was 20/300 (Fig. 2B).
Fig. 2.
2A: Wide field color fundus photo with vitreous hemorrhage and inferior subretinal and suprachoroidal hemorrhage.
2B: Wide field color fundus photo after proton beam irradiation of inferotemporal choroidal melanoma and repair of recurrent retinal detachment with a 360 retinectomy.
2C: B-scan ultrasound at time of presentation showing choroidal hemorrhage and underlying dome-shaped mass (outlined for emphasis).
2D: B scan ultrasound three weeks after presentation showing marked reduction in mass size.
2E: MRI showing pre-gadolinium T1 hyperintensity in the lesion and surrounding choroidal hemorrhage.
2F: MRI showing post-gadolinium T1 shows no any evidence of contrast enhancement within the lesion.
2G: Fine needle aspiration biopsy of the choroidal mass showing crowded cluster of atypical melanocytes and (2H) atypical epithelioid cells with intracytoplasmic brown pigment. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
Intraocular hemorrhage is a rare presenting sign of uveal melanoma. In a review of 450 eyes enucleated due to uveal melanoma, only 13 eyes (2.9 %) had intraocular hemorrhage as the initial clinical sign, and of those, only one had subretinal hemorrhage. No patients had choroidal hemorrhage.1 Published cases of uveal melanoma causing intraocular hemorrhage often describe large tumors greater than 9mm in height, and/or filling most of the vitreous cavity as demonstrated on histopathology after enucleation.3, 4, 5
The two cases we describe are unusual not only due to their initial presentation of significant choroidal hemorrhage, but also that the causative choroidal melanomas were both smaller than previous reports. Pathologic staging in Case 1 showed a height of 4.0mm and largest base of 13mm while clinical staging in Case 2 revealed a height of 3.1mm and largest base of 9.0mm, both consistent with stage IIA disease. In both cases there was no initial view of the choroidal mass and ultrasound was required for evaluation. When there is overlying retinal, subretinal, or choroidal hemorrhage, the ultrasound image is difficult to discern and an underlying choroidal mass may not be immediately evident. Choroidal hemorrhage in particular can obscure a choroidal mass as illustrated in Fig. 2C. Physicians and sonographers must have a high index of suspicion for a choroidal mass when a patient presents with massive, spontaneous choroidal hemorrhage. The lack of acoustic hollowness within the lesion with intratumoral hemorrhage resulting in heterogenous signals may lead to a non-classic appearance of choroidal melanoma on B-scan ultrasound (Fig. 1A). There are some clinical findings that would support the diagnosis of an underlying melanoma in an eye with choroidal hemorrhage including the presence of sentinel vessels, a transillumination shadow, or intrinsic vascularity with ultrasound. However, sentinel vessels are usually not present in posterior tumors and choroidal hemorrhage can also cause a transillumination shadow. In both cases in this report, the initial ultrasound did not show intrinsic vascularity at the time of presentation, though this is an important clinic test that might be able to differentiate the two diagnoses.
In both cases, the initial tumor size decreased significantly with time. In Case 1 the tumor decreased from a height of 7.7mm–4.0mm in one month. In the second case the tumor decreased from 5.1mm to 3.0mm in two weeks. In the presence of choroidal hemorrhage, the initial size of a choroidal tumor is likely exaggerated due to intra-tumoral bleeding. If a diagnosis of melanoma is made with concomitant choroidal hemorrhage, more accurate tumor measurements should be obtained after a period of observation to allow time for the hemorrhage to absorb. Falsely elevated measurements due to intratumoral hemorrhage may adversely affect the treatment plan when planning for surgical intervention or radiation dose.
Orbital imaging with MRI is not routinely used in evaluation of choroidal melanoma, as clinical exam features and B-scan ultrasound are typically sufficient to arrive at the diagnosis. However, when media opacity limits the view to the fundus and there is concern for melanoma, MRI may prove useful in diagnosis. Uveal melanoma is often T1-hyperintense and T2-hypointense on precontrast MRI, and usually demonstrates contrast enhancement.7 However, in the cases presented in this study, concomitant intra-tumoral and choroidal hemorrhage made interpretation of the images more challenging. Hemorrhage itself can be T1 hyperintense and limit the ability to assess for the intrinsic T1-hyperintensity signal of melanin and mask post-contrast tumor enhancement. While the hemorrhagic lesions in both cases could be visualized with MRI, and questionable heterogeneous enhancement was present in the second case, overall assessment for enhancement of the lesions was limited by the hemorrhage.
Both cases in this paper presented in young (ages 39 and 42), otherwise healthy men who were younger than the mean age for choroidal melanoma. Neither patient was taking any systemic medication such as an anticoagulant that would increase their likelihood of significant hemorrhage. Both tumors were similar in size and location, and both melanomas caused initial choroidal hemorrhage and subsequent vitreous hemorrhage. However, the tumors had vastly different presenting signs: angle closure glaucoma in Case 1 and scleritis with orbital inflammation in Case 2. While angle closure glaucoma is a well-established complication of a ciliary body melanoma,8 Case 1 demonstrates that if a patient has acute angle closure glaucoma and spontaneous choroidal hemorrhage, a careful examination with B-scan ultrasound should be completed of the posterior segment to evaluate for an underlying choroidal neoplasm. Pathologic examination in this case revealed an otherwise normal iridocorneal angle, suggesting the angle closure was mechanical due to hemorrhage posterior to the iris.
Inflammation is also a rare presenting sign of uveal melanoma; in the review of 450 eyes enucleated for uveal melanoma by Fraser et al., fewer than 5 % demonstrated ocular inflammation as the initial clinical sign.1 In previous cases of uveal melanoma presenting with scleritis, it was theorized that intratumoral necrosis might be responsible for the inflammatory response. However, the tumor in Case 2 was small and showed no clinical nor cytologic evidence of necrosis. It is also unclear if the inflammatory reaction was related to the underlying choroidal hemorrhage, or due to the tumor alone. We expect the reduction in size of the tumor in Case 2 was result of improvement in intratumoral hemorrhage, similar to Case 1. However, the release of inflammatory mediators such as tumor necrosis factor, interleukin-1 and interlukin-6 in the picture of ocular inflammation has been hypothesized to have treatment effect on melanoma, resulting in incomplete regression of the tumor9,10
The precise cause of hemorrhage in these cases is unknown; it is theorized that tumor necrosis can rupture the posterior ciliary arteries, leading to massive intraocular hemorrhage, or that localized vascular compression and impaired venous return in the setting of tumor invasion through Bruch's membrane can yield secondary hemorrhage.3,4 Pathologic examination of the globe in Case 1 revealed no evidence of intravascular melanoma or thrombi, though the choroidal vessels did appear congested and dilated. Bruch's membrane also appeared normal throughout the pathologic section other than a focal defect from which the melanoma grew into the subretinal space. The pagetoid growth pattern in Case 1 was atypical of choroidal melanoma; it is possible that the diffuse spread of the melanoma could have affected the choroidal vasculature, leading to hemorrhage, however there was no evidence of intravascular invasion seen in any section of the enucleated specimen. Though no vascular invasion was seen in the enucleated eye, it may be important to note that both of these medium-sized tumors were located in the equatorial region close to the presumed location of a vortex vein. It is possible the location may have played a role in the choroidal hemorrhage.
Choroidal melanoma is a clinical diagnosis, rarely requiring the use of choroidal biopsy. However, in the event of diagnostic uncertainty, choroidal biopsy may be necessary.11 In both of these cases, the underlying tumor could not be visualized on exam, the B-scan quality was suboptimal due to concomitant choroidal hemorrhage, and the MRI did not clearly show enhancement within the lesion. Although a diagnosis of choroidal melanoma was considered likely in both cases, it could not be confirmed clinically. Due to the posterior location and surrounding choroidal hemorrhage, a transscleral needle biopsy was not possible. Instead, a 25-gauge needle was used to obtain a transvitreous aspiration biopsy in both cases after pars plana vitrectomy to remove vitreous hemorrhage. Histopathologic evaluation confirmed in both cases the diagnosis of a melanocytic malignancy. A small needle-based biopsy approach was sufficient and provided adequate tissue for diagnosis in these cases despite the presence of choroidal hemorrhage. More substantial incisional choroidal biopsies or vitrectomy assisted chorioretinal biopsies have higher complication rates and can be initially deferred even in complex cases until small needle biopsies have been attempted.11
Uveal melanoma is an important diagnosis to consider in patients who present with spontaneous intraocular hemorrhage. These cases demonstrate that it is important to maintain a high index of suspicion for choroidal melanoma specifically in any patient with unexplained choroidal hemorrhage. In situations of spontaneous choroidal hemorrhage, attention must be taken to evaluate the entire posterior segment completely with a combination of clinical examination, ultrasonography, and orbital imaging where necessary. Small, posterior choroidal melanomas may be the underlying cause but may be very difficult to identify during an acute hemorrhagic event. When appropriate, a choroidal needle biopsy may be needed to obtain the correct diagnosis.
Patient consent
Subjects in this case reports have given their informed consent to publish their case and images.
Funding
Research was supported by an unrestricted departmental grant for the University of Washington Department of Ophthalmology from Research to Prevent Blindness.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
Dr. Andrew W. Stacey is a consultant for Immunocore. The remaining authors have no conflicts of interest to declare.
Acknowledgements
None.
References
- 1.Fraser D.J. Ocular inflammation and hemorrhage as initial manifestations of uveal malignant melanoma: incidence and prognosis. Arch Ophthalmol. 1979;97(7):1311. doi: 10.1001/archopht.1979.01020020053012. [DOI] [PubMed] [Google Scholar]
- 2.Chan W.-M., Kwok A.K.H., Liu D.T.L., Choi P.C.L., Lam S.-W., Lam D.S.C. Hemorrhagic complication in an unsuspected macular choroidal melanoma. Am J Ophthalmol. 2004;137(3):574–577. doi: 10.1016/j.ajo.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 3.Oellers P., Wolkow N., Jakobiec F.A., Kim I.K. Hemorrhagic choroidal melanoma. Am J Ophthalmol Case Rep. 2018;10:105–107. doi: 10.1016/j.ajoc.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peddada K, Dalvin LA, Mashayekhi A, Shields CL. DIAGNOSTIC CHALLENGES IN NECROTIC UVEAL MELANOMA: Retin Cases Brief Rep. Published online August 2019:1. doi:10.1097/ICB.0000000000000913. [DOI] [PubMed]
- 5.Perry H.D., Hsieh R.C., Evans R.M. Malignant melanoma of the choroid associated with spontaneous expulsive choroidal hemorrhage. Am J Ophthalmol. 1977;84(2):205–208. doi: 10.1016/0002-9394(77)90853-4. [DOI] [PubMed] [Google Scholar]
- 6.Amin M.B., American Joint Committee on Cancer, American Cancer Society, editors. AJCC Cancer Staging Manual. Springer; 2017. Eight edition/editor-in-chief, Mahul B. Amin, MD, FCAP ; editors, Stephen B. Edge, MD, FACS [and 16 others] ; Donna M. Gress, RHIT, CTR-Technical editor ; Laura R. Meyer, CAPM-Managing editor. American Joint Committee on Cancer. [Google Scholar]
- 7.Mafee M.F., Peyman G.A., Peace J.H., Cohen S.B., Mitchell M.W. Magnetic resonance imaging in the evaluation and differentiation of uveal melanoma. Ophthalmology. 1987;94(4):341–348. doi: 10.1016/S0161-6420(87)33440-2. [DOI] [PubMed] [Google Scholar]
- 8.Othman I.S., Assem M., Zaki I.M.A. Secondary glaucoma as initial manifestation of uveal melanoma. Saudi J Ophthalmol Off J Saudi Ophthalmol Soc. 2013;27(3):203–208. doi: 10.1016/j.sjopt.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields C.L., Shields J.A., Santos M.C., Gündüz K., Singh A.D., Othmane I. Incomplete spontaneous regression of choroidal melanoma associated with inflammation. Arch Ophthalmol Chic Ill 1960. 1999;117(9):1245–1247. [PubMed] [Google Scholar]
- 10.Yap E.Y., Robertson D.M., Buettner H. Scleritis as an initial manifestation of choroidal malignant melanoma. Ophthalmology. 1992;99(11):1693–1697. doi: 10.1016/s0161-6420(92)31744-0. [DOI] [PubMed] [Google Scholar]
- 11.Eide N., Walaas L. Fine-needle aspiration biopsy and other biopsies in suspected intraocular malignant disease: a review. Acta Ophthalmol. 2009;87(6):588–601. doi: 10.1111/j.1755-3768.2009.01637.x. [DOI] [PubMed] [Google Scholar]