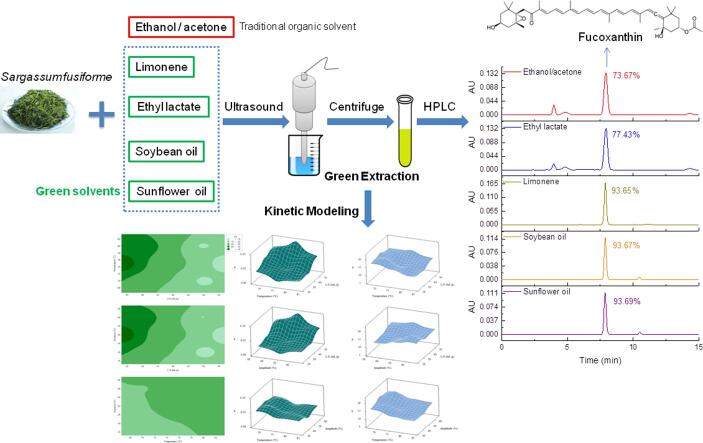
Graphical abstract
Keywords: Sargassum fusiforme, Fucoxanthin, Green solvent, Ultrasonic-assisted extraction, Kinetic modeling
Highlights
-
•
Ultrasonic-assisted extraction of fucoxanthin from S. fusiforme was investigated.
-
•
Ethyl lactate, limonene, and vegetable oils were compared for effective extraction.
-
•
The extraction conditions were optimized by single-factor and response surface tests.
-
•
The second-order kinetic model was fit to describe the dynamic extraction process.
Abstract
The development of green and sustainable extraction technologies for various naturally active biomaterials is gaining increasing attention due to their environmentally friendly advantages. In this work, the ultrasonic-assisted extraction of fucoxanthin from edible brown algae Sargassum fusiforme using different green solvents was presented. Ethyl lactate, limonene, soybean oil, and sunflower oil were used in place of traditional organic solvents. Ethyl lactate showed similar performance to organic solvents, whereas limonene and vegetable oil exhibited higher selectivity for fucoxanthin. Moreover, the effects of various extraction factors, including liquid/solid ratio, extraction time, extraction temperature, as well as amplitude were studied. The optimal conditions were optimized as follows: liquid/solid ratio, 40 mL/g; extraction time, 27 min; extraction temperature, 75 ℃; amplitude, 53%; and solvent, ethyl lactate. Optimal model of second-order kinetic parameters (rate constant, equilibrium concentration, and initial extraction rate) was successfully developed for describing the dynamic ultrasonic extraction process under different operating conditions.
1. Introduction
Brown algae are a rich source of carotenoids and other bioactive compounds such as polysaccharides, polyunsaturated fatty acids, and phycocolloids. Moreover, it is a highly sustainable food rich in fibers. Recently, brown algae have been used to produce fucoxanthin on an industrial scale because of potential advantages of lower cost, larger scale, shorter time, and higher yield as compared with terrestrial plants [1]. Sargassum fusiforme, an edible brown macroalga belongs to the class Phaeophyceae, subclass Cyclosporeae, order Fucales, and family Sargassaceae. It is an endemic species found in the temperate regions of the Pacific Northwest, mainly distributed along the coast of China, Japan, and Korea [2], [3]. S. fusiforme is composed of holdfasts, trunks, branches, and algae leaves, and contains complex nutrients, such as polysaccharides, proteins, lipids, pigments, and trace elements [2], [4]. S. fusiforme is called an “anti-aging vegetable” that has been used as an important food and therapeutic drug for thousands of years [5], [6]. Therefore, S. fusiforme is an important economic alga that is cultivated on a large scale in Asian countries [6].
Recently, increasing consideration for public health and environmental safety has promoted the extraction of natural products using highly efficient green technologies. Green extraction technology should have the characteristics of high efficiency, low energy consumption and reducing the use of organic solvents [7], such as pulsed electric fields, pressurized solvents, supercritical fluids, microwaves, ultrasound, and high-pressure homogenization. These extraction techniques have been used to extract carotenoids from microalgae and seaweeds. Poojary et al. [1] compared the differences in yield, selectivity, economics, and environmental sustainability of these innovative technologies in extracting carotenoids. Ultrasonic-assisted extraction (UAE) is a modern extraction technology that uses the cavitation effect, mechanical vibration, and thermal effect produced by ultrasound to destroy plant cell walls, thereby promoting the diffusion of the solvent and accelerating the dissolution of the compound [8]. This method has the advantages of simple instrumentation, easy operation, and high efficiency. Ultrasonic devices are divided into two types: water bath type and probe type [9]. The probe type ultrasonic has higher power and can provide stronger wall breaking effect, thereby shortening the extraction time and reducing the use of solvents.
Fucoxanthin is a natural carotenoid, which is abundant in brown algae, including in S. fusiforme. It has a unique structure consisting of an allenic bond and oxygenic functional groups, such as epoxy, hydroxyl, carbonyl, and carboxyl groups [10]. This unique structure imparts several biological properties to fucoxanthin, such as antioxidant, antiobesity, antidiabetes, anticancer, and anti-inflammatory properties [11]. High-efficiency extraction of fucoxanthin not only helps to reduce costs, but also helps to increase the utilization value of raw materials. The existing traditional methods for extracting fucoxanthin, such as Soxhlet extraction, reflux, shaking, and stirring, suffer from limitations such as cumbersome operation, time consumption, and low efficiency. Therefore, the high efficient ultrasonic-assisted extraction technology was used to extract fucoxanthin in this study. Fucoxanthin is a fat-soluble pigment and is often extracted with organic solvents, such as ethanol, methanol, ethyl acetate, chloroform, etc. [12]. But most organic solvents are toxic, causing environmental pollution and potential harm to human health. In addition, the organic solvents have a low boiling point and are easy to volatilize into the air during the process of UAE, especially the probe type ultrasonic which cannot be sealed. Thus, the combination of organic solvent and ultrasound is not satisfactory. At present, researchers have discovered several green solvents that can successfully extract carotenoids, such as vegetable oils [13], bio-based solvents [14], [15], and ionic liquids [16]. Green solvents are renewable, non-toxic or low-toxic, and have good extraction efficiency [17]. More importantly, the boiling point of these solvents is generally higher than that of organic solvents, and they are not easily lost due to volatilization, so they are more suitable for UAE.
Kinetics is a very popular analytical method in scientific research. It can intuitively describe the dynamic changes in the research process, such as the extraction of polysaccharide [18], inactivation of PPO [19], and degradation of red pigments and ascorbic acid [20]. A second-order kinetic model is often introduced to describe the dynamic extraction process of the compound. The model can well predict the extraction rate of compounds under certain conditions [13]. At present, the literature on the extraction of fucoxanthin from S. fusiforme using an ultrasound-assisted process and green solvents and its kinetic analysis is scarce. In this study, we used organic solvents as a control to study the differences in several green solvents used in the UAE of fucoxanthin from S. fusiforme. The extraction process was optimized using single-factor and response surface tests. A second-order adsorption model was used to describe the extraction kinetics of fucoxanthin, and an optimal model of kinetic parameters was deduced by a central composite design method.
2. Materials and methods
2.1. Materials and reagents
Fresh S. fusiforme was purchased from Dongtou (Zhejiang, China) and stored at −20 ℃. After thawing, S. fusiforme was dried and ground into powder. Refined soybean oil and sunflower oil (100% purity) were purchased from a local supermarket (Hangzhou, China). L-ethyl lactate and D-limonene were purchased from Wenzhou Shoucheng Chemical Technology Ltd. (Wenzhou, China) and Shanghai Citrus Import Corporation Ltd. (Shanghai, China), respectively. Ethanol, acetone, n-hexane, and ammonium acetate were analytically grade, whereas methanol and acetonitrile were chromatographically grade. These chemical reagents were purchased from Zhejiang Changqing Chemical Ltd. (Hangzhou, China). Fucoxanthin standard (≥95.0%) was purchased from Sigma-Aldrich (Shanghai, China).
2.2. Ultrasonic-assisted extraction
UAE was performed using a probe-type ultrasonic device (VCX500, Sonics & Materials Inc., USA) with an ultrasonic power of 500 W and frequency of 20 kHz, and equipped with a time, temperature, amplitude, and pulse control system. S. fusiforme was weighed according to the liquid/solid ratio (L/S), and the materials were mixed evenly in the beaker. In order to make the solvent fully infiltrate the raw material, the beaker was wrapped with a tin foil and placed in the dark for 2 h. Afterward, the beaker was placed in a water bath preheated for 5 min, so that the temperature reached the set ultrasonic temperature. The ultrasonic parameters were adjusted first, and next the drill was extended a bit below the liquid level. The distance between the tip and the bottom of the cup was controlled by 1 cm. After the ultrasound was completed, the sample was centrifuged (Sorvall RC 6 Plus high-speed refrigerated centrifuge, Thermo, USA) at 9000 g/min for 10 min at 4 °C. The supernatant was collected and supplemented with an extracted solvent to 100 mL. It was used as a sample solution for later use.
2.3. Fucoxanthin detection
The sample solutions were diluted four times before testing. Ethanol/acetone, ethyl lactate, and limonene were diluted with ethanol, whereas soybean oil and sunflower oil were diluted with n-hexane. The diluent (2 mL) was drawn with a syringe and filtered into an injection bottle with a 0.45 µm syringe filter. Next, fucoxanthin in the extract was detected by high-pressure liquid chromatography (HPLC; Waters e2695, USA). Fucoxanthin was quantified by comparing the peak area with a standard curve.
The following HPLC detection conditions were used: Welchrom C18 column (250 mm × 4.6 mm, 5 µm, Welch Materials Inc., China), detection wavelength of 449 nm, acetonitrile:methanol: 0.1% aqueous ammonium acetate solution (v:v:v, 75:15:10) as mobile phase under isocratic elution mode, flow rate of 1 mL/min, injection volume of 20 µL, column temperature of 30 °C, and running time of 15 min.
The extraction yield of fucoxanthin Y (µg/g) is given by Eq. (1).
| (1) |
where C is the fucoxanthin concentration of the sample solution (C = 176603S – 105594, R2 = 0.9991, see the supplementary materials for the details of fucoxanthin standard curve), L is the volume of the fucoxanthin extract, and M is the mass of S. fusiforme used to extract fucoxanthin.
2.4. Kinetic model
The solid/liquid extraction process can be considered as the reverse of an adsorption operation. Therefore, the second-order law can be used to define the extraction rate. The general second-order model [13] can be written as:
| (2) |
where k is the second-order extraction rate constant (mL/µg min), Ce is the equilibrium concentration of fucoxanthin in the liquid extract (extraction capacity, µg/mL), and Ct is the fucoxanthin concentration (µg/mL) in the extract at any time t (min).
The integrated rate law for a second-order extraction under the boundary conditions t = 0 to t and Ct = 0 to Ct, can be written as an Eq. (3) or a linearized Eq. (4) [21]:
| (3) |
| (4) |
The plots of Eq. (4) corresponded to a linear equation y = b+ at, where a = 1/Ce and b = 1/kCe2, allowing determination of k and Ce. The initial extraction rate, h (µg/mL min), when t approaches 0, can be defined as:
| (5) |
2.5. Experimental design
2.5.1. Screening of various green solvent
Ethanol/acetone (v/v = 3:1), ethyl lactate, limonene, soybean oil, and sunflower oil were used as extraction solvents to extract fucoxanthin from S. fusiforme. The extraction efficiency of five solvents were compared by UAE. The extraction conditions were as follows: L/S of 10 mL/g, temperature of 65 °C, amplitude of 40%, running time for 10 s with an interval of 10 s, and the total time of 20 min.
2.5.2. Single-factor experiment design
Ethyl lactate was selected as the extraction solvent. Single-factor experiments were designed to determine the effects of various factors on the UAE of fucoxanthin. When the L/S (10, 20, 30, 40, 50, and 60 mL/g) was used as the control factor, other conditions were time, 20 min; temperature, 65℃; and amplitude, 40%. When time (10, 15, 20, 25, 30, and 35 min) was used as the control factor, other conditions were L/S, 30 mL/g; temperature, 65℃; and amplitude, 40%. When the temperature (55, 60, 65, 70, 75, and 80℃) was used as the control factor, other conditions were L/S, 30 mL/g; time, 20 min; and amplitude, 40%. When the amplitude (20, 30, 40, 50, 60, and 70%) was used as the control factor, other conditions were L/S, 30 mL/g; time, 20 min; and temperature, 65℃.
2.5.3. Response surface optimization
The central composite design method was used to optimize the extraction parameters of fucoxanthin from S. fusiforme. Moreover, it evaluated the correlation and effect of each operating parameter on the extraction process. According to the single-factor experiment, the L/S was fixed at 40 mL/g, and time, temperature, and amplitude were selected as variables. As shown in Table S1, the effects were studied at five experimental levels −a, −1, 0, +1, and +a, where a = 2n/4 and n was the number of variables.
2.5.4. Construction of kinetic parameter models
A group of experiments was designed using the central composite design method to construct a model for three kinetic parameters (Ce, k, and h) in the UAE process. According to the conclusion of a single factor, temperature (X2), amplitude (X3), and L/S (X4) were selected as variables; their level designs are shown in Table S1. In each extraction process, 1 mL of the material/liquid mixture was drawn from the beaker at 5-min intervals, a total of 6 times. To not affect the L/S as much as possible, each suction was operated under ultrasonic operation and the materials were mixed evenly. The mixture was centrifuged (DLAB-D1008; Dalong Xingchuang Experimental Instrument Ltd., China) for 5 min. The supernatant was diluted 4 times and detected by HPLC.
2.6. Statistical analysis
All data are presented as a mean of three determinations. Response surface design and multiple regression analysis were performed using Minitab 13.32. Linear regression analysis was performed using Origin 7.5 to calculate the kinetic parameters. The fit between the model and the experimental data was evaluated using the correlation coefficient (R2). A higher value of R2 indicated that the model fitted better to the experimental data.
3. Results and discussion
3.1. Evaluation of extraction efficiency of different green solvents.
Both ethyl lactate and limonene are recognized as safe (GRAS) solvents approved by the U.S. Food and Drug Administration (FDA) and Environmental Protection Agency (EPA) [17], [22]. They are commonly used as essential oils in the cosmetics and food industries. Soybean oil and sunflower oil are used as daily cooking oils. These are completely non-toxic and harmless green solvents. Therefore, these four solvents were considered as a substitute for conventional organic solvents to extract fucoxanthin. We compared the extraction efficiency of four green solvents and a organic solvent on S. fusiforme fucoxanthin with UAE. The results are shown in Fig. 1 and Fig. S1.
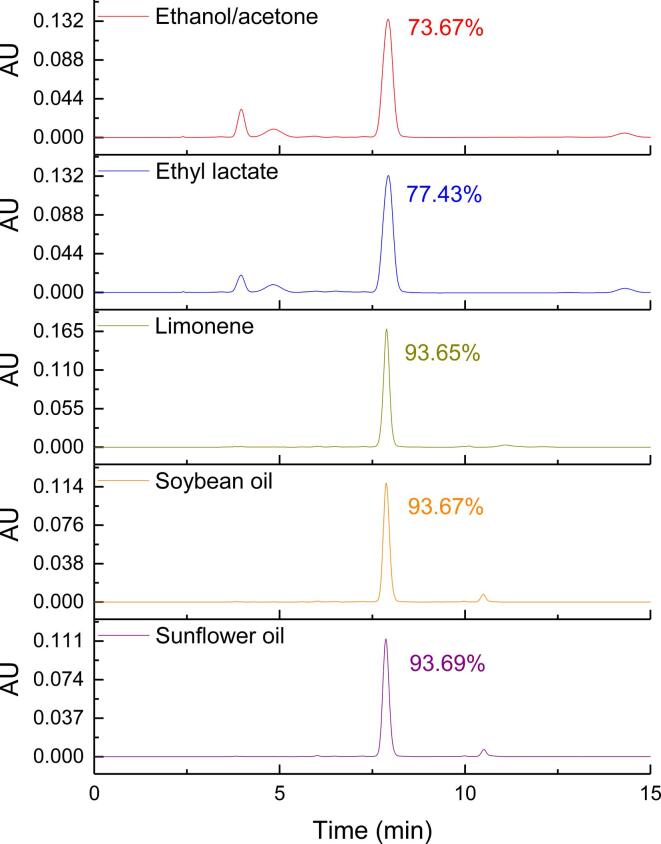
Fig. 1.
HPLC chromatograms of fucoxanthin extracted by different solvents.
The peaks of several substances in the chromatogram of ethyl lactate extract were almost same to those of ethanol/acetone extract. The peak area of fucoxanthin accounted for 77.43% of all peak areas by ethyl lactate extraction, slightly higher than that by ethanol/acetone extraction (73.67%) (Fig. 1). The extraction rate of ethyl lactate was 599.47 µg/g, which was not statistically different from the extraction rate of ethanol/acetone (600.02 µg/g). This indicated that ethyl lactate had similar efficiency to ethanol/acetone in the extraction of fucoxanthin from S. fusiforme, and therefore could be used as a substitute for conventional solvents. Furthermore, ethyl lactate exhibited a good extraction efficiency on other carotenoids. Strati et al. [23] who studied the extraction of carotenoids from tomato waste with ethyl lactate and several other conventional organic solvents, found that the extraction rate of ethyl lactate was 243.00 mg/kg. However, the extraction rate by acetone, ethyl acetate, n-hexane, and ethanol were below 60 mg/kg, indicating that the high extraction efficiency of ethyl lactate is attributed to the fact that ethyl lactate could be dissolved in both water (polar) and hydrocarbon solvents (non-polar). Similarly, Wu et al. [24] also found that ethyl lactate showed a stronger extraction ability in extracting astaxanthin from red yeast Xanthophyllomyces dendrorhous compared with acetone and ethanol. The content of astaxanthin extracted by acetone (ethanol) was 85% (82%) of that of ethyl lactate.
The chromatography of limonene extract was cleaner than the chromatography of ethyl lactate and traditional solvent extracts. The peak area of fucoxanthin accounted for 93.65%. This indicated that limonene could selectively extract fucoxanthin from S. fusiforme, which would facilitate subsequent purification. However, the extraction rate of fucoxanthin was 485.02 µg/g for limonene, which was lower than that of ethyl lactate (599.47 µg/g). This result was similar to that obtained by Andrea et al. [15], who studied the green method of selectively extracting fucoxanthin from Phaeodactylum tricornutum. It was found that compared with ethyl acetate (1.22, 1.69 mg/g), ethyl lactate (0.54, 1.69 mg/g), and ethanol (0.52, 1.56 mg/g), limonene had the highest selectivity (3.54 mg/g) but the lowest extraction rate (0.92 mg/g). Furthermore, Aissou et al. [22] used Hansen solubility parameters (HSPs) and Conductor-like Screening Model for Real Solvents (COSMO-RS) to speculate that limonene had a high solubility for carotenoids and proved it through experiments.
The chromatograms of extracts of soybean oil and sunflower oil were similar to those of limonene. The proportions of peak area of fucoxanthin were 93.67% and 93.69%, respectively; thus, showing a high selectivity for fucoxanthin. However, there was an unknown small peak of approximately 10.50 min in the chromatogram of vegetable oil. This might be a substance which has a higher solubility in oil compared with several other solvents. Fucoxanthin extraction rates of soybean oil and sunflower oil were 349.68 µg/g and 340.19 µg/g, respectively, which were lower than those of ethyl lactate (599.47 µg/g) and limonene (485.02 µg/g). The main purpose of extracting carotenoids from animals and plants using vegetable oil was to obtain an oil rich in carotenoids, so it was not necessary to achieve such a high extraction rate [13]. The extraction rate of vegetable oil was faster than that of conventional organic solvents when β-carotene was extracted from carrots by ultrasound [25]. For example, if the yield of n-hexane was obtained within 60 min, that of sunflower seed oil could be obtained within 20 min. Goula et al. [13] compared the differences between soybean oil and sunflower seed oil in the UAE of carotenoids from pomegranate waste. They found that the carotenoid concentration in the obtained extract was better in soybean oil than that in sunflower oil. The difference in the extraction efficiency between different vegetable oils could be attributed to the viscosity of the oil. Lower viscosity oils migrate faster in the matrix; thus, improving the extraction efficiency. At different temperatures, the viscosity of sunflower oil was generally higher than that of soybean oil. For example, at 65 °C, the viscosity of sunflower oil and soybean oil are 16.90 and 15.73 mPa s, respectively [26]. However, sunflower oil showed a higher extraction capacity than soybean oil when extracting astaxanthin from shrimp waste [27]. Therefore, the extraction ability of different vegetable oils was related to the type of carotenoid extracted.
In summary, the extraction efficiency of ethyl lactate was equivalent to that of traditional organic solvent. The boiling points of ethyl lactate and limonene were 154℃ and 176℃, respectively. Compared with limonene, ethyl lactate was easier to separate from fucoxanthin. Moreover, ethyl lactate was cheaper than limonene and was more suitable for industrial applications. Therefore, ethyl lactate was selected as the solvent for subsequent experiments.
3.2. Optimization of the ultrasonic-assisted extraction process
3.2.1. Results of the single-factor experiments
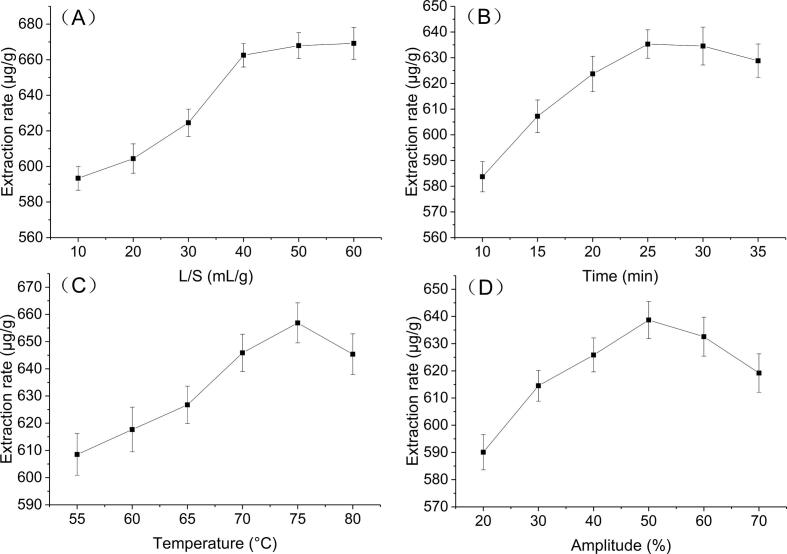
The relevant literature was searched to select factors significantly affecting UAE process, including L/S, extraction time, extraction temperature, and amplitude. We performed a single-factor test to clarify the relationship between each factor and the extraction efficiency. As shown in Fig. 2A, L/S significantly affected the fucoxanthin extraction rate. When L/S increased from 10 to 40 mL/g, the extraction rate gradually increased to 662.51 µg/g, a total increase of 11.65%. The increased L/S promoted the contact between the solvent and the raw materials, whereas the increased concentration gradient of fucoxanthin inside and outside the cells promoted the dissolution of fucoxanthin. However, when L/S exceeded 40 mL/g, the rate of increase in extraction rate gradually decreases. When the L/S increased from 40 to 60 mL/g, the extraction rate only increased by 1.01%. When the L/S reached 40 mL/g, the majority of fucoxanthin in S. fusiforme was extracted. Therefore, the extraction rate did not change significantly with the increase in the solvent dosage. Furthermore, Zou et al. [28] reported the effect of L/S on the extraction rate of astaxanthin from seaweed Haematococcus pluvialis. The maximum extraction rate of astaxanthin was achieved at the L/S value of 20 mL/g. Further increase in the amount of solvent slightly reduced the extraction rate. Considering solvent wastage, L/S at 40 mL/g was selected for subsequent experiments.
Fig. 2.
Effect of different UAE parameters on the extraction rate. (A) Effect of L/S on the extraction rate of fucoxanthin. (B) Effect of time on the extraction rate of fucoxanthin. (C) Effect of temperature on the extraction rate of fucoxanthin. (D) Effect of amplitude on the extraction rate of fucoxanthin.
Between 10 and 25 min, the extraction rate of fucoxanthin increased with time. The maximum value of 635.32 µg/g was reached at 25 min; however, when the time exceeded 25 min, the extraction rate decreased slightly (Fig. 2B). Raguraman et al. [29] found that before the ultrasound time reached 30 min, the yield of Padina tetrastromatica fucoxanthin showed an upward trend, beyond which the yield displayed a downward trend. Moreover, Pasquet et al. [30] and Delbrut et al. [31] reported a similar relationship between extraction time and extraction rate. The reason could be that the concentration of fucoxanthin inside and outside the cell did not reach equilibrium before 25 min. With further increase in time, the fucoxanthin gradually dissolved. The concentration of fucoxanthin in the extract no longer increased when the equilibrium state was established inside and outside the cell. After the equilibrium was reached, the fucoxanthin degraded rapidly under the long-term effect of ultrasound due to its unstable structure [28]. Therefore, the extraction time of 25 min was selected for subsequent experiments.
As shown in Fig. 2C, the extraction rate of fucoxanthin gradually increased between 55 and 75℃ and reached the maximum value of 656.88 µg/g at 75℃. The solubility and diffusion rate of fucoxanthin increased with increasing temperature. Moreover, higher temperature destroyed the cell wall; thus, promoting the dissolution of fucoxanthin in S. fusiforme [23]. However, when the temperature reached 80℃, the extraction rate of fucoxanthin was lower than that at 75℃, indicating that the amount of degradation of fucoxanthin was higher than the dissolution amount when the temperature was increased to 80℃. According to Ying et al. [32] and Strati et al. [23], the mechanical and thermal effects of ultrasound increased with the increase in the temperature, elevating the temperature near the ultrasonic drill than the set temperature. Fucoxanthin was easily oxidized or decomposed under the influence of high temperature. In addition, the high temperature reduces the surface tension of microbubbles and increases the vapor pressure in the bubbles, resulting in ultrasonic attenuation. Therefore, 75℃ was used in subsequent experiments.
The effect of amplitude change on the extraction rate of fucoxanthin is shown in Fig. 2D. The extraction rate of 590.10 µg/g was the lowest when the amplitude was 20%, as lower amplitude could not provide sufficient energy to destroy the cell wall, and thus fucoxanthin could not be completely dissolved. With the increase in the amplitude, the temperature and pressure inside the bubble increased, causing it to burst in a shorter time and producing a violent shock wave. Consequently, a strong cavitation effect was produced, and the cell wall was destroyed. [13] This, in turn, enhanced the penetration of the solvent into the cell tissue and accelerated the dissolution of fucoxanthin. The extraction rate increased with the increase in the amplitude. However, when the amplitude exceeded 50%, the extraction rate of fucoxanthin continued to decline. At extremely high amplitudes, an excessive cavitation effect is produced, increasing the temperature near the probe, generating more hydroxyl radicals, and resulting in excessive fucoxanthin degradation. [32] This could explain the decline in the extraction rate of fucoxanthin. Therefore, 50% amplitude was selected for subsequent experiments.
3.2.2. Response surface optimization
Based on the above single-factor experiment, the fixed L/S was 40 mL/g, with time (X1), temperature (X2), and amplitude (X3) as variables. The central composite design was used to analyze the effects of linear, quadratic, and their interactions on the extraction rate of fucoxanthin to obtain the maximum extraction rate. The experimental data were analyzed by multiple regression. The response and experimental variables were related by the following regression equation:
| (6) |
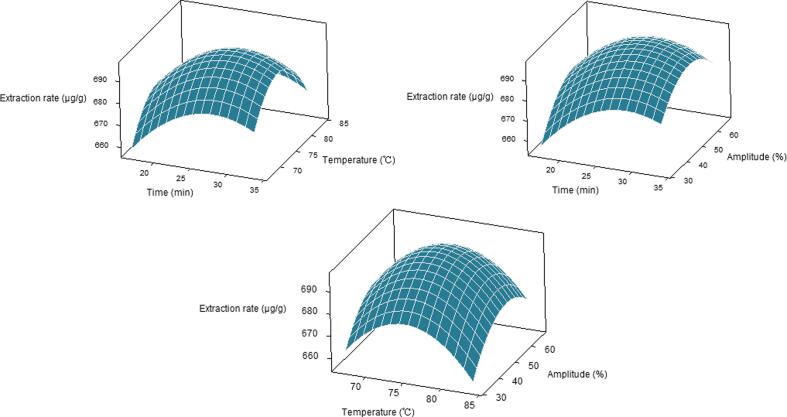
The results of the ANOVA are shown in Table 1. The p-value of the model was less than 0.001, indicating that the regression model was highly significant. The lack of fit associated with a p-value of 0.156 (>0.05) indicated that the model could adequately fit the experiment data. The value of the correlation coefficient R2 of the model was 0.9908, indicating a high correlation between observed and predicted values. The adjusted R2 was 0.9825, implying that only 0.83% of the total variation could not be explained by the model. The above data proved that the model was suitable and could be used for optimization and monitoring. According to the F-value, all three factors had a significant effect on the extraction rate of fucoxanthin from S. fusiforme (p < 0.05). The order of the effect of various factors on the extraction rate was time > amplitude > temperature. Moreover, three factors displayed significant quadratic effects. However, for the interaction between factors, only the interaction between time and amplitude was significant. The three-dimensional (3D) response surface graphs are shown in Fig. 3. A similar relationship between the factors and the extraction rate of fucoxanthin was present. With the increase in time, amplitude, and temperature, the extraction rate first increased and subsequently decreased.
Table 1.
ANOVA for the regression equation.
| Source | DF | Adj SS | Adj MS | F-Value | P-Value |
|---|---|---|---|---|---|
| Model | 9 | 1400.19 | 155.577 | 119.49 | 0.000 |
| Linear | 3 | 398.46 | 132.821 | 102.01 | 0.000 |
| X1 | 1 | 246.25 | 246.247 | 189.12 | 0.000 |
| X2 | 1 | 9.68 | 9.682 | 7.44 | 0.021 |
| X3 | 1 | 142.53 | 142.533 | 109.47 | 0.000 |
| Square | 3 | 991.09 | 330.364 | 253.72 | 0.000 |
| X12 | 1 | 256.56 | 256.561 | 197.04 | 0.000 |
| X22 | 1 | 615.80 | 615.805 | 472.95 | 0.000 |
| X32 | 1 | 300.66 | 300.660 | 230.91 | 0.000 |
| 2-Way Interaction | 3 | 10.64 | 3.547 | 2.72 | 0.100 |
| X1X2 | 1 | 1.02 | 1.015 | 0.78 | 0.398 |
| X1X3 | 1 | 8.80 | 8.799 | 6.76 | 0.027 |
| X2X3 | 1 | 0.83 | 0.826 | 0.63 | 0.444 |
| Error | 10 | 13.02 | 1.302 | ||
| Lack of Fit | 5 | 9.43 | 1.887 | 2.63 | 0.156 |
| Pure Error | 5 | 3.59 | 0.717 | ||
| Total | 19 | 1413.22 |
Fig. 3.
Surface diagrams showing the effects of different interactions on the extraction of fucoxanthin from S. fusiforme.
The desirability profile for optimum fucoxanthin extraction rate (Fig. S2) indicated the following conditions: time, 27.29 min; temperature, 74.58 °C; and amplitude, 52.89%. Under these conditions, the predicted value of the fucoxanthin extraction rate was 697.14 µg/g. To confirm this prediction, three repeated tests were performed under optimized conditions. The actual operating conditions were: time, 27 min; temperature, 75 °C; and amplitude 53%. The average extraction rate of fucoxanthin was 696.85 ± 2.84 µg/g, which was in good agreement with the predicted value (the error was only 0.6%). These results further validated the model, indicating that the model had a good predictive ability.
3.3. Kinetic modeling of the ultrasonic-assisted extraction process
With time as the variable, results of the single-factor test revealed that the linear relationship between t/Ct and t (Fig. S3) was consistent with the second-order leaching model expressed by Eq. (4). This indicated that there could be a suitable kinetic model in the UAE of fucoxanthin from S. fusiforme. Therefore, with the L/S, temperature, and amplitude as variables, a set of experiments was designed using the central composite design method to determine the best model of kinetic parameters, such as k, Ce, and h. A kinetic model was obtained to quantify the extraction rate of fucoxanthin. Moreover, the effect of different variables on the kinetic parameters could be determined, thus evaluating the extraction ability of the UAE of fucoxanthin from S. fusiforme using ethyl lactate as a solvent. For the 20 sets of different extraction conditions designed, there was a linear relationship between t/Ct and t, and they are all in agreement with the second-order leaching model expressed by Eq. (4). The three kinetic parameters Ce (Ce = 1/a), k (k = 1/bCe2), and h (h = kCe2) could be determined through the slope (a) and intercept (b) of the function. The results are shown in Table 2.
Table 2.
Parameters of the second-order kinetic model for fucoxanthin extraction from S. fusiforme under different conditions.
| Run | X4 | X2 | X3 | a (mL/μg) | b (min mL/μg) | Ce (μg/mL) | k (mL/μg min) | h (μg/mL min) | R2 (y = at + b) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 0.0447 | 0.0657 | 22.3714 | 0.0304 | 15.2117 | 0.9996 |
| 2 | 1 | −1 | −1 | 0.0724 | 0.0557 | 13.8122 | 0.0940 | 17.9374 | 0.9989 |
| 3 | −1 | 1 | −1 | 0.0371 | 0.1681 | 26.9358 | 0.0082 | 5.9499 | 0.9982 |
| 4 | 1 | 1 | −1 | 0.0702 | 0.0694 | 14.2450 | 0.0710 | 14.4171 | 0.9997 |
| 5 | −1 | −1 | 1 | 0.0399 | 0.0440 | 25.0415 | 0.0362 | 22.7083 | 0.9998 |
| 6 | 1 | −1 | 1 | 0.0674 | 0.0629 | 14.8368 | 0.0723 | 15.9082 | 0.9992 |
| 7 | −1 | 1 | 1 | 0.0331 | 0.0749 | 30.2115 | 0.0146 | 13.3474 | 0.9973 |
| 8 | 1 | 1 | 1 | 0.0660 | 0.0789 | 15.1515 | 0.0552 | 12.6695 | 0.9995 |
| 9 | −α | 0 | 0 | 0.0291 | 0.0683 | 34.3134 | 0.0124 | 14.6413 | 0.9973 |
| 10 | α | 0 | 0 | 0.0757 | 0.0591 | 13.2100 | 0.0970 | 16.9331 | 0.9998 |
| 11 | 0 | −α | 0 | 0.0559 | 0.0543 | 17.8891 | 0.0576 | 18.4216 | 0.9994 |
| 12 | 0 | α | 0 | 0.0450 | 0.0918 | 22.2222 | 0.0220 | 10.8888 | 0.9975 |
| 13 | 0 | 0 | −α | 0.0542 | 0.0791 | 18.4592 | 0.0371 | 12.6413 | 0.9968 |
| 14 | 0 | 0 | α | 0.0456 | 0.0723 | 21.9298 | 0.0288 | 13.8350 | 0.9997 |
| 15 | 0 | 0 | 0 | 0.0473 | 0.0951 | 21.1416 | 0.0235 | 10.5152 | 0.9989 |
| 16 | 0 | 0 | 0 | 0.0470 | 0.0847 | 21.2766 | 0.0261 | 11.8111 | 0.9991 |
| 17 | 0 | 0 | 0 | 0.0492 | 0.0899 | 20.3333 | 0.0269 | 11.1235 | 0.9975 |
| 18 | 0 | 0 | 0 | 0.0454 | 0.0926 | 22.0054 | 0.0223 | 10.7950 | 0.9987 |
| 19 | 0 | 0 | 0 | 0.0474 | 0.0942 | 21.0970 | 0.0239 | 10.6157 | 0.9991 |
| 20 | 0 | 0 | 0 | 0.0479 | 0.0960 | 20.8768 | 0.0239 | 10.4157 | 0.9994 |
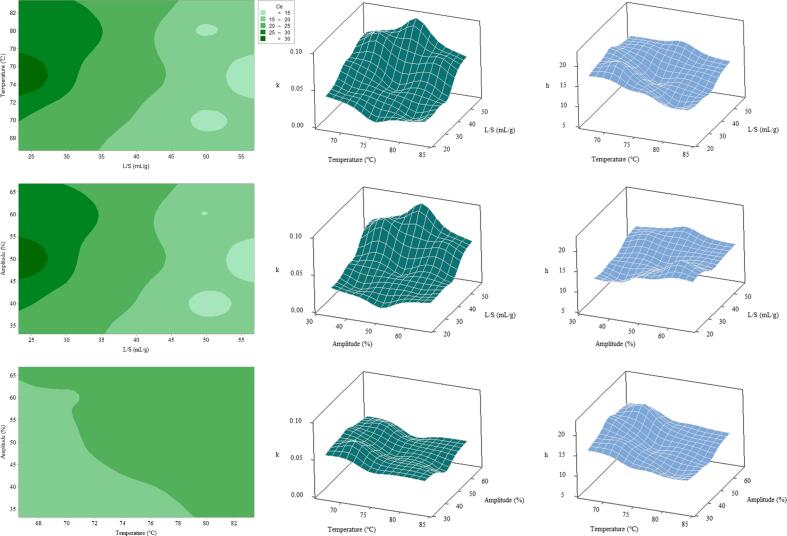
Combined with Fig. 4 and Table 3, the L/S, temperature, and amplitude significantly affected Ce, k, and h. The interaction of L/S and temperature or amplitude had a similar effect on Ce. The lower the L/S, the higher the Ce because when the volume of the solvent was less, fucoxanthin was nearly saturated in the extract with an increase in the volume. The equilibrium concentration was gradually diluted. This was similar to the phenomenon observed by Qu et al., who reported that the equilibrium concentration of antioxidants in pomegranate marc decreased with increasing L/S [33]. With the increase in temperature and amplitude, Ce first increased and subsequently decreased, reaching the maximum Ce at approximately 75℃ and an amplitude of about 50%. Thus, the single-factor test could explain this phenomenon at extremely high temperature and amplitude, causing degradation of fucoxanthin. The interaction of temperature and amplitude had no significant effect on Ce (P > 0.05).
Fig. 4.
The three-dimensional surface or two-dimensional contour plots of the interaction of different conditions on kinetic parameters.
Table 3.
Regression analysis of central composite design.
| Variable | Ce |
k |
h |
|||
|---|---|---|---|---|---|---|
| Coefficient | P-Value | Coefficient | P-Value | Coefficient | P-Value | |
| Constant | 21.155 | 0.000 | 0.024 | 0.000 | 10.880 | 0.000 |
| X4 | −6.005 | 0.000 | 0.025 | 0.000 | 0.554 | 0.045 |
| X2 | 1.301 | 0.000 | −0.010 | 0.000 | −2.786 | 0.000 |
| X3 | 1.004 | 0.001 | −0.003 | 0.003 | 0.961 | 0.003 |
| X42 | 0.717 | 0.005 | 0.012 | 0.000 | 1.732 | 0.000 |
| X22 | −0.593 | 0.013 | 0.006 | 0.000 | 1.332 | 0.000 |
| X32 | −0.544 | 0.020 | 0.004 | 0.000 | 0.831 | 0.005 |
| X4X2 | −1.123 | 0.002 | 0.000 | 0.644 | 1.483 | 0.001 |
| X4X3 | −0.502 | 0.087 | −0.006 | 0.000 | −2.334 | 0.000 |
| X2X3 | 0.061 | 0.823 | 0.001 | 0.422 | 0.023 | 0.944 |
The effect of each factor on k was also similar to the phenomenon of the single-factor test. For example, the k value gradually approached the maximum value as L/S increased because the concentration gradient increased with the amount of solvent, increasing the mass transfer rate of fucoxanthin. The effect of temperature and amplitude on k was similar to the results obtained by Goula et al. [13]. As the temperature increased, the kinetic parameter k of carotenoid extraction gradually increased, reaching the highest value at 40 to 50℃. However, when the temperature was higher than 50℃, the k value decreased.
Temperature and amplitude significantly affected h (p < 0.01). Fig. 4 showed a trend of higher h value when the temperature was low, and the amplitude was high. This could be attributed to the fact that in a short extraction time, fucoxanthin degradation was less at low temperatures. The high amplitude was beneficial to instantaneously destroy the cell wall and rapidly dissolved fucoxanthin. The interactions between L/S and temperature or amplitude significantly affected h (p < 0.01). The maximum h value could be obtained when the temperature was between 65 and 75℃ and L/S between 20 and 40 mL/g, indicating that the initial extraction rate of fucoxanthin in this section was high. If a higher fucoxanthin yield was required in a shorter time, this section could be selected as the extraction condition. This was more in line with the trend of rapid extraction in modern industry. Goula [21] used a central composite design method to establish a model for each kinetic parameter of ultrasound-based extraction of pomegranate seed oil. It was found that several kinetic parameters increased regularly with the increase in L/S, decrease in temperature, and increase in the amplitude. However, when Rabesiaka et al. [34] studied the kinetic model of constant temperature stirring to extract Fumaria officinalis protopine, Ce and h showed a downward trend, and k showed an upward trend with the increase in L/S. This showed that the kinetic parameters were affected by the thermal stability of the substance itself, the nature of raw materials, and the extraction method.
Qu et al. [33] fitted the relationship between kinetic parameters and extraction factors (particle size, water/sample ratio, and temperature) through linear, power, or second-order polynomial functions. However, they developed a different model for each factor. We used multiple regression analysis to establish the equation which could simultaneously predict the effect of all factors on the extraction rate of fucoxanthin, as shown in Eqs. (7), (8), (9). A kinetic model for predicting the extraction of fucoxanthin from S. fusiforme could be obtained by substituting Eqs. (7), (8), (9) into Eq. (4) or (3).
| (7) |
| (8) |
| (9) |
Even if the models in Eqs. (7), (8), (9) cannot completely explain the phenomena governing extraction processes, they could be used to determine the effects of L/S, temperature, and amplitude on the ability of UAE of fucoxanthin from S. fusiforme when using ethyl lactate as solvent. In addition, an empirical model for a given unit process was developed for the food processing, which optimized the extraction process and provided guidance for reducing cost and time. Moreover, it could predict the possible causes of fluctuation in the quality of a batch of products in time.
4. Conclusion
UAE is a proven green biorefining technology with advantages of lower cost, shorter time, lesser energy, and higher yield. Ethyl lactate, limonene, and vegetable oil are recognized as renewable green solvents. We proposed the use of a combination of ultrasound and these green solvents to extract fucoxanthin. Ethyl lactate has a high extraction capacity for fucoxanthin like organic solvents, and limonene and vegetable oil have high selectivity for fucoxanthin. Single-factor test and response surface methodology showed that L/S, time, temperature, and amplitude were the key factors affecting the extraction rate of fucoxanthin. Furthermore, an optimal process for extracting fucoxanthin from S. fusiforme was determined. The second-order kinetic model was introduced to describe the dynamic process of fucoxanthin extraction under different parameters of UAE that provided a reference for selecting green extraction technology for natural bioactive compounds.
CRediT authorship contribution statement
Jinggui Nie: Investigation, Methodology, Data curation, Writing - original draft. Danting Chen: Investigation, Methodology. Jing Ye: Investigation, Methodology. Yanbin Lu: Conceptualization, Supervision, Investigation, Writing - review & editing. Zhiyuan Dai: Investigation, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Y.L. thanks the Department of Science and Technology of Zhejiang Province for financial support through Grant No. LGN18C200015. J.Y. thanks the Department of Science and Technology of Zhejiang Province for financial support through Grant No. LGN18C200016.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105671.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Poojary M.M., Barba F.J., Aliakbarian B., Donsì F., Pataro G., Dias D.A., Juliano P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs. 2016;14:214. doi: 10.3390/md14110214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yende S.R., Harle U.N., Chaugule B.B. Therapeutic potential and health benefits of Sargassum species. Pharmacogn. Rev. 2014;8:1–7. doi: 10.4103/0973-7847.125514. 125514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kokubu S., Nishihara G.N., Watanabe Y., Tsuchiya Y., Amamo Y., Terada R. The effect of irradiance and temperature on the photosynthesis of a native alga Sargassum fusiforme (Fucales) from Kagoshima, Japan. Phycologia. 2015;54(3):235–247. doi: 10.2216/15-007.1. [DOI] [Google Scholar]
- 4.Zhang R., Zhang X., Tang Y., Mao J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: a review. Carbohyd. Polym. 2020;228:115381. doi: 10.1016/j.carbpol.2019.115381. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z., Liu J., Fu Z., Ye C., Zhang R., Song Y., Zhang Y., Li H., Ying H., Liu H. 24(s)-saringosterol from edible marine seaweed Sargassum fusiforme is a novel selective lxrβ agonist. J. Agric. Food Chem. 2014;62(26):6130–6137. doi: 10.1021/jf500083r. [DOI] [PubMed] [Google Scholar]
- 6.Wang W., Lu J.B., Wang C., Wang C.S., Zhang H.H., Li C.Y., Qian G.Y. Effects of Sargassum fusiforme polysaccharides on antioxidant activities and intestinal functions in mice. Int. J. Biol. Macromol. 2013;58:127–132. doi: 10.1016/j.ijbiomac.2013.03.062. [DOI] [PubMed] [Google Scholar]
- 7.Chemat F., Vian M.A., Cravotto G. Green extraction of natural products: concept and principles. Int. J. Mol. Sci. 2012;13:8615–8627. doi: 10.3390/ijms13078615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirsath S.R., Sonawane S.H., Gogate P.R. Intensification of extraction of natural products using ultrasonic irradiations—a review of current status. Chem. Eng. Process. 2012;53:10–23. doi: 10.1016/j.cep.2012.01.003. [DOI] [Google Scholar]
- 9.Lima R.D.S., Nunes I.L., Block J.M. Ultrasonic-assisted extraction for the recovery of carotenoids from guava's pulp and waste powders. Plant Food Hum Nutr. 2020;75:63–69. doi: 10.1016/j.jfoodeng.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 10.Pádua D., Rocha E., Gargiulo D., Ramos A.A. Bioactive compounds from brown seaweeds: Phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem. Lett. 2015;14:91–98. doi: 10.1016/j.phytol.2015.09.007. [DOI] [Google Scholar]
- 11.D’Orazio N., Gemello E., Gammone M., de Girolamo M., Ficoneri C., Riccioni G. Fucoxantin: a treasure from the sea. Mar. Drugs. 2012;10:604–616. doi: 10.3390/md10030604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyashita K., Beppu F., Hosokawa M., Liu X., Wang S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020;686:108364. doi: 10.1016/j.abb.2020.108364. [DOI] [PubMed] [Google Scholar]
- 13.Goula A.M., Ververi M., Adamopoulou A., Kaderides K. Green ultrasonic-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2016;34:821–830. doi: 10.1016/j.ultsonch.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Kua Y.L., Gan S., Morris A., Ng H.K. Ethyl lactate as a potential green solvent to extract hydrophilic (polar) and lipophilic (non-polar) phytonutrients simultaneously from fruit and vegetable by-products. Sustain. Chem. Pharm. 2016;4:21–31. doi: 10.1016/j.scp.2016.07.003. [DOI] [Google Scholar]
- 15.Del Pilar Sánchez-Camargo A., Pleite N., Herrero M., Cifuentes A., Ibáñez E., Gilbert-López B. New approaches for the selective extraction of bioactive compounds employing bio-based solvents and pressurized green processes. J. Supercrit. Fluid. 2017;128:112–120. doi: 10.1016/j.supflu.2017.05.016. [DOI] [Google Scholar]
- 16.Larangeira P.M., de Rosso V.V., da Silva V.H.P., de Moura C.F.G., Ribeiro D.A. Genotoxicity, mutagenicity and cytotoxicity of carotenoids extracted from ionic liquid in multiples organs of wistar rats. Exp. Toxicol. Pathol. 2016;68(10):571–578. doi: 10.1016/j.etp.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 17.F. Chemat, M. A. Vian, Ethyl Lactate Main Properties, Production Processes, and Applications, Alternative Solvents for Natural Products Extraction, Springer: Berlin Heidelberg, 2014, pp. 107–125, doi: 10.1007/978-3-662-43628-8.
- 18.Wang Y., Liu J., Liu X., Zhang X., Xu Y., Leng F., Avwenagbiku M.O. Kinetic modeling of the ultrasonic-assisted extraction of polysaccharide from Nostoc commune and physicochemical properties analysis. Int. J. Biol. Macromol. 2019;128:421–428. doi: 10.1016/j.ijbiomac.2018.12.247. [DOI] [PubMed] [Google Scholar]
- 19.Ziabakhsh Deylami M., Abdul Rahman R., Tan C.P., Bakar J., Olusegun L. Effect of blanching on enzyme activity, color changes, anthocyanin stability and extractability of mangosteen pericarp: a kinetic study. J. Food Eng. 2016;178:12–19. doi: 10.1016/j.jfoodeng.2016.01.001. [DOI] [Google Scholar]
- 20.Wang J., Yang X., Mujumdar A.S., Fang X., Zhang Q., Zheng Z., Gao Z., Xiao H. Effects of high-humidity hot air impingement blanching (HHAIB) pretreatment on the change of antioxidant capacity, the degradation kinetics of red pigment, ascorbic acid in dehydrated red peppers during storage. Food Chem. 2018;259:65–72. doi: 10.1016/j.foodchem.2018.03.123. [DOI] [PubMed] [Google Scholar]
- 21.Goula A.M. Ultrasonic-assisted extraction of pomegranate seed oil - kinetic modeling. J. Food Eng. 2013;117:492–498. doi: 10.1016/j.jfoodeng.2012.10.009. [DOI] [Google Scholar]
- 22.Aissou M., Chemat-Djenni Z., Yara-Varón E., Fabiano-Tixier A.-S., Chemat F. Limonene as an agro-chemical building block for the synthesis and extraction of bioactive compounds. C. R. Chime. 2017;20(4):346–358. doi: 10.1016/j.crci.2016.05.018. [DOI] [Google Scholar]
- 23.Strati I.F., Oreopoulou V. Effect of extraction parameters on the carotenoid recovery from tomato waste. Int. J. Food Sci. Tech. 2011;46:23–29. doi: 10.1111/j.1365-2621.2010.02496.x. [DOI] [Google Scholar]
- 24.Wu W., Lu M., Yu L. A new environmentally friendly method for astaxanthin extraction from Xanthophyllomyces dendrorhous. Eur. Food Res. Technol. 2011;232(3):463–467. doi: 10.1007/s00217-010-1414-4. [DOI] [Google Scholar]
- 25.Li Y., Fabiano-Tixier A.S., Tomao V., Cravotto G., Chemat F. Green ultrasonic-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013;20:12–18. doi: 10.1016/j.ultsonch.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Fasina O.O., Colley Z. Viscosity and specific heat of vegetable oils as a function of temperature: 35℃ to 180℃. Int. J. Food Prop. 2008;11:738–746. doi: 10.1080/10942910701586273. [DOI] [Google Scholar]
- 27.Sachindra N.M., Mahendrakar N.S. Process optimization for extraction of carotenoids from shrimp waste with vegetable oils. Bioresource Technol. 2005;96(10):1195–1200. doi: 10.1016/j.biortech.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Zou T., Jia Q., Li H., Wang C., Wu H. Response surface methodology for ultrasonic-assisted extraction of Astaxanthin from Haematococcus pluvialis. Mar. Drugs. 2013;11:1644–1655. doi: 10.3390/md11051644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raguraman V., Stanley A.L., Mubarakali D., Narendrakumar G., Thirugnanasambandam R., Kirubagaran R., Thajuddin N. Unraveling rapid extraction of fucoxanthin from Padina tetrastromatica: purification, characterization and biomedical application. Process Biochem. 2018;73:211–219. doi: 10.1016/j.procbio.2018.08.006. [DOI] [Google Scholar]
- 30.Pasquet V., Chérouvrier J.-R., Farhat F., Thiéry V., Piot J.-M., Bérard J.-B., Kaas R., Serive B., Patrice T., Cadoret J.-P., Picot L. Study on the microalgal pigments extraction process: performance of microwave assisted extraction. Process Biochem. 2011;46(1):59–67. doi: 10.1016/j.procbio.2010.07.009. [DOI] [Google Scholar]
- 31.Delbrut A., Albina P., Lapierre T., Pradelles R., Dubreucq E. Fucoxanthin and polyunsaturated fatty acids co-extraction by a green process. Molecules. 2018;23:874. doi: 10.3390/molecules23040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ying Z., Han X., Li J. Ultrasonic-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011;127:1273–1279. doi: 10.1016/j.foodchem.2011.01.083. [DOI] [PubMed] [Google Scholar]
- 33.Qu W., Pan Z., Ma H. Extraction modeling and activities of antioxidants from pomegranate marc. J. Food Eng. 2010;99(1):16–23. doi: 10.1016/j.jfoodeng.2010.01.020. [DOI] [Google Scholar]
- 34.Rakotondramasy-Rabesiaka L., Havet J.-L., Porte C., Fauduet H. Solid-liquid extraction of protopine from Fumaria officinalis L.—kinetic modelling of influential parameters. Ind. Crop. Prod. 2009;29(2-3):516–523. doi: 10.1016/j.indcrop.2008.10.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.