Bamlanivimab and etesevimab are IgG1 monoclonal antibodies (mAbs) that bind to SARS-CoV-2 spike receptor-binding domain. Treatment with bamlanivimab 2800 mg and etesevimab 2800 mg has been shown to significantly reduce SARS-CoV-2 viral load at day 11 in mild to moderate COVID-19 patients.1 In the phase III part of the BLAZE-1 trial, the risk of COVID-related hospitalization or death was reduced by 70% in patients treated with this combination versus placebo.2
Subsequently, a temporary use authorization for bamlanivimab 700 mg and etesevimab 1400 mg was approved in France to treat patients presenting with high risk of developing severe COVID-19 infection.
Therefore, we treated 34 cancer patients presenting with a mild to moderate form of SARS-CoV-2 infection and no need for oxygen therapy, within 5 days of their first symptoms, with bamlanivimab 700 mg plus etesevimab 1400 mg. Median age was 62.5 (31-83) years old. Most patients presented with a solid tumor (n = 24, 71%); 10 (29%) had hematological malignancies and 47.1% were receiving chemotherapy.
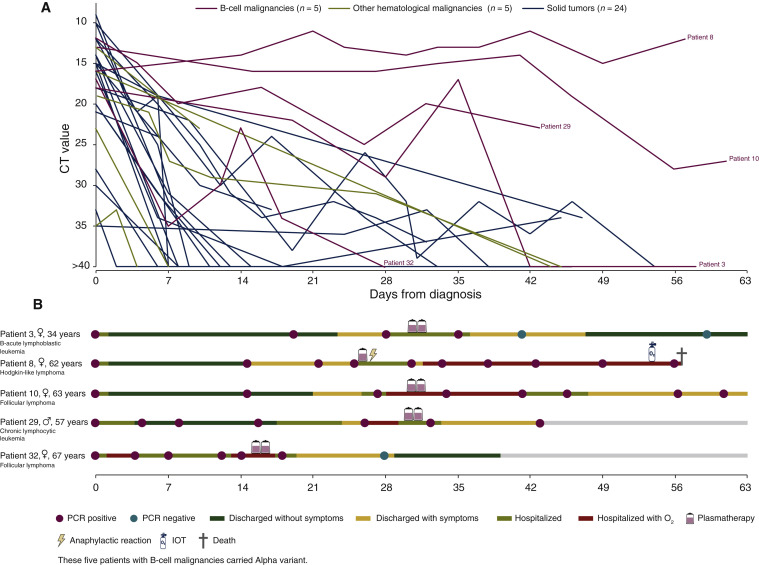
COVID-19 outcomes were mostly favorable with supplemental oxygenation required in only 29% of patients versus 41% in our historical cohort (unpublished data). Two patients died of COVID-19 pneumonia but were afterwards found out to be infected by variants with the spike E484K mutation. These favorable issues were correlated with increasing cycle threshold of nasopharyngeal PCR over time (Figure 1 ).
Figure 1.
CT and clinical evolution in patients according to cancer type.
CT, cycle threshold; IOT, orotracheal intubation.
Strikingly, we noticed that patients with B-cell malignancies (n = 5, 15%) displayed a worse clinical evolution, with delayed COVID-19 symptoms from day 14 to day 30 following the mAbs therapy. All of them needed to be hospitalized again after day 14. We carried out viral spike gene sequencing on pre-and post-bitherapy nasopharyngeal swabs. Four of these five patients acquired a mutation in the receptor binding domain: three had a Q493R mutation and one had a E484D mutation. Those five patients were rescued with convalescent plasma therapy: four patients recovered and were subsequently discharged, and one died from COVID-19.
Patients with hematological malignancies are at high risk of developing severe COVID-19, due to the inherent immune defect caused by the disease and therapy. In a meta-analysis including 3377 hematological malignancy patients, the risk of death among adult patients was 34% (95% CI, 28% to 39%; n = 3240).3 Also, CD8+ T cells might play a key role in COVID-19 patients with impaired humoral immunity.4
Lohr and colleagues reported the emergence of E484K and Q493R mutations after bamlanivimab monotherapy in an immunocompromised patient with the Alpha variant.5
In the Gottlieb study, putative treatment-emergent variants were detected in 1% of the patients (1/102) in the bamlanivimab and etesevimab combination group, and in 4.8% of the patients (7/145) in the placebo group.1 Our data shows that this risk could be much higher in patients with B-cell malignancies.
Blunting viral replication might be sufficient to avoid severe complications in immunocompetent patients, as the mAbs may initiate host immune response.6
Patients with impaired humoral and cellular immune response may have prolonged viral replication and higher viral load.7 Thus, pre-existing viral strains with escaping mAb-neutralization mutation could be selected. Higher doses of IgG1 mAbs, polyclonal antibodies or a combination of several mAbs should be interesting in such B-depleted patients.
Finally, while bamlanivimab 700 mg associated with etesevimab 1400 mg is effective in patients with solid tumors, patients with B-cell malignancies present a secondary clinical deterioration associated with immune escape mutations. Our observation highlights the importance of clinical trials in this specific population, including comprehensive clinical and virological follow-up.
Acknowledgments
Funding
None declared.
Disclosure
AB is a Principal/sub-Investigator of Clinical Trials for Abbvie, Adaptimmune, Adlai Nortye USA Inc, Aduro Biotech, Agios Pharmaceuticals, Amgen, Argen-X Bvba, Astex Pharmaceuticals, Astra Zeneca Ab, Aveo, Basilea Pharmaceutica International Ltd, Bayer Healthcare Ag, Bbb Technologies Bv, Beigene, BicycleTx Ltd, Blueprint Medicines, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Ca, Celgene Corporation, Chugai Pharmaceutical Co, Clovis Oncology, Cullinan-Apollo, Curevac, Daiichi Sankyo, Debiopharm, Eisai, Eisai Limited, Eli Lilly, Exelixis, Faron Pharmaceuticals Ltd, Forma Tharapeutics, Gamamabs, Genentech, Glaxosmithkline, H3 Biomedicine, Hoffmann La Roche Ag, Imcheck Therapeutics, Innate Pharma, Institut De Recherche Pierre Fabre, Iris Servier, Iteos Belgium SA, Janssen Cilag, Janssen Research Foundation, Kura Oncology, Kyowa Kirin Pharm. Dev, Lilly France, Loxo Oncology, Lytix Biopharma As, Medimmune, Menarini Ricerche, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Molecular Partners Ag, Nanobiotix, Nektar Therapeutics, Novartis Pharma, Octimet Oncology Nv, Oncoethix, Oncopeptides, Orion Pharma, Ose Pharma, Pfizer, Pharma Mar, Pierre Fabre, Medicament, Roche, Sanofi Aventis, Seattle Genetics, Sotio A.S, Syros Pharmaceuticals, Taiho Pharma, Tesaro, Turning Point Therapeutics, Xencor, received Research Grants from Astrazeneca, BMS, Boehringer Ingelheim, GSK, INCA, Janssen Cilag, Merck, Novartis, Pfizer, Roche, Sanofi, and Non-financial support (drug supplied) from Astrazeneca, Bayer, BMS, Boringher Ingelheim, GSK, Medimmune, Merck, NH TherAGuiX, Pfizer, Roche. JMM LA Pfizer, Novartis, Merck, Astra Zeneca, MSD, Roche, Ipsen, Bristol Myer Squibb, Astellas, Exeliquis, Amgen, Peloton therapeutics, Corvus Pharmaceuticals, all outside the submitted work. JMM is a principal/sub-investigator of clinical trials for Abbvie, Agios, Amgen, Astex, AstraZeneca, BMS, Boeringer Ingelheim, Celgene, Debiopharm, Forma, Genentech, GSK, Incyte, Innate Pharma, Janssen, Lilly, Loxo, Medimmune, MSD, Novartis, Pfizer, Pharmamar, Roche, Sanofi and Xencor; and reports non-financial support (drugs, equipment supplied by the entity, travel paid by the entity, writing assistance, administrative support, etc.) from AstraZeneca, Roche, Novartis, Gilead, Celgene and Bristol-Myers Squibb, all outside the submitted work. LA received grants and honoraria from Pfizer, Novartis, BMS, Ipsen, Roche, AstraZeneca, Amgen, Astellas, Exelixis, Corvus Pharmaceuticals, Peloton therapeutics, MSD, and Merck, outside the submitted work. KL received Advisory boards, travels grants, educational activities from Gilead, MSD, Abbvie, ViiV Healthcare, all outside the submitted work. PT is employed by Etablissement Français du Sang, the French transfusion public service in charge of collecting and issuing blood components in France. FB has personal financial interests in AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Eli Lilly Oncology, F Hoffmann-La Roche, Novartis, Merck, MSD, Pierre Fabre, Pfizer, and Takeda; and institutional financial interests in AbbVie, ACEA, Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Eisai, Eli Lilly Oncology, F. Hoffmann-La Roche, Genentech, Ipsen, Ignyta, Innate Pharma, Loxo, Novartis, Medimmune, Merck, MSD, Pierre Fabre, Pfizer, Sanofi-Aventis, and Takeda. FB is an advisory board member for Amgen, Bayer, BMS, CheckMab, Celgene, Daiichi Sankyo, Incyte, Merck, Novartis, F. Hoffmann-La Roche, and Seattle Genetics, all outside the submitted work. EC has acted as a consultant and participated in advisory boards for Ipsen, BMS, Sanofi, and Tesaro and Clovis, all outside the submitted work. All other authors have declared no conflicts of interest.
References
- 1.Gottlieb R.L., Nirula A., Chen P., et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dougan, M. Bamlanivimab+etesevimab for treatment of COVID-19 in high-risk ambulatory patients. Paper presented at the 28th Conference on Retroviruses and Opportunistic Infections. March 9, 2021; Virtual.
- 3.Vijenthira A., Gong I.Y., Fox T.A., et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bange E.M., Han N.A., Wileyto P., et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021;27(7):1280–1289. doi: 10.1038/s41591-021-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohr B., Niemann D., Verheyen J. Bamlanivimab treatment leads to rapid selection of immune escape variant carrying E484K mutation in a B.1.1.7 infected and immunosuppressed patient. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab392. [DOI] [PubMed] [Google Scholar]
- 6.Corti D., Purcell L.A., Snell G., Veesler D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell. 2021;184(12):3086–3108. doi: 10.1016/j.cell.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michot J.M., Hueso T., Ibrahimi N., et al. Severe COVID-19 in patients with hematological cancers presenting with viremia. Ann Oncol. 2021 doi: 10.1016/j.annonc.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]