Highlights
-
•
Genetic studies led to a deeper knowledge of the pathophysiological processes in CAD and the identification of novel treatment targets (e.g., in lipid metabolism).
-
•
The interplay between genetic factors and traditional risk factors, such as obesity, hypertension, smoking, or hyperlipidemia, but also noise and air pollution, is the subject of extensive research.
-
•
In the future, genetic risk scores may improve risk prediction and lead to the development of individualized treatment strategies, the cornerstone of precision medicine.
Key Words: cardiovascular diseases, coronary artery disease, genome-wide association studies, polygenic risk scores, precision medicine
Abbreviations and Acronyms: CAD, coronary artery disease; CXCL1, chemokine (C-X-C motif) ligand 1; GWAS, genome-wide association study; LDLR, low-density lipoprotein receptor; lncRNA, long non-coding RNA; LPL, lipoprotein lipase; MI, myocardial infarction; PCSK9, proprotein convertase subtilisin/kexin type 9
Summary
Many cardiovascular diseases are facilitated by strong inheritance. For example, large-scale genetic studies identified hundreds of genomic loci that affect the risk of coronary artery disease. At each of these loci, common variants are associated with disease risk with robust statistical evidence but individually small effect sizes. Only a minority of candidate genes found at these loci are involved in the pathophysiology of traditional risk factors, but experimental research is making progress in identifying novel, and, in part, unexpected mechanisms. Targets identified by genome-wide association studies have already led to the development of novel treatments, specifically in lipid metabolism. This review summarizes recent genetic and experimental findings in this field. In addition, the development and possible clinical usefulness of polygenic risk scores in risk prediction and individualization of treatment, particularly in lipid metabolism, are discussed.
Central Illustration
Cardiovascular diseases are the leading killers worldwide (1). Among these, coronary atherosclerosis and its complications, chronic or acute coronary syndromes, and ischemic heart failure are leading entities. A positive family history has been recognized for many years to be a risk factor for coronary artery disease (CAD), whereas the specific genetic variants underlying this observation remained obscure (2). Fifteen years ago, mutations leading to familial hypercholesterolemia were the only known genetic causes of CAD. Recently, advances in biotechnology facilitated large-scale genomic studies, which have led to remarkable discoveries. Currently, the complex etiology of CAD results from an interplay of hundreds of genetic risk variants that infiltrate the entire population, and in a systems biology sense, lay the cellular foundation for disease development. Based on this foundation, several (exogenous) risk factors, such as hypertension, diabetes mellitus, and smoking (3), aggravate the situation. This opens up new vistas on the pathophysiological processes in CAD and raises the hope for novel therapeutic concepts that are able to interfere with these, so far unknown, biological processes. In this review, we discuss recent findings in discovery and mechanistic exploration, as well as the therapeutic exploration of genetic risk variants for cardiovascular diseases with a focus on CAD.
Genetics of CAD and/or Myocardial Infarction
Marenberg et al. (4) reported in 1994 that monozygotic and dizygotic twins had a higher risk of dying from CAD if their twins died themselves from CAD at young age. The same is true for subjects with a relative who had a myocardial infarction (MI) (5). Early studies designed to unravel the underlying genetic risk factors had limited success. Particularly, candidate gene studies focusing on traditional risk factors or unrelated pathways (e.g., the angiotensin-converting enzyme) (6) resulted in dead-end streets. For a long time, only mutations in the low-density lipoprotein cholesterol receptor (LDLR) (7,8) or proprotein convertase subtilisin/kexin type 9 (PCSK9) (e.g., those leading to hypercholesterolemia) were considered as causal via this intermediate phenotype (9,10).
Since 2007, mainly 2 factors have changed the scenario: first, the introduction of arrays that enabled genome-wide genotyping of an increasing number of genomic variants with subsequent imputation of millions of further variants; and second, the formation of large international consortia jointly investigating the genetics of CAD. As a result, the last several years have provided deep insights into the genetic architecture of the disease with implications for future prevention and treatment strategies (11,12). In the beginning, most of the large-scale genetic studies mainly focused on subjects who had been recruited in Europe and the United States. With efforts as the UK Biobank (13), the CARDIoGRAMplusC4D 1M+ Hearts project (14), and the Million Veteran Program (15), the discovery of genetic risk factors for CAD and/or MI in subjects of European ancestry will continue. Meanwhile, however, several genome-wide association studies (GWASs) in other ethnic groups [e.g., Han Chinese (16) and Japanese (17)] have been performed. A recently published large-scale GWAS also revealed 43 novel loci in Japanese, highlighting the ongoing search for population-specific and trans-ancestry risk factors (18). These studies are of outmost importance, because the implementation of polygenic risk scores in clinical practice will largely depend on the accuracy of predicting effect sizes of risk alleles, which varies with the genetic background (19).
Genetics of CAD: overview
The first discovery in modern CAD genetics research is exemplary in its success and challenges, the chromosome 9p21 locus. It was unraveled independently by 3 research consortia at the same time (20, 21, 22, 23), and together with the LPA locus, represents the locus with the strongest effect on CAD risk (24). This is remarkable because how it exerts its molecular effects is not completely understood. The locus has been initially called a “gene desert” because an obvious traditional candidate gene could not be determined. Close-by genes encode the cyclin-dependent kinase inhibitors 2A/2B (CDKN2A/CDKN2B), which have been thoroughly investigated but not proven to be involved (25). Another interesting candidate is the long noncoding RNA (lncRNA) ANRIL because the risk allele associates with its expression, and increased expression of linear ANRIL was linked to enhanced atherosclerosis (26). In contrast, circular ANRIL seems to be protective (27). A recent elegant study used genetic engineering in induced pluripotent stem cells and showed that replacement of the non-risk genotype by the risk genotype led to overexpression of ANRIL and pro-atherogenic phenotypes in vascular smooth muscle cells (28).
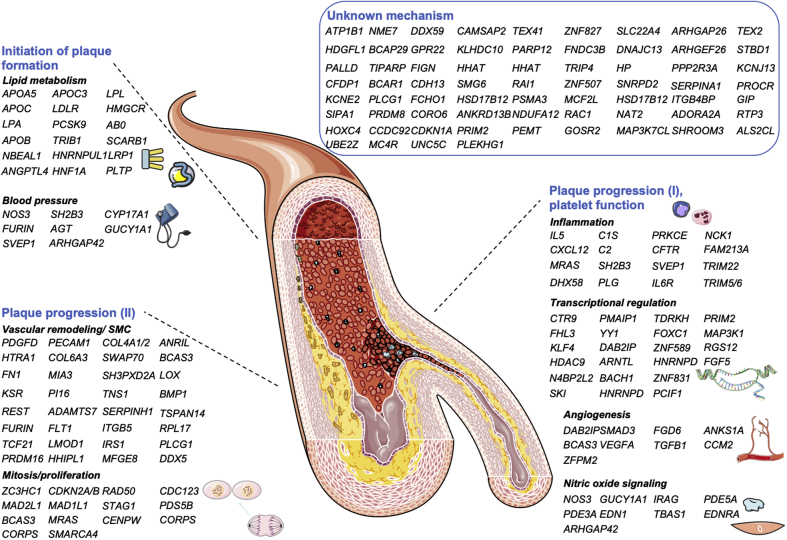
Since 2007, >200 loci like the one at chromosome 9p21 locus have been reported to be associated with CAD and MI. Comprehensive lists of these loci, including the risk alleles, allele frequencies, and candidates at the loci have been published previously [for an overview, please see (12,29)]. Although initially only a handful of loci could be explained by an influence on classical risk factors such as hypertension or hypercholesterolemia, currently, approximately one-half of the loci can be attributed to pathophysiological pathways, many of them being new “drivers” of CAD and/or MI (Figure 1). One example is nitric oxide signaling (43, 44, 45), which has been well characterized for modulating vascular tone but also plays a major role in precipitating genetic CAD risk (46,47).
Figure 1.
Currently Known CAD Genes and Supposed Mechanisms
Mechanisms were grouped to initiation, plaque progression, and platelet function but are not limited to this rough classification. Previously unknown genes were grouped to the proposed pathways if experimental data (using the queries “gene name” and “atherosclerosis” in PubMed) was published in the meantime: LMOD1 (30); NBEAL1 (31); IRS1 (32); PLCG1 (33); NCK1 (34,35); PRDM16 (36); HHIPL1 (37); MFGE8 (38); HNRNPUL1 (39); RAC1 (40,41); and DDX5 (42). CAD = coronary artery diseased. Modified after and updated from Erdmann et al. (12). Modified image material available at Servier Medical Art under a Creative Commons Attribution 3.0 Unsupported License.
Challenges in the identification of the mechanisms linking phenotype and genotype
GWASs detected numerous variants that are associated with CAD. However, the elucidation of the underlying mechanisms remains challenging. Usually, the ‘closest gene approach’ was used, that is, the genes that are located nearby the detected variant were reported as candidates. This strategy can be complemented by various bioinformatics approaches (e.g., expression data from datasets like GTEx (48)). The GTEx database includes genome-wide genotyping and transcriptome data, making it possible to report expression quantitative trait loci. Because a GWAS variant is associated with differential expression of a more distantly located gene, the closest gene might not be causal. A plethora of studies used such strategies or a combination of strategies to predict the causal genes at GWAS loci (49). Nevertheless, there may be a need to prove causal effects experimentally. A prominent example is the chromosome 6p24 locus. It harbors the PHACTR1 gene, which, intriguingly, was not only associated with CAD (50) but also with spontaneous coronary artery dissection (51) and coronary artery calcification (52). There are also experimental data that support association of calcification with PHACTR1 (53). However, an elegant experimental study revealed a rather surprising result. Using CRISPR-based genome editing in stem cell−derived endothelial cells, alteration of a variant at the chromosome 6p24 locus led to expression changes of EDN1 rather than PHACTR1 (54). EDN1 encodes endothelin-1, a well-studied vasoconstrictor and modulator of vascular smooth muscle cell function. Even if the results from genome editing are in doubt, the studied variant was also associated with endothelin-1 precursor protein levels in plasma, which validated the finding (54).
Another strategy might be the investigation of transgenic mouse models regarding atherosclerotic plaque formation. Although the limitations of this approach, because of the differences between mice and humans, are obvious, a recent review provided evidence that most of the genes identified by CAD GWASs that were already studied in atherosclerosis-prone mouse models revealed consistent results (55). Nevertheless, results from such studies need to be interpreted with caution because the approach of deleting genes might be too artificial. One example is the GUCY1A1 locus, which harbors private mutations leading to a loss of function (43), and common noncoding variants that are associated with CAD (56). However, the knockout of the murine counterpart Gucy1a1 led to enhanced atherosclerotic plaque formation (57), a finding that opposes the genetic associations. However, in a population-based approach in mice, a variant that was associated with reduced Gucy1a1 expression was also linked to enhanced atherosclerotic plaque formation (44). The approach of investigating genetic variation in mice (e.g., using the Hybrid Mouse Diversity Panel) (58) might be particularly useful. Taken together, these examples illustrate the hurdles on the way from genotype to phenotype that need to be considered when interpreting findings from such analyses.
A novel view on lipid metabolism
Although most of the genomic loci were not linked to CAD before, GWASs also identified genes that are associated with both CAD and traditional risk factors (e.g., lipid metabolism and blood pressure). While both hypertension and hypercholesterolemia increase CAD risk, lipid metabolism remains unique. Important examples of early associations are PCSK9 and LDLR, as discussed previously (59). However, there are more genes that expanded our view on lipid metabolism and might represent future treatment targets. In this section, we want to discuss 3 promising examples. In addition, Table 1 lists CAD risk genes in lipid metabolism that are targets of current and future treatment strategies.
Table 1.
CAD Genes Associated With CAD and Lipid Phenotypes in GWASs That Are Used as Therapeutic Targets
| Gene | Protein/Mechanism | Clinical Use/Perspective | Ref. # |
|---|---|---|---|
| APOC3 | Apolipoprotein C-3/lipoprotein lipase activity ⇓, plasma triglycerides ⇑ → increased CAD risk | Antisense APOC3 inhibitor (Volanesorsen) leads to a dose-dependent 31%–71% reduction in triglycerides, effective triglyceride reduction in familial chylomicronemia syndrome | (60, 61, 62, 63) |
| ANGPTL3/4 | Angiopoietin-like protein 3/4/Lipoprotein lipase activity ⇓, plasma triglycerides ⇑ → increased CAD risk | Evinacumab, a monoclonal antibody against ANGPTL3, reduced plasma LDL-cholesterol levels in patients with familial hypercholesterolemia by 47% | (60,64, 65, 66, 67, 68, 69, 70) |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9/LDL-cholesterol receptor recycling on the hepatocellular surface ⇓, LDL-cholesterol receptor density ⇓, LDL-cholesterol ⇑ → increased CAD risk | Monoclonal antibodies and antisense molecules reduce PCSK9 function and LDL-cholesterol and cardiovascular events | (9,50,71, 72, 73, 74, 75) |
| LPA | Lipoprotein(a)/lipoprotein with prothrombotic and proinflammatory properties → increased CAD risk | Lipoprotein(a) can be reduced by lipid apheresis; TQJ230, an antisense oligomer, is currently investigated in clinical trials (NCT04023552∗) | (76, 77, 78, 79, 80) |
| NPC1L1 | Niemann-Pick C1-Like 1/Involved in the resorption of cholesterol from the intestine, LDL-cholesterol ⇑ → increased CAD risk | Ezetimibe, a NPC1L1 inhibitor, was able to reduce LDL-cholesterol and cardiovascular events when added to statin therapy | (78,81,82) |
| HMGCR | 3-Hydroxy-3-methylglutaryl-CoA reductase/pivotal in endogenous cholesterol biosynthesis, LDL-cholesterol ⇑ → increased CAD risk | Statins targeting 3-hydroxy-3-methylglutaryl-CoA reductase have been repeatedly shown to reduce cardiovascular events | (83,84) |
CAD = coronary artery disease; CoA = coenzyme A; GWASs = genome-wide association studies; LDL = low-density lipoprotein; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Assessing the Impact of Lipoprotein (a) Lowering With TQJ230 on Major Cardiovascular Events in Patients With CVD [Lp(a)HORIZON].
SORT1
The chromosome 1p13.3 locus was identified early in the GWAS era (21,50). Subsequently, experimental studies identified sortilin 1 (SORT1) (85), a protein that plays an important role in the regulation of plasma LDL cholesterol by interacting with APOB in the Golgi apparatus in hepatocytes (85), as the most likely candidate gene at this locus. The risk variant affects gene expression with the risk allele being linked to lower SORT1 mRNA levels (86). Its use as a therapeutic target might be complicated because the enzyme affects a variety of phenotypes [for an overview, see (87,88)], including proper function of neurotrophins and neuron viability (89). However, SORT1 has also been linked with frontotemporal dementia, and clinical trials using a monoclonal antibody targeting SORT1 in this indication (e.g., A Phase 2 Study to Evaluate Safety of Long-term AL001 Dosing in Frontotemporal Dementia [FTD] Patients [INFRONT-2]; NCT03987295; A Phase 3 Study to Evaluate Efficacy and Safety of AL001 in Frontotemporal Dementia [INFRONT-3]; NCT04374136) are currently underway.
Lipoprotein lipase and its modulators
Lipoprotein lipase (LPL) is a vascular enzyme critically involved in metabolizing triglyceride-rich lipoproteins. The locus harbors both common, noncoding, and rare variants that lead to a loss of function and increased risk of CAD. Other variants lead to a gain of function and reduced CAD risk, respectively (90). Importantly, not only the LPL gene but also pivotal endogenous regulators of LPL activity are associated with CAD, including APOA5 (8,60), APOC3 (60, 61, 62), ANGPTL4 (91), and ANGPTL3 (67). For most of these, treatment approaches are currently being investigated (Table 1). ANGPTL3 in particular revealed promising results: evinacumab, an anti-Angptl3 antibody, reduces plasma triglyceride levels and atherosclerotic plaque formation in mice. In humans, the administration of the humanized counterpart confirmed the effect on plasma triglyceride levels (68), and recently, a beneficial effect was also shown for LDL cholesterol (70).
TRIB1
A less mature candidate is represented by the TRIB1 locus (56,78,92). TRIB1 also seems to influence lipid metabolism with an inverse correlation of hepatocellular TRIB1 expression and the expression of lipogenic genes; in line targeted overexpression of Trib1 in wild-type mice reduced cholesterol levels (93). Whether this approach can ultimately be transferred to human treatment will be interesting to follow.
Targeting the vascular interface
A number of risk genes have been classified to influence processes in the vascular wall. With increasing knowledge about the involvement of immune cells [for an overview, see (94)] and platelets [for an overview, see (95)] in atherosclerosis, it seems prudent to investigate the vascular interface where resident, recruited, and circulating cells, and lipids interact to beget atherosclerotic plaque formation. Some recently investigated genetic examples are discussed in the following section.
Genes involved in vascular smooth muscle cell function
Vascular smooth muscle cells can influence atherosclerosis by at least 2 mechanisms: first, via vascular tone, and thereby, modulation of blood pressure; second, via local processes including inflammation, vascular remodeling, and so on, leading either to stabilization or destabilization of the plaque. As expected, there is significant genetic overlap between these 2 phenotypes. An important example is the previously mentioned nitric oxide signaling pathway, in which, upon release of nitric oxide, vascular smooth muscle cells form the second messenger cyclic guanosine monophosphate (96). This leads to relaxation of vascular smooth muscle cells. Vice versa, mice that lack the nitric oxide receptor in vascular smooth muscle cells show a hypertensive phenotype (97). However, an intracellular increase in cyclic guanosine monophosphate levels also inhibits migration of vascular smooth muscle cells (98), which itself is a hallmark of atheroprogression (99). Another genetic locus mediating its effects most likely through alterations of vascular smooth muscle cell biology is the 9p21 locus (27,28). Further promising putative targets were identified and are discussed in this section.
TCF21
Recently, a comprehensive view on the role of the CAD gene TCF21 (79) in atherosclerosis was published. A disease-associated variant in the 3′ untranslated region of TCF21 alters its mRNA stability by differential binding of a microRNA (100), which allows insight into the fascinating interaction of disease genetics and gene regulation by noncoding RNAs. Wirka et al. (101) reported that a loss of Tcf21 resulted in an inhibition of the phenotype switch of vascular smooth muscle cells, and, as a consequence, there were fewer fibromyocytes in the fibrous cap of atherosclerotic plaques, which indicated reduced stability. It is now also known that the molecular mechanism, for example, involves an interaction of TCF21 with the myocardin-serum response factor pathway (102). Increasing the availability of TCF21 might have beneficial effects in the prevention and stabilization of atherosclerotic plaques, but further information about the production and fate of TCF21 is needed. Therapeutic targeting of the mRNA−microRNA interaction by oligomers might be even more complicated by the need for delivering such substances to the vascular smooth muscle cell.
ADAMTS7
A more convenient target in this regard might be the extracellular matrix protease ADAMTS-7 (79,103,104). It is produced by endothelial cells and vascular smooth muscle cells and has been shown to degrade multiple members of the thrombospondin family (105,106). Mice lacking Adamts-7 develop less atherosclerosis and are resistant to neointima formation secondary to vascular injury (106,107). The downstream mechanisms in atherosclerosis so far remain unknown. However, in vitro, ADAMTS-7 degrades the sushi, von Willebrand factor type A, EGF, and pentraxin domain containing 1 (SVEP1) protein (106), another extracellular matrix protein with an important role in development and lymphatic vessel formation (108). SVEP1 was identified as a CAD gene by an exome-wide association study (91). First results now render an atheroprotective role of SVEP1 possible with Svep1 haploinsufficiency promoting atherosclerotic plaque formation and recruitment of leukocytes to the vascular wall (109). Although there are still open questions regarding the role of circulating SVEP1 as a biomarker or risk modifier and the multiple sources of SVEP1 in the body, SVEP1 further indicates the need for the identification of ADAMTS-7 inhibitors as a possible upstream target to increase therapeutic effects.
HHIPL1
Novel players in the vascular wall with specific drugs available include hedgehog interacting protein-like 1 (HHIPL1) (79). HHIPL1 has been annotated to hedgehog signaling, which is indispensable in embryonic development, in particular for the coronary vasculature, and ischemia-driven neoangiogenesis (110,111). In atherosclerosis, HHIPL1 represents an interactor of hedgehog signaling in vascular smooth muscle cells with a positive influence on vascular smooth muscle cell migration and proliferation. Mice lacking Hhipl1 present smaller atherosclerotic plaques (37). Further evidence can be drawn from a study in which antagonizing hedgehog signaling led to the opposite effect with larger atherosclerotic plaques (112). Of note, in a recently published analysis of genetic variants that influence atherogenic phenotypes in vascular smooth muscle cells in vitro, there was a considerable overlap of genetic variants that influenced such vascular smooth muscle cell phenotypes and genetic variants that are associated with CAD (113).
Circulating cells and vascular inflammation
In the process of atherosclerotic plaque formation and progression, lipid-laden monocytes are recruited to the intimal layer, giving rise to foam cells, cell necrosis, and apoptosis, as well as plaque progression and/or destabilization (99). The benefits from lipid-lowering therapies are well documented. The inflammatory part of this process was controversially discussed in epidemiology and clinical trials, albeit experimental evidence on the role of inflammation in atherosclerosis was overwhelming. The CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study) (114) and studies using colchicine in patients with CAD (115,116), however, showed that independent of lipid metabolism, targeting the inflammatory cytokines reduced cardiovascular events in high-risk subjects. This and the experimental evidence, summarized in Schloss et al. (117), is now complemented by genetic evidence. Interestingly, genes with GWAS evidence for association with CAD being classified as playing a role in inflammation do not contain the usual suspects such as interleukin-1β (118), components of the NLRP3 inflammasome (119), or classic proatherogenic chemokines (e.g., chemokine [C-X-C motif] ligand 1 [CXCL1]) (120). However, another member of the CXCL family, CXCL12, as well as IL6R, are CAD genes (21,79). CXCL12 represents the ligand for CXCR4 and signaling via this receptor influences atherosclerosis phenotypes. In particular, endothelial cell−derived CXCL12 seems to have pro-atherosclerotic properties (121), whereas the role of CXCR4 is more complex (122), which limits its current value in prevention and therapy.
The role of platelets in mediating the effects of the GWAS loci remains more controversial. However, the observation that antiplatelet therapy with aspirin, a strategy that has no value in primary prevention, reduces the incidence of cardiovascular events in subjects homozygous for the risk allele at the GUCY1A1 locus will fuel further research (45).
Polygenic Risk Scores
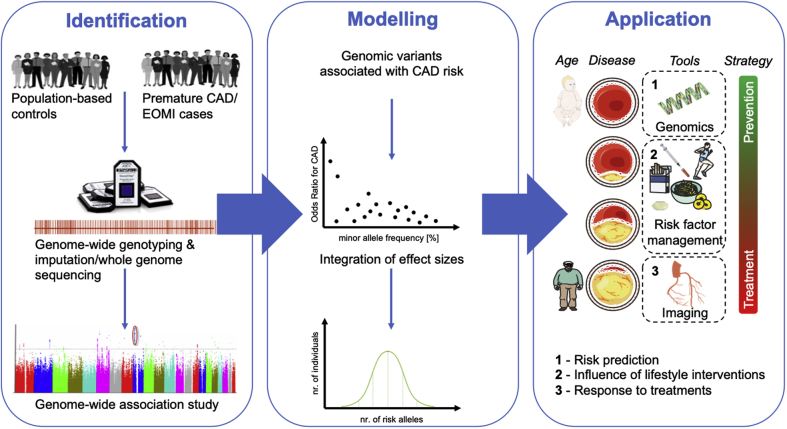
Promises of genetic studies of adding knowledge to the pathophysiology of CAD and identifying novel therapeutic targets seem to be fulfilled. Another promise is to identify subjects at risk with a higher precision than conventional risk scores. Undoubtedly, a polygenic risk score can predict information already at birth (i.e., long before risk factors or imaging modalities may be used in this respect) (Figure 2). Therefore, genetic information offers the only tool to guide primordial prevention (i.e., targeted interventions) before onset of traditional risk factors or visible disease manifestations. However, initial results were disappointing [for an overview, see (123)]. The discovery of many more genomic variants that are associated with CAD, the possibility to impute millions of variants throughout the human genome that have not been directly genotyped, biostatistical modeling, and the availability of large cohorts as the UK Biobank (13) have enabled the construction of (genome-wide) polygenic risk scores that changed the scenario. In addition, this recent approach is used to rather discriminate differences in absolute risk between subjects than to differentiate healthy and diseased subjects, which might be more appropriate. The methodological and statistical background cannot be fully addressed in this review and was recently summarized by Aragam and Natarajan (124). In brief, a polygenic risk score is calculated as the sum of a number of genomic variants weighted for their effect estimate, which has been determined by GWASs (125) (Figure 2).
Figure 2.
Generation and Use of Polygenic Risk Scores
Identification: genome-wide association studies are required to identify variants that are associated with CAD and early-onset myocardial infarction (EOMI). Bioinformatic tools enable the imputation of not directly genotyped variants to increase the numbers of variants that can then be compared between healthy control subjects and cases. Statistical analysis leads to the identification of variants that are associated with CAD at the genome-wide level of significance (p < 10−8). Modeling: in a second step, polygenic risk scores are based on modeling the sum of particular risk alleles, integrating their effect sizes. Polygenic risk scores follow a Gaussian distribution with most subjects carrying an intermediate number of risk alleles and a small portion either carrying a small or a large number of risk alleles. Application: genetic information is the only tool in risk prediction and management that is basically available at birth and could be used to predict risk and tailor prevention strategies. During life, the influence of risk factors and their management becomes increasingly important. In older adults, imaging to detect atherosclerosis and its complications remains the main diagnostic tool. Over time, the strategy shifts from prevention in young and middle-aged subjects to treatment and secondary prevention. However, genetic information could, be used at all stages to predict risk in young and middle-aged individuals (1), evaluate the beneficial effects of lifestyle but also pharmacological interventions (2), and predict the response to a given treatment strategy (e.g., statins or PCSK9 inhibitors) (3). For details, see text. Modified image material available at Servier Medical Art under a Creative Commons Attribution 3.0 Unsupported License. Abbreviations as in Figure 1.
Currently, such scores are often used in research: 1) to predict the risk of experiencing CAD or major complications; 2) to identify subjects who may display strong benefit from a given intervention or therapy; and 3) to study the inter-relationship between the risk of CAD and other phenotypes.
Prediction of risk
One of the first approaches used the chromosome 9p21 locus to predict CAD. In middle-aged men, the genetic information on this locus was added to the Framingham Risk Score and compared the combination to the Framingham Risk Score alone. Although there was an improvement in reclassification (i.e., approximately 13% of men could be more accurately classified to their risk group), there was no substantial value of adding chromosome 9p21 information (126). The same was basically observed for further studies that incorporated >10 risk loci (127). In comparison, a polygenic risk score may incorporate many more variants that do not necessarily meet the criterion of being associated with CAD at the genome-wide significance threshold. Such scores consist of up to 6 million variants. This concept revealed that although all subjects have a given number of risk alleles, only a few subjects have very high or very low numbers. Nevertheless, this means for those at the extreme ends that the risk of suffering from CAD is markedly decreased or increased. In a study published by Khera et al. (128), the risk for those subjects who represented the 8%, 2.3%, and 0.5% of the largest number of risk alleles was increased >3-, 4-, and 5-fold, respectively. This prompted an interesting comparison. Because familial hypercholesterolemia has a prevalence of 0.4% in the population with a 3-fold increase in CAD risk, and the subjects at the top 0.5% of the polygenic risk score display a >5-fold increased CAD risk, the polygenic risk score has a higher predictive power than diagnosis of a monogenic disease as familial hypercholesterolemia (128). Furthermore, this seems to be independent of the information of a positive family history (129). In a cohort of patients who experienced early-onset MI, the prevalence of familial hypercholesterolemia was 1.7%, and the estimated increase in MI risk was estimated as 3.8-fold. A comparable increase in risk of MI was observed for those subjects in the top 5% polygenic risk score. However, with 17%, the prevalence was 10 times higher. Mean LDL cholesterol levels were 206 and 132 mg/dl in those with familial hypercholesterolemia and subjects in the top 5% polygenic risk score, respectively, which indicated independent mechanisms (130). Along this line, genetic risk scores performed better when they were compared with traditional risk factors (e.g., smoking or hypercholesterolemia) (131). Importantly, this concept does not only work in subjects of European ancestry. In a study focusing on South Asian subjects, the top 5% polygenic risk score was also associated with an approximately 3- to 4 times increased risk of CAD (132). Nevertheless, most of the contemporary polygenic risk scores for CAD are derived from studies in subjects of European ancestry and are not necessarily transferable to populations of African ancestry (133). Together with uncovering novel risk factors, ancestral differences remain a major challenge in this field of genome-driven precision medicine.
A step towards precision medicine?
Personalized medicine incorporating genetic information is often regarded as a cornerstone of the term “precision medicine.” As 2 examples, we aim to discuss: 1) what polygenic risk scores could provide for subjects to change their lifestyle and behavior; and 2) how polygenic risk could inform decision-making in the use of lipid-lowering drugs specifically in primary prevention.
Genetics and lifestyle
Knowledge of genetic CAD and/or MI risk could tailor prevention and treatment strategies to specific patient groups. Subjects carrying a high genetic risk could more profoundly reduce risk if they follow a healthy lifestyle with regular exercise, healthy diet, and absence of smoking (134). In the UK Biobank, compared with subjects with a low genetic risk and a poor lifestyle, risk of CAD was increased >4-fold in subjects with high genetic risk and a poor lifestyle. Despite this impressive interplay of independent risk factors, subjects with a high genetic risk but a healthy lifestyle also displayed a lower risk of CAD as subjects with a low genetic risk but a poor lifestyle (135). However, the power of genetic risk scores to beneficially influence behavior is challenged by observations that indicate that the communication of genetic risk does not influence smoking, physical activity, or diet (136). Most of the recent studies were small and found no or little effect. For example, Jouni et al. (137) reported no improvement in metrics as participation in decision-making or perception of the quality of discussion in 207 subjects, one-half of whom were informed about traditional risk factors alone or the combination of traditional risk factors with genetic risk. Further prospective studies are needed to determine whether knowledge of genetic risk might translate to a reduction in CAD risk.
Individualized treatment
Genetic risk factors might also influence response to therapeutic strategies. An exciting example is that subjects with a high genetic risk score had larger benefit from statin treatment, both in secondary and primary prevention (138). A genetic score including 57 variants identified subjects at high genetic risk for CAD who also displayed the strongest reduction in CAD risk, with 46% compared with 26% in subjects with a lower genetic risk (139). In addition, knowledge of genetic risk might help to identify undertreated individuals. In patients with high cardiovascular risk (e.g., in patients with hypercholesterolemia), the use of statins is well established and recommended by current guidelines (140). Although a high polygenic risk score is associated with an increase in risk comparable to traditional risk factors, statins were not prescribed as often in such subjects (141,142). Two further studies revealed that polygenic risk scores were also able to identify subjects who benefitted most from treatment with the PCSK9 inhibitors evolocumab and alirocumab (143,144). In a post-hoc analysis of FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) trial (74), the absolute risk reduction by the monoclonal antibody evolocumab was 1.4% in participants with multiple traditional risk factors but low genetic risk; in contrast, subjects with a high genetic risk displayed an absolute risk reduction of 4.0%, regardless of traditional risk factors (143). Likewise, alirocumab treatment led to an absolute risk reduction of 1.5% in participants with low genetic risk compared with 6% in subjects with a high genetic risk (144). Whether these post hoc results, although encouraging in identifying treatment responders, can be replicated in prospective clinical trials on subjects without previous disease manifestation in line with an acute coronary syndrome is awaited. In addition, we so far only have limited knowledge how genetic risk, which inherently includes alterations in a variety of pathophysiological pathways, is modulated by 1 single therapy. Therefore, although the data on statins are encouraging, we need to be aware that this class of drugs rather represents a role model because the studies and the necessary data are available. Because only a small number of the genes that have been tagged by GWASs variants are involved in lipid metabolism, we currently do not know which other drugs might reveal comparable benefits.
Risk of CAD and other phenotypes
Quantifying the genetic risk of CAD using a genetic score can also be used to study the connection between CAD and other traits on the basis of genetics. A recent example by Ntalla et al. (145) is based on a genetic risk score that incorporated 300 CAD-related variants and found an association with a plethora of cardiovascular phenotypes: 1) traditional CAD risk factors, such as hypercholesterolemia, hypertension, obesity, and type 2 diabetes; 2) diseases that occur as complications of CAD, such as atrial fibrillation, heart failure, and premature death; and 3) other cardiovascular diseases, such as migraine, abdominal aortic aneurysm, aortic stenosis, peripheral artery disease, and stroke. Although the overlap in genetic risk does not necessarily prove causality, it powerfully demonstrates the potential of genetic scores to investigate complex interactions in large-scale datasets. Education, for example, is known to influence lifestyle behavior, such as smoking, diet, or physical activity (146). As for CAD, a strong genetic component was also shown for intelligence and educational attainment (i.e., the length of school education). In Europe, educational attainment was inversely correlated with the incidence of CAD, meaning that least educated subjects had the highest CAD incidence and mortality rates (147). A genetic score for educational attainment was also correlated with CAD with an inverse relationship. The score was also associated with the traditional risk factors of smoking, obesity, and hypertension. When the model was adjusted for these risk factors, the association between the educational attainment genetic score and risk of CAD was attenuated, indicating that educational attainment mediates CAD risk via lifestyle. Another important finding was that the association was not altered by adjustment for the number of years spent in school (148). Taken together, it was hypothesized that some genetic variants affect lifestyle behavior via educational attainment, and some genetic variants directly influence these lifestyle factors, culminating in increased risk of CAD. Therefore, increasing the duration of school education as a consequence might not be the clue to reduce the impact of education on CAD risk (149).
Limitations and challenges of polygenic risk scores
As mentioned previously, transferability of results from subjects of European ancestry to other ethnicities remains a challenge. In addition, the search for genetic risk variants is still ongoing. The heritability of CAD, which is explained by the currently known risk variants, is still believed to be below approximately 30%, and the concept of “missing heritability” remains an open issue (150). It remains questionable whether it is theoretically possible to identify variants that explain all or even a large portion of the heritability of complex traits. However, is this even necessary, particularly in risk prediction? Perhaps the genetic basis of complex traits as CAD needs to be understood as probabilistic rather than deterministic. In addition, even the risk, which is believed to be determined by monogenic risk variants, seems to be modulated by polygenic risk (151). Besides the hurdles in the medical community to adequately implement genetic risk scores in clinical practice, there are also psychosocial factors that need to be considered. Knowledge of genetic risk could lead to fear, but also, in cases of low genetic risk, to the feeling of invulnerability. In addition, there are also studies that pour some water into the wine. In another analysis of the UK Biobank data that compared standard risk models and models, including polygenic risk scores, the reclassification improvement was present but modest (152). A further comparison of a standard model to determine CAD risk compared with a model that also included a polygenic risk score in the ARIC (Atherosclerosis Risk In Communities) study and the MESA (Multi-Ethnic Study of Atherosclerosis) failed to show improved reclassification of patients (153). Guidelines that address the indication, implementation, and proper genetic counseling are needed before genetic risk scores will enter clinical routine. The basis for this need is prospective clinical trials that definitively evaluate the benefit of genetic risk scores in prevention and treatment.
Summary and Conclusions
Within the past 15 years, tremendous success has been made in the elucidation of CAD genetics. Although genetic studies often face the critique that they have not led to a breakthrough in a reduction of CAD incidence, they have certainly led to identification of novel treatment targets. Some of these are already addressed in clinical practice (e.g., PCSK9), whereas others are still a focus of translational studies but may be ready for prime time in the near future. In addition, GWASs give an exciting insight into the pathophysiology of CAD—that most of the identified loci are not associated with traditional risk factors and that the role of almost one-half of the loci in the pathophysiology still remains unknown. It is obvious that our understanding of coronary atherosclerosis remains incomplete and requires further experimental research. Lastly, the rise of polygenic risk scores demonstrates the power of genetics to improve risk prediction and personalize prevention and treatment strategies. The Central Illustration summarizes the impact of CAD genetics on these different fields.
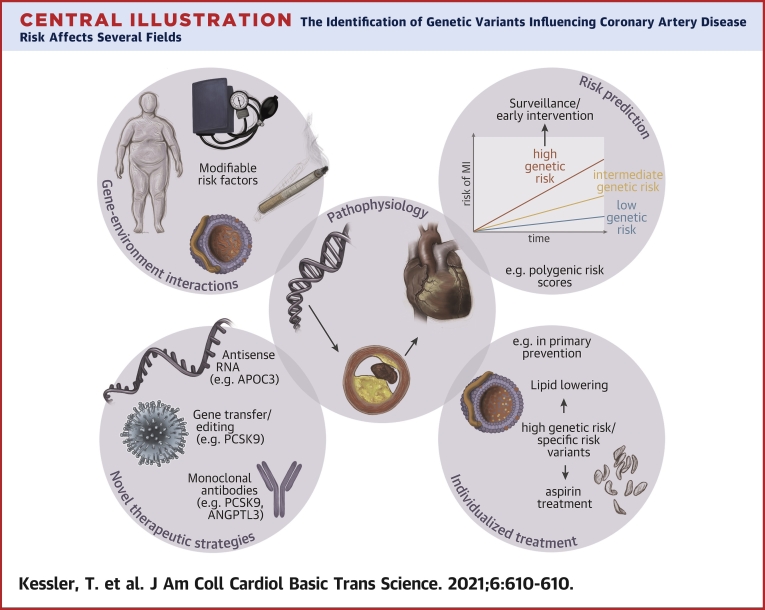
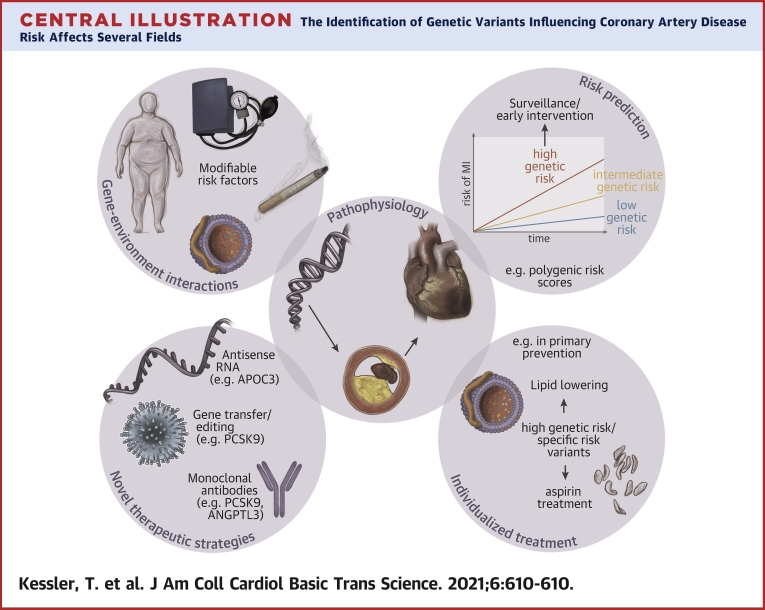
Central Illustration.
The Identification of Genetic Variants Influencing Coronary Artery Disease Risk Affects Several Fields
From a deeper knowledge of the: 1) pathophysiological processes, 2) novel treatment targets were identified; 3) the interplay between genetic factors and traditional risk factors such as obesity, hypertension, smoking, or hyperlipidemia but also noise and air pollution is the subject of extensive research. Finally, genetic risk scores may in the future 4) improve risk prediction and lead to the development of 5) individualized treatment strategies, the cornerstone of precision medicine. PCSK9 = proprotein convertase subtilisin/kexin type 9. Modified image material available at Servier Medical Art under a Creative Commons Attribution 3.0 Unsupported License.
Outlook
What can we expect from the near future? Results from ongoing efforts of large international consortia to identify further CAD risk variants are awaited. The opportunity to perform exome or even whole-genome sequencing at low cost will probably lead to the identification of further rare variants with large effects and thereby reduce the “missing heritability.” It will furthermore be exciting to see whether polygenic risk scores can overcome the discussed hurdles and be implemented into clinical practice. Lastly, can we expect the discovery of novel therapeutic targets? So far, medications inspired by genetic findings all interact with lipid metabolism. Although the drugs are new, and the clinical results are promising, they do not represent entirely new therapeutic concepts. This basically also applies to inflammation-related targets. It will be exciting to see whether we will witness the introduction of therapies that address vascular remodeling or cell proliferation to reduce atherosclerotic plaque formation and progression. However, even if this is not the case, findings from genetic studies in CAD and other cardiovascular diseases will continue to expand our view on the biology underlying the diseases, thereby also improving prevention and therapy.
Funding Support and Author Disclosures
Dr. Kessler was supported by the Corona Foundation as part of the Junior Research Group Translational Cardiovascular Genomics (S199/10070/2017) and the German Research Foundation as part of the collaborative research center SFB 1123 (B02) Dr. Schunkert was supported by German Research Foundation as part of the collaborative research centers SFB 1123 TRR 267 (B06) and also supported by additional grants received from the German Federal Ministry of Education and Research within the framework of ERA-NET on Cardiovascular Disease (ERA-CVD: grant JTC2017_21-040) and within the scheme of target validation (BlockCAD: 6GW0198K). Further support was received from the British Heart Foundation/DZHK collaborative project “Genetic discovery-based targeting of the vascular interface in atherosclerosis.” Dr. Schunkert has received personal fees from MSD Sharp & Dohme, Amgen, Bayer Vital GmbH, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, Servier, Brahms, Bristol-Myers-Squibb, Medtronic, Sanofi Aventis, Synlab, Pfizer, and Vifor T as well as grants and personal fees from Astra-Zeneca outside the submitted work. Drs. Schunkert and Kessler are named inventors on a patent application for prevention of restenosis after angioplasty and stent implantation outside the submitted work.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Benjamin E.J., Muntner P., Alonso A. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Schunkert H., Scheidt von M., Kessler T., Stiller B., Zeng L., Vilne B. Genetics of coronary artery disease in the light of genome-wide association studies. Clin Res Cardiol. 2018;120:963–968. doi: 10.1007/s00392-018-1324-1. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S., Hawken S., Ounpuu S. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 4.Marenberg M.E., Risch N., Berkman L.F., Floderus B., de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 5.Mayer B., Erdmann J., Schunkert H. Genetics and heritability of coronary artery disease and myocardial infarction. Clin Res Cardiol. 2007;96:1–7. doi: 10.1007/s00392-006-0447-y. [DOI] [PubMed] [Google Scholar]
- 6.Cambien F., Poirier O., Lecerf L. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature. 1992;359:641–644. doi: 10.1038/359641a0. [DOI] [PubMed] [Google Scholar]
- 7.Brown M.S., Goldstein J.L. Expression of the familial hypercholesterolemia gene in heterozygotes: mechanism for a dominant disorder in man. Science. 1974;185:61–63. doi: 10.1126/science.185.4145.61. [DOI] [PubMed] [Google Scholar]
- 8.Do R., Stitziel N.O., Won H.-H. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2015;518:102–106. doi: 10.1038/nature13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abifadel M., Varret M., Rabès J.-P. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J.C., Boerwinkle E., Mosley T.H., Hobbs H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 11.Khera A.V., Kathiresan S. Genetics of coronary artery disease: discovery, biology and clinical translation. Nat Rev Genet. 2017;18:331–344. doi: 10.1038/nrg.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdmann J., Kessler T., Munoz Venegas L., Schunkert H. A decade of genome-wide association studies for coronary artery disease: the challenges ahead. Cardiovasc Res. 2018;114:1241–1257. doi: 10.1093/cvr/cvy084. [DOI] [PubMed] [Google Scholar]
- 13.Littlejohns T.J., Sudlow C., Allen N.E., Collins R. UK Biobank: opportunities for cardiovascular research. Eur Heart J. 2019;40:1158–1166. doi: 10.1093/eurheartj/ehx254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CARDIoGRAMplusC4D 1M+ Hearts project. https://www.phpc.cam.ac.uk/ceu/1m-hearts-study Available at:
- 15.Gaziano J.M., Concato J., Brophy M. Million veteran program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214–223. doi: 10.1016/j.jclinepi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Lu X., Wang L., Chen S. Genome-wide association study in Han Chinese identifies four new susceptibility loci for coronary artery disease. Nat Genet. 2012;44:890–894. doi: 10.1038/ng.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi F., Yokota M., Yamamoto K. Genome-wide association study of coronary artery disease in the Japanese. Eur J Hum Genet. 2012;20:333–340. doi: 10.1038/ejhg.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyama S., Ito K., Terao C. Population-specific and trans-ancestry genome-wide analyses identify distinct and shared genetic risk loci for coronary artery disease. Nat Genet. 2020;1–27 doi: 10.1038/s41588-020-0705-3. [DOI] [PubMed] [Google Scholar]
- 19.Gola D., Erdmann J., Läll K. Population bias in polygenic risk prediction models for coronary artery disease. Circ Genom Precis Med. 2020;3:468. doi: 10.1161/CIRCGEN.120.002932. [DOI] [PubMed] [Google Scholar]
- 20.Helgadottir A., Thorleifsson G., Manolescu A. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 21.Samani N.J., Erdmann J., Hall A.S. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McPherson R., Pertsemlidis A., Kavaslar N. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke R., Peden J.F., Hopewell J.C. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 25.Visel A., Zhu Y., May D. Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature. 2010;464:409–412. doi: 10.1038/nature08801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holdt L.M., Teupser D. Recent studies of the human chromosome 9p21 locus, which is associated with atherosclerosis in human populations. Arterioscler Thromb Vasc Biol. 2012;32:196–206. doi: 10.1161/ATVBAHA.111.232678. [DOI] [PubMed] [Google Scholar]
- 27.Holdt L.M., Stahringer A., Sass K. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. 2016;7:12429. doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sardo Lo V., Chubukov P., Ferguson W. Unveiling the role of the most impactful cardiovascular risk locus through haplotype editing. Cell. 2018;175:1796–1810.e20. doi: 10.1016/j.cell.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kessler T., Schunkert H. Genomic strategies toward identification of novel therapeutic targets. Handb Exp Pharmacol. 2020;491:56. doi: 10.1007/164_2020_360. [DOI] [PubMed] [Google Scholar]
- 30.Perisic Matic L., Rykaczewska U., Razuvaev A. Phenotypic modulation of smooth muscle cells in atherosclerosis is associated with downregulation of LMOD1, SYNPO2, PDLIM7, PLN, and SYNM. Arterioscler Thromb Vasc Biol. 2016;36:1947–1961. doi: 10.1161/ATVBAHA.116.307893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bindesbøll C., Aas A., Ogmundsdottir M.H. NBEAL1 controls SREBP2 processing and cholesterol metabolism and is a susceptibility locus for coronary artery disease. Sci Rep. 2020;10:4528. doi: 10.1038/s41598-020-61352-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xi G., Shen X., Wai C., White M.F., Clemmons D.R. Hyperglycemia induces vascular smooth muscle cell dedifferentiation by suppressing insulin receptor substrate-1-mediated p53/KLF4 complex stabilization. J Biol Chem. 2019;294:2407–2421. doi: 10.1074/jbc.RA118.005398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma F., Li T., Zhang H., Wu G. MiR-30s family inhibit the proliferation and apoptosis in human coronary artery endothelial cells through targeting the 3'UTR region of ITGA4 and PLCG1. J Cardiovasc Pharmacol. 2016;68:327–333. doi: 10.1097/FJC.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 34.Wines-Samuelson M., Chowdhury S., Berk B.C. Nck1 is a critical adaptor between proatherogenic blood flow, inflammation, and atherosclerosis. J Clin Invest. 2020;130:3968–3970. doi: 10.1172/JCI138536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alfaidi M., Acosta C.H., Wang D., Traylor J.G., Orr A.W. Selective role of Nck1 in atherogenic inflammation and plaque formation. J Clin Invest. 2020;130:4331–4347. doi: 10.1172/JCI135552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu D., Song J., Ji X., Liu Z., Li T., Hu B. PRDM16 upregulation induced by microRNA-448 inhibition alleviates atherosclerosis via the TGF-β signaling pathway inactivation. Front Physiol. 2020;11:846. doi: 10.3389/fphys.2020.00846. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Aravani D., Morris G.E., Jones P.D. HHIPL1, a gene at the 14q32 coronary artery disease locus, positively regulates hedgehog signaling and promotes atherosclerosis. Circulation. 2019;140:500–513. doi: 10.1161/CIRCULATIONAHA.119.041059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viola J.R., Lemnitzer P., Paulin N. Deletion of MFGE8 inhibits neointima formation upon arterial damage. Thromb Haemost. 2018;118:1340–1342. doi: 10.1055/s-0038-1649522. [DOI] [PubMed] [Google Scholar]
- 39.Wang D., Liu B., Xiong T., Yu W., She Q. Investigation of the underlying genes and mechanism of familial hypercholesterolemia through bioinformatics analysis. BMC Cardiovasc Disord. 2020;20:419. doi: 10.1186/s12872-020-01701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bandaru S., Ala C., Ekstrand M. Lack of RAC1 in macrophages protects against atherosclerosis. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0239284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Healy A., Berus J.M., Christensen J.L. Statins disrupt macrophage RAC1 regulation leading to increased atherosclerotic plaque calcification. Arterioscler Thromb Vasc Biol. 2020;40:714–732. doi: 10.1161/ATVBAHA.119.313832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan Y., Chen Y., Zhang J. Protective role of RNA helicase DEAD-box protein 5 in smooth muscle cell proliferation and vascular remodeling. Circ Res. 2019;124:e84–e100. doi: 10.1161/CIRCRESAHA.119.314062. [DOI] [PubMed] [Google Scholar]
- 43.Erdmann J., Stark K., Esslinger U.B. Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature. 2013;504:432–436. doi: 10.1038/nature12722. [DOI] [PubMed] [Google Scholar]
- 44.Kessler T., Wobst J., Wolf B. Functional characterization of the GUCY1A3 coronary artery disease risk locus. Circulation. 2017;136:476–489. doi: 10.1161/CIRCULATIONAHA.116.024152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall K.T., Kessler T., Buring J.E. Genetic variation at the coronary artery disease risk locus GUCY1A3 modifies cardiovascular disease prevention effects of aspirin. Eur Heart J. 2019;40:3385–3392. doi: 10.1093/eurheartj/ehz384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wobst J., Schunkert H., Kessler T. Genetic alterations in the NO-cGMP pathway and cardiovascular risk. Nitric Oxide. 2018;76:105–112. doi: 10.1016/j.niox.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Dang T.A., Schunkert H., Kessler T. cGMP signaling in cardiovascular diseases: linking genotype and phenotype. J Cardiovasc Pharmacol. 2020;75:516–525. doi: 10.1097/FJC.0000000000000744. [DOI] [PubMed] [Google Scholar]
- 48.GTEx Consortium The genotype-tissue expression (GTEx) project. Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braenne I., Civelek M., Vilne B. Prediction of causal candidate genes in coronary artery disease loci. Arterioscler Thromb Vasc Biol. 2015;35:2207–2217. doi: 10.1161/ATVBAHA.115.306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myocardial Infarction Genetics Consortium. Voight B.F., Purcell S. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adlam D., Olson T.M., Combaret N. Association of the PHACTR1/EDN1 genetic locus with spontaneous coronary artery dissection. J Am Coll Cardiol. 2019;73:58–66. doi: 10.1016/j.jacc.2018.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Donnell C.J., Kavousi M., Smith A.V. Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation. 2011;124:2855–2864. doi: 10.1161/CIRCULATIONAHA.110.974899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aherrahrou R., Aherrahrou Z., Schunkert H., Erdmann J. Coronary artery disease associated gene Phactr1 modulates severity of vascular calcification in vitro. Biochem Biophys Res Commun. 2017;491:396–402. doi: 10.1016/j.bbrc.2017.07.090. [DOI] [PubMed] [Google Scholar]
- 54.Gupta R.M., Hadaya J., Trehan A. A genetic variant associated with five vascular diseases is a distal regulator of endothelin-1 gene expression. Cell. 2017;170:522–533.e15. doi: 10.1016/j.cell.2017.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Scheidt M., Zhao Y., Kurt Z. Applications and limitations of mouse models for understanding human atherosclerosis. Cell Metab. 2017;25:248–261. doi: 10.1016/j.cmet.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.CARDIoGRAMplusC4D Consortium. Deloukas P., Kanoni S. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segura-Puimedon M., Mergia E., Al-Hasani J. Proatherosclerotic effect of the α1-subunit of soluble guanylyl cyclase by promoting smooth muscle phenotypic switching. Am J Pathol. 2016;186:2220–2231. doi: 10.1016/j.ajpath.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 58.Bennett B.J., Davis R.C., Civelek M. Genetic architecture of atherosclerosis in mice: a systems genetics analysis of common inbred strains. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenson R.S., Hegele R.A., Koenig W. Cholesterol-lowering agents. Circ Res. 2019;124:364–385. doi: 10.1161/CIRCRESAHA.118.313238. [DOI] [PubMed] [Google Scholar]
- 60.Willer C.J., Sanna S., Jackson A.U. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pollin T.I., Damcott C.M., Shen H. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.The TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaudet D., Alexander V.J., Baker B.F. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373:438–447. doi: 10.1056/NEJMoa1400283. [DOI] [PubMed] [Google Scholar]
- 64.Kathiresan S., Melander O., Guiducci C. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lafferty M.J., Bradford K.C., Erie D.A., Neher S.B. Angiopoietin-like protein 4 inhibition of lipoprotein lipase: evidence for reversible complex formation. J Biol Chem. 2013;288:28524–28534. doi: 10.1074/jbc.M113.497602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helgadottir A., Gretarsdottir S., Thorleifsson G. Variants with large effects on blood lipids and the role of cholesterol and triglycerides in coronary disease. Nat Genet. 2016;48:634–639. doi: 10.1038/ng.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stitziel N.O., Khera A.V., Wang X. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol. 2017;69:2054–2063. doi: 10.1016/j.jacc.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dewey F.E., Gusarova V., Dunbar R.L. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377:211–221. doi: 10.1056/NEJMoa1612790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmad Z., Banerjee P., Hamon S. Inhibition of Angiopoietin-Like Protein 3 With a Monoclonal Antibody Reduces Triglycerides in Hypertriglyceridemia. Circulation. 2019;140:470–486. doi: 10.1161/CIRCULATIONAHA.118.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raal F.J., Rosenson R.S., Reeskamp L.F. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383:711–720. doi: 10.1056/NEJMoa2004215. [DOI] [PubMed] [Google Scholar]
- 71.Robinson J.G., Farnier M., Krempf M. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 72.Sabatine M.S., Giugliano R.P., Wiviott S.D. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 73.Ridker P.M., Revkin J., Amarenco P. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 2017;376:1527–1539. doi: 10.1056/NEJMoa1701488. [DOI] [PubMed] [Google Scholar]
- 74.Sabatine M.S., Giugliano R.P., Keech A.C. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz G.G., Steg P.G., Szarek M. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 76.Tregouet D.-A., König I.R., Erdmann J. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet. 2009;41:283–285. doi: 10.1038/ng.314. [DOI] [PubMed] [Google Scholar]
- 77.Emerging Risk Factors Collaboration. Erqou S., Kaptoge S. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teslovich T.M., Musunuru K., Smith A.V. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schunkert H., König I.R., Kathiresan S. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu R.Z., Gunawan R., Post N. Disposition and Pharmacokinetics of a GalNAc3-Conjugated Antisense Oligonucleotide Targeting Human Lipoprotein (a) in Monkeys. Nucleic Acid Ther. 2016;26:372–380. doi: 10.1089/nat.2016.0623. [DOI] [PubMed] [Google Scholar]
- 81.Myocardial Infarction Genetics Consortium Investigators. Stitziel N.O., Won H.-H. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N Engl J Med. 2014;371:2072–2082. doi: 10.1056/NEJMoa1405386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lauridsen B.K., Stender S., Frikke-Schmidt R., Nordestgaard B.G., Tybjaerg-Hansen A. Genetic variation in the cholesterol transporter NPC1L1, ischaemic vascular disease, and gallstone disease. Eur Heart J. 2015;36:1601–1608. doi: 10.1093/eurheartj/ehv108. [DOI] [PubMed] [Google Scholar]
- 83.Aragam K.G., Jiang T., Kanoni S. Genome-wide association study of over one million participants identifies 49 novel loci associated with coronary artery disease. Circulation. 2019;140(Suppl 1):A15391. [Google Scholar]
- 84.Chou R., Dana T., Blazina I., Daeges M., Jeanne T.L. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008–2024. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 85.Kjolby M., Andersen O.M., Breiderhoff T. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab. 2010;12:213–223. doi: 10.1016/j.cmet.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 86.Musunuru K., Strong A., Frank-Kamenetsky M. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466:714–719. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diemert P., Samani N.J., Schunkert H. Sorting out cholesterol and coronary artery disease. N Engl J Med. 2010;363:2462–2463. doi: 10.1056/NEJMcibr1010765. [DOI] [PubMed] [Google Scholar]
- 88.Diemert P., Heeren J., Aherrahrou Z. Genetic variation at chromosome 1p13.3 affects sortilin mRNA expression, cellular LDL-uptake and serum LDL levels which translates to the risk of coronary artery disease. Atherosclerosis. 2010;208:183–189. doi: 10.1016/j.atherosclerosis.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 89.Nykjaer A., Lee R., Teng K.K. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 90.Khera A.V., Won H.-H., Peloso G.M. Association of rare and common variation in the lipoprotein lipase gene with coronary artery disease. JAMA. 2017;317:937–946. doi: 10.1001/jama.2017.0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators Coding variation in ANGPTL4,LPL,and SVEP1and the risk of coronary disease. N Engl J Med. 2016;374:1134–1144. doi: 10.1056/NEJMoa1507652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.IBC 50K CAD Consortium Large-scale gene-centric analysis identifies novel variants for coronary artery disease. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burkhardt R., Toh S.-A., Lagor W.R. Trib1 is a lipid- and myocardial infarction–associated gene that regulates hepatic lipogenesis and VLDL production in mice. J Clin Invest. 2010;120:4410–4414. doi: 10.1172/JCI44213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Swirski F.K., Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018;18:733–744. doi: 10.1038/s41577-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 95.Kessler T., Schunkert H., Hundelshausen von P. Novel Approaches to Fine-Tune Therapeutic Targeting of Platelets in Atherosclerosis: A Critical Appraisal. Thromb Haemost. 2020;366:54–113. doi: 10.1055/s-0040-1714352. [DOI] [PubMed] [Google Scholar]
- 96.Feil R., Kemp-Harper B. cGMP signalling: from bench to bedside. Conference on cGMP generators, effectors and therapeutic implications. EMBO Rep. 2006;7:149–153. doi: 10.1038/sj.embor.7400627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Groneberg D., König P., Wirth A., Offermanns S., Koesling D., Friebe A. Smooth muscle-specific deletion of nitric oxide-sensitive guanylyl cyclase is sufficient to induce hypertension in mice. Circulation. 2010;121:401–409. doi: 10.1161/CIRCULATIONAHA.109.890962. [DOI] [PubMed] [Google Scholar]
- 98.Dubey R.K., Jackson E.K., Luscher T.F. Nitric oxide inhibits angiotensin II-induced migration of rat aortic smooth muscle cell. Role of cyclic-nucleotides and angiotensin1 receptors. J Clin Invest. 1995;96:141–149. doi: 10.1172/JCI118014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 100.Miller C.L., Haas U., Diaz R. Coronary heart disease-associated variation in TCF21 disrupts a miR-224 binding site and miRNA-mediated regulation. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wirka R.C., Wagh D., Paik D.T. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat Med. 2019;25:1280–1289. doi: 10.1038/s41591-019-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagao M., Lyu Q., Zhao Q. Coronary disease-associated gene TCF21 inhibits smooth muscle cell differentiation by blocking the myocardin-serum response factor pathway. Circ Res. 2020;126:517–529. doi: 10.1161/CIRCRESAHA.119.315968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coronary Artery Disease C4D Genetics Consortium A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43:339–344. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 104.Reilly M.P., Li M., He J. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. 2011;377:383–392. doi: 10.1016/S0140-6736(10)61996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu C.-J., Kong W., Ilalov K. ADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matrix protein. FASEB J. 2006;20:988–990. doi: 10.1096/fj.05-3877fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kessler T., Zhang L., Liu Z. ADAMTS-7 inhibits re-endothelialization of injured arteries and promotes vascular remodeling through cleavage of thrombospondin-1. Circulation. 2015;131:1191–1201. doi: 10.1161/CIRCULATIONAHA.114.014072. [DOI] [PubMed] [Google Scholar]
- 107.Bauer R.C., Tohyama J., Cui J. Knockout of Adamts7, a novel coronary artery disease locus in humans, reduces atherosclerosis in mice. Circulation. 2015;131:1202–1213. doi: 10.1161/CIRCULATIONAHA.114.012669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karpanen T., Padberg Y., van de Pavert S.A. An evolutionarily conserved role for Polydom/Svep1 during lymphatic vessel formation. Circ Res. 2017;120:1263–1275. doi: 10.1161/CIRCRESAHA.116.308813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Winkler M.J., Müller P., Sharifi A.M. Functional investigation of the coronary artery disease gene SVEP1. Basic Res Cardiol. 2020;115:67. doi: 10.1007/s00395-020-00828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lavine K.J., Long F., Choi K., Smith C., Ornitz D.M. Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development. 2008;135:3161–3171. doi: 10.1242/dev.019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lavine K.J., White A.C., Park C. Fibroblast growth factor signals regulate a wave of hedgehog activation that is essential for coronary vascular development. Genes Dev. 2006;20:1651–1666. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beckers L., Heeneman S., Wang L. Disruption of hedgehog signalling in ApoE - /- mice reduces plasma lipid levels, but increases atherosclerosis due to enhanced lipid uptake by macrophages. J Pathol. 2007;212:420–428. doi: 10.1002/path.2193. [DOI] [PubMed] [Google Scholar]
- 113.Aherrahrou R., Guo L., Nagraj V.P. Genetic regulation of atherosclerosis-relevant phenotypes in human vascular smooth muscle cells. Circ Res. 2020;52:48. doi: 10.1161/CIRCRESAHA.120.317415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 115.Tardif J.-C., Kouz S., Waters D.D. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 116.Nidorf S.M., Fiolet A.T.L., Mosterd A. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 117.Schloss M.J., Swirski F.K., Nahrendorf M. Modifiable cardiovascular risk, hematopoiesis, and innate immunity. Circ Res. 2020;126:1242–1259. doi: 10.1161/CIRCRESAHA.120.315936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vromman A., Ruvkun V., Shvartz E. Stage-dependent differential effects of interleukin-1 isoforms on experimental atherosclerosis. Eur Heart J. 2019;40:2482–2491. doi: 10.1093/eurheartj/ehz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grebe A., Hoss F., Latz E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018;122:1722–1740. doi: 10.1161/CIRCRESAHA.118.311362. [DOI] [PubMed] [Google Scholar]
- 120.Zernecke A., Shagdarsuren E., Weber C. Chemokines in atherosclerosis: an update. Arterioscler Thromb Vasc Biol. 2008;28:1897–1908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- 121.Döring Y., van der Vorst E.P.C., Duchene J. CXCL12 derived from endothelial cells promotes atherosclerosis to drive coronary artery disease. Circulation. 2019;139:1338–1340. doi: 10.1161/CIRCULATIONAHA.118.037953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Döring Y., Noels H., van der Vorst E.P.C. Vascular CXCR4 limits atherosclerosis by maintaining arterial integrity: evidence from mouse and human studies. Circulation. 2017;136:388–403. doi: 10.1161/CIRCULATIONAHA.117.027646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kessler T., Schunkert H. Clinical validation of genetic markers for improved risk estimation. Eur J Prev Cardiol. 2012;19:25–32. doi: 10.1177/2047487312448993. [DOI] [PubMed] [Google Scholar]
- 124.Aragam K.G., Natarajan P. Polygenic scores to assess atherosclerotic cardiovascular disease risk: clinical perspectives and basic implications. Circ Res. 2020;126:1159–1177. doi: 10.1161/CIRCRESAHA.120.315928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chatterjee N., Shi J., García-Closas M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet. 2016;17:392–406. doi: 10.1038/nrg.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Talmud P.J., Cooper J.A., Palmen J. Chromosome 9p21.3 coronary heart disease locus genotype and prospective risk of CHD in healthy middle-aged men. Clin Chem. 2008;54:467–474. doi: 10.1373/clinchem.2007.095489. [DOI] [PubMed] [Google Scholar]
- 127.Hughes M., Saarela O., Stritzke J. Genetic markers enhance coronary risk prediction in men: the MORGAM Prospective Cohorts. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0040922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khera A.V., Chaffin M., Aragam K.G. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50:1219–1224. doi: 10.1038/s41588-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tada H., Melander O., Louie J.Z. Risk prediction by genetic risk scores for coronary heart disease is independent of self-reported family history. Eur Heart J. 2016;37:561–567. doi: 10.1093/eurheartj/ehv462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Khera A.V., Chaffin M., Zekavat S.M. Whole-genome sequencing to characterize monogenic and polygenic contributions in patients hospitalized with early-onset myocardial infarction. Circulation. 2019;139:1593–1602. doi: 10.1161/CIRCULATIONAHA.118.035658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Inouye M., Abraham G., Nelson C.P. Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J Am Coll Cardiol. 2018;72:1883–1893. doi: 10.1016/j.jacc.2018.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang M., Menon R., Mishra S. Validation of a genome-wide polygenic score for coronary artery disease in South Asians. J Am Coll Cardiol. 2020;76:703–714. doi: 10.1016/j.jacc.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martin A.R., Gignoux C.R., Walters R.K. Human demographic history impacts genetic risk prediction across diverse populations. Am J Hum Genet. 2017;100:635–649. doi: 10.1016/j.ajhg.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khera A.V., Emdin C.A., Drake I. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375:2349–2358. doi: 10.1056/NEJMoa1605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Said M.A., Verweij N., van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank Study. JAMA Cardiol. 2018;3:693–702. doi: 10.1001/jamacardio.2018.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hollands G.J., French D.P., Griffin S.J. The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ. 2016;352:i1102. doi: 10.1136/bmj.i1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jouni H., Haddad R.A., Marroush T.S. Shared decision-making following disclosure of coronary heart disease genetic risk: results from a randomized clinical trial. J Investig Med. 2017;65:681–688. doi: 10.1136/jim-2016-000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mega J.L., Stitziel N.O., Smith J.G. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385:2264–2271. doi: 10.1016/S0140-6736(14)61730-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Natarajan P., Young R., Stitziel N.O. Polygenic risk score identifies subgroup with higher burden of atherosclerosis and greater relative benefit from statin therapy in the primary prevention setting. Circulation. 2017;135:2091–2101. doi: 10.1161/CIRCULATIONAHA.116.024436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grundy S.M., Stone N.J., Bailey A.L. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 141.Aragam K.G., Dobbyn A., Judy R. Limitations of Contemporary Guidelines for Managing Patients at High Genetic Risk of Coronary Artery Disease. J Am Coll Cardiol. 2020;75:2769–2780. doi: 10.1016/j.jacc.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lechner K., Kessler T., Schunkert H. Should We Use Genetic Scores in the Determination of Treatment Strategies to Control Dyslipidemias? Curr Cardiol Rep. 2020;22:146. doi: 10.1007/s11886-020-01408-9. [DOI] [PubMed] [Google Scholar]
- 143.Marston N.A., Kamanu F.K., Nordio F. Predicting benefit from evolocumab therapy in patients with atherosclerotic disease using a genetic risk score: results from the FOURIER Trial. Circulation. 2020;141:616–623. doi: 10.1161/CIRCULATIONAHA.119.043805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Damask A., Steg P.G., Schwartz G.G. Patients with high genome-wide polygenic risk scores for coronary artery disease may receive greater clinical benefit from alirocumab treatment in the ODYSSEY OUTCOMES Trial. Circulation. 2020;141:624–636. doi: 10.1161/CIRCULATIONAHA.119.044434. [DOI] [PubMed] [Google Scholar]
- 145.Ntalla I., Kanoni S., Zeng L. Genetic risk score for coronary disease identifies predispositions to cardiovascular and noncardiovascular diseases. J Am Coll Cardiol. 2019;73:2932–2942. doi: 10.1016/j.jacc.2019.03.512. [DOI] [PubMed] [Google Scholar]
- 146.Brunello G., Fort M., Schneeweis N., Winter-Ebmer R. The causal effect of education on health: what is the role of health behaviors? Health Econ. 2016;25:314–336. doi: 10.1002/hec.3141. [DOI] [PubMed] [Google Scholar]
- 147.Li L., Pang S., Zeng L., Güldener U., Schunkert H. Genetically determined intelligence and coronary artery disease risk. Clin Res Cardiol. 2021;110:211–219. doi: 10.1007/s00392-020-01721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zeng L., Ntalla I., Kessler T. Genetically modulated educational attainment and coronary disease risk. Eur Heart J. 2019;40:2413–2420. doi: 10.1093/eurheartj/ehz328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lager A.C.J., Torssander J. Causal effect of education on mortality in a quasi-experiment on 1.2 million Swedes. Proc Natl Acad Sci U S A. 2012;109:8461–8466. doi: 10.1073/pnas.1105839109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Manolio T.A., Collins F.S., Cox N.J. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fahed A.C., Wang M., Homburger J.R. Polygenic background modifies penetrance of monogenic variants for tier 1 genomic conditions. Nat Commun. 2020;11:3635. doi: 10.1038/s41467-020-17374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Elliott J., Bodinier B., Bond T.A. Predictive accuracy of a polygenic risk score-enhanced prediction model vs a clinical risk score for coronary artery disease. JAMA. 2020;323:636–645. doi: 10.1001/jama.2019.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mosley J.D., Gupta D.K., Tan J. Predictive accuracy of a polygenic risk score compared with a clinical risk score for incident coronary heart disease. JAMA. 2020;323:627–635. doi: 10.1001/jama.2019.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]