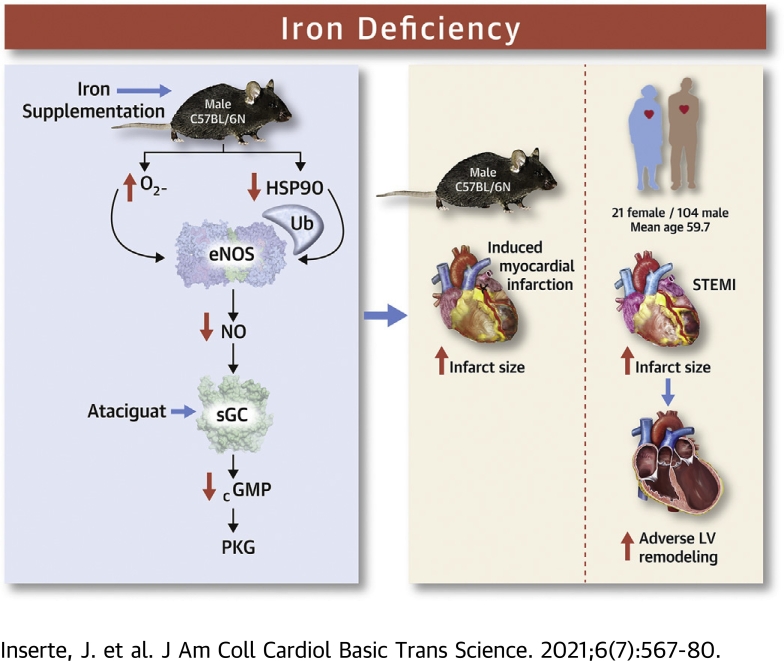
Visual Abstract
Key Words: acute myocardial infarction, endothelial nitric oxide synthase, iron deficiency, myocardial reperfusion, soluble guanylate cyclase
Abbreviations and Acronyms: CK-MB, creatine kinase-myocardial band; CMR, cardiac magnetic resonance; eNOS, endothelial nitric oxide synthase; HSP90, heat-shock protein 90; ID, iron deficiency; iNOS, inducible nitric oxide synthase; LV, left ventricular; MVO, microvascular obstruction; PKG, protein kinase G; sGC, soluble guanylyl cyclase; STEMI, ST-segment elevation acute myocardial infarction; STIR, short tau inversion recovery; VASP, vasodilator-stimulated phosphoprotein
Highlights
-
•
In patients with STEMI treated with primary percutaneous coronary intervention, iron deficiency is associated with larger infarcts, more extensive microvascular obstruction, and a higher frequency of adverse left ventricular remodeling.
-
•
An iron-deficient diet reduces the tolerance to ischemia/reperfusion in mice at least in part by interfering with the cardioprotective pathway eNOS/soluble guanylate cyclase/protein kinase G.
-
•
An iron-deficient diet reduces eNOS activity by increasing oxidative/nitrosative stress and its proteasome-dependent degradation.
-
•
Not only iron excess but also iron deficiency may have deleterious effects in the context of acute myocardial ischemia.
Summary
In patients with a first anterior ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention, iron deficiency (ID) was associated with larger infarcts, more extensive microvascular obstruction, and higher frequency of adverse left ventricular remodeling as assessed by cardiac magnetic resonance imaging. In mice, an ID diet reduced the activity of the endothelial nitric oxide synthase/soluble guanylate cyclase/protein kinase G pathway in association with oxidative/nitrosative stress and increased infarct size after transient coronary occlusion. Iron supplementation or administration of an sGC activator before ischemia prevented the effects of the ID diet in mice. Not only iron excess, but also ID, may have deleterious effects in the setting of ischemia and reperfusion.
Iron is essential for normal cardiovascular function. In patients with chronic heart failure, iron deficiency (ID) is common irrespective of anemia, it portends a worse prognosis, and its correction improves symptoms and quality of life (1,2). The significance of ID in acute coronary syndromes has been less investigated. Recent studies reported a high prevalence of ID in this setting but were inconsistent regarding its association with outcomes (3, 4, 5, 6).
Both iron excess and ID may negatively influence cardiomyocyte survival after transient ischemia. Because of its ability to redox cycle, iron excess promotes oxidative stress via Fenton-type reactions (7), and iron chelation has been shown to reduce experimental ischemia/reperfusion injury (8,9). Conversely, ID may affect the activity of essential iron-based enzymes, leading to reactive oxygen species production (10). Enhanced myocardial oxidative/nitrosative stress has been found in animals fed with low-iron diets, an effect attributed to increased superoxide production caused by mitochondrial dysfunction and inducible nitric oxide synthase (iNOS) expression (11) or to reduced antioxidant activity (12).
ID-induced oxidative stress may affect the activity of signaling pathways involved in cardiomyocyte survival after reperfusion (13,14). The endothelial nitric oxide synthase (eNOS)/soluble guanylyl cyclase (sGC)/protein kinase G (PKG) axis influences cell fate after restoration of flow, and increasing cGMP synthesis or reducing its degradation attenuates cardiomyocyte death in this setting (15,16). However, oxidative stress dramatically affects cGMP production (17, 18, 19). Because eNOS and sGC are iron-based enzymes, ID might also directly affect their activity (20). Finally, ID could influence myocardial healing and post-infarction left ventricular (LV) remodeling, because of its effect on infarct size (21,22), directly through the aforementioned mechanisms (23) or by enhancing inflammation (24,25). However, the implications of ID in acute myocardial infarction remain unclear, and the involvement of the eNOS/sGC/PKG pathway in this setting is unknown.
Accordingly, we aimed to assess whether ID is associated with infarct size and with adverse LV remodeling in patients with ST-segment elevation acute myocardial infarction (STEMI), whether it modifies myocardial tolerance to ischemia/reperfusion injury by altering the activity of the eNOS/sGC/PKG pathway in mice, and whether in this latter model its effects are antagonized by iron supplementation or sGC activation.
Methods
The protocol was approved by the Ethics Committees on Clinical Research (JAB-HIE-2017-01) and Animal Experimentation (CEEA-13.17) of Hospital Vall d’Hebron. All patients gave written informed consent to participate. The experimental procedures conformed to the EU directive 2010/63EU.
Patients
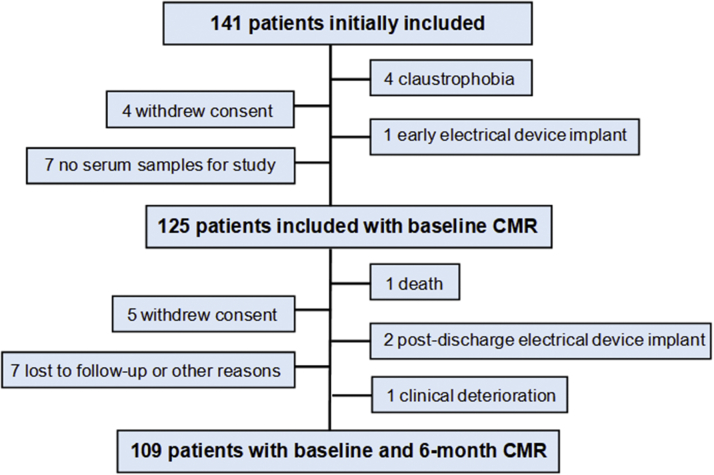
The study cohort consisted of 141 patients admitted to our acute cardiac care unit between October 2013 and May 2019 with a first anterior STEMI treated with primary percutaneous coronary intervention. Anterior STEMI was diagnosed by the presence of prolonged anginal symptoms associated with ST-segment elevation ≥0.2 mV in ≥2 anterior leads and with a significant elevation of cardiac biomarkers. Patients were not included if they had a history of previous infarction, significant valvular or myocardial disease, persistent clinical instability, persistent arrhythmia, or contraindications for cardiac magnetic resonance (CMR). Sixteen patients were excluded for different reasons, and 125 were finally included, of whom 109 underwent a second CMR at 6 months (Figure 1). Patients’ management was in accordance with practice guidelines.
Figure 1.
Patient Flowchart
CMR = cardiac magnetic resonance.
Clinical and laboratory variables
Demographic data, cardiovascular risk factors, comorbidities, hospitalization data, and pharmacologic therapy were prospectively collected. Anterograde flow in the culprit vessel was characterized using the TIMI (Thrombolysis In Myocardial Infarction) scale. The extent of coronary artery disease was assessed as the number of vessels with lesions ≥50%. Revascularization of significant distant lesions was often performed in a staged procedure. Blood levels of creatine kinase-myocardial band (CK-MB) were determined upon admission, at 4- to 6-hour intervals during the first 24 hours, and at 12-hour intervals between 24-48 hours after admission. Troponin values were disregarded because different assays were used throughout the study period.
The morning after admission, a fasting blood sample was obtained for standard hematological and biochemical determinations. An additional sample of 10 mL of blood was collected, and serum was obtained by centrifugation and stored at −80 °C. Free iron, ferritin, transferrin, and transferrin saturation were measured in the stored samples. ID was defined as ferritin level <100 μg/L or 100-299 μg/L if transferrin saturation was <20% (1,2).
CMR imaging
Studies were performed with 1.5-T equipment (Siemens Sonata or Avanto), as described (22,26). Electrocardiogram-gated breath-hold short-axis cine views were performed to quantify volumes and ejection fraction (steady-state free procession sequences; slice thickness 6 mm; space between slices 67%; matrix 256 × 256; field of view 300 mm-370 mm; temporal resolution <50 ms). Additional 2-chamber, 3-chamber, and 4-chamber views were obtained. Short tau inversion recovery (STIR) sequences were used in the same view as the cine sequences, in mid-diastole, to evaluate edema and hemorrhage (slice thickness 8 mm; space between slices 20%; matrix 256 × 256; field of view 300-370 mm; temporal resolution <50 ms; repetition time 2 R-R intervals; echo time 100 ms; inversion time 170 ms; flip angle 160°; bandwidth 781 Hz/pixel). Late gadolinium enhancement images were acquired at identical slice positions to the cine images after administration of 0.2 mmol/kg gadolinium-DTPA (Berlex). A segmented inversion-recovery gradient-echo sequence was acquired starting 10-15 min after contrast administration (matrix 256 × 197; voxel size 2.0 × 1.6 × 6 mm; TE 4.91 ms; TR 700 ms; flip angle 30°; bandwidth 140 Hz/pixel). Studies were analyzed by an experienced operator unaware of laboratory results. LV end-diastolic and end-systolic volumes and ejection fraction were calculated. In the initial study, myocardial edema was quantified in STIR sequences delineating the areas of hyperintensity with 2 SD above average, obtained from the remote healthy myocardium and normalized by LV mass. Intramyocardial hemorrhage was identified as the hypointense core within the hyperintense regions in STIR images, and its mass was calculated as published elsewhere (21). Infarct size was quantified by delineating the enhanced areas in the late gadolinium enhancement sequences with 5 SD above average, obtained from the remote healthy myocardium and normalized by LV mass. Microvascular obstruction (MVO) was defined by the presence of hypoenhanced zones inside hyperenhanced areas. Adverse LV remodeling was defined as an increase in LV end-diastolic volume ≥20% at 6 months with respect to baseline (26).
Mouse model of ID and monitoring
Male C57BL/6N mice (Charles River Laboratories) aged 6 weeks were kept on a control diet (control group; D10012M, Research Diets Inc), or after 250- to 300-μL blood withdrawal, on an ID diet (ID group; 3 mg/kg Fe; D03062702, Research Diets Inc) for 4 weeks, as previously described (27). To determine the effect of subsequent iron repletion, an additional group fed with ID diet received 2 mg/kg intravenous iron hydroxide in sucrose (Vifor Pharma) once a week (ID+Fe group). On week 4, plasma hemoglobin concentration was determined, and ferritin, transferrin, and iron concentrations were measured using an immunoturbidimetric and colorimetric assay (AU5800 Beckman Coulter analyzer). Myocardial iron content was determined in digested tissue samples using inductively coupled plasma-mass spectrometry (Nexion 350x, PerkinElmer) and was normalized to the dry weight of myocardium. Transthoracic echocardiography was performed at baseline and at week 4 using a Vivid-Q portable ultrasound system equipped with a i12L-RS 13 MHz transducer (GE Healthcare) as described earlier (28).
Quantification of oxidative and nitrosative stress
Production of superoxide was detected in myocardial cryosections by oxidation of dihydroethidium (Thermo Fisher Scientific) as previously described (13). Myocardial extracts were used to measure protein carbonyl, 8-isoprostane, and 3-nitrotyrosine as markers of oxidative and nitrosative stress (Cayman, items 10005020 and 516351, and Elabscience, respectively), and total antioxidant capacity (Cayman, item 709001) and superoxide dismutase-1 activity (Cayman, item 706002). Production of H2O2 in isolated fresh mitochondria was monitored in a spectrofluorimeter (SpectraMax GeminiXS, Molecular Devices) using malate, glutamate or succinate substrates as previously described (28).
Analysis of the eNOS/sGC/PKG pathway
Total myocardial nitrate/nitrite and cGMP were measured in myocardial homogenates by using, respectively, a nitrate/nitrite colorimetric assay and a competitive enzyme-linked immunosorbent assay (Cayman, items 780001 and 581021, respectively) following the manufacturer’s instructions.
Proteins were separated by Western blot as previously described (13). Primary antibodies used were raised against eNOS and pS1177-eNOS (BD Transduction Laboratories), iNOS (Abcam), heat-shock protein 90 (HSP90, Abcam), PKG (Enzo Life Sciences), ubiquitin (Abcam), vasodilator-stimulated phosphoprotein (VASP) and pS237-VASP (Cell Signaling Technology), and GAPDH (Genetex). Dimeric and monomeric forms of eNOS were separated by low-temperature electrophoresis as previously described (13). Protein bands were detected by chemiluminescence (ECL, Amersham) and quantified using a charge-coupled device system (Image Reader LAS-3000, Fujifilm) and image analysis software (Image Gauge, version 4.0 Fujifilm).
Changes in total and dimeric eNOS protein levels were also evaluated in isolated cardiomyocytes obtained as previously described (29) and incubated with the iron chelator deferasirox (Selleckchem) at 200 μmol/L for 4 hours in Hanks’ balanced salt solution (Gibco).
Polyubiquitination levels of eNOS were evaluated by using agarose-tandem ubiquitin-binding entities (LifeSensors) following the manufacturer’s protocol. Pulled-down myocardial samples were analyzed by immunoblotting using an eNOS antibody (BD Transduction Laboratories) and a polyubiquitin FK2 monoclonal antibody (Enzo Life Sciences).
In situ myocardial infarction
Mice were anesthetized (40 mg/kg pentobarbital–40 mg/kg ketamine) and subjected to 45-min left anterior descendent coronary artery ligation followed by 24-h reperfusion. Hearts were removed and retrogradely perfused with Krebs-Henseleit buffer for 1 min. The coronary artery was relegated at the same point, and the heart was perfused with 5% Evans blue and sliced in 6 short-axis sections (Zivic Instruments heart slicer matrix) that were incubated with 1% triphenyltetrazolium chloride (Sigma Chemical) for 10 min at 37 °C. The slices were weighed and photographed from both sides. The area at risk (Evans blue–negative) and the area of necrosis (Evans blue–negative and triphenyltetrazolium chloride–negative) were semiautomatically determined (Image ProPlus software version 4.5, Media Cybernetics) in the digital images and expressed as average percent area from both sides of each slice and corrected for slice weight: area at risk weight = (weight slice 1 × % area at risk slice 1) + … + (weight slice N × % area at risk slice N). Area of necrosis weight was calculated in the same manner. Finally, infarct size was calculated as percentage of the area at risk (28). To explore the effect of sGC activation on myocardial infarction, a subgroup of animals from the control and ID groups received 10 μg/kg ataciguat (HMR1766, Sigma-Aldrich) intraperitoneally 10 min before ischemia.
Statistical analysis
Data analysis was performed using SPSS software, version 20.0.0. (IBM). Continuous variables are described as mean ± SEM or as median with 25th and 75th percentiles (Q1-Q3) if not normally distributed. Categorical variables are described as counts (percentages). Comparisons between continuous variables were performed by means of Student’s t-tests, Mann-Whitney U-test or by 1-way analysis of variance with Tukey’s post hoc test applied for multiple pairwise comparisons, when appropriate. Changes in CMR endpoints from baseline to 6 months were assessed by paired Student’s t-tests. Values of P < 0.05 were considered statistically significant.
Results
Clinical and laboratory data
Mean age of the 125 patients included was 59.7 ± 1.1 years and 104 (89.2%) were male. ID was present in 54 (43.2%). Demographic characteristics and frequency of cardiovascular risk factors and comorbidities were comparable in patients with and without ID but for a higher frequency of female sex in the former (Table 1). Clinical features on presentation, angiographic data, complications, and in-hospital management were also similar in both groups but for a significantly higher frequency of glycoprotein IIb/IIIa inhibitor use in patients with ID (Table 2). No patient received iron supplements during hospitalization or at discharge.
Table 1.
Demographic and Baseline Clinical Characteristics of Patients With and Without ID
| No ID (n =71) | ID (n = 54) | P Value | |
|---|---|---|---|
| Age, y | 58.8 ± 1.4 | 60.8 ± 1.7 | 0.359 |
| Male | 64 (90.1) | 40 (74.1) | 0.017 |
| Body mass index, kg/m2 | 27.6 ± 0.5 | 27.5 ± 0.6 | 0.908 |
| Active smoking | 38 (53.5) | 22 (40.7) | 0.157 |
| Hypertension | 29 (40.8) | 30 (55.6) | 0.103 |
| Diabetes mellitus | 11 (15.5) | 12 (22.2) | 0.336 |
| Dyslipidemia | 41 (57.7) | 27 (50.0) | 0.389 |
| Chronic angina or previous PCI | 3 (4.2) | 1 (1.9) | 0.419 |
| Previous heart failure | 0 (0) | 0 (0) | — |
| Previous stroke | 0 (0) | 0 (0) | — |
| Peripheral artery disease | 0 (0.0) | 3 (5.6) | 0.078 |
| Severe chronic renal failure | 1 (1.4) | 0 (0.0) | 0.568 |
| Pulmonary disease | 4 (5.6) | 3 (5.6) | 1.000 |
| Previous antiplatelet agents | 5 (7.0) | 7 (13.0) | 0.266 |
| Previous beta blockers | 7 (9.9) | 3 (5.6) | 0.585 |
| Previous ACE inhibitors/ARB | 19 (26.8) | 21 (38.9) | 0.150 |
| Previous aldosterone inhibitors | 0 (0) | 0 (0) | — |
| Previous diuretics | 9 (12.7) | 12 (22.2) | 0.157 |
| Previous oral antidiabetic drugs | 7 (9.9) | 7 (13.0) | 0.586 |
| Previous insulin | 3 (4.2) | 2 (3.7) | 0.628 |
| Previous statins | 16 (22.5) | 14 (25.9) | 0.660 |
Values are means ± SEM or n (%).
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blockers; ID = iron deficiency; PCI = percutaneous coronary intervention.
Table 2.
Clinical and Angiographic Characteristics and Hospital Management of Patients With and Without ID
| No ID (n = 71) | ID (n = 54) | P Value | |
|---|---|---|---|
| Time symptom onset-balloon, min | 169 (138-257) | 189 (134-273) | 0.942 |
| Initial systolic blood pressure, mm Hg | 146 ± 3 | 146 ± 4 | 0.932 |
| Initial heart rate, beats/min | 75 (65-86) | 76 (62-87) | 0.822 |
| Killip class ≥2 | 10 (14.1) | 8 (14.8) | 0.908 |
| GRACE score | 107 ± 4 | 112 ± 4 | 0.328 |
| Initial TIMI flow grade 0-1 | 51(85.0) | 47 (95.9) | 0.118 |
| Final TIMI flow grade 3 | 68 (97.1) | 54 (100) | 0.317 |
| Multivessel disease | 24 (34.3) | 22 (42.3) | 0.366 |
| Reinfarction | 1 (1.4) | 2 (3.7) | 0.398 |
| Worse Killip class ≥3 | 1 (1.4) | 3 (5.6) | 0.214 |
| Ventricular fibrillation | 7 (9.9) | 10 (18.5) | 0.162 |
| Paroxysmal atrial fibrillation | 3 (4.2) | 2 (3.7) | 0.628 |
| Advanced auriculoventricular block | 0 (0.0) | 2 (3.7) | 0.185 |
| Glycoprotein IIb/IIIa inhibitors | 24 (34.3) | 29 (53.7) | 0.030 |
| Dual antiplatelet therapy at discharge | 71 (100) | 52 (96.3) | 0.185 |
| Oral anticoagulants at discharge | 5 (7.0) | 4 (7.4) | 1.000 |
| Beta-blockers at discharge | 68 (95.8) | 48 (88.9) | 0.260 |
| ACE inhibitors/ARB at discharge | 70 (98.6) | 52 (96.3) | 0.398 |
| Aldosterone inhibitors at discharge | 18 (25.4) | 12 (22.2) | 0.685 |
| Statins at discharge | 70 (98.6) | 51 (94.4) | 0.214 |
Values are median (25th-75th percentiles), mean ± SEM, or n (%).
GRACE = Global Registry of Acute Coronary Events; TIMI = Thrombolysis In Myocardial Infarction; other abbreviations in Table 1.
Table 3 summarizes the main laboratory data. Hemoglobin levels were comparable in patients with and without ID. Twelve patients (9.6%) had mild anemia (lowest hemoglobin value, 11.4 g/dL). Blood cell counts, glucose levels, and renal function were comparable among groups. Serum levels of iron and ferritin and transferrin saturation were lower in patients with ID than in the rest. Remarkably, CK-MB peak was significantly higher in patients with ID than in those without ID (median [Q1-Q3]: 335 ng/mL [249-477 ng/mL] vs 246 ng/mL [139-335 ng/mL], respectively [P = 0.003]).
Table 3.
Laboratory Data in Patients With and Without ID
| No ID (n = 71) | ID (n = 54) | P Value | |
|---|---|---|---|
| Hemoglobin levels, g/dL | 14.4 ± 0.1 | 14.2 ± 0.2 | 0.381 |
| Hematocrit value, % | 43.1 ± 0.4 | 42.8 ± 0.5 | 0.665 |
| Mean corpuscular volume, fL | 91.7 ± 0.6 | 91.2 ± 0.8 | 0.646 |
| Leukocyte count/μL | 10.7 (9.4-13.4) | 12.3 (9.2-14.9) | 0.224 |
| Platelet count/μL | 226.7 ± 7.5 | 230.2 ± 8.6 | 0.759 |
| Serum iron, μg/dL | 70.9 ± 3.2 | 55.9 ± 4.1 | 0.004 |
| Serum ferritin, mg/dL | 233 (168-383) | 96 (62-171) | <0.001 |
| Serum transferrin, mg/dL | 224.3 ± 3.4 | 234.6 ± 5.0 | 0.081 |
| Transferrin saturation, % | 31.9 ± 1.5 | 24.0 ± 1.8 | 0.001 |
| Glucose levels, mg/dL | 130 (113-159) | 138 (111-170) | 0.360 |
| Creatinine levels, mg/dL | 0.88 ± 0.03 | 0.89 ± 0.03 | 0.768 |
| eGFR, mL/min/1.73 m2 | 99.7 ± 3.2 | 91.4 ± 3.2 | 0.071 |
| Peak creatine kinase-MB, ng/mL | 246 (139-335) | 335 (249-477) | 0.003 |
Values are mean ± SEM or median (25th-75th percentiles).
eGFR = estimated glomerular filtration rate; ID = iron deficiency; MB = myocardial band.
CMR imaging
In the initial exam (median [Q1-Q3]: 5 days [3 days-8 days] after admission), LV mass averaged 126.0 ± 3.1 g (64.9 ± 1.4 g/m2), the area at risk (n = 90) averaged 41.9 ± 16.8 g (34.1% ± 12.0% of LV mass), infarct size averaged 24.7 ± 1.6 g (19.4% ± 1.0% of LV mass, 59.6% ± 24.7% of the area at risk), and 64 patients (54.7%) had MVO, which involved a median (Q1-Q3) of 1 segments (0 segments-3 segments). Nineteen patients (17.3%) had intramyocardial hemorrhage, which when present, averaged 2.7 ± 0.5 g (2.2% ± 0.4% of LV mass). Among patients with a follow-up CMR, LV end-diastolic volume increased at a median (Q1-Q3) of 6 months (5 months-6 months) by 8.7% ± 2.3% (from 77.5 ± 1.6 mL/m2 to 82.9 ± 1.9 mL/m2; P = 0.001), end-systolic volume did not change significantly (from 42.0 ± 1.2 mL/m2 to 41.6 mL/m2; P = 0.817), and ejection fraction increased by 5.4% ± 0.7% (from 46.0% ± 0.9% to 51.4% ± 1.0%; P < 0.001). Twenty-six patients (23.9%) had adverse LV remodeling as defined before.
Patients with ID had significantly larger mean infarct size (22.8% ± 1.4% vs 16.8% ± 1.2% of LV mass; P = 0.002, 68.0% ± 3.6% vs 52.8% ± 3.5% of the area at risk; P = 0.004 [n = 90]) and more frequent and extensive MVO than those without ID, though a similar extent of myocardial hemorrhage (Table 4). They also showed significantly greater increases in ventricular volumes at 6 months than patients without ID, with similar changes in ejection fraction. Adverse LV remodeling occurred in 37.8% of patients with ID and in 14.1% of those without ID (P = 0.004). Compared with the remaining patients, those developing adverse remodeling had larger mean infarct size (24.8% ± 1.9% vs 17.0% ± 1.1% of LV mass; P = 0.001), more extensive MVO (median [Q1-Q3]: 3 segments [1 segments-4 segments] vs 0 segment [0 segment-2 segments]; P < 0.001) and a higher percentage (38.1% vs 12.8%; P = 0.019) and extent (1.0% ± 0.3% vs 0.2% ± 0.1% of LV mass; P = 0.042) of intramyocardial hemorrhage.
Table 4.
Main Results of CMR Imaging in Patients With and Without ID
| No ID (n = 71) | ID (n = 54) | P Value | |
|---|---|---|---|
| Area at risk, ga | 43.7 ± 2.7 | 39.6 ± 2.2 | 0.266 |
| Area at risk, % of LV massa | 35.3 ± 1.9 | 32.6 ± 1.7 | 0.296 |
| Infarct size, g | 20.7 ± 1.6 | 30.1 ± 2.9 | 0.003 |
| Infarct size, % of LV mass | 16.8 ± 1.2 | 22.8 ± 1.4 | 0.002 |
| Infarct size, % of the area at riska | 52.8 ± 3.5 | 68.0 ± 3.6 | 0.004 |
| No. segments with MVO | 0 (0-2) | 2 (0-3) | 0.002 |
| ≥3 segments with MVO, % | 12 (17.9) | 20 (40.0) | 0.008 |
| Any hemorrhage, % | 9 (13.6) | 10 (22.7) | 0.217 |
| Hemorrhage, % of LV mass | 0.3 ± 0.1 | 0.5 ± 0.2 | 0.238 |
| Relative increase in LVEDV, %b | 3.7 ± 2.5 | 15.9 ± 4.1 | 0.012 |
| Relative increase in LVESV, %b | -7.2 ± 3.0 | 9.3 ± 4.7 | 0.002 |
| Increase in LVEF, %b | 6.4 ± 0.9 | 3.9 ± 1.1 | 0.080 |
| Adverse LV remodeling, %b | 9 (14.1) | 17 (37.8) | 0.004 |
Values are mean ± SEM or median (25th-75th percentiles).
CMR = cardiac magnetic resonance; ID = iron deficiency; LV = left ventricular; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume.
Values from 90 patients with adequate measurements.
Values from the 109 patients (45 with ID and 64 without ID) with cardiac magnetic resonance imaging repeated at 6 months.
Baseline characteristics and echocardiographic data in mice
Hematological parameters, myocardial iron content, and hemodynamic and echocardiographic data in the experimental groups are summarized in Table 5. Plasma hemoglobin was mildly reduced by ID diet with respect to control subjects, although values remained within the normal range. Serum ferritin, serum iron content, and transferrin saturation were reduced by ID diet, whereas serum transferrin was increased. Most importantly, ID diet induced a significant decrease in myocardial iron content. All these parameters were normalized after weekly administration of iron-sucrose. ID diet lacked any effects on hemodynamic or transthoracic echocardiography measurements.
Table 5.
Hematological Parameters, Cardiac Iron Content, and Hemodynamic and Echocardiographic Data in Mice
| Control Diet (n = 8) | ID Diet (n = 8) | ID Diet+Fe (n = 8) | P Value | |
|---|---|---|---|---|
| Body weight, g | 34.6 ± 1.8 | 35.4 ± 1.1 | 36.0 ± 1.5 | 0.710 |
| Hemoglobin, g/dL | 13.8 ± 0.2 | 12.6 ± 0.4∗ | 13.7 ± 0.4 | 0.024 |
| Serum ferritin, ng/mL | 527 ± 37 | 343 ± 14‡ | 581 ± 36 | <0.001 |
| Serum iron, μmol/L | 36.7 ± 3.0 | 24.4 ± 2.5‡ | 45.5 ± 4.2 | 0.001 |
| Cardiac iron, μg/g d.w.‖ | 124.4 ± 3.1 | 84.0 ± 8.6† | 143.9 ± 11.2 | 0.010 |
| Serum transferrin, g/L | 0.66 ± 0.08 | 1.24 ± 0.10§ | 0.80 ± 0.09 | <0.001 |
| Transferrin saturation, % | 55.7 ± 5.2 | 19.4 ± 1.9§ | 52.3 ± 3.4 | <0.001 |
| Heart rate, beats/min | 421 ± 12 | 427 ± 16 | 412 ± 15 | 0.654 |
| MBP, mm Hg | 84 ± 3 | 89 ± 5 | 81 ± 4 | 0.547 |
| LVEDD, mm | 4.23 ± 0.17 | 4.32 ± 0.15 | 4.18 ± 0.13 | 0.712 |
| LVESD, mm | 3.04 ± 0.17 | 3.06 ± 0.10 | 2.99 ± 0.15 | 0.550 |
| IVS, mm | 0.74 ± 0.05 | 0.78 ± 0.09 | 0.70 ± 0.08 | 0.274 |
| LVPW, mm | 0.73 ± 0.04 | 0.74 ± 0.07 | 0.72 ± 0.08 | 0.651 |
| LVEF, % | 66.3 ± 3.2 | 63.6 ± 4.5 | 67.1 ± 7.6 | 0.198 |
Values are mean ± SEM.
d.w. = dry weight; ID = iron deficiency; IVS = interventricular septum. LVEDD = left ventricular end-diastolic diameter. LVEF; left ventricular ejection fraction. LVESD = left ventricular end-systolic diameter. LVPW = left ventricular posterior wall; MBP = mean blood pressure.
P < 0.05 vs control diet.
P < 0.05.
P < 0.01.
P < 0.001 versus control diet and ID diet+Fe.
Cardiac iron was measured in 6 additional mice. Statistical significance tested with the use of analysis of variance with Tukey’s post hoc test applied for multiple pairwise comparisons.
Activity of the eNOS/sGC/PKG pathway
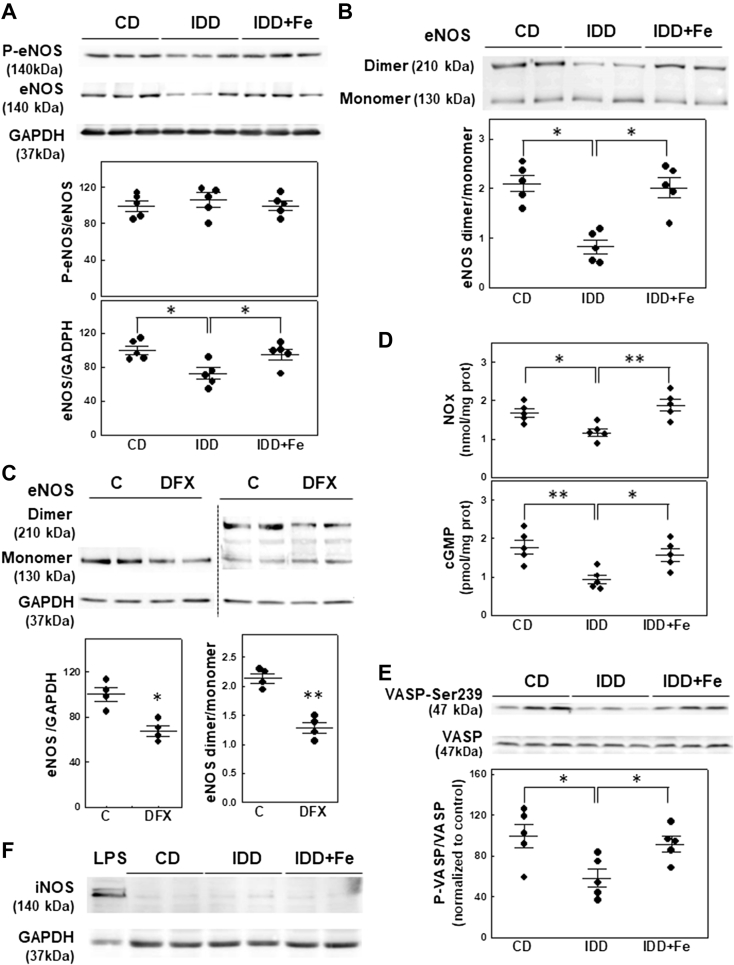
Western blot analysis of eNOS demonstrated a significant decrease in total protein without changes in the extent of its phosphorylation in ID mice (P = 0.023) (Figure 2A). The stability of eNOS, calculated as the ratio between its dimeric and monomeric forms, was reduced in myocardial homogenates from ID mice (P = 0.020) (Figure 2B). The effect of ID on total and dimeric eNOS protein levels was reproduced in isolated cardiomyocytes incubated with the iron chelator deferasirox (P = 0.005 and P < 0.001, respectively) (Figure 2C). Measurement of myocardial nitrate/nitrite concentration confirmed the decreased eNOS activity in iron-depleted hearts (P = 0.005) (Figure 2D). Attenuation of eNOS activity correlated with the inhibition of the sGC/PKG pathway as indicated by the reduction in the myocardial cGMP content (P = 0.005) (Figure 2D) and by the reduced phosphorylation of the PKG substrate VASP (P = 0.023) (Figure 2E). No significant differences in the myocardial expression of iNOS were observed (Figure 2F). No changes in total PKG levels were observed (data not shown). Iron administration effectively restored the activity of the eNOS/sGC/PKG pathway (Figure 2).
Figure 2.
ID Inhibits the eNOS/cGMP/PKG Pathway
Representative Western blot and quantification of (A) total eNOS and its phosphorylated form and (B) the sodium dodecyl sulfate–resistant eNOS dimer in cardiac lysates obtained from the different experimental groups, n = 5 per group. (C) Representative Western blot and quantification of total eNOS and its dimeric form in isolated cardiomyocytes treated with deferasirox (DFX), n = 4 per group. (D) Quantification of myocardial content of nitrate/nitrite (NOx) and cGMP, n = 5 per group. (E) protein kinase G (PKG) activity evaluated by Western blot as PKG-dependent phosphorylation of its substrate vasodilator-stimulated phosphoprotein (VASP), n = 5 per group. (F) Representative Western blot of inducible nitric oxide synthase (iNOS). LPS corresponds to a mouse spleen sample obtained 6 h after lipopolysaccharide injection (10 mg/kg intraperitoneally). ∗P < 0.05, ∗∗P < 0.01 vs IDD group. CD = control diet; eNOS = endothelial nitric oxide synthase; IDD = iron-deficient diet; IDD+Fe = IDD plus iron supplementation. Data are mean ± SEM.
Cytosolic oxidative and nitrosative stress
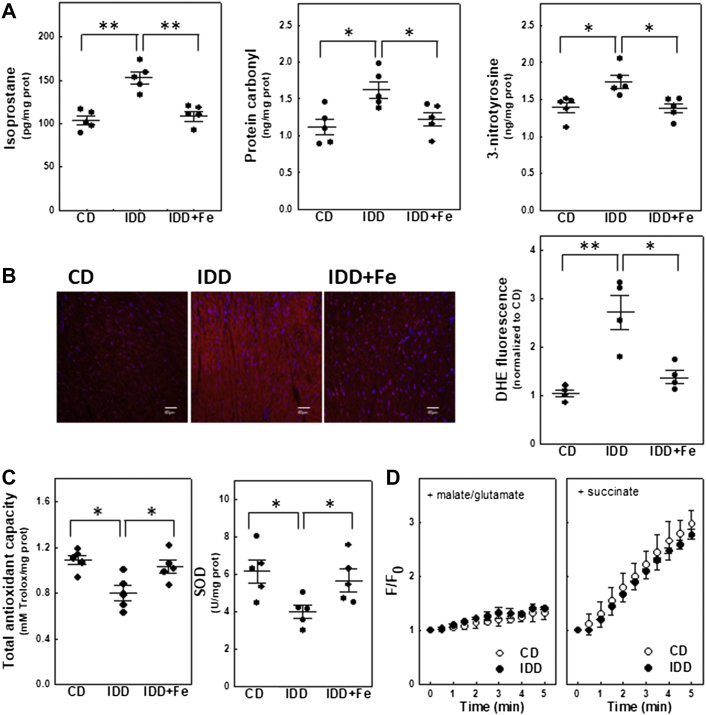
Mice fed with the ID diet showed increased oxidative and nitrosative stress as indicated by the enhanced production of isoprostane (P = 0.008), protein carbonyl (P = 0.011), and tyrosine nitration (P = 0.043) in myocardial extracts (Figure 3A). Increased levels of cytosolic O2− in ID mice was confirmed by measuring the fluorescence produced by dihydroethidium in heart sections (P = 0.002) (Figure 3B). Oxidative stress was associated with a significant reduction in the total myocardial antioxidant capacity (P = 0.008) and in the activity of the antioxidant enzyme superoxide dismutase (P = 0.032) (Figure 3C). The effect of ID on mitochondrial H2O2 production was analyzed in isolated mitochondria incubated with complex-I substrates (malate and glutamate) or succinate. ID diet did not affect H2O2 production driven by malate plus glutamate. Hydrogen peroxide increased linearly with the addition of succinate, but no significant differences associated with diet were observed after 5 min of incubation (Figure 3D). Iron supplementation prevented both oxidative/nitrosative stress and reduced antioxidant capacity (Figure 3).
Figure 3.
ID Increases Oxidative/Nitrosative Stress by Reducing Myocardial Antioxidant Capacity
(A) Quantification of markers of oxidative (8-isoprostane and protein carbonyl) and nitrosative (nitrotyrosine) stress, n = 5 per group. (B) Representative ventricular cross-sections incubated with dihydroethidium (DHE). DHE fluorescence is expressed as arbitrary units, n = 4 per group. (C) Quantification of total antioxidant capacity and superoxide dismutase-1 (SOD1) activity, n =5 per group. (D) Time course of H2O2 production from isolated mitochondria after the addition of malate plus glutamate or succinate. F/F0 corresponds to arbitrary fluorescence values normalized with respect to baseline (excitation at 320 nm, emission at 440 nm), n = 3 per group. Data are mean ± SEM. ∗P < 0.05, ∗∗P < 0.01 vs IDD groups. Abbreviations as in Figure 2.
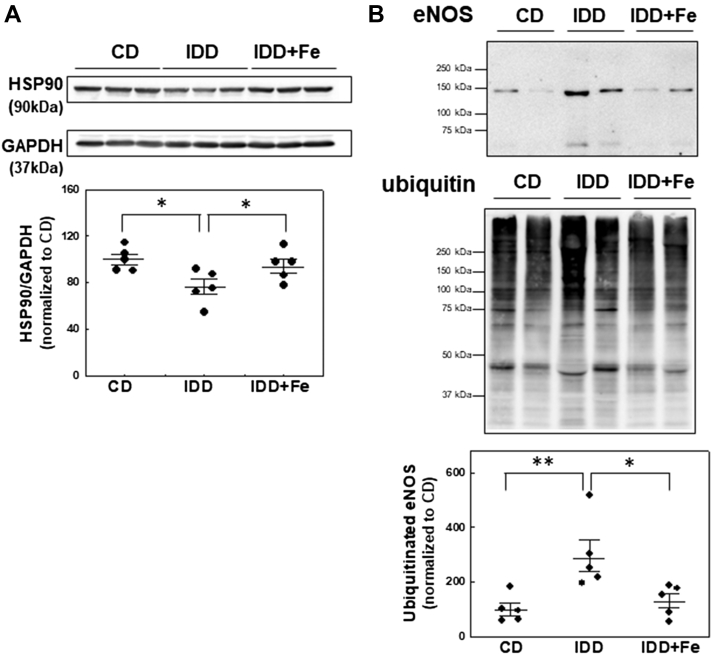
eNOS ubiquitination
Because total eNOS protein levels were reduced in ID mice, we next examined whether ID downregulates eNOS by increasing its degradation through the ubiquitin/proteasome pathway. We observed a significant reduction in the levels of the chaperon protein HSP90 in myocardial samples from ID-mice (P = 0.031) (Figure 4A). Because HSP90 has been proposed to modulate eNOS stability in a ubiquitin-dependent manner (30), polyubiquitinated proteins were pulled down from cardiac extracts using the agarose-tandem ubiquitin-binding entities molecular tool and probed with eNOS antibody. The results showed that ID diet increases myocardial eNOS ubiquitination (P = 0.008) (Figure 4B) and reduces eNOS protein levels (P = 0.007) (data not shown). Iron supplementation prevented the decrease in HSP90 protein content and the enhanced eNOS ubiquitination and degradation (Figure 4).
Figure 4.
ID Reduces HSP90 Levels and Increases the Extent of eNOS Ubiquitination
(A) Representative Western blot and quantification of HSP90. (B) Polyubiquitination levels of eNOS in cardiac lysates analyzed by using agarose-tandem ubiquitin-binding entities pull-downs. eNOS bands were normalized to their corresponding ubiquitin smears and ratios were normalized to ubiquitinated eNOS level in control samples. Representative Western blots are shown in all panels. Data are mean ± SEM. ∗P < 0.05, ∗∗P < 0.01 vs IDD group. Abbreviations as in Figure 2.
Myocardial tolerance to ischemia/reperfusion
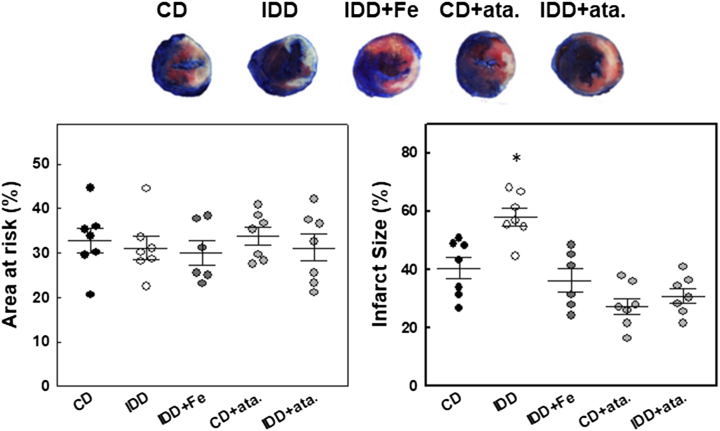
In mice subjected to 45-min coronary ligation, mortality rate during the procedure was 15% (2 animals from the ID+Fe group and 1 from each of the remaining groups). The mass of myocardium at risk was 32.8% ± 2.8% of LV in the control group and was similar among groups (Figure 5). By contrast, infarct size measured after 24 h of reperfusion was significantly larger in mice fed with ID diet than in controls (58.1% ± 3.0% vs 40.4% ± 3.7% of the area at risk; P = 0.004), and iron supplementation prevented this effect (36.3% ± 4.0%; P = 0.407 vs control group). The sGC activator ataciguat induced a trend toward reduced infarct size in mice fed with control diet (27.5% ± 2.8%; P = 0.061 vs control group) and prevented the increase in infarct size observed in ID-mice (31.0% ± 2.5%; P = 0.287 vs control+ataciguat group).
Figure 5.
ID Increases Myocardial Susceptibility to Ischemia/Reperfusion Injury
Area at risk, expressed as a percentage of left ventricular mass, and infarct size, measured by triphenyltetrazolium chloride staining and expressed as a percentage of the area at risk, in mouse hearts after 24-h of reperfusion in the different experimental groups. Representative examples are shown. Data are mean ± SEM. n = 6-7 per group. ∗P < 0.05 vs the CD group. Ata = ataciguat; other abbreviations as in Figure 2.
Discussion
In this study, 43% of patients with a first anterior STEMI undergoing primary percutaneous coronary intervention had ID. Patients with ID had larger infarcts and more severe MVO acutely and a higher frequency of adverse LV remodeling at 6 months than the remaining patients. Mice fed with the ID diet had larger infarcts after transient coronary occlusion than control mice and iron supplementation prevented this effect. ID diet inhibited the activity of the eNOS/sGC/PKG pathway, which was associated with increased oxidative/nitrosative stress linked to altered antioxidant defense and to eNOS degradation via ubiquitin/proteasome system. sGC activators reverted the detrimental effects of ID in mice.
Few studies have assessed the prevalence and significance of ID in patients with acute coronary syndromes. ID was found in 29% to 60% of patients, but its clinical implications have been inconsistent between studies (3, 4, 5, 6). ID was related to a worse functional recovery (3) or to a higher long-term occurrence of myocardial infarction/cardiovascular death or total mortality in unselected acute coronary syndrome patients (4,5). By contrast, in a study in STEMI patients undergoing mechanical reperfusion, those with ID had higher admission troponin levels but a more benign in-hospital clinical course, and among those with a CMR performed, similar infarct size, higher myocardial salvage index, and less MVO as compared with those without ID (6). The reasons for these discrepancies are unclear but might be related in part to the diverse patient populations and the different definitions of ID used.
To facilitate interpretation and comparability of our results, we defined ID using the criteria that have become standard in the setting of acute heart failure, aimed to account in part for the nonspecific L-ferritin increase occurring in acute disorders (1,2). In our series, ID was present in 43% of cases and was significantly associated with larger infarcts—either estimated by CK-MB peak or by CMR—and with a more extensive MVO. Of note, this association would still be present if ID were defined as a transferrin saturation <20% irrespective of ferritin levels (data not shown). Because patients with and without ID had comparable baseline characteristics and were treated similarly, the results suggest that ID enhanced myocardial injury. The higher use of glycoprotein IIb/IIIa inhibitors in patients with ID is in line with this interpretation. On the one hand, it might reflect a higher thrombotic burden in these patients, and on the other hand, these drugs are potentially protective against ischemia/reperfusion injury (31).
To the best of our knowledge, no previous experimental studies have analyzed the effect of dietary ID on myocardial tolerance to transient ischemia. In our experiments in mice, we used a model of ID without anemia recently described by Rineau et al (27) to isolate the effects of ID from those of anemia, usually present in dietary models of ID or in models with repeated blood withdrawal (11,32). In contrast to the Rineau et al (27) study, ours showed a slight decline in hemoglobin levels, although values remained within the physiological range. Blood analyses and myocardial iron quantification confirmed significant ID that was fully prevented by iron supplementation. ID did not induce ventricular dilatation or dysfunction, in agreement with the Rineau et al (27) study and in contrast with the myocardial remodeling described in models of long-term ID anemia (11).
The larger infarct size observed in ID mice with respect to control mice concurs with our findings in STEMI patients. As expected (10, 11, 12), this effect coincided with increased myocardial oxidative/nitrosative stress. Our data indicated that ID increases cytosolic O2− levels in association with reduced total myocardial antioxidant capacity caused, at least partially, by the altered activity of antioxidant enzymes with no evidence of changes in the mitochondrial contribution to oxidative stress. These alterations were previously documented in blood from patients with ID anemia and in the myocardium from animals with dietary ID and were reverted by iron therapy (12,33). Similarly, our results showed reduced oxidative stress coupled with enhanced antioxidant capacity in mice receiving ID diet plus iron supplementation.
Abundant evidence demonstrates that preservation of the eNOS/sGC/PKG pathway attenuates myocardial cell death after ischemia/reperfusion (15,16). This pathway is highly susceptible to oxidation (13,17, 18, 19) and can be affected by ID at different levels. First, O2− combines with NO to generate ONOO−, which apart from reducing NO availability, induces nitro-oxidative stress toxicity (34). Second, O2− and ONOO− can oxidize tetrahydrobiopterin, a cofactor essential for proper eNOS function, leading to eNOS uncoupling (19). Third, O2− oxidizes the heme group of sGC, decreasing its responsiveness to NO and reducing cGMP production and activation of PKG, the final effector of this pathway (17,18). Finally, both eNOS and sGC contain a prosthetic heme group and their enzymatic activity may be inhibited independently of oxidative stress by severe ID (20). In our study, ID increased myocardial nitrotyrosine, a fingerprint of the in vivo presence of ONOO−, and reduced the dimeric form of eNOS, reflecting its uncoupling, and myocardial nitrates and nitrites levels, used as surrogate markers for NO. As a result, myocardial cGMP content and the phosphorylation of VASP, a protein substrate of PKG, but not total PKG levels, were reduced. Further supporting the inhibition of the eNOS/sGC/PKG pathway and its contribution to the effects of ID, administration of the sGC activator ataciguat prevented the increased susceptibility to ischemia in ID mice.
Our data show that ID, in addition to its oxidation, decreased myocardial eNOS protein content and did not induce iNOS expression. These results differ from other studies describing eNOS and iNOS up-regulation and increased nitro-oxidative stress in the heart of anemic rats in parallel with the development of ventricular dilatation (11). Recently, a study described reduced eNOS function and increased reactive oxygen species formation in red blood cells from anemic mice, which was compensated by enhanced expression of eNOS in aorta, although no significant differences in myocardial eNOS expression were observed (32). Altogether, these results suggest that eNOS response to ID may vary depending on the presence of anemia or tissue hypoxia. The reduction in myocardial eNOS content in ID mice correlated with lower HSP90 protein levels and increased eNOS ubiquitination. In endothelial cells, HSP90 associates with eNOS, maintains its functional dimeric structure, and regulates its degradation by ubiquitination (30). Iron chelators downregulate HSP90 expression in vitro (35) and oxygen radicals cleave HSP90, disrupting its chaperoning function (36). Based on our results and this previous evidence, it is tempting to speculate that ID-induced oxidative stress may result in a defective proteasomal degradation of eNOS by reducing HSP90 protein content.
In agreement with previous studies (21,22), infarct size, MVO, and intramyocardial hemorrhage predicted adverse remodeling. It is therefore possible that the association between ID and adverse LV remodeling in our STEMI patients was mainly driven by their more severe damage acutely, and a direct effect of ID on ventricular healing can be neither supported nor excluded based on our data. Previous evidence in this respect is contradictory, as some observational studies in STEMI patients suggested that ID could be detrimental to the remodeling process (25,37), whereas other studies reported an attenuated LV dilatation in experimental animals treated with post-infarction dietary iron restriction (38) or iron chelation (39). Erythropoietin administration has not improved clinical outcomes or LV performance after STEMI (40), indicating that increasing erythropoiesis does not ameliorate post-infarction remodeling, but these results do not exclude that ID is detrimental to this process. In fact, erythropoietin mobilizes iron from storage sites for hemoglobin synthesis, potentially favoring absolute ID, and lowers percent transferrin saturation in the presence of adequate iron stores, favoring functional ID (41).
Study limitations
Our relatively low-risk patients may not be representative of the overall STEMI population, but the inclusion criteria were selected to ensure homogeneity and to avoid any confounding factors for CMR analysis. Nine percent had mild anemia, but none of the main results would have differed had these patients been excluded (data not shown). Measuring the area at risk based on edema quantification in STIR images is subject to limitations (42). No adjustment for multiple comparisons was applied in the statistical differences depicted in the tables. The observational nature of our clinical study prevents us from establishing a cause-effect relationship between ID and the increased post-ischemic damage in STEMI patients. However, this interpretation concurred with the hypothesis of the study and was strongly supported by the results of our experiments in mice. Although only male mice were used, increased oxidative stress in response to ID (12) and the cardioprotective role of eNOS/sGC pathway (43) have been demonstrated in both sexes, and no sex differences in infarct size were observed in previous studies from our group (28), suggesting that the proposed effect of ID on acute myocardial tolerance to ischemia is independent of sex. Despite that our data obtained in isolated cardiomyocytes support a direct effect of ID on these cells, our study does not rule out the contribution of endothelial cells to the myocardial effects of ID. Finally, although we provide a plausible mechanism for the detrimental effect of ID in STEMI patients, we cannot exclude that others are involved.
Conclusions
This study shows that ID is associated with larger infarcts and more frequent adverse LV remodeling in patients with a reperfused first anterior STEMI and that dietary ID without anemia reduces the myocardial tolerance to ischemia/reperfusion in mice. This effect is produced at least in part by inhibiting the eNOS/sGC/PKG pathway and involves increased oxidative/nitrosative stress and the proteasome-dependent degradation of eNOS. Our findings highlight that not only iron excess but also ID may have deleterious effects on myocardial salvage and on ventricular remodeling in patients with acute myocardial infarction and justify a word of caution on the use of iron-lowering therapies in this setting.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In patients with STEMI, ID is associated with larger infarcts and with a higher frequency of LV adverse remodeling. ID enhances the susceptibility to myocardial ischemia/reperfusion injury, and this effect is produced at least in part by inhibiting the eNOS/sGC/PKG pathway.
TRANSLATIONAL OUTLOOK: In patients with acute myocardial infarction, the definition of ID needs to be improved and the optimal iron status clarified. Meanwhile, a word of caution is justified on the use of interventions addressed to either increase or decrease iron levels in this clinical context.
Funding Support and Author Disclosures
This study was funded by Instituto de Salud Carlos III, Spain, through the project PI16/00232 and the research network CIBERCV (CB16/11/00479), both cofunded by European Regional Development Fund, and by the Sociedad Española de Cardiología (Proyecto de Investigación Traslacional en Cardiología 2016). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Contributor Information
Javier Inserte, Email: javier.inserte@vhir.org.
José A. Barrabés, Email: jabarrabes@vhebron.net.
References
- 1.Anker S.D., Comin Colet J., Filippatos G. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2438. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 2.Rocha B.M.L., Cunha G.J.L., Menezes Falcão L.F. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol. 2018;71:782–793. doi: 10.1016/j.jacc.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Meroño O., Cladellas M., Ribas-Barquet N. Iron deficiency is a determinant of functional capacity and health-related quality of life 30 days after an acute coronary syndrome. Rev Esp Cardiol. 2017;70:363–370. doi: 10.1016/j.rec.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Zeller T., Waldeyer C., Ojeda F. Adverse outcome prediction of iron deficiency in patients with acute coronary syndrome. Biomolecules. 2018;8:60. doi: 10.3390/biom8030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González-D'Gregorio J., Miñana G., Núñez J. Iron deficiency and long-term mortality in elderly patients with acute coronary syndrome. Biomark Med. 2018;12:987–999. doi: 10.2217/bmm-2018-0021. [DOI] [PubMed] [Google Scholar]
- 6.Cosentino N., Campodonico J., Pontone G. Iron deficiency in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol. 2020;300:14–19. doi: 10.1016/j.ijcard.2019.07.083. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura T., Naguro I., Ichijo H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim Biophys Acta Gen Subj. 2019;1863:1398–1409. doi: 10.1016/j.bbagen.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Neckář J., Boudíková A., Mandíková P. Protective effects of dexrazoxane against acute ischaemia/reperfusion injury of rat hearts. Can J Physiol Pharmacol. 2012;90:1303–1310. doi: 10.1139/y2012-096. [DOI] [PubMed] [Google Scholar]
- 9.Ghafourian K., Shapiro J.S., Goodman L., Ardehali H. Iron and heart failure: diagnosis, therapies, and future directions. J Am Coll Cardiol Basic Trans Science. 2020;5:300–313. doi: 10.1016/j.jacbts.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koskenkorva-Frank T.S., Weiss G., Koppenol W.H., Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013;65:1174–1194. doi: 10.1016/j.freeradbiomed.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Dong F., Zhang X., Culver B., Chew H.G., Jr., Kelley R.O., Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci. 2005;109:277–286. doi: 10.1042/CS20040278. [DOI] [PubMed] [Google Scholar]
- 12.Kurtoglu E., Ugur A., Baltaci A.K., Undar L. Effect of iron supplementation on oxidative stress and antioxidant status in iron-deficiency anemia. Biol Trace Elem Res. 2003;96:117–123. doi: 10.1385/BTER:96:1-3:117. [DOI] [PubMed] [Google Scholar]
- 13.Inserte J., Hernando V., Vilardosa Ú., Abad E., Poncelas-Nozal M., Garcia-Dorado D. Activation of cGMP/protein kinase G pathway in postconditioned myocardium depends on reduced oxidative stress and preserved endothelial nitric oxide synthase coupling. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.112.005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granger D.N., Kvietys P.R. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bice J.S., Burley D.S., Baxter G.F. Novel approaches and opportunities for cardioprotective signaling through 3',5'-cyclic guanosine monophosphate manipulation. J Cardiovasc Pharmacol Ther. 2014;19:269–282. doi: 10.1177/1074248413518971. [DOI] [PubMed] [Google Scholar]
- 16.Inserte J., Garcia-Dorado D. The cGMP/PKG pathway as a common mediator of cardioprotection: translatability and mechanism. Br J Pharmacol. 2015;172:1996–2009. doi: 10.1111/bph.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stasch J.P., Schmidt P.M., Nedvetsky P.I. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006;116:2552–2561. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z., Pyriochou A., Kotanidou A. Soluble guanylyl cyclase activation by HMR-1766 (ataciguat) in cells exposed to oxidative stress. Am J Physiol Heart Circ Physiol. 2008;295:H1763–H1771. doi: 10.1152/ajpheart.51.2008. [DOI] [PubMed] [Google Scholar]
- 19.Chen W., Druhan L.J., Chen C.A. Peroxynitrite induces destruction of the tetrahydrobiopterin and heme in endothelial nitric oxide synthase: transition from reversible to irreversible enzyme inhibition. Biochemistry. 2010;49:3129–3137. doi: 10.1021/bi9016632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldblatt M.I., Choi S.H., Swartz-Basile D.A., Nakeeb A., Sarna S.K., Pitt H.A. Iron deficiency suppresses ileal nitric oxide synthase activity. J Gastrointest Surg. 2001;5:393–399. doi: 10.1016/s1091-255x(01)80068-8. [DOI] [PubMed] [Google Scholar]
- 21.Ganame J., Messalli G., Dymarkowski S. Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J. 2009;30:1440–1449. doi: 10.1093/eurheartj/ehp093. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Palomares J.F., Gavara J., Ferreira-González I. Prognostic value of initial left ventricular remodeling in patients with reperfused STEMI. J Am Coll Cardiol Img. 2019;12:2445–2456. doi: 10.1016/j.jcmg.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Fraccarollo D., Galuppo P., Motschenbacher S., Ruetten H., Schäfer A., Bauersachs J. Soluble guanylyl cyclase activation improves progressive cardiac remodeling and failure after myocardial infarction. Cardioprotection over ACE inhibition. Basic Res Cardiol. 2014;109:421. doi: 10.1007/s00395-014-0421-1. [DOI] [PubMed] [Google Scholar]
- 24.Lee S.D., Huang C.Y., Shu W.T. Pro-inflammatory states and IGF-I level in ischemic heart disease with low or high serum iron. Clin Chim Acta. 2006;370:50–56. doi: 10.1016/j.cca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Huang C.H., Chang C.C., Kuo C.L. Serum iron concentration, but not hemoglobin, correlates with TIMI risk score and 6-month left ventricular performance after primary angioplasty for acute myocardial infarction. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miranda B., Barrabés J.A., Figueras J. Plasma bilirubin values on admission and ventricular remodeling after a first anterior ST-segment elevation acute myocardial infarction. Ann Med. 2016;48:1–9. doi: 10.3109/07853890.2015.1112027. [DOI] [PubMed] [Google Scholar]
- 27.Rineau E., Gaillard T., Gueguen N. Iron deficiency without anemia is responsible for decreased left ventricular function and reduced mitochondrial complex I activity in a mouse model. Int J Cardiol. 2018;266:206–212. doi: 10.1016/j.ijcard.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Inserte J., Aluja D., Barba I. High-fat diet improves tolerance to myocardial ischemia by delaying normalization of intracellular PH at reperfusion. J Mol Cell Cardiol. 2019;133:164–173. doi: 10.1016/j.yjmcc.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Inserte J., Hernando V., Ruiz-Meana M. Delayed phospholamban phosphorylation in post-conditioned heart favours Ca2+ normalization and contributes to protection. Cardiovasc Res. 2014;103:542–553. doi: 10.1093/cvr/cvu163. [DOI] [PubMed] [Google Scholar]
- 30.Chen W., Xiao H., Rizzo A.N., Zhang W., Mai Y., Ye M. Endothelial nitric oxide synthase dimerization is regulated by heat shock protein 90 rather than by phosphorylation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrabés J.A., Inserte J., Mirabet M. Antagonism of P2Y12 or GPIIb/IIIa receptors reduces platelet-mediated myocardial injury after ischaemia and reperfusion in isolated rat hearts. Thromb Haemost. 2010;104:128–135. doi: 10.1160/TH09-07-0440. [DOI] [PubMed] [Google Scholar]
- 32.Wischmann P., Kuhn V., Suvorava T. Anaemia is associated with severe RBC dysfunction and a reduced circulating NO pool: vascular and cardiac eNOS are crucial for the adaptation to anaemia. Basic Res Cardiol. 2020;115:43. doi: 10.1007/s00395-020-0799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo J.H., Maeng H.Y., Sun Y.K. Oxidative status in iron-deficiency anemia. J Clin Lab Anal. 2009;23:319–323. doi: 10.1002/jcla.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabó C., Ischiropoulos H., Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 35.Sidarovich V., Adami V., Gatto P. Translational downregulation of HSP90 expression by iron chelators in neuroblastoma cells. Mol Pharmacol. 2015;87:513–524. doi: 10.1124/mol.114.095729. [DOI] [PubMed] [Google Scholar]
- 36.Beck R., Dejeans N., Glorieux C. Hsp90 is cleaved by reactive oxygen species at a highly conserved N-terminal amino acid motif. Plos One. 2012;7 doi: 10.1371/journal.pone.0040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Florian A., Ludwig A., Rösch S. Positive effect of intravenous iron-oxide administration on left ventricular remodelling in patients with acute ST-elevation myocardial infarction - a cardiovascular magnetic resonance (CMR) study. Int J Cardiol. 2014;173:184–189. doi: 10.1016/j.ijcard.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Eguchi A., Naito Y., Iwasaku T. Association of dietary iron restriction with left ventricular remodeling after myocardial infarction in mice. Heart Vessels. 2016;31:222–229. doi: 10.1007/s00380-014-0621-5. [DOI] [PubMed] [Google Scholar]
- 39.Behrouzi B., Weyers J.J., Qi X. Action of iron chelator on intramyocardial hemorrhage and cardiac remodeling following acute myocardial infarction. Basic Res Cardiol. 2020;115:24. doi: 10.1007/s00395-020-0782-6. [DOI] [PubMed] [Google Scholar]
- 40.Yang H.T., Xiu W.J., Zheng Y.Y., Ma Y.T., Xie X. Effects of erythropoietin on the clinical outcomes of patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: a meta-analysis. Int J Clin Pharmacol Ther. 2018;56:277–279. doi: 10.5414/CP203225. [DOI] [PubMed] [Google Scholar]
- 41.Adamson J. Erythropoietin, iron metabolism, and red blood cell production. Semin Hematol. 1996;33(2 Suppl 2):5–7. discussion 8-9. [PubMed] [Google Scholar]
- 42.Ibanez B., Aletras A.H., Arai A.E. Cardiac MRI endpoints in myocardial infarction experimental and clinical trials: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;74:238–256. doi: 10.1016/j.jacc.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.du Toit E.F., Genade S., Carlini S., Moolman J.A., Brunner F., Lochner A. Efficacy of ischaemic preconditioning in the eNOS overexpressed working mouse heart model. Eur J Pharmacol. 2007;556:115–120. doi: 10.1016/j.ejphar.2006.11.004. [DOI] [PubMed] [Google Scholar]