Abstract
Objective:
Aims were to assess 1.) whether odds for incident radiographic osteoarthritis (ROA) differ between men and women in regard to body mass index (BMI) and inflammatory magnetic resonance imaging (MRI) markers one and two years prior and 2.) whether presence of inflammation on MRI differs between normal-weight, and overweight/obese persons that develop ROA up to four years prior.
Methods:
We studied 355 knees from the Osteoarthritis Initiative study that developed incident ROA and 355 matched controls. MRIs were read for effusion-synovitis and Hoffa-synovitis for up to four consecutive annual time points. Subjects were classified as normal-weight (BMI < 25), overweight (BMI ≥25/<30) or obese (BMI ≥30). Conditional logistic regression was used to assess odds of incident ROA for effusion-synovitis and Hoffa-synovitis at one and two years prior ROA incidence (i.e. “P-1” and “P-2”) considering BMI category. Bivariate logistic regression was used to assess odds of inflammation for cases only.
Results:
178 (25.1%) participants were normal-weight, 283 (39.9%) overweight and 249 (35.1%) obese. At P-2 being overweight with Hoffa-synovitis (OR 3.26, 95%CI 1.39,7.65) or effusion-synovitis (3.56, 95%CI 1.45,8.75) was associated with greater odds of incident ROA in women. For those with incident ROA there were no increased odds of synovitis in the overweight/obese subgroup for most time points but increased odds for effusion-synovitis were observed at P-2 (OR 2.21, 95%CI 1.11,4.43).
Conclusions:
Presence of inflammatory markers seems to play a role especially in overweight women while obese women have increased odds for ROA also in the absence of these markers.
Keywords: MRI, obesity, knee, osteoarthritis, inflammation
Introduction
Obesity is one of the key risk factors for the development of knee osteoarthritis (OA) (1). Associations linking OA development to components of the so-called metabolic syndrome beyond obesity have been suggested. These include chronic low-grade inflammation, a feature shared by OA and metabolic disorders that may contribute to the genesis of both (2, 3). While studies have reported that the metabolic syndrome is clearly associated with increased risk of knee OA (4), a recent meta-analysis suggested that this may only be indirect, and that there was insufficient evidence that the metabolic syndrome was associated with incident knee OA independent of body mass index (BMI) (5).
Beyond pro-inflammatory systemic factors, local intra-articular adipose tissues such as Hoffa’s fat pad produce inflammatory and catabolic mediators that may contribute to OA pathogenesis (6). Further, it is unclear whether women and men show differences regarding the presence of metabolic syndrome and incident knee OA. While one study did not report any sex-specific differences (3), others have highlighted that inflammation and metabolic syndrome may have a larger impact on OA incidence in women compared to men (7). We hypothesize that persons with high body mass index (BMI) and local inflammation as assessed by MRI, considered as surrogates for some of the components of the metabolic syndrome (8), may be at increased odds for incident knee OA and that overweight and obese persons are at increased odds for exhibiting signs of local joint inflammation as assessed by MRI up to four years prior the incidence of ROA.
Thus, aims of the study were 1.) to assess whether odds for incident radiographic OA (ROA) differ between men and women in regard to BMI and inflammatory MRI markers one and two years prior to ROA incidence using a matched case-control sample of subjects that developed or did not develop incident ROA and 2.) to analyze whether odds of presence of MRI-features of inflammation such as effusion-synovitis (‘effusion’) and Hoffa-synovitis (‘synovitis’) differ between normal-weight, and overweight/obese persons that develop incident ROA over a period of up to four years prior.
Methods
The Osteoarthritis Initiative
The Osteoarthritis Initiative (OAI) is a longitudinal cohort study designed to identify biomarkers of the onset and/or progression of knee OA. Both knees of 4,796 participants were studied using 3 Tesla MRI and fixed-flexion radiography at baseline, 12, 24, 36, and 48 months of follow-up (9). The Institutional Review Boards at each of the sites approved the study, and all participants gave informed consent.
Radiography
OAI knee radiographs were acquired using the posterior-anterior fixed-flexion weight-bearing protocol using a positioning frame. The Kellgren-Lawrence (K-L) grade was determined by central readings of baseline serial fixed-flexion knee radiographs (10).
Case and Control Knee Selection
Cases were defined as study participants who had at least one knee that developed incident ROA during the four years of follow-up. Incident ROA was defined as the first occurrence of radiographic findings compatible with OA (K-L grade of ≥2 on the p.a. view based on central readings) during the course of study. This time point was called P0 with P-1 being defined as the time point one year before ROA was detected, P-2 defined as two years prior, P-3 three years prior and P-4 four years prior incident radiographic OA was read. All participants fulfilling the case definition were included. An identical number of control knees were selected from knees that did not develop incident radiographic OA during the study period. The controls were matched to case knees according to K-L grade, sex, age (within five years), and contralateral knee OA status (i.e. K-L grade = 0, 1, or 2+ in the other knee). Each case was matched to those who were at risk at the time of case occurrence and those with available images at relevant time points, whether this was at 12, 24, 36 or 48 months of follow-up. Both cases and control knees were either K-L 0 or 1 at baseline based on central readings. Only one knee per subject was used as a case knee. A flowchart of the inclusion of cases and controls is included as Appendix 1.
MRI Acquisition and Assessment
MRI of both knees was performed on identical 3T systems (Siemens Trio, Erlangen, Germany) at the four OAI clinical sites. The OAI pulse sequence protocol and the sequence parameters have been published in detail (9).
Two musculoskeletal radiologists with 11 (F.W.R.) and 14 (A.G.) years’ experience of semiquantitative assessment of knee OA at the time of reading, blinded to clinical data and case-control status, read the MRIs according to the MRI Osteoarthritis Knee Score (MOAKS) system (11). Baseline and follow-up MRIs were read with the chronological order known to the readers. Diffuse hyperintense signal on the sagittal IW fat-suppressed sequence in the intercondylar region of Hoffa’s fat pad were scored from 0 to 3 as a surrogate for synovial thickening termed Hoffa-synovitis (i.e. ‘synovitis’). The degree of hyperintensity is assessed according to the following grades: 0=normal; 1=mild, 2=moderate and 3=severe. Joint effusion (also called effusion-synovitis as it is not possible to discern joint fluid from synovial thickening on non-contrast-enhanced MRI) was graded from 0 to 3 in terms of the estimated maximal distention of the synovial cavity (i.e. ‘effusion’) as follows: Grade 0=none, grade 1=small, grade 2=medium and grade 3=large. (11, 12). Examples of the different grades of Hoffa- and effusion-synovitis are presented in Figure 1. Detailed reliability data of MRI assessment is presented in Appendix 2.
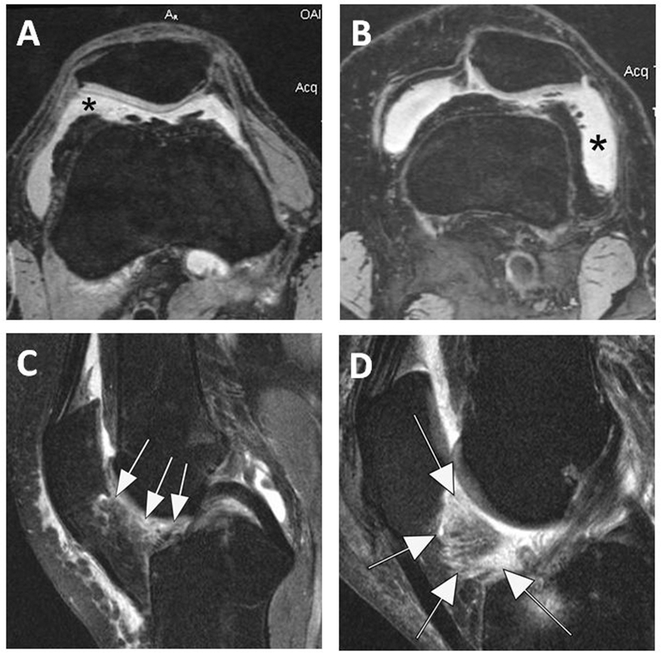
Figure 1.
MRI markers of inflammation in OA. Fluid sensitive sequences are capable of delineating intraarticular joint fluid. However, a distinction between true joint effusion and synovial thickening is not possible as both are visualized as hyperintense signal within the joint cavity. For this reason the term effusion-synovitis was introduced, which in the MOAKS system is scored based on the distension of the joint capsule and is graded from 0 to 3 in terms of the estimated maximal distention of the synovial cavity with 0=normal, grade 1=<33% of maximum potential distention, grade 2=33%–66% of maximum potential distention and grade 3=>66% of maximum potential distention. Axial dual-echo at steady-state (DESS) MR images show A. Grade 2 effusion-synovitis (asterisk), and B. Grade 3 effusion-synovitis (asterisk). In addition, signal changes in Hoffa’s fat pad are commonly used as a surrogate for synovitis on non-contrast enhanced MRI. Although synovitis can only be visualized directly on contrast-enhanced sequences, it has been shown that Hoffa’s signal changes are a sensitive but non-specific surrogate of synovitis. C. Sagittal intermediate-weighted fat-suppressed MRI shows a discrete ill-defined hyperintense signal alteration in Hoffa’s fat pad consistent with grade 2 Hoffa-synovitis (arrow). D. Severe, grade 3 signal alterations almost occupying the entire fat pad are seen in this image (arrows).
Statistical Analysis
Subjects were classified as normal weight (BMI <25 kg/m2), overweight (BMI ≥25 kg/m2 and <30 kg/m2) or obese (BMI ≥ 30 kg/m2) at OAI enrollment. In the case-control design part of the study, conditional logistic regression was used to assess the risk of incident ROA stratified by presence of synovitis and effusion focusing on the time points P-1 and P-2 only. Presence of synovitis and effusion was defined as “any”, i.e. knees that exhibited grades 1 to 3 of synovitis or effusion on MRI. The time points P-3 and P-4 were not considered as low numbers did not allow meaningful interpretation of the interactions (for P-3 only 59 cases and for P-4 only 53 cases were available). For the case-control analysis stratification by sex was undertaken, and BMI, synovitis, and effusion or the interaction were used as exposure variables. First, the bivariate associations of ROA and the different synovitis and effusion categories and BMI were estimated. After this initial analysis, the risk of ROA for the interaction of BMI and effusion/synovitis was examined. The category of normal weight, especially in men, was sufficiently uncommon that we used the overweight category as the referent for the BMI analysis because it was the norm.
Bivariate logistic regression was used to assess the odds of the presence of synovitis and effusion at time points P-1, P-2, P-3, P-4 and baseline in subjects that developed radiographic OA (i.e. only cases) comparing overweight and obese subjects combined to normal-weight subjects as the reference. We considered a two-tailed p-value of less than 0.05 as statistically significant. All statistical calculations were performed using Stata/IC 11.2 for Windows (StataCorp, College Station, TX) and SAS 9.3 (SAS Institute Inc, Cary, NC).
Results
A total of 355 case knees and 355 matched control knees were included. Participants had a mean age of 60.2 ± 8.6 years, 66.5% were female. Cases had a slightly higher BMI compared to controls (28.9 kg/m2 versus 27.7 kg/m2; p<0.001). No significant differences with regard to ethnicity between cases and controls were observed (84% percent of the subjects white). The case-defining visit of radiographic OA incidence was 12 months for 119 knees (33.5%), 24 months for 83 knees (23.4%), 36 months for 103 knees (29.0%), and 48 months for 50 knees (14.1%). 178 (25.1%, 138/77.5% women) participants were normal-weight, 283 (39.9%, 166/58.7% women) were overweight and 249 (35.1%, 170/68.3% women) obese at baseline. Details of the demographics regarding cases and controls are presented in Appendix 3.
Regarding the interaction of BMI with synovitis and effusion, using overweight women and men without synovitis or effusion as the reference, obesity without synovitis was associated with greater odds of ROA in women (OR 2.87, 95% CI [1.21,6.83]) at P-2, as was being overweight with synovitis (OR 3.26, 95% CI [1.39, 7.65]). Being obese with synovitis was not associated with increased odds at P-2. For men, there were no combinations of synovitis and BMI that were associated with increased odds of ROA compared to those being overweight without synovitis at P-2. Furthermore, being overweight with joint effusion at P-2 was associated with increased OA odds in women (OR 3.56, 95% CI [1.45,8.75]), which was the case also for the obese category in women (OR 3.46, 95% CI [1.38,8.72]).
At P-1 and combining all BMI categories, having any synovitis or any effusion was associated with increased odds of ROA in both, men and women. Further, presence of synovitis was associated with incident ROA in overweight and obese women and men, with the latter association also seen for normal weight men, which was not the case for normal weight women. Positive associations of effusion with incident OA were only seen in overweight (OR 3.14, 95% CI [1.55 6.36]) and obese women (OR 3.03, 95% CI [1.50 6.15]) but not normal weight women or in men. Table 1 gives a detailed overview of these results regarding the interactions between BMI, sex and severity of inflammation at P-2 and P-1.
Table 1.
Demographics of sample
| Cases (N = 355) | Controls (N = 355) | p value | |||
|---|---|---|---|---|---|
| Mean | Std. Dev. | Mean | Std. Dev. | ||
|
| |||||
| Age (years) | 60.1 | 8.6 | 60.0 | 8.4 | NA |
| BMI (kg/m2) | 28.9 | 4.5 | 27.7 | 4.4 | 0.0003 |
| WOMAC knee pain1 | 2.6 | 3.3 | 1.4 | 2.5 | <.0001 |
| WOMAC functioning1 | 8.4 | 10.8 | 4.3 | 7.8 | <.0001 |
|
| |||||
| N | % | N | % | ||
| Sex | NA | ||||
| Female | 237 | 66.8 | 237 | 66.8 | |
| Male | 118 | 33.2 | 118 | 33.2 | |
|
| |||||
| BMI (kg/m2) | 0.0032 | ||||
| Normal/underweight | 70 | 19.7 | 108 | 30.4 | |
| Overweight | 147 | 41.4 | 136 | 38.3 | |
| Obese | 138 | 38.9 | 111 | 31.3 | |
|
| |||||
| Race | 0.2143 | ||||
| White | 283 | 79.7 | 299 | 84.2 | |
| African American | 61 | 17.2 | 47 | 13.2 | |
| Asian | 6 | 1.7 | 2 | 0.6 | |
| Other | 5 | 1.4 | 7 | 2 | |
|
| |||||
| KL grade | NA | ||||
| 0 | 133 | 37.5 | 133 | 37.5 | |
| 1 | 222 | 62.5 | 222 | 62.5 | |
|
| |||||
| Knee Injury at OAI baseline2 | 136 | 38.3 | 70 | 19.7 | <.0001 |
| Knee Surgery at OAI baseline3 | 54 | 15.2 | 24 | 6.8 | 0.0004 |
Std. Dev. – standard deviation, BMI – body mass index, KL - Kellgren-Lawrence, OAI – Osteoarthritis Initiative, NA – not applicable
WOMAC knee pain is on a scale from 1 to 20 and WOMAC functioning from 1–96, higher values representing more pain/less functioning.
Knee injury defined as one inhibiting ability to walk for at least two days
Knee surgery includes arthroscopy.
P values for differences by Fisher’s exact test for categorical variables and t-tests for ordinal variables and were not calculated for variables used in matching.
For those knees that developed ROA there were no increased odds of synovitis in the combined overweight/obese (i.e. categories combined) BMI subgroup compared to the normal weight subgroup at any of the four time points prior the case visit or the baseline visit. However, being overweight/obese was associated with an increased odds of effusion at P-2 (OR 2.21, 95% CI [1.11, 4.43]). Albeit not statistically significant, increased odds for effusion were also observed for the visit P-1 (OR 1.68, 95% CI [0.98, 2.88]). Table 2 presents details for the case knees and associated odds for synovitis or effusion at several time points prior to the incidence of ROA.
Table 2.
Odds for developing radiographic OA at OAI visits two (P-2) or one (P-1) year prior the case-defining visit in matched cases and controls
| P-2 | P-1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| N (all) | N (men) | Men n=136 | N (women) | Women n=300 | N (all) | N (men) | Men n=224 | N (women) | Women n=436 | ||
| (%) | (%) | OR (95% confidence interval | (%) | OR (95% confidence interval | (%) | (%) | OR (95% confidence interval | (%) | OR (95% confidence interval | ||
|
| |||||||||||
| Normal weight | 116 (26.6) | 21 (15.4) | 1.32 (0.42,4.15) | 95 (31.7) | 0.57 (0.31,1.04) | Normal weight | 166 (25.2) | 38 (17.0) | 0.96 (0.44,2.10) | 128 (29.4) | 0.52 (0.31,0.87) |
| Overweight | 169 (38.8) | 63 (46.3) | Ref | 106 (35.3) | Ref | Overweight | 264 (40.0) | 114 (50.9) | Ref | 150 (34.4) | Ref |
| Obese | 151 (34.6) | 52 (38.24) | 0.89 (0.44,1.78) | 99 (33.0) | 1.37 (0.77,2.43) | Obese | 230 (34.8) | 72 (32.1) | 0.92 (0.52,1.64) | 158 (36.2) | 1.44 (0.89,2.35) |
|
| |||||||||||
| No synovitis | 221 (50.7) | 59 (43.4) | Ref | 162 (54.0) | Ref | No synovitis at P-1 | 337 (51.1) | 99 (44.4) | Ref | 238 (54.6) | Ref |
| Synovitis | 215 (49.3) | 77 (56.6) | 1.79 (0.91,3.50) | 138 (46.0) | * 1.75 (1.05,2.91) | Synovitis at P-1 | 322 (48.9) | 124 (55.6) | * 3.62 (1.94,6.74) | 198 (45.4) | * 1.97 (1.29,3.01) |
| No effusion | 234 (53.7) | 72 (52.9) | Ref | 162 (54.0) | Ref | No effusionat P-1 | 338 (51.2) | 107 (47.8) | Ref | 231 (53.0) | Ref |
| Effusion | 202 (46.3) | 64 (47.1) | 0.75 (0.36,1.59) | 138 (46.0) | * 2.88 (1.64,5.03) | Effusion at P-1 | 322 (48.8) | 117 (52.2) | * 1.88 (1.05,3.37) | 205 (47.0) | * 2.89 (1.87,4.47) |
|
| |||||||||||
| No Synovitis, BMI Normal | 66 (15.1) | 11 (8.1) | 1.45 (0.31,6.77) | 55 (18.3) | 0.67 (0.29,1.59) | No Synovitis, BMI Normal | 91 (13.8) | 18 (8.1) | 1.57 (0.42,5.81) | 73 (16.7) | 0.75 (0.35 1.59) |
| No Synovitis, BMI Overweight | 84 (19.3) | 25 (18.4) | Ref | 59 (19.7) | Ref | No Synovitis, BMI Overweight | 130 (19.7) | 47 (21.1) | Ref | 83 (19.0) | Ref |
| No Synovitis, BMI Obese | 71 (16.3) | 23 (16.9) | 1.03 (0.31,3.42) | 48 (16.0) | * 2.87 (1.21,6.83) | No Synovitis, BMI Obese | 116 (17.6) | 34 (15.2) | 1.63 (0.58,4.57) | 82 (18.8) | * 2.30 (1.17,4.56) |
| Synovitis, BMI Normal | 50 (11.5) | 10 (7.4) | 3.25 (0.69,15.29) | 40 (13.3) | 1.52 (0.61,3.77) | Synovitis, BMI Normal | 74 (11.2) | 19 (8.5) | * 4.10 (1.46,11.55) | 55 (12.6) | 1.23 (0.57,2.64) |
| Synovitis, BMI Overweight | 85 (19.5) | 38 (27.9) | 1.99 (0.66,6.00) | 47 (15.7) | * 3.26 (1.39,7.65) | Synovitis, BMI Overweight | 134 (20.3) | 67 (30.0) | * 5.69 (2.06,15.67) | 67 (15.4) | * 3.66 (1.74,7.69) |
| Synovitis, BMI Obese | 80 (18.3) | 29 (21.3) | 1.63 (0.58,4.60) | 51 (17.0) | 1.86 (0.80,4.34) | Synovitis, BMI Obese | 114 (17.3) | 38 (17.0) | * 3.72 (1.39,9.98) | 76 (17.4) | * 2.71 (1.24,5.92) |
|
| |||||||||||
| No Effusion, BMI Normal | 69 (15.8) | 15 (11.0) | 3.86 (0.83 18.05) | 54 (18.0) | 0.68 (0.28,1.63) | No Effusion, BMI Normal | 105 (15.9) | 26 (11.6) | 1.00 (0.35,2.86) | 79 (18.1) | 0.50 (0.23,1.09) |
| No Effusion, BMI Overweight | 92 (21.1) | 31 (22.8) | ref | 61 (20.3) | Ref | No Effusion, BMI Overweight | 125 (18.9) | 46 (20.5) | Ref | 79 (18.1) | Ref |
| No Effusion, BMI Obese | 73 (16.7) | 26 (19.1) | 1.41 (0.46 4.33) | 47 (15.7) | 1.16 (0.50,2.67) | No Effusion, BMI Obese | 108 (16.4) | 35 (15.6) | 0.61 (0.25,1.51) | 73 (16.7) | 1.71 (0.85,3.47) |
| Effusion, BMI Normal | 47 (10.8) | 6 (4.4) | 10.00 (0.00,0.00) | 41 (13.7) | 1.30 (0.51,3.30) | Effusion, BMI Normal | 61 (9.2) | 12 (5.4) | 1.75 (0.46,6.58) | 49 (11.2) | 1.78 (0.81,3.89) |
| Effusion, BMI Overweight | 77 (17.7) | 32 (23.5) | 1.64 (0.53,5.12) | 45 (15.0) | * 3.56 (1.45,8.75) | Effusion, BMI Overweight | 139 (21.1) | 68 (30.4) | 1.41 (0.60,3.30) | 71 (16.3) | * 3.14 (1.55,6.36) |
| Effusion, BMI Obese | 78 (17.9) | 26 (19.1) | 1.10 (0.36,3.37) | 52 (17.3) | * 3.46 (1.38,8.72) | Effusion, BMI Obese | 122 (18.5) | 37 (16.5) | 1.94 (0.77,4.86) | 85 (19.5) | * 3.03 (1.50,6.15) |
No case knees in this category
statistically significant at p<0.05
OR- Odds Ratio; P-1 – OAI visit one year prior the case defining visit when incident radiographic osteoarthritis was diagnosed/read; P-2 – OAI visit two years prior the case defining visit when incident radiographic osteoarthritis was diagnosed/read; BMI – body mass index
Discussion
The presence of synovitis increased the odds of developing ROA in overweight women at the time point two years before ROA was detected, while obese women had an increased risk for ROA also without synovitis. At the time point one year prior OA incidence, we observed increased odds for incident ROA in overweight and obese women with presence of joint effusion but not in men. At the same time point, increased odds for ROA incidence were seen in both overweight and obese women and men in the presence of synovitis, but not for normal weight women with synovitis, suggesting the presence of effusion seems to play a role particularly in overweight or obese women. In knees that developed ROA, increased odds of effusion were observed for the combined overweight/obese group at P-2 but not for Hoffa-synovitis or any of the other time points suggesting a possible link between high BMI, presence of joint effusion and ROA development two years later.
While the role of body weight and knee ROA incidence is well established, its interactions with local inflammation have been less clear (1). Reported associations between obesity and OA development also for non-weight bearing joints suggest a more complex interaction beyond increased biomechanical loading. In a population-based cohort study it has been reported that metabolic syndrome may be prevalent in 59% of patients with knee OA and in 23% without (13). On the other hand, Niu et al. found in a population-based study that among women abdominal obesity and high blood pressure were associated with incident radiographic OA, but metabolic syndrome was not (3). We have shown previously a strong association between the presence of joint inflammatory markers based on MRI and subsequent ROA incidence and this current work expands this taking also into account sex and BMI differences (14). The fact that two years prior ROA incidence in women obesity without synovitis exhibited increased risk for ROA as well as being overweight with synovitis but obesity with synovitis did not, was not an expected finding. We can only speculate that potentially in obese persons other factors including direct results of increased loading due to higher BMI resulting in structural changes like bone marrow alterations, cartilage damage or meniscal lesions and extrusion, may be more relevant than inflammatory manifestations like effusion or synovitis.
Concerning the second part of our analysis focusing on cases only regarding prevalence of inflammatory markers in the different subgroups we found that up to four years prior ROA incidence in general the combined overweight/obese subgroup did not show significantly increased rates of local inflammation with the exception of effusion two years prior ROA incidence while at 1 year prior the association was close to being significant. A recent study also from the OAI reported a significantly greater prevalence and severity of synovial inflammation imaging biomarkers in knees of overweight and obese participants compared to those that have normal weight (15). In contrast to our study, however, almost 20% of included subjects exhibited ROA grades 2 and 3 and for those without ROA it is not known how many developed ROA at later time points. Thus, we speculate based on our findings that for case knees only i.e. for those that developed ROA, other factors beyond obesity including local structural damage such as meniscal or cartilage lesions may have additional impact on presence of synovial inflammation and thus diluting possible impact of increased BMI.
We acknowledge that in this exploratory study we did not analyze subjects with defined metabolic syndrome as we only analyzed interactions of BMI and MRI markers of inflammation, which limits extrapolation of our findings to patients with metabolic syndrome (3). An additional limitation of our study includes the absence of information on symptomatic OA. We do not know if subjects that developed ROA also developed symptoms and if subjects developed symptoms prior to the diagnosis of ROA. Further, the OAI study does not include contrast-enhanced MRI sequences, the gold standard for synovitis assessment (16). However, we used an established surrogate for whole joint synovitis that has been used in multiple studies applying MRI (11). Inter-and intra-reader agreement was almost perfect for effusion grading but only substantial for synovitis assessment, which is a limitation and likely reflecting the non-specificity of non-contrast-enhanced MRI (17).
In summary, the presence of MRI-defined Hoffa-synovitis seems to play a role for incident ROA development, especially in overweight women, whereas obese women have increased odds for ROA even in the absence of Hoffa-synovitis. Presence of joint effusion has an impact on ROA development particularly in overweight and obese women but not men. Being overweight/obese increased odds for joint effusion in the knees that developed incident ROA at time points one and two years prior. These results suggest that both mechanical load and inflammation have a role in OA incidence for overweight and obese women while for men the role of inflammation in conjunction with high BMI seems to be less relevant.
Supplementary Material
Significance and Innovations.
In women, being overweight with Hoffa-synovitis and being overweight or obese with effusion-synovitis increases odds for incident radiographic osteoarthritis (ROA) 2 years later
Presence of effusion-synovitis increases odds for incident ROA in overweight and obese women but not in men
For persons that develop incident ROA increased odds for effusion-synovitis were observed two years prior (OR 2.21, 95%CI 1.11,4.43).
Both mechanical load and inflammation seem to have a role in OA incidence for overweight and obese women while for men the role of inflammation in conjunction with high body mass index seems to be less relevant
Acknowledgments
We would like to thank the OAI participants, OAI investigators, OAI clinical and technical staff, the OAI coordinating center and the OAI funders for providing this unique public data base.
Funding
The study and image acquisition were funded by the OAI, a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners of the OAI include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
The image analyses were funded by a contract with the University of Pittsburgh (Pivotal OAI MRI Analyses [POMA]: NIH/NHLBI Contract No. HHSN2682010000 21C), and in part by a vendor contract from the OAI coordinating center at University of California, San Francisco (N01-AR-2-2258). The statistical data analysis was funded by a contract with the University of Pittsburgh (Pivotal OAI MRI Analyses [POMA]: NIH/NHLBI Contract No. HHSN2682010000 21C). The sponsors were not involved in the design and conduct of this particular study, in the analysis and interpretation of the data, and in the preparation, review, or approval of the manuscript.
Michael J. Hannon, MA: Has received an institutional grant by the NIH and has received consulting fees from EMD Serono (less than $10,000)
Felix Eckstein, MD: Has received consulting fees, speaking fees, and/or honoraria from MerckSerono, Synarc and Servier (less than $10,000 each, and has held educational lectures for Medtronic (less than $10,000). He has received funding support (for studies not related to the current one) from Pfizer, Eli Lilly, Stryker, Novartis, MerckSerono, Glaxo Smith Kline, Wyeth, Centocor, Abbvie, Kolon, Synarc, Ampio, and Orthotrophix.
Disclosure of interests
Frank W. Roemer, MD: Has received consulting fees, speaking fees, and/or honoraria from California Institute for Biomedical Research – Calibr (less than $10,000) and owns stock or stock options in Boston Imaging Core Lab. (BICL),LLC. a company providing image assessment services.
Ali Guermazi, MD, PhD: Has received consulting fees, speaking fees, and/or honoraria from AstraZeneca, Galapagos, Roche (less than $10,000 each), Pfizer Merck Serono, and TissuGene (more than $10,000 each) and owns stock or stock options in Boston Imaging Core Lab (BICL), LLC.
Tomoko Fujii, MS, MPH: No disclosures reported.
Patrick Omoumi, MS, Ph: No disclosures reported.
David J. Hunter, MBBS, PhD: Has received consulting fees, speaking fees, and/or honoraria from Pfizer, Merck Serono, TissueGene, and TLC (less than $10,000 each).
C. Kent Kwoh, MD: Has received consulting fees, speaking fees, and/or honoraria from Astellas, Fidia, GlaxoSmithKline, Kolon TissueGene, Regeneron, Regulus, Taiwan Liposome Company, and Thusane (less than 10,000 each) and from EMD Serono, and Express Scripts (more than $10,000 each).
References
- 1.Niu J, Zhang YQ, Torner J, Nevitt M, Lewis CE, Aliabadi P, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61(3):329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum 2012;64(2):443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu J, Clancy M, Aliabadi P, Vasan R, Felson DT. Metabolic Syndrome, Its Components, and Knee Osteoarthritis: The Framingham Osteoarthritis Study. Arthritis Rheumatol 2017;69(6):1194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S, Felson DT. What Is the Evidence to Support the Association Between Metabolic Syndrome and Osteoarthritis? A Systematic Review. Arthritis Care Res (Hoboken). 2019;71(7):875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Cheng Y, Shao D, Chen J, Sang Y, Gui T, et al. Metabolic Syndrome Increases the Risk for Knee Osteoarthritis: A Meta-Analysis. Evid Based Complement Alternat Med 2016;2016:7242478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clockaerts S, Bastiaansen-Jenniskens YM, Runhaar J, Van Osch GJ, Van Offel JF, Verhaar JA, et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage. 2010;18(7):876–82. [DOI] [PubMed] [Google Scholar]
- 7.Lee BJ, Yang S, Kwon S, Choi KH, Kim W. Association between metabolic syndrome and knee osteoarthritis: A cross-sectional nationwide survey study. J Rehabil Med 2019;51(6):464–70. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006;23(5):469–80. [DOI] [PubMed] [Google Scholar]
- 9.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appendix A. Project 15: reader discrepancies and adjudication procedures. OAI central reading of knee x-rays for K-L grade and individual features of knee OA. 2013; p. 8. URL: http://oai.epi-ucsf.org/datarelease/forms/kXR_SQ_BU_Descrip.pdf?V01XRKL. [Google Scholar]
- 11.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage. 2011;19(8):990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis 2007;66(12):1599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimura N, Muraki S, Oka H, Kawaguchi H, Nakamura K, Akune T. Association of knee osteoarthritis with the accumulation of metabolic risk factors such as overweight, hypertension, dyslipidemia, and impaired glucose tolerance in Japanese men and women: the ROAD study. J Rheumatol 2011;38(5):921–30. [DOI] [PubMed] [Google Scholar]
- 14.Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ, Eckstein F, Fujii T, et al. What comes first? Multitissue involvement leading to radiographic osteoarthritis: magnetic resonance imaging-based trajectory analysis over four years in the osteoarthritis initiative. Arthritis Rheumatol 2015;67(8):2085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanthawang T, Bodden J, Joseph GB, Lane NE, Nevitt M, McCulloch CE, et al. Obese and overweight individuals have greater knee synovial inflammation and associated structural and cartilage compositional degeneration: data from the osteoarthritis initiative. Skeletal Radiol 2020. Jul 23. doi: 10.1007/s00256-020-03550-5. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guermazi A, Roemer FW, Hayashi D, Crema MD, Niu J, Zhang Y, et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann Rheum Dis 2011;70(5):805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roemer FW, Jarraya M, Felson DT, Hayashi D, Crema MD, Loeuille D, et al. Magnetic resonance imaging of Hoffa’s fat pad and relevance for osteoarthritis research: a narrative review. Osteoarthritis Cartilage. 2016;24(3):383–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.