Highlights
-
•
Metastatic SCC arising from the ovary is rare, and the optimal treatment is unknown.
-
•
Pembrolizumab successfully treated a patient with metastatic SCC.
-
•
Patients on pembrolizumab should be monitored for immune-related adverse events.
Keywords: Ovarian squamous cell carcinoma, Mature cystic teratoma, Immune checkpoint inhibitor, Pembrolizumab
1. Introduction
Squamous cell carcinoma (SCC) of the ovary is a rare clinical entity that most commonly arises from a mature cystic teratoma (MCT). While MCT is generally a benign ovarian germ cell tumor, malignant transformation occurs in <2% of cases, with the majority (>80%) of these events being SCC (Li et al., 2019, Hackethal et al., 2008). The prognosis is poor for patients diagnosed at International Federation of Gynecology and Obstetrician (FIGO) stage II or higher. While adjuvant chemotherapy has evidence of a survival benefit in advanced disease, the optimal first-line regimen is unclear, and management is often adapted from treatment for epithelial ovarian cancer or SCC of other sites (Li et al., 2019, Hackethal et al., 2008).
In recent years, the advent of immune checkpoint inhibitors (ICIs) has vastly expanded the oncological armamentarium. Among these agents, pembrolizumab (Keytruda®, Merck and Co., Inc.) is a humanized monoclonal antibody that blocks the binding of programmed death-1 (PD-1) receptor to programmed death ligand-1 and 2 (PD-L1 and PD-L2) and has shown efficacy against a wide variety of solid tumors. For treatment of gynecological malignancies, it has been approved by the United States Food and Drug Administration for use in recurrent or metastatic cervical cancer and as part of combination therapy for advanced endometrial cancer (Merck Sharp & Dohme, 2020).
To our knowledge, there have been no reports in the literature of SCC arising from an ovarian MCT treated with an ICI. We report here the first case of a patient with metastatic SCC presumed to arise from an ovarian MCT who was successfully treated with pembrolizumab after progressing through platinum-based combination chemotherapy.
2. Case report
A 36-year-old multiparous woman with a past medical history significant for uterine fibroids presented to her local gynecologist with symptoms of left flank pain, urinary urgency, and pelvic pain. She had an Eastern Cooperative Oncology Group (ECOG) score of 0. Six weeks prior to surgery, she underwent computerized tomography (CT) and magnetic resonance imaging (MRI) studies that revealed a 6 cm pelvic mass thought to be arising from the uterus and moderate left-sided hydronephrosis due to mass effect. A CA 125 level was normal at 27 U/mL. Due to initial concern for uterine fibroids, she received one dose of leuprolide, but did not tolerate the side-effects. She self-referred to gynecologic oncology and was recommended for surgical evaluation. The patient underwent a diagnostic laparoscopy converted to a laparotomy. Intraoperatively, she was found to have a pelvic mass densely adherent to the left pelvic sidewall, completely obstructing the left ureter and adherent to the sigmoid colon (Fig. 1). Palpable liver lesions were also present. The left ovary was unable to be identified. She had an en bloc resection of the uterus, cervix, left pelvic mass, and a portion of the sigmoid colon, and underwent a liver biopsy. Frozen pathology was consistent with a poorly differentiated epithelioid neoplasm, and the contralateral ovary was also removed. At the completion of the case she had residual disease remaining in the bilateral pelvic sidewalls and liver. A ureteral stent was unable to be inserted intraoperatively due to complete obstruction, and a percutaneous nephrostomy tube was placed postoperatively. Her preoperative creatinine was 1.1 mg/dL.
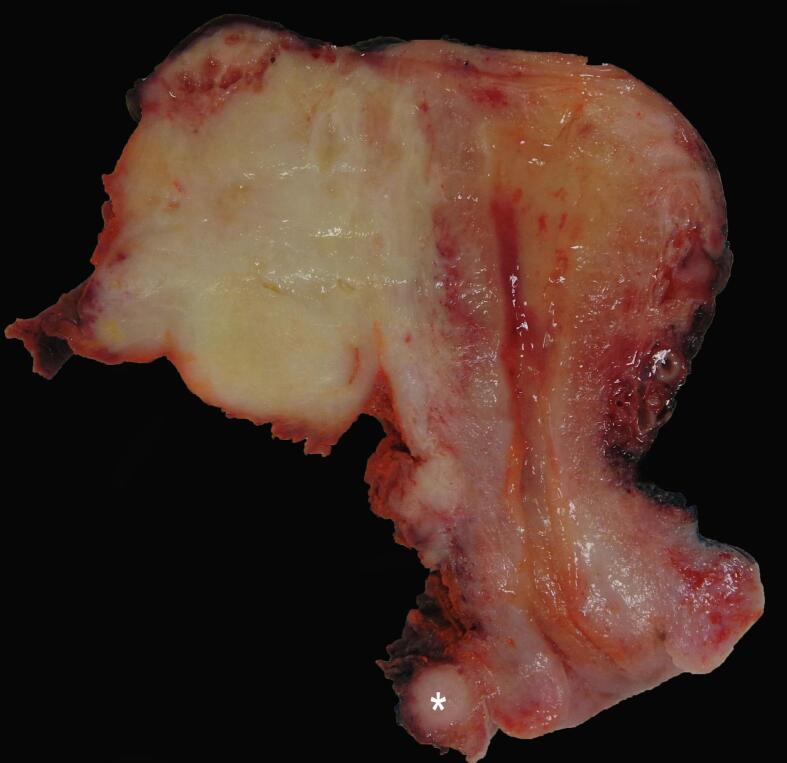
Fig. 1.
White-yellow firm mass in the left adnexal region invading into the myometrium. Note an additional nodule in the left parametrium (*). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Final pathology showed a metastatic poorly differentiated SCC (Fig. 2a) involving the left parametrium, cervical stroma, myometrium, pelvic sidewall, colon, liver, and two lymph nodes that was positive for p40 (diffuse), GATA-3 (patchy), and CK20 (weak, non-specific), but negative for p16 and CK7. Multiple sections were examined of the mass in the left adnexal region, but the tissue was completely infarcted and no viable tumor, residual ovary, nor fallopian tube were identified. While morphology and immunoprofile were compatible with SCC, the origin of the tumor was unclear. The disease distribution (i.e., tumor growing into the uterus), lack of overlying squamous dysplasia, and a negative p16 argued against a cervical origin. Similarly, the absence of a vaginal, vulvar, or anogenital mass, and a negative p16 made an HPV-associated lower genital tract SCC unlikely. Due to the encasement of the ureter, a urothelial cell carcinoma with extensive squamous differentiation was considered but was improbable as CK7 was negative. Thus, in the absence of a known primary elsewhere, an ovarian origin was favored. Ovarian SCC are uncommon; they most commonly arise from a MCT, and less likely from a malignant Brenner tumor with extensive squamous differentiation. As the latter are generally CK7 positive, development from a MCT was favored as the primary lesion. Tumor PD-L1 expression was 50–60% (Fig. 2b). Next generation sequencing showed that the tumor was microsatellite stable with TP53, NF2, and NOTCH1 mutations, CDKN2A/2B loss, and RAD21 amplification.
Fig. 2.
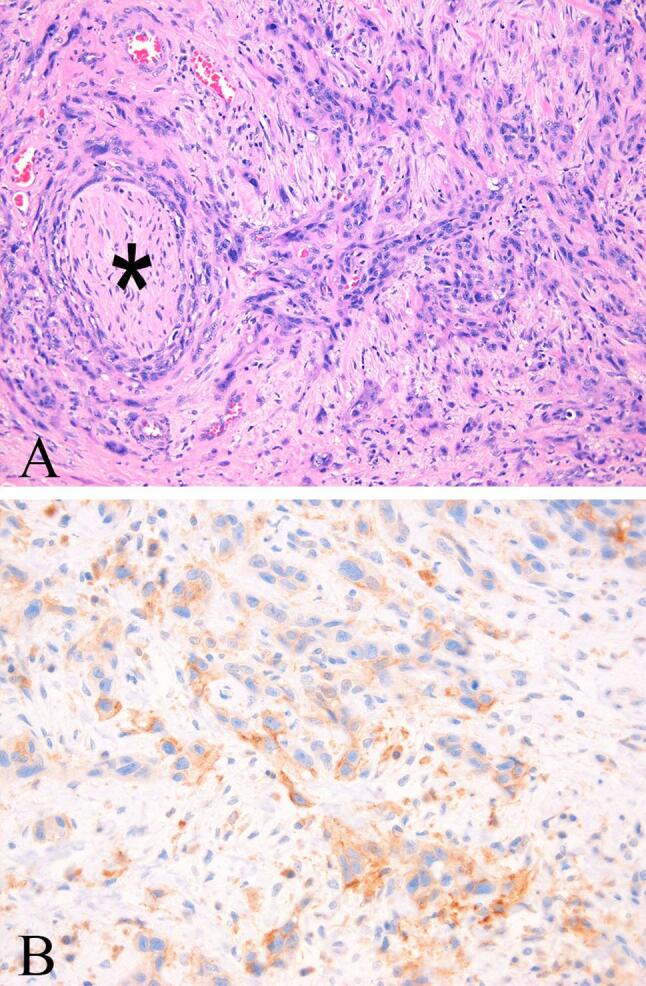
(A) Poorly differentiated squamous cell carcinoma with perineural invasion (*) (Hematoxylin and eosin stain, 400X). (B) A subset of tumor cells showed partial or complete membranous staining of moderate intensity for PD-L1 (PD-L1 immunostain, 400X).
The case was discussed at multidisciplinary tumor board, and she was recommended for adjuvant carboplatin and paclitaxel with addition of bevacizumab at cycle two. A baseline postoperative CT scan of the chest, abdomen, and pelvis prior to initiation of chemotherapy showed interval development of metastases to the lungs, liver, retroperitoneum, mesentery, and omentum. Serum levels of CA 125, CEA, CA 19–9, and AFP obtained prior to chemotherapy initiation were all within normal limits.
Five weeks after surgery, the patient was initiated on carboplatin at intravenously (IV) AUC 6 dose and paclitaxel IV 175 mg/m2. During her second cycle, bevacizumab 15 mg/kg was added. The patient tolerated three cycles of this regimen, however, restaging CT showed progression of disease. Unfortunately, the patient’s ECOG performance status declined to a score of 2 due to rapid disease progression. As such, she was not eligible for any available clinical trials.
Given the tumor’s PD-L1 positivity, the decision was made to pursue therapy with pembrolizumab IV 200 mg every three weeks. She began to symptomatically improve after cycle one and a restaging CT scan after cycle three showed a decreased size of all lesions with the exception of a new pleural lesion. Imaging after six months on therapy showed a decreased size of most reference lesions including the pleural lesion (Fig. 3).
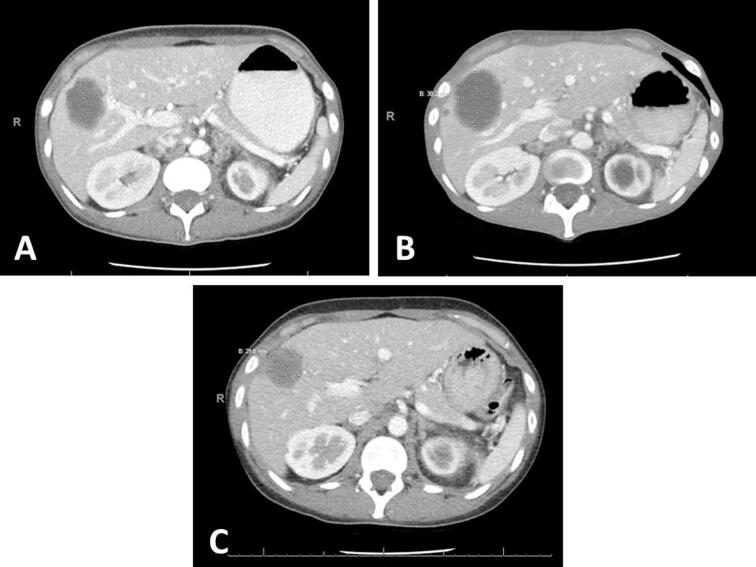
Fig. 3.
Axial CT scans of the chest, abdomen, and pelvis. (A) Imaging prior to adjuvant chemo. Reference hepatic lesion measures 4.4 × 3.2 cm. (B) Imaging after cycle 3 of carboplatin, paclitaxel, and bevacizumab. Hepatic lesion measures 3.8 × 5.1 cm. (C) Imaging after cycle 6 of pembrolizumab. Hepatic lesion measures 3.5 × 3.0 cm.
At cycle six, the patient was transitioned to pembrolizumab 400 mg dosed every six weeks. However, she experienced grade 3 arthritis with significant, prolonged morning stiffness of her hands along with grade 2 sicca syndrome. She consequently resumed her previous dosing schedule. After cycle eight of treatment, she experienced grade 4 hypercalcemia with a maximum serum calcium up to 14.1 mg/dL and required hospital admission. Other laboratory work-up showed normal serum albumin (3.8 g/dL), low parathyroid hormone (PTH) (<6 pg/mL), normal 25-hydroxy vitamin D (28 ng/mL), and normal PTH-related protein (1.1 pmol/L). The etiology was thought to be an immune-related adverse event (irAE) secondary to pembrolizumab therapy. Pembrolizumab was held for one cycle and the patient received intravenous fluids, but her calcium level rose again despite initial improvement. With hypercalcemia, arthritis, and sicca, the clinical picture had features of sarcoidosis although her anti-angiotensin antibodies were unremarkable. She then received a systemic corticosteroid taper in addition to zolendronic acid with significant improvement in her serum calcium level and arthritis and was subsequently able to resume pembrolizumab.
At the time of this publication, the patient continues on pembrolizumab at 15 months with stable disease. She is more than 18 months out from her initial diagnosis and has returned to an ECOG performance status of 0.
3. Discussion
Ovarian SCC arising from MCT is a rare clinical entity that exhibits a poor prognosis when diagnosed at advanced stages. In the largest systematic review of ovarian SCC to date, Li et al. (2019) examined data from 435 cases and showed that while patients diagnosed at stage I had a 5-year overall survival of 85.8%, those diagnosed at stages II, III, and IV had significantly reduced survival rates of 39.1%, 26.2%, and 0% respectively. Our patient was found to have stage IVb disease, and the extent of her tumor burden prevented full resection at time of surgery. In cases of advanced disease, chemotherapy has been shown in retrospective studies to confer survival benefit, although given the rarity of ovarian SCC, prospective randomized data on optimal adjuvant treatment do not exist (Li et al., 2019). In a Gynecologic Cancer InterGroup consensus review, Glasspool et al. (2014) noted that platinum-based combination therapy was frequently used. Our patient’s initial regimen of carboplatin, paclitaxel, and bevacizumab was selected based on first-line therapy for advanced ovarian cancer, in addition to activity seen in cervical SCC. Unfortunately, she showed rapid disease progression without a clear second-line option recognized within the literature.
Given positive tumor PD-L1 expression, it was hypothesized that the patient’s disease may be responsive to ICI therapy. There are no data regarding prevalence of PD-L1 expression in ovarian SCC, but studies of SCC of other primary sites have shown PD-L1 positivity ranging from 33 to 59% in squamous non-small cell lung cancer to 83.7% in cervical cancer (Chung et al., 2019, Yu et al., 2016). A recent comprehensive molecular analysis of carcinomas arising from MCT from Tamura et al. (2020) found that, compared to SCC of other sites, SCC arising from MCT resulted in tumor cells that stably overexpress the gene XCL1, which produces a cytokine that contributes to activation of CD8+ cytotoxic T cells. XCL1 overexpression was significantly associated with both CD8+ T cell tumor infiltration and PD-L1 tumor expression. Although we do not have information regarding XCL1 expression in our patient, this may be one mechanism that explains her durable response to pembrolizumab therapy.
Although pembrolizumab has a relatively favorable safety profile compared to cytotoxic chemotherapy agents, our patient experienced grade 2 sicca syndrome, grade 3 arthritis, and grade 4 hypercalcemia potentially attributable to pembrolizumab. While hypercalcemia of all grades occurred in 14% of cervical cancer patients who received pembrolizumab in the KEYNOTE-158 trial, only 2.6% were grades 3–4 (Merck Sharp & Dohme, 2020). Our patient also presented with a clinical picture suggestive of a more systemic autoimmune process during the episode of hypercalcemia, prompting treatment with a steroid taper for possible sarcoid-like reaction secondary to ICI therapy. IrAEs secondary to ICIs are well-described, and there have been reports of sarcoidosis exacerbated or induced by pembrolizumab therapy (Gkiozos et al., 2018). Our patient ultimately responded well to a course of systemic corticosteroids and zoledronic acid. She was able to continue on pembrolizumab without recurrence of her hypercalcemia to date.
4. Conclusion
The prognosis for ovarian SCC remains grim in cases diagnosed at stage II and greater, and the optimal second line treatment regimen is unclear. Given the success of ICIs in multiple solid tumors, patients who progress through or fail to respond to an initial line of chemotherapy should be considered for immunotherapy, preferably on a clinical trial if one is available. Further investigation into the genomic characteristics of ovarian SCC may help guide selection of those patients who may most benefit most from ICI therapy.
5. Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
Meng Wu: Writing - original draft. Jennifer A. Bennett: Writing - review & editing. Pankti Reid: Writing - review & editing. Gini F. Fleming: Conceptualization, Writing - review & editing. Katherine C. Kurnit: Conceptualization, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chung H.C., Ros W., Delord J.P. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 2019;37(17):1470–1478. doi: 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- Gkiozos I., Kopitopoulou A., Kalkanis A., Vamvakaris I.N., Judson M.A., Syrigos K.N. Sarcoidosis-like reactions induced by checkpoint inhibitors. J. Thorac. Oncol. 2018;13(8):1076–1082. doi: 10.1016/j.jtho.2018.04.031. [DOI] [PubMed] [Google Scholar]
- Glasspool R.M., González Martín A., Millan D. Gynecologic Cancer InterGroup (GCIG) consensus review for squamous cell carcinoma of the ovary. Int. J. Gynecol. Cancer. 2014;24(9 Suppl 3):S26–S29. doi: 10.1097/IGC.0000000000000209. [DOI] [PubMed] [Google Scholar]
- Hackethal A., Brueggmann D., Bohlmann M.K., Franke F.E., Tinneberg H.R., Münstedt K. Squamous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data [published correction appears in Lancet Oncol. 2009 May;10(5):446] Lancet Oncol. 2008;12(9):1173–1180. doi: 10.1016/S1470-2045(08)70306-1. [DOI] [PubMed] [Google Scholar]
- Keytruda (pembrolizumab) [package insert]. Whitehouse Station, NJ, Merck Sharp & Dohme, 2020.
- Li C., Zhang Q., Zhang S. Squamous cell carcinoma transformation in mature cystic teratoma of the ovary: a systematic review. BMC Cancer. 2019;19(1):217. doi: 10.1186/s12885-019-5393-y. Published 2019 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura R., Yoshihara K., Nakaoka H. XCL1 expression correlates with CD8-positive T cells infiltration and PD-L1 expression in squamous cell carcinoma arising from mature cystic teratoma of the ovary. Oncogene. 2020;39(17):3541–3554. doi: 10.1038/s41388-020-1237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Boyle T.A., Zhou C., Rimm D.L., Hirsch F.R. PD-L1 Expression in Lung Cancer [published correction appears in J Thorac Oncol. 2017 Jan;12 (1):157-159] J. Thorac. Oncol. 2016;11(7):964–975. doi: 10.1016/j.jtho.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]