Abstract
Background
In Southeast Asia, Gynura procumbens has been utilized as a traditional medicinal herb for many diseases. The nontoxic effects of the leaves of G procumbens can be consumed safely for the treatment of many diseases, especially diabetes mellitus.
Objectives
This study aimed to assess the antibacterial, anti-inflammatory, and cytotoxic effects of G procumbens leaves through different extracts.
Methods
Cold extraction was employed for G procumbens leaves. Disc diffusion, bovine serum albumin denaturation, brine shrimp lethality assays, and microscopic examination of tissues (ie, liver, kidney, and heart) were performed to measure antibacterial, anti-inflammatory, and cytotoxic activities, and histopathologic analysis, respectively.
Results
The distinct concentrations of aqueous, ethanol, and n-hexane extracts showed prominent antibacterial activity against four pathogenic bacterial strains (Chromobacterium sp, Staphylococcus aureus, Enterococcus faecium, and Escherichia coli 0157:H7:LT). The aqueous extract of G procumbens at a concentration of 200 µg/mL showed potential antibacterial activities against S aureus and E faecium, with mean (SD) zones of inhibition of 15 (1.0) mm and 10 (0.55) mm, respectively. At a concentration of 40 µg/mL, the aqueous extract of G procumbens exhibited significant (P < 0.01) anti-inflammatory activity compared with that of the other 2 extracts in the bovine serum albumin denaturation assay. The n-hexane extract showed moderate cytotoxic activity compared with that of vincristine sulfate. The median lethal concentration values of the aqueous, ethanol, and n-hexane extracts were 427.66, 218.56, and 114.51 μg/mL, respectively. The aqueous extract exhibited lower cytotoxicity than that exhibited by the ethanol and n-hexane extracts. The histopathologic analysis results showed that the aqueous extract of G procumbens had no harmful effects on the different organs of the experimental mice.
Conclusions
The aqueous extract of G procumbens could be a potential source for treating various infectious and chronic diseases. (Curr Ther Res Clin Exp. 2021; 82:XXX–XXX)
Key words: Antibacterial activity, anti-inflammatory activity, cytotoxicity, Gynura procumbens, histopathology, lethality bioassay
Introduction
Gynura procumbens belongs to the family Asteraceae and is a fast-growing evergreen herb that commonly grows in Malaysia, Vietnam, Thailand, Indonesia, and China.1 It is widely used as an effective alternative medicine. The leaves of G procumbens are exploited as a traditional medicinal plant to treat many diseases, such as diabetes, hypertension, hyperlipidemia, inflammation, and cancer.2 The phytochemicals (ie, flavonoids, terpenoids, tannins, and saponins) present in G procumbens are responsible for its health benefits.3 The polysaccharide compounds present in G procumbens possess effective biological activities for the treatment of diabetes mellitus, hypertension, rheumatism, constipation, wound healing, and kidney diseases.1,4 Currently, the intake of conventional drugs with herbal medicines has been reported to increase the efficacy of traditional drugs.3
Plant products have attracted increasing attention for the development of alternative and novel drugs because some human and animal infective bacteria have become increasingly resistant to conventional medicines and some conventional medicines have negative side effects. Most medicinal plants have diverse remedial influence on different types of diseases and are cost-effective, have high efficacy, and high availability.2,5 Among medicinal plants, G procumbens has the potential to decrease cholesterol levels and high blood pressure. In addition, G procumbens can be used to treat dysentery, kidney failure, bleeding, throat infection, and menstrual cycle disturbances.6 Glycosides and flavonoids present in G procumbens have beneficial health effects.4 For primary health care, around 90% of rural people in developing countries are wholly dependent on traditional medicines, and G procumbens can defend against many diseases. However, the dose-dependent antibacterial and anti-inflammatory effects of different extracts of G procumbens have not yet been investigated. Thus, this study focused on evaluating the bioactivity of multiple doses of the novel extracts of G procumbens leaves.
Materials and Methods
Materials and chemicals
The reagents and chemicals used in this study were procured from Thermo Fisher Scientific (Waltham, Massachusetts). Vincristine sulfate, dimethyl sulfoxide (DMSO) 78.13, and methanol were purchased from Merck (Kenilworth, New Jersey) and BDH (Purite-Suez Water, U.K), respectively. Ethanol, ether, anhydrous sodium sulfate, sodium chloride, bovine serum albumin (BSA), and salicylic acid were purchased from Sigma Aldrich (St Louis, Missouri). Nutrient broth and nutrient agar media were procured from Merck. Whatman No. 1 filter paper was obtained from the Merck.
Sample collection and preparation
Leaves of G procumbens were collected from the Daratana nursery in Jashore, Bangladesh, and identified by the Bangladesh National Herbarium, Bangladesh. After washing, the leaves were dried at room temperature (25°C), crushed into a fine powder, and stored at 4°C.
Preparation of G procumbens leaf extracts
The extraction of G procumbens leaf was carried out according to the method as described with slight modification.7 The powdered plant material (200 g) was first extracted via maceration in 1.5 L water and kept at 50°C in a water bath for 3 hours, followed by stirring (160 rpm) of the extract at 20-minute intervals. The aqueous extract was freeze-dried using a BR Biochem Life Sciences (New Delhi, India) freeze dryer to obtain the powder, and the powdered extract was stored at 4°C until further use. For ethanolic and n-hexane extraction, 200 g G procumbens powder was added to 95% ethanol (1.5 L) and n-hexane (1.5 L) for 7 days at room temperature with shaking at 250 rpm in a shaking incubator. A rotary vacuum evaporator (260,0000 Witeg, Germany) was used to concentrate the extracted solvent at 40°C to yield the ethanolic and n-hexane extracts of G procumbens leaves, which were then stored at 4°C.
Phytochemical analysis of G procumbens extracts
Qualitative phytochemical screening
The extracts (ie, aqueous, ethanolic, and n-hexane) of G procumbens were subjected to qualitative chemical tests for identification of various phytoconstituents following the methodology of Harborne and Kokate.8,9
Test for alkaloids
Dragendorff's test. Dragendorff's reagent (1 mL) was added to the extract. An orange-red precipitate indicated the presence of alkaloids.
Test for carbohydrates
Molisch test. α-Naphthol solution (1 mL) was added to the extract, and concentrated sulfuric acid was added along the sides of the test tube. A purple or reddish-violet color at the junction between the 2 liquids indicated the presence of carbohydrates.
Test for proteins
Biuret test. The extract was added to 1 mL 40% sodium hydroxide and 2 drops 1% copper sulfate solution. A violet color indicated the presence of proteins.
Test for phenols
Distilled water (2 mL) followed by several drops of 10% ferric chloride was added to 1 mL extract. A green color indicated the presence of phenols.
Test for steroids
Salkowski test. The extracts were dissolved in chloroform, and an equal volume of concentrated sulfuric acid was added. A bluish-red to cherry red color is observed in the chloroform layer, and the acid layer is marked with green fluorescence, indicating the presence of steroids.
Test for glycosides
Keller Killiani test. The extracts were dissolved in acetic acid containing a trace of ferric chloride and transferred to the surface of concentrated sulfuric acid. At the junction, a reddish-brown color is formed, which gradually becomes blue, indicating the presence of glycosides.
Test for flavonoids
Magnesium turnings were added to the extract, followed by the addition of concentrated hydrochloric acid. A red color indicated the presence of flavonoids.
Test for tannins
Ferric chloride was added to the extract. Dark-blue or greenish-black colors indicated the presence of tannins.
Test for terpenoids
A mixture of chloroform (2 mL) and concentrated tetraoxosulfate acid (3 mL) was added to 5 mL of each extract to form a layer. The presence of a reddish-brown coloration at the interface indicated the presence of terpenoids.
Test for saponin
A mixture of distilled water (5 mL) and each extract (10 mL) was vigorously agitated to form a stable, persistent foam. The formation of an emulsion upon the addition of 3 drops of olive oil indicated a positive result.
Test for caffeine
Murexide test. Several drops of G procumbens extracts and 3 to 5 drops of nitric acid were added in a small porcelain dish and evaporated to dryness. After adding 2 drops of ammonium hydroxide, the development of a purple color indicated the presence of caffeine.
Antimicrobial activity measurement
Preparation of the bacterial strains
The bacterial strains (Escherichia coli H7:LT04:0157, Staphylococcus aureus, Enterococcus faecium, and Chromobacterium sp) were acquired from the Department of Microbiology, Jashore University of Science and Technology, Jashore, Bangladesh. Luria-Bertani (LB) broth and nutrient agar media were used to culture the bacterial strains. For antibacterial testing, the frozen stock culture was thawed and then 100 μL was spread onto LB agar medium, and the single colony was cultured on LB agar medium. A single colony was then inoculated into 25 mL LB broth and incubated at 37°C with constant agitation at 250 rpm for bacterial growth, unless the mid-log phase of absorbance at 600 nm was obtained at 0.4 (3.2 × 108 cells/mL) using a spectrophotometer (V-1100; Mapada Instruments Co, Ltd, Shanghai, China).
Disc diffusion assay
The antibacterial action was implemented by disc diffusion techniques presented previously by Rahman et al10 with a slight alteration. A 6-mm-diameter disc of Whatman No. 1 filter paper was placed into a vial followed by autoclaving for 15 minutes at 121°C and 15 lb/in2 pressure. After autoclaving, the discs were dried in a drying oven. The different extracts of G procumbens leaves were dissolved in DMSO to a final concentration of 100 mg/mL. Each disc was soaked with 10 μL 100 mg/mL (1000 μg/disc) of the respective extracts and aseptically air-dried on a clean bench for use in the antibacterial assay. The negative control discs were soaked in 10 μL DMSO only. The bacterial broth culture of the mid-log phase was dispersed onto a nutrient agar plate, followed by incubation for 24 hours at 37°C. The zones of inhibition were calculated in millimeters to determine the antibacterial activity. Ampicillin was used as the positive control.
Assessment of in vitro anti-inflammatory activity
Inhibition albumin denaturation assay
The anti-inflammatory activity of G procumbens extracts (ie, aqueous, ethanolic, and n-hexane) was determined in vitro to inhibit the denaturation of BSA according to previously described methods with some modifications.11,12 In this assay, the reaction mixture consisted of test extracts of G procumbens leaves at different concentrations and 1% BSA solution prepared in phosphate-buffered saline (pH 7.4). The pH of the reaction mixture was adjusted to 6.8 using a small amount of 1 N hydrochloride. The reaction mixtures were incubated at 72°C for 5 minutes and allowed to cool. The absorbance of the control and test samples was determined using a spectrophotometer (V-1100; Mapada Instruments Co) at 660 nm. The experiment was conducted in triplicate, and the percent inhibition of BSA denaturation was calculated as follows:
where AC is the absorbance of the control sample and AS is the absorbance of the test sample.
In vitro cytotoxicity assay
Brine shrimp lethality bioassay
The in vitro cytotoxicity of G procumbens extracts (ie, aqueous, ethanolic, and n-hexane) was determined by a brine shrimp lethality bioassay as described by Naz et al13 with some modifications. Brine shrimp (Artemia salina) eggs were acquired from an aquarium shop in Dhaka, Bangladesh, and hatched in a vessel with simulated seawater. Simulated seawater was prepared using 38 g sodium chloride in 1 L water, and hatching was continued for 48 hours under constant aeration with direct light that warmed the vessel to approximately 24°C to 25°C. After hatching, active nauplii were collected and transferred to fresh seawater. Different concentrations of the various solvent extracts of G procumbens leaves and positive control (vincristine sulfate) were prepared in DMSO (not more than 50 μL 10% DMSO in 5 mL solution) and seawater to attain diluted solutions at concentrations of 500, 250, 125, 75, 62.25, 50, 31.25, 30, and 20 µg/mL. A vial containing 50 μL 10% DMSO diluted to a total volume of 5 mL simulated seawater was used as a negative control. Ten nauplii were placed in each vial, and the number of surviving nauplii in each vial was calculated by observation using a magnifying glass after 24 hours. The following formula was used to calculate the percent mortality of brine shrimp nauplii at each concentration:
where NT is the number of nauplii taken (n = 10) and NL is the number of nauplii alive after 24 hours of incubation. The LC50 values were calculated by linear regression analysis of the percent mortality versus the concentrations of test samples using Microsoft Office Excel 2010 (Microsoft Corp, Redmond, Washington).
Histopathologic analysis for toxicity test
Experimental animals
Swiss albino mice aged 6 weeks (weight 25–30 g) were obtained from Jahangirnagar University, Bangladesh. The mice were acclimatized to the laboratory environment for 7 days before the experiments.
Induction of diabetes in mice
Alloxan was administered intraperitoneally at a concentration of 150 mg/kg to induce diabetes, which was confirmed by measuring blood glucose levels above 13 mmol/L using a glucometer.14
Experimental design
Eighteen mice were randomly divided into 3 groups (n = 6). Mice in the normal control group (negative control) were fed normal saline. Mice in the alloxan-induced diabetic mice group were treated with G procumbens aqueous extracts (treatment group) and fed an aqueous extract of G procumbens (200 mg/kg weight). The alloxan-induced diabetic mouse group (the positive control) were fed normal saline. The body weight and fasting blood glucose level of each mouse in each group were measured at day 0 and after 28 days of treatment. After 28 days of treatment, overnight-fasted mice were euthanized to obtain heart, liver, and kidney samples for analysis.
Histopathologic examination
Histopathologic examination of the vital organs of the mice in all groups was performed as previously described.15
Statistical analyses
Data were analyzed using Origin Lab version 7.0. One Round house plaza Northampton, MA 01060 USA Values are presented as mean (SEM). Analysis of variance followed by Tukey post-hoc test was used to calculate the statistical significance of the results. The level of significance was expressed as P < 0.05, P < 0.01, and P < 0.001.
Results and Discussion
The diverse solvent extracts of G procumbens leaves containing various phytochemicals are shown in Table 1. Compared with the other 2 extracts (ie, ethanolic and n-hexane), the aqueous extract contained some pharmacologically important phytoconstituents. Phytoconstituents, as active components in plant extracts, are amenable to multifarious pharmacologic actions. Plant secondary metabolites influence the therapeutic and pharmacologic activities of medicinal herbs. Preliminary phytochemical screening experiments showed that the aqueous extracts of G procumbens leaves contained phenolic compounds, such as tannins and flavonoids. In contrast, the ethanolic and n-hexane extracts of G procumbens leaves showed only the presence of flavonoids. This may be why the aqueous solvent has a strong capacity for extracting phenolic compounds.
Table 1.
Preliminary phytochemical screening of the various extracts of Gaultheria procumbens leaves.
| Phytochemical compound | Extract* |
||
|---|---|---|---|
| Aqueous | Ethanol | n-hexane | |
| Carbohydrates | + | + | + |
| Proteins | + | + | + |
| Alkaloids | + | + | + |
| Phenols | + | _ | _ |
| Tannins | + | _ | _ |
| Saponins | + | + | + |
| Steroids | + | + | + |
| Flavonoids | + | + | + |
| Terpenoids | _ | + | _ |
| Glycosides | _ | + | _ |
| Caffeine | _ | _ | _ |
(+) indicates present and (–) indicates absence of the phytochemical compounds in the aqueous, ethanol, and n-hexane extracts.
Plant phenolic compounds possess potent antimicrobial16,17 and anti-inflammatory activity.12,18 Flavonoid compounds and their derivatives, such as isovitexin 2′'-O-xyloside, 6,8-Di-C-beta-D-arabinopyranosylapigenin, and homoesperetin 7-rutinoside, have been identified in the aqueous extracts of G procumbens,19 and flavonoids from plant extracts possess antimicrobial and anti-inflammatory properties.20,21 Saponins and glycosides are vital classes of secondary metabolites because some of them are cardioactive and used in the treatment of heart disease,22 and saponins have antimicrobial23 and anti-inflammatory activity.24 The strong presence of tannins in plant extracts allows them to possess potent antimicrobial activities16 and anti-inflammatory properties.25 In this study, we found that aqueous extracts were enriched with phenols and tannins. Therefore, the aqueous extracts of G procumbens leaves may have a potential health benefit.
The antibacterial activities of different extracts of G procumbens leaves against the gram-positive and gram-negative bacteria were examined on the basis of a clear zone of inhibition. As shown in Table 2, among the 3 different concentrations (200, 250, and 500 µg/mL) of the aqueous, ethanol, and n-hexane extracts, the 500 μg/disc exhibited significant antibacterial activity against the gram-negative bacteria E coli-H7, -LT04, and -0157 with zones of inhibition ranging from 3 to 21 mm. Moreover, E coli-H7 had the highest mean (SEM) zone of inhibition (10.00 [0.46] mm) in the aqueous extract; E coli LT04 had a men (SEM) zone of inhibition of 21.00 (1.25 mm) in n-hexane, and E coli-0157 had a mean (SEM) zone of inhibition of 20.00 (1.16) mm in the ethanol extract. For gram-positive bacteria (Chromobacterium sp, S aureus, and E faecium), the aqueous, ethanolic, and n-hexane extracts at 500 μg/disc exhibited high potential antibacterial activity with a mean (SEM) inhibition zone ranging from 10 (0.65) mm to 19 (1.45) mm. Among them, S aureus and E faecium had the highest mean (SEM) zone of inhibition at 18 (1.25) mm and 19 (1.45) mm, respectively, in both the aqueous and n-hexane extracts, whereas Chromobacterium sp showed the highest mean (SEM) zone of inhibition at 18 (1.35) mm in both ethanol and n-hexane extracts. Unexpectedly, S aureus and E faecium exhibited mean (SEM) zones of inhibition ranging from 10 (0.55) to 16 (1.35) mm at the 200 and 250 μg/disc concentrations, which is comparable to those at the 500 μg/disc concentration. As a positive control, ampicillin was employed and displayed a mean (SEM) zone of inhibition extending from 10.00 (0.65) to 21.00 (1.86) mm. Antibacterial activity results obtained for Cucurbita maxima by the disc diffusion assay indicated that indigenous pumpkin seed oil has more effective antibacterial activity against all pathogenic bacteria.26 In addition, G procumbens extract exhibits antibacterial efficacy against both gram-positive (Bacillus cereus) and gram-negative (Pseudomonas aeruginosa, Vibrio parahaemolyticus, and Salmonella typhi) bacteria.27,28 However, in this study, the antibacterial activity of G procumbens extract was observed against the gram-negative bacteria E coli-H7 and -0157 and gram-positive bacteria (S aureus, E faecium, and Chromobacterium sp).
Table 2.
Antibacterial activity of the various extracts of Gaultheria procumbens leaf with different concentrations.
| Different bacterial strain | Zone of inhibition* (mm) |
Ampicillin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aqueous extractconcentration(µg/mL) | Ethanol extractconcentration(µg/mL) | n- hexane extractconcentration(µg/mL) | ||||||||
| Gram negative bacteria | 200 | 250 | 500 | 200 | 250 | 500 | 200 | 250 | 500 | 10 µg/disc |
| Escherichia coli H7 | 1 | 2.2 (0.0) | 10 (0.460 | 2 (0.01) | 4 (0.08) | 7.5 (0.1) | 0 | 0 | 3 (0.04) | 10.00 (0.65) |
| E coli LT 04 | 0 | 0 | 6 (0.6) | 2.1 (0.01) | 2.6 (0.02) | 8 (0.6) | 9 (0.65) | 10 (0.7) | 21 (1.25) | 15.25 (1.05) |
| E coli 0157 | 0 | 0 | 10 (0.5) | 5 (0.02) | 7 (0.45) | 20 (1.16) | 2 (0.01) | 5 (0.85) | 19 (1.05) | 18.32 (1.44) |
| Gram positive bacteria | ||||||||||
| Staphylococcus aureus | 15 (1.0) | 16 (1.35) | 18 (1.45) | 0 | 0 | 10 (0.65) | 7 (0.05) | 10 (0.55) | 18 (1.35) | 12.66 (1.06) |
| Enterococcus faecium | 10 (0.55) | 11 (0.65) | 19 (01.45) | 0 | 0 | 10 (0.65) | 2 (0.02) | 5 (0.05) | 19 (1.42) | 21.00 (1.86) |
| Chromobacterium sp | 0 | 0 | 10 (0.48) | 7 (0.68) | 7.5 (0.72) | 18 (1.26) | 2 (0.01) | 7 (0.75) | 18 (1.15) | 16.12 (1.56) |
The diameter of zones of inhibition of different doses of G procumbens leaf extracts with different concentration. Standard antibiotic ampicillin (10 μg/disc) was employed as a positive control. Values are presented as mean (SEM) of 3 independent experiments.
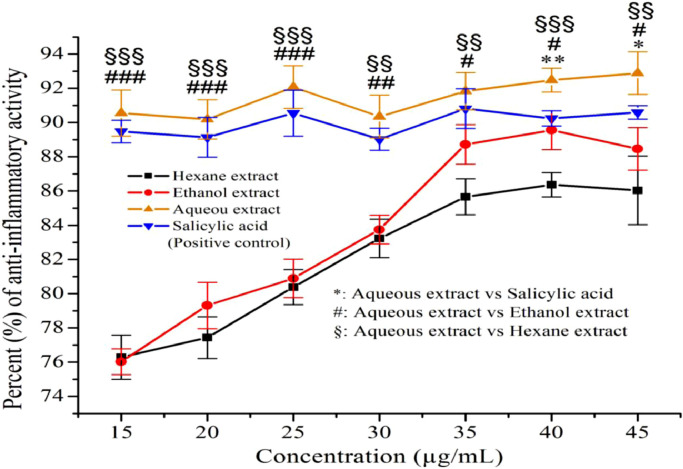
As shown in Figure 1, the n-hexane extracts of G procumbens leaves at concentrations of 15, 20, 25, 30, 35, 40, and 45 µg/mL exhibited anti-inflammatory activity ranging from mean (SEM) 76.32% (0.74%) to 86.05% (1.16%), whereas at the same concentrations, the ethanol extracts of G procumbens leaves showed mean (SEM) 76.08% (0.43%) to 88.90% (0.68%) anti-inflammatory activity. The aqueous extracts of G procumbens leaves showed anti-inflammatory activity ranging from mean (SEM) 90.12% (0.66%) to 93.00% (0.72%). The positive control (salicylic acid) exhibited anti-inflammatory activity, extending from mean (SEM) 89.46% (0.38%) to 90.65% (0.24%). The anti-inflammatory activity of G procumbens was dose-dependent. The present study showed that all three tested extracts of G procumbens leaves markedly inhibited BSA denaturation. However, at a concentration of 40 µg/mL, aqueous extracts exhibited the highest (92.13%) anti-inflammatory activity compared with that of the reference standard salicylic acid (90.40%; P < 0.01), which was significantly higher than that of ethanol (89.02%; P < 0.05) and n-hexane (86.05%; P < 0.001) extracts (Figure 1). Therefore, the BSA denaturation assay results revealed that the aqueous extract of G procumbens has more protective anti-inflammatory activity than that of the other 2 extracts. Bailey-Shaw et al29 also reported BSA denaturation inhibition, wherein aspirin was used as a standard anti-inflammatory drug. These results may indicate the existence of a high level of biologically active phytoconstituents in the aqueous extracts compared with that in the n-hexane and ethanolic extracts of G procumbens leaves.
Figure 1.
Bovine serum albumin (BSA) denaturation inhibitory activity of the extracts (ie, n-hexane, ethanol, and aqueous) of Gynura procumbens leaves. The anti-inflammatory effects of n-hexane, ethanol, and aqueous extracts of G procumbens leaves were examined by BSA denaturation inhibitory activity. The anti-inflammatory activity of the aqueous extracts and reference (salicylic acid) was substantially distinct from that of the n-hexane and ethanol extracts. The significance level was specified as *P < 0.05, **P < 0.01, and ***P < 0.001 for aqueous extract versus salicylic acid (standard). The anti-inflammatory activity of the aqueous extract was substantially distinct from that of the ethanol and n-hexane extracts. The significance level was set at #P < 0.05, ##P < 0.01, and ###P < 0.001 for aqueous extract versus ethanol extract and $P < 0.05, $$P < 0.01, and $$$P < 0.001 for aqueous extract versus n-hexane extract. Values are presented as mean (SD) (n = 3 experiments).
The brine shrimp assay is a fast, cost-effective, and effortless bioassay for analyzing plant extract mortality, which is related to cytotoxicity and antitumor activities. Usually, the required biological activity does not depend on a single component but instead depends on a combination of heterogeneous bioactive plant constituents. Therefore, it is necessary to screen novel extracts for determining their biological activity. The brine shrimp lethality bioassay is a secure method for observing the biological activities of natural products.30 None of the 3 extracts (ie, aqueous, ethanol, and n-hexane) from 20 to 50 µg/mL exhibited a significant effect on cell viability (no death of nauplii after 12 hours) (Table 3). Increasing the concentration of the extract proportionally increased the percentage cytotoxicity. Notably, at concentrations of 125, 250, and 500 µg/mL, the aqueous extract exhibited 30%, 40%, and 50% mortality, respectively. At the same concentration, the ethanol and n-hexane extracts showed 60%, 70%, and 80% and 80%, 90%, and 100% mortality, respectively. Therefore, the aqueous extract showed 50% mortality at 500 μg/mL, but the other 2 extracts (ie, ethanol and n-hexane) exhibited 80% and 100% mortality. The LC50 values of aqueous, ethanol, and n-hexane extracts were 427.66, 218.56, and 114.51 μg/mL, respectively. Therefore, the n-hexane extract was more toxic than the others, and the aqueous extract exhibited the lowest mortality. The LC50 value for the reference standard vincristine sulfate was 2.16 μg/mL. However, no mortality was observed in the negative control (1% DMSO) group. The cytotoxic activity assay of the methanolic extracts of Caesalpinia pulcherrima wood on brine shrimp larvae has little effect, but aqueous extracts have a relatively toxic effect.31 However, in this study, the cytotoxicity results suggested that compared with the other 2 extracts (ie, ethanol and n-hexane), up to 500 µg/mL aqueous extract of G procumbens is safe for intake as medication.
Table 3.
Brine shrimp lethality bioassay of the various extracts of Gaultheria procumbens leaf
| Test materials | Percent mortality at the concentration studied (μg/mL) |
LC50 (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| w/v | 10 | 20 | 31.25 | 50 | 62.5 | 75 | 125 | 250 | 500 | |
| Aqueous extract | 0 | 0 | 0 | 10 | 20 | 20 | 30 | 40 | 50 | 427.66 |
| Ethanol extract | 0 | 0 | 10 | 30 | 30 | 40 | 60 | 70 | 80 | 218.86 |
| n-hexane extract | 0 | 0 | 20 | 50 | 60 | 70 | 80 | 90 | 100 | 114.51 |
| w/v | 0.125 | 0.25 | 0.5 | 1 | 5 | 10 | ||||
| Vincristine sulfate* | 20 | 30 | 40 | 50 | 90 | 100 | 2.16 | |||
| v/v (%) | 0.1 | 0.5 | 1 | 2.5 | ||||||
| Dimethyl sulfoxide† | 0 | 0 | 0 | 0 | 0 | |||||
Values are presented as mean (SEM) of 3 independent experiments.
Positive control.
Negative control.
To observe the organ-level toxic effects of G procumbens leaf extract on alloxan-induced diabetic mice, a photomicrographical histopathology experiment was conducted. The collected mice were induced by an intraperitoneal injection of alloxan (180 mg/kg body weight), and diabetes was confirmed by testing fasting blood glucose levels exceeding 13 mmol/L using a glucometer. The blood glucose levels of the normal control mice, alloxan-induced diabetic mice, and alloxan-induced diabetic mice treated with G procumbens aqueous extracts are presented in Table 4. In this study, only G procumbens aqueous extracts at 200 mg/mL was used to treat diabetes mellitus because of the effective results of the aqueous extracts of G procumbens leaves (200 mg/mL) compared with the other 2 extracts (ethanol and n-hexane).
Table 4.
Measurement of blood glucose level of normal control mice (NCM), alloxan-induced diabetic mice (ADM), and GPAE-200 treated diabetic mice (GPAETDM) (after first and 28th days).
| Treatment group | Body weight* (g) |
Blood sugar level* (mmol/L) |
||
|---|---|---|---|---|
| Day 1 | Day 28 | Day 1 | Day 28 | |
| NCM | 26.8 (0.23) | 23.8 (0.17) | 6.6 (0.12) | 5.1 (0.06) |
| ADM | 27.2 (0.12) | 21.2 (0.12) | 16.0 (0.06) | 14.0 (0.12) |
| GAETDM | 25.1 (0.12)† | 22.5 (0.06) | 6.9 (0.12)‡ | 4.36 (0.01)‡ |
Values are presented as mean (SEM) of the 3 experiments.
Indicates significant difference between day 0 and day 28 of same treatment group at P < 0.05.
Indicates highly significant difference between day 0 and day 28 of same treatment group at P < 0.001.
The toxicities of the aqueous extracts of G procumbens leaves toward the liver, kidneys, and heart of the tested mice are shown in Figure 2. Microscopic histopathologic analysis of the liver, kidneys, and heart of the mice in the administrative group fed with normal dried rice and cereal showed no changes (Figure 2A–C). In contrast, in diabetes mellitus-induced experimental mice treated with the aqueous extracts of G procumbens leaves (5 g), no deteriorating effect was observed in the heart, kidneys, or liver (Figure 2A1, 2B1, and 2C1). These results indicate that the aqueous extracts of G procumbens leaves have no toxic effects on the heart, kidneys, or liver of mice. These results are consistent with those reported by Ahmed et al.32
Figure 2.
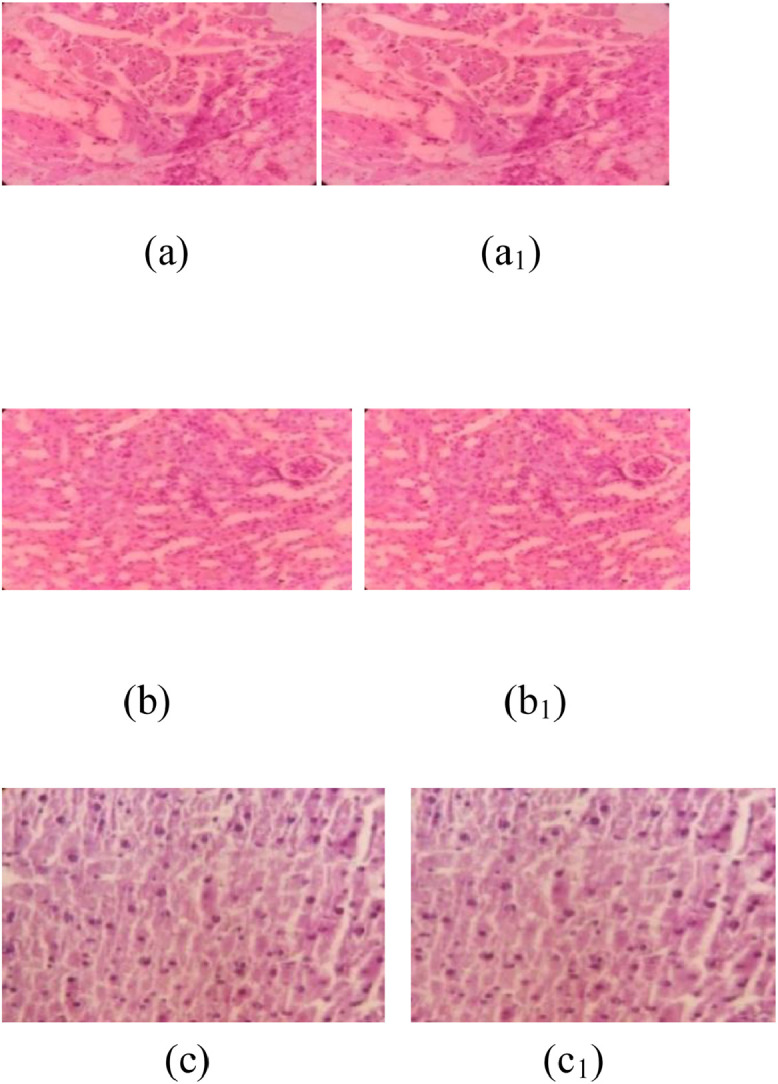
Cytotoxic effect of the aqueous extract of Gynura procumbens leaves on the heart, kidneys, and liver of experimental mice (Swiss albino) determined through histopathologic analysis. (A) Photograph of a heart segment from a normal mouse (fed with 5 g common dried rice for 4 weeks) exhibiting no deteriorating effect. (a1) Photomicrograph of a heart segment from a mouse (fed with 5 g of the aqueous extract of G procumbens leaves for 4 weeks) exhibiting no deteriorating effect. (B) Photograph of the kidney segment from a normal mouse (fed with 5 g common dried rice for 4 weeks) exhibiting no deteriorating effect. (B1) Photomicrograph of a kidney segment from a mouse (fed with 5 g aqueous extract of G procumbens leaves for 4 weeks) exhibiting no deteriorating effect. (C) Photograph of a liver segment from a control mouse (fed with 5 g normal dried rice for 4 weeks) exhibiting no deteriorating effect. (C1) Photomicrograph of a liver segment from a mouse (fed with 5 g aqueous extract of G procumbens leaves for 4 weeks) exhibiting no deteriorating effect. Each value represents the mean (SEM) (n = 3 experiments). *Indicates significant differences between days 0 and 28 in the same treatment group at P < 0.05. **Indicates highly significant differences between days 0 and 28 in the same treatment group at P < 0.001.
Conclusions
In this study, among the 3 distinct extracts (ie, aqueous, ethanol, and n-hexane) of G procumbens leaves, significant antibacterial and anti-inflammatory activities were observed for different concentrations of aqueous extracts that showed strong positive correlations with phenolic and tannin contents. The aqueous extract of G procumbens at a concentration of 200 µg/mL showed potential antibacterial activities against S aureus and E faecium, with mean (SEM) zones of inhibition of 15 (1.0) mm and 10 (0.55) mm, respectively. At a concentration of 40 µg/mL, the aqueous extract of G procumbens exhibited significant (P < 0.01) anti-inflammatory activity compared with that of the other 2 extracts in the BSA denaturation assay. Compared with the ethanol and n-hexane extracts, the aqueous extracts also exhibited low cytotoxicity (LC50 values of aqueous, ethanol, and n-hexane extracts were 427.66, 218.56, and 114.51 μg/mL, respectively). The histopathologic analysis results also showed that the aqueous extract of G procumbens had no harmful effects on the different organs of the experimental mice. Finally, the results of this study indicate that the aqueous extract of G procumbens leaves can be a promising source of a lead compound.
Declaration of Competing Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
CRediT authorship contribution statement
M. Ziaul Amin: Study design, data interpretation, writing.Mitu Afrin: data collection. do experiment.Nigar Sultana Meghla: literature search.Ashaduzzaman Nur: literature search.Mashiar Rahman: Figure creation, data interpretation, writing.M. Jashim Uddin: data interpretation.
Acknowledgments
The authors thank the Animal Center, Jahangirnagar University, Savar, Dhaka, Bangladesh, for providing Swiss Albino mice for this study. The authors also thank the Department of Nutrition & Food Technology and Microbiology, Jashore University of Science and Technology, Jashore, Bangladesh, for providing technical support and bacterial strains; Dr Md. Amirul Islam, Department of Biochemistry and Molecular Biology, Rajshahi University, Rajshahi-7200, Bangladesh, for helping with the histopathologic analysis; and Mr Phillip Thomas for critically reading the manuscript.
References
- 1.Tan HL, Chan KG, Pusparajah P, Lee LH, Goh BH. Gynura procumbens: An overview of the biological activities. Front Pharmacol. 2018;7:52. doi: 10.3389/fphar.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mou KM, Dash PR. A Comprehensive Review on Gynura Procumbens Leaves. Int J Pharmacognosy. 2016;3(4):167–174. doi: 10.13040/IJPSR.0975-8232. doi.org/ [DOI] [Google Scholar]
- 3.Ernst E. Herb-drug interactions: Potentially important but woefully under-researched. Eur. J. Clin. Pharmacol. 2000;56(8):523–524. doi: 10.1007/s002280000194. [DOI] [PubMed] [Google Scholar]
- 4.Akowuah G, Sadikun A, Mariam A. Flavonoid Identification and hypoglycaemic Studies of the butanol fraction from Gynura procumbens. Pharm. Biol. 2002;40:405–410. doi: 10.1076/phbi.40.6.405.8440. [DOI] [Google Scholar]
- 5.Khan R, Islam B, Akram M, Shakil S, Ahmad A, Ali SM, Siddiqui M, Khan AU. Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) of bacteria and fungus of clinical origin. Molecules. 2009;14:586–597. doi: 10.3390/molecules14020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkill IH. A Dictionary of the Economic Product of Malay Peninsular. Kuala Lumpur;” Ministry of Agriculture and Co-operatives, Kuala Lumpur, Vol. 1 and 11, p2444, 1966W. In: Chen K., editor. Linear Networks and Systems (Book style) Wadsworth; Belmont, CA: 1993. pp. 123–135. [Google Scholar]
- 7.Karim A., Islam M.A., Islam M.M., Rahman M.S, Sultana S., Biswas S., Hosen M.J., Rahman M.M., Hasan M.Z. Evaluation of antioxidant, anti-hemolytic, cytotoxic effects and antibacterial activity of selected mangrove plants (Bruguieragymnorrhiza and Heritiera littoralis) in Bangladesh. Clin Phytosci. 2020;6(8) doi: 10.1186/s40816-020-0152-9. K, doi.org/ [DOI] [Google Scholar]
- 8.Harborne JB. 2nd ed. Chapman and Hall; London: 1998. Phytochemical methods: A guide to modern techniques of plant analysis; pp. 54–84. [Google Scholar]
- 9.Kokate KC. 4th ed. Vallabh Prakashan; Delhi: 1997. Practical pharmacognosy; p. 218. [Google Scholar]
- 10.Rahman M.M., Kabir M.M., Noman M.A.A., Islam M.R., Dash B.K., Akhter S., Uddin M.J., Rahman A. Mikania cordata leaves extract promotes activity against pathogenic bacteria and anticancer activity in EAC cell-bearing swiss albino mice. Journal of Applied Pharmaceutical Science. 2020;10(2):112–122. doi: 10.7324/JAPS.2020.102017. [DOI] [Google Scholar]
- 11.Mizushima Y, Kobayashi M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biological active proteins. J of Pharma Pharmacol. 1968;20:169–173. doi: 10.1111/j.2042-7158.1968.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 12.Sakat SS, Juvekar AR, Gambhire MN. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. International Journal of Pharma and Pharmacological Sciences. 2010;2:146–155. [Google Scholar]
- 13.Naz R, Ayub H, Nawaz S, Islam ZU, Yasmin T, Bano A, Wakeel A, Zia S, Roberts TH. Antimicrobial activity, toxicity and anti-inflammatory potential of methanolic extracts of four ethnomedicinal plant species from Punjab, Pakistan. BMC Complementary and Alternative Medicine. 2017;17:302. doi: 10.1186/s12906-017-1815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunarwidhi AL, Sudarsono S, Nugroho AE. Hypoglycemic Effect of Combination of Azadirachta indica A. Juss. and Gynura procumbens (Lour.) Merr. Ethanolic Extracts Standardized by Rutin and Quercetin in Alloxan-induced Hyperglycemic Rats. Adv Pharm Bull. 2014;4(Suppl 2):613–618. doi: 10.5681/apb.2014.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munshi M, Tumu KN, Hasan MN, Amin MZ. Biochemical effects of commercial feedstuff's on the biochemical composition of the fry of climbing perch (Anabas testudineus) and it's impact on Swiss albino mice as an animal model. Toxicology Reports. 2018;5:521–530. doi: 10.1016/j.toxrep.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur GJ, Arora DS. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Compl. Altern. Med. 2009;9:30. doi: 10.1186/1472-6882-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai HY, Yau YY, Kim KH. Blechnum orientale Linn - a fern with potential as antioxidant, anticancer and antibacterial agent. BMC Complem. Altern. Med. 2010;10:15. doi: 10.1186/1472-6882-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg VKR, Jain M, Sharma PKR, Garg G. Anti inflammatory activity of Spinacia oleracea. Intern. J. Pharma Prof. Res. 2010;1(1):1–4. [Google Scholar]
- 19.Manogaran M, Lim V, Mohamed R. Phytoconstituents of the Gynura procumbens ethanol leaf extract and its fractions and their effects on viability of macrophages. J Herbmed Pharmacol. 2019;8(3) doi: 10.15171/jhp.2019.xx. x-x. [DOI] [Google Scholar]
- 20.Lopez-Lazaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 21.Amaral S, Mira L, Nogueira JM, da Silva AP, Florencio MH. Plant extracts with anti-inflammatory properties—a new approach for characterization of their bioactive compounds and establishment of structure-antioxidant activity relationships. Bioorg. Med. Chem. 2009;17(5):1876–1883. doi: 10.1016/j.bmc.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 22.Oloyede OI. Chemical profile of unripe pulp of Carica papaya. Pak. J. Nutr. 2005;4:379–381. [Google Scholar]
- 23.Mandal P, Babu SSP, Mandal NC. Antimicrobial activity of saponins from Acacia auriculiformis. Fitoterapia. 2005;76(5):462–465. doi: 10.1016/j.fitote.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Gepdireman A, Mshvildadze V, Suleyman H, Elias R. Acute anti-inflammatory activity of four saponins isolated from ivy: alphahederin, hederasaponin-C, hederacolchiside-E and hederacolchiside F in carrageenan-induced rat paw edema. Phytomedicine. 2005;12(6-7):440–444. doi: 10.1016/j.phymed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Fawole OA, Amoo SO, Ndhlala AR, Light ME, Finnie JF, Van Staden J. Anti-inflammatory, anticholinesterase, antioxidant and phytochemical properties of medicinal plants used for pain-related ailments in South Africa. J. Ethnopharmacol. 2010;127(2):235–241. doi: 10.1016/j.jep.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Amin MZ, Islam T, Uddin MR, Rahman MM, Uddin MJ. A Comparative assessment of anti-inflammatory, anti-oxidant and anti-microbial activities of hybrid and indigenous varieties of pumpkin (Cucurbita maxima. Linn.) seed oil. Biocatalysis and Agricultural Biotechnology. 2020;28 doi: 10.1016/j.bcab.2020.101767. [DOI] [Google Scholar]
- 27.Rahman AFMM, Al Asad MS. Chemical and biological investigation of the leaves of Gynura procumbens. Int. J.Bio. 2013;3:36. 43. [Google Scholar]
- 28.Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. [Google Scholar]
- 29.Bailey-Shaw YA, Williams LAD, Green CE, Rodney S, Smith AM. In-Vitro Evaluation of the Anti-Inflammatory Potential of Selected Jamaican Plant Extracts using the Bovine Serum Albumin Protein Denaturation Assay. Int. J. Pharm. Sci. Rev. Res. 2017;47(1):145–153. [Google Scholar]
- 30.Chanda S, Baravalia Y. Brine shrimp cytotoxicity of Caesalpinia pulcherrima aerial parts, antimicrobial activity and characterization of isolated active fractions. Nat.Prod. Res. 2011;25(20):1955–1964. doi: 10.1080/14786419.2010.530600. [DOI] [PubMed] [Google Scholar]
- 31.Pawar CR, Mutha RE, Landge AD, Jadhav RB, Surana SJ. Antioxidant and cytotoxic activities of Caesalpinia pulcherrima wood. Indian J. Biochem. Biophys. 2009;46(2):198–200. [PubMed] [Google Scholar]
- 32.Ahmad B, Khan IS, Bashir S, Azam S, Hussain F. Screening of Zizyphus jujube for antibacterial, phytotoxic and haemagglutination activities. Afr. J. Biotechnol. 2011;10:2514–2519. doi: 10.5897/AJB10.768. [DOI] [Google Scholar]