Abstract
Background
Patiromer and sodium zirconium cyclosilicate (SZC) are newer options for hyperkalemia treatment. This systematic review and meta-analysis were conducted to assess the safety and side effect profile of patiromer and SZC compared with placebo or other standards of care in the management of hyperkalemia.
Methods
We searched electronic databases for relevant articles. The screening was performed independently and data were extracted among the selected studies. We performed a statistical analysis on Revman 5.4 software. The odds ratio (OR) was used for outcome estimation with a 95% CI.
Results
Patiromer had lower rates of hyperkalemia (OR = 0.44; 95% CI, 0.22–0.89) compared with standard of care. The analysis showed no significant differences between the 2 groups in terms of overall adverse effects, any serious/specific adverse effects, or treatment discontinuation as a result of adverse effects. Comparing the SZC-10 group with standard of care showed no significant differences in the occurrence of hyperkalemia during treatment, overall adverse effects, any serious/specific adverse effects, or treatment discontinuation as a result of adverse effects but showed a higher rate of edema in the treatment group (OR = 6.77; 95% CI, 1.03–44.25). Similarly, no significant differences were seen between the 2 SZC doses for the occurrence of any adverse effects, hyperkalemia, constipation, diarrhea, or urinary tract infection, whereas edema was higher among patients receiving SZC-10 (OR = 3.13; 95% CI, 1.19–8.27).
Conclusions
In patients with acute hyperkalemia, SZC is the drug of choice due to its more rapid reduction of serum potassium level, whereas in patients with chronic hyperkalemia, patiromer appears to be the drug of choice because SZC is associated with an increase in edema, likely due to an increase in sodium absorption, which could have important adverse consequences in patients with chronic kidney disease and or heart failure. Thus, both drugs were found to be safe while treating hyperkalemia. (Curr Ther Res Clin Exp. 2021; 82:XXX–XXX)
Key words: hyperkalemia, patiromer, potassium, sodium zirconium cyclosilicate
Introduction
Hyperkalemia is defined as serum potassium level ≥5 mEq/L1 and is a potentially life-threatening condition associated with ventricular arrhythmias and sudden cardiac arrest.1 For decades, sodium polystyrene sulfonate (SPS) was the only US Food and Drug Administration (FDA)-approved treatment for hyperkalemia.2 However, the variable time to onset of effect and high sodium content of SPS made it a poor choice of agent in sodium-restricted patients, such as those with congestive heart failure and chronic kidney disease. SPS lacks robust, randomized, controlled clinical trial efficacy data and has well-known gastrointestinal (GI) adverse effects such as bowel ischemia and electrolyte disorders.3
Patiromer and sodium zirconium cyclosilicate (SZC) have emerged as new treatment options for hyperkalemia due to the unknown efficacy and safety concerns related to SPS. Patiromer for oral suspension, formerly known as RLY5016, was approved by the FDA for the treatment of hyperkalemia in 2015.4 Patiromer works by binding free potassium ions in the GI tract, mainly in the distal colon lumen, and releasing calcium ions for exchange, lowering the amount of potassium available for absorption and increasing the amount excreted via the feces.5 The most common side effects are hypomagnesemia, and GI-related concerns, including constipation, diarrhea, and nausea. SZC, formerly known as ZS-9, is an insoluble, inorganic, nonpolymer zirconium silicate compound comprising units of oxygen-linked zirconium and silicon atoms in the form of a microporous cubic lattice framework.5 It works as a selective cation exchange agent, primarily releasing hydrogen and sodium and preferentially capturing potassium, increasing fecal excretion.6 The most common side effects include edema, constipation, and headache. Patiromer calcium sorbitex (patiromer) and SZC (ZS-9) are believed to be effective treatments for hyperkalemia that overcome the limitations of other available therapies. Clinical trials for both patiromer and SZC appear to provide evidence for efficacy in lowering potassium levels.7, 8, 9
The objective of this systematic review was to assess the safety profile and side effects of patiromer and SZC compared with placebo or other standards of care (SOC) in hyperkalemia.
Materials and Methods
We followed the guidelines of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) for conducting the meta-analysis.10
Study protocol
We did a preliminary search and literature review on our research question. Then we prepared our protocol according to the PRISMA protocol. We then submitted our protocol in Prospero on November 20, 2020. Our protocol is registered in Prospero with ID CRD42020223468.11
Information sources
We used electronic databases such as PubMed, PubMed Central, Scopus, and Embase for searching relevant articles with key words hyperkalemia, patiromer, sodium zirconium cyclosilicate, and ZS-9. Electronic search details are available in Supplemental Appendix 1 in the online version.
Study records
Data management
All identified articles were imported into Mendeley software (Elsevier, London, United Kingdom) where duplicates were removed and those files, after removing duplicates, were imported into Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) where further removal of duplicates was done.
Selection process
All the stages of data extraction were done according to the PRISMA flow diagram. Two of our reviewers (S.B. and Y.A.) independently screened the articles based on title and abstract and conflicts were resolved by the next reviewer (A.M.). Full-text reviews were performed independently by 2 reviewers (A.M. and S.B.) and conflicts were resolved by the next reviewer (Y.A.) in Covidence software based on inclusion and exclusion criteria.
Our inclusion criteria were all comparative studies (eg, cross-sectional, cohort, randomized controlled trial, and case-control) comparing patiromer and/or SZC versus placebo or other SOC in hyperkalemia without language restriction and in published articles. Exclusion criteria were editorials, comments, and viewpoint articles with no proper data regarding the safety, cost-effectiveness, and treatment success rate between patiromer and SZC.
Data collection process
Data relating to patiromer and SZC in hyperkalemia were extracted using a tailored form and checked by other reviewers. The form included study identifier, study year, population/participants characteristics (eg, total number, sex, age, and other relevant parameters of participants such as to cause of admission, presentation, and comorbidities), intervention, comparator (placebo or SOC), and outcomes such as changes in baseline potassium value, adverse effects, and mortality.
Intervention
Either patiromer or SZC individually or SZC-5/SZC-10 along with SOC was taken in the treatment arm. Placebo alone or placebo along with SOC was in the control arm. SOC provided was a combination of insulin with glucose.
Outcomes
Primary outcomes were safety, side effects, cost-effectiveness, and treatment success rate of patiromer and SZC, and secondary outcomes were subgroup analysis of primary outcomes such as the occurrence of different common adverse effects.
Risk of bias in individual studies
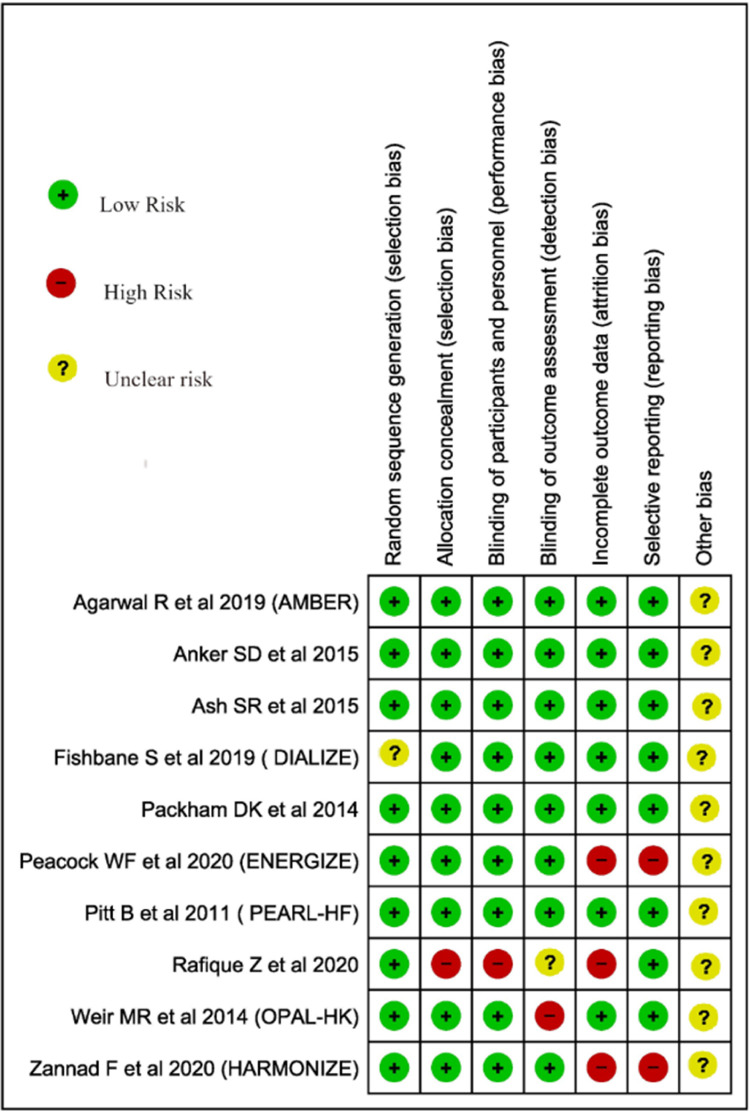
The quality of individual articles will be evaluated using the Joanna Briggs Institute critical appraisal for observational studies12 and Cochrane ROB 2.0 for trials.13 See Figure 1 and Table 1.
Figure 1.
Cochrane ROB 2.0 for bias assessment.
Table 1.
Joanna Briggs Institute bias assessment of observational studies.
| SN | Desai NR, et al14 | |
|---|---|---|
| 1 | Were the 2 groups similar and recruited from the same population? | Yes |
| 2 | Were the exposures measured similarly to assign people to both exposed and unexposed groups? | Yes |
| 3 | Was the exposure measured in a valid and reliable way? | Yes |
| 4 | Were confounding factors identified? | No |
| 5 | Were strategies to deal with confounding factors stated? | No |
| 6 | Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? | Yes |
| 7 | Were the outcomes measured in a valid and reliable way? | Yes |
| 8 | Was the follow-up time reported and sufficient to be long enough for outcomes to occur? | Yes |
| 9 | Was follow-up complete, and if not, were the reasons to loss to follow-up described and explored? | Yes |
| 10 | Were strategies to address incomplete follow-up utilized? | NA |
| 11 | Was appropriate statistical analysis used? | Yes |
| Overall appraisal | Include |
SN = Serial Number.
Data synthesis
We performed a statistical analysis on Revman 5.4 software (Cochrane Training, London, United Kingdom). Odds ratio (OR) was used for outcome estimation with a 95% CI. A random or fixed-effect model was used based on the heterogeneities.
Assessment of heterogeneity
The I2 test was used for the assessment of heterogeneity using the Cochrane Handbook for Systematic Review of Interventions.15
Subgroup analysis and investigation of heterogeneities
Subgroup analysis was carried out for adverse effects based on a specific type of commonly reported adverse effects.
Sensitivity analysis
To evaluate any changes in results due to mild-to-moderate heterogeneities, we employed a random effect model in addition to the fixed-effect model.
Results
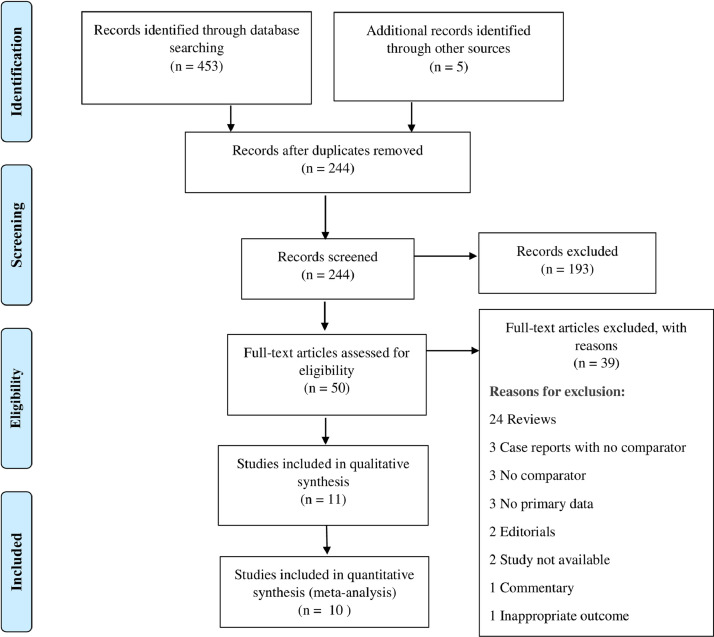
Our database search resulted in a total of 458 studies. After the removal of 214 duplicates, we screened the title and abstract of 244 studies. A total of 193 studies were excluded and we assessed the full text of 50 studies for eligibility (Figure 2). Of these, we excluded an additional 39 studies resulting in 11 studies being included in the qualitative analysis (Table 2). Basic study details such as place of study, inclusion and exclusion criteria, and limitations are provided in Supplemental Appendix 2 in the online version. A total of 10 studies were included in the quantitative analysis. Among the selected studies, 4 examined patiromer while 6 evaluated SZC.
Figure 2.
Preferred reporting items for systematic reviews and meta-analyses flow diagram.
Table 2.
Included studies for analysis.
| Study ID | Type of study | Place of study | Study period | Primary outcome |
|---|---|---|---|---|
| Agarwal R, et al,16 2019 | Phase II, double-blind, placebo-controlled RCT | 62 outpatient centers in 10 countries (Bulgaria, Croatia, Georgia, Hungary, Ukraine, France, Germany, South Africa, United Kingdom, and the United States) | February 13, 2017, and August 20, 2018 | Patients taking spironolactone at week 12: T = 126/147; C = 98/148 |
| Anker DS, et al,17 2015 | Phase III randomized, double-blind, placebo-controlled trial | 44 sites from cardiology, nephrology, and general research sites in United States, Australia, and South Africa | March-August 2014 | 28-day withdrawal phase; mean serum potassium (mmol/L) SZC-5: 4.7 (95% CI, 4.5–4.9), SZC-10: 4.5 (95% CI, 4.3–4.6), SZC –15: 4.4 (95% CI, 4.2–4.5), C: 5.2 (95% CI, 5.0–5.4) |
| Ash RS18 et al. 2015 | Phase II RCT | 9 US sites | November 2011-May 2012 | Mean reductions in serum potassium were seen on day 2 (hour 28 to 48) with 10-g SZC vs placebo |
| Desai NR, et al,14 2020 | Descriptive observational study | Optum's Clinformatics Data Mart (Eden Prairie, MN) | January 1, 2016, to December 31, 2017 | Rate difference of ED visits (postindex-preindex) on ITT: T= –0.12 (–0.29 to 0.07); C = 0.75 (0.71 to 0.79) |
| Fishbane S, et al,8 2018 | Phase IIIb, randomized, double-blind, placebo controlled trial | 54 sites across Japan, Russia, the United States, and the United Kingdom | December 14, 2017-November 7, 2018 | Proportion of responders 41.2% (n = 40 of 97) in SZC group vs 1.0% (n = 1 of 99) |
| Packham DK, et al,19 2014 | Phase III, 2-stage, double-blind, randomized, placebo controlled study | 65 sites in the United States, Australia, and South Africa | November 2012-November 2013 | In maintenance phase, both the 5-g and 10-g daily doses of SZC were superior to placebo in maintaining normokalemia |
| Peacock WF, et al,7 2020 | Phase II, randomized, double-blind, placebo-controlled trial | 33 sites in Denmark, Italy, Russia, and the United States | February 13, 2018-December 21, 2018 | Greater reduction in serum potassium at 4 h in the SZC group than the placebo group: –0.36 (0.57) for SZC versus –0.25 (0.63) mmol/L for placebo |
| Pitt B, et al,9 2011 | Phase II, randomized, double-blind, placebo-controlled trial | Conducted in 38 centers in United States, Germany, the Czech Republic, Poland, the Ukraine, Russia, and Georgia | June 2009-November 2009 | Change in serum potassium from baseline to day 28 (mEq/L) T = 20.34 + 0.08; C = 0.09 + 0.10 |
| Rafique Z, et al, 202020 | Single-center, single-blinded, randomized, open-label, pilot study | Innercity ED, USA | August 2016-August 2017 | Change in serum potassium (mEq/L) from baseline to 6-h posttreatment T = 6.32 (95% CI 6.0–6.63) C = 5.81(95% CI 5.48–6.14) |
| Weir MR, et al,21 2014 | Phase III randomized, single blind, placebo controlled study | Sites in Eastern Europe (n = 24 sites), the European Union (n = 21), and the United States (n = 14) | February 2013-July 2013 | Initial phase; Change in serum potassium (mmol/L) from baseline to week 4: −1.01 (0.03) |
| Zannad F, et al,22 2019 | Phase III, randomized, double-blind, placebo-controlled study | 45 investigational sites in Japan, Russia, South Korea, and Taiwan | March 3, 2017-February 14, 2018 | Geometric LSM (95% CI) (mmol/L) SZC-10: 4.38 95% CI 4.27–4.50); SZC-5: 4.81 (95% CI 4.69–4.94); C: 5.32 (95% CI 5.16–5.49) |
ED = emergency department; C = Control group; ITT = Intention to Treat; LSM = least square mean; RCT = randomized controlled trial; SZC = sodium zirconium cyclosilicate; T = Treatment group.
Quantitative analysis
Patiromer versus placebo
Pitt et al9 prevention of hyperkalemia in patients with heart failure (Pearl-HF), Agarwal et al16 Spironolactone With Patiromer in the Treatment of Resistant Hypertension in Chronic Kidney Disease (AMBER), Weir et al21 Phase 3 Study Evaluating the Efficacy and Safety of Patiromer for the Treatment of Hyperkalemia (OPAL-HK), DIALIZE: A Study to Test Whether ZS (Sodium Zirconium Cyclosilicate) Can Reduce the Incidence of Increased Blood Potassium Levels Among Dialized Patients. ENERGIZE: A Study to Evaluate a Potassium Normalization Treatment Regimen Including Sodium Zirconium Cyclosilicate (ZS) Among Patients With S-K ≥5.8. Safety & Efficacy of Zirconium Silicate Dosed for 28 Days in Hyperkalemia (HARMONIZE) and Rafique et al20 were included in the quantitative analysis comparing patiromer with placebo for hyperkalemia treatment.
Adverse effects among patiromer versus placebo
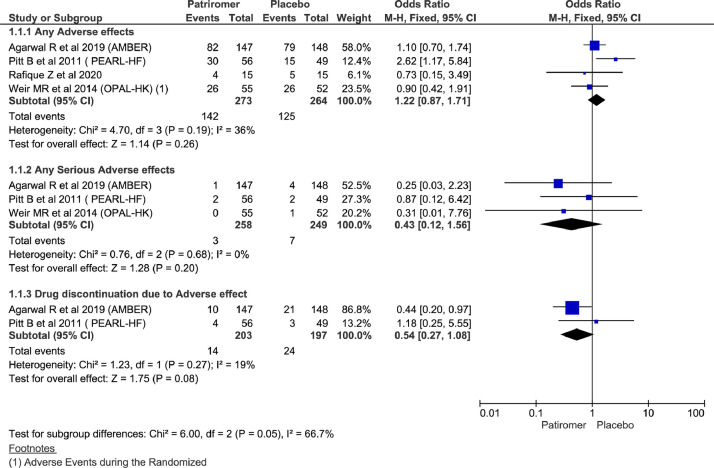
Analysis showed no statistically significant differences between the 2 groups in terms of any adverse effects (OR = 1.22; 95% CI, 0.87–1.71; n = 537; I2 = 36%), any serious adverse effects (OR = 0.43; 95% CI, 0.12–1.56; n= 507; I2 = 0%), and treatment discontinuation as a result of adverse effects (OR = 0.54; 95% CI, 0.27–1.08; n = 400; I2 = 19%) (Figure 3). Considering mild heterogeneity for any adverse effects and running analysis using random effect also did not show significant changes (Supplemental Appendix 3 in the online version and Figure 1).
Figure 3.
Forest plots comparing adverse effects between patiromer and placebo group.
Commonly reported specific adverse effects
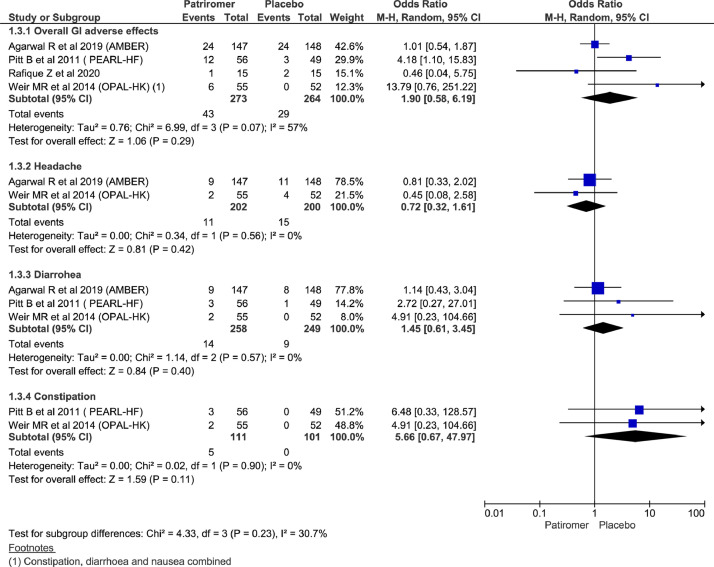
There were no significant differences between the 2 groups in overall GI adverse effects (OR = 1.90; 95% CI, 0.58–6.19; n = 537; I2 = 57%). Among commonly reported adverse effects, there was no significant difference between the 2 groups for reporting of headache (OR = 0.72; 95% CI, 0.32 to 1.61; n = 402; I2 = 0%); diarrhea (OR = 1.45; 95% CI, 0.61–3.45; n = 507; I2 = 0%), and constipation (OR = 5.66; 95% CI, 0.67–47.97; n= 212; I2 = 0%) (Figure 4).
Figure 4.
Forest plots comparing commonly adverse effects between patiromer and placebo group.
Mortality
Three studies reported mortality and there was 1 mortality event in the placebo arm in all 3 studies; there was no significant difference among groups when pooled together (OR = 0.31, 95% CI, 0.05–1.98; n = 507; I2 = 0%) (Supplemental Appendix 3 in the online version and Figure 2).
SZC-10 g versus placebo
Adverse effects among SZC versus placebo
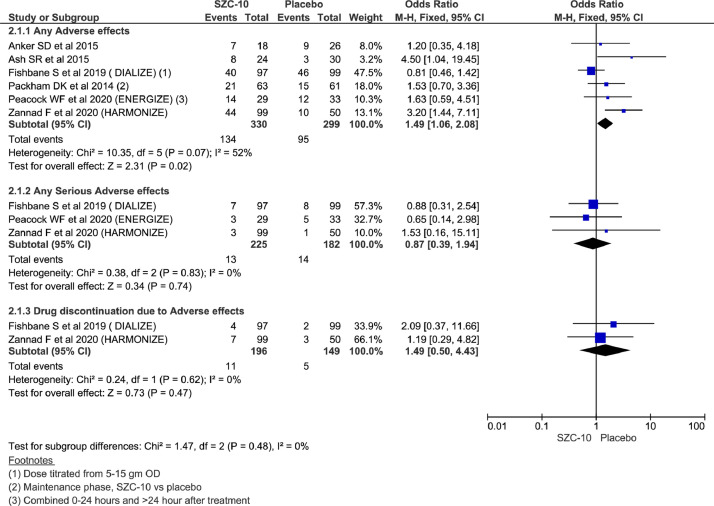
Analysis showed statistically significant higher reporting of any adverse effects in the SZC-10 group than placebo arm using a fixed-effect model (OR = 1.49; 95% CI, 1.06–2.08; n = 629; I2 = 52%), whereas there were no significant differences between the 2 groups for any serious adverse effects (OR = 0.87; 95% CI, 0.39–1.94; n = 407; I2 = 0%) and treatment discontinuation as a result of adverse effects (OR = 1.49; 95% CI, 0.50–4.43; n= 345; I2 = 0%) (Figure 5). Considering mild heterogeneity for any adverse effects and running analysis using random effect could not reach statistical significance for overall adverse effects as well (OR = 1.64; 95% CI, 0.96–2.79; n = 629; I2 = 52%) (Supplemental Appendix 3 in the online version and Figure 3).
Figure 5.
Forest plots comparing adverse effects between sodium zirconium cyclosilicate (SZC)-10 and placebo group.
Commonly reported specific adverse effects
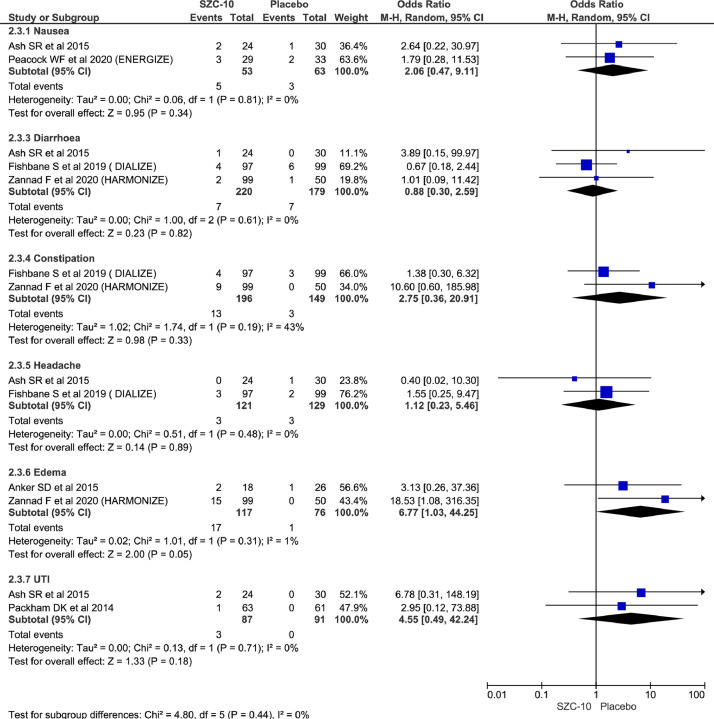
Among commonly reported adverse effects, there was no significant difference between the 2 groups for reporting of nausea (OR = 2.06; 95% CI, 0.47–9.11; n = 116; I2 = 0%), diarrhea (OR = 0.88; 95% CI, 0.30–2.59; n = 399; I2 = 0%), constipation (OR = 2.75; 95% CI, 0.36–20.91; n= 345; I2 = 43%), headache (OR = 1.12; 95% CI, 0.23–5.46; n= 250; I2 = 0%), and urinary tract infection (OR = 4.55; 95% CI, 0.49–42.24; n = 178; I2 = 0%) but showed higher rate of edema in the treatment group (OR = 6.77; 95% CI, 1.03–44.25; n = 193; I2 = 1%) (Figure 6).
Figure 6.
Forest plots comparing commonly adverse effects between SAC-10 and placebo group
Mortality outcome
Two studies reported mortality and there was 1 mortality event in the placebo arm 1 in the treatment arm in another study with no significant differences across the 2 groups (OR = 1.08; 95% CI, 0.15–7.88; n = 258; I2 = 0%) (Supplemental Appendix 3 in the online version and Figure 4).
Comparing SZC-10 with SZC-5
We compared 2 different doses of SZC at 10 g and 5 g for the treatment of hyperkalemia. There were no significant differences between the 2 groups in the occurrence of any adverse effects (OR = 1.71; 95% CI, 0.97–3.01; n = 410; I2 = 32%). Further, there were no significant differences between the 2 groups in incidence of hyperkalemia (OR = 0.40; 95% CI, 0.05–3.09; n = 326; I2 = 0%), constipation (OR = 2.24, 95% CI, 0.08–62.43; n = 246; I2 = 67%), diarrhea (OR = 2.36, 95% CI, 0.34–16.42; n= 246; I2 = 0%), and urinary tract infection (OR = 0.79, 95% CI, 0.13–4.74; n = 176; I2 = 12%), whereas edema was higher among SZC-10 group (OR = 3.13; 95% CI, 1.19–8.27; n = 234; I2 = 0%) (Supplemental Appendix 3 in the online version and Figure 5).
Publication bias
To assess publication bias of included studies, we constructed a funnel plot using the MD (mean differences); and 1/SE (Standard errors) values obtained from trials measuring the adverse effects across studies comparing patiromer versus SOC and SZC-10 versus placebo/SOC.23 The generated funnel plot suggested the possibility of publication bias in the analysis (Supplemental Appendix 3 in the online version and Figures 6 and 7).
Discussion
SPS was the only FDA-approved agent for the treatment of hyperkalemia until 2015. SPS lacks robust, randomized, controlled clinical trial efficacy data and has well-known GI and electrolyte-related adverse effects. As such, the management of hyperkalemia is challenged by unknown efficacy and lingering safety concerns with SPS. Patiromer and SZC have been developed in an attempt to overcome the gaps and limitations. Here, we systemically reviewed the safety profile and adverse effects related to these agents in the published literature.
Our analysis showed patiromer to be a relatively well-tolerated medication because no significant differences were seen between the 2 groups in terms of adverse effects. Patiromer was associated with a lower odds of hyperkalemia during treatment, shown by the previous meta-analysis by Das et al24 and Meaney et al.25 The most common side effects observed with patiromer were constipation and hypomagnesemia in the Patiromer in the Treatment of Hyperkalemia in Patients With Hypertension and Diabetic Nephropathy (AMETHYST-DN) trial. Although GI-related side effects such as nausea, diarrhea, and constipation are reported in the clinical trials included in our analysis, no significant differences between the 2 groups were observed in the meta-analysis of the individual adverse effects.9,16,20,21 There were no serious side effects of treatment discontinuation among patients treated with patiromer compared with placebo.
Patiromer clinical trials showed a dose-dependent potassium reduction with efficacy through follow-up periods of 12 weeks and 52 weeks. This makes patiromer well suited for management for chronic hyperkalemia19, 20, 21, 22 alleviating concerns of SPS such as variable potassium-lowering effect and side effects such as intestinal necrosis.26 Also, patiromer in combination with spironolactone is cost-effective and thus useful to increase compliance on guideline-directed medical therapy and improve outcomes of patients with heart failure and hyperkalemia.27 One important consideration when using patiromer is the potential for drug–drug interactions with metformin, clopidogrel, thyroxine, ciprofloxacin, metoprolol, and furosemide.28,29 This can be avoided by taking other medications at least 3 hours before, or after, patiromer. This may be a challenge for some patients and affect medication adherence.
SZC is useful for prompt reduction of potassium levels within 48 hours in cases of hyperkalemia.19,30 A previous meta-analysis done by Meaney et al25 showed potassium reduction by –0.17 mEq/L with SZC compared with placebo and a mean potassium reduction of –0.4 mEq/L was seen in the analysis done by Kosiborod et al.30 The most prominent side effect noted with SZC was edema, likely related to the high sodium content of the drug. An increase in edema, due to an increase in sodium absorption, suggests an increase in blood volume. In patients with chronic kidney disease and or heart failure who already have an increase in blood volume any further increase in blood volume as a result of an increase in sodium absorption would be accompanied by an activation of the renin-angiotensin-aldosterone system, including an increase in aldosterone. A chronic increase in aldosterone would have detrimental effects on myocardial and vascular fibrosis. Thus, in patients with chronic hyperkalemia, patiromer will be the drug of choice. Meaney et al25 found lower risks of GI side effects and hypomagnesemia in patients receiving SZC compared with placebo. There were no differences in GI side effects, headache, and urinary tract infections in patients receiving SZC compared with placebo, although these findings were reported in our included trials.18,19 We found no difference in side effects between 10 mg and 5 mg SZC. Interpretation of the data from 3 SZC clinical trials indicates the onset of effect is 1 hour, with a dose-dependent, predictable potassium-lowering response. Data from patients with mild-to-moderate hyperkalemia indicate a predictable decline in potassium concentration of –0.11 to –0.2 mEq/L by hour 1 and –0.73 to –1.1 mEq/L by 48 hours although none of the SZC clinical trials studied patients with acute hyperkalemia, so further research in this population is necessary.18,19,30 Patiromer was studied for the treatment of acute hyperkalemia although it had a delayed onset of action of 7 hours and had a mean potassium reduction measuring 0.21 mEq/L.31 Based on the results, SZC appears to be the preferred agent for the treatment of hyperkalemia to reduce potassium acutely when compared with patiromer and SPS.
Our study analyzes the role of novel potassium binders like patiromer and SZC in the treatment of hyperkalemia. SZC is the drug of choice in patients with acute hyperkalemia, whereas patiromer appears to be the drug of choice in patients with chronic hyperkalemia due to aforementioned reasons. Most of our included studies were Phase II or Phase III randomized clinical trials and pooling the results of these analyses adds to the validity and strength of our study. Most of the outcomes had low heterogeneity in the analysis. Our meta-analysis had several limitations, such as the small number of studies included and the heterogeneity in the study designs and populations. Most of the included studies enrolled patients with chronic kidney disease, diabetes mellitus, and congestive heart failure; however, excluded patients receiving dialysis or with renal transplants who are at high risk of developing hyperkalemia. The studies included in our analysis had a heterogeneous patient population with diversity in age groups, etiology of hyperkalemia, and coadministration of diuretics and angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists that can influence the reduction of serum potassium. The included studies had their limitations such as small sample size, open-label nature, short follow-up time, and exclusion of hospitalized patients.
Conclusions
In patients with acute hyperkalemia, SZC is the drug of choice due to its more rapid reduction of serum potassium. However, among patients with chronic hyperkalemia, patiromer appears to be the drug of choice because SZC increases sodium absorption leading to an increase in edema. Both patiromer and SZC were found to be safe in the treatment of hyperkalemia.
Acknowledgments
Acknowledgments
D. Shrestha, P. Budhathoki, and Y. R. Sedhai contributed to the concept and design, analysis, and interpretation of data. D. Shrestha, P. Budhathoki, Y. Adhikari, A. Marasini, and S. Bhandari contributed to the literature search, data extraction, review, and initial manuscript drafting. Y. R. Sedhai, R. Baniya, C. A. Cable, M. G. Kashiouris, D. L. Dixon, and J. M. Kidd participated in interpretation of data, revising the manuscript for important intellectual content, and approval of the final manuscript. All authors were involved in drafting and revising the manuscript and approved the final version.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.curtheres.2021.100635.
Appendix. Supplementary materials
References
- 1.Yusuf A.A., Hu Y., Singh B., Menoyo J.A., Wetmore J.B. Serum Potassium Levels and Mortality in Hemodialysis Patients: A Retrospective Cohort Study. Am J Nephrol. 2016;44(3):179–186. doi: 10.1159/000448341. [DOI] [PubMed] [Google Scholar]
- 2.Elliott M.J., Ronksley P.E., Clase C.M., Ahmed S.B., Hemmelgarn B.R. Management of patients with acute hyperkalemia. CMAJ. 2010;182(15):1631–1635. doi: 10.1503/cmaj.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harel Z., Harel S., Shah P.S., Wald R., Perl J., Bell C.M. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: A systematic review. Am J Med. 2013;126(3) doi: 10.1016/j.amjmed.2012.08.016. 264.e9-264.e24. [DOI] [PubMed] [Google Scholar]
- 4.Veltassa (patiromer) FDA Approval History - Drugs.com. Accessed December 18, 2020. https://www.drugs.com/history/veltassa.html
- 5.Esposito P., Conti N.E., Falqui V. New Treatment Options for Hyperkalemia in Patients with Chronic Kidney Disease. J Clin Med. 2020;9(8):2337. doi: 10.3390/jcm9082337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stavros F., Yang A., Leon A., Nuttall M., Rasmussen H.S. Characterization of Structure and Function of ZS-9, a K+ Selective Ion Trap. Bansal V, ed. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peacock W.F., Rafique Z., Vishnevskiy K. Emergency Potassium Normalization Treatment Including Sodium Zirconium Cyclosilicate: A Phase II, Randomized, Double-blind, Placebo-controlled Study (ENERGIZE). Heard KJ, ed. Acad Emerg Med. 2020;27(6):475–486. doi: 10.1111/acem.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishbane S., Ford M., Fukagawa M. A phase 3B, randomized, double-blind, placebo-controlled study of sodium zirconium cyclosilicate for reducing the incidence of predialysis hyperkalemia. J Am Soc Nephrol. 2019;30(9):1723–1733. doi: 10.1681/ASN.2019050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitt B., Anker S.D., Bushinsky D.A., Kitzman D.W., Zannad F., Huang I.-Z. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32(7):820–828. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(No Title). Accessed January 26, 2021. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020223468
- 12.Critical Appraisal Tools, Joanna Briggs Institute. Accessed December 18, 2020. https://joannabriggs.org/critical-appraisal-tools.
- 13.Sterne J.A.C., Savović J., Page M.J. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 14.Desai N.R., Rowan C.G., Alvarez P.J., Fogli J., Toto R.D. Hyperkalemia treatment modalities: A descriptive observational study focused on medication and healthcare resource utilization. Seguro AC, ed. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0226844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.9.5.2 Identifying and measuring heterogeneity. Accessed December 18, 2020. https://handbook-5-1.cochrane.org/chapter_9/9_5_2_identifying_and_measuring_heterogeneity.htm
- 16.Agarwal R., Rossignol P., Romero A. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2019;394(10208):1540–1550. doi: 10.1016/S0140-6736(19)32135-X. [DOI] [PubMed] [Google Scholar]
- 17.Anker S.D., Kosiborod M., Zannad F. Maintenance of serum potassium with sodium zirconium cyclosilicate (<scp>ZS</scp>-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail. 2015;17(10):1050–1056. doi: 10.1002/ejhf.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ash S.R., Singh B., Lavin P.T., Stavros F., Rasmussen H.S. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015;88(2):404–411. doi: 10.1038/ki.2014.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Packham D.K., Rasmussen H.S., Lavin P.T. Sodium Zirconium Cyclosilicate in Hyperkalemia. N Engl J Med. 2015;372(3):222–231. doi: 10.1056/nejmoa1411487. [DOI] [PubMed] [Google Scholar]
- 20.Rafique Z., Liu M., Staggers K.A., Minard C.G., Peacock W.F. Patiromer for Treatment of Hyperkalemia in the Emergency Department: A Pilot Study. Baumann BM, ed. Acad Emerg Med. 2020;27(1):54–60. doi: 10.1111/acem.13868. [DOI] [PubMed] [Google Scholar]
- 21.Weir M.R., Bakris G.L., Bushinsky D.A. Patiromer in Patients with Kidney Disease and Hyperkalemia Receiving RAAS Inhibitors. N Engl J Med. 2015;372(3):211–221. doi: 10.1056/nejmoa1410853. [DOI] [PubMed] [Google Scholar]
- 22.Zannad F., Hsu B., Maeda Y. Efficacy and safety of sodium zirconium cyclosilicate for hyperkalaemia: the randomized, placebo-controlled HARMONIZE-Global study. ESC Hear Fail. 2020;7(1):55–65. doi: 10.1002/ehf2.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das S., Dey J.K., Sen S., Mukherjee R. Efficacy and Safety of Patiromer in Hyperkalemia: A Systematic Review and Meta-Analysis. J Pharm Pract. 2018;31(1):6–17. doi: 10.1177/0897190017692921. [DOI] [PubMed] [Google Scholar]
- 25.Meaney C.J., Beccari M.V.., Yang Y., Zhao J. Systematic Review and Meta-Analysis of Patiromer and Sodium Zirconium Cyclosilicate: A New Armamentarium for the Treatment of Hyperkalemia. Pharmacotherapy. 2017;37(4):401–411. doi: 10.1002/phar.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGowan C.E., Saha S., Chu G., Resnick M.B., Moss S.F. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J. 2009;102(5):493–497. doi: 10.1097/SMJ.0b013e31819e8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bounthavong M., Butler J., Dolan C.M. Cost-Effectiveness Analysis of Patiromer and Spironolactone Therapy in Heart Failure Patients with Hyperkalemia. Pharmacoeconomics. 2018;36(12):1463–1473. doi: 10.1007/s40273-018-0709-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brew C.T., Blake J.F., Mistry A. Use of QSPR Modeling to Characterize In Vitro Binding of Drugs to a Gut-Restricted Polymer. Pharm Res. 2018;35(4):1–10. doi: 10.1007/s11095-018-2356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesko L.J., Offman E., Brew C.T. Evaluation of the Potential for Drug Interactions with Patiromer in Healthy Volunteers. J Cardiovasc Pharmacol Ther. 2017;22(5):434–446. doi: 10.1177/1074248417691135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosiborod M., Peacock W.F., Packham D.K. Sodium Zirconium Cyclosilicate for Urgent Therapy of Severe Hyperkalemia. N Engl J Med. 2015;372(16):1577–1578. doi: 10.1056/nejmc1500353. [DOI] [PubMed] [Google Scholar]
- 31.Bushinsky D.A., Williams G.H., Pitt B. Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney dkalemia. Kidney Int. 2015;88(6):1427–1433. doi: 10.1038/ki.2015.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.