Abstract
Neuroendocrine neoplasms (NENs) constitute a heterogeneous group of tumors. In this review, we summarize the results of various clinical trials that have been conducted to investigate the efficacy and safety of various therapeutic options for NENs. Based on the encouraging results obtained from these trials, various therapeutic options have been established for the treatment of NENs, including somatostatin analogs (SSAs), molecularly targeted drugs and cytotoxic agents. In addition, peptide receptor radionucleotide therapy has recently been evaluated for the treatment of various NENs. We also discuss the approach for selecting the appropriate drugs and sequence of treatment with the various drug classes, as recommended by different treatment guidelines. Finally, we discuss the scope for future research in this field, especially into the merits of combination therapy with molecularly targeted drugs plus SSAs, along with ongoing studies.
Keywords: Pancreatic neuroendocrine tumors, gastroenteropancreatic neuroendocrine neoplasms, somatostatin analog, molecular-targeted agent, cytotoxic agent, treatment strategy
Introduction
Research on evidence-based therapies for unresectable gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) had fallen behind; however, since 2010, several randomized controlled clinical trials of various therapies have been conducted. Based on the promising results of these trials, multiple therapeutic options are now available for GEP-NENs. In this review, we discuss the progress in medical as well as other modalities of therapy for unresectable/recurrent GEP-NENs.
Epidemiology
Neuroendocrine neoplasm (NEN) is a general term that is used to refer to neoplasms that arise from the neuroendocrine cells (1). While NENs cover a broad spectrum, they have been roughly divided into two types: neuroendocrine tumors (NETs) and neuroendocrine carcinomas (NECs) (2). Gastrointestinal NENs are broadly divided based on their site of origin, as foregut, midgut or hindgut NENs. According to the Surveillance, Epidemiology and End Results (SEER) database in the USA, foregut (41%) and midgut (26%) NENs account for the majority of gastrointestinal NENs, with hindgut NENs accounting for only 19% of all gastrointestinal NENs; in the remaining 13%, the primary tumor site remained unknown (3). According to the national cancer registry in Japan, a total of 6735 individuals were diagnosed with GEP-NEN in Japan in 2016 (4). Annual onset incidence was 0.70/100 000 for pancreatic NEN and 2.84/100 000 for gastrointestinal NEN. In contrast with USA, there was little frequency of the ileum NET (only 1% of total GEP-NENs) (4). Thus, hindgut gastrointestinal NENs appear to be far more common, and midgut NENs far less common, in Japan than in the USA (5).
Gastrointestinal NENs are further divided into pancreatic NENs and NENs of the gastrointestinal tract, both of which are currently classified according to the 2019 WHO Classification of Tumors of the Digestive System, 5th ed. (2).
Standard and promising therapies for unresectable/recurrent GEP-NETs and NECs
As stated above, since 2010, numerous randomized controlled clinical trials of various therapies for NETs have reported encouraging results, and multiple therapeutic options are now available for NETs (Table 1). Three major classes of drugs have been demonstrated to show promising antitumor effects against GEP-NETs: somatostatin analogs [SSAs; e.g. lanreotide and octreotide long-acting release (LAR)] (6, 7), molecularly targeted drugs (e.g. everolimus and sunitinib) and cytotoxic agents [e.g. streptozotocin (STZ)]. In Japan, octreotide LAR is covered by insurance only for NETs of the gastrointestinal tract, while sunitinib is covered by insurance only for pancreatic NETs. In the West, peptide receptor radionuclide therapy (PRRT) and Temozolomide are also used for the treatment of GEP and pancreatic NETs, respectively (8). In addition, immune checkpoint inhibitors (ICIs) have recently attracted attention as potentially beneficial agents for the treatment of GEP-NETs.
Table 1.
Principal prospective phase III trials of unresectable, recurrent GEP-NETs
| Study | Study design | Setting | Treatment and dose | No. of patients | Primary endpoint | Outcome of primary endpoint | HR | OS |
|---|---|---|---|---|---|---|---|---|
| Somatostatin analogs | ||||||||
| PROMID Rinke A et al. (5,15) |
Phase III | Midgut or unknown origin NET (Functional and non-functional) | Octreotide LAR 30 mg every 28 days vs. placebo |
N = 85 42 vs. 43 |
TTP | OCT: 14.3 m vs. placebo: 6.0 m | 0.34 (0.20–0.59) P < 0.001 |
84.7 vs. 83.7 m |
| CLARINET Martyn E et al. (6,19) |
Phase III | Ki67 < 10% enteropancreatic or unknown origin NET (non-functioning) | Lanreotide LAR vs. placebo |
N = 204 101 vs. 103 |
PFS | NR vs. 18 0.0 m (32.8 vs. 18.0 in open-label extension study) |
0.47 (2.1–24.0) P < 0.001 |
Not reported |
| Molecularly targeted agents | ||||||||
| SUN1111 Raymond E et al. (25,31) |
Phase III | Progressive disease pancreatic NET |
Sunitinib vs. placebo |
N = 171 86 vs. 85 |
PFS | 11.4 m vs. 5.5 m | 0.42 (0.26–0.66) P = 0.0001 |
38.6 vs. 29.1 m |
| RADIANT3 Yao JC et al. (38,39) |
Phase III | Progressive disease pancreatic NET |
Everolimus vs. placebo | 207 vs. 203 | PFS | 11.0 vs. 4.6 m | 0.35 (0.27–0.45) P < 0.0001 |
44.0 vs. 37.7 m |
| RADIANT4 Yao JC et al. (40) |
Phase III | Progressive disease lung or GI NET |
Everolimus vs. placebo | 205 vs. 97 | PFS | 11 vs 3.9 m | 0.48 (0.35–0.67) P < 0.001 |
Not reported |
| SANEP-p Xu J et al. (30) |
Phase III | Progressive disease pancreatic NET |
Surufatinib vs. placebo | 113 vs. 59 | 10.9 vs 3.7 m | 0.49 (0.32–0.76) P = 0.0011 |
Not reported | |
| SANEP-ep Xu J et al. (36) |
Phase III | Progressive disease extrapancreatic NET |
Surufatinib vs. placebo | 129 vs. 69 | 9.2 vs. 3.8 m | 0.33 (0.22–0.50) P < 0.001 |
Not reported | |
| Cytotoxic anticancer agents | ||||||||
| STZ study Moertel CG et al. (45) |
Phase III | pancreatic NET | STZ + doxorubicin vs. STZ + 5-FU vs. chlorozotocin |
N = 105 38 vs. 34 vs. 33 |
OS | 2.2 vs. 1.5 vs. 1.4 years | Not reported | 2.2 vs. 1.5 vs. 1.4 years |
| PRRT | ||||||||
| NETTER-1 (69) | Phase III | Midgut NET progressive to SSA |
PRRT (177Lu-Dotate) vs. Octreotide LAR 60 mg |
N = 229 116 vs. 113 |
PFS | NR vs. 8.4 m |
0.21 (0.13–0.33) | OS data immature |
*Include subgroup analysis.
NET, neuroendocrine tumor; n, number of patients; HR, hazard ratio; RR, response rate; PFS, progression free survival; TTP, time to progression; OS, overall survival; m, months; STZ, streptozocin; 5-FU, 5-fluorouracil; DOX, doxorubicin; PRRT, peptide receptor radionuclide therapy; NR, not reached; −, no data.
Somatostatin analogs
NETs have been shown to express somatostatin receptors (SSTRs) 2 and 5 (9–11), which serve as the therapeutic targets for certain SSAs. SSA was originally used for symptom control which was caused by functional pancreatic NET or Carcinoid syndrome with the majority of patients displaying a midgut primary. Although SSAs were developed primarily for symptomatic control of NETs (antisecretory effect), SSA were also evaluated for antiproliferatice effects in GEP-NET.
The direct antitumor effects of SSTR agonists include SSTR activation, phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin (mTOR) pathway inhibition and downregulation of the mitogen-activated protein kinase (MAPK) pathway (12–14). In addition, some of the indirect effects of this class of drugs include inhibition of angiogenesis, alterations of the tumor immunity and suppression of growth factors (14, 15).
Octreotide LAR
Four publications have reported the results of comparison of octreotide LAR vs. placebo/no treatment; these include reports of two prospective studies, including a randomized, double-blind, placebo-controlled trial of octreotide LAR for the control of tumor growth in patients with metastatic midgut NETs (PROMID) (6, 16) and two retrospective studies from the SEER database (17, 18). In the PROMID trial, 85 patients with functionally active or inactive metastatic well-differentiated midgut NETs received either long-acting octreotide or placebo (6). The median time-to-tumor progression (TTP) was 14.3 months in the long-acting octreotide group (n = 42) as compared with 6.0 months in the placebo group (n = 43) (hazard ratio [HR], 0.34; 95% confidence interval [CI], 0.20–0.59; P = 0.000072). After 6 months of treatment, the response was classified as ‘stable disease’ in 66.7% of patients in the long-acting octreotide group vs. 37.2% of patients in the placebo group (6). Rinke et al. (16) reported that the final median overall survival times (OS) in the long-acting octreotide and placebo groups in the PROMID trial were 84.7 and 83.7 months, respectively (HR, 0.83; 95% CI, 0.47–1.46; P = 0.51). There was a trend toward improved survival in patients with a low hepatic tumor load (HTL) receiving long-acting octreotide vs. placebo (median not reached vs. 87.2 months; HR, 0.59; 95% CI, 0.29–1.2; P = 0.142). Crossover of a majority of patients of the placebo group to the long-acting octreotide group may have confounded the results pertaining to the OS (16).
One prospective study examined the patients who had received 30 mg of standard-dose long-acting octreotide every 28 days vs. those of the same patients after they were switched to 30 mg of long-acting octreotide administered every 21 days. The shorter dose interval was associated with a longer TTP (30 vs. 9 months; P < 0.0001), and 93% of the patients on the 21-day schedule were rated as having stable disease (19).
Lanreotide
The CLARINET study (7) evaluated the SSA lanreotide in patients with advanced, grade 1 or 2 (G1/G2) differentiated, non-functioning, SSTR-positive NETs [diagnosed as moderately differentiated to well-differentiated, pancreatic, gastrointestinal tract or unknown-origin NETs with a Ki-67 labeling index (LI) of <10%] and documented the disease progression status. The primary endpoint was the progression-free survival (PFS). The PFS was significantly prolonged in the lanreotide group as compared with the placebo group (median PFS not reached in the lanreotide group vs. 18.0 months in the placebo group; HR, 0.47; 95% CI, 0.3–0.73; P < 0.001). A subgroup analysis of patients with pancreatic NETs also showed improvement of the PFS in the drug treatment arm, indicating the effectiveness of lanreotide (median PFS not reached in the lanreotide group vs. 12.1 months in the placebo group; HR, 0.58; 95% CI, 0.32–1.04).
Recently, an open-label extension study of CLARINET (20) was reported. In this study, 88 patients with stable disease were selected; 41 were continued on treatment with lanreotide, while 47 were switched from placebo to lanreotide (120 mg/28 days). The results of this study showed a clear prolongation of the PFS (32.8 vs. 18 months) for those in the placebo group reported in the CLARINET study (7). Ito et al. (21) reported that long-term safety and efficacy of lanreotide was confirmed by phase II open-label extension study in Japanese patients.
The ELECT study was a phase III study of the efficacy and safety of lanreotide for the treatment of carcinoid syndrome in patients with NETs (22); a total of 150 patients with carcinoid syndrome were randomized to lanreotide (120 mg) or placebo administered every 4 weeks, with access to octreotide rescue. The results revealed that lanreotide treatment was associated with a 15% lower need for rescues (P = 0.036) and reduced the frequency of diarrhea and flushing by 76 and 73%, respectively. These results demonstrated the effectiveness of lanreotide in controlling the symptoms of carcinoid syndrome (22).
An observational retrospective-prospective analysis was conducted to investigate the usefulness of SSAs as first-line treatment for GEP-NETs, which identified primary pancreatic tumor, distant extrahepatic metastasis and non-resection of the primary tumor as predictors of a negative response to SSAs (23). Another study identified tumor Ki67 LI <5%, tumor stability and HTL <25% as predictors of a positive response to SSAs (24). The response to octreotide LAR in the PROMID study was higher in patients in whom the primary tumor had been resected and the HTL was <10% (6). In summary, SSAs are invaluable as front-line therapy for both functioning and non-functioning GEP-NETs, especially in patients with indolent tumors and a low HTL (25).
Molecularly targeted drugs
Targeted agents have been introduced in the therapeutic landscape for pancreatic NETs. Considering the high expression levels of vascular endothelial growth factor receptors (VEGFR), platelet-derived growth factor receptors α and β and stem cell factor receptor (c-kit) in pancreatic NETs, antiangiogenic agents such as sunitinib (26, 27), pazopanib (28, 29), cabozantinib (30), lenvatinib (31) and surufatinib (32) have been investigated for their effectiveness in patients with pancreatic NETs and been demonstrated to show significant antitumor activity (33). Among these, sunitinib and surufatinib have been evaluated in phase III studies.
Sunitinib
SUN1111 was a phase III randomized study conducted to investigate the efficacy of sunitinib (27). The results showed improved PFS in the drug treatment arm as compared with the placebo arm (11.4 vs. 5.5 months; HR, 0.42; 95% CI, 0.26–0.66), based on which sunitinib was approved by regulatory agencies for the treatment of pancreatic NETs. Long-term data indicated positive, but statistically non-significant survival effects (34). Subsequent long-term survival analysis reported a median OS of 38.6 months for the sunitinib treatment arm vs. 29.1 months for the placebo arm (HR 0.73; P = 0.09) with a lack of statistical significance attributed to substantial crossover from placebo to sunitinib arm upon progression (34).
A phase II trial to assess the efficacy of sunitinib was also conducted in Japan (35). The clinical benefit rate, which was the primary endpoint of this trial, was 75%. Although the median PFS was not reached, the PFS rates at 6 and 12 months were 91 and 71%, respectively, demonstrating the efficacy of sunitinib also in Japanese patients.
Surufatinib
Surufatinib is an orally active, potent, selective inhibitor of VEGFR-1, -2 and -3, fibroblast growth factor receptor (FGFR)-1 and colony-stimulating factor-1 receptor (CSF-1R). Based on these observations, two randomized, double-blind, placebo-controlled phase III studies recently investigated the safety and efficacy of surufatinib in Chinese patients with well-differentiated, progressive, advanced pancreatic (SANET-p trial) (32) and extrapancreatic (SANET-ep trial) NETs (36). Both studies showed an improvement of the PFS in the surufatinib arm (SANET-p trial: 10.9 vs. 3.7 months; HR, 0.49; 95% CI, 0.32–0.76; SANET-ep trial: 9.2 vs. 3.8 months; HR, 0.33; 95% CI, 0.22–0.50). Both trials met the early termination criteria at the time of the interim analysis and were terminated on the recommendation of the Independent Data Monitoring Committee. Therefore, surufatinib is expected to become a useful addition to the treatment armamentarium available for patients with well-differentiated NETs as the option of the second line or later, regardless of the specific origin.
Everolimus
Excessive activation of the mTOR pathway is known to be involved in the development and progression of pancreatic NETs. Therefore, the efficacy/safety of everolimus, a rapamycin analog mTOR inhibitor, has been investigated in patients with pancreatic NETs, based on preclinical data (37). The results of RADIANT-3, a phase III randomized controlled clinical trial, showed that the use of everolimus had a favorable impact on the median PFS (11.4 vs. 5.4 months; HR, 0.35; P < 0.0001) as compared with placebo in patients with advanced pancreatic NETs (38). The median OS was 44.0 months in the treatment arm vs. 37.7 months in the placebo arm (HR, 0.94; 95% CI, 0.73–1.20), and the difference was not statistically significant (39). This lack of a significant difference was probably due to the crossing over of 84.7% of the patients to the treatment arm from the placebo arm.
RADIANT-4, a randomized controlled phase III trial of everolimus for NETs of the gastrointestinal tract (foregut/midgut/hindgut) and lung, with the same study design as the RADIANT-3 trial (40), showed a significantly prolonged median PFS in the everolimus arm as compared with the placebo arm (11.0 vs. 3.9 months; HR, 0.48; 95% CI, 0.35–0.67, P < 0.001). A subgroup analysis for midgut NETs also showed a favorable trend in the everolimus arm (median PFS, 8.11 vs. 1.94 months; HR, 0.27; 95% CI, 0.15–0.51) (41). The HR of everolimus treatment for rectal NETs was 0.17 (95% CI, 0.00–0.38), the highest efficacy ever reported until now for everolimus.
Currently, everolimus is widely used for the treatment of GEP-NETs. However, it is generally recommended that treatment with everolimus be initiated only in patients with progressive or symptomatic disease, due to the indolent nature of many NETs and the inclusion criteria of the RADIANT trials considering the documented progression (42). The optimal sequence of use of the available treatment options remains undefined.
Cytotoxic agents
Chemotherapy using cytotoxic agents for the treatment of pancreatic NETs has been adopted for a long time. Alkylating agents, prototypes of which are streptozotocin (STZ) and temozolomide, are proactively used as anticancer agents in patients with pancreatic NETs.
Streptozotocin
STZ is taken up into the cells via glucose transporter 2 (GLUT2) and cause alkylation of DNA and suppression of tumor growth by inhibiting DNA synthesis (43). The major sites of GLUT2 expression are the pancreatic β-cells (endocrine cells), small intestine, liver and kidneys (44). This suggests that STZ may be expected to exert an antitumor effect not only on pancreatic NETs but also on NETs of the gastrointestinal tract.
Moertel et al. (45) reported that a combination of STZ plus 5-fluorouracil (5-FU) was superior to 5-FU monotherapy in 84 patients with advanced islet carcinoma, with response rates (RRs) in the two treatment arms of 63 and 36%, respectively. In addition, the MST was also significantly prolonged (26 vs. 16.4 months). However, no statistically significant improvement of the PFS was observed. A phase III study of STZ + 5-FU and STZ + doxorubicin or chlorozotocin monotherapy was reported in 1992. This study showed that treatment with STZ + doxorubicin yielded a higher RR (69 vs. 54%) and a significant improvement of the OS (2.2 vs. 1.4 years) (46). However, subsequent retrospective studies failed to reproduce the effect of STZ + doxorubicin (47, 48). In addition, due to the risk of cardiotoxicity and other adverse drug reactions associated with doxorubicin, opinion is divided regarding the clinical usefulness of STZ + doxorubicin (49). Recently, it was reported from one study that STZ + 5-FU was more favorable for use than STZ + doxorubicin because of its more acceptable toxicity profile (50).
In Japan, STZ is frequently used not only daily regimen but also weekly regimen (51, 52). This has an advantage to be able to give treatment in an outpatient. There are still unanswered questions regarding the optimal sequence of use of the drug classes and impact of the O6-methylguanine-DNA methyltransferase (MGMT) status as a biomarker for the treatment response to STZ. The currently ongoing SEQTOR trial is evaluating the optimal sequence of use of STZ + 5-FU and everolimus for the treatment of pancreatic NETs, and further prospective data are expected (NCT02246127). A recent report showed a high concordance between negative expression of MGMT and response to STZ treatment, suggesting that MGMT expression could indeed be a potential biomarker of the response to STZ treatment (53).
As for the outcomes of streptozocin treatment for NETs of the gastrointestinal tract, in 2005, the results for a randomized controlled trial of STZ + 5-FU vs. doxorubicin+5FU in patients with gastrointestinal tract NETs (54) showed that STZ + 5-FU yielded a significantly longer MST (24.3 vs. 15.7 months; P = 0.0267). However, due to the wide range of primary sites (small intestine, rectum, pancreas, lung, multiple organs, unknown, other) and the absence of subgroup analyses, the National Comprehensive Cancer Network (NCCN) guidelines and ENETS guidelines (55) maintain that STZ + 5-FU shows poor efficacy against NETs of the gastrointestinal tract.
Temozolomide
The orally administered alkylator-, temozolomide- and temozolomide-based therapies have been widely used for the treatment of pancreatic NETs in the West. The most promising results have been reported for the combination of temozolomide plus capecitabine. In a randomized phase II trial (E2211 study) conducted by Kunz et al. in 2018 ASCO Annual Meeting (56), temozolomide + capecitabine (CAPTEM) was compared with temozolomide monotherapy in patients with pancreatic NET-G1/G2. Although there were imbalances and lack of stratification by tumor grade, the study demonstrated for the first time that the combination (CAPTEM) was generally active and more effective than temozolomide monotherapy (median PFS 22.7 vs. 14.4 months; HR, 0.58; 95% CI, 0.36–0.93, P = 0.023) (56). This median PFS of CAPTEM (22.7 months) showed much longer than that of Molecularly targeted drugs (Everolimus and sunitinib; 11.4 months). Consistent with PFS data, the median OS was 38 months in patients receiving temozolomide monotherapy, while it was not reached in patients treated with CAPTEM, which was significantly longer than temozolomide monotherapy [HR 0.58 (95% CI, 0.36–0.93), P = 0.023]. In addition, this study showed a higher RR (temozolomide monotherapy 27.8% vs. CAPTEM 33.3%; P = 0.47), compared with conventional standard treatment.
Another potential strategy of interest is combining cytotoxic agents with PRRT. In a prospective phase II single-center study of 30 patients with pancreatic NET-G1/G2, PRRT (four doses of 177Lu-Dotatate) in combination with CAPTEM (14 days of chemotherapy every 8 weeks during PRRT) yielded an objective RR of 80%, with a median PFS of 48 months (57). Although CAPTEM is expected to be an important treatment strategy for pancreatic NETs going forward, it still remains to be approved in Japan. In addition, there are still unanswered questions, including those on the optimal line of use and impact of MGMT-methylation status as a biomarker of the treatment response (42).
Treatment for NET-G3
The WHO pathological classification was revised based on the 2017 WHO classification, and pancreatic NEN-G3 (NEC in the 2010 WHO classification) was classified into pancreatic NET-G3 and pancreatic NEC. The NORDIC NEC study (58) showed significantly inferior platinum-based regimens treatment RRs with a Ki67 LI of <55% vs. higher grade tumors (15 vs. 42%), despite the better OS. In addition, the Japanese pancreatic NEN-G3 study, a multicenter retrospective case series involving 70 patients with pancreatic NENs, revealed that pancreatic NET-G3 showed some differences in clinicopathological features, such as lower values of the Ki67 LI, absence of KRAS mutations, loss of immunostaining for the retinoblastoma (Rb) protein (Rb loss) and absence of response to platinum-based regimens as compared with pancreatic NEC (51.3 vs. 0%; P < 0.001) (59). There are no available prospective treatment data for NET-G3. Based on the available data, STZ + 5-FU and CAPTEM appear to be effective treatment options and are currently preferred for patients with advanced pancreatic NET-G3 (42, 60). There are some phase2 clinical trails targeting mainly NET-G3, single arm eveloimus (EVINEC:NCT02113800), single arm Cabozantinib (CABONEN: NCT04524208) and carboplatin or Cisplatin+etoposide vs. Capecitabine+Temozolomide (NCT02595424). By results of these clinical trails, the actual situation of NET-G3 is expected to be found more.
Treatment for NEC
Studies have shown that NEC responds well to platinum-based regimens, and platinum-based chemotherapy is now recommended for small-cell lung carcinomas (54, 60). However, the only existing studies on the effects of platinum-based drugs against NEC are small-scale retrospective studies. In Japan, a phase III clinical trial (JCOG1213) is being conducted to compare the efficacy of irinotecan + cisplatin and etoposide + cisplatin for pancreatic and other NECs (jRCTs031180005). Platinum-based drugs are highly effective in cases of pancreatic NEC with KRAS mutations and Rb loss; therefore, KRAS mutations and Rb loss were determined as predictors of the efficacy of platinum-based drugs in patients with pancreatic NEC (61). Patients with pancreatic NEC show varied outcomes of treatment with platinum-based regimens. In the future, it would be useful to classify pancreatic NEC into more detailed distinct groups based on immunohistochemical and genetic findings.
Immune checkpoint inhibitors
ICIs have been demonstrated to be effective against melanoma (61, 62) and non-small-cell lung cancer (63) and so on. Emerging evidence has shown the possibility of targeting the tumor immune microenvironment as novel therapeutic option for NENs (64, 65). Besides PD-L1/L2 expression has been identified in GEP-NETs (66), in metastatic GEP-NENs, the expression of PD-L1 is associated with high-grade NET (67) and has both predictive and prognostic value for survival of patients (66, 68). Besides, the expression of PD-L1 has been reported in around 100% of well-differentiated G3 GEP-NETs (69).
The KEYNOTE-028 study is a phase Ib study that examined the safety and efficacy of the anti-PD-1 inhibitor, pembrolizumab, in patients with PD-L1-positive (≥1%) solid tumors. This study included 16 patients with pancreatic NETs. However, pembrolizumab was found to exert limited efficacy, with an RR of 6%, PFS of 4.5 months and OS time of 21.0 months (70). In addition, in the KEYNOTE-158 study, a phase II basket trial conducted to examine the efficacy of pembrolizumab in patients with advanced solid cancers (71). Among the 107 patients with NETs in this study, 40 had pancreatic NETs. The RR was only 3.7%, median PFS was 4.1 months, and a median OS time was 24.2 months. In addition, grade 3–5 adverse events occurred in 20.6% of the patients.
Some phase II studies of ICI for GEP-NET were published. Spartalizumab, PD-1 monoclonal antibody, was evaluated in patients with well-differentiated NET G1/2 and GEP-NEC. The ORR was 7.4% in the NET group (thoracic, 16.7%; gastrointestinal, 3.1%; pancreatic, 3.0%), which was below the predefined success criterion of ≥10%, and 4.8% (95% CI: 0.1, 23.8) in the GEP-NEC group. The efficacy of spartalizumab was limited in GEO-NET population; however, the results in the thoracic cohort are encouraging and warrant further investigation (72).
Currently, phase II studies are under way to investigate the efficacy of ICIs in patients with advanced NETs: Avelumab for metastatic/unresectable well-differentiated NET-G2/G3 (NCT03278379), avelumab for progressive NEC after chemotherapy (NCT03352934), durvalumab and tremelimumab for GEP-NEN-G3 (NCT03095274) (73) and pembrolizumab for metastatic high-grade NETs (NCT02939651).
In addition, normalizing blood vessels with angiogenesis inhibitors have recently been demonstrated to improve the immune responses to tumors and thus boost the effect of ICIs. Therefore, the combination of ICIs plus angiogenesis inhibitors is increasingly attracting attention. For example, combination immunotherapy with Ipilimumab and Nivolumab was administrated for Rare Cancers in CA209–538 Clinical Trial. Of this study, 29 patients with advanced NENs received treatment of this study. The objective RR of showed 24% with a CBR of 72% and demonstrated significant clinical activity in subgroups of patients with advanced NETs (74). In addition, combined atezolizumab plus bevacizumab therapy has been attempted for the treatment of NETs (73). Similarly, cabozantinib and atezolizumab have also been used for the treatment of advanced and progressive NETs (NCT04400474).
Some evidence suggests that tumor mutation burden (TMB) may be a useful biomarker to select patients who could respond to immunotherapy, independently from the microsatellite instability status of the tumor (64). According to the subanalysis of the Keynote158 study, this tendency is thought to be similar for GEP-NET (75). Also, it is reported that high-grade NET was significantly higher in TMB when we divide GEP-NEN into high grade and low grade in the recent study (9.5 mut/MB vs. 5.1, P ≤ 0.0001) (67).
Peptide receptor radionucleotide therapy
PRRT, using radiolabeled SSAs, is an effective and well-tolerated treatment modality that extends the concept of targeting SSRs in NETs (76). The superior efficacy of PRRT (177Lu-Dotatate) to octreotide LAR 60 mg (high dose) was demonstrated in a prospective phase III study (NETTER-1 trial) in patients with advanced well-differentiated midgut NETs. The PFS was clearly favorable, with an estimated PFS at 20 months of 65.2% in the PRRT group vs. 10.8% in the control group (HR, 0.21; 95% CI, 0.13–0.33), suggesting a new standard for patients in this setting.
Although the NETTER-1 trial was conducted in patients with midgut NETs, PRRT has also been reported to be effective against pancreatic NETs. A total of 8 studies (2 prospective, 6 retrospective) have reported the effectiveness of PRRT against pancreatic NETs (8, 77–84) (Table 2). The reported median PFS ranged from 20 to 39 months, and the median OS times ranged from 37 to 79 months. It is worthy of note that no significant difference in the median PFS or OS was found when the treatment outcomes for pancreatic NETs and NETs at other sites were compared. It should also be noted that these studies were quite heterogeneous in terms of the treatments used in previous lines of therapy as well as in terms of whether the patients had progressive disease during treatment (85). Thus, PRRT appears to be effective not only against midgut NETs, but also against pancreatic NETs, and has therefore been approved for the treatment of NETs by the United States Food and Drug Administration. However, PRRT is not yet approved in Japan.
Table 2.
Outcomes of PRRT in pancreatic NET
| Author/year (ref) | Radiopharmaceutical | Study type | No. of panNET patients | ORR | mPFS months (95% CI) | mOS months (95% CI) |
|---|---|---|---|---|---|---|
| Baum et al. 2016 (68) | 177Lu-PRRT (36%), 90Y-PRRT (15%) or both (49%) | Retrospective | 384 | NS | 20 (17–23) | 44 (38–50) |
| Campana et al. 2013 (69) | 90Y-DOTATOC or 177Lu-DOTATATE | Retrospective | 45 | 31% | 23 | NS |
| Pfeifer et al. 2011 (70) | 90Y-DOTATOC (77%), 177Lu-DOTATOC or both (23%) | Retrospective | 21 | 33% | 27 | NR |
| Dumont et al. 2015 (72) | 90Y-DOTATOC (80%) or 90Y-DOTATOC & 177Lu-DOTATOC (20%) | Prospective | 36 | 33% | NS | 40 |
| Hörsch et al. 2016 (73) | 177Lu-PRRT (54%), 90Y-PRRT (17%), both (29%) | Retrospective | 172 | NS | 39 (29–49) | 53 (37–69) |
| Bertani et al. 2016 (74) | 90Y-DOTATOC (37%), 177Lu-DOTATATE (28%), both (35%) | Prospective | 90 | 26% | 36 (24–44) | 75 (64–104) |
| Kunikowska et al. 2017 (73) | 90Y-DOTATATE & 177Lu-DOTATATE | Prospective | 19 | NS | 30 | 79 |
| Sharma et al. 2017 (75) | 90Y-PRRT (83%), 177Lu-PRRT (15%) | Retrospective | 35 | NS | NS | 37 (18–48) |
panNET, pancreatic neuroendocrine tumor; ORR, overall response rate; mPFS, median progression-free survival; mOS, median overall survival; NS, not stated; NR, not reached; CI, confidence interval.
PRRT is an established treatment for NET-G1/G2, with an increased uptake on SSTR imaging (SRI). However, there is promising evidence to support the effectiveness of PRRT against SRI-positive G3 tumors (86, 87). A review of four studies conducted to determine the efficacy of PRRT against NEN-G3 revealed promising RRs (31–41%) and disease control rates (69–78%) in three of the studies. The median PFS (11–16 months) and survival (22–46 months) times were the best in patients with tumors showing a Ki-67 LI of <55% (86, 87). These results suggest that PRRT could be considered for patients with increased uptake on SRI, both those with NET-G3 and those with NEC with a Ki67 LI of <55% (86).
The NETTER-2 trial (NCT03972488) is an ongoing phase III, randomized study of 177Lu-Dotatate with 30-mg octreotide LAR vs. 60-mg octreotide LAR for first-line treatment of advanced GEP-NET-G2/G3. Another study of interest is the COMPETE trial (NCT03049189), which is a phase III, randomized trial investigating the effect of PRRT (177Lu-DOTATOC) vs. everolimus for first-line treatment of advanced GEP-NETs (all grades included).
Radiotherapy has been shown to increase the tumor antigenicity as well as increase antigen presentation, which, in turn, can enhance T-cell destruction in tumor cells (88). Therefore, investigation of the efficacy of PRRT in combination with immunotherapy may be warranted in the future (85). In addition, PRRT plus cytotoxic agents or PRRT plus targeted therapy are expected to be examined in future prospective studies that will draw great attention.
Selection of the optimal drug and sequence of use of the drug classes for pancreatic NETs
In general, the tumor burden and tumor aggressiveness are considered as the two most important factors for selecting the optimal treatment agents for patients with pancreatic NETs. The ENETS (55), ESMO (89) and NCCN guidelines (90) propose the following algorithm to select the drugs/sequence of use of the drug classes for patients with pancreatic NETs: when the Ki-67 LI or tumor volume is low, SSAs may be used first, and if the tumor progresses after this treatment, molecularly targeted drugs or cytotoxic agents could then be used. The NCCN guidelines (90) mention that in selected cases, it may be appropriate to proceed to front-line systemic therapy or liver-directed therapy prior to or concurrently with octreotide or lanreotide. No standard therapy has been established yet for NET-G3, however, despite the limited evidence, the ENETS and ESMO guidelines recommend the use of STZ + 5-FU and CAPTEM as first-line therapies for NET-G3 (89, 91). Yao and Phan (92) have proposed an algorithm for pancreatic NETs based on the pattern of disease progression and tumor volume. According to this algorithm, for patients with a relatively small tumor burden and slow disease progression, SSAs would be selected as the first-line treatment agents. For those with a relatively large tumor burden and rapid tumor progression, cytotoxic agents would be selected for achieving tumor shrinkage.
However, clear criteria have not been established for disease progression and tumor volume. Thus, there is no clear scientific basis for determining the sequence of drugs to be used for the treatment of pancreatic NETs.
Recently, Ikeda et al. (93) proposed a tentative map of optimal treatment selection for patients with unresectable pancreatic NETs, based on a discussion by Japanese experts of the results of previously published studies (Fig. 1). They considered classifying tumors according to the Ki-67 LI (5, 10 or 20%) and liver metastasis tumor volumes (10, 25 and 50%). Although this study requires further validation, Ki-67 LI appears to be a promising indicator for therapy selection.
Figure 1.
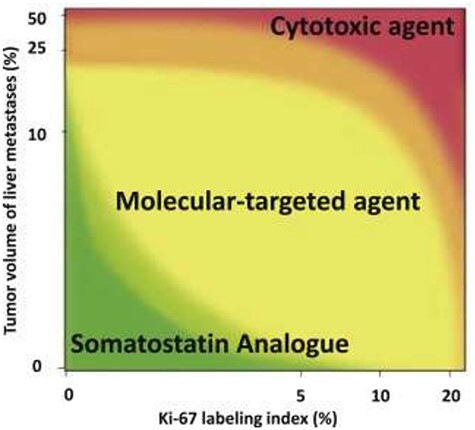
Treatment selection MAP of the first-line systemic treatment for patients with unresectable pancreatic neuroendocrine tumors. (Permission to reprint from Pancreatology license number 5014700331143) As the first-line treatment agent, patients in the green, yellow and red areas would be recommended a somatostatin analog, molecular-targeted agent and cytotoxic agent, respectively; x-axis shows Ki-67 labeling index (LI) (%) of tumor tissue sample, and y-axis shows tumor volume (%) of liver metastases which is calculated in the whole liver by pretreatment CT or MRI.
Selection of the optimal drugs and sequence of use of the drug classes for GEP-NETs
The ESMO guidelines (91) recommend the following algorithm to determine the sequence of use of the available drugs for small intestinal NETs: treatment could be started with SSAs for NET-G1/G2, SSTR-positive, Ki-67 LI <10% and slow-growing tumors; if the tumor progresses after this treatment, therapy could be continued with local treatments, PRRT, or everolimus: for NET-G2, Ki-67 > 10–15%, or rapidly growing tumors, therapy could be started with everolimus; for SSTR-negative small-intestinal NETs, therapy could be started with locoregional treatment options. The NCCN guidelines (90) state that everolimus, PRRT, local treatments, interferon α-2b and cytotoxic agents may be considered for tumors that are resistant to SSAs. However, as for pancreatic NETs, the NCCN guidelines also mention that in selected cases, it may be appropriate to proceed to front-line systemic therapy or liver-directed therapy prior to or concurrently with octreotide or lanreotide.
It must be noted that the ENETS guidelines (55) also state that the majority of the NETs of the gastrointestinal tract investigated in phase III studies, the results of which served as the basis for the use of SSAs were small-intestinal (midgut) NETs; moreover, there is little scientific evidence to support the efficacy of SSAs against foregut and hindgut NETs, and hindgut NETs which are prevalent among Asians, including the Japanese, are often considered to have a relatively poor prognosis (55). Therefore, new evidence is required for selecting the optimal drugs and sequence of treatment for hindgut NETs.
Further areas of research
The standard treatment for NET-G1/G2 is considered to be monotherapy with an SSA or molecularly targeted drug, starting with the less toxic SSAs and switching to molecularly targeted drugs if the disease progresses after treatment with SSAs (5, 6). However, it is suggested that alternative front-line systemic therapy or liver-directed therapy should be considered for more aggressive cases, as specified in the NCCN guidelines (90). In NETs, the mTOR pathway is activated; therefore, use of everolimus in combination with SSAs, which inhibit the PI3K/mTOR pathway, would be expected to yield a superior antitumor effect to everolimus alone (94–96). In fact, in expectation of this synergistic effect, trials are being conducted of combined everolimus plus SSA therapy for pancreatic NETs and NETs of the gastrointestinal tract (Table 3).
Table 3.
Summary of studies on everolimus combined with SSAs
| Study | Objects | Setting | Treatment and dose | mPFS |
|---|---|---|---|---|
| RADIANT-1 (86) | Pancreatic NET | Non-randomized phaseIIstudy | Everolimus plus octreotide LAR vs. everolimus | 16.7 vs. 9.7 m |
| RADIANT-2 (89) | GI-NET (functioning) | Randomized phase III study | Everolimus plus octreotide LAR vs. everolimus | 16.0 m |
| EVERLAR (85) | GI-NET | Single-arm phaseIIstudy | Everolimus plus octreotide LAR | 20.3 m |
| ITMO (86) | Pancreas/GI/lung | Single-arm phaseIIstudy | Everolimus plus octreotide LAR | 33.6 m |
| STARTER-NET (88) | GEP-NET | Randomized phase III study | Everolimus vs. everolimus plus lanreotide | Ongoing |
SSAs, somatostatin analogs; GI, gastrointestinal; GEP-NET, gastroenteropancreatic neuroendocrine tumor; LAR, long-acting release.
In the RADIANT-1 study (97), which was a non-randomized phase II study, treatment with everolimus at 10 mg/day plus octreotide LAR yielded a median PFS of 16.7 months, whereas that with everolimus at 10 mg/day alone yielded a median PFS of 9.7 months in patients with metastatic pancreatic NETs. In the EVERLAR study (98), which was a single-arm phase II study, treatment with everolimus at 10 mg/day plus octreotide LAR yielded a median PFS of 20.3 months in patients with advanced, nonfunctional, well-differentiated gastrointestinal NETs. In the ITMO study (99), treatment with everolimus at 10 mg/day plus octreotide LAR yielded a median TTP of 33.6 months in patients with advanced, well-differentiated gastrointestinal NETs. The phase III RADIANT-2 trial (100) compared everolimus plus octreotide LAR with placebo in patients with advanced NETs associated with carcinoid syndrome. The P value of the improved median PFS was marginally over the prespecified threshold for statistical significance.
Based on this background, the Japan Clinical Oncology Group (JCOG) is conducting a multicenter, randomized, controlled, phase III trial named STARTER-NET (JCOG1901) to confirm the superiority of combined everolimus plus lanreotide therapy over everolimus monotherapy for unresectable or recurrent non-functioning GEP-NETs with poor prognostic factors (Ki-67 LI 5–20% or Ki-67 LI <5% with diffuse bilobar liver metastases) in terms of the PFS (jRCT1031200023) (101) (Fig. 2). If this trial proves the superiority of everolimus plus lanreotide in terms of the PFS, it will lead to the establishment of a novel standard therapy both in Japan and elsewhere and enable efficient use of different therapies.
Figure 2.
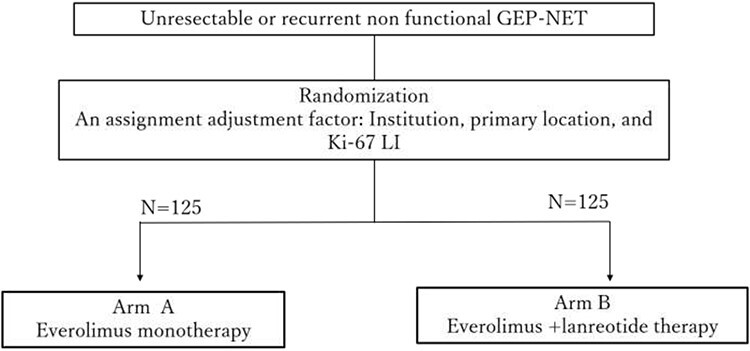
STARTER-NET study scheme This randomized phase III trial in Japan is to confirm the superiority of combined everolimus plus lanreotide therapy over everolimus monotherapy in patients with unresectable or recurrent gastroenteropancreatic NETs with poor prognostic factors (Ki-67 LI: LI 5–20% or Ki-67 LI < 5% with diffuse liver metastases).
After curative surgery, there is no indication for specific medical treatment (102). Even though there are some known factors associated with a higher risk for relapse, there is no approved strategy at present to improve the outcomes in patients with these risk factors. Therefore, a discussion of adjuvant treatment(s) for GEP-NETs is of paramount importance (103). Gao et al. (104) established risk stratification for recurrence of patients with resected pancreatic NETs and recommend clinical trials of post-operative adjuvant treatments in patients with a very high risk of recurrence. Until date, there is one retrospective study of adjuvant therapy for resected GEP-NETs using SSAs or cytotoxic agents (105); this study concluded that adjuvant therapy for resected GEP-NETs is negatively associated with the RFS and confers no OS benefit. However, this was a retrospective cohort study, and some selection bias could have affected the results. Similar results for adjuvant chemotherapy using streptozotocin +5FU, including the low number of survival events after resection of liver metastases in patients with digestive tract NETs, were reported in another retrospective registry-based series, in which multivariate analysis revealed no improvement of the RFS or OS (106). Future studies should focus on patient subsets who may benefit from adjuvant therapy. Additional data regarding time-to-recurrence and OS of patients with resected NETs will be necessary to design adequately powered studies in this setting (107). However, the most promising chemotherapy regimen for the adjuvant setting still remains unknown.
In addition, there is no known role for systemic treatment in the neoadjuvant setting for resectable disease or surgical resection after systemic treatment for unresectable GEP-NETs, the so-called conversion surgery. In regard to conversion surgery, there are some reports that cytotoxic neoadjuvant chemotherapy allowed resection in some cases of highly locally advanced GEP-NETs (108). However, there are no reports of cases of GEP-NETs with distant metastasis in which systemic neoadjuvant treatment resulted in conversion to resectable disease (102). The absence of any regimens for GEP-NETs that have been shown to elicit high RRs of over 30% may be one of the reasons. In order to establish conversion surgery as the standard of care for unresectable GEP-NETs, systemic treatments that can elicit higher RRs would need to be developed.
Conclusion
NETs constitute a clinically heterogeneous group of tumors and even experts face challenges in selecting the optimal multidisciplinary approach. We would like to emphasize the wide spectrum of clinical behaviors of these tumors, ranging from indolent to aggressive, so that personalized treatment approaches, based on risk–benefit evaluation on a per-patient basis, are needed.
Contributor Information
Susumu Hijioka, Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital, Tokyo, Japan.
Chigusa Morizane, Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital, Tokyo, Japan.
Masafumi Ikeda, Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital East, Kashiwa, Japan.
Hiroshi Ishii, Department of Gastroenterology, Chiba Cancer Center, Chiba, Japan.
Takuji Okusaka, Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital, Tokyo, Japan.
Junji Furuse, Department of Medical Oncology, Kyorin University Faculty of Medicine, Tokyo, Japan.
Funding
This study was supported by the National Cancer Center Research and Development Fund and provided by the Japan Pancreas Society.
Conflict of interest statement
None declared.
References
- 1. Klöppel G, Lloyd RV, Rosai J, Osamura RY. WHO Classification of Tumours of Endocrine Organs. In: 4th edn. Lyon, France: World Health Organization, 2017.
- 2. Board WCoTE . WHO Classification of Tumours of the Digestive System. In: 5th edn. Lyon, France: World Health Organization, 2019.
- 3. Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–72. [DOI] [PubMed] [Google Scholar]
- 4. Masui T, Ito T, Komoto I, Uemoto S. Recent epidemiology of patients with gastro-entero-pancreatic neuroendocrine neoplasms (GEP-NEN) in Japan: a population-based study. BMC Cancer 2020;20:1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol 2015;50:58–64. [DOI] [PubMed] [Google Scholar]
- 6. Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut Tumors: a report from the PROMID study group. J Clin Oncol 2009;27:4656–63. [DOI] [PubMed] [Google Scholar]
- 7. Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224–33. [DOI] [PubMed] [Google Scholar]
- 8. Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med 2017;376:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stueven AK, Kayser A, Wetz C, et al. Somatostatin analogues in the treatment of neuroendocrine tumors: past, present and future. Int J Mol Sci 2019;20:3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mizutani G, Nakanishi Y, Watanabe N, et al. Expression of somatostatin receptor (SSTR) subtypes (SSTR-1, 2A, 3, 4 and 5) in neuroendocrine tumors using real-time RT-PCR method and immunohistochemistry. Acta Histochem Cytochem 2012;45:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hasskarl J, Kaufmann M, Schmid HA. Somatostatin receptors in non-neuroendocrine malignancies: the potential role of somatostatin analogs in solid tumors. Future Oncol 2011;7:895–913. [DOI] [PubMed] [Google Scholar]
- 12. Sideris L, Dube P, Rinke A. Antitumor effects of somatostatin analogs in neuroendocrine tumors. Oncologist 2012;17:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strosberg J, Kvols L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J Gastroenterol 2010;16:2963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. K O. Antitumor effects of octreotide LAR, a somatostatin analog. Nat Rev Endocrinol 2010;6:188–9. [DOI] [PubMed] [Google Scholar]
- 15. Theodoropoulou M, Stalla GK. Somatostatin receptors: from signaling to clinical practice. Front Neuroendocrinol 2013;34:228–52. [DOI] [PubMed] [Google Scholar]
- 16. Rinke A, Wittenberg M, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): results of long-term survival. Neuroendocrinology 2017;104:26–32. [DOI] [PubMed] [Google Scholar]
- 17. Shen C, Shih YC, Xu Y, Yao JC. Octreotide long-acting repeatable use among elderly patients with carcinoid syndrome and survival outcomes: a population-based analysis. Cancer 2014;120:2039–49. [DOI] [PubMed] [Google Scholar]
- 18. Shen C, Shih YC, Xu Y, Yao JC. Octreotide long-acting repeatable among elderly patients with neuroendocrine tumors: a survival analysis of SEER-Medicare data. Cancer Epidemiol Biomark Prev 2015;24:1656–65. [DOI] [PubMed] [Google Scholar]
- 19. Ferolla P, Faggiano A, Grimaldi F, et al. Shortened interval of long-acting octreotide administration is effective in patients with well-differentiated neuroendocrine carcinomas in progression on standard doses. J Endocrinol Investig 2012;35:326–31. [DOI] [PubMed] [Google Scholar]
- 20. Caplin ME, Pavel M, Ćwikła JB, et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr Relat Cancer 2016;23:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ito T, Fujimori N, Honma Y, et al. Long-term safety and efficacy of lanreotide autogel in Japanese patients with neuroendocrine tumors: final results of a phase II open-label extension study. Asia Pac J Clin Oncol 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vinik AI, Wolin EM, Liyanage N, Gomez-Panzani E, Fisher GA. Evaluation of LANREOTIDE depot/AUTOGEL efficacy and safety as a carcinoid syndrome treatment (elect): a randomized, double-blind, placebo-controlled trial. Endocr Pract 2016;22:1068–80. [DOI] [PubMed] [Google Scholar]
- 23. Faggiano A, Carratu AC, Guadagno E, et al. Somatostatin analogues according to Ki67 index in neuroendocrine tumours: an observational retrospective-prospective analysis from real life. Oncotarget 2016;7:5538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palazzo M, Lombard-Bohas C, Cadiot G, et al. Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumors. Eur J Gastroenterol Hepatol 2013;25:232–8. [DOI] [PubMed] [Google Scholar]
- 25. Gomes-Porras M, Cárdenas-Salas J, Álvarez-Escolá C. Somatostatin analogs in clinical practice: a review. Int J Mol Sci 2020;21:1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 2008;26:3403–10. [DOI] [PubMed] [Google Scholar]
- 27. Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501–13. [DOI] [PubMed] [Google Scholar]
- 28. Grande E, Capdevila J, Castellano D, et al. Pazopanib in pretreated advanced neuroendocrine tumors: a phase II, open-label trial of the Spanish task force Group for Neuroendocrine Tumors (GETNE). Ann Oncol 2015;26:1987–93. [DOI] [PubMed] [Google Scholar]
- 29. Bergsland EK, Mahoney MR, Asmis TR, et al. Prospective randomized phase II trial of pazopanib versus placebo in patients with progressive carcinoid tumors (CARC) (Alliance A021202). J Clin Oncol 2019;37:4005. [Google Scholar]
- 30. Chan JA, Faris JE, Murphy JE, et al. Phase II trial of cabozantinib in patients with carcinoid and pancreatic neuroendocrine tumors (pNET). J Clin Oncol 2017;35:228. [Google Scholar]
- 31. Capdevila J, Fazio N, Lopez CL, et al. Final results of the TALENT trial (GETNE1509): a prospective multicohort phase II study of lenvatinib in patients (pts) with G1/G2 advanced pancreatic (panNETs) and gastrointestinal (giNETs) neuroendocrine tumors (NETs). J Clin Oncol 2019;37:4106. [Google Scholar]
- 32. Xu J, Shen L, Bai C, et al. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:1489–99. [DOI] [PubMed] [Google Scholar]
- 33. Pozas J, San Román M, Alonso-Gordoa T, et al. Targeting angiogenesis in pancreatic neuroendocrine tumors: resistance mechanisms. Int J Mol Sci 2019;20:4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Faivre S, Niccoli P, Castellano D, et al. Sunitinib in pancreatic neuroendocrine tumors: updated progression-free survival and final overall survival from a phase III randomized study. Ann Oncol 2017;28:339–43. [DOI] [PubMed] [Google Scholar]
- 35. Ito T, Okusaka T, Nishida T, et al. Phase II study of sunitinib in Japanese patients with unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumor. Investig New Drugs 2013;31:1265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu J, Shen L, Zhou Z, et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:1500–12. [DOI] [PubMed] [Google Scholar]
- 37. Yao JC. Neuroendocrine tumors. Molecular targeted therapy for carcinoid and islet-cell carcinoma. Best Pract Res Clin Endocrinol Metab 2007;21:163–72. [DOI] [PubMed] [Google Scholar]
- 38. Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yao JC, Pavel M, Lombard-Bohas C, et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 study. J Clin Oncol 2016;34:3906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016;387:968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singh S, Carnaghi C, Buzzoni R, et al. Everolimus in neuroendocrine tumors of the gastrointestinal tract and unknown primary. Neuroendocrinology 2018;106:211–20. [DOI] [PubMed] [Google Scholar]
- 42. Kiesewetter B, Raderer M. How I treat neuroendocrine tumours. ESMO Open 2020;5:e000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes 1994;43:1326–33. [DOI] [PubMed] [Google Scholar]
- 44. Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics 2007;8:113–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med 1980;303:1189–94. [DOI] [PubMed] [Google Scholar]
- 46. Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 1992;326:519–23. [DOI] [PubMed] [Google Scholar]
- 47. Cheng PN, Saltz LB. Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer 1999;86:944–8. [PubMed] [Google Scholar]
- 48. McCollum AD, Kulke MH, Ryan DP, et al. Lack of efficacy of streptozocin and doxorubicin in patients with advanced pancreatic endocrine tumors. Am J Clin Oncol 2004;27:485–8. [DOI] [PubMed] [Google Scholar]
- 49. Delaunoit T, Ducreux M, Boige V, et al. The doxorubicin-streptozotocin combination for the treatment of advanced well-differentiated pancreatic endocrine carcinoma; a judicious option? Eur J Cancer 2004;40:515–20. [DOI] [PubMed] [Google Scholar]
- 50. Bundschuh RA, Habacha B, Lütje S, Essler M. Therapy of patients with neuroendocrine neoplasia-evidence-based approaches and new horizons. J Clin Med 2019;8:1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shibuya H, Hijioka S, Sakamoto Y, et al. Multi-center clinical evaluation of streptozocin-based chemotherapy for advanced pancreatic neuroendocrine tumors in Japan: focus on weekly regimens and monotherapy. Cancer Chemother Pharmacol 2018;82:661–8. [DOI] [PubMed] [Google Scholar]
- 52. Aoki T, Kokudo N, Komoto I, et al. Streptozocin chemotherapy for advanced/metastatic well-differentiated neuroendocrine tumors: an analysis of a multi-center survey in Japan. J Gastroenterol 2015;50:769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hijioka S, Sakuma K, Aoki M, et al. Clinical and in vitro studies of the correlation between MGMT and the effect of streptozocin in pancreatic NET. Cancer Chemother Pharmacol 2019;83:43–52. [DOI] [PubMed] [Google Scholar]
- 54. Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: eastern cooperative oncology group study E1281. J Clin Oncol 2005;23:4897–904. [DOI] [PubMed] [Google Scholar]
- 55. Pavel M, O'Toole D, Costa F, et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 2016;103:172–85. [DOI] [PubMed] [Google Scholar]
- 56. Kunz PLCP, Nimeiri H, Fisher GA, Longacre TA, Suarez CJ. A trial of the ECOG-ACRIN cancer research group (E2211). J Clin Oncol 2018;36:4004. [Google Scholar]
- 57. Claringbold PG, Turner JH. Pancreatic neuroendocrine tumor control: durable objective response to combination 177Lu-Octreotate-Capecitabine-Temozolomide Radiopeptide Chemotherapy. Neuroendocrinology 2016;103:432–9. [DOI] [PubMed] [Google Scholar]
- 58. Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol 2013;24:152–60. [DOI] [PubMed] [Google Scholar]
- 59. Hijioka S, Hosoda W, Matsuo K, et al. Rb loss and KRAS mutation are predictors of the response to platinum-based chemotherapy in pancreatic neuroendocrine neoplasm with grade 3: a Japanese Multicenter Pancreatic NEN-G3 Study. Clin Cancer Res 2017;23:4625–32. [DOI] [PubMed] [Google Scholar]
- 60. Hijioka S, Hosoda W, Morizane C, Mizuno N, Hara K, Okusaka T. The diagnosis and treatment of pancreatic NEN-G3—a focus on clinicopathological difference of NET-G3 and NEC G3. JOP S 2018;3:346–53. [Google Scholar]
- 61. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 64. Borga C, Businello G, Murgioni S, et al. Treatment personalization in gastrointestinal neuroendocrine tumors. Curr Treat Options in Oncol 2021;22:29. [DOI] [PubMed] [Google Scholar]
- 65. Weber MM, Fottner C. Immune checkpoint inhibitors in the treatment of patients with neuroendocrine neoplasia. Oncol Res Treat 2018;41:306–12. [DOI] [PubMed] [Google Scholar]
- 66. Kim ST, Ha SY, Lee S, et al. The impact of PD-L1 expression in patients with metastatic GEP-NETs. J Cancer 2016;7:484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Puccini A, Poorman K, Salem ME, et al. Comprehensive genomic profiling of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs). Clin Cancer Res 2020;26:5943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cavalcanti E, Armentano R, Valentini AM, Chieppa M, Caruso ML. Role of PD-L1 expression as a biomarker for GEP neuroendocrine neoplasm grading. Cell Death Dis 2017;8:e3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. da Silva A, Bowden M, Zhang S, et al. Characterization of the neuroendocrine tumor immune microenvironment. Pancreas 2018;47:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mehnert JM RH, O'Neil BH, Santoro A, Schellens JHM, Cohen RB. 427OPembrolizumab for patients with PD-L1–positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Ann Oncol 2020;126:3021–30. [DOI] [PubMed] [Google Scholar]
- 71. Strosberg J, Mizuno N, Doi T, et al. Efficacy and safety of pembrolizumab in previously treated advanced neuroendocrine tumors: results from the phase II KEYNOTE-158 study. Clin Cancer Res 2020;26:2124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yao JC, Strosberg J, Fazio N, et al. Spartalizumab in metastatic, well/poorly-differentiated neuroendocrine neoplasms. Endocr Relat Cancer 2021;ERC–20–0382.R1. doi: 10.1530/ERC-20-0382. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 73. Halperin DM, Liu S, Dasari A, et al. A phase II trial of atezolizumab and bevacizumab in patients with advanced, progressive neuroendocrine tumors (NETs). J Clin Oncol 2020;38:619. [Google Scholar]
- 74. Klein O, Kee D, Markman B, et al. Immunotherapy of ipilimumab and nivolumab in patients with advanced neuroendocrine tumors: a subgroup analysis of the CA209-538 clinical trial for rare cancers. Clin Cancer Res 2020;26:4454–9. [DOI] [PubMed] [Google Scholar]
- 75. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353–65. [DOI] [PubMed] [Google Scholar]
- 76. Kwekkeboom DJ, Krenning EP. Peptide receptor radionuclide therapy in the treatment of neuroendocrine tumors. Hematol Oncol Clin North Am 2016;30:179–91. [DOI] [PubMed] [Google Scholar]
- 77. Baum RP, Kluge AW, Kulkarni H, et al. [(177)Lu-DOTA](0)-D-Phe(1)-Tyr(3)-octreotide ((177)Lu-DOTATOC) for peptide receptor radiotherapy in patients with advanced neuroendocrine tumours: a phase-II study. Theranostics 2016;6:501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pfeifer AK, Gregersen T, Grønbæk H, et al. Peptide receptor radionuclide therapy with Y-DOTATOC and (177)Lu-DOTATOC in advanced neuroendocrine tumors: results from a Danish cohort treated in Switzerland. Neuroendocrinology 2011;93:189–96. [DOI] [PubMed] [Google Scholar]
- 79. Campana D, Capurso G, Partelli S, et al. Radiolabelled somatostatin analogue treatment in gastroenteropancreatic neuroendocrine tumours: factors associated with response and suggestions for therapeutic sequence. Eur J Nucl Med Mol Imaging 2013;40:1197–205. [DOI] [PubMed] [Google Scholar]
- 80. Dumont RA, Seiler D, Marincek N, et al. Survival after somatostatin based radiopeptide therapy with (90)Y-DOTATOC vs. (90)Y-DOTATOC plus (177)Lu-DOTATOC in metastasized gastrinoma. Am J Nucl Med Mol Imaging 2015;5:46–55. [PMC free article] [PubMed] [Google Scholar]
- 81. Hörsch D, Ezziddin S, Haug A, et al. Effectiveness and side-effects of peptide receptor radionuclide therapy for neuroendocrine neoplasms in Germany: a multi-institutional registry study with prospective follow-up. Eur J Cancer 2016;58:41–51. [DOI] [PubMed] [Google Scholar]
- 82. Bertani E, Fazio N, Radice D, et al. Resection of the primary tumor followed by peptide receptor radionuclide therapy as upfront strategy for the treatment of G1-G2 pancreatic neuroendocrine tumors with unresectable liver metastases. Ann Surg Oncol 2016;23:981–9. [DOI] [PubMed] [Google Scholar]
- 83. Kunikowska J, Pawlak D, Bąk MI, Kos-Kudła B, Mikołajczak R, Królicki L. Long-term results and tolerability of tandem peptide receptor radionuclide therapy with (90)Y/(177)Lu-DOTATATE in neuroendocrine tumors with respect to the primary location: a 10-year study. Ann Nucl Med 2017;31:347–56. [DOI] [PubMed] [Google Scholar]
- 84. Sharma N, Naraev BG, Engelman EG, et al. Peptide receptor radionuclide therapy outcomes in a North American cohort with metastatic well-differentiated neuroendocrine tumors. Pancreas 2017;46:151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Starr JS, Sonbol MB, Hobday TJ, Sharma A, Kendi AT, Halfdanarson TR. Peptide receptor radionuclide therapy for the treatment of pancreatic neuroendocrine tumors: recent insights. Onco Targets Ther 2020;13:3545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sorbye H, Kong G, Grozinsky-Glasberg S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr Relat Cancer 2020;27:R67–r77. [DOI] [PubMed] [Google Scholar]
- 87. Pellat A, Coriat R. Well differentiated grade 3 neuroendocrine tumors of the digestive tract: a narrative review. J Clin Med 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys 2005;63:655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pavel M, Öberg K, Falconi M, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:844–60. [DOI] [PubMed] [Google Scholar]
- 90.NCCN Guidelines v. Neuroendocrine and Adrenal Tumors.
- 91. Pavel M, O' Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, et al. Consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (nen) and nen of unknown primary site. Neuroendocrinology 2016. [DOI] [PubMed] [Google Scholar]
- 92. Yao J, Phan AT. Optimising therapeutic options for patients with advanced pancreatic neuroendocrine tumours. Eur Oncol Haematol 2012;8:217–23. [Google Scholar]
- 93. Ikeda M, Morizane C, Hijioka S, et al. Optimal strategy of systemic treatment for unresectable pancreatic neuroendocrine tumors based upon opinion of Japanese experts. Pancreatology 2020;20:944–50. [DOI] [PubMed] [Google Scholar]
- 94. O'Reilly KE. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 2006;66:1500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Missiaglia E, Dalai I, Barbi S, et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol 2010;28:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (Everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol 2008;26:4311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol 2010;28:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Capdevila J, Teule A, Barriuso J, et al. Phase II study of everolimus and octreotide LAR in patients with nonfunctioning gastrointestinal neuroendocrine tumors: the GETNE1003_EVERLAR study. Oncologist 2019;24:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bajetta E, Catena L, Fazio N, et al. Everolimus in combination with octreotide long-acting repeatable in a first-line setting for patients with neuroendocrine tumors: an ITMO group study. Cancer 2014;120:2457–63. [DOI] [PubMed] [Google Scholar]
- 100. Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 2011;378:2005–12. [DOI] [PubMed] [Google Scholar]
- 101. Shimoyama R, Hijioka S, Mizuno N, et al. Study protocol for a multi-institutional randomized phase III study comparing combined everolimus plus lanreotide therapy and everolimus monotherapy in patients with unresectable or recurrent gastroenteropancreatic neuroendocrine tumors; Japan clinical oncology group study JCOG1901 (STARTER-NET study). Pancreatology 2020;20:1183–8. [DOI] [PubMed] [Google Scholar]
- 102. Yamamoto M, Yoshida M, Furuse J, et al. Clinical practice guidelines for the management of liver metastases from extrahepatic primary cancers 2021. J Hepatobiliary Pancreat Sci 2020. [DOI] [PubMed] [Google Scholar]
- 103. Lamberti G, Manuzzi L, Maggio I, Campana D. Should we lose hope in adjuvant therapy for neuroendocrine tumors?-in response to: adjuvant therapy following resection of gastroenteropancreatic neuroendocrine tumors provides no recurrence or survival benefit. J Surg Oncol 2020;122:570–1. [DOI] [PubMed] [Google Scholar]
- 104. Gao H, Liu L, Wang W, et al. Novel recurrence risk stratification of resected pancreatic neuroendocrine tumor. Cancer Lett 2018;412:188–93. [DOI] [PubMed] [Google Scholar]
- 105. Barrett JR, Rendell V, Pokrzywa C, et al. Adjuvant therapy following resection of gastroenteropancreatic neuroendocrine tumors provides no recurrence or survival benefit. J Surg Oncol 2020;121:1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Maire F, Hammel P, Kianmanesh R, et al. Is adjuvant therapy with streptozotocin and 5-fluorouracil useful after resection of liver metastases from digestive endocrine tumors? Surgery 2009;145:69–75. [DOI] [PubMed] [Google Scholar]
- 107. Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor Clinical Trials Planning Meeting. J Clin Oncol 2011;29:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Prakash L, Bhosale P, Cloyd J, et al. Role of fluorouracil, doxorubicin, and streptozocin therapy in the preoperative treatment of localized pancreatic neuroendocrine tumors. J Gastrointest Surg 2017;21:155–63. [DOI] [PubMed] [Google Scholar]


