Abstract
Interleukins (ILs) are cytokines with crucial functions in innate and adaptive immunity. IL genes are only found in vertebrates, except for IL-16, which has been cloned in some arthropod species. However, the function of this gene in invertebrates is unknown. In the present study, an IL-16–like gene (EsIL-16) was identified from the Chinese mitten crab Eriocheir sinensis. EsIL-16 was predicted to encode a precursor (proEsIL-16) that shares similarities with pro-IL-16 proteins from insects and vertebrates. We show that caspase-3 processes proEsIL-16 into an approximately 144-kDa N-terminal prodomain with nuclear import activity and an approximately 34-kDa mature peptide that might be secreted into the extracellular region. EsIL-16 mRNA could be detected in all analyzed tissues and was significantly upregulated after immune challenge both in vitro and in vivo. T7 phage display library screening suggested potential binding activity between EsIL-16 and integrin, which was confirmed by coimmunoprecipitation assay. Interestingly, EsIL-16 promoted cell proliferation via integrin β1 in primary cultured crab hemocytes and Drosophila S2 cells. Furthermore, the interaction between EsIL-16 and integrin β1 was necessary to efficiently protect the host from bacterial infection. To our knowledge, this study revealed integrin β1 as a receptor for IL-16 and the function of this interaction in hemocyte proliferation in invertebrates for the first time. These results provide new insights into the regulation of innate immune responses in invertebrates and shed the light on the evolution of ILs within the animal kingdom.
Keywords: interleukin-16, integrin β1, cell proliferation, invertebrate
Abbreviations: BSA, bovine serum albumin; cDNA, complementary DNA; CFSE, carboxyfluorescein diacetate succinimidyl ester; Co-IP, coimmunoprecipitation; EdU, 5-ethynyl-2′-deoxyuridine; HA, hemagglutinin; HEK293T, human embryonic kidney 293T; IL, interleukin; PBST, PBS with Tween-20; qRT-PCR, quantitative RT-PCR; TBS, Tris-buffered saline
It has become the central dogma of evolutionary immunologists that invertebrates, in the absence of “true” lymphocytes and functional antibodies, rely entirely on innate immunity as their primary mechanism of defense against parasites and pathogens (1). Arthropods, which comprise insects, crustaceans, and related forms, are a highly successful group of protostomate invertebrates about which a great deal is known concerning their immune systems and diseases (2). In general, arthropods use a range of cellular and humoral defenses to protect themselves from disease agents that manage to gain access to their internal tissues by penetrating the exoskeleton/cuticle or alimentary canal (3). By contrast, vertebrates have both an acquired response and an innate system of defense; however, even within innate defense systems, extensive differences exist between vertebrates and invertebrates (4). For example, the interleukin (IL) superfamily members are cytokines produced and secreted mainly by CD3+ and CD4+ T lymphocytes, which promote development and differentiation of immune-related cells, are involved in systemic inflammation and immune system modulation, and thus play crucial roles in fighting infections and other diseases (5, 6, 7). To date, only a few IL genes have been traced evolutionarily back to invertebrates.
IL-16 is a cytokine originally designated as a lymphocyte chemoattractant factor and is characterized as an essential regulator of various cellular processes, including cell recruitment and activation (8, 9). IL-16 is generated as a precursor molecule (pro-IL-16) that appears to be predominantly produced by CD8+ and CD4+ T lymphocytes; however, other cells, including eosinophils, dendritic cells, mast cells, macrophages, B cells, and monocytes, also show high levels of IL-16 secretion under certain conditions (10, 11). IL-16 contains PDZ domains in the C-terminal region, which plays a key role in the formation and function of signal transduction, and might represent the signature of IL-16 molecules (12). IL-16 homologs have been well characterized in vertebrates, which show common functions associated with the recruitment of CD4+ or CD9+ immune cells to sites of inflammation, and function as the ligand for CD4 molecule (13, 14, 15). However, few reports have clarified the existence and function of IL-16 in arthropods. An IL-16–like gene was characterized and revealed to respond to bacteria and virus infection in shrimp (Litopenaeus vannamei) (16). Recently, pro-IL-16 was found to be cleaved to its bioactive form, mature IL-16, after bacterial challenge in crab (Scylla paramamosain) (17). Collective data verify the existence of IL-16 in arthropods; however, the immunological functions of this important molecule, as well as the receptor for IL-16 in arthropods and other invertebrates, still remain largely unclear.
Recent studies identified the expression pattern of IL-16 in mud crab (17) and suggested that IL-16 might regulate antibacterial immune responses positively in shrimp (16). The Chinese mitten crab (Eriocheir sinensis) represents a good model to study innate immunity within arthropods, mainly because of its clear genomic background (18, 19) and high commercial value (approximately US $9.54 billion per year). In the present study, we identified an IL-16–like gene in Chinese mitten crab, demonstrated its induced expression after bacterial challenge, revealed the maturation process of pro-IL-16, and showed that mature IL-16 could promote hemocyte proliferation via interaction with its cell-membrane receptor, which indeed protected host from bacterial infection. These findings provide new insights into how IL-16 regulates innate immune responses in invertebrates and provide a better understanding of the evolution of ILs from invertebrates to vertebrates.
Results
Complementary DNA cloning and bioinformatics analysis of IL-16
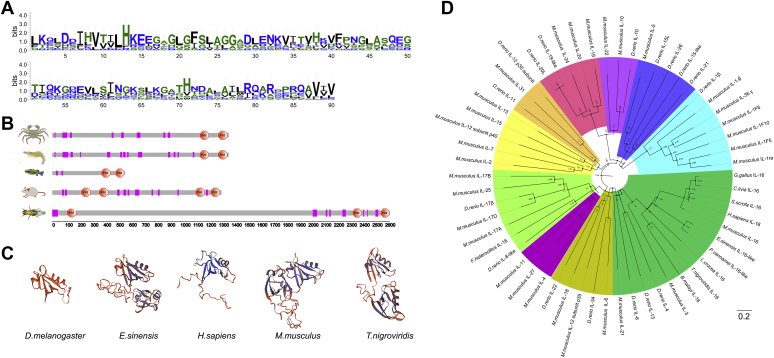
The full-length EsIL-16 complementary DNA (cDNA) was cloned from hemocytes of crab E. sinensis. The EsIL-16 gene contains a short 5′ UTR, a relatively long 3′ UTR, and an ORF. The ORF was predicted to encode a 150.31-kDa protein comprising 1379 amino acids. The EsIL-16 protein contains a potential caspase cleavage site and two PDZ domains at the C-terminal region (Fig. S1). After comparing the EsIL-16 amino acid sequence with its homologs across vertebrates and invertebrates, comparatively high similarities were found among the C-terminal regions (Fig. 1A and Fig. S2), which comprise the mature IL-16 protein, suggesting that IL-16–regulated immunological functions might be partially conserved in different species. The PDZ domain acts as a signature for vertebrate IL-16 proteins, and analysis of the five vertebrate and invertebrate species showed that IL-16 in vertebrates and its homolog IL-16–like protein in invertebrates carry a PDZ domain, mainly at the C terminus, which will be preserved in the mature IL-16 protein. However, mouse IL-16 has four PDZ domains located at the N terminus and C terminus, while the length of Drosophila melanogaster IL-16 amino acid sequence is obviously longer than that of the other species (Fig. 1B). Moreover, the IL-16 protein of D. melanogaster, E. sinensis, Homo sapiens, Mus musculus, and Tetraodon nigroviridis shared comparative similar structure (Fig. 1C). The phylogenetic analysis including numerous IL superfamily members from different vertebrate and invertebrate species showed EsIL-16 clustered with IL-16 from other nine different species (Fig. 1D). However, IL-16 from fish Fundulus heteroclitus was not clustered with IL-16 from the other species, which indicate its different functions, but because of the limited information of IL-16 from F. heteroclitus, perhaps we cannot provide an explanation at this time.
Figure 1.
Pro-IL-16 domain architecture and protein structure.A, differential sequence conservation of IL-16. Sequence logo representation of the conservation of N-terminal region of pro-IL-16 from vertebrate and invertebrate species. Upper panel, bits (y-axis) indicate units of evolutionary conservation. B, diagram of the domain architectures of pro-IL-16 proteins from Eriocheir sinensis, Penaeus vannamei, Tetraodon nigroviridis, Mus musculus, and Drosophila melanogaster. C, a comparison of the 3D structures of pro-IL-16 proteins from aforementioned species. D, phylogenetic analysis of pro-IL-16 and some representative proteins from vertebrate and invertebrate species. The red star indicates Es-pro-IL-16. IL-16, interleukin 16.
Expression pattern of IL-16
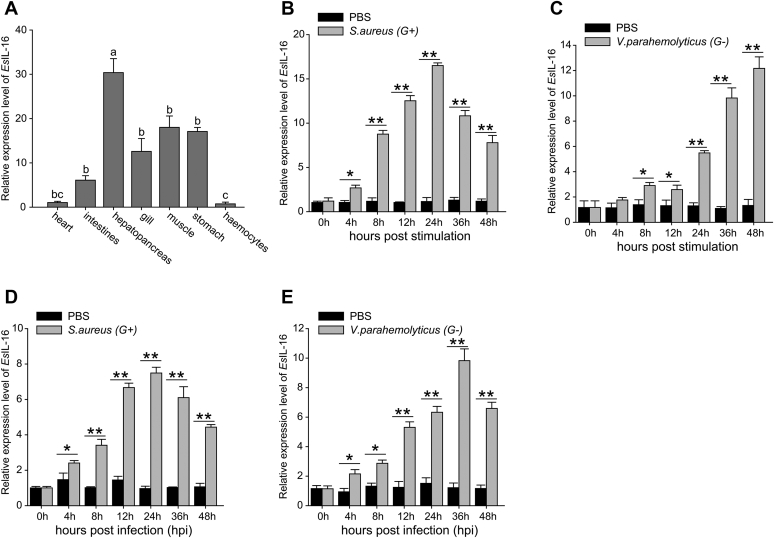
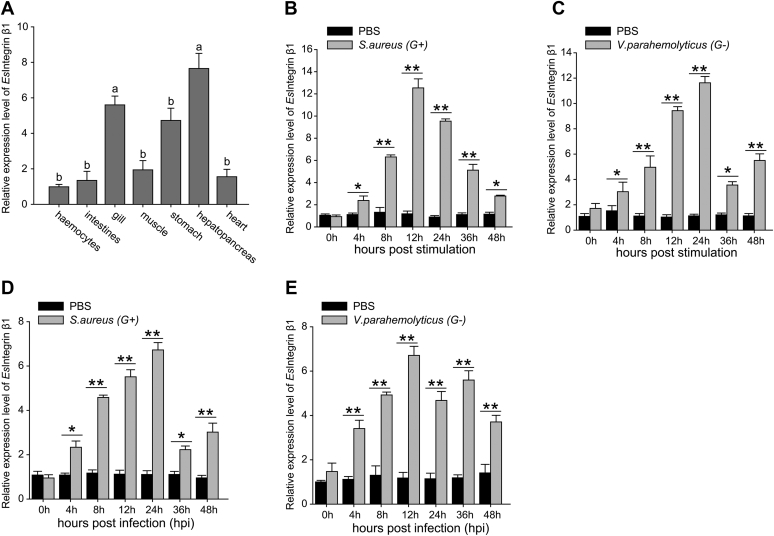
The distribution of the EsIL-16 mRNA in tissues was determined using quantitative RT-PCR (qRT-PCR) (Fig. 2A). EsIL-16 expression was detected in all the examined tissues in E. sinensis. Among these tissues, hemocytes and heart expressed the lowest level of EsIL-16, whereas the hepatopancreas expressed the highest level, which was 30.7 times that in hemocytes. To test whether EsIL-16 expression could be induced after challenge, Staphylococcus aureus and Vibrio parahaemolyticus were used to stimulate primary cultured crab hemocytes in vitro (Fig. 2, B and C) or infect crab in vivo (Fig. 2, D and E). The results showed significantly induced EsIL-16 mRNA expression after challenge with both bacteria, thus demonstrating the potential participation of EsIL-16 in innate immunity.
Figure 2.
Expression pattern of IL-16.A, tissue distribution of IL-16. RNA samples were extracted from healthy Eriocheir sinensis, and EsIL-16 expression was analyzed by quantitative RT-PCR with β-actin as the internal reference. Total RNA was extracted from seven different tissues of crab. Each sample was taken from at least three crabs; data shown are the means ± SD. Three independent repeats were performed (≥5 crabs per sample), with different lowercase letters indicating the significance (one-way ANOVA). B–E, mRNA expression of EsIL-16 in hemocytes as assessed by quantitative RT-PCR over a time course after Staphylococcus aureus (B) or Vibrio parahaemolyticus (C) stimulation in vitro and S. aureus (D) or V. parahaemolyticus (E) infection in vivo. RNA was extracted at 0, 4, 8, 12, 24, 36, and 48 h after bacterial challenge. Bars represent the mean ± SD from three independent samples, with at least five crabs per sample. ∗p < 0.05, ∗∗p < 0.01 (Student's t test). IL-16, interleukin 16.
Detection of IL-16 cytoplasmic fragments and its nuclear or extracellular translocation
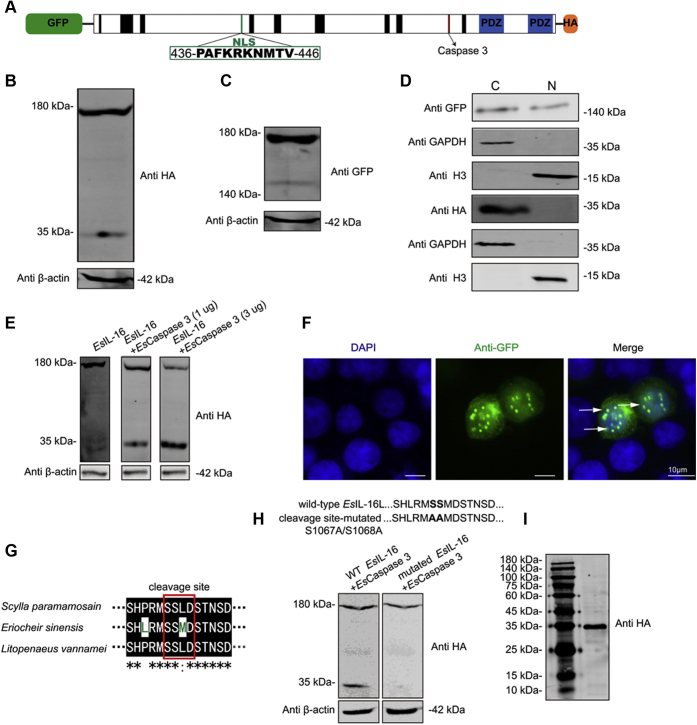
The vertebrate pro-IL-16 is cleaved by caspase-3 following cell activation into an N-terminal prodomain and a C-terminal peptide. The former can translocate into nuclei, whereas the latter is secreted from the cell to form mature IL-16 (20). However, whether the invertebrate IL-16–like protein shares a similar mechanism is unclear. Therefore, we screened the EsIL-16 amino acid sequence and found a conserved nuclear localization signal in the N terminus that might have an essential role in IL-16 prodomain nuclear translocation and predicted a caspase cleavage site at the C terminus, cleavage of which might result in mature IL-16 release (Fig. 3A). After expressing N-terminal GFP-tagged and C-terminal hemagglutinin (HA)-tagged Espro-IL-16 in human embryonic kidney 293T (HEK293T) cells, Western blotting using antibodies against the C-terminal HA showed that the Espro-IL-16 protein comprised a prominent approximately 178-kDa fragment and a smaller fragment of 34 kDa (Fig. 3B), which coincided with the predicted molecular weight of Espro-IL-16 and C-terminal mature IL-16, respectively. Meanwhile, Western blotting using antibodies against N-terminal GFP showed that the Espro-IL-16 protein comprised a prominent approximately 178-kDa fragment as well as a smaller fragment of 144 kDa (Fig. 3C), which coincided with the predicted molecular weight of Espro-IL-16 and the N-terminal EsIL-16 prodomain, respectively. To detect the nuclear or extracellular translocation of EsIL-16 fragments, we analyzed their subcellular localizations and quantified their protein content in the cytoplasm, nucleus, or cell culture medium of HEK293T cells. The 144-kDa N-terminal EsIL-16 prodomain was found in the cytoplasm and nucleus, whereas the 34-kDa C-terminal mature EsIL-16 was found only in the cytoplasm (Fig. 3D). Western blotting showed that increased Escaspase-3 expression level in HEK293T cells led to upregulated cleavage fragment of EsIL-16 (Fig. 3E), and the immunostaining result showed that the EsIL-16 prodomain localized to the nucleus (Fig. 3F). The alignment of amino acids between IL-16 from E. sinensis and the other two crustacean species demonstrated a conserved caspase cleavage site (Fig. 3G). After expressing the C-terminal HA-tagged wildtype EsIL-16 and C-terminal HA-tagged cleavage site–mutated EsIL-16 in HEK293T cells, the Western blot assay showed that the wildtype EsIL-16 (178 kDa) can generate a small protein fragment with the molecular weight of about 34 kDa, but this band was not detected in cleavage site–mutated EsIL-16 (Fig. 3H), which confirm the critical role of these amino acid residues in caspase cleavage, and also suggest the common features of caspase cleavage crustacean IL-16. Moreover, Western blotting revealed secreted mature EsIL-16 in the cell culture medium of HEK293T cells (Fig. 3I).
Figure 3.
Caspase-dependent pro-IL-16 cleavage and secretion.A, schematic representation of HEK293T cells expressed N-terminal GFP-tagged and C-terminal HA-tagged pro-EsIL-16 protein. B and C, Western blotting showing the cleavage products of the (B) C-terminal FLAG-tagged mature EsIL-16 and (C) the N-terminal GFP-tagged EsIL-16 prodomain. D, Western blotting showed the N-terminal GFP-tagged EsIL-16 translocated into nuclei, rather than C-terminal FLAG-tagged mature EsIL-16. E, Western blotting showed that Escaspase-3 is responsible for EsIL-16 cleavage. Cells that only transfected with EsIL-16 cannot generate a small fragment, cells that cotransfected with EsIL-16 and low concentration (1 μg) of Escaspase-3 generate clearly ~34 kDa small fragment, cells that cotransfected with EsIL-16 and high concentration (3 μg) of Escaspase-3 generate a strong ~34 kDa fragment. F, immunostaining shown nuclear imported N-terminal GFP-tagged EsIL-16. G, sequence alignment between caspase-3 cleavage site from three crustacean species showed comparative similar regions from Eriocheir sinensis S1062 to D1075. H, mutated E. sinensis caspase-3 cleavage site with indicated point mutants that displayed in bold and named wildtype EsIL-16 and cleavage site-mutated, respectively (upper panel). Western blot analysis showed after Escaspase-3 (1 μg) cotransfected with wildtype EsIL-16 or mutated EsIL-16, the wildtype EsIL-16 can generate a cleavage protein fragment with molecular weight of about ~34 kDa, but this fragment was absent in cleavage site–mutated EsIL-16 (latter panel). I, Western blotting showed that the C-terminal FLAG-tagged mature EsIL-16 is secreted into the extracellular region. Data are representative of three independent repeats. HA, hemagglutinin; HEK293T, human embryonic kidney 293T; IL-16, interleukin 16.
Interaction between non-PDZ region of IL-16 and integrin beta subunits, N-terminal portion of extracellular region domain of integrin β1
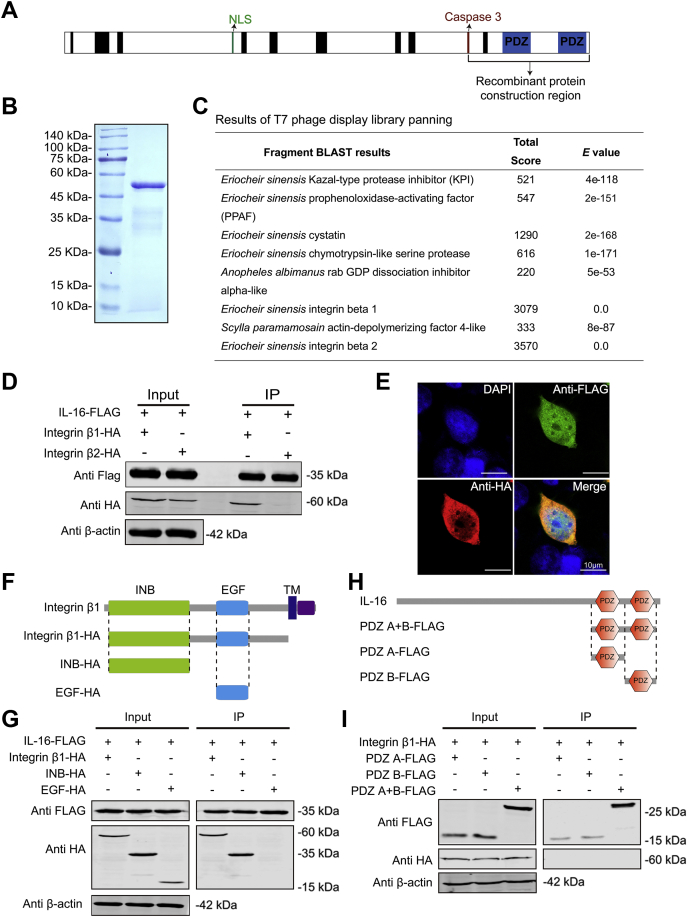
In vertebrates, the receptor of IL-16 has been identified as CD4 molecule (13, 14, 15); however, whole genome sequencing data suggested that there are no CD4 genes within insects (21) and crustacea (22), which indicated that IL-16 might bind with another, as yet unknown, cell-membrane receptor in invertebrates, which motivated us to screen for the IL-16 receptor in E. sinensis. After constructing a recombinant mature EsIL-16 protein (Fig. 4B) that covered the region from the caspase 3 cleavage site to the end of the C terminus (Fig. 4A), a T7 phage display library expressing crab genes was used to screen potential EsIL-16-binding proteins. The results identified some possible binding proteins (Fig. 4C), with integrin β1 and β2 of particular interest because of their roles as cell-membrane receptors, as predicted by the transmembrane region by the TMHMM (Fig. S2) and the signal peptide by the signal-NM methods (Fig. S2).
Figure 4.
Interaction between IL-16 and integrin β1.A, schematic diagram of regions ranging from the caspase 3 cleavage site to the last PDZ domain to construct the recombinant EsIL-16 protein. B and C, recombinant EsIL-16 protein (B)–based T7 phage library (C) screening suggesting the potential interaction between IL-16 and integrin β1. Results for T7 phage library panning, two of eight sequenced fragments were identified as Esintegrin β1/2 based on BLAST analysis. D, coimmunoprecipitation assay results showing the possible interaction between Esintegrin β1, Esintegrin β2, and mature EsIL-16 in HEK293T cells. Anti-HA antibodies were used to detect Esintegrin β1 or Esintegrin β2, anti-Flag antibodies were used to detect EsIL-16 in cotransfected HEK293T cells. The anti-HA and anti-FLAG antibodies were incubated with the cell lysates and then isolated using Protein G-FLAG-m-beads. E, colocalization of EsIL-16 and Esintegrin β1 in HEK293T cells by confocal analysis. F, illustration of different Esintegrin β1 expression plasmids. G, coimmunoprecipitation assay results showing the interaction between different domains of Esintegrin β1 and EsIL-16 in HEK293T cells. Anti-FLAG and anti-HA antibodies were used to detect EsIL-16 and Esintegrin β1 in cotransfected HEK293T cells, respectively. H, illustration of different EsIL-16 expression plasmids. I, coimmunoprecipitation assay results showing the interaction between different domains of EsIL-16 and Esintegrin β1 in HEK293T cells. Anti-FLAG and anti-HA antibodies were used to detect EsIL-16 and Esintegrin β1 in cotransfected HEK293T cells, respectively. Data are representative of three independent repeats. HA, hemagglutinin; HEK293T, human embryonic kidney 293T; IL-16, interleukin 16.
To verify the potential interaction between EsIL-16 and Esintegrin β1 and β2, we conducted coimmunoprecipitation (Co-IP) assays in HEK293T cells by expressing Flag-tagged mature EsIL-16, HA-tagged Esintegrin β1, and HA-tagged Esintegrin β2. The results showed that EsIL-16 bound to Esintegrin β1 rather than to Esintegrin β2 (Fig. 4D). This result was confirmed by confocal analysis that EsIL-16 colocalized with Esintegrin β1 in HEK293T cells (Fig. 4E). To determine the role of the signature domains within Esintegrin β1 for the protein–protein interaction, plasmids that expressed the extracellular region of Esintegrin β1 and their truncated sequences were constructed (Fig. 4F) and expressed in HEK293T cells. Subsequently, Co-IP results showed that EsIL-16 could bind to the integrin beta subunits, N-terminal portion of extracellular region domain of Esintegrin β1 rather than the epidermal growth factor domain (Fig. 4G). To determine the role of the signature PDZ domains within EsIL-16 for the protein–protein interaction, plasmids that expressed each or both PDZ domain were constructed (Fig. 4H) and expressed in HEK293T cells. Subsequently, Co-IP results showed that Esintegrin β1 could not bind with the PDZ domain of EsIL-16 (Fig. 4I), which suggest that the N-terminal region of mature EsIL-16 may act as the binding site for Esintegrin β1.
Expression pattern of integrin β1
The distribution of Esintegrin β1 mRNA in tissues was determined using qRT-PCR (Fig. 5A). The expression of Esintegrin β1 was detected in all examined tissues in E. sinensis. Among these tissues, hemocytes expressed a comparatively lower level of Esintegrin β1, whereas hepatopancreas expressed the highest level, being 7.6 times higher than that in hemocytes. To test whether Esintegrin β1 expression could be induced after challenge, S. aureus and V. parahaemolyticus were used to stimulate primary cultured hemocytes in vitro (Fig. 5, B and C) or infect crab in vivo (Fig. 5, D and E). The results showed significantly induced Esintegrin β1 expression after challenge with both bacteria, thus suggesting its function in innate immunity.
Figure 5.
Expression pattern of integrin β1.A, tissue distribution of Esintegrin β1. RNA samples were extracted from healthy Eriocheir sinensis, and Esintegrin β1 expression was studied by using quantitative RT-PCR with β-actin as the internal reference. Total RNA was extracted from seven different tissues of crab. Each sample was taken from at least three crabs. Data are shown as the means ± SD. Three independent repeats were performed (≥5 crabs per sample), with different lowercase letters indicating the significance (one-way ANOVA). B–E, mRNA expression of Esintegrin β1 in hemocytes was assessed using quantitative RT-PCR over a time course after Staphylococcus aureus (B) or Vibrio parahaemolyticus (C) stimulation in vitro and S. aureus (D) or V. parahaemolyticus (E) infection in vivo. RNA was extracted at 0, 4, 8, 12, 24, 36, and 48 h after bacterial challenge. The bars represent the mean ± SD from three independent samples, with at least five crabs per sample. ∗p < 0.05, ∗∗p < 0.01 (Student's t test).
IL-16 promotes cell proliferation via integrin β1
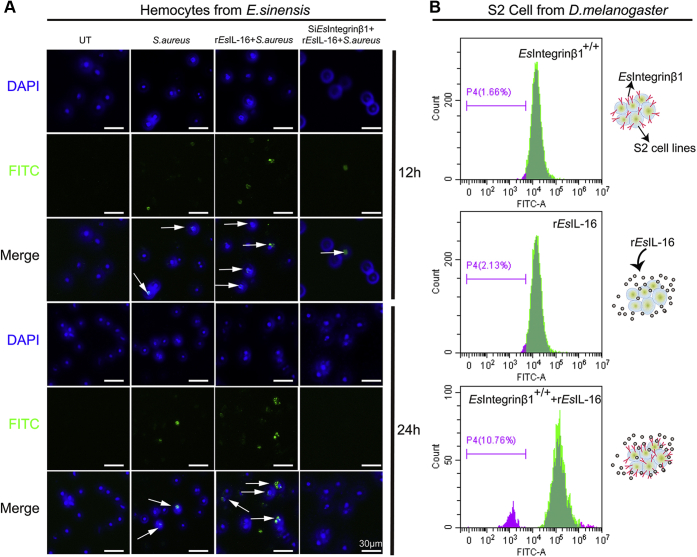
The IL family members have important regulatory functions in cell differentiation and proliferation (6). Therefore, we carried out 5-ethynyl-2′-deoxyuridine (EdU) incorporation assays to assess whether cell proliferation in arthropod immune cells were controlled by IL-16 and integrin β1. The expression of integrin β1 in crab hemocytes was very low; therefore, hemocytes were pretreated with S. aureus to induce high expression of integrin β1. After discarding the original cell culture medium and adding new medium without bacteria, recombinant IL-16 protein was added to the wildtype hemocytes or integrin β1–silenced hemocytes. The levels of EdU incorporation were analyzed at 12 and 48 h after stimulation (Fig. 6A). The results demonstrated that IL-16 significantly promoted hemocyte proliferation, whereas this phenotype was significantly suppressed in integrin β1–silenced hemocytes, which suggested that IL-16 promotes hemocyte proliferation via integrin β1. To confirm the capacity of EsIL-16 to promote cell proliferation via Esintegrin β1, Esintegrin β1 was stably expressed in S2 cells, and the cell proliferation regulated by EsIL-16 was harvested and analyzed for carboxyfluorescein diacetate succinimidyl ester (CFSE) by flow cytometry (Fig. 6B). The results showed that Esintegrin β1–expressing S2 cells or EsIL-16–stimulated wildtype S2 cells did not show cell proliferation, whereas Esintegrin β1–expressing S2 cells that received EsIL-16 stimulation had significantly induced CFSE signal peaks, which demonstrated that IL-16 could also promote S2 cell proliferation via integrin β1.
Figure 6.
IL-16 promotes cell proliferation via integrin β1 in different cell types.A, crab hemocytes proliferation detected by EdU labeling. EdU was applied to untreated hemocytes, Staphylococcus aureus stimulated hemocytes, S. aureus plus rEsIL-16 stimulated hemocytes, and S. aureus plus rEsIL-16 stimulated Esintegrin β1–silenced hemocytes at time points indicated (12 and 48 h). Hemocytes were labeled anti-EdU (green) and anti-DAPI (blue). B, S2 cells proliferation monitored using CFSE labeling. CFSE was used to label the Esintegrin β1–expressed S2 cells, rEsIL-16–stimulated S2 cells, or rEsIL-16–stimulated S2 cells that express Esintegrin β1. After 24 h, cells were harvested and analyzed by flow cytometry. Cells at the right side represent nondivided CFSE-labeled responder cells, whereas the cells at the left side indicate daughter cell population that represent divided CFSE-labeled cells. Data are representative of three independent repeats. CFSE, carboxyfluorescein diacetate succinimidyl ester; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; EdU, 5-ethynyl-2′-deoxyuridine; IL-16; interleukin 16.
IL-16–integrin β1 protects the host from bacterial infection
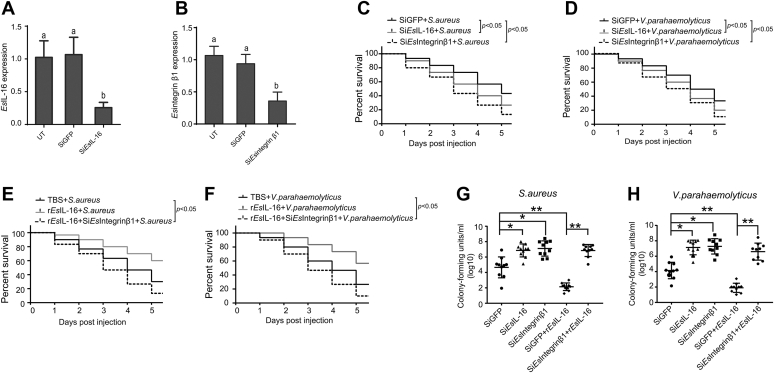
To verify the role of the IL-16–integrin β1 interaction in innate immunity, siEsIL-16 (Fig. 7A) or siEsintegrin β1 (Fig. 7B) was injected into crab hemolymph to knockdown their expression. A crab survival assay showed that EsIL-16– or Esintegrin β1–silenced crab has significantly suppressed survival rate after S. aureus (Fig. 7C) or V. parahaemolyticus (Fig. 7D) infection. Moreover, the two kinds of bacteria were coated with rEsIL-16, in the presence or the absence of siEsintegrin β1, and injected into crabs in vivo. A crab survival assay showed that rEsIL-16 significantly improved survival rate of crabs after both S. aureus (Fig. 7E) or V. parahaemolyticus (Fig. 7F) infection in an Esintegrin β1–dependent manner. Correspondingly, rEsIL-16 significantly suppressed the hemolymph bacterial concentration in an Esintegrin β1–dependent manner after S. aureus (Fig. 7G) or V. parahaemolyticus (Fig. 7H) infection. These results suggested that the IL-16–integrin β1 axis plays critical roles in innate immunity by promoting hemocyte proliferation in vivo.
Figure 7.
The IL-16–integrin β1 axis protects crabs from bacterial infection.A, RNAi of the expression of EsIL-16 in crabs. B, RNAi of the expression of Esintegrin β1 in crabs. Each sample was taken from at least three crabs, and the results are shown as means ± SD. Three independent repeats were performed (≥5 crabs per sample), with different lowercase letters indicating the significance (one-way ANOVA). C and D, EsIL-16 or Esintegrin β1–silenced crabs showed increased susceptibility to Staphylococcus aureus and Vibrio parahaemolyticus infection. Crabs were each injected with siEsIL-16 or siEsintegrin β1 with siGFP as the control, and their 5-day survival post-S. aureus (C) and post-V. parahaemolyticus (D) infection was recorded from three independent repeats of 30 crabs per sample. E and F, EsIL-16 protects crabs from S. aureus or V. parahaemolyticus infection via Esintegrin β1. Recombinant EsIL-16 was used to stimulate crabs, with or without siEsintegrin β1, with TBS as the control, and their 5-day survival post-S. aureus (E) and post-V. parahaemolyticus (F) infection was recorded from three independent repeats of 30 crabs per sample. G and H, EsIL-16 reduces bacterial proliferation via Esintegrin β1 in crabs treated as described previously. From each crab, the hemolymph was drawn at day 4 post-S. aureus (G) and post-V. parahaemolyticus (H) infection and plated onto agar plates for bacterial counting. Shown are the means ± SD. Three independent repeats were performed (≥10 crabs per sample). ∗p < 0.05, ∗∗p < 0.01 (Student's t test). IL-16, interleukin 16; TBS, Tris-buffered saline.
Discussion
Cytokines are important factors to regulate the immune response and inflammation (23). As a proinflammatory cytokine, IL-16 plays a pivotal role in initiating innate and adaptive immune responses and assists in generating a local inflammatory response in vertebrates (8, 11). At present, IL-16–like genes have been widely predicted in the genomes of arthropods; however, currently, there are very limited data on the functional analyses of these genes (16, 17). Here, an IL-16–like gene was identified in crab, and its function in regulating hemocyte proliferation and in protecting the host from bacterial infection was revealed.
The amino acid sequences of the putative arthropod IL-16 proteins, in particular the C-terminal region, analyzed in this study share high similarities with each other. The C-terminal region of EsIL-16 was high homologous to those of the mature vertebrate IL-16 proteins, suggesting that IL-16 could be evolutionarily conserved within vertebrates and invertebrates. Interestingly, at present, IL-16–like genes have not been found in invertebrates other than arthropods, and thus, the evolutionary relationships between vertebrate and arthropod IL-16 genes and the reason why the IL-16 gene is absent in other phyla of invertebrates still remains unclear. In mammals, both the lymphocyte-derived and neuronal IL-16 precursors are processed by caspase-3 to generate a mature IL-16 protein containing a single C-terminal PDZ domain (20). In contrast, EsIL-16 has two PDZ domains in the C-terminal region, and bioinformatics analysis suggested that the potential caspase-3 cleavage site could be located before the first PDZ domain, indicating that processing by caspase-3 could generate a mature EsIL-16 peptide containing two C-terminal PDZ domains. Our study showed that expression of the full-length EsproIL16 cDNA in HEK293T cells also generated a 178-kDa band representing the N-terminal EsIL-16 prodomain and a 34-kDa band representing the C-terminal mature EsIL-16, possibly as a result of caspase-3 cleavage, which has been confirmed in L. vannamei (16), and we also confirmed that caspase-3–mediated IL-16 cleavage in crab uncovered conserved amino acid residues between different crustacean species may act as a critical target for caspase cleavage. Moreover, the EsIL-16 prodomain can translocate into the nucleus, and mature EsIL-16 can be secreted into the extracellular region, which agreed with the results of studies in vertebrates. Taken together, the aforementioned results suggested that although the lengths and domain compositions of the mature IL-16 products were different between arthropods and vertebrates, they employ similar processes to produce the mature protein.
In mammals, IL-16 modulates T cell–mediated inflammation via interaction with CD4 or CD9 in the target cell surface (13, 14, 15). However, genome sequencing data of D. melanogaster (21), L. vannamei (22), and E. sinensis (19) suggested that there is no CD4 gene in the arthropod genome, tsp2A gene in D. melanogaster is a homolog of human CD9 (24), but there are no report on tsp2A in E. sinensis, and we could not find this gene in the genome database of E. sinensis. Thus, we speculated that another receptor might exist to bind arthropod IL-16 and activate downstream signal transduction. Our study is the first to demonstrate the interaction between the transmembrane receptor integrin β1 and IL-16 in invertebrates, which indicated that arthropod IL-16 might have different immune functions compared with those in mammals. Integrins are cell adhesion molecules that mediate cell–cell, cell–extracellular matrix, and cell–pathogen interactions (25). They play critical roles in the immune system, such as leukocyte trafficking and migration, immunological synapse formation, costimulation, and phagocytosis (25). Twenty-four integrin chains consisting of 18 alpha and eight beta subunits have been discovered in humans (26, 27), whereas only five alpha and two beta subunits have been found in fruit fly (28). Human immune cells express at least ten members of the integrin family, belonging to the β2, β7, and β1 subfamilies, among which the β2 and β7 integrins are exclusively expressed on leukocytes, and the β1 integrins are expressed on a wide variety of cells throughout the body. Nevertheless, their diversity in subunit composition contributes to the diversity in ligand recognition, binding to cytoskeletal components, and coupling to downstream signaling pathways (26, 27). Within invertebrate, integrin was first found in the hemocytes of the freshwater crayfish Pacifastacus leniusculus (29), and the later studies are focused on their multiple functions that include embryonic development, gut morphogenesis, and linkage of epithelia cells to extracellular matrix and cuticle (30). Furthermore, integrins have been proved to regulate the innate immune responses in invertebrates. For instance, D. melanogaster integrins play a key role in regulating cell adhesion and proliferation (31, 32, 33), whereas the β integrin domains from Manduca sexta and Pseudoplusia showed strong relationship with cell adhesion, encapsulation, and phagocytosis (34, 35). The interaction of IL-16 and integrin β1 that was found in this study may suggest a different mechanism for IL-16 binding with the receptor between vertebrate and invertebrate and also may shed the light on integrin-regulated multiple immune responses in invertebrate.
Hemocytes play crucial roles in tissue remodeling during embryogenesis and metamorphosis and in immune defenses of arthropod. Drosophila contains three distinct hemocyte types including plasmatocytes, lamellocytes, and crystal cells, which generate distinct immune functions, such as antimicrobial peptide expression, encapsulation, phagocytosis, and melanization (36). Significant progress has been made recently in understanding the genetic control of arthropod hemocyte differentiation during development (37). However, it is still unclear whether crustacean mature hemocytes have the ability to proliferate, whereas of course hematopoietic cells do (38, 39, 40), and information on the mechanisms by which hemocyte proliferation and differentiation are triggered during an immune response within invertebrate have so far remained elusive. Most other studies on hematopoiesis in insects demonstrated that maintenance of hemocyte populations during the larval stage also depends on both the proliferation of cells already in circulation and the release of hemocytes from hematopoietic organs. However, maintenance of mature hemocyte populations via proliferation of cells already in circulation appears to be more important during early stage of infection, whereas the production and release of hemocytes from the hematopoietic organs occurs delay. Several studies implicate Janus kinase/signal transducer and activator of transcription (41) and Pvr signaling (42) in the proliferation of prohemocytes, platelet-derived growth factor/vascular endothelial-like growth factor induces hemocyte proliferation in Drosophila larvae, and differentiation of prohemocytes into plasmatocytes, crystal cells, or lamellocytes requires downregulation of Dome (43). The phenomenon of IL-16 promoting hemocyte proliferation by interacting with integrin β1 has been revealed in this study; however, the downstream molecules, including transcription factors and effectors that contribute to cell proliferation, still remain elusive, and identifying the key molecules that are activated by IL-16/integrin β1 after pathogen infection requires further study.
In summary, under conditions of bacterial infection, the expression levels of IL-16 and integrin β1 were significantly induced to activate immune reactions efficiently. Pro-IL-16 is cleavage by a caspase to generate an N-terminal IL-16 prodomain and a C-terminal mature IL-16 peptide; the former may be translocated into the nucleus, whereas the latter will be secreted into the extracellular region. The secreted mature IL-16 interacts with the integrin beta subunits, N-terminal portion of extracellular region domain of integrin β1, and this ligand/receptor interplay at the cell membrane could promote hemocyte proliferation by activating signaling transduction on the one hand and may induce antimicrobial peptide expression on the other hand (16), which has a synergistic action or an additive action to protect the host from bacterial infection.
Experimental procedures
Animals and primary cultured hemocytes
Healthy Chinese mitten crabs (E. sinensis, 100 ± 10 g per adult) were purchased from the Songjiang aquatic farm. The crabs were transferred rapidly to the laboratory and maintained in aerated and filtered freshwater with an abundance of oxygen and fed daily with a commercially formulated antibiotic-free diet.
Primary culture of E. sinensis hemocytes was performed following established techniques (44). We collected hemolymph from the nonsclerotized membrane of the posterior walking leg using a 10-ml sterile syringe preloaded with 5 ml of precooled sterile anticoagulant (0.14 M NaCl, 0.1 M glucose, 30 mM trisodium citrate, 26 mM citric acid, 10 mM EDTA, and pH 4.6) at a ratio of 1:1. The hemolymph was centrifuged immediately at 300g at 4 °C for 10 min. The supernatant was removed, and the pellet was washed with PBS. The obtained hemocytes were resuspended gently in pure Leibovitz's L-15 medium (Sigma–Aldrich) containing 1% antibiotics (10,000 units/ml penicillin and 10,000 μg/ml streptomycin; Gibco) and 0.2 mM NaCl (676 ± 5.22 mOsm/kg), at pH 7.2 to 7.4, and then counted in an automated cell counter (Countess; Thermo Fisher Scientific). Then, 4 ml (1 × 106 cells/ml) were seeded in 60-mm dishes without any more additives.
D. melanogaster S2 cells were cultured in Drosophila Schneider's medium (Gibco) supplemented with 10% fetal bovine serum and antibiotics. HEK293T cells were cultured in Dulbecco's modified Eagle's medium (Life Technologies) in a humidified incubator at 37 °C and a 5% CO2 atmosphere.
Protein annotation and phylogenetic analysis
The full-length cDNAs of encoding EsIL-16 and Esintegrin β1 were amplified using gene-specific primers based on the sequences in National Center for Biotechnology Information and then resequenced to confirm their accuracy. Similarity analysis was performed using Clustalx from EMBL. SignalP, version 4.1 server (http://www.cbs.dtu.dk/services/SignalP/) and SMART (http://smart.embl-heidelberg.de/) were used to predict the signal peptide and domain architecture, respectively. Swiss-PdbViewer (https://spdbv.vital-it.ch/) predicted the protein 3D structure. A phylogenic tree was constructed based on the deduced amino acid sequences of EsIL-16 and other known IL-16 within vertebrates and invertebrates (Table S2) using the neighbor-joining algorithm in MEGA (Mega Limited, Auckland, New Zealand), version 6.0 software, and bootstrap trials were replicated 1000 times.
Immune stimulation and sample collection
S. aureus and V. parahaemolyticus were obtained from the National Pathogen Collection Center for Aquatic Animals (nos. BYK0113 and BYK00036, respectively; Shanghai Ocean University). S. aureus is used commonly for bacterial infection in vertebrates and invertebrates, and V. parahaemolyticus is one of the most dangerous pathogens for aquatic animals (45). The two bacteria strains were cultured, collected, and resuspended in sterile PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, and pH 7.4). Bacterial counts were determined using the agar plating method.
In vitro and in vivo bacterial challenges were carried following a previously described method (46). For in vitro bacterial stimulation, cultured bacteria (1 × 107 cells per dish, 50 μl) in 50 μl sterile PBS were added separately to a hemocyte-culture dish to serve as the control. Total RNA was collected from hemocytes at specific time points after bacterial stimulation. At least three crabs were used per sample. First-strand cDNA was synthesized using a Reverse Transcriptase Kit (Takara) following the manufacturer's protocol. For in vivo bacterial infection, bacteria (1 × 108 colony-forming units per crab, 200 μl) were injected into the hemolymph from the nonsclerotized membrane of a posterior walking leg that differed from the site for hemolymph collection; sterile PBS (200 μl) was used injected as the control. Total RNA was collected from hemocytes at specific time points after infection. At least three crabs were used per sample.
RNAi assay
The cDNA fragments of EsIL-16 and Esintegrin β1 were first amplified using PCR with primers containing a T7 promoter (Table S1). The cDNAs were then used as the templates to produce siRNA using an in vitro T7 Transcription Kit (Fermentas). The corresponding control GFP siRNA was purchased from GenePharma. For in vitro RNAi, the siRNAs were dissolved in RNase-free water and transfected into E. sinensis primary-cultured hemocytes with the aid of 10 nM Lipofectamine 3000 (Thermo Fisher Scientific). For in vivo RNAi, the siRNAs (1 μg/g) were injected into hemolymph from the nonsclerotized membrane of the posterior walking leg of each crab. The RNAi efficiency was assessed using real-time qRT-PCR, with double-stranded GFP RNA as the control. After setting up the RNAi assay, S. aureus was used to stimulate the gene-silenced hemocytes in vitro, and S. aureus or V. parahaemolyticus was used to infect the gene-silenced crabs in vivo.
Gene expression profile analysis
The relative gene expression levels in treated hemocytes were determined using qRT-PCR and SYBR Premix Ex Taq (Tli RNaseH Plus) and the CFX96 Real-Time System (Bio-Rad) and with gene-specific primers (Table S1). The reaction conditions comprised 94 °C for 3 min; followed by 40 cycles of 94 °C for 10 s and 60 °C for 1 min; and then melting curve analysis from 65 to 95 °C. The 2−ΔΔCT method was used to normalize the obtained data to that of the control group. Three independent experiments were conducted, with results expressed as the mean ± SD.
Western blotting
Protein samples obtained from HEK293T cells were quantified using the Coomassie Plus protein assay reagent (Thermo Fisher Scientific). HEK293T cells were lysed in radioimmunoprecipitation assay lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, sodium orthovanadate, sodium fluoride, EDTA, and leupeptin) containing protease and phosphatase inhibitor mixtures (Roche Applied Science). Cytoplasmic and nuclear proteins were extracted using a Nuclear Protein Extraction Kit (Beyotime) according to the manufacturer's instructions. Protein concentrations were quantified using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). The proteins were then separated using 10% SDS-PAGE and then transferred to a nitrocellulose membrane. The membrane was blocked for 1 to 2 h using 3% nonfat milk in Tris-buffered saline (TBS) (10 mM Tris–HCl, pH 8.0, 150 mM NaCl) and then incubated with commercial antibodies against HA, GAPDH, histone H3, GFP, the Flag tag, or β-actin (all from Abcam) in TBS-3% nonfat milk for 3 h. The membrane was washed three times in TBS, and alkaline phosphatase–conjugated goat anti-rabbit/antimouse IgG (1:10,000 dilution in TBS) was added and incubated for 3 h, followed by washing to remove the unbound IgG. The membrane was incubated with the reaction system and visualized in the dark using 4-chloro-1-naphthol oxidation for 5 min. All images were captured using the Odyssey CLx system (LI-COR).
Immunocytochemical staining
Immunocytochemical staining was used to examine the subcellular location of the N-terminal EsIL-16 prodomain in HEK293T cells. Briefly, pretreated cells were blocked with 3% bovine serum albumin (BSA) for 30 min at 37 °C and then incubated with antibodies recognizing GFP-tagged EsIL-16 overnight at 4 °C. After washing with PBS, the cells were incubated with 3% BSA for 10 min, after which the goat antimouse Alexa Fluor 488 secondary antibody (1:1000 dilution in 3% BSA) was added. The reaction was performed in the dark for 1 h at 37 °C, and then the cells were washed with PBS. Finally, the cells were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (AnaSpec) for 10 min at room temperature, washed, and observed under a Revolve Hybrid Microscope (Echo).
Panning of the T7 phage display library
Panning was performed based on previously published method (47) with some modifications. Briefly, 40 μg of purified mature EsIL-16 protein was added to the wells of a 96-well plate and incubated at 4 °C overnight. The unbound protein was removed by washing with PBS twice, and 10 μl of T7 phage display library derived from Chinese mitten crab hemocytes was added to the wells. Incubation was performed overnight. After three washes with TBST (50 mM Tris–HCl, 0.5 M NaCl, 0.02% Tween 20, and pH 7.5), EsIL-16-bound phages were eluted using 200 μl of 1% SDS and then centrifuged at 6000g for 5 min. Then, 10 μl of the supernatant was inoculated into 1 ml of BLT5403 cell culture (absorbance at 600 nm = 0.5) and cultured at 37 °C for 2 h. The culture was centrifuged, and the supernatant was plated on an agarose plate containing the BLT5403 cells. The plates were cultured at 37 °C for 3 h. Extraction buffer (100 mM NaCl, 6 mM MgSO4, 20 mM Tris–HCl, and pH 8.0) was added to the wells, and the plates were incubated at 4 °C overnight. The extraction buffer was then collected for the next panning. After three repeats, single plaques appeared, and the ten longest (>500 bp) fragments were sequenced.
Co-IP
A Co-IP assay was performed according to a previously described method (44) to detect the interaction between EsIL-16 and Esintegrin β1. HEK293T cells were seeded in 60-mm dishes (1 × 107 cells/dish) overnight and transfected with 10 mg of empty plasmid or various expression plasmids. At 48 h posttransfection, the medium was removed carefully, and the cell monolayer was washed twice with ice-cold PBS. The cells were then lysed using 500 μl of radioimmunoprecipitation assay lysis buffer (Beyotime) containing a protease cocktail (Yeasen). Lysates were centrifuged at 14,000g for 15 min. The supernatant was transferred to a fresh tube and precipitated for 2 h at 4 °C with 30 μl of either an anti-HA or anti-Flag affinity gel (Biotool). The affinity gel was washed with cold TBS four times and eluted by boiling for 10 min in TBS and a 6× SDS loading buffer (2% SDS, 60 mM Tris–HCl, pH 6.8, 10% glycerol, 0.001% bromophenol blue, and 0.33% 2-mercaptoethanol). The cell lysates were also eluted using this 6× SDS loading buffer and boiled. Proteins isolated from the beads and cell lysates were separated using SDS-PAGE and analyzed by Western blotting using the indicated antibodies. All images were captured by the Odyssey CLx system (LI-COR).
Laser scanning confocal analysis
Confocal was used to examine the possible interaction between EsIL-16 and Esintegrin β1. HEK293T cells were seeded onto 24 × 24 mm glass cover slips in 35-mm dishes overnight and transfected with 1 μg of empty or expression plasmids. At 36 h after transfection, the medium was removed and the cells were washed twice with PBS and fixed immediately with 4% paraformaldehyde in sterile PBS for 15 min. The cells were then permeabilized with 0.5% Triton X-100 in PBS for 10 min, washed with PBS three times, and blocked with 3% BSA for 2 h and then incubated with anti-Flag antibody (1:200) and anti-HA antibody (1:200) overnight at 4 °C. Subsequently, unbound antibody was removed by washing with PBS with Tween-20 (PBST), cells were stained with goat IgG H&L (FITC conjugated) (1:100) and goat anti-rabbit IgG H (Rhodamine Red-X conjugated) (1:100) for 2 h at 37 °C. After washing with PBST, cells were stained with 4′,6-diamidino-2-phenylindole dihydrochloride for 5 min. After a final wash in PBST, cover slips were sealed with nail polish on the edges and then removed for observation under a laser scanning confocal microscope (Leica).
Recombinant expression and purification
A PCR fragment representing the mature EsIL-16 was amplified using specific primers (Table S1). Subsequently, the PCR products were purified, digested with restriction enzymes (EcoRI and XhoI), and ligated into the pET-28a (+) vector (Novagen). The recombinant plasmid pET-28a (+)-EsIL-16 was transformed into Eriocheir coli Rosetta (DE3) cells (Transgene) for expression of the recombinant mature EsIL-16 protein. Fusion protein expression was induced using four different sets of conditions: 1 mM IPTG for 3 h at 37 °C; 0.25 mM IPTG for 3 h at 37 °C; 1 mM IPTG for 3 h at 30 °C; and 0.25 mM IPTG for 3 h at 30 °C. The fusion protein was purified using nickel–nitrilotriacetic acid resin (Transgene) according to the manufacturer's instructions.
Cell proliferation
Crab hemocyte and S2 cell proliferation was detected using the EdU and CFSE methods, respectively. EdU incorporation was analyzed according to the manufacturer's instructions using the Click-iT Plus EdU Imaging Kit (Life Technologies). Briefly, half of the cell culture media was replaced with fresh media containing 20 μM EdU and incubated for another 24 h. Cells were fixed with formaldehyde, permeabilized with 0.5% Triton X-100, and stained with a reaction cocktail and Hoechst according to the manufacturer's instructions. Cells were imaged with fluorescence microscopy, and the percentage of EdU-positive cells was evaluated by fluorescence microscopy. For the CFSE method, a CFSE stock solution was prepared (10 mM in dimethyl sulfoxide; Invitrogen) and stored at −20 °C. The stock solution was thawed and diluted in PBS to the desired working concentrations. In pilot experiments, we tested different CFSE labeling conditions (final concentrations: 0.2, 0.5, 1, 2, 5, and 10 μM) to obtain a high cell viability and a broad CFSE signal measurement toward antigen or polyclonal stimuli. S2 cells were resuspended in PBS (with 0.1% BSA) at 2 × 106 cells/ml and incubated with CFSE (final concentration: 1 μM) for 7 min at 37 °C. The cells were washed and resuspended in culture medium for 15 min to stabilize the CFSE staining. After a final wash step, the cells were resuspended in culture medium at the indicated cell concentrations. Thereafter, the CFSE-labeled S2 cells were transfected with the Esintegrin β1 overexpression plasmid with or without EsIL-16 (200 ng/ml; 100 μl) stimulation. After 48 h, the S2 cells were harvested, and the CFSE signal of gated cells was analyzed using flow cytometry (Beckman).
Assessment of crab survival and bacterial clearance assay
Crabs were divided randomly into eight groups of 30 animals each. After pretreatment with the EsIL-16 siRNA, Esintegrin β1 siRNA, GFP siRNA, recombinant EsIL-16 protein, Esintegrin β1 siRNA plus the recombinant EsIL-16 protein or TBS, all crabs were injected with S. aureus or V. parahaemolyticus (1 × 109 colony-forming units per crab, 200 μl). Dead crabs in each group were counted daily, and survival was tabulated over 5 days. Four days after the bacterial injections, the hemolymph from each crab was collected, diluted, and cultured overnight on solid LB plates, and then the bacterial colonies on each plate were counted.
Data availability
All data are contained within the article.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article. All authors agree with the submission. This work has not been published or submitted for publication elsewhere, either completely or in part, or in another form or language. No material has been reproduced from another source. Experiments using invertebrate animal were approved by local authorities.
Acknowledgments
We thank the Experimental Platform for Molecular Zoology (East China Normal University, Shanghai, China) for providing instruments essential for conducting our study.
Author contributions
Y.-H. Z. data curation; Y.-H. Z., H. L., and W.-W. L. validation; Y.-H. Z., H. L., H. Z., W.-K. S., W.-W. L. investigation; Y.-H. Z. and H. L. visualization; Y.-H. Z., H. L., H. Z., and W.-K. S. methodology; Y.-H. Z. and W.-W. L. writing–original draft; Q. W. and W.-W. L. funding acquisition; Q. W. and W.-W. L. project administration; W.-W. L. conceptualization; W.-W. L. supervision.
Funding and additional information
This work was supported by the National Natural Science Foundation of China (Grants 31972820 to W.-W. L. and 31970490 to Q. W.) and Shanghai Rising-Star Program (Grant 20QA1403000 to W.-W. L.).
Edited by Peter Cresswell
Contributor Information
Qun Wang, Email: qwang@bio.ecnu.edu.cn.
Wei-Wei Li, Email: wwli@bio.ecnu.edu.cn.
Supporting information
References
- 1.Little T.J., Hultmark D., Read A.F. Invertebrate immunity and the limits of mechanistic immunology. Nat. Immunol. 2005;6:651–654. doi: 10.1038/ni1219. [DOI] [PubMed] [Google Scholar]
- 2.Buchon N., Silverman N., Cherry S. Immunity in Drosophila melanogaster--from microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 2014;14:796–810. doi: 10.1038/nri3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowley A.F., Powell A. Invertebrate immune systems specific, quasi-specific, or nonspecific? J. Immunol. 2007;179:7209–7214. doi: 10.4049/jimmunol.179.11.7209. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann J.A., Kafatos F.C., Janeway C.A., Ezekowitz R.A. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 5.Brocker C., Thompson D., Matsumoto A., Nebert D.W., Vasiliou V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum. Genomics. 2010;5:30–55. doi: 10.1186/1479-7364-5-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello C.A., Mier J.W. Interleukins. Annu. Rev. Med. 1986;37:173–178. doi: 10.1146/annurev.me.37.020186.001133. [DOI] [PubMed] [Google Scholar]
- 7.Secombes C.J., Wang T., Bird S. The interleukins of fish. Dev. Comp. Immunol. 2011;35:1336–1345. doi: 10.1016/j.dci.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Cruikshank W., Center D.M. Modulation of lymphocyte migration by human lymphokines. II. Purification of a lymphotactic factor (LCF) J. Immunol. 1982;128:2569–2574. [PubMed] [Google Scholar]
- 9.Richmond J., Tuzova M., Cruikshank W., Center D. Regulation of cellular processes by interleukin-16 in homeostasis and cancer. J. Cell. Physiol. 2014;229:139–147. doi: 10.1002/jcp.24441. [DOI] [PubMed] [Google Scholar]
- 10.Conti P., Kempuraj D., Kandere K., Di Gioacchino M., Reale M., Barbacane R.C., Castellani M.L., Mortari U., Boucher W., Letourneau R., Theoharides T.C. Interleukin-16 network in inflammation and allergy. Allergy Asthma Proc. 2002;23:103–108. [PubMed] [Google Scholar]
- 11.Sharma V., Sparks J.L., Vail J.D. Human B-cell lines constitutively express and secrete interleukin-16. Immunology. 2000;99:266–271. doi: 10.1046/j.1365-2567.2000.00959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponting C.P. Evidence for PDZ domains in bacteria, yeast, and plants. Protein Sci. 1997;6:464–468. doi: 10.1002/pro.5560060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruikshank W., Little F. lnterleukin-16: The ins and outs of regulating T-cell activation. Crit. Rev. Immunol. 2008;28:467–483. doi: 10.1615/critrevimmunol.v28.i6.10. [DOI] [PubMed] [Google Scholar]
- 14.Liu C., Mills J., Dixon K., Vennarini J., Cunningham M., Del Vecchio A., Das A., Glass W. IL-16 signaling specifically induces STAT6 activation through CD4. Cytokine. 2007;38:145–150. doi: 10.1016/j.cyto.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Skundric D.S., Cai J., Cruikshank W.W., Gveric D. Production of IL-16 correlates with CD4+ Th1 inflammation and phosphorylation of axonal cytoskeleton in multiple sclerosis lesions. J. Neuroinflammation. 2006;3:13. doi: 10.1186/1742-2094-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang Q., Zheng J., Zuo H., Li C., Niu S., Yang L., Yan M., Weng S.P., He J., Xu X. Identification and characterization of an interleukin-16-like gene from pacific white shrimp Litopenaeus vannamei. Dev. Comp. Immunol. 2017;74:49–59. doi: 10.1016/j.dci.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Gu W.B., Zhou Y.L., Tu D.D., Zhou Z.K., Zhu Q.H., Chen Y.Y., Shu M.A. Identification and characterization of pro-interleukin-16 from mud crab Scylla paramamosain: The first evidence of proinflammatory cytokine in crab species. Fish Shellfish Immunol. 2017;70:701–709. doi: 10.1016/j.fsi.2017.09.057. [DOI] [PubMed] [Google Scholar]
- 18.Song L., Bian C., Luo Y., Wang L., You X., Li J., Qiu Y., Ma X., Zhu Z., Ma L., Wang Z., Lei Y., Qiang J., Li H., Yu J. Draft genome of the Chinese mitten crab, Eriocheir sinensis. Gigascience. 2016;5:5. doi: 10.1186/s13742-016-0112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang B., Wang Z., Liu Q., Zhang H., Jiang S., Li X., Wang Z., Sun Y., Sha Z., Jiang H., Wu X., Ren Y., Li H., Xuan F., Ge B. High-quality genome assembly of Eriocheir japonica sinensis reveals its unique genome evolution. Front. Genet. 2019;10:1340. doi: 10.3389/fgene.2019.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Center D.M., Wu D.M., Cruikshank W.W., Yuan J., Andrews D.W., Kornfeld H. Processing and activation of pro-interleukin-16 by caspase-3. J. Biol. Chem. 1998;273:1144–1149. doi: 10.1074/jbc.273.2.1144. [DOI] [PubMed] [Google Scholar]
- 21.Myers E.W., Sutton G.G., Delcher A.L., Dew I.M., Fasulo D.P., Flanigan M.J., Kravitz S.A., Mobarry C.M., Reinert K.H., Remington K.A., Anson E.L., Bolanos R.A., Chou H.H., Jordan C.M., Halpern A.L. A whole-genome assembly of Drosophila. Science. 2000;287:2196–2204. doi: 10.1126/science.287.5461.2196. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X., Yuan J., Sun Y., Li S., Gao Y., Yu Y., Liu C., Wang Q., Lv X., Zhang X., Ma K.Y., Wang X., Lin W., Wang L., Zhu X. Penaeid shrimp genome provides insights into benthic adaptation and frequent molting. Nat. Commun. 2019;10:356. doi: 10.1038/s41467-018-08197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neurath M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 24.Jeibmann A., Halama K., Witte H.T., Kim S.N., Eikmeier K., Koos B., Klämbt C., Paulus W. Involvement of CD9 and PDGFR in migration is evolutionarily conserved from Drosophila glia to human glioma. Lab. Invest. 2015;124:373–383. doi: 10.1007/s11060-015-1864-4. [DOI] [PubMed] [Google Scholar]
- 25.Luo B.H., Carman C.V., Springer T.A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell I.D., Humphries M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seetharaman S., Etienne-Manneville S. Integrin diversity brings specificity in mechanotransduction. Biol. Cell. 2018;110:49–64. doi: 10.1111/boc.201700060. [DOI] [PubMed] [Google Scholar]
- 28.Humphries M.J. Integrin structure. Biochem. Soc. Trans. 2000;28:311–339. [PubMed] [Google Scholar]
- 29.Holmblad T., Thörnqvist P.O., Söderhäll K., Johansson M.W. Identification and cloning of an integrin beta subunit from hemocytes of the freshwater crayfish Pacifastacus leniusculus. J. Exp. Zool. 1997;277:255–261. [PubMed] [Google Scholar]
- 30.Burke R.D. Invertebrate integrins: Structure, function, and evolution. Int. Rev. Cytol. 1999;191:257–284. doi: 10.1016/s0074-7696(08)60161-8. [DOI] [PubMed] [Google Scholar]
- 31.Dinkins M.B., Fratto V.M., Lemosy E.K. Integrin alpha chains exhibit distinct temporal and spatial localization patterns in epithelial cells of the Drosophila ovary. Dev. Dyn. 2008;237:3927–3939. doi: 10.1002/dvdy.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Reilly A.M., Lee H.H., Simon M.A. Integrins control the positioning and proliferation of follicle stem cells in the Drosophila ovary. J. Cell Biol. 2008;182:801–815. doi: 10.1083/jcb.200710141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yee G.H., Hynes R.O. A novel, tissue-specific integrin subunit, beta nu, expressed in the midgut of Drosophila melanogaster. Development. 1993;118:845–858. doi: 10.1242/dev.118.3.845. [DOI] [PubMed] [Google Scholar]
- 34.Lavine M.D., Strand M.R. Haemocytes from Pseudoplusia includens express multiple alpha and beta integrin subunits. Insect Mol. Biol. 2003;12:441–452. doi: 10.1046/j.1365-2583.2003.00428.x. [DOI] [PubMed] [Google Scholar]
- 35.Levin D.M., Breuer L.N., Zhuang S., Anderson S.A., Nardi J.B., Kanost M.R. A hemocyte-specific integrin required for hemocytic encapsulation in the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2005;35:369–380. doi: 10.1016/j.ibmb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Lanot R., Zachary D., Holder F., Meister M. Postembryonic hematopoiesis in Drosophila. Dev. Biol. 2001;230:243–257. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- 37.Lebestky T., Chang T., Hartenstein V., Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–149. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- 38.Junkunlo K., Söderhäll K., Söderhäll I. Transglutaminase inhibition stimulates hematopoiesis and reduces aggressive behavior of crayfish, Pacifastacus leniusculus. J. Biol. Chem. 2019;294:708–715. doi: 10.1074/jbc.RA118.005489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Junkunlo K., Söderhäll K., Söderhäll I., Noonin C. Reactive oxygen species affect transglutaminase activity and regulate hematopoiesis in a crustacean. J. Biol. Chem. 2016;291:17593–17601. doi: 10.1074/jbc.M116.741348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin X., Söderhäll I. Crustacean hematopoiesis and the astakine cytokines. Blood. 2011;117:6417–6424. doi: 10.1182/blood-2010-11-320614. [DOI] [PubMed] [Google Scholar]
- 41.Luo H., Hanratty W.P., Dearolf C.R. An amino acid substitution in the Drosophila hopTum-l Jak kinase causes leukemia-like hematopoietic defects. EMBO J. 1995;14:1412–1420. doi: 10.1002/j.1460-2075.1995.tb07127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munier A.I., Doucet D., Perrodou E., Zachary D., Meister M., Hoffmann J.A., Janeway C.A., Jr., Lagueux M. PVF2, a PDGF/VEGF-like growth factor, induces hemocyte proliferation in Drosophila larvae. EMBO Rep. 2002;3:1195–1200. doi: 10.1093/embo-reports/kvf242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown S., Hu N., Hombría J.C. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr. Biol. 2001;11:1700–1705. doi: 10.1016/s0960-9822(01)00524-3. [DOI] [PubMed] [Google Scholar]
- 44.Li D., Wan Z., Li X., Duan M., Yang L., Ruan Z., Wang Q., Li W. Alternatively spliced down syndrome cell adhesion molecule (Dscam) controls innate immunity in crab. J. Biol. Chem. 2019;294:16440–16450. doi: 10.1074/jbc.RA119.010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos H.M., Tsai C.Y., Maquiling K.R.A., Tayo L.L., Mariatulqabtiah A.R., Lee C.W., Chuang K.P. Diagnosis and potential treatments for acute hepatopancreatic necrosis disease (AHPND): A review. Aquac. Int. 2020;28:169–185. doi: 10.1007/s10499-019-00451-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L., Li X., Qin X., Wang Q., Zhou K., Li H., Zhang X., Wang Q., Li W. Deleted in azoospermia-associated protein 2 regulates innate immunity by stimulating Hippo signaling in crab. J. Biol. Chem. 2019;294:14704–14716. doi: 10.1074/jbc.RA119.009559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X.W., Zhao X.F., Wang J.X. C-type lectin binds to β-integrin to promote hemocytic phagocytosis in an invertebrate. J. Biol. Chem. 2014;289:2405–2414. doi: 10.1074/jbc.M113.528885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the article.