Abstract
Sevoflurane affects on the A1 receptor in the central nervous system and potentiates the action of neuromuscular blocking agents. In the present study, we investigated whether sevoflurane (SEVO) has the ability to potentiate the neuromuscular blocking effect of rocuronium and if the specific antagonist of adenosine receptor (SLV320) can reverse this effect. In this study, phrenic nerve–hemidiaphragm tissue specimens were obtained from 40 Sprague–Dawley (SD) rats. The specimens were immersed in an organ bath filled with Krebs buffer and stimulated by a train‐of‐four (TOF) pattern using indirect supramaximal stimulation at 20 s intervals. The specimens were randomly allocated to control, 2‐chloroadenosine (CADO), SEVO, or SLV320 + SEVO groups. In the CADO and SLV320 + SEVO groups, CADO and SLV320 were added to the organ bath from the start to a concentration of 10 μM and 10 nM, respectively. We then proceeded with rocuronium‐induced blockade of >95% depression of the first twitch tension of TOF (T1) and TOF ratio (TOFR). In the SEVO and SLV320 + SEVO groups, SEVO was added to the Krebs buffer solution to concentration of 400–500 μM for 10 min. Sugammadex‐induced T1 and TOFR recovery was monitored for 30 min until >95% of T1 and >0.9 of TOFR were confirmed, and the recovery pattern was compared by plotting these data. T1 recovery in the SEVO and CADO groups was significantly delayed compared with the control and SLV320 + SEVO groups (p < .05). In conclusion, sevoflurane affects on the A1 receptor at the neuromuscular junction and delays sugammadex‐induced recovery from neuromuscular blockade.
Keywords: acetylcholine, neuromuscular blockade, neuromuscular blocking agent, rocuronium, sevoflurane, sugammadex
Effects of sevoflurane and adenosine receptor antagonist on the sugammadex‐induced recovery from rocuronium‐induced neuromuscular blockade in rodent phrenic nerve‐hemidiaphragm tissue specimens.
![]()
1. INTRODUCTION
Sevoflurane is a commonly used inhalation anesthetic agent in clinical settings. Although the effects of inhalation anesthetic agents cannot be explained by a single molecular mechanism, it is known that sevoflurane has a tendency to activate adenosine A1 receptor. 1 The hypnotic or soporific effects of adenosine have been described in animals 2 and humans. 3 Furthermore, specific antagonists of the adenosine receptor, such as theophylline, can decrease the sedation effect of propofol 4 and sevoflurane. 5 Such effects are due to the blockade of effects of sevoflurane or propofol on adenosine receptors. At the neuromuscular junction, adenosine receptors, together with presynaptic muscarinic acetylcholine receptors, modulate acetylcholine (ACh) release upon neuronal firing and regulate each other as a facilitatory or inhibitory receptor. 6 , 7 , 8 When these receptors are modulated and their functions are affected by specific agonists and antagonists, spontaneous and evoked release of ACh at the neuromuscular junction have been observed. These changes, in turn, affect the responses of muscle tension to variable neuronal stimulation. 9
Sugammadex, a γ‐cyclodextrin derivative, can reverse the effect of aminosteroidal neuromuscular blocking agents by encapsulating and inactivating them. 10 , 11 , 12 This effect does not influence the amount of ACh at the neuromuscular synaptic junction and this is different from the mechanism of classic reversal strategy to increase ACh using cholinesterase inhibitors such as neostigmine. 13 At the end of the surgery, sevoflurane does not completely wash out from the body and still has effects in the neuromuscular junction, especially on receptors which are responsible for the modulation of ACh release. When cholinesterase inhibitors are used as reversal agents, ACh is accumulated in the neuromuscular junction because cholinesterases near the AChR are blocked by their antagonists and degradation of ACh is delayed. In that environment, subtle changes in ACh amount made by sevoflurane might have little or no effect on muscle tension and recovery from neuromuscular blockade because there are plenty of ACh molecule in the neuromuscular junction. On the contrary, if sugammadex is used instead of cholinesterase inhibitors, amount of ACh and metabolism are not affected during recovery from neuromuscular blockade. As such, subtle changes in ACh release may present changes in muscle tension during recovery from neuromuscular blockade which might be hindered during anticholinesterase‐induced recovery. We hypothesized that, during sugammadex‐induced recovery from neuromuscular blockade, the muscle tension might be influenced by modulation of the presynaptic release of ACh. We also hypothesized that the modulation of ACh release might be responsible for the potentiation of the neuromuscular blockade effect of sevoflurane. To confirm these hypotheses, here, we performed ex vivo experiments using rodent phrenic nerve/hemidiaphragm tissue specimens. We investigated the time course of sugammadex‐induced recovery from rocuronium‐induced neuromuscular blockade in tissue specimens in an organ bath with adenosine A1 receptor agonist, sevoflurane, or sevoflurane and adenosine A1 receptor‐specific blocker.
2. METHODS
2.1. Basic study design and sample preparation
The study protocol was approved by the Ethics Committee of the Laboratory of Animal Research, Asan Institute of Life Science (Seoul, Republic of Korea), on September 1, 2018 (Protocol No. 2018‐13‐198). Forty male Sprague–Dawley (SD) rats with average weight of 245.45 g (range 225.0–296.9 g) were used in the study. All rats were bred at a constant temperature of 22℃ under a regular diurnal cycle. They were provided food and water ad libitum. We performed surgical procedures for harvesting tissue specimen after confirming that the rats did not respond by pinching their hindlimb. Additional sacrifice methods for SD rats were not necessary in this study because rats were expired as soon as the thoracic cages were harvested during the preparation. Inclusion criteria were that the initial T1 twitch tension showed more than 100 mN and maintained for more than 20 min. Data were excluded when the T1 twitch tensions were gradually decreased before adding study drugs or when the maximum recovery of T1 twitch tension was less than 50 mN within 10 min. Urethane, 2‐chloroadenosine (CADO), and SLV320 were purchased from Sigma‐Aldrich Korea Ltd. CADO stock solution was stored at −20℃ in a refrigerator and thawed before use. Stock solutions were discarded 2 weeks after preparation if unused. Concentrations of CADO and SLV320 during the experiment were set on the basis of our previous study, 14 pilot study, and other reported articles. 15 , 16 The dose of sevoflurane was selected as 400–500 μM at the point of initiation of recovery. This was determined before the main experiment by performing a pilot study and by referring to a previous study. 17
2.2. Study protocol
Each rat was anesthetized with intraperitoneal injection of urethane (500 μg/kg). The thoracic cage was immediately isolated, and the phrenic nerve/hemidiaphragm tissue was obtained. The sampled tissues were immersed in Krebs buffer solution (120 mM NaCl, 2.5 mM CaCl2, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, and 11 mM α‐d‐glucose) maintained at 35℃ and 95% O2 and 5% CO2 with continuous bubbling to ensure tissue viability throughout the experimental session. Sizes and weights of each tissue were measured and compared between groups (Table 1). The tissues were fixed to a frame with electrodes and then immersed in a 100 mL organ bath containing 75 mL of oxygenated Krebs buffer solution. Subsequently, 40 mN resting tension was applied to the tendinous portion of the diaphragm of each sample hooked to a Grass FT03 Force Transducer (Grass Technologies). The phrenic nerve was fixed to a platinum bipolar electrode and stimulated using a Grass S88 Stimulator (Grass Technologies). Supramaximal stimulation with a square wave pulse of 0.2 ms was administered every 20 s at train‐of‐four (TOF) stimulation with 2 Hz impulse. All waveforms were acquired and stored using the PowerLab 4/26 Data Acquisition System (AD Instruments) and LabChart 7 Software (AD Instruments), respectively.
TABLE 1.
Characteristics of Sprague–Dawley rats and tissue specimen
|
Control (n = 10) |
SEVO (n = 10) |
SEVO + SLV320 (n = 10) |
CADO (n = 10) |
|
|---|---|---|---|---|
| BW (g) | 246.36 ± 13.78 | 239.38 ± 5.94 | 255.70 ± 16.26 | 240.48 ± 6.23 |
| wWt (mg) | 123.91 ± 18.53 | 123.08 ± 18.34 | 121.00 ± 13.70 | 122.78 ± 18.64 |
| Size (mm2) | 133.04 ± 28.94 | 125.38 ± 24.63 | 127.20 ± 33.93 | 128.51 ± 23.52 |
Data are expressed as mean ±SD. There were no significant differences among the groups (p > .05).
Abbreviations: BW, body weight of rats; CADO, 2‐chloroadenosine group; SEVO, sevoflurane; size, size (width × length) of the hemidiaphragm; wWt, weight of the hemidiaphragm.
The phrenic nerve/hemidiaphragm tissues were randomly allocated to the control, CADO (CADO 10 μM), SEVO (sevoflurane 400–500 μM), or SEVO + SLV320 group (sevoflurane 400–500 μM + SLV320 10 nM) after generating random number by using following equation in Microsoft Excell 2010 program (Microsoft Office®, Microsoft Corporation); f x = (INT(RAND() × 4)) + 1. Group blinding was not available because SEVO is volatile and its scent is pungent. Twitch tensions were serially monitored during a 30 min stabilization time. Thereafter, Krebs buffer solution, SLV320, or CADO stock solution was added to the organ bath. For double blinding, the prime investigator was handed over unlabeled study drugs (Krebs buffer solution for control and SEVO group, SLV320 for SEVO + SLV320 group, and CADO for CADO group). A 500 µg loading dose of rocuronium (Esmeron®; MSD Korea) and 250 μg boost dose of rocuronium were subsequently added into the organ bath. The subsequent boost dose was added 10 min after the previous dose or when five consecutive T1 twitch tension depressions were <3% of the previous T1 twitch tension. The boost doses were stopped when 95% or more depression of the T1 was achieved. The loading dose was set as the dose at which the T1 twitch tension did not change after adding the loading dose and the TOF ratio (TOFR) changes were within 3% of those achieved before adding the loading dose. The boost dose was set as the first dose at which the T1 twitch tension changed and the total boost doses were within 10. After confirming that the T1 twitch tensions disappeared, sevoflurane was added to the SEVO and SEVO + SLV320 groups for 10 min to a concentration of 400–500 μM, whereas the other groups were allowed to rest for 10 min. After 10 min of exposure time of sevoflurane, equimolar doses of sugammadex, which were to react 1:1 to the rocuronium used to create >95% depression of T1 twitch tension, were added to each group to recover from rocuronium‐induced neuromuscular blockade. Recovery patterns were monitored and plotted for 30 min or until the T1 twitch tension recovery was >95% and TOFR was ≥0.9. The study protocol is summarized in Figure 1.
FIGURE 1.
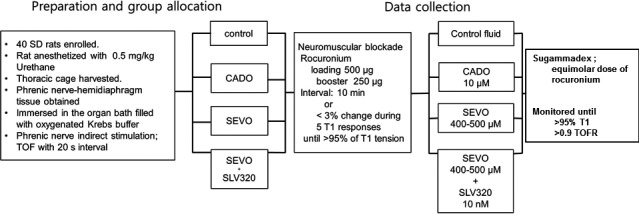
Study protocol. Study drugs (CADO, SLV320) were administered from the start of the experiment. Sevoflurane exposure time was set as 10 min. CADO, 2‐chloroadenosine; SD, Sprague–Dawley rats; SEVO, sevoflurane; T1, the first twitch tension of train‐of‐four stimulation; TOF, train‐of four stimulation
2.3. Statistical analysis
The first aim of this study was to compare the time required in each group to attain >95% recovery in T1. To achieve this goal, the T1 twitch tensions were serially monitored and recorded; these responses were plotted as regression curves and compared among the groups. T1 recovery and TOFR recovery were achieved simultaneously until TOFR was >0.9. The second aim was to compare the variables obtained during sugammadex‐induced recovery from neuromuscular blockade. In a clinical practice, the recovery index (RI, the interval of T1 recovery from 25% to 75%) is a good marker for comparing the recovery rates from neuromuscular blockade. We performed inter‐group comparisons of the time interval of T1 twitch tension recovery at 10%, 25%, 50%, 75%, and 95%.
There are many issues with performing sample size estimation in ex vivo neuromuscular studies. Previous studies have suggested that about 10 animals per group are needed to achieve statistically significant results. 14 , 18 In the present study, the sample size was calculated based on the previous experiment and pilot study, which suggested that 10 samples per group were sufficient at α = .05, power = 0.80, and a dropout rate of 10%.
Results are expressed as mean ± standard deviation (SD). All doses were converted and expressed in μmol/L (μM). Time is expressed in min. Graphs were plotted, and statistical analyses were carried out using SPSS 13.0 software (SPSS Inc.). Rocuronium EC50 and EC95 values for twitch tension were calculated by fitting nonlinear regression curves to group data. We used the following equation for T1 depression; y = 50 + 50sin(Ωx + b), where y represents the T1 tension, x represents the recovery time, b represents half of the time required to achieve T1 >95%, and Ω represents the slope of the regression curve (R 2 = .87). Start points were set as the time of administration of sugammadex and the time when T1 twitch tension recovered to 10%, and the slopes are represented as Ω 1 and Ω 2, respectively. We considered the RI as the time interval from 25% to 75% of T1 twitch tension recovery, and compared the RI among the groups. We also used the following equation for TOFR; y = λ(x−b) 3 + c, where y and x represent TOFR and recovery time, respectively, while λ represents the slope of the regression curve (R 2 = .805). Differences in continuous variables among the groups were analyzed using analysis of variance, followed by the Bonferroni method for multiple pairwise comparisons. The mean group values of Ωs and λ were compared using the Mann–Whitney U test. Statistical significance was set at p < .05.
3. RESULTS
There were no differences in the average body weight of rats, wet weight of tissue specimens, and dose of rocuronium used among the groups (Table 1). We plotted the recovery data of T1 and TOFR of each group and compared their slopes (represented as Ω and λ, respectively). When T1 twitch tension recovery was plotted with the start point as the time of sugammadex administration, there were no significant differences on Ω 1 between groups (Figure 2A,B). The data of the CADO group were not included in this comparison because most of the T1 twitch tensions in the CADO group were reappeared 5 min after the administration of sugammadex and did not recover within 30 min (Table 2). When the start point was set as the time when the T1 twitch tension recovered to 10%, there were significant differences in Ω 2 of the CADO and SEVO groups compared with that of control and SLV320 + SEVO groups (Figure 3A,B). When Ω 2 of control group was compared with those of SEVO and CADO group, there were significant differences between groups (p = .008 and .010, respectively, Figure 3B, Table 2). When Ω 2 of SEVO + SLV320 group was compared with those of SEVO and CADO group, we also found significant differences between groups (p = .035 and .044, respectively, Figure 3B, Table 2). We also compared the recovery time interval from 25% to 75% recovery of T1 (RI) among the groups. The RIs of the CADO and SEVO groups were significantly different from those of the other groups (p = .005 with control vs. SEVO, p = .000 with control vs. other groups, Table 2), although there were no significant differences between the control and SEVO + SLV320 group (Table 2, p > .050). A comparison of TOFR recovery revealed no significant differences among the groups except CADO group (Table 2) when T1 twitch tension recovery was plotted with the start point as the reappearance of T1. TOFR recovery in CADO group had failed to reach to 0.9 within 1 h. As such, the data obtained from CADO group were not included in this comparison.
FIGURE 2.

Progression of T1 twitch tension recovery of control (●, solid line), SEVO (△, dashed line), and SEVO + SLV320 (◇, dash‐dot line) group when the zero point was set as the time of sugammadex administration (A). Data obtained from CADO group were not included because most of the data failed to reach 100% twitch height recovery within 30 min. Comparisons of omegas of T1 recovery are shown at (B). We set the equation for T1 recovery as y = 50 + 50sin(Ωx + b), where y is the % recovery of T1 twitch tension and x is time. It was inclined to show delayed recovery progression of T1, although we failed to obtain statistical significances of Ω between the groups. CADO, 2‐chloroadenosine; SEVO, sevoflurane; T1, the first twitch tension of TOF; TOF, train‐of‐four stimulation; TOFR, TOF ratio
TABLE 2.
Comparison of recovery progression and recovery index.
|
Control (n = 10) |
SEVO (n = 10) |
SEVO + SLV320 (n = 10) |
CADO (n = 10) |
|
|---|---|---|---|---|
| Ω 1 (×10−3) | 0.975 ± 0.237 | 1.027 ± 0.104 | 0.954 ± 0.234 | — |
| Ω 2 (×10−3) | 0.877 ± 0.202 | 1.002 ± 0.092* | 0.898 ± 0.202 | 0.999 ± 0.056* |
| λ (×10−3) | 0.569 ± 0.721 | 0.266 ± 0.179 | 0.384 ± 0.376 | — |
| RI (min) | 6.034 ± 1.791 | 8.531 ± 2.113 † | 5.534 ± 1.852 | 14.949 ± 4.498 † |
Data are expressed as mean ± SD.
Abbreviations: CADO, 2‐chloroadenosine group; SEVO, sevoflurane; Ω 1, regression slope when the zero point was set as the time of sugammadex administration; Ω 2, regression slope when the zero point was set as the time of 10% T1 twitch tension recovery.
p < .050 compared with the control or SEVO + SLV320 group (p = .008 with control vs. SEVO; p = .010 with control vs. CADO; p = .035 with SEVO + SLV320 vs. SEVO; and p = .044 with SEVO + SLV320 vs. CADO).
p < .050 compared with the control or SEVO + SLV320 group (p = .005 with control vs. SEVO; p = .000 with control vs. CADO; and p = .000 with SEVO + SLV320 vs. SEVO or CADO).
FIGURE 3.

Progression of T1 twitch tension recovery of control, and CADO (□, dash‐dot‐dot line) group when the zero point was set as the time when the recovery of the T1 twitch tension was 10% (A). Comparisons of omegas of T1 recovery are shown at (B). We set the equation for T1 recovery as y = 50 + 50sin(Ωx + b), where y is the % recovery of T1 twitch tension and x is time. *p < .050 compared with the control (p = .008 with control vs. SEVO; p = .010 with control vs. CADO). † p < .050 compared with SEVO + SLV320 group (p = .035 with SEVO + SLV320 vs. SEVO; p = .044 with SEVO + SLV320 vs. CADO). CADO, 2‐chloroadenosine; SEVO, sevoflurane; T1, the first twitch tension of TOF; TOF, train‐of‐four stimulation; TOFR, TOF ratio
4. DISCUSSION
In the present study, we demonstrated that sevoflurane affects the A1 receptor at the neuromuscular junction and delays sugammadex‐induced recovery from rocuronium‐induced neuromuscular blockade. Adenosine acts as an A1 agonist at a low concentration (300 nM), and as an A2A agonist at high concentration (>1 μM). 19 Furthermore, it has been demonstrated that enflurane and sevoflurane have the ability to activate adenosine A1 receptors in an in vitro culture of rat hippocampus. 1 Aminophylline is a nonselective antagonist of the adenosine receptor, 20 and it can decrease the sedation effects of sevoflurane, 5 , 21 but not the anesthesia induced by desflurane. 4 Sevoflurane is one of the most potent volatile anesthetics that potentiates the effect of neuromuscular blocking agents. 22 The present study results are in consistent with these findings. Sugammadex has no effect on the neuromuscular junction and ACh release. Sugammadex‐induced recovery from rocuronium‐induced neuromuscular blockade is dependent on the relative concentration of rocuronium and ACh at the neuromuscular junction. Thus, the T1 twitch response to the indirect nerve stimulation reappears in the presence of a large amount of ACh molecules at the neuromuscular junction because the ACh molecules have a greater chance to attach to AChR than rocuronium. When the action of the receptor or channel related to the release of ACh was modulated and the release of ACh in the neuromuscular presynaptic membrane was reduced, there was a delay in recovery from neuromuscular blockade. 14 In the present study, the modulation of adenosine receptor with CADO and sevoflurane delayed T1 recovery and resulted in a low recovery index compared with those of the control (no receptor modulation). We speculated that the modulation of the A1 receptor at the presynaptic membrane of the neuromuscular junction by sevoflurane could partially induce delayed recovery from neuromuscular blockade. We did not change the current for phrenic nerve stimulation and, thus, the magnitude of indirect stimulation to the phrenic nerve might not have changed. However, in this study, the amount of ACh released per indirect stimulation might have reduced during sugammadex‐induced recovery from neuromuscular blockade because of the activation of the A1 receptor in the neuromuscular junctions by sevoflurane. Furthermore, sugammadex binds to rocuronium only outside the neuromuscular junction, and there is a concentration difference in rocuronium between the neuromuscular junction and organ bath; consequently, rocuronium is removed from the neuromuscular junction. In the present study, the decrease in rocuronium concentration at the neuromuscular junction was thought to be similar among all groups because we used equimolar dose of sugammadex and rocuronium in the organ bath. Rocuronium molecules were able to bind to the AChRs at the postsynaptic membrane because the amount of ACh released was less at the same stimuli that caused presynaptic A1 receptor modulation by sevoflurane. This might have resulted in the delayed recovery from rocuronium‐induced neuromuscular blockade in the present study.
The present study had some limitations. First, this was an ex vivo study. We extracted phrenic nerve–hemidiaphragm tissue specimens after sacrificing SD rats. During this phase, although we handled the specimen in the Krebs buffer solution with 95% O2/CO2 gas bubbling, transient hypoxemia and tissue damage were inevitable. Furthermore, pharmacokinetic component was abolished during all phases of the experiment. To compensate for these limitations, we cautiously performed the following steps: (1) tissue specimens were extracted and immersed in a Petri dish and organ bath containing oxygenated Krebs buffer solutions throughout the experiment to minimize tissue hypoxia; and (2) maintaining the concentration of sevoflurane in the organ bath was another challenge. In the clinical setting, sevoflurane is supplied using an exclusive vaporizer and a closed‐circuit system, and its concentration is expressed as vol%. However, in the present study, it was difficult to develop a closed‐circuit system because frames, electrodes, and strings connected to the force transducer were out from the orifice of organ bath. Furthermore, scavenging system of our laboratory was not suitable for use with volatile anesthetics. Instead, to minimize air pollution in the laboratory, we had to find alternative method simulating a closed‐circuit system to apply sevoflurane. We sealed the organ bath with a flexible film and added sevoflurane intermittently to achieve the desired concentration in the Krebs buffer solution. In a clinical experiment, it took about 40 min for sevoflurane to equilibrate between blood and muscle component and make effect on muscle. 23 , 24 However, in some studies, only 10 min was needed for sevoflurane to make effect. 22 In our experiment, we shortened this reaction time to 10 min by applying sevoflurane directly to the environment of tissue specimen. This was quite a different approach compared with the clinical setting. Moreover, as sevoflurane is volatile, it is very difficult to maintain the designated concentration of sevoflurane in the organ bath, and fluctuation in its concentration was inevitable. The concentration of sevoflurane was maintained 400–500 μM until the point of initiation of recovery. This was determined by referring to a previous study of Kharasch et al. 15 In their study, blood concentrations of sevoflurane increased rapidly after administration of 1.3 MAC of sevoflurane. During end‐tidal, sevoflurane was maintained at about 2.5% for 3 h, and blood concentration of sevoflurane was increased from 400 to 700 μM. Blood concentration of sevoflurane was then decreased to about 200 μM for 1 h after discontinuation of sevoflurane. As such, we tried to maintain sevoflurane concentration at 400–500 μM during the experiment of SEVO and SEVO + SLV320 groups. Second, this was a “functional” study, not an immunochemistry study. We performed this experiment by measuring the tension generated by diaphragm contraction. That is, we deduced the results in an indirect manner, as the tension is thought to be driven by the ACh concentration differences at the neuromuscular junction. For accurate data, timely measurement of the amount of ACh released during serial indirect stimulation is required. However, we could not find ideal method for this. The ACh concentrations should be measured repeatedly at 20 s interval. We performed a conventional functional study that is commonly used in neuromuscular studies. To obtain more convincing results, a more suitable immunohistochemical study should be conducted in the future.
In conclusion, sevoflurane potentiates the effects of neuromuscular blocking agent partially by modulating the adenosine A1 receptor at the neuromuscular junction. As sevoflurane affects the A1 receptor and decreases ACh release, it affects sugammadex‐induced neuromuscular recovery after rocuronium‐induced neuromuscular blockade. Although the results were obtained in an ex vivo environment, they suggest that recovery from neuromuscular blockade can be delayed at the end of surgery if sevoflurane is used as the main anesthetic drug and, unlike expected, that sugammadex‐induced recovery from neuromuscular blockade can also be delayed.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in association with this work.
AUTHORS’ CONTRIBUTIONS
YBK and HSY designed the study and wrote the manuscript; YBK, JC, HC, and HSY analyzed and interpreted the data. YBK JC, HC, JI, and CP collected and statistically analyzed the data. YBK wrote manuscript and all authors read and revised manuscript body. JC and HSY performed political and external relation work.
ACKNOWLEDGMENTS
These authors are members of the Neuromuscular Physiology Research Team in the Laboratory of Animal Research, Asan Institute of Life Science, Seoul, Republic of Korea.
Kim YB, Choi J‐M, Park C, Choi H‐R, In J, Yang H‐S. Effects of sevoflurane and adenosine receptor antagonist on the sugammadex‐induced recovery from rocuronium‐induced neuromuscular blockade in rodent phrenic nerve–hemidiaphragm tissue specimens. Pharmacol Res Perspect. 2021;9:e00827. 10.1002/prp2.827
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials. The data that support the findings of this study are available from the corresponding author (fred0314@gilhospital.com) upon reasonable request.
REFERENCES
- 1. Tas PW, Eisemann C, Roewer N. Indirect activation of adenosine A1 receptors in cultured rat hippocampal neurons by volatile anaesthetics. Eur J Anaesthesiol. 2005;22:694‐702. [DOI] [PubMed] [Google Scholar]
- 2. Haulilca I, Ababei L, Branisteanu D, Topoliceanu F. Preliminary data on the possible hypnogenic role of adenosine. J Neurochem. 1973;21:1019‐1020. [DOI] [PubMed] [Google Scholar]
- 3. Segerdahl M, Ekblom A, Sandelin K, Wickman M, Sollevi A. Peroperative adenosine infusion reduces the requirements for isoflurane and postoperative analgesics. Anesth Analg. 1995;80:1145‐1149. [DOI] [PubMed] [Google Scholar]
- 4. Turan A, Kasuya Y, Govinda R, et al. The effect of aminophylline on loss of consciousness, bispectral index, propofol requirement, and minimum alveolar concentration of desflurane in volunteers. Anesth Analg. 2010;110:449‐454. [DOI] [PubMed] [Google Scholar]
- 5. Turan A, Memiş D, Karamanlioğlu B, Çolak A, Pamukçu Z, Turan N. Effect of aminophylline on recovery from sevoflurane anaesthesia. Eur J Anaesth. 2002;19:452‐454. [PubMed] [Google Scholar]
- 6. Oliveira L, Timoteo MA, Correia‐de‐Sa P. Modulation by adenosine of both muscarinic M1‐facilitatory and M2‐inhibition of [3H]‐acetylcholine release from the rat motor nerve terminals. Eur J Neurosci. 2002;15:1728‐1736. [DOI] [PubMed] [Google Scholar]
- 7. Pereira MW, Bornia ECS, Correia‐de‐Sa P, Alves‐Do‐Prado W. Presynaptic muscarinic and adenosine receptors are involved in 2 Hz‐induced train‐of‐four fade caused by antinicotinic neuromuscular relaxants in the rat. Clin Exp Pharmacol Physiol. 2011;38:764‐770. [DOI] [PubMed] [Google Scholar]
- 8. Bornia ECS, Correia‐de‐Sa P, Alves‐Do‐Prado W. Presynaptic facilitatory adenosine A2A receptors mediate fade induced by neuromuscular relaxants that exhibit anticholinesterase activity. Clin Exp Pharmacol Physiol. 2011;38:164‐169. [DOI] [PubMed] [Google Scholar]
- 9. Tomàs J, Santafé MM, Garcia N, et al. Presynaptic membrane receptors in acetylcholine release modulation in the neuromuscular synapse. J Neurosci Res. 2014;92:543‐554. [DOI] [PubMed] [Google Scholar]
- 10. Beny K, Piriou V, Dussart C, Hénaine R, Aulagner G, Armoiry X. Impact of sugammadex on neuromuscular blocking agents use: a multicentric pharmaco‐epidemiologic study in French university hospital and military hospitals. Ann Fr Anesth Reanim. 2013;32:838‐843. [DOI] [PubMed] [Google Scholar]
- 11. Pavoni V, Gianesello L, De Scisciolo G, et al. Reversal of profound and “deep” residual rocuronium‐induced neuromuscular blockade by sugammadex: a neurophysiological study. Minerva Anestesiol. 2012;78:542‐549. [PubMed] [Google Scholar]
- 12. Woo T, Kim KS, Shim YH, et al. Sugammadex versus neostigmine reversal of moderate rocuronium‐induced neuromuscular blockade in Korean patients. Korean J Anesthesiol. 2013;65:501‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fuchs‐Buder T, Meistelman C, Raft J. Sugammadex: clinical development and practical use. Korean J Anesthesiol. 2013;65:495‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim YB, Lee S, Choi HR, et al. Effects of adenosine receptor agonist on the rocuroniuminduced neuromuscular block and sugammadex‐induced recovery. Korean J Anesthesiol. 2018;71:476‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kharasch ED, Karol MD, Lanni C, Sawchuk R. Clinical sevoflurane metabolism and disposition. I. Sevoflurane and metabolite pharmacokinetics. Anesthesiology. 1995;82:1369‐1378. [DOI] [PubMed] [Google Scholar]
- 16. Kalk P, Eggert B, Relle K, et al. The adenosine A1 receptor antagonist SLV320 reduces myocardial fibrosis in rats with 5/6 nephrectomy without affecting blood pressure. Br J Pharmacol. 2007;151:1025‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xue FS, Liao X, Tong SY, Liu JH, An G, Luo LK. Dose‐response and time‐course of the effect of rocuronium bromide during sevoflurane anaesthesia. Anaesthesia. 1998;53:25‐30. [DOI] [PubMed] [Google Scholar]
- 18. Kiesman WF, Elzen E, Zablocki J. A1 adenosine receptor antagonists, agonists, and allosteric enhancers. Handb Exp Pharmacol. 2009;193:25‐58. [DOI] [PubMed] [Google Scholar]
- 19. Garcia N, Priego M, Obis T, et al. Adenosine A1 and A2A receptor‐mediated modulation of acetylcholine release in the mice neuromuscular junction. Eur J Neurosci. 2013;38:2229‐2241. [DOI] [PubMed] [Google Scholar]
- 20. Stone TW, Hollins C, Lloyd H. Methylxanthines modulate adenosine release from slices of cerebral cortex. Brain Res. 1981;31:352‐354. [DOI] [PubMed] [Google Scholar]
- 21. Fredholm BB, Ijzerman AP, Jacobson KA, Linden J, Müller CE. International union of basic and clinical pharmacology. LXXXI. Nomenclature and classification of adenosine receptors‐an update. Pharmacol Rev. 2011;63:1‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bock M, Klippel K, Nitsche B, Bach A, Martin E, Motsch J. Rocuronium potency and recovery characteristics during steady‐state desflurane, sevoflurane, isoflurane or propofol anaesthesia. Br J Anaesth. 2000;84:43‐47. [DOI] [PubMed] [Google Scholar]
- 23. Lowry DW, Mirakhur RK, McCarthy GJ, Carroll MT, McCourt KC. Neuromuscular effects of rocuronium during sevoflurane, isoflurane, and intravenous anesthesia. Anesth Analg. 1998;87:936‐940. [DOI] [PubMed] [Google Scholar]
- 24. Haerter F, Simons JCP, Foerster U, et al. Comparative effectiveness of calabadion and sugammadex to reverse nondepolarizing neuromuscular blocking agents. Anesthesiology. 2015;123:1337‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials. The data that support the findings of this study are available from the corresponding author (fred0314@gilhospital.com) upon reasonable request.


