Abstract
The renin-angiotensin-aldosterone system (RAS) is a vital hormone-receptor system that regulates cardiovascular and renal functions. In this article, we discuss exciting new findings in the RAS field. Recently solved active state crystal structures of Angiotensin II type 1 (AT1R) and type 2 receptor (AT2R) helped in understanding receptor activation mechanisms in detail. Also, considerable attention is given to the developments in characterizing the counter-regulatory RAS axis due to current hope for harnessing this axis for the development of protective therapies against various cardiovascular diseases. We describe the RAS component, angiotensin-converting enzyme 2 (ACE2) functioning as cellular entry receptor for the causative agent of COVID-19 pandemic, SARS-CoV-2. Altogether, these discoveries paved the way for developing novel therapies targeting different components of the RAS in the future.
Keywords: ACE, ACE2, Ang II, Ang IV, Ang(1−7), Angiotensin II type 1 receptor (AT1R), Angiotensin II type 2 receptor (AT2R), ARBs, AT1R signaling, Classical RAS, Counter-regulatory RAS, Crystal structures of GPCRs, Dual-acting angiotensin-receptor/NEP inhibitor (ARNI), Mas Receptor (MasR), Mas-related G-protein–coupled receptor D (MrgD), Pro-renin Receptor, Renin inhibitor, Renin-angiotensin system (RAS), The COVID-19 receptor
In this article, we focus on one of the most intensely studied hormone-receptor systems in humans responsible for the regulation of blood pressure and water-electrolyte homeostasis. Since the last review chapter on Angiotensin Receptor (Inagami, 2004), there have been significant advances made in understanding the signal transduction mechanisms, pharmacology, and structural biology of these receptors as well description of a counter-regulatory arm of the renin-angiotensin system (RAS). There are four angiotensin (Ang) peptides, Ang II, Ang III, Ang IV, and Ang (1−7), which meet the definition of an endogenous hormone that act through specific receptors. These cell surface Ang receptors, also called “interpreters of angiotensinergic signals”, have been described in great detail in previous reviews (Forrester et al., 2018, Karnik et al., 2015). Angiotensin II, the classical blood pressure hormone binds and activates the type 1 (AT1R) and type 2 (AT2R) receptors. The Ang (1−7) peptide is paired with multiple receptors, of which Mas receptor (MasR) and the Mas-related receptor (MRGD) are better studied. These four Ang receptors are seven-transmembrane helical G protein-coupled receptors (GPCRs) that rely on heterotrimeric G proteins for mediating their cellular effects. These GPCRs are also capable of mediating β-arrestin biased signaling. The receptors for Ang IV (AT4Rs) are not GPCRs, instead they are type II single transmembrane Zn metalloproteases, IRAP (insulin regulated aminopeptidase), and its paralog AP-N (aminopeptidase-N). Ang III, Ang (1−7) and Ang IV also function as surrogate ligands for AT1R and AT2R.
Careful studies have been performed by researchers for more than a century to understand several pathological states that result from RAS dysregulation in order to develop effective therapies. Indeed these efforts have resulted in two classes of highly successful and safe drugs, the angiotensin-converting enzyme inhibitors (ACEIs) and the AT1 receptor blockers (ARBs). Along with the importance of classical RAS, this article will highlight recent advancement in the field such as the role of counter-regulatory RAS, recent breakthroughs in defining the active state crystal structures of AT1R and AT2R; RAS components as targets of new diseases, the clinical potential of the approved drugs, and RAS targeting drugs under clinical trials.
Generation of Hormone Peptides of RAS and Consequences of Inhibition
All four angiotensin hormones are produced from the ten residues long amino-terminal segment (called Ang I) of angiotensinogen, a ≈ 65 kd serpin produced predominantly in the liver. Angiotensinogen gene knockout is lethal in mice, and it is the only source for the production of Ang I in the human proteome. The cascade of enzymes that are not localized to any one tissue but act on circulating and locally produced Ang I or its derivative fragments to generate the hormone peptides constitute RAS.
The initiating enzyme of the RAS cascade is renin, an aspartyl protease predominantly produced in the kidney that reacts exclusively with angiotensinogen, and cleaves only one peptide bond between residues 10 and 11 generating the decapeptide Ang I (Fig. 1 ). No hormonal activity is detected for Ang I, although its ubiquitous distribution may be attributed to Ang I-binding carrier proteins that show no receptor-like function to transmit any signal. The physiological significance of Ang I is that it is the only source for the hormone-generation role of RAS in body fluids and the intra- or inter-cellular apace. Many isoforms of renin exist that show tissue-specific variation in expression. Renin gene knockout in mice is not lethal.
Fig. 1.
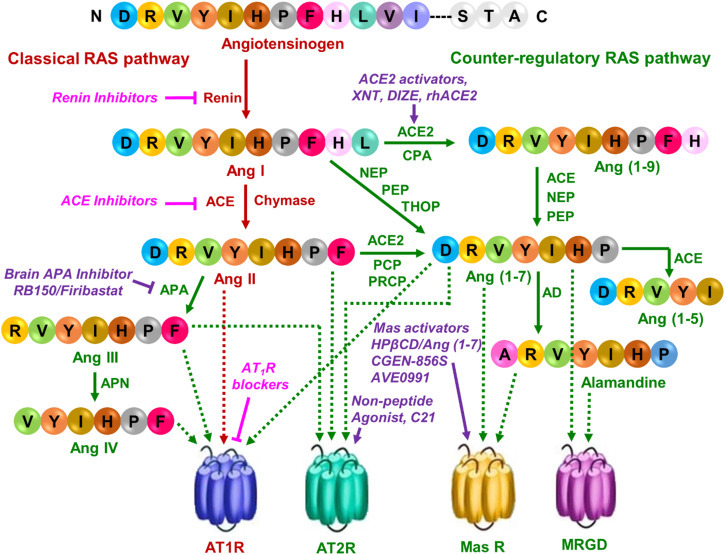
The classical and counter-regulatory renin-angiotensin pathways with currently available and developmental drugs. The classical RAS pathway is shown in dark red. Currently available antihypertensive drugs are shown in pink with italics. The counter-regulatory RAS pathway is shown in dark green. Compounds targeting this pathway are shown in violet, italics. Solid arrow indicates the synthesis of peptides, while the dotted arrow shows peptides targeting to their respective receptors. APA, aminopeptidase A; APN, aminopeptidase N; ACE2, angiotensin-converting enzyme 2; CPA, carboxypeptidase A; NEP, neutral endopeptidase; PEP, prolyl endopeptidase; THOP, thimet oligopeptidase; PCP, prolyl carboxyendopeptidase; PRCP, prolyl carboxypeptidase; AD, aspartate decarboxylase; AT1R, Angiotensin II type 1 receptor; AT2R, Angiotensin II type 2 receptor; Mas R, Mas receptor; MRGD, Mas-related G protein-coupled receptor member D.
An orally active non-peptide, a direct renin inhibitor, Aliskiren (Fig. 2 ) was FDA approved for the treatment of hypertension. It reduced plasma renin activity and hypertension, which are effects similar to those produced by ACEIs and ARBs. Aliskiren is well-tolerated with a relatively lower incidence of cough and angioedema (life-threatening airway swelling and obstruction) than those taking ACEIs. Gastrointestinal adverse effects upon taking Aliskiren is reported in some patients. The ALTITUDE trial studies (Novartis, 2014) reported increased adverse events (non-fatal stroke, renal complications, hyperkalemia, hypotension) with no apparent additional benefits in diabetic patients.
Fig. 2.

Chemical structures of the approved drugs targeting different components of the renin-angiotensin system. AT1R, Angiotensin II type 1 receptor; ACE, Angiotensin-converting enzyme; NEP, Neprilysin.
Release of renin by the kidney is under feedback control of circulating Ang II levels as well as many physiological factors, including ambient blood pressure, fluid volume, and electrolyte balance. Higher levels of prorenin than renin is released to plasma in disease states such as hypertension and diabetes. The question, how prorenin is activated outside the kidney led to the discovery of the prorenin receptor (PRR) (Batenburg et al., 2004, Nguyen et al., 2002). PRR is a ubiquitously expressed 350-amino acid protein, previously described as Na/H+ ATPase, that binds prorenin and induces Ang I generation without proteolytic activation of prorenin. PRR expression levels are high in brain regions, which could be particularly relevant because the expression of classic RAS components is low. The PRR may contribute to angiotensin surges, and drugs to control renin activity by targeting PRR may be developed in the future.
The prohormone Ang I serves as the substrate for two prominent Zn protease angiotensin-converting enzymes, ACE and ACE2 abundantly expressed in the lungs but also in other tissues. Classical ACE is the same protein known as the kininase II in the kinin-kallikrein system. Thus, ACE converts Ang I to the vasoconstrictor octa-peptide hormone Ang II (Fig. 1), and it also degrades vasodilator bradykinins. ACE activity increases blood pressure by producing Ang II, which induces constriction of blood vessels. This enzyme is the target of one of the most successful antihypertensive drugs, with a minimum of side effects and complications. Starting with captopril, a series of long-acting ACE inhibitors such as ramipril, enalapril, lisinopril (Fig. 2) have been highly successful drugs, primarily used for the treatment of high blood pressure, but have been effectively repurposed for treatment in heart failure, kidney damage in diabetes and fibrotic tissue damage.
ACE2 is a late addition to RAS, after the characterization of AT1 and AT2 receptors. It is a smaller molecule with high homology and functional similarity to ACE. However, it’s enzymatic mechanism is different, being a carboxyl mono-peptidase it degrades Ang II to produce Ang (1−7) (Fig. 1). Removal of the C-terminal residues from Ang I to form Ang (1−9) combined then with the carboxyl di-peptidase activity of ACE also produces Ang (1−7) and Ang (1−5) (Fig. 1). Of these, Ang (1−9) and Ang (1−5) are inert degradation products. There are a few reports of the biological effects of these peptides that need further confirmation. Whereas Ang (1−7) was first shown to activate phospholipase A2 to release arachidonic acid from phospholipids leading to the formation of prostaglandins through a GPCR, MasR, and later through the Mas related GPCR, MRGD. Both inhibitory and potentiating pharmacological agents targeting ACE2 have been disappointing in experimental systems without leading to clinically useful drugs as yet.
In recent literature actions of Ang II through AT1R is described as classical RAS axis (Fig. 1) presumably because most of the known Ang II functions are mediated by the AT1R. Further, AT1R gene null mice do not survive, an outcome similar to knocking out the angiotensinogen gene. In contrast, knocking out genes for all other components of RAS is not lethal but demonstrate adaptive changes. Thus, the second axis of RAS, a counter regulator of the classical RAS axis (Fig. 1), is proposed consisting of AT2R, MasR, and MRGD, their cognate ligands, and enzymes that produce these ligands. We will summarize recent advances concerning these two arms of RAS.
Classical RAS, Signaling Mechanisms and Topics of Current Interest in AT1R Research
Classical RAS consisting of ACE/Ang II/AT1R promotes almost all of the homeostatic regulatory functions of Ang II on cardiovascular, renal, and cerebral systems. The physiological levels of the hormone Ang II maintains normal blood pressure through regulation of peripheral resistance of vasculature, heart rate, cardiac output, neuronal control, and body fluid homeostasis through activation of the AT1R. Whereas, overstimulation of AT1R is associated with disease states such as hypertension, heart failure, cardiac hypertrophy, coronary artery disease, stroke, ischemic heart, diabetic nephropathy, arrhythmia and renal diseases (Khan, 2011, Lee et al., 2012, Vejakama et al., 2012, Vijayaraghavan and Deedwania, 2011).
Upon Ang II binding to AT1R intracellular signals are mediated by heterotrimeric G-proteins (Gq/11, G12/13, and Gi), which interact with the receptor, followed by the production of second messengers such as inositol trisphosphate, diacylglycerol, reactive oxygen species (ROS), and arachidonic acid. These molecules trigger the activation of downstream effectors like phospholipases C, A, and D. Furthermore, AT1R activates various protein kinases intracellularly, including serine/threonine kinases such as mitogen-activated protein kinase (MAPK) family kinases, Protein kinase C (PKC), and Protein kinase B (PKB or Akt), receptor tyrosine kinases (RTKs) and non-receptor tyrosine kinases (nRTKs). AT1R also produces G protein-independent signals, such as β-arrestin signaling (Wei et al., 2003). Some novel and expanding Ang II signaling pathways investigated are Wnt/ β-catenin pathway, Notch pathway, NLRP3 (Nucleotide-binding domain and leucine-rich repeat-containing PYD-3) inflammasome and Hippo pathway which plays a variety of roles during developmental processes. Further, the key expanding areas include intracellular as well as extracellular organelle signal communications, signaling through posttranslational protein modification, cellular and tissue metabolic modulation, microRNAs, and long noncoding RNAs (Forrester et al., 2018).
Non-canonical AT1R functions have been described that include ligand-independent activation of AT1R through either mechanical stress or AT1R -directed agoniztic autoantibodies or via receptor mutations (Liu et al., 2016, Mederos y Schnitzler et al., 2011, Storch et al., 2012, Unal et al., 2012, Wallukat and Schimke, 2014). These modes of activation may occur clinically, as in hypertension or preeclampsia (Wei et al., 2011, Zou et al., 2004). Candesartan and other inverse agonizts may show increased therapeutic effects in these disease conditions (Wei et al., 2011, Zou et al., 2004). AT1R signaling properties could be altered in pathology when AT1R forms both homodimers and heterodimers (AbdAlla et al., 2004, AbdAlla et al., 2000, Ayoub et al., 2015, Bellot et al., 2015, de Lourdes Gonzalez-Hernandez et al., 2010, Goupil et al., 2015, Martinez-Pinilla et al., 2015, Rozenfeld et al., 2011, Siddiquee et al., 2013, Wnorowski and Jozwiak, 2014) in disease states like atherosclerosis, preeclampsia, and chronic kidney disease (AbdAlla et al., 2001, AbdAlla et al., 2004, de Lourdes Gonzalez-Hernandez et al., 2010).
β-arrestin biased signaling in GPCRs is thought to be a relatively protective long-term effect of hormonal action. The discovery of β-arrestin biased AT1R ligands preferentially activating the β-arrestin-mediated signaling pathway (Violin et al., 2010, Wei et al., 2003) has gained much attention due to therapeutic potential in treating cardiovascular diseases (Boerrigter et al., 2011, Boerrigter et al., 2012). The size of the side chain of eighth residue present in different Ang II analogs determines the relative strength of AT1R signaling bias through G-protein and β-arrestin pathways (Domazet et al., 2015, Rajagopal et al., 2011, Zimmerman et al., 2012). Ang II and other full agonizts with Phe8 efficiently activate the Gq pathway. Whereas, Ile8 substitution in S1I8 changes it to a partial agonist for Gq signaling. It has been reported that strong β-arrestin biased ligands (which functions through β-arrestin while deficient in Gq-mediated functions) have smaller side chains (e.g., Ala) or even deletion of eighth residue (Rajagopal et al., 2011, Strachan et al., 2014).
Crystal Structures of AT1R
Crystallographic structures of both active and inactive conformational states are now available for AT1R, which provide insights into the conformational dynamics of the receptor in agonist and antagonist bound states.
Two inactive state structures were solved first, with an experimentally used antagonist ZD7155 (PDB ID: 4YAY) and clinically used inverse agonist olmesartan (PDB ID: 4ZUD) (Zhang et al., 2015a, Zhang et al., 2015b). For solving the structure, the human AT1R was engineered for thermal stability by truncating at the N-terminal region (delete Met1, Thr7-Asp16) and C-terminal tail at the end of helix 8, and by inserting a thermos stabilized apocytochrome, b562RIL (BRIL) at N-terminus. The final structure contains 289 out of 359 full-length residues of AT1R. However, these modifications did not alter the pharmacological and functional properties of the receptor for antagonist binding and signaling (Zhang et al., 2015b). High-accuracy computer model of hAT1R that included deleted portions were generated (Singh et al., 2018). The binding poses of experimentally used antagonists, and eight clinically used ARBs were found to be similar. Molecular dynamics simulation studies validated experimental Ki differences of ARBs as well as discriminatory mutagenesis data. Differences in spatiotemporal interactions of different ARBs was observed, which could account for efficacy differences reported for ARBs in various clinical trials. Nature and bonding energy contribution of some critical residues involved in binding all ARBs significantly differ. For example, Arg167ECL2 sidechain interacted with both imidazole and tetrazole groups in olmesartan but not in candesartan, losartan, and irbesartan. Instead, Lys199TM5 interacted with losartan and irbesartan. Valsartan interactions with Lys199TM5 is a weak water-mediated interaction. Both Arg167ECL2 and Lys199TM5 bond with two carboxylic groups of eprosartan, but mutating either of these residues do not affect eprosartan binding (Singh et al., 2018). Initial structural papers also predicted that Ang II binding mode to differ from that of ARBs, but sharing critical contact residues (Tyr353.91, Trp842.60, Tyr872.63, Arg167ECL2, Lys1995.42, Ile2887.39, and Tyr2927.43) which can account for the competitive antagonistic relationship of ARBs with Ang II (Singh et al., 2018).
The active state structure of AT1R bound to the Ang II analog [Sar1, Ile8]Ang II (S1I8) was elucidated at 2.9 Å resolution (PDB ID: 6DO1) (Wingler et al., 2019a, Wingler et al., 2019b). The crystallization construct included all N-terminal residues and BRIL inserted into ICL3 as has been done for solving structures of many other GPCRs. In this configuration, the engineered receptor construct increased the affinity for Ang II ~2-fold and also displayed strong binding to an active-state conformation-specific AT1R nanobody, Nb. AT110i1. This study demonstrated unequivocally the critical residues of AT1R that bond with various sidechains in the peptide ligand and provided a structural interpretation for the movement of different segments of the receptor, which enable signaling. For instance, Pro7 and Ile8 of S1I8 (Phe8 in Ang II) interact with Trp842.60, Val1083.32, Leu1123.36, and Ile2887.38, which are located at the bottom of the orthosteric binding pocket. Ile8 of the peptide also forms a hydrophobic contact with His2566.51, a residue critical for AT1R activation (Noda et al., 1995). Unlike ARBs, the peptide ligand is involved in extracellular interactions with hydrophobic residues (Ile172ECL2, Tyr92ECL1, Val179ECL2, and Ala181ECL2) and charged residues (Asp2817.32 and Asp2636.58) confirming prior mutagenesis results (Feng et al., 1995, Fillion et al., 2013). The terminal carboxylate of the peptide interacts with Lys1995.42. This interaction has been observed as a key contact in AT1R activation by mutagenesis studies (Noda et al., 1995, Fillion et al., 2010). Due to inward shifts of TM5 and TM7, and changes in ELC2 conformation, the ligand-binding pocket is substantially constricted around the peptide in the active state receptor compared to the inactive structure. The peptide-binding site of the AT1R showing S1I8-AngII binding residues of the receptor are shown in Fig. 3 .
Fig. 3.

The peptide-binding site of the AT1R showing S1I8-AngII binding residues of the receptor and position of helix 8 in an active state of AT1R. Sarcosine1, Isoleucine8-AngII is abbreviated as S1I8-AngII. The S1I8-AngII-binding residues of AT1R are shown in green. S1I8-AngII is shown in orange.
While comparing the active and inactive structures of AT1R, the most notable conformational changes were observed on the intracellular side of the receptor, as observed in almost all activated GPCR structures (Manglik and Kruse, 2017). Outward displacement of TM6 by 11 Å, along with the rotation of TM5 away from the G protein/β-arrestin binding pocket and inward rotation TM7, was clearly observed. Additionally, a short α helix is formed by the reorganization of the ICL2, and a significant repositioning of helix 8 was also observed. Helix 8 in the inactive AT1R structure (Fig. 4 ) follows an atypical conformation bent away from the membrane plane. On the other hand, the active structure (Fig. 3) displays the conventional position of Helix 8 parallel to the membrane. The structural findings validate several functional studies suggesting that the Helix 8 of AT1R may be a critical motif for generating G protein independent signals.
Fig. 4.

The ARB’s binding site of the AT1R showing ZD7155 (antagonist) binding residues of the receptor and position of helix 8 in an inactive state of AT1R. The antagonist ZD7155 binding residues of AT1R are shown in orange. ZD7155 is shown in violet.
The activation mechanism of AT1R suggested by Wingler et al. suggests the movement of Trp2536.48 and Tyr2927.43 to avoid a steric clash with Phe8 sidechain of Ang II, resulting ultimately in the breakage of a hydrogen bond between Asn1113.35-Asn2957.46 present in the inactive structure (Wingler et al., 2019a, Wingler et al., 2019b). A slightly different scheme for activation is suggested by MD simulation studies. This model suggests that Phe8 in Ang II is involved in a van der Waals “grasp” interaction with Ile2887.39 in AT1R. An induced mechanical strain pulls Tyr2927.43 and breaks critical inter-helical H-bonds, first between Tyr2927.43 and Val1083.32 and second between Asn1113.35 and Asn2957.46 (Singh et al., 2019). The consensus step in both schemes, breakage of hydrogen bond(s) between Asn1113.35-Asn2957.46 was shown to activate AT1R ligand independently by mutagenesis studies (Noda et al., 1995, Singh et al., 2019, Wingler et al., 2019b).
Approved ARBs and Their Clinical Potential
Eight ARBs, azilsartan, candesartan, eprosartan, olmesartan, irbesartan, losartan, telmisartan, and valsartan are in clinical use as safe drugs primarily to control blood pressure. These drugs vary in their duration of action and efficacy when repurposed in the treatment of disorders of kidney, heart, lungs, fibrogenesis of liver, kidney, or heart and stroke prevention. Additional indications have been recommended. For example, telmisartan can improve glucose metabolism and lipid profile (Nedogoda et al., 2013). Losartan shows uricosuric activity (Nedogoda, 2011). Reduction in inflammatory processes in vessel walls was observed in patients with arterial hypertension and metabolic syndrome when treated with ARBs, thus decreasing their risk for cardiovascular disease and development of diabetes mellitus (Savoia and Schiffrin, 2007). Based on the experimental data, sartans are reported to have possible therapeutic effects in the treatment of autoimmune diseases such as rheumatoid arthritis (Silveira et al., 2013) and multiple sclerosis (Lanz et al., 2010).
AT1 receptors are abundantly expressed on the endothelial cells of the blood-brain barrier, and these have been benefited by the neuroprotective actions of the ARBs (Saavedra et al., 2011). ACE inhibitors captopril and perindopril, and ARB’s losartan, telmisartan, and candesartan have shown neuroprotective effects in animal Parkinson’s disease models. These effects seem to be mediated by a decrease in the overproduction of reactive oxygen species (ROS) (Perez-Lloret et al., 2017). Preclinical and clinical data support potential antidepressant properties of ACEIs and ARBs. For example, captopril (Costall et al., 1990), losartan (Llano Lopez et al., 2012, Srinivasan et al., 2003), valsartan (Ping et al., 2014), irbesartan (Ayyub et al., 2017), telmisartan (Aswar et al., 2017), and Candesartan (Benicky et al., 2011, Saavedra et al., 2006) had positive effects on depression and reduced anxiety behavior, whereas other antihypertensive agents did not (Vian et al., 2017).
Dual-Acting Angiotensin-Receptor/NEP Inhibitor (ARNI)
Sacubitril/valsartan or LCZ696, called Entresto (developed by Novartis), is a novel dual-acting drug formulation comprising equimolar amounts of the ARB valsartan and the neprilysin inhibitor sacubitril (Sacubitril is a prodrug that is activated to sacubitrilat by de-ethylation via esterases). This drug combination increases neprilysin activity while inhibiting the harmful effects of the RAS, without affecting bradykinin and other neprilysin-derived vasoprotective factors. Sacubitril/ valsartan has demonstrated clinical efficacy in lowering blood pressure in patients with primary hypertension and patients with or without heart failure. The landmark clinical trial named PARADIGM-HF [Prospective comparison of ARNI (LCZ696) with ACEI (Enalapril) to Determine Impact on Global Mortality and morbidity in chronic Heart Failure] showed that LCZ696 was significantly more effective than enalapril in treating heart failure (McMurray et al., 2014, Mogensen et al., 2018). Sacubitril/valsartan was approved by the FDA in 2015. This drug is now included in European (Ponikowski et al., 2016) and American (Yancy et al., 2016) clinical practice guidelines for the treatment of heart failure.
Counter-Regulatory RAS With its Importance and Limitations
Counter- regulatory or the non-canonical or the non-classical RAS primarily consists of Ang II/Ang III-AT2R, and the ACE2-Ang-(1−7)-MasR axis. Generally, it is believed that the counter-regulatory RAS functions in a manner antagonistic to the deleterious effects of a dysregulated classical RAS, the Ang II-AT1R axis. This includes actions of extensively studied ligand/receptor actions such as Ang (1−7)/MasR and Ang II or Ang III/AT2R, as described earlier (Forrester et al., 2018). There are also reports suggesting the actions of Ang (1−9)/AT2R on the cardiovascular system (Mendoza-Torres et al., 2018). Potential roles of ligands, alamandine, and Angiotensin A remain poorly characterized. Ang-(1−7) has also been suggested to function as a β-arrestin-biased agonist of AT1R (Teixeira et al., 2017). Alamandine functions through MRGD and activates the AMP-activated protein kinase (AMPK)–nitric oxide (NO) pathway, which prevents Ang II-induced hypertrophy (Jesus et al., 2018). Angiotensin 1–5 has been shown to induce atrial natriuretic peptide (ANP) secretion through the Mas receptor and activating the PI3K–Akt–endothelial NO synthase pathway (Yu et al., 2016).
Different research groups have different opinions about whether Ang-(1−7) binds directly to the Mas receptor or not, and this has been reflected from the contradictory results published. Some showed that Ang-(1−7) can oppose the Ang II signaling as a result of heterodimerization between AT1R and Mas receptor but not by direct binding to the Mas receptor (Gaidarov et al., 2018, Kostenis et al., 2005). However, others demonstrated the binding of radiolabeled or fluorescent Ang-(1−7) to the Mas receptor-expressing tissue (Santos et al., 2003). In summary, more research is required to confirm whether Ang-(1−7) acts as an endogenous agonist of the Mas receptor.
Crystal Structures of AT2R
AT2R structures have been solved with either small-molecule ligands (quinazolinone-biphenyl tetrazole derivatives 1 and 2) named as compound 1 and compound 2 (Zhang et al., 2017b). The compound 1 is AT2R selective, while compound 2 is AT1R/AT2R dual ligand [PDB ID: 5UNF; 5UNG; 5UNH]. Following this study, the peptide ligand-bound AT2R structures were solved with S1I8 (PDB ID: 5XJM) or the endogenous peptide, Ang II (PDB ID: 6JOD) (Asada et al., 2018, Asada et al., 2020).
A nearly similar AT2R construct was used to obtain both S1I8-Ang II-bound and Ang II bound crystal structures. The AT2R-specific antibody, Fab4A03, was added during the purification of both the structures to increase thermal stability and facilitate crystallization. Both structures lack the putative C-terminal palmitoylation site (residue range: 35–346) and N-terminal glycosylation sites. The difference lies in the type and position of BRIL that was inserted in the third intracellular loop in S1I8 bound AT2R and fused at N-terminus in Ang II-bound AT2R. The S1I8-Ang II bound AT2R sequence includes 35–346 of the 363 residues, while the Ang II bound AT2R sequence includes 1–346 of 363 residues.
The structures demonstrated AT2R to accommodate both peptides in a very similar binding mode. The Ang II sidechains interacted with AT2R residues whose position and chemical characteristics are conserved, as seen in the AT1R structure. Arg2 forms salt bridges with Asp2796.58 and Asp2977.32, which are conserved as Asp2636.58 and Asp2817.32 in AT1R. The guanidinium group of Arg182ECL2, which is conserved as Arg167ECL2 in AT1R bonds with carbonyl oxygens of His6 and Pro7 in Ang II. Phe8C-terminal carboxyl group forms a salt bridge with the side chain of Lys2155.42, which is conserved as Lys1995.42 in AT1R. Phe8 of Ang II also interacts with Leu1243.32, Met1283.36, Trp2696.48, Phe2726.51, and Phe3087.43. Ligand binding and mutagenesis experiments proved that Tyr1042.64, Met1283.36, Lys2155.42, Trp2696.48, Phe2726.51, Arg182ECL2, Asp2977.32, and Phe3087.43 play vital roles in Ang II binding. Although Fab4A03, AT2R specific antibody used during crystallization, does not affect the conformation of the side chains critical for ligand binding in both Ang II-bound and S1I8-Ang II-bound AT2R, ECL2 conformation on the surface is affected in both structures. The peptide-binding site of the AT2R with Ang II binding residues of the receptor are shown in Fig. 5 .
Fig. 5.
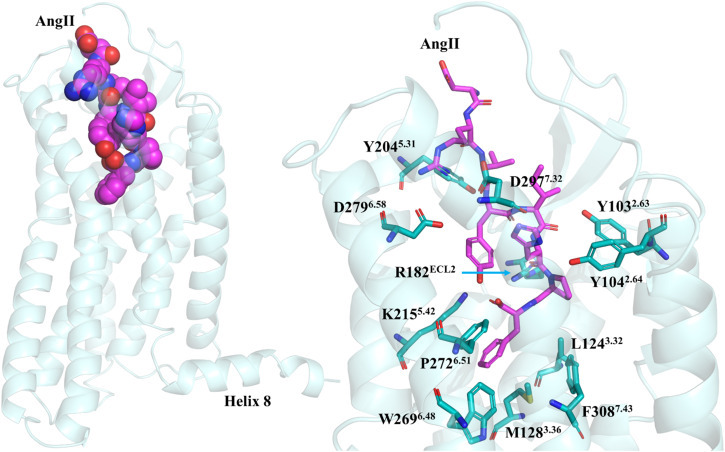
The peptide-binding site of the AT2R showing Ang II binding residues of the receptor and position of helix 8. The Ang II-binding residues of AT2R are shown in teal. Ang II is shown in magenta.
The residues at the bottom of the ligand-binding pocket and Met1283.36 (Leu1123.36 for AT1R), seems to play a key role in AT2R activation. Upon Ang II binding, Met1283.36 moves toward Phe3087.43 (Phe3087.43 for AT1R) to make room for Phe8 side chain of Ang II, leading to the rotation of TM7 possessing Phe3087.43. These residues form hydrophobic core at the bottom of the ligand-binding cavity of AT2R, as observed in AT1R. The insertion of Ang II Phe8 residue into this hydrophobic core appears to trigger the activation of the receptor, where the conformational change is transferred through the region containing the internal lock, Asn1113.35-Asn2957.46 leads to the breakage of the hydrogen bond between these residues during AT1R activation. In AT2R, Ser3117.46 is present in place of Asn2957.46, and a hydrogen bond between Asn1273.35 and Ser3117.46 is not observed in the Ang II-bound AT2R structure. It is not clear whether these residues form hydrogen bond in the inactive conformation since AT2R inactive structure is not available. However, AT2R has been described as a constitutively active receptor (Miura and Karnik, 2000), therefore it may naturally lack the hydrogen bond between Asn1273.35 and Ser3117.46.
In support of this view, an active like conformation of AT2R was captured (Asada et al., 2018, Asada et al., 2020, Zhang et al., 2017b). Structures showed that AT2R lacks the key conformational locks seen in AT1R: 1) TM5/TM6 phenylalanine cluster (Phe2085.51/Phe2496.44/Phe2506.45) in AT1R are replaced by aliphatic residues (Leu2245.51/Phe2656.44/Ile2666.45) in AT2R. In all the three crystal structures available for AT2R (mentioned under structure of AT2R section), these residues align with active AT1R structure rather than inactive AT1R. 2) The Asn1113.35-Asn2957.46 hydrogen bond in the AT1R inactive state is not present in the AT2R structure. Instead, AT2R has Ser3117.46 (in place of Asn2957.46), and Asn3.35 has moved away from the receptor core. 3) In AT1R, T292F mutation increases the affinity towards Ang II while decreases towards antagonists. Whereas Tyr2927.43 is substituted by Phe3087.43 in the AT2R, denoting a mutation-induced-stabilization of an active-like state. 4) Finally, AT2R shows highly unusual behavior as it does not seem to signal through traditional G-protein- and β-arrestin-mediated signaling pathways, and this is potentially due to helix 8 overlap over the transducer-binding site of AT2R (Zhang et al., 2017a).
The MasR and Mas-related GPCRs exhibit structural features of AT2R that cause constitutive activation. In signaling experiments, high constitutive signaling by MasR is reported. Therefore intrinsic constitutive activation is likely a feature of these receptors. However, there has been no structural investigations focused on MasR and its activation by ligands such as Ang (1−7) or related peptides.
Targeting the Counter-regulatory Axis of RAS and Novel Therapeutic Approaches
AT2R signaling and functions are better understood now and being translated to develop potential drugs targeting AT2R. The developmental drugs are AT2R agonizts with primary indications for use in fibrotic diseases and diabetic nephropathy. A non-peptide AT2R agonist, Compound 21 (C21) (Fig. 6 ), has successfully completed phase I clinical testing and entered Phase II clinical study in patients with Pulmonary fibrosis in systemic sclerosis (SSc) and Idiopathic pulmonary fibrosis (IPF). In addition, a phase II study of C21 in patients with COVID-19 proposed. The rationale behind this study is to address an imbalance in the local RAS caused by ACE2 inactivation due to COVID-19 binding. C21 may suppress inflammatory mediators by acting directly on the AT2R and bypass the way by which the virus disables the system (See Relevant Websites Section). Selective AT2R agoniztic lanthipeptide MOR107 (MorphoSys, See Relevant Websites Section) previously known as LP-2 (by Lanthio Pharma See Relevant Websites Section) is currently being tested in a phase I clinical trial with a focus on oncology indications. The AT2R antagonist EMA401 (Fig. 6) has completed a phase II clinical trial for the treatment of neuropathic pain (Rice et al., 2014). It is currently undergoing additional phase II studies (Novartis, 2020).
Fig. 6.

Chemical structures of the drugs which are under clinical trials targeting different components of the renin-angiotensin system. AT2R, Angiotensin II type 2 receptor; MasR, Mas Receptor; APA, Aminopeptidase A.
Central acting Aminopeptidase A (APA) inhibitors are in consideration for the treatment of neurogenic hypertension. Overproduction of Ang III in the brain exerts a tonic stimulatory control over blood pressure in experimental models of hypertension. Brain Ang III is generated by brain APA, and hence brain APA represents a promising target for the development of potent and selective central-acting antihypertensive agents. RB150/QGC001 (4,4-dithio-{bis[(3S)−3-aminobutyl sulfonic acid]}), a prodrug of selective and specific APA inhibitor EC33, later renamed as Firibastat (Fig. 6), was developed for clinical studies (Fournie-Zaluski et al., 2004). Orally administered RB150 crosses the hepatic, intestinal, and blood-brain barriers. The prodrug RB150 is cleaved by brain reductases on entry into the brain to generate two active molecules of EC33. This active molecule then inhibits the brain APA activity, block the formation of brain Ang III (Fournie-Zaluski et al., 2004), thus decrease BP and arginine-vasopressin release in deoxycorticosterone acetate salt rats and spontaneously hypertensive rats (Bodineau et al., 2008, Marc et al., 2012, Marc et al., 2018). A phase Ia clinical studies have shown that Firibastat is well tolerated in healthy human volunteers (Balavoine et al., 2014). A phase IIa trial was carried out in grade I and II hypertensive patients (Azizi et al., 2017), which suggests that firibastat treatment is very effective in decreasing daytime ambulatory systolic blood pressure (SBP) without causing hypotension. Later, a large phase IIb studies, NEW-HOPE (Novel Evaluation with QGC001 in Hypertensive Overweight Patients of Multiple Ethnic Origins), was carried out in overweight hypertensive patients and a significant BP-lowering efficacy and a safe tolerability profile were observed (NCT03198793) (Ferdinand et al., 2018) in those patients. If the proposed phase III trials to determine the efficacy succeed, firibastat could be the first of a new class of centrally acting antihypertensive agents. Based on all the clinical studies, firibastat may be especially effective in African Americans who are poor responders to blockers of the systemic RAS.
A recent study has shown that an oral formulation of hydroxypropyl-β-cyclodextrin/Ang-(1−7) (Fig. 6) is effective in humans, as demonstrated by attenuating eccentric overload muscle damage (Becker et al., 2018). The effects of Ang-(1−7) oral formulation in humans may open new possibilities for evaluating the actions Ang-(1−7) and its therapeutic effects in patients.
RAS Components as Targets of New Diseases Including COVID-19
RAS is a complex, multifunctional system that possesses important roles beyond the cardiovascular system. Alterations in the expression of these components were shown to be involved in various diseases. For example, brain RAS involving the ACE2/Ang-(1−7)/Mas receptor axis and the Ang IV/insulin-regulated aminopeptidase pathways may play a role in Parkinson’s and Alzheimer’s diseases (Wright et al., 2013). ACE, Ang-(1−7), AT2 receptors, and N-acetyl-Ser-Asp-Lys-Pro may have a role in hematopoiesis (Rodgers and Dizerega, 2013). Aldosterone produced locally may have a pathogenic role (Aroor et al., 2013, De Mello and Frohlich, 2014), and the ACE2/Ang-(1−7)/Mas receptor pathway may take part in reproduction, fetal programming, and cancer (Chappell et al., 2014, Herr et al., 2013). Activation of skeletal RAS plays a major role in bone diseases, such as arthritis, osteoporosis, and deterioration, as well as in fracture healing (Zhao et al., 2019).
The recently emerged disease involving the RAS component is COVID-19 (Coronavirus Disease 2019) pandemic. The virus is named SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2). The virus binds to the ACE2 receptor for entry into target cells. ACE2 prevents adverse effects of Ang II by degrading it to Ang-(1−7) (Donoghue et al., 2000, Hamming et al., 2007) and has been implicated in hypertension (Allred et al., 2002, Crackower et al., 2002), diabetes (Tikellis et al., 2003), Ang-(1−7) regulation during pregnancy (Brosnihan et al., 2003), heart failure and ventricular remodeling (Donoghue et al., 2003, Zisman et al., 2003). ACE2 predominantly expressed on epithelial cells of lungs, heart, kidney, and intestine (Donoghue et al., 2000, Zhang et al., 2020, Zhao et al., 2020) is a functional receptor for the coronavirus (Li et al., 2003). Interaction between the SARS-CoV-2 spike receptor-binding domain (RBD) and ACE2 has been shown in atomic-resolution by structural studies (Lan et al., 2020, Wang et al., 2020). These findings shed light on virus recognition, infection, and provide important structural information about the development of therapeutic treatment against this emerging virus.
Several comorbidities, including cardiovascular diseases, diabetes mellitus, and hypertension, are involved in COVID-19 patients with a severe course of progression and contributing to higher mortality risk (The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, china, 2020). Some research groups reported that both ACEI and ARBs could substantially increase the mRNA expression of cardiac ACE2 (Ferrario et al., 2005), which may increase virus susceptibility. However, due to lack of evidence about the potential negative impact of these medications on COVID-19 infection, European and American Societies of Cardiology expressed that ACEIs and ARBs are safe to continue and should be prescribed according to established guidelines (Sommerstein et al., 2020). In conclusion, cardiovascular diseases and/or their therapy, by influencing ACE2 levels, may play a crucial role in infectiveness and outcome of COVID-19. Whether treatment or disease triggered upregulation of ACE2 affects the course of COVID-19 needs to be determined.
Acknowledgment
We thank Karnik Laboratory members, Drs. Dhanachandra S. Khuraijam and Terri Harford for critical reading and suggestions for improving this manuscript. This work was supported by National Institutes of Health grants, HL132351, and HL142091 to SSK.
This is an update of T. Inagami, Angiotensin Receptors, in Encyclopedia of Biological Chemistry (Second Edition), Edited by: William J. Lennarz, M. Daniel Lane, Elsevier Inc., 2013, https://doi.org/10.1016/B978-0-12-378630-2.00489-8.
References
- AbdAlla S., Lother H., Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature. 2000;407:94–98. doi: 10.1038/35024095. [DOI] [PubMed] [Google Scholar]
- AbdAlla S., Lother H., el Massiery A., Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nature Medicine. 2001;7:1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- AbdAlla S., Lother H., Langer A., el Faramawy Y., Quitterer U. Factor XIIIA transglutaminase crosslinks AT1 receptor dimers of monocytes at the onset of atherosclerosis. Cell. 2004;119:343–354. doi: 10.1016/j.cell.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Allred A.J., Donoghue M., Acton S., Coffman T.M. Regulation of blood pressure by the angiotensin converting enzyme homologue ACE2. American Journal of Nephrology. 2002;13:52A. [Google Scholar]
- Aroor A.R., Demarco V.G., Jia G., et al. The role of tissue renin-angiotensin-aldosterone system in the development of endothelial dysfunction and arterial stiffness. Frontiers in Endocrinology. 2013;4:161. doi: 10.3389/fendo.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada H., Horita S., Hirata K., et al. Crystal structure of the human angiotensin II type 2 receptor bound to an angiotensin II analog. Nature Structural & Molecular Biology. 2018;25:570–576. doi: 10.1038/s41594-018-0079-8. [DOI] [PubMed] [Google Scholar]
- Asada H., Inoue A., Ngako Kadji F.M., et al. The Crystal structure of angiotensin II type 2 receptor with endogenous peptide hormone. Structure. 2020;28:418–425. doi: 10.1016/j.str.2019.12.003. (e414) [DOI] [PubMed] [Google Scholar]
- Aswar U., Chepurwar S., Shintre S., Aswar M. Telmisartan attenuates diabetes induced depression in rats. Pharmacological Reports: PR. 2017;69:358–364. doi: 10.1016/j.pharep.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Ayoub M.A., Zhang Y., Kelly R.S., et al. Functional interaction between angiotensin II receptor type 1 and chemokine (C-C motif) receptor 2 with implications for chronic kidney disease. PloS One. 2015;10:e0119803. doi: 10.1371/journal.pone.0119803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyub M., Najmi A.K., Akhtar M. Protective effect of Irbesartan an angiotensin (AT1) receptor antagonist in unpredictable chronic mild stress induced depression in mice. Drug Research. 2017;67:59–64. doi: 10.1055/s-0042-118172. [DOI] [PubMed] [Google Scholar]
- Azizi M., Courand P., Denolle T., et al. [OP.4A.08] A randomized double-blind placebo controlled crossover study to compare QGC001, a brain aminopeptidase A inhibitor, with placebo in patients with grade I/II essential hypertension. Journal of Hypertension. 2017;35:e36. [Google Scholar]
- Balavoine F., Azizi M., Bergerot D., et al. Randomised, double-blind, placebo-controlled, dose-escalating phase I study of QGC001, a centrally acting aminopeptidase a inhibitor prodrug. Clinical Pharmacokinetics. 2014;53:385–395. doi: 10.1007/s40262-013-0125-y. [DOI] [PubMed] [Google Scholar]
- Batenburg W.W., Garrelds I.M., Bernasconi C.C., et al. Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation. 2004;109:2296–2301. doi: 10.1161/01.CIR.0000128696.12245.57. [DOI] [PubMed] [Google Scholar]
- Becker L.K., Totou N., Moura S., et al. Eccentric overload muscle damage is attenuated by a novel angiotensin- (1-7) treatment. International Journal of Sports Medicine. 2018;39:743–748. doi: 10.1055/a-0633-8892. [DOI] [PubMed] [Google Scholar]
- Bellot M., Galandrin S., Boularan C., et al. Dual agonist occupancy of AT1-R-alpha2C-AR heterodimers results in atypical Gs-PKA signaling. Nature Chemical Biology. 2015;11:271–279. doi: 10.1038/nchembio.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benicky J., Sanchez-Lemus E., Honda M., et al. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2011;36:857–870. doi: 10.1038/npp.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodineau L., Frugiere A., Marc Y., et al. Orally active aminopeptidase A inhibitors reduce blood pressure: A new strategy for treating hypertension. Hypertension. 2008;51:1318–1325. doi: 10.1161/HYPERTENSIONAHA.107.098772. [DOI] [PubMed] [Google Scholar]
- Boerrigter G., Lark M.W., Whalen E.J., et al. Cardiorenal actions of TRV120027, a novel ss-arrestin-biased ligand at the angiotensin II type I receptor, in healthy and heart failure canines: A novel therapeutic strategy for acute heart failure. Circulation. Heart Failure. 2011;4:770–778. doi: 10.1161/CIRCHEARTFAILURE.111.962571. [DOI] [PubMed] [Google Scholar]
- Boerrigter G., Soergel D.G., Violin J.D., Lark M.W., Burnett J.C., Jr. TRV120027, a novel beta-arrestin biased ligand at the angiotensin II type I receptor, unloads the heart and maintains renal function when added to furosemide in experimental heart failure. Circulation. Heart Failure. 2012;5:627–634. doi: 10.1161/CIRCHEARTFAILURE.112.969220. [DOI] [PubMed] [Google Scholar]
- Brosnihan K.B., Neves L.A., Joyner J., et al. Enhanced renal immunocytochemical expression of ANG-(1-7) and ACE2 during pregnancy. Hypertension. 2003;42:749–753. doi: 10.1161/01.HYP.0000085220.53285.11. [DOI] [PubMed] [Google Scholar]
- Chappell M.C., Marshall A.C., Alzayadneh E.M., Shaltout H.A., Diz D.I. Update on the angiotensin converting enzyme 2-angiotensin (1-7)-MAS receptor axis:Fetal programing, sex differences, and intracellular pathways. Frontiers in Endocrinology. 2014;4:201. doi: 10.3389/fendo.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B., Domeney A.M., Gerrard P.A., et al. Effects of captopril and SQ29,852 on anxiety-related behaviours in rodent and marmoset. Pharmacology, Biochemistry, and Behavior. 1990;36:13–20. doi: 10.1016/0091-3057(90)90118-2. [DOI] [PubMed] [Google Scholar]
- Crackower M.A., Sarao R., Oudit G.Y., et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- De Mello W.C., Frohlich E.D. Clinical perspectives and fundamental aspects of local cardiovascular and renal renin-angiotensin systems. Frontiers in Endocrinology. 2014;5:16. doi: 10.3389/fendo.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus I.C.G., Scalzo S., Alves F., et al. Alamandine acts via MrgD to induce AMPK/NO activation against ANG II hypertrophy in cardiomyocytes. American Journal of Physiology-Cell Physiology. 2018;314:C702–C711. doi: 10.1152/ajpcell.00153.2017. [DOI] [PubMed] [Google Scholar]
- de Lourdes Gonzalez-Hernandez M., Godinez-Hernandez D., Bobadilla-Lugo R.A., Lopez-Sanchez P. Angiotensin-II type 1 receptor (AT1R) and alpha-1D adrenoceptor form a heterodimer during pregnancy-induced hypertension. Autonomic & Autacoid Pharmacology. 2010;30:167–172. doi: 10.1111/j.1474-8673.2009.00446.x. [DOI] [PubMed] [Google Scholar]
- Domazet I., Holleran B.J., Richard A., et al. Characterization of angiotensin II molecular determinants involved in AT1 receptor functional selectivity. Molecular Pharmacology. 2015;87:982–995. doi: 10.1124/mol.114.097337. [DOI] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circulation Research. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Donoghue M., Wakimoto H., Maguire C.T., et al. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. Journal of Molecular and Cellular Cardiology. 2003;35:1043–1053. doi: 10.1016/s0022-2828(03)00177-9. [DOI] [PubMed] [Google Scholar]
- Feng Y.H., Noda K., Saad Y., et al. The docking of Arg2 of angiotensin II with Asp281 of AT1 receptor is essential for full agonism. The Journal of Biological Chemistry. 1995;270:12846–12850. doi: 10.1074/jbc.270.21.12846. [DOI] [PubMed] [Google Scholar]
- Ferdinand K., Balavoine F., Besse B., et al. Efficacy and safety of a novel antihypertensive pharmacotherapy approach in a high-risk diverse population (abstract) Circulation. 2018;138:e766–e767. [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Fillion D., Lemieux G., Basambombo L.L., et al. The amino-terminus of angiotensin II contacts several ectodomains of the angiotensin II receptor AT1. Journal of Medicinal Chemistry. 2010;53:2063–2075. doi: 10.1021/jm9015747. [DOI] [PubMed] [Google Scholar]
- Fillion D., Cabana J., Guillemette G., et al. Structure of the human angiotensin II type 1 (AT1) receptor bound to angiotensin II from multiple chemoselective photoprobe contacts reveals a unique peptide binding mode. The Journal of Biological Chemistry. 2013;288:8187–8197. doi: 10.1074/jbc.M112.442053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester S.J., Booz G.W., Sigmund C.D., et al. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiological Reviews. 2018;98:1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournie-Zaluski M.C., Fassot C., Valentin B., et al. Brain renin-angiotensin system blockade by systemically active aminopeptidase A inhibitors: a potential treatment of salt-dependent hypertension. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7775–7780. doi: 10.1073/pnas.0402312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarov I., Adams J., Frazer J., et al. Angiotensin (1-7) does not interact directly with MAS1, but can potently antagonize signaling from the AT1 receptor. Cellular Signalling. 2018;50:9–24. doi: 10.1016/j.cellsig.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Goupil E., Fillion D., Clement S., et al. Angiotensin II type I and prostaglandin F2alpha receptors cooperatively modulate signaling in vascular smooth muscle cells. The Journal of Biological Chemistry. 2015;290:3137–3148. doi: 10.1074/jbc.M114.631119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Cooper M.E., Haagmans B.L., et al. The emerging role of ACE2 in physiology and disease. The Journal of Pathology. 2007;212:n1–n11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr D., Bekes I., Wulff C. Local renin-angiotensin system in the reproductive system. Frontiers in Endocrinology. 2013;4:150. doi: 10.3389/fendo.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagami T. Angiotensin receptors. Encyclopedia of Biological Chemistry. 2004:111–115. [Google Scholar]
- Karnik S.S., Unal H., Kemp J.R., et al. International union of basic and clinical pharmacology. XCIX. Angiotensin receptors: Interpreters of pathophysiological angiotensinergic stimuli (corrected) Pharmacological Reviews. 2015;67:754–819. doi: 10.1124/pr.114.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan B.V. The effect of amlodipine besylate, losartan potassium, olmesartan medoxomil, and other antihypertensives on central aortic blood pressure and biomarkers of vascular function. Therapeutic Advances in Cardiovascular Disease. 2011;5:241–273. doi: 10.1177/1753944711420464. [DOI] [PubMed] [Google Scholar]
- Kostenis E., Milligan G., Christopoulos A., et al. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111:1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- Lan J., Ge J., Yu J., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Lanz T.V., Ding Z., Ho P.P., et al. Angiotensin II sustains brain inflammation in mice via TGF-β. The Journal of Clinical Investigation. 2010;120:2782–2794. doi: 10.1172/JCI41709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., Saver J.L., Hong K.S., et al. Renin-angiotensin system modulators modestly reduce vascular risk in persons with prior stroke. Stroke. 2012;43:113–119. doi: 10.1161/STROKEAHA.111.632596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Wang Y., Wang X., et al. Role of agonistic autoantibodies against type-1 angiotensin II receptor in the pathogenesis of retinopathy in preeclampsia. Scientific Reports. 2016;6:29036. doi: 10.1038/srep29036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano Lopez L.H., Caif F., Garcia S., et al. Anxiolytic-like effect of losartan injected into amygdala of the acutely stressed rats. Pharmacological Reports: PR. 2012;64:54–63. doi: 10.1016/s1734-1140(12)70730-2. [DOI] [PubMed] [Google Scholar]
- Manglik A., Kruse A.C. Structural basis for G protein-coupled receptor activation. Biochemistry. 2017;56:5628–5634. doi: 10.1021/acs.biochem.7b00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc Y., Gao J., Balavoine F., et al. Central antihypertensive effects of orally active aminopeptidase A inhibitors in spontaneously hypertensive rats. Hypertension. 2012;60:411–418. doi: 10.1161/HYPERTENSIONAHA.112.190942. [DOI] [PubMed] [Google Scholar]
- Marc Y., Hmazzou R., Balavoine F., Flahault A., Llorens-Cortes C. Central antihypertensive effects of chronic treatment with RB150: an orally active aminopeptidase A inhibitor in deoxycorticosterone acetate-salt rats. Journal of Hypertension. 2018;36:641–650. doi: 10.1097/HJH.0000000000001563. [DOI] [PubMed] [Google Scholar]
- Martinez-Pinilla E., Rodriguez-Perez A.I., Navarro G., et al. Dopamine D2 and angiotensin II type 1 receptors form functional heteromers in rat striatum. Biochemical Pharmacology. 2015;96:131–142. doi: 10.1016/j.bcp.2015.05.006. [DOI] [PubMed] [Google Scholar]
- McMurray J.J., Packer M., Desai A.S., et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. The New England Journal of Medicine. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- Mederos y Schnitzler M., Storch U., Gudermann T. AT1 receptors as mechanosensors. Current Opinion in Pharmacology. 2011;11:112–116. doi: 10.1016/j.coph.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Mendoza-Torres E., Riquelme J.A., Vielma A., et al. Protection of the myocardium against ischemia/reperfusion injury by angiotensin-(1-9) through an AT2R and Akt-dependent mechanism. Pharmacological Research. 2018;135:112–121. doi: 10.1016/j.phrs.2018.07.022. [DOI] [PubMed] [Google Scholar]
- Miura S., Karnik S.S. Ligand-independent signals from angiotensin II type 2 receptor induce apoptosis. The EMBO Journal. 2000;19:4026–4035. doi: 10.1093/emboj/19.15.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen U.M., Gong J., Jhund P.S., et al. Effect of sacubitril/valsartan on recurrent events in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) European Journal of Heart Failure. 2018;20:760–768. doi: 10.1002/ejhf.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedogoda S.V. Lozartan potential in hyperuricemia correction. Therapeutic Archive. 2011;83:45–48. [PubMed] [Google Scholar]
- Nedogoda S.V., Salasyuk A.S., Chalyabi T.A., et al. Effect of angiotensin II receptor antagonists on left ventricular hypertrophy, vascular elasticity, and lipid and carbohydrate metabolic parameters in metabolic syndrome. Systemic Hypertension. 2013;10:27–33. [Google Scholar]
- Nguyen G., Delarue F., Burckle C., et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. The Journal of clinical investigation. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K., Saad Y., Karnik S.S. Interaction of Phe8 of angiotensin II with Lys199 and His256 of AT1 receptor in agonist activation. The Journal of Biological Chemistry. 1995;270:28511–28514. doi: 10.1074/jbc.270.48.28511. [DOI] [PubMed] [Google Scholar]
- Novartis, 2014. Aliskiren trial in type 2 diabetes using cardiovascular and renal disease endpoints (core and extension phases) (ALTITUDE). ClinicalTrials.gov Identifier: NCT00549757. Available at: https://clinicaltrials.gov/ct2/show/NCT00549757. (accessed 20.04.20).
- Novartis, 2020. Safety and efficacy of EMA401 in patients with painful diabetic neuropathy (PDN) (EMPADINE). ClinicalTrials.gov Identifier: NCT03297294. Available at: https://clinicaltrials.gov/ct2/show/NCT03297294. (accessed 20.04.20).
- Perez-Lloret S., Otero-Losada M., Toblli J.E., Capani F. Renin-angiotensin system as a potential target for new therapeutic approaches in Parkinson’s disease. Expert opinion on Investigational Drugs. 2017;26:1163–1173. doi: 10.1080/13543784.2017.1371133. [DOI] [PubMed] [Google Scholar]
- Ping G., Qian W., Song G., Zhaochun S. Valsartan reverses depressive/anxiety-like behavior and induces hippocampal neurogenesis and expression of BDNF protein in unpredictable chronic mild stress mice. Pharmacology, Biochemistry, and Behavior. 2014;124:5–12. doi: 10.1016/j.pbb.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- Rajagopal S., Ahn S., Rominger D.H., et al. Quantifying ligand bias at seven-transmembrane receptors. Molecular pharmacology. 2011;80:367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A.S.C., Dworkin R.H., McCarthy T.D., et al. EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: A randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet. 2014;383:1637–1647. doi: 10.1016/S0140-6736(13)62337-5. [DOI] [PubMed] [Google Scholar]
- Rodgers K.E., Dizerega G.S. Contribution of the local RAS to hematopoietic function: A novel therapeutic target. Frontiers in Endocrinology. 2013;4:157. doi: 10.3389/fendo.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R., Gupta A., Gagnidze K., et al. AT1R-CB(1)R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. The EMBO Journal. 2011;30:2350–2363. doi: 10.1038/emboj.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J.M., Armando I., Bregonzio C., et al. A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharmacology: Official publication of the American College of Neuropsychopharmacology. 2006;31:1123–1134. doi: 10.1038/sj.npp.1300921. [DOI] [PubMed] [Google Scholar]
- Saavedra J.M., Sanchez-Lemus E., Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: therapeutic implications. Psychoneuroendocrinology. 2011;36:1–18. doi: 10.1016/j.psyneuen.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R.A., Simoes e Silva A.C., Maric C., et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia C., Schiffrin E.L. Vascular inflammation in hypertension and diabetes: Molecular mechanisms and therapeutic interventions. Clinical science. 2007;112:375–384. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- Siddiquee K., Hampton J., McAnally D., May L., Smith L. The apelin receptor inhibits the angiotensin II type 1 receptor via allosteric trans-inhibition. British Journal of Pharmacology. 2013;168:1104–1117. doi: 10.1111/j.1476-5381.2012.02192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira K.D., Coelho F.M., Vieira A.T., et al. Mechanisms of the anti-inflammatory actions of the angiotensin type 1 receptor antagonist losartan in experimental models of arthritis. Peptides. 2013;46:53–63. doi: 10.1016/j.peptides.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Singh K.D., Unal H., Desnoyer R., Karnik S.S. Divergent Spatiotemporal Interaction of Angiotensin Receptor Blocking Drugs with Angiotensin Type 1 Receptor. Journal of Chemical Information and Modeling. 2018;58:182–193. doi: 10.1021/acs.jcim.7b00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.D., Unal H., Desnoyer R., Karnik S.S. Mechanism of Hormone Peptide Activation of a GPCR: angiotensin II Activated State of AT1R Initiated by van der Waals Attraction. Journal of Chemical Information and Modeling. 2019;59:373–385. doi: 10.1021/acs.jcim.8b00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerstein R., Kochen M.M., Messerli F.H., Grani C. Coronavirus disease 2019 (COVID-19): Do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? Journal of the American Heart Association. 2020;9:e016509. doi: 10.1161/JAHA.120.016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan J., Suresh B., Ramanathan M. Differential anxiolytic effect of enalapril and losartan in normotensive and renal hypertensive rats. Physiology & Behavior. 2003;78:585–591. doi: 10.1016/s0031-9384(03)00036-2. [DOI] [PubMed] [Google Scholar]
- Storch U., Mederos y Schnitzler M., Gudermann T. G protein-mediated stretch reception. American Journal of Physiology-Heart and Circulatory Physiology. 2012;302:H1241–H1249. doi: 10.1152/ajpheart.00818.2011. [DOI] [PubMed] [Google Scholar]
- Strachan R.T., Sun J.P., Rominger D.H., et al. Divergent transducer-specific molecular efficacies generate biased agonism at a G protein-coupled receptor (GPCR) The Journal of Biological Chemistry. 2014;289:14211–14224. doi: 10.1074/jbc.M114.548131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira L.B., Parreiras E.S.L.T., Bruder-Nascimento T., et al. Ang-(1-7) is an endogenous beta-arrestin-biased agonist of the AT1 receptor with protective action in cardiac hypertrophy. Scientific Reports. 2017;7:11903. doi: 10.1038/s41598-017-12074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikellis C., Johnston C.I., Forbes J.M., et al. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension. 2003;41:392–397. doi: 10.1161/01.HYP.0000060689.38912.CB. [DOI] [PubMed] [Google Scholar]
- Unal H., Jagannathan R., Karnik S.S. Mechanism of GPCR-directed autoantibodies in diseases. Advances in Experimental Medicine and Biology. 2012;749:187–199. doi: 10.1007/978-1-4614-3381-1_13. [DOI] [PubMed] [Google Scholar]
- Vejakama P., Thakkinstian A., Lertrattananon D., et al. Reno-protective effects of renin-angiotensin system blockade in type 2 diabetic patients: a systematic review and network meta-analysis. Diabetologia. 2012;55:566–578. doi: 10.1007/s00125-011-2398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vian J., Pereira C., Chavarria V., et al. The renin-angiotensin system: A possible new target for depression. BMC Medicine. 2017;15:144. doi: 10.1186/s12916-017-0916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan K., Deedwania P. Renin-angiotensin-aldosterone blockade for cardiovascular disease prevention. Cardiology Clinics. 2011;29:137–156. doi: 10.1016/j.ccl.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Violin J.D., DeWire S.M., Yamashita D., et al. Selectively engaging beta-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. The Journal of Pharmacology and Experimental Therapeutics. 2010;335:572–579. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- Wallukat G., Schimke I. Agonistic autoantibodies directed against G-protein-coupled receptors and their relationship to cardiovascular diseases. Seminars in Immunopathology. 2014;36:351–363. doi: 10.1007/s00281-014-0425-9. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhang Y., Wu L., et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F., Jia X.J., Yu S.Q., et al. Candesartan versus imidapril in hypertension: A randomised study to assess effects of anti-AT1 receptor autoantibodies. Heart. 2011;97:479–484. doi: 10.1136/hrt.2009.192104. [DOI] [PubMed] [Google Scholar]
- Wei H., Ahn S., Shenoy S.K., et al. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler L.M., Elgeti M., Hilger D., et al. Angiotensin analogs with divergent bias stabilize distinct receptor conformations. Cell. 2019;176:468–478. doi: 10.1016/j.cell.2018.12.005. (e411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler L.M., McMahon C., Staus D.P., Lefkowitz R.J., Kruse A.C. Distinctive activation mechanism for angiotensin receptor revealed by a synthetic nanobody. Cell. 2019;176:479–490. doi: 10.1016/j.cell.2018.12.006. (e412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wnorowski A., Jozwiak K. Homo- and hetero-oligomerization of beta2-adrenergic receptor in receptor trafficking, signaling pathways and receptor pharmacology. Cellular Signalling. 2014;26:2259–2265. doi: 10.1016/j.cellsig.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Wright J.W., Kawas L.H., Harding J.W. A role for the brain RAS in Alzheimer’s and Parkinson’s dseases. Frontiers in Endocrinology. 2013;4:158. doi: 10.3389/fendo.2013.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy C.W., Jessup M., Bozkurt B., et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: An update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Journal of the American College of Cardiology. 2016;68:1476–1488. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Yu L., Yuan K., Phuong H.T., Park B.M., Kim S.H. Angiotensin-(1-5), an active mediator of renin-angiotensin system, stimulates ANP secretion via Mas receptor. Peptides. 2016;86:33–41. doi: 10.1016/j.peptides.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Zhang H., Unal H., Desnoyer R., et al. Structural basis for ligand recognition and functional selectivity at angiotensin receptor. The Journal of Biological Chemistry. 2015;290:29127–29139. doi: 10.1074/jbc.M115.689000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Unal H., Gati C., et al. Structure of the angiotensin receptor revealed by serial femtosecond crystallography. Cell. 2015;161:833–844. doi: 10.1016/j.cell.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Han G.W., Batyuk A., et al. Structural basis for selectivity and diversity in angiotensin II receptors. Nature. 2017;544:327–332. doi: 10.1038/nature22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kang Z., Gong H., et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv. 2020 doi: 10.1101/2020.01.30.927806. [DOI] [Google Scholar]
- Zhang Y., Sun B., Feng D., et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature. 2017;546:248–253. doi: 10.1038/nature22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Yang H., Chen B., Zhang R. The skeletal renin-angiotensin system: A potential therapeutic target for the treatment of osteoarticular diseases. International Immunopharmacology. 2019;72:258–263. doi: 10.1016/j.intimp.2019.04.023. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhao Z., Wang Y., et al. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv. 2020 doi: 10.1101/2020.01.26.919985. [DOI] [Google Scholar]
- Zimmerman B., Beautrait A., Aguila B., et al. Differential beta-arrestin-dependent conformational signaling and cellular responses revealed by angiotensin analogs. Science Signaling. 2012;5:ra33. doi: 10.1126/scisignal.2002522. [DOI] [PubMed] [Google Scholar]
- Zisman L.S., Keller R.S., Weaver B., et al. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: Evidence for upregulation of the angiotensin-converting enzyme homologue ACE2. Circulation. 2003;108:1707–1712. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- Zou Y., Akazawa H., Qin Y., et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nature Cell Biology. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
Relevant Websites
- https://www.lanthiopharma.com/–Lanthio Pharma
- https://www.morphosys.com/–MorphoSys AG
- https://vicorepharma.com/–Vicore Pharma: Home


