Abstract
Background
Early antiretroviral therapy (ART) restricts the size of the human immunodeficiency virus (HIV) reservoir in infants. However, whether antiretroviral (ARV) prophylaxis given to exposed vertically infected children exerts similar effects remains unknown.
Methods
We measured total and integrated HIV DNA, as well as the frequency of CD4 T cells producing multiply spliced RNA (msRNA) after stimulation (inducible reservoir) in vertically infected Thai infants. Eighty-five infants were followed longitudinally for up to 3 years. We compared the size of the reservoir in children who received continuous ARV prophylaxis since birth vs those who never received or discontinued prophylaxis before initiating ART. We used samples from a cross-sectional cohort of 37 Thai children who had initiated ART within 6 months of life to validate our findings.
Results
Before ART, levels of HIV DNA and the frequencies of cells producing msRNA were significantly lower in infants who received continuous ARV prophylaxis since birth compared to those in whom ARV prophylaxis was discontinued or never initiated (P < .020 and P < .001, respectively). Upon ART initiation, total and integrated HIV DNA levels decayed significantly in both groups (P < .01 in all cases). Interestingly, the initial differences in the frequencies of infected cells persisted during 3 years on ART. The beneficial effect of prophylaxis on the size of the HIV reservoir was confirmed in the cross-sectional study. Importantly, no differences were observed between children who discontinued prophylactic ARVs before starting ART and those who delayed ART initiation without receiving prior prophylaxis.
Conclusions
Neonatal ARV prophylaxis with direct transition to ART durably limits the size of the HIV reservoir.
Keywords: prophylaxis, paediatric, HIV reservoir, vertical infection, early antiretroviral therapy
Continuous antiretroviral prophylaxis since birth restricts the seeding of cellular Human Immunodeficiency Virus (HIV) reservoirs. In addition, a direct transition from prophylaxis to fully suppressive antiretroviral therapy limits the persistence of the HIV reservoir.
(See the Editorial Commentary by Dhummakupt and Persaud on pages 439–40.)
Although interventions to prevent vertical human immunodeficiency virus (HIV) transmission are extremely successful, around 180 000 new infections occurred among infants globally in 2017 [1]. Compared to adults, acute HIV infection in infants is characterized by higher peak and set point viral loads (VLs) in the presence of a developing immune system, leading to a higher risk of rapid progression to AIDS and death [2, 3]. Therefore, early diagnosis of vertically infected newborns and rapid initiation of antiretroviral therapy (ART) are critical interventions to limit disease progression and promote child health [4]. Diagnosis in infants relies on HIV DNA polymerase chain reaction (PCR) testing during the first weeks of life [5]. Infants with detectable HIV DNA at birth were likely to be infected in utero, while newborns infected at delivery may not display detectable levels of HIV DNA for several days to weeks. Despite the critical importance of prompt diagnosis, HIV DNA PCR testing usually requires a centralized reference laboratory, presenting challenges for rapid result reporting and prompt initiation of ART. Therefore, the World Health Organization (WHO) guidelines recommend dual prophylaxis (zidovudine and nevirapine) for a period of 4–6 weeks in HIV-exposed infants at high risk of acquiring HIV to prevent vertical transmission [5]. Meanwhile in Thailand, the national guidelines recommend a combination of zidovudine, lamivudine, and nevirapine as a neonatal prophylaxis regimen [6, 7].
Early ART initiation during the first year of life not only reduces morbidity and mortality [8], but also limits the size of the HIV reservoir [9–18]. Similar to adults, there is rapid viral rebound if ART is interrupted, even in infants treated within days of birth [9, 19]. However, a few early-treated infants have controlled viral replication after treatment cessation [20–23]. Therefore, there is a need to better understand the dynamics of the HIV reservoir in vertically infected early-treated children to achieve a functional cure.
In 2015, Thailand achieved the WHO targets for the elimination of vertical transmission (< 2% rate). The public health infrastructure is strong with near 100% antenatal visit and HIV testing rates among pregnant women [24]. There are about 5000 pregnant women with HIV annually with > 95% prevention of mother-to-child transmission coverage and 120 infants infected with HIV annually.
This observational study is uniquely embedded in the Thai Ministry of Public Health (MoPH) Active Case Management Network that offered the opportunity to engage mothers with HIV and their infants from birth [25]. We measured the establishment and persistence of the HIV reservoir in a relatively large number of Thai children vertically infected with HIV (RV475/HIVNAT209) who initiated ART within the first 6 months of life. To evaluate the impact of ARV prophylaxis at birth, we compared infants who directly transitioned from prophylaxis to fully suppressive ART with those who discontinued prophylaxis prior to ART initiation. We further validated our findings using samples from a cross-sectional study of Thai children with HIV (RV474/HIVNAT194).
MATERIALS AND METHODS
Study Participants
Longitudinal Study
Thai children vertically infected with HIV (N = 85) who initiated ART within the first 6 months of life were enrolled in the RV475/HIVNAT209 study and followed longitudinally for up to 3 years (Supplementary Figure 1 and Table 1). The study was designed in conjunction with the Thai MoPH Network, which ensures that every infant with a positive HIV DNA PCR result promptly starts ART. Following Thai guidelines, high-risk infants received zidovudine/lamivudine and nevirapine from birth for 6 weeks whereas low-risk infants received 4 weeks of zidovudine [6]. HIV DNA PCR testing was performed on whole blood at birth and/or between 1 and 4 weeks of life. ART was initiated promptly after the first positive HIV DNA PCR result while pending for the confirmation of a second HIV DNA PCR test result. The recommended first-line ART regimen for infants in Thailand was zidovudine/lamivudine and lopinavir/ritonavir. All infants were formula-fed per standard of care. See the Supplementary Methods for additional information.
Table 1.
RV475/HIVNAT209 Study Participant Characteristics
| RV475/HIVNAT 209 | ||||
|---|---|---|---|---|
| Characteristic | All (N = 85) | Direct Transition From Prophylaxis to ART (n = 34) | Discontinued Prophylaxis Before ART Initiation (n = 51) | P Value Between Groupsa |
| Sex, female, No. (%) | 46 (54) | 18 (53) | 28 (55) | 1.0 |
| HIV transmission, No. (%) | < .0001 | |||
| In utero | 26 (31) | 23 (68) | 3 (6) | |
| Peripartum | 34 (40) | 7 (21) | 27 (53) | |
| Unknown | 25 (29) | 4 (12) | 21 (41) | |
| Prophylactic ARV history, No. (%) | < .0001 | |||
| None | 5 (6) | 0 (0) | 5 (10) | |
| ZDV | 18 (21) | 2 (6) | 16 (31) | |
| ZDV + 3TC + NVP | 61 (72) | 31 (91) | 30 (59) | |
| ZDV + NVP single dose | 1 (1) | 1 (3) | 0 (0) | |
| Duration of ARV prophylaxis, d, median (IQR) | 33 (28–44) | 34 (26–49) | 33 (28–42) | .61 |
| Time between end of ARV prophylaxis and ART initiation, wk, median (IQR) | 3.9 (0–8.4) | 0 (0–0) | 7 (4.1–13) | < .0001 |
| Percentage of lifetime without ARVs, median (IQR) | 43.2 (0–65) | 0 (0-0) | 62 (49–72) | < .0001 |
| Age at ART initiation, mo, median (IQR) | 2.1 (1.3–3.4) | 1.1 (0.9–1.6) | 2.8 (2.2–4.1) | < .0001 |
| ART regimen, No. (%) | 1.0 | |||
| PI-based | 82 (96) | 33 (97) | 49 (96) | |
| NNRTI-based | 3 (4) | 1 (3) | 2 (4) | |
Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; ARV, antiretroviral; HIV, human immunodeficiency virus; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; ZDV, zidovudine.
a P values between groups: Wilcoxon tests for continuous variables and Fisher test for categorical variables. Significant P values are indicated in bold.
Reservoir measures were performed on blood samples collected after confirmation of HIV diagnosis—that is, just prior to ART initiation (visit 1). At this first visit, the median age of the participants was 2.1 months, which was the time required to confirm HIV infection by PCR. Blood was then collected after 1, 2 and 3 years of ART (visits 3, 5 and 7; median ages = 1.3, 2.3 and 3.3 years, respectively).
Cross-sectional Study for Validation
Vertically infected children (n = 37, RV474/HIVNAT194 study; Table 2) were eligible if they were older than 2 years of age, had positive HIV DNA PCR, initiated ART during the first 6 months of life, and had sustained suppressed plasma viremia (< 400 HIV RNA copies/mL).
Table 2.
RV474/HIVNAT 194 Study Participant Characteristics
| RV474/HIVNAT 194 | ||||
|---|---|---|---|---|
| Characteristic | Direct Transition From Prophylaxis to ART (n = 6) | Discontinued Prophylaxis Before the Initiation of ART (n = 10) | Delayed ART (n = 21) | P Value Between Groupsa |
| Age, y, median (IQR) | 4.7 (3.1–6.8) | 3.1 (2.5–4.7) | 6.4 (4.9–8.3) | .008 |
| Sex, female, No. (%) | 3 (50) | 6 (60) | 13 (62) | 1.0 |
| CD4 count, wk, cells/µL, median (IQR) | 1485 (1183–1970) | 1497 (1178–2496) | 1117 (783–1420) | .04 |
| Prophylactic ARV history, No. (%) | < .001 | |||
| None | 0 (0) | 0 (0) | 21 (100) | |
| ZDV | 0 (0) | 4 (40) | 0 (0) | |
| ZDV + 3TC | 3 (50) | 0 (0) | 0 (0) | |
| ZDV + 3TC + NVP | 3 (50) | 6 (60) | 0 (0) | |
| Duration of ARV prophylaxis, wk, median (IQR) | 5.2 (2.2–8.5) | 5.2 (4–6) | 0 (0–0) | < .001 |
| Time between end of ARV prophylaxis and ART initiation, wk, median (IQR) | 0 (0–0) | 9.4 (6.4–13.1) | 18.4 (15.3–22.3) | < .001 |
| Age of ART initiation, wk, median (IQR) | 5.2 (2.2–8.6) | 15.3 (11.2–17.5) | 18.4 (15.3–22.3) | < .001 |
| Time on ART, y, median (IQR) | 4.6 (3–6.7) | 2.9 (2.2–4.2) | 6.1 (4.3–8.2) | .01 |
| ART regimen, No. (%) | ||||
| PI-based | 1 (17) | 7 (70) | 9 (43) | |
| NNRTI-based | 5 (83) | 3 (30) | 12 (57) | |
Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; ARV, antiretroviral; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; ZDV, zidovudine.
a P values between groups: Wilcoxon tests for continuous variables and Fisher test for categorical variables. Significant P values are indicated in bold.
These studies were conducted at 8 sites in Thailand: the HIV Netherlands Australia Thailand Research Collaboration (King Chulalongkorn Memorial Hospital), Siriraj Hospital, HatYai Hospital, Srinagarind Hospital, Chiangrai Prachanukroh Hospital, Nakornping Hospital, Prachomklao Hospital, and Queen Sirikit National Institute of Child Health. Caregivers gave their consent, and both studies were approved by the Thai MoPH and all participating ethical committees, and as well as by the Institutional Review Board of the Centre Hospitalier de l’Université de Montréal.
Total and Integrated HIV DNA
Total CD4+ T cells were isolated by negative magnetic selection (EasySep Human CD4+ T Cell Enrichment Kit, Stemcell) from frozen peripheral blood mononuclear cells. Pellets of enriched CD4+ T cells were digested with proteinase K to measure total (5′-LTR-gag) and integrated (3′-LTR-alu) HIV DNA by real-time PCR as previously described [26]. Samples for which < 50 000 cells were analyzed were excluded from the analysis.
Frequency of CD4+ T Cells With Multiply Spliced HIV RNA
Productively infected cells and inducible HIV reservoir were measured by the Tat/rev induced limiting dilution assay (TILDA) ex vivo and after stimulation, respectively, as previously described, using primers adapted for clade CRF01_AE [27]. To ensure that TILDA negative results were not due to a primer or probe mismatch, we quantified viral production in the culture supernatants, as previously described [28]. Of note, the primers for viral production quantification were designed to efficiently amplify the B and CRF01_AE HIV clades. Samples with a negative TILDA value and from which HIV production in the supernatant was detected were excluded (see Supplementary Methods).
Statistical Analysis
All reservoir data were log10 (X + 1) transformed to better meet the assumption of normality. Bivariate associations between continuous variables were assessed by Spearman rank correlation coefficient. Comparisons between groups were done with Wilcoxon tests for continuous variables and Fisher test for categorical variables. Longitudinal analyses were performed using a linear mixed model with the group of prophylaxis (discontinuous vs continuous), visits (V1, V3, V5, and V7), and the interaction group × visit as independent variables, with children identification number as a random variable. For the decay analysis, the 3 measures of HIV reservoir were standardized and appended in a new variable. A linear mixed model was then performed on this new outcome, and the coefficient of the interaction visit × reservoir measurement was used to test the equality of the slopes between the different types of reservoir measurement. Statistical analyses were performed using R software, version 3.6.1 and Prism 7.
RESULTS
Establishment of the HIV Reservoir Before ART Initiation
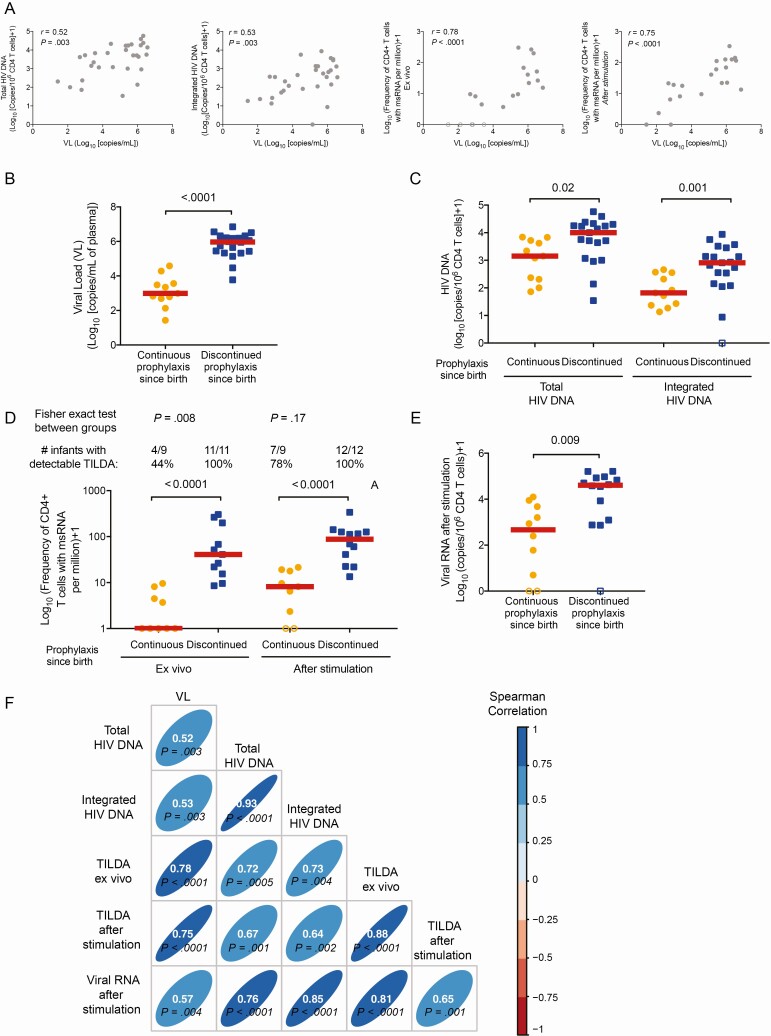
To assess the HIV reservoir seeding, we measured total and integrated HIV DNA, as well as the active and inducible reservoirs in 31 infants vertically infected with HIV (median age, 2.1 months; range, 0.83–5.3) at the time of HIV diagnosis (RV475/HIVNAT209-V1; Table 3). Plasma VL strongly correlated with the frequency of cells harboring total and integrated HIV DNA, the frequency of productively infected cells measured by TILDA without stimulation and the size of the inducible reservoir measured by TILDA after stimulation (P = .003, P = .003, P < .001, and P < .001, respectively; Figure 1A). Hence, the size of the pool of infected cells was reflected by the magnitude of HIV replication, suggesting that prophylactic ARVs could limit the seeding of the HIV reservoir. To test this hypothesis, we compared infants who were still receiving prophylactic ARVs (continuous prophylaxis ARV group, n = 11) with those who had already completed prophylaxis (or never received it) and were not receiving ARV at the time of sampling (discontinued prophylaxis ARV, n = 20; Table 3). As expected, newborns who were still on prophylactic ARVs showed significantly lower VL compared to infants who had completed prophylaxis (P < .001; Figure 1B). Hence, infants who were still on prophylactic ARVs showed significantly lower frequencies of cells carrying total and integrated HIV DNA compared to those who discontinued prophylaxis (P = .02 and P = .001, respectively; Figure 1C). TILDA results further confirmed that newborns on continuous prophylaxis displayed lower frequencies of productively infected cells and lower inducible reservoirs (Figure 1D). Despite these differences, the frequency of cells expressing multiply spliced RNA (msRNA) increased between the ex vivo and the stimulated TILDA conditions in both groups, suggesting that a latent reservoir had already been established, even in infants on continuous ARV since birth. Similar differences between groups were observed when we measured the production of viral particles after stimulation of CD4+ T cells (P = .009; Figure 1E). Importantly, all measures of HIV reservoir strongly correlated with each other and with plasma viremia (Figure 1F), suggesting that VL could be used as a surrogate marker of the HIV reservoir size before ART initiation. Interestingly, a longer time between the end of prophylactic ARVs and sample collection (measured in days or as a percentage of lifetime) positively correlated with the levels of integrated HIV DNA as well as with the inducible reservoir, and there was a trend for correlation with levels of total HIV DNA (Supplementary Figure 2). These results indicated that continuous ARV since birth was associated with a relatively small pool of infected cells.
Table 3.
RV475/HIVNAT209 Newborns’ Characteristics From Before Antiretroviral Therapy Initiation
| RV475/HIVNAT 209 Visit 1 Subgroups | ||||
|---|---|---|---|---|
| Characteristic | RV475/HIVNAT 209 Visit 1 (n = 31) | Continuous Prophylaxis (n = 11) | Discontinued ARV (n = 20) | P Value Between Subgroupsa |
| Age, mo, median (IQR) | 2.1 (1.5–2.8) | 1 (1–1.5) | 2.8 (2–3.8) | < .001 |
| Sex, female, No. (%) | 19 (61) | 7 (64) | 12 (59) | 1.0 |
| CD4 T-cell counts, cells/µL, median (IQR)b | 2921 (2131–3447) | 2856 (2514–4179) | 2985 (1852–3285) | .24 |
| CD8 T-cell counts, cells/µL, median (IQR)c | 2415 (1872–2858) | 2526 (1664–3355) | 2415 (1687–2721) | .95 |
| Ratio CD4/CD8, median (IQR) | 1.2 (0.9–1.4) | 1.4 (1.1–2.1) | 0.96 (0.8–1.4) | .17 |
| Detectable VL, log10 HIV RNA copies/mL, median (IQR) | 5.3 (3.5–6.1) | 3 (2.7–3.6) | 6 (5.4–6.3) | < .001 |
| VL < 50 copies/mL, No. (%) | 1 (3) | 1 (9) | 0 (0) | .37 |
| VL < 1000 copies/mL, No. (%) | 7 (23) | 7 (55) | 0 (0) | < .001 |
| HIV transmission, No. (%) | < .001 | |||
| In utero | 9 (29) | 8 (73) | 1 (5) | |
| Peripartum | 13 (42) | 3 (27) | 10 (50) | |
| Unknown | 9 (29) | 0 (0) | 9 (45) | |
| Prophylactic ARVs, No. (%) | .01 | |||
| None | 3 (10) | 0 (0) | 3 (15) | |
| ZDV | 7 (22) | 0 (0) | 7 (35) | |
| ZDV + 3TC + NVP | 21 (68) | 11 (100) | 10 (50) | |
| Duration of ARV prophylaxis, d, median (IQR) | 31 (28–42) | 31 (28–44) | 37 (28–42) | .7 |
| Time between end of ARV prophylaxis and visit 1 sampling, wk, median (IQR) | 3.9 (0–6.9) | 0 (0–0) | 6.1 (3.9–9.9) | < .001 |
| Percentage of lifetime without ARVs, median (IQR) | 43 (0–62) | 0 (0–0) | 50 (44–71) | < .001 |
Abbreviations: 3TC, lamivudine; ARV, antiretroviral; HIV, human immunodeficiency virus; IQR, interquartile range; NVP, nevirapine; VL, viral load; ZDV, zidovudine.
a P values between groups: Wilcoxon tests for continuous variables and Fisher test for categorical variables. Significant P values are indicated in bold.
bCD4 T-cell counts: only 22 samples available.
cCD8 T-cells counts: only 15 samples available.
Figure 1.
Markers of human immunodeficiency virus (HIV) persistence in infants before antiretroviral therapy (ART) initiation in the RV475/HIVNAT209 study, visit 1 (n = 31). A, Correlations between plasma viral load (VL) and the levels of total (left panel) and integrated (middle left) HIV DNA, active replication (Tat/rev induced limiting dilution assay [TILDA] ex vivo, middle right panel), and inducible reservoir (TILDA after stimulation, right panel). B–E, Virological markers in infants who were still on prophylactic antiretroviral (ARV) (continuous prophylaxis ARV, orange circles, n = 11) vs those who had already completed prophylaxis and were not taking ARV drug(s) at the time of sample collection (discontinued ARV prophylaxis, blue squares, n = 20). Plasma viral load (B), frequency of cells harboring total and integrated HIV DNA (C), frequency of cells producing multiply spliced RNA (msRNA) spontaneously (TILDA ex vivo) or after 12 hours of stimulation (inducible reservoir) (D), and production of viral particles after stimulation (E) are shown. F, Correlation matrix between the markers of HIV persistence. In all panels, samples below the limit of the detection of the assay (which varies according to the number of cells analyzed) were considered as 0 for statistical analysis and are indicated as open circles in all graphs. P values were obtained from Spearman correlation (A and F) or Mann-Whitney U test (B, C, D, E). For (D), the number of samples (and corresponding percentages) with measurable levels of msRNA ex vivo or after stimulation is indicated. Fisher exact test was used to compare groups.
Persistence of the HIV Reservoir After 1 Year on ART
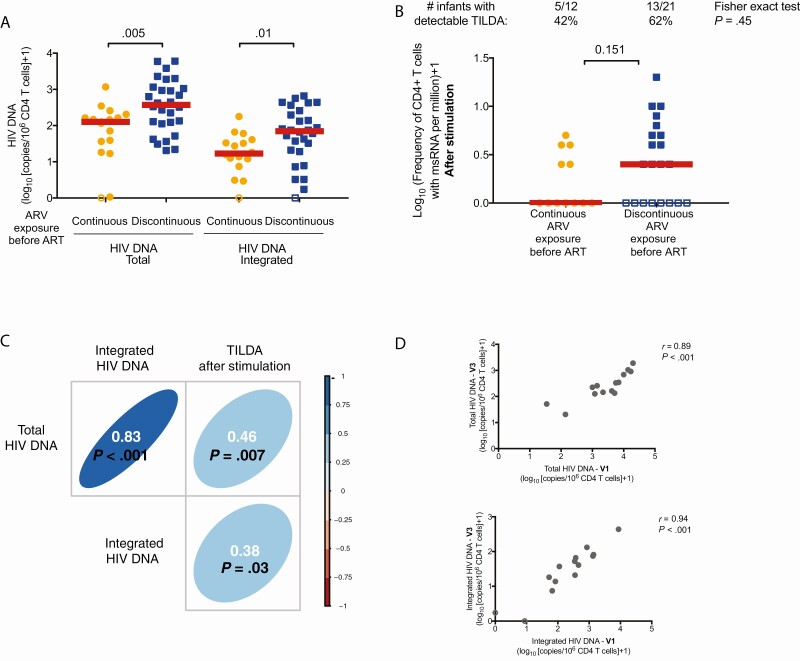
We stratified children by prophylaxis history to determine if continuous ARV exposure since birth had a sustained impact on the size of HIV reservoir after 1 year of ART (RV475/HIVNAT209-V3; Table 4). Initiation of ART led to a significant decrease in the frequency of cells harboring total and integrated HIV DNA in both groups (Supplementary Figure 3A and 3B; P < .01 in both cases). However, after 1 year of ART, children on continuous ARV exposure since birth showed significantly lower levels of total and integrated HIV DNA compared to those who discontinued prophylaxis before ART initiation (P = .005 and P = .010, respectively, Figure 2A). The size of the inducible reservoir diminished rapidly after ART initiation (Supplementary Figure 3C) and reached values below the limit of detection of the assay in a large fraction of the samples tested in both groups (Figure 2B; P = .45). The levels of total and integrated HIV DNA as well as the inducible reservoir correlated with each other after 1 year of ART (Figure 2C). Importantly, measures of total and integrated HIV DNA before (RV475/HIVNAT209-V1) and after 1 year (RV475/HIVNAT209-V3) of ART initiation were strongly correlated (Figure 2D), indicating that the frequency of infected cells at the time of ART initiation predicted the size of the reservoir after 1 year of ART. In addition, a longer time between the end of prophylactic ARVs and initiation of ART positively correlated with the frequency of cells harboring total and integrated HIV DNA after 1 year of ART (Supplementary Figure 4). The age of ART initiation also correlated with the levels of integrated HIV DNA after 1 year of ART (Supplementary Figure 4C), confirming the benefit of early ART on the size of the HIV reservoir [9, 10, 16, 29].
Table 4.
RV475/HIVNAT209 Infant Characteristics From After 1 Year of Antiretroviral Therapy
| RV475/HIVNAT 209 Visit 3 (on ART for 1 y) | |||
|---|---|---|---|
| Characteristic | Direct Transition From Prophylaxis to ART (n = 17) | Discontinued Prophylaxis Before the Initiation of ART (n = 28) | P Value Between Groupsa |
| Age, mo, median (IQR) | 14 (13–15) | 16 (15–18) | < .001 |
| Sex, female, No. (%) | 10 (56) | 11 (39) | .4 |
| CD4 counts, cells/µL, median (IQR) | 2251 (1822–3350) | 2327 (1678–3386) | .74 |
| CD8 counts, cells/µL, median (IQR) | 1612 (1218–2132) | 1872 (1367–2428) | .29 |
| Ratio CD4/CD8, median (IQR) | 1.7 (1–2.2) | 1.5 (1.1–1.9) | .7 |
| VL at visit 1, HIV RNA copies/mL, median (IQR) | 3.5 (3.2–4.4) | 6 (5.4–6.5) | < .001 |
| VL at visit 2 < 50 HIV RNA copies/mL, No./samples evaluated (%)b | 11/16 (69) | 13/28 (46) | .21 |
| VL at visit 3 < 50 HIV RNA copies/mL, No. (%) | 16 (94) | 26 (93) | 1 |
| VL at visit 3 < 100 HIV RNA copies/mL, No. (%) | 17 (100) | 28 (100) | 1 |
| HIV transmission, No. (%) | < .001 | ||
| In utero | 11 (65) | 0 (0) | |
| Peripartum | 3 (18) | 14 (50) | |
| Unknown | 3 (18) | 14 (50) | |
| Prophylactic ARV history, No. (%) | .01 | ||
| None | 0 (0) | 3 (11) | |
| ZDV | 1 (6) | 10 (36) | |
| ZDV + 3TC + NVP | 16 (94) | 15 (54) | |
| Duration of ARV prophylaxis, d, median (IQR) | 34 (25–51) | 42 (28–42) | .5 |
| Time between end of ARV prophylaxis and ART initiation, wk, median (IQR) | 0 (0–0) | 8 (4–15) | < .001 |
| Percentage of lifetime without ARVs, median (IQR) | 0 (0–0) | 60 (46–74) | < .001 |
| Age at ART initiation, mo, median (IQR) | 1.2 (0.8–1.6) | 2.9 (2.3–4.2) | < .001 |
| Time on ART, mo, median (IQR) | 12 (11–14) | 13 (12–14) | .36 |
| ART regimen | .51 | ||
| PI-based | 17 (100) | 26 (93) | |
| NNRTI-based | 0 (0) | 2 (7) | |
Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; ARV, antiretroviral; HIV, human immunodeficiency virus; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; VL, viral load; ZDV, zidovudine.
a P values between groups: Wilcoxon tests for continuous variables and Fisher test for categorical variables. Significant P values are indicated in bold.
bVL at visit 2: only 16 samples evaluated for continuous prophylaxis group.
Figure 2.
Markers of human immunodeficiency virus (HIV) persistence in infants after 1 year of antiretroviral therapy (ART) initiation in the RV475/HIVNAT209 study, visit 3 (n = 45). Infants were subgrouped according to their prophylactic history: continuous antiretroviral (ARV) exposure since birth (direct transition from ARV prophylaxis to ART, orange circles, n = 17) vs discontinuous ARV exposure before ART initiation (blue squares, n = 28). A, Frequency of total and integrated HIV DNA in enriched CD4+ T cells. P values were obtained from Mann-Whitney U test. B, Frequency of cells producing multiply spliced RNA (msRNA) after stimulation (inducible reservoir) in enriched CD4+ T cells. Since > 15% of the samples were below the limit of detection, Tat/rev induced limiting dilution assay (TILDA) measurements were considered as categorical values (detectable vs undetectable). The numbers of samples (and corresponding percentages) in which msRNA was detected are indicated. P values were obtained from Fisher exact test. C, Correlation matrix between markers of HIV persistence at visit 3. D, Correlation between the frequency of total and integrated HIV DNA at visit 1 and visit 3. C and D, P values were obtained from the Spearman correlation test. In all panels, samples below the limit of detection of the assay (which varies according to the number of cells analyzed) were considered as 0 for statistical analysis and are indicated as open circles in all graphs.
Longitudinal Analysis of the HIV Reservoir
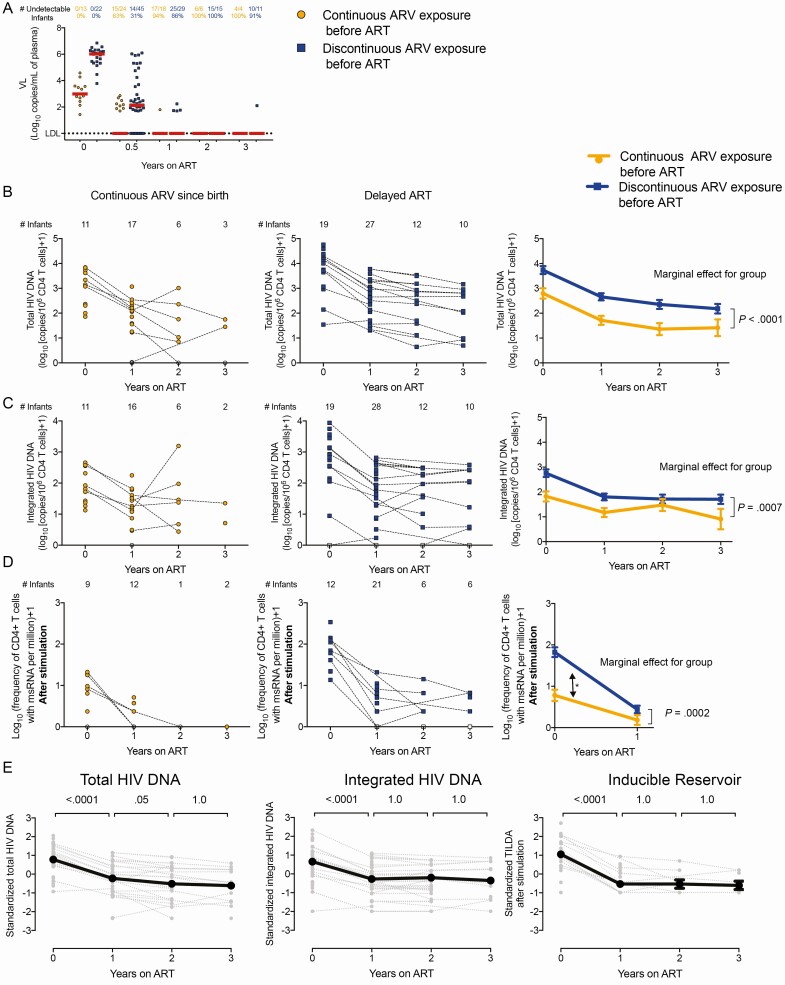
We longitudinally assessed the impact of ARV prophylaxis on the persistence of the HIV reservoir for up to 3 years (V7) of ART (Table 1 and Supplementary Figure 1B). After 6 months of ART, 63% of the children with continuous ARV since birth reached undetectable VL, whereas only 31% achieved undetectability among those who had discontinuous ARV exposure before ART. After 1 year of therapy, most of the children from both groups achieved viral suppression. With the exception of rare viral blips (< 100 copies/mL), all children displayed undetectable VL after 2–3 years of ART (Figure 3A). The initial differences in the levels of total and integrated HIV DNA observed between groups persisted over time (marginal effect for group: P < .001 for both total and integrated HIV DNA; Figure 3B and 3C). Despite these sustained differences, the levels of total and integrated HIV DNA decayed at similar rates in the 2 groups, suggesting that the size of the reservoir after prolonged ART was essentially driven by the frequencies of infected cells prior to ART initiation. The initial differences in the size of the inducible reservoir decreased to reach undetectable values in the majority of children (marginal effect for group: P = .0002; Figure 3D).
Figure 3.
Longitudinal analysis of markers of human immunodeficiency virus (HIV) persistence in the RV475/HIVNAT209 study (n = 85). Infants were subgrouped according to their prophylactic history: continuous antiretroviral (ARV) exposure since birth (direct transition from prophylactic ARVs to antiretroviral therapy [ART], orange circles, n = 34) vs discontinuous ARV exposure before ART initiation (blue squares, n = 51). Samples collected immediately prior to ART initiation (time point 0) were included. Participants were followed for 3 years on ART. A, Plasma viral loads (VLs). The numbers of samples in which HIV RNA in plasma were detected (and the corresponding percentages) are indicated for each group. B–D, Levels of total (B) and integrated (C) HIV DNA, and frequencies of cells expressing multiply spliced RNA after stimulation (Tat/rev induced limiting dilution assay after stimulation, D) in enriched CD4+ T cells from infants who received continuous ARV since birth (left panels, in orange) and those who discontinued prophylactic treatment before ART initiation (middle panels, in blue). The numbers of samples tested at each time point are indicated. Right panels show the estimated means and standard errors, using a linear mixed model with the group of prophylaxis (continuous vs discontinuous ARV exposure before ART), visits (V1, V3, V5 and V7), and the interaction group × visit as independent variables. Participant identifier was used as a random variable, to take into account the within-children correlation. Because the interaction was not significant, the additive model was used as the final model with visit and group of prophylaxis as independent variables (no interaction included). For each model, the P value associated to the marginal effect for the group is indicated. E, HIV persistence markers were standardized to compare the decay rates. P values reported are Bonferroni-adjusted and calculated from pairwise post hoc comparisons between visits. The model used was similarly the additive mixed model with the group of prophylaxis, with the visit (V1–V7) as fixed effect and participant identifier as random effect.
All 3 reservoir measures displayed a rapid initial decay during the first year of ART (P < .001 in all cases), with the inducible reservoir showing the steepest decay (slopes = −1.66, −1.07, and −0.94, for TILDA, total HIV DNA, and integrated HIV DNA, respectively). All measures remained relatively stable after 1 year of therapy (Figure 3E).
Persistence of the HIV Reservoir in a Cross-sectional Validation Study
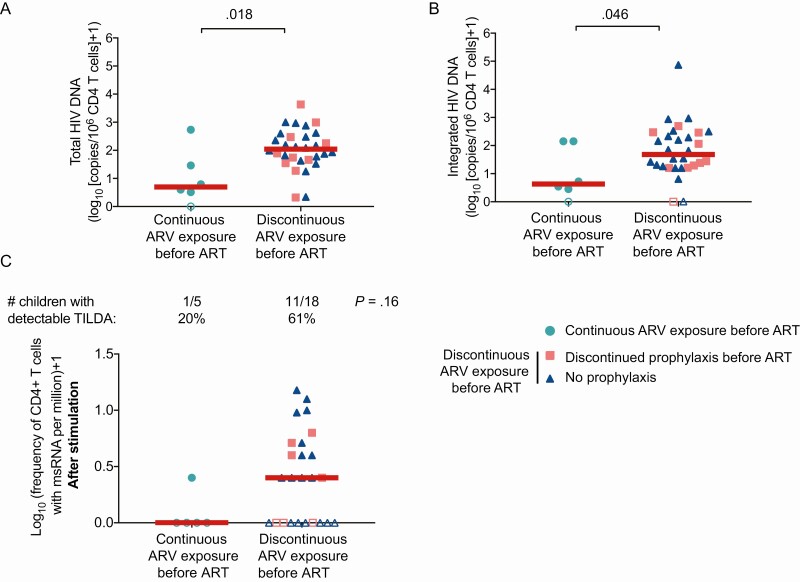
We used samples from the cross-sectional study RV474/HIVNAT194 to further validate our findings, again subgrouping children between those who received continuous ARV since birth (prophylaxis or ART) and those who discontinued prophylaxis before ART (Table 2). The frequencies of cells harboring total and integrated HIV DNA were lower in children who received continuous ARV since birth compared to the other group (P = .018 and P = .046, respectively; Figure 4A and 4B). Importantly, no differences were observed between children who discontinued prophylactic treatment before starting ART and those who had late ART initiation without receiving prophylaxis (P = .56 and P = .47, for total and integrated HIV DNA, respectively). These results suggested that there was no beneficial effect of prophylaxis on the size of the HIV reservoir if treatment was discontinued. A similar trend was observed when we measured the size of the inducible reservoir: Only 20% of children who received continuous ARV since birth had detectable frequencies of cells producing msRNA, whereas 61% of the children in the other group displayed detectable TILDA values (P = .16; Figure 4C).
Figure 4.
Markers of human immunodeficiency virus (HIV) persistence in early-treated children (within 6 months) from the RV474/HIVNAT194 study (n = 37). Children were subgrouped according to their prophylactic history: continuous antiretroviral (ARV) or antiretroviral therapy (ART) since birth (turquoise circles, n = 6) vs discontinuous ARV exposure before ART. Within this last group, we subdivided between children who discontinued prophylaxis before ART initiation (pink squares, n = 10) and those who never received prophylaxis (blue triangles, n = 21). Levels of total (A) and integrated (B) HIV DNA in enriched CD4+ T cells. P values were obtained from Mann-Whitney U test. C, Frequency of cells producing multiply spliced RNA (msRNA) after stimulation (inducible reservoir) in enriched CD4+ T cells. Numbers of samples (and corresponding percentages) in which msRNA was detected by Tat/rev induced limiting dilution assay (TILDA) are indicated. P values were obtained from Fisher exact test.
A longer delay between the end of prophylactic ARVs and initiation of ART positively correlated with the frequency of cells harboring total HIV DNA and tended to correlate with integrated HIV DNA and TILDA values (P = .03, P = .06, and P = .08, respectively; Supplementary Figure 5A) as seen in the RV475/HIVNAT209 study. The age of ART initiation also positively correlated with the levels of total and integrated HIV DNA (P = .02 and P = .05, respectively; Supplementary Figure 5B) and tended to correlate with TILDA values (P = .06). These results confirmed that continuous exposure to ARV and the age at which ART is started are critical factors to limit HIV persistence.
DISCUSSION
Early ART initiation in vertically infected children limits the size of the HIV reservoir [9–14, 18, 30–32]. However, ART initiation is rarely initiated during the first days or weeks of life because of the delay in obtaining a diagnosis of HIV infection. In this study, we show that controlling viral replication with continuous prophylactic ARVs before ART initiation limits the size of the initial reservoir and that this benefit is maintained for at least the first 3 years of therapy.
Newborns infected in utero and tested at birth are more likely to be diagnosed earlier and to benefit from a direct transition from prophylaxis to fully suppressive ART at a younger age. This resulted in a significantly smaller reservoir compared to children who did not directly transition from prophylaxis to ART. Our study suggests that continuous exposure to ARVs, rapid reporting of HIV DNA PCR results, and closer follow-up visits and testing by primary care providers are needed to ensure prompt ART initiation after discontinuation of prophylaxis in order to limit the size of the persistent reservoir. Additional studies will be needed to confirm if a 3-drug prophylactic regimen is required to restrict the viral reservoir, or if dual therapy is sufficient.
This study is unique in that it addresses the impact of ARV prophylaxis on the size of the viral reservoir in vertically infected children. Since drug doses used for prophylaxis are lower than those used for treatment, VL was detectable in all children at baseline. Nonetheless, we observed that ARV prophylaxis partially controlled viral replication, which was associated with a lower frequency of infected cells and to a shorter time to viral suppression upon ART initiation. Importantly, the time during which children were not exposed to any ARV was positively correlated with the frequency of infected cells, indicating that the beneficial effects of prophylaxis were limited if it was interrupted.
The initial differences in the size of the HIV reservoir between the children on continuous ARV since birth and those who discontinued prophylaxis before ART were maintained for at least 3 years. However, the decay in the different reservoir markers was similar between groups, suggesting that ART initiation within 6 months of life limits the size of the persistent reservoir by restricting its seeding rather than by modulating its half-life, in agreement with previous studies [13, 31, 32].
Several studies reported a rapid initial decay in the levels of total HIV DNA upon ART initiation, followed by a slower decay after 6–9 months of therapy [30, 31]. Our study confirms and extends these observations, with a rapid decay in the levels of HIV DNA during the first year of ART, followed by a slower decay, likely reflecting heterogenous populations of infected cells characterized by different clearance rates [33]. Interestingly, different markers of HIV persistence displayed different decay rates during the first year of ART, with the inducible reservoir showing the steepest slope compared to total and integrated HIV DNA, possibly reflecting the preferential clearance of inducible viral genomes compared to latent proviruses or genomes containing large defects. Studies including a larger sample size are required to confirm these results.
This study has some limitations, including the small sample size in the continuous ARV group at years 2 and 3 of follow-up. In addition, since Thailand has a no-breastfeeding policy for women living with HIV, we could not assess the impact of ARV prophylaxis on the establishment and persistence of HIV reservoirs in children who acquired HIV postnatally.
We used TILDA as a surrogate measure of the transcriptional-competent and inducible reservoir, which tends to correlate with the quantitative viral outgrowth assay [27]. We observed low inducibility of the HIV reservoir as early as 1 year after ART initiation in all children. These results are in agreement with other studies that reported low levels of replication-competent HIV in early-treated children [10, 29, 34]. Indeed, Persaud et al reported that 40%–60% of early-treated children (within 6 weeks of birth) had undetectable levels of replication competent virus after 1–2 years of ART [29]. These results are comparable to those obtained with the TILDA, which were negative in 40% of early-treated children, despite the fact that HIV DNA was detected in most participants. Further studies are needed to determine if these low frequencies of inducible proviruses are due to a large proportion of defective viral genomes or to the inefficient reactivation of intact genomes in children.
Taken together, our results demonstrate the importance of continuous prophylaxis with direct transition to ART to increase the likelihood of achieving sustained viral suppression within 6 months of life and to restrict the seeding of the HIV reservoir.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
RV474/HIVNAT194 and RV475/HIV-NAT209 Study Groups. Research sites: (1) HIV Netherlands Australia Thailand Research Collaboration (HIV-NAT), Thai Red Cross AIDS Research Group/Chulalongkorn University: Torsak Bunupuradah, Stephen Kerr, Sasiwimol Ubolyam, Apicha Mahanontharit, Naphassanant Laopraynak, Preeyarach Klaytong, Tulathip Suwanlerk, Thita Pitimahajanaka, Naruporn Kasipong, Thornthan Noppakaorattanamanee, Kesdao Nanthapisal, Thatri Iampornsin, Sasithorn Burichai, Yupawadee Jummanee, Sudarat Soongpankeeree, Monta Intawan, Tuangthip Theerawat, Juthamanee Moonwong, Patchareeyawan Srimuen, Chutima Saisaengjan, Wasana Prasitsuebsai, Watsamon Jantarabenjakul, Suvaporn Anugulruengkitt, Umaporn Methanggool; (2) Queen Sirikit National Institute of Child Health: Pugpen Sirikutt, Pimsiri Leowsrisook, Yosawadee Na Nakorn, Naruemon Sassungnune; (3) Siriraj Hospital, Mahidol University: Kanokkarn Wongmayurachat; (4) Srinagarind Hospital, Khon Kaen University: Chanasda Kakkaew, Somjai Rattanamanee; (5) Prachomklao Hospital: Manee Yentang, Patcha Panyim, Janyarak Punyim; (6) Nakornping Hospital: Thida Namwong, Siripim Kamphaengkham, Supanpilat Chaisri; (7) Chiangrai Prachanukroh Hospital: Areerat Khongponoi; (8) Hat Yai Hospital: Ratchanee Saksawad, Usa Sukhaphan, Arena Laeyuheem. Laboratory and network: (1) Program for HIV Prevention and Treatment: Gonzague Jourdain, Nicole Ngo-Giang-Huong; (2) Vaccine and Cellular Immunology laboratory–Chulalongkorn University: Sunee Sirivichayakul; (3) National Cancer Institute, National Institutes of Health (NIH): Frank Maldarelli; (4) University of Sydney: Sarah Palmer. Thai Ministry of Public Health (MoPH) and Active Case Management Network to promote early ART initiation Aiming for Cure: (1) Thailand MoPH–US Centers for Disease Control and Prevention Collaboration: Michael Martin, Rangsima Lolekha, Thananda Naiwatanakul, Worawan Faikratok, Benjamas Baipluthong; (2) Bureau of Health Promotion, MoPH: Danai Teewunda, Sarawut Boonsuk, Chaweewan Tonputsa, Pariwat Tangpong; (3) Clinical Research Center, MoPH: Archawin Rojanawiwat, Hansa Thaisri, Wiroi Puangtubtim, Chaidan Boonrossak; (4) Bureau of AIDS, Tuberculosis and Sexually Transmitted Infections, MoPH: Sumet Ongwandee, Walairat Chaifoo, Cheewanan Lertpiriyasuwat, Patcharaporn Pawapootarnont, Jiraporn Chucherd, Juthamanee Moonwong; (5) Faculty of Associated Medical Sciences, Chiang Mai University: Tanawan Samleerat; (6) National Health Security Office: Suchada Chaiwut; US Military HIV Research Program: Suteeraporn Pinyakorn, Oratai Butterworth, Madelaine Ouellette, Nelson Michael, Robert Gramzinski. Search: Chutima Saisaengja.
Acknowledgments. The authors thank the children and their parents for their time and participation in this study and also the RV474/HIVNAT194 and RV475/HIV-NAT209 Study Groups. The authors thank Dominique Gauchat and Philippe St-Onge (Flow Cytometry Core Facility, Centre de Recherche du Centre hospitalier de l’Université de Montréal [CRCHUM], Montréal, Canada) and Olfa Debbeche (Biosafety Level 3 Core Facility, CRCHUM, Montréal, Canada). The authors thank Paule Bodson-Clermont of the Center for the Integration and Analysis of Medical Data (CITADEL) core facility of the CRCHUM for assisting with the methodology of this study and performing the statistical analysis.
Disclaimer. The views expressed are those of the authors and should not be construed to represent the positions of the United States Army, the Department of Defense, the NIH of Health, the Department of Health and Human Services, the Thai Red Cross, or the Henry M. Jackson Foundation for the Advancement of Military Medicine. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Financial support. This work was supported by the NIH (award number R01 AI114236); the Canadian Institutes of Health Research (grant number 364408); and the Réseau SIDA et maladies infectieuses du Fonds de Recherche du Quebec–Santé (FRQS). M. M. is supported by a Beatriu de Pinós (BP) Postdoctoral Fellowship from Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR), Generalitat de Catalunya. N. C. is supported by a Research Scholar Career Award of the Quebec Health Research Fund (FRQS, number 253292). M. R. is supported by the NIH (grant number R01 HD 080435-01).
Potential conflicts of interest. J. A. has received honorarium from Merck, ViiV Healthcare, Gilead, AbbVie, and Roche for participating in advisory meetings. P. S. reports a research grant from the HIV-NAT Research Collaboration. N. C. reports grants from EMD Serono and Gilead, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
RV474/HIVNAT194 and RV475/HIVNAT 209 Study Groups:
Torsak Bunupuradah, Stephen Kerr, Sasiwimol Ubolyam, Apicha Mahanontharit, Naphassanant Laopraynak, Preeyarach Klaytong, Tulathip Suwanlerk, Thita Pitimahajanaka, Naruporn Kasipong, Thornthan Noppakaorattanamanee, Kesdao Nanthapisal, Thatri Iampornsin, Sasithorn Burichai, Yupawadee Jummanee, Sudarat Soongpankeeree, Monta Intawan, Tuangthip Theerawat, Juthamanee Moonwong, Patchareeyawan Srimuen, Chutima Saisaengjan, Wasana Prasitsuebsai, Watsamon Jantarabenjakul, Suvaporn Anugulruengkitt, Umaporn Methanggool, Pugpen Sirikutt, Pimsiri Leowsrisook, Yosawadee Na Nakorn, Naruemon Sassungnune, Kanokkarn Wongmayurachat, Chanasda Kakkaew, Somjai Rattanamanee, Manee Yentang, Patcha Panyim, Janyarak Punyim, Thida Namwong, Siripim Kamphaengkham, Supanpilat Chaisri, Areerat Khongponoi, Ratchanee Saksawad, Usa Sukhaphan, Arena Laeyuheem, Gonzague Jourdain, Nicole Ngo-Giang-Huong, Sunee Sirivichayakul, Frank Maldarelli, Sarah Palmer, Michael Martin, Rangsima Lolekha, Thananda Naiwatanakul, Worawan Faikratok, Benjamas Baipluthong, Danai Teewunda, Sarawut Boonsuk, Chaweewan Tonputsa, Pariwat Tangpong, Archawin Rojanawiwat, Hansa Thaisri, Wiroi Puangtubtim, Chaidan Boonrossak, Sumet Ongwandee, Walairat Chaifoo, Cheewanan Lertpiriyasuwat, Patcharaporn Pawapootarnont, Jiraporn Chucherd, Juthamanee Moonwong, Tanawan Samleerat, Suchada Chaiwut, Suteeraporn Pinyakorn, Oratai Butterworth, Madelaine Ouellette, Nelson Michael, and Robert Gramzinski
References
- 1. Joint United Programme on HIV/AIDS. 2017 global HIV statistics. Available at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed 1 December 2019.
- 2. Zijenah LS, Moulton LH, Iliff P, et al. ZVITAMBO Study Group . Timing of mother-to-child transmission of HIV-1 and infant mortality in the first 6 months of life in Harare, Zimbabwe. AIDS 2004; 18:273–80. [DOI] [PubMed] [Google Scholar]
- 3. Bourne DE, Thompson M, Brody LL, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS 2009; 23:101–6. [DOI] [PubMed] [Google Scholar]
- 4. Lilian RR, Kalk E, Technau KG, Sherman GG. Birth diagnosis of HIV infection in infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. Pediatr Infect Dis J 2013; 32:1080–5. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach.2016. Available at: https://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 10 June 2020.
- 6. Anugulruengkitt S, Suntarattiwong P, Ounchanum P, et al. CIPHER_AEPEP Study Team . Safety of 6-week neonatal triple-combination antiretroviral postexposure prophylaxis in high-risk HIV-exposed infants. Pediatr Infect Dis J 2019; 38:1045–50. [DOI] [PubMed] [Google Scholar]
- 7. Lolekha R, Chokephaibulkit K, Phanuphak N, et al. Thai National Prevention of Mother-to-Child Transmission (PMTCT) Guidelines Working Group . Thai national guidelines for the prevention of mother-to-child transmission of human immunodeficiency virus 2017. Asian Biomed (Res Rev News) 2017; 11:145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Violari A, Cotton MF, Gibb DM, et al. CHER Study Team . Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359:2233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martínez-Bonet M, Puertas MC, Fortuny C, et al. Establishment and replenishment of the viral reservoir in perinatally HIV-1-infected children initiating very early antiretroviral therapy. Clin Infect Dis 2015; 61:1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luzuriaga K, Tabak B, Garber M, et al. HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis 2014; 210:1529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Zyl GU, Bedison MA, van Rensburg AJ, Laughton B, Cotton MF, Mellors JW. Early antiretroviral therapy in South African children reduces HIV-1-infected cells and cell-associated HIV-1 RNA in blood mononuclear cells. J Infect Dis 2015; 212:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuhn L, Paximadis M, Da Costa Dias B, et al. Age at antiretroviral therapy initiation and cell-associated HIV-1 DNA levels in HIV-1-infected children. PLoS One 2018; 13:e0195514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McManus M, Mick E, Hudson R, et al. PACTG 356 Investigators . Early combination antiretroviral therapy limits exposure to HIV-1 replication and cell-associated HIV-1 DNA levels in infants. PLoS One 2016; 11:e0154391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ananworanich J, Puthanakit T, Suntarattiwong P, et al. HIV-NAT 194 Study Group . Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS 2014; 28: 1015–20. [DOI] [PubMed] [Google Scholar]
- 15. Moragas M, Distefano M, Mecikovsky D, et al. Impact of the time to achieve viral control on the dynamics of circulating HIV-1 reservoir in vertically infected children with long-term sustained virological suppression: a longitudinal study. PLoS One 2018; 13:e0205579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tagarro A, Chan M, Zangari P, et al. Early and highly suppressive antiretroviral therapy are main factors associated with low viral reservoir in European perinatally HIV-infected children. J Acquir Immune Defic Syndr 2018; 79:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bitnun A, Ransy DG, Brophy J, et al. Clinical correlates of HIV-1 DNA and inducible HIV-1 RNA reservoirs in peripheral blood in children with perinatally acquired HIV-1 infection with sustained virologic suppression for at least 5 years. Clin Infect Dis 2020; 70:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Veldsman KA, Rensburg A, Isaacs S, et al. HIV‐1 DNA decay is faster in children who initiate ART shortly after birth than later. J Int AIDS Soc 2019; 22:68–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giacomet V, Trabattoni D, Zanchetta N, et al. No cure of HIV infection in a child despite early treatment and apparent viral clearance. Lancet 2014; 384:1320. [DOI] [PubMed] [Google Scholar]
- 20. Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 2013; 369:1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luzuriaga K, Gay H, Ziemniak C, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med 2015; 372:786–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frange P, Faye A, Avettand-Fenoël V, et al. ANRS EPF-CO10 Pediatric Cohort and the ANRS EP47 VISCONTI Study Group . HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 2016; 3:e49–54. [DOI] [PubMed] [Google Scholar]
- 23. Violari A, Cotton MF, Kuhn L, et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat Commun 2019; 10:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lolekha R, Boonsuk S, Plipat T, et al. Elimination of mother-to-child transmission of HIV—Thailand. MMWR Morb Mortal Wkly Rep 2016; 65:562–6. [DOI] [PubMed] [Google Scholar]
- 25. Lolekha R, Pavaputanon P, Puthanakit T, et al. Implementation of an active case management network to identify HIV-positive infants and accelerate the initiation of antiretroviral therapy, Thailand 2015–2018. J Int AIDS Soc 2020; 23:e25450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vandergeeten C, Fromentin R, Merlini E, et al. Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. J Virol 2014; 88:12385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Procopio FA, Fromentin R, Kulpa DA, et al. A novel assay to measure the magnitude of the inducible viral reservoir in HIV-infected individuals. EBioMedicine 2015; 2:872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vandergeeten C, Fromentin R, DaFonseca S, et al. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood 2013; 121:4321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Persaud D, Palumbo PE, Ziemniak C, et al. Dynamics of the resting CD4(+) T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. AIDS 2012; 26:1483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zanchetta M, Walker S, Burighel N, et al. Long-term decay of the HIV-1 reservoir in HIV-1-infected children treated with highly active antiretroviral therapy. J Infect Dis 2006; 193:1718–27. [DOI] [PubMed] [Google Scholar]
- 31. Uprety P, Chadwick EG, Rainwater-Lovett K, et al. Cell-associated HIV-1 DNA and RNA decay dynamics during early combination antiretroviral therapy in HIV-1-infected infants. Clin Infect Dis 2015; 61:1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uprety P, Patel K, Karalius B, et al. Pediatric HIV/AIDS Cohort Study (PHACS) . Human immunodeficiency virus type 1 DNA decay dynamics with early, long-term virologic control of perinatal infection. Clin Infect Dis 2017; 64:1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strain MC, Günthard HF, Havlir DV, et al. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: intrinsic stability predicts lifelong persistence. Proc Natl Acad Sci U S A 2003; 100:4819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bitnun A, Samson L, Chun TW, et al. Early initiation of combination antiretroviral therapy in HIV-1-infected newborns can achieve sustained virologic suppression with low frequency of CD4+ T cells carrying HIV in peripheral blood. Clin Infect Dis 2014; 59:1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.