Abstract
Background
Chlamydia trachomatis is the most common nationally notifiable sexually transmitted infection in the United States; however, the seroprevalence of C. trachomatis infection is unknown.
Methods
This cross-sectional study was conducted among 1725 females aged 18 to 39 years who provided serum and urine samples in the 2013 through 2016 National Health and Nutrition Examination Surveys. Presence of anti-C. trachomatis Pgp3 immunoglobulin G (IgG) was determined using both an enzyme-linked immunosorbent assay (ELISA) and multiplex bead array (MBA). Weighted seroprevalence estimates were calculated. Correlates of seroprevalence were examined by multivariable Poisson regression.
Results
In 2013 through 2016, overall seroprevalence of C. trachomatis Pgp3 IgG was 30.0% (95% confidence interval [CI], 25.5-35.0) as measured by ELISA and 29.4% (95% CI, 25.8-33.0) as measured by the MBA assay. Overall agreement between tests was 87.1% (1503/1725). There was a high positive agreement by the MBA assay with current detection of chlamydia in urine (86% [36/42]), a past-year diagnosis of chlamydia (81.8% [27/33]), and a history of treatment for pelvic inflammatory disease (60.7% [37/61]). Seroprevalence of C. trachomatis Pgp3 IgG, as measured by MBA, was significantly higher among non-Hispanic Blacks (68.0%; adjusted prevalence ratio (aPR) = 2.7 [95% CI, 2.3-3.3]), Mexican Americans (30.9%; aPR = 1.5 [95% CI, 1.2-1.9]), and other Hispanics (35.0%; aPR = 1.9 [95% CI, 1.4-2.5]) compared with non-Hispanic Whites (21.4%). A higher lifetime number of sexual partners and a younger age at sexual debut was also associated with higher seroprevalence.
Conclusion
Both the ELISA and MBA serologic assays revealed a high prevalence of antibodies to C. trachomatis Pgp3 in young adult females in the US household population. There were major racial/ethnic disparities in exposure to C. trachomatis, with increased vulnerability among non-Hispanic Black females.
Keywords: Chlamydia trachomatis, Pgp3 antibody, National Health and Nutrition Examination Surveys (NHANES), seroprevalence, enzyme-linked immunosorbent assay (ELISA), multiplex bead array (MBA)
Data from the cross-sectional 2013 through 2016 National Health and Nutrition Examination Surveys indicate that the seroprevalence of Chlamydia trachomatis pgp3 immunoglobulin G as measured by a multiplex bead array among US females was 29.4% (95% CI, 25.8-33.0), with seroprevalence disproportionately higher among non-Hispanic Blacks.
Chlamydia trachomatis is a nationally notifiable infection in the United States and is reported more than any other sexually transmitted infectious disease, with more than 1.7 million infections in 2018 [1, 2]. Although easily treated, C. trachomatis infection is often asymptomatic. When left untreated, however, C. trachomatis infection can lead to negative health outcomes, such as pelvic inflammatory disease (PID) in women, congenital C. trachomatis infection via in utero transmission, and increased risk of human immunodeficiency virus acquisition [2–5]. People younger than age 25 years compose the majority of reported infections, and cases are higher in Black, Hispanic, American Indian/Native Alaskan, and Native Hawaiian/Other Pacific Islander persons compared with White persons [2, 6]. Over the past 20 years, C. trachomatis screening has substantially increased in the United States. It is currently recommended by the US Centers for Disease Control and Prevention (CDC) that women younger than age 25 years be screened annually, in addition to high-risk women 25 years or older [2]. Because infection is frequently asymptomatic, people may not know they are infected and therefore would not seek sexually transmitted infection testing and treatment [2, 7]. Therefore, surveillance-based case reporting of C. trachomatis infection may be biased.
Because tests for C. trachomatis infection by nucleic acid cannot determine past infection, serological assays are preferable to determine prevalence of prior exposure [8]. Serological testing for C. trachomatis can prove challenging because of cross-reactivity with other pathogens, especially other Chlamydia species, such as Chlamydia pneumoniae [9]. Immunoglobulin G (IgG) antibodies to plasmid gene product 3 (Pgp3) have been identified as the most reliable marker of exposure to C. trachomatis because Pgp3’s genetic code is generally highly conserved across isolates and rarely found in C. pneumoniae [9–11]. In addition, C. trachomatis Pgp3 antibodies appear to persist for > 10 years in women [9, 12, 13]. Sensitivity and specificity of C. trachomatis Pgp3 antibody enzyme-linked immunosorbent assays (ELISAs) also tend to outperform ELISAs for other C. trachomatis antigens [8, 9, 12].
Estimating prevalence of prior exposure to C. trachomatis has many applications to C. trachomatis control, including determining potential vaccination strategies, as well as estimating the contribution of C. trachomatis for a variety of negative health outcomes [3, 8]. Nationally representative seroprevalence estimates of C. trachomatis in the United States have not been calculated previously. Therefore, the goal of this study was to estimate C. trachomatis seroprevalence among adult females in the noninstitutionalized, civilian population in the United States and describe the agreement of 2 serological assays that detect C. trachomatis Pgp3 antibodies.
METHODS
Data Source and Population
Data for this study are from the continuous National Health and Nutrition Examination Surveys (NHANES), a cross-sectional, complex survey conducted by the National Center for Health Statistics (NCHS) [14]. NHANES uses a stratified, multistage probability sampling design to generate representative estimates of the noninstitutionalized, civilian US population. The survey includes both in-person household interviews, where demographic and health-related information is collected through in person computer-assisted personal interviews and in-person visit to a medical examination center (MEC) with collection of additional health-related information via audio computer-assisted self-interviews, physical examinations, and biological specimens [15]. The overall response rate for the MEC component among females was 68.8% in 2013 and 2014 and 60.0% in 2015 and 2016 [16].
This analysis used data from females aged 18 to 39 years who participated in the MEC component of the 2013 through 2016 NHANES. Serum samples for C. trachomatis antibody testing were collected only among females who also provided urine samples for C. trachomatis nucleic acid detection. A total of 2250 females aged 18 to 39 years participated in the MEC component, of which 2195 provided urine samples, and 1725 females provided both urine and serum samples (Supplemental Figure 1).
Laboratory Testing
Urine and blood samples were frozen at –30°C and –80°C, respectively, and shipped to the CDC laboratories in Atlanta, Georgia, where they were tested [17]. Current C. trachomatis infections were detected with BDProbeTec Chlamydia trachomatis Amplified DNA Assay on urine samples [18, 19].
Detection of IgG antibody to C. trachomatis Pgp3 was performed on serum samples that had not undergone previous freeze thaw cycles, with 2 separate assays: the Luminex MAGPIX multiplex bead array (MBA [Luminex Corp., Austin, Texas]) and an indirect ELISA [3, 20–22]. Both assays were verified in house by the CDC’s Laboratory Reference and Research Branch in the Division of Sexually Transmitted Disease Prevention. MBA assays were run once; unreadable results or tests that experienced error were rerun and the second test results were used. ELISA assays were run twice and the results averaged. In the case of error during the ELISA, it was rerun twice again, with the results averaged. Serum specimens were shipped to and analyzed at the CDC’s Laboratory Reference and Research Branch [23].
Seropositivity for herpes simplex virus 2 (HSV-2) was detected at Emory University using a solid-phase enzymatic ImmunoDOT assay for gG-2, a glycoprotein that is not cross-reactive with HSV-1 [24–26].
Questionnaire Data
Self-reported data on age, sex, race/ethnicity, marital status, education, place of birth, and household income were all collected in the participant’s household. Marital status was asked only of participants age 20 years or older; therefore a “not asked” category was created for participants ages 18 to 19 years. Participants age 20 years and older were categorized as either “never married,” “married/living with partner,” and “widowed divorced/separated.” Poverty status was defined as either having an annual household income that was below the federal poverty line or having a household income that was at or above the poverty line. History of treatment for PID was collected at the MEC through computer-assisted personal interview. A sexual behavior questionnaire was administered in the MEC using an audio computer-assisted self-interview, including data on a self-reported diagnosis of chlamydia in the past 12 months. Lifetime and past-year sexual partners included both same and opposite sex partners, as well as anal, vaginal, and oral sex partners. If someone responded they had never had sex, values of “0” were imputed for sex partner in the past year and “no” for having a new sex partner in the past year. For those who reported not having a partner in the past year, but were sexually experienced, values of “no” were imputed for having a new sex partner in the past year.
Statistical Analysis
Concordance and discordance of results on the MBA and ELISA were described using unweighted observations. Positivity of the tests among those who had current detection, who reported being diagnosed with chlamydia in the past 12 months or been treated for PID previously were also explored to evaluate test performance (ie, percent positive agreement). In a supplemental analysis, a univariable multinomial logistic regression was performed among females who were positive on at least 1 assay to explore factors associated with positive concordance and discordance on anti-C. trachomatis Pgp3 IgG assays. The possible outcomes were: ELISA positive and MBA negative, ELISA negative and MBA positive, and positive on both the ELISA or MBA.
Prevalence of anti-C. trachomatis Pgp3 IgG and factors associated with seropositivity were examined using MEC weights, which were calculated by the NCHS to account for nonresponse and unequal probability of selection. Additionally, NCHS poststratifies the weights to match the population counts from the Census Bureau’s American Community Survey. Because this analysis combines data from 2 NHANES cycles, MEC weights were adjusted according to NCHS guidelines. Taylor series linearization was used for variance estimation. Korn-Graubard 95% confidence intervals (95% CI) were calculated for prevalence estimates [27].
Prevalence of anti-C. trachomatis Pgp3 IgG was defined 2 separate ways: (1) positive on ELISA and (2) positive on MBA. Sociodemographic and sexual behavioral factors associated with ELISA and MBA anti-Pgp3 IgG positivity were examined using univariable and multivariable Poisson regression. Prevalence estimates as well as prevalence ratios for age of sexual debut were calculated among only those who were sexually experienced, whereas all other prevalence estimates and prevalence ratios were estimated among all participants. As an ancillary analysis, age- and race-specific prevalence estimates of anti-C. trachomatis Pgp3 IgG (as measured by MBA) and anti-HSV-2 IgG were comparatively examined.
A sensitivity analysis was performed examining prevalence of anti-C. trachomatis Pgp3 IgG defined 2 additional ways: (1) positive on both the ELISA and MBA, and (2) positive on either the ELISA and MBA. Prevalence of being positive on both assays provides the most conservative (specific) population-level estimates for anti-C. trachomatis Pgp3 IgG positivity, whereas prevalence by being positive on either provides the most liberal (sensitive) population-level estimates. A third sensitivity analysis was performed using multiply imputed data to account for item nonresponse among female participants aged 18 to 39 years who participated in the MEC component, particularly for anti-C. trachomatis Pgp3 IgG and other variables of interest. Imputation for missing variables was done using predictive mean matching for continuous variables, logistic regression for binary variables, and random forests for categorical variables. Missingness by inclusion and exclusion in the main analysis is shown in Supplementary Table 1. All variables used in the primary analysis were added to the imputation model as well as additional auxiliary variables (smoking, alcohol use, drug use, insurance status, body mass index, healthcare use, pregnancy status, family size, military service, time of year of interview). Survey weights and stratum were also used in the imputation model.
All weighted analyses were performed in R version 3.6.1. using the “survey” package, whereas imputation was performed using the “mice” package [28, 29].
Ethics Statement
Data collection was approved by the NCHS Research Ethics Board. The analysis was conducted using deidentified publicly available data and was waived from review by Johns Hopkins University School of Medicine institutional review board.
RESULTS
Assay Concordance/Discordance
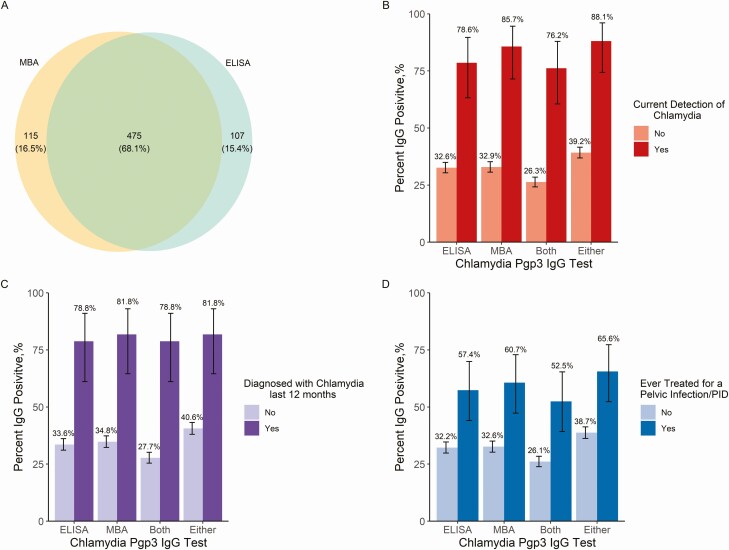
Of the 1725 females who were tested for anti-C. trachomatis Pgp3 IgG by both ELISA and MBA assays, 40.4% (697/1725) tested positive on at least 1 assay, 33.7% (582/1725) tested positive on the ELISA, whereas 34.2% (590/1725) tested positive on the MBA and 27.5% (475/1725) were positive on both. Among all participants, 87.1% (1503/1725) had concordant results, whereas 68.1% (475/697) of participants who tested positive on at least 1 assay, tested positive on both (Figure 1).
Figure 1.
Chlamydia trachomatis seroprevalence among US females aged 18 to 39 years. A, Concordance and discordance of 2 anti-C. trachomatis Pgp3 IgG assays among females age 18–39 (n = 697). MBA positivity is represented in yellow, ELISA positivity represented in blue and concordance of MBA and ELISA in green. B, Anti-C. trachomatis Pgp3 IgG positivity among females with current chlamydia detection by urine NAAT test (n = 42) and without chlamydia detection (n = 1683). C, Anti-C. trachomatis Pgp3 IgG positivity among females diagnosed with chlamydia in the past 12 months (n = 33) and those not diagnosed (n = 1391). D, anti-C. trachomatis Pgp3 IgG positivity among females ever treated for PID or pelvic infection (n = 61) and those not diagnosed (n = 1471). Note: Data are unweighted. 95% confidence intervals are exact binomial confidence intervals. Definitions of positivity: ELISA, positive on the anti-pgp3 ELISA; MBA, positive on the anti-pgp3 MBA; Both, positive on both an anti-pgp3 MBA and ELISA; Either, positive on either the anti-pgp3 MBA and ELISA. Abbreviations: IgG, immunoglobin G; ELISA, enzyme-linked immunosorbent assay; MBA, multiplex bead format assay; NAAT, nucleic acid amplification test; Pgp3, plasmid gene product 3; PID, pelvic inflammatory disease.
Among those who had current C. trachomatis infection (n = 42), the MBA assay had a slightly higher positive percentage agreement as opposed to the ELISA (85.7% [n = 36] vs. 78.6% [n = 33]). Across all definitions of anti-C. trachomatis Pgp3 IgG, a significantly higher prevalence of anti-C. trachomatis Pgp3 IgG was found among those who had current C. trachomatis infection than those who did not have current C. trachomatis infection (MBA, 85.7% vs. 32.9%, respectively). Similar findings were observed with a self-reported diagnosis of chlamydia in the past 12 months and those who have ever been treated for PID.
Distribution of sociodemographic and behavioral characteristics by results on both assays, including those who were concordant negative, are shown in Supplemental Table 2. Factors associated with being positive by only 1 assay compared with both assays are shown in Supplemental Table 3. Those who were married/living with partner were more likely to be positive on 1 assay, but not both (discordant), whereas non-Hispanic Black females and those with a higher number of lifetime sexual partners were more likely to be positive on both (concordant).
Seroprevalence by Sociodemographics
The overall population-level prevalence of anti-C. trachomatis Pgp3 IgG in females aged 18 to 39 years was similar when measured by ELISA (30.0% [95% CI, 25.5-35.0] and by MBA (29.4% [95% CI, 25.8-33.0]) (Table 1). Seroprevalence measured by being positive on both ELISA and MBA was expectedly lower at 23.0% (95% CI, 19.7-27.0), whereas it was expectedly higher when examined by being positive on either ELISA or MBA (36.3% [95% CI, 32.0-41.0]). Seroprevalence estimated by ELISA and MBA were fairly similar when stratified by individual characteristics.
Table 1.
Seroprevalence of Chlamydia trachomatis Overall and Stratified by Individual-Level Characteristics in US Females Ages 18-39, as Measured by 4 Definitions of Seropositivitya
| ELISA | MBA | Both | Either | |
|---|---|---|---|---|
| Characteristics | Weighted % (95% CI) | Weighted % (95% CI) | Weighted % (95% CI) | Weighted % (95% CI) |
| Overall | 30.0 (25.5–35.0) | 29.4 (25.8–33.0) | 23.0 (19.7–27.0) | 36.3 (32.0–41.0) |
| Age, y | ||||
| 18–24 | 27.4 (22.4–32.9) | 27.9 (22.9–33.2) | 21.7 (17.8–25.9) | 33.6 (27.8–39.8) |
| 25–29 | 29.3 (23.3–35.8) | 27.4 (22.6–32.6) | 22.1 (17.3–27.4) | 34.6 (28.8–40.8) |
| 30–34 | 31.5 (24.6–39.1) | 30.4 (23.9–37.5) | 21.3 (16.0–27.4) | 40.6 (34.4–46.9) |
| 35–39 | 32.3 (25.3–40.0) | 32.4 (25.2–40.3) | 27.5 (21.2–34.5) | 37.3 (29.1–45.9) |
| Race/ethnicity | ||||
| Non-Hispanic White | 23.0 (18.1–28.5) | 21.4 (17.5–25.7) | 14.8 (11.6–18.5) | 29.6 (24.9–34.6) |
| Non-Hispanic Black | 60.5 (54.4–66.5) | 68.0 (61.1–74.5) | 59.7 (53.5–65.8) | 68.8 (61.9–75.2) |
| Mexican American | 31.8 (25.9–38.2) | 30.9 (26.1–36.0) | 25.4 (20.6–30.7) | 37.4 (31.5–43.5) |
| Other Hispanic | 35.1 (27.8–42.9) | 35.0 (28.1–42.4) | 28.7 (21.8–36.6) | 41.3 (34.7–48.2) |
| Non-Hispanic Asian | 17.0 (11.0–24.4) | 15.5 (10.3–22.1) | 9.9 (5.6–15.8) | 22.6 (16.1–30.2) |
| Other race, including multiracial | 43.0 (23.2–64.5)a | 35.9 (22.5–51.2) | 33.4 (21.2–47.6) | 45.5 (24.2–68.1)a |
| Born in the 50 US states | ||||
| No | 30.3 (25.3–35.7) | 29.8 (25.5–34.5) | 23.5 (19.7–27.6) | 36.7 (31.6–42.0) |
| Yes | 28.5 (23.0–34.4) | 27.4 (23.4–31.7) | 21.0 (16.7–26.0) | 34.8 (29.7–40.2) |
| Poverty status | ||||
| Below the poverty level | 39.2 (33.0–45.6) | 42.4 (35.8–49.2) | 35.1 (29.6–41.0) | 46.4 (39.5–53.4) |
| At or above poverty level | 26.3 (21.7–31.4) | 25.0 (21.0–29.3) | 19.0 (15.6–22.7) | 32.4 (27.8–37.2) |
| Education | ||||
| Less than high school | 36.8 (30.2–43.8) | 41.6 (34.7–48.7) | 32.2 (26.0–38.9) | 46.2 (38.9–53.6) |
| GED/high school degree/some college | 34.0 (27.2–41.2) | 34.5 (27.5–42.1) | 28.2 (21.9–35.2) | 40.3 (33.2–47.8) |
| College degree of higher | 27.4 (22.6–32.5) | 25.4 (22.0–29.0) | 19.6 (16.7–22.8) | 33.1 (28.5–38.1) |
| Marital status | ||||
| Never married | 34.7 (29.5–40.3) | 33.9 (29.1–38.8) | 29.2 (25.1–33.6) | 39.4 (33.7–45.3) |
| Married/living with partner | 25.8 (21.3–30.6) | 26.2 (21.7–31.1) | 18.6 (15.0–22.7) | 33.3 (28.7–38.1) |
| Widowed/divorced/separated | 48.6 (35.2–62.2) | 45.7 (35.5–56.1) | 38.2 (28.6–48.6) | 56.1 (42.9–68.6) |
| Not askedb | 23.4 (15.9–32.4) | 18.9 (12.4–26.8) | 15.3 (10.7–20.8) | 27.0 (18.5–37.0) |
| Lifetime sexual partners | ||||
| 0–1 | 11.2 (7.0–16.8) | 7.4 (4.9–10.7) | 4.8 (3.0–7.1) | 13.9 (9.1–20.0) |
| 2–3 | 23.9 (17.3–31.4) | 18.6 (13.4–24.9) | 14.3 (10.3–19.1) | 28.2 (21.0–36.4) |
| 4–7 | 37.1 (30.4–44.3) | 40.7 (33.7–48.0) | 33.1 (27.3–39.4) | 44.7 (37.0–52.6) |
| 8–15 | 37.4 (28.8–46.7) | 38.5 (31.7–45.8) | 29.8 (23.0–37.3) | 46.2 (37.8–54.7) |
| > 15 | 43.2 (31.0–56.0) | 47.2 (36.9–57.6) | 38 (27.9–48.9) | 52.3 (40.3–64.2) |
| Sexual partner over past 12 mo | ||||
| 0 | 18.6 (10.0–30.3) | 14.5 (8.8–21.9) | 9.6 (4.9–16.3) | 23.5 (14.3–34.9) |
| 1 | 28.1 (23.8–32.8) | 27.8 (23.9–32.0) | 22.1 (18.8–25.7) | 33.8 (29.2–38.8) |
| 2 | 38.7 (30.4–47.5) | 40.2 (34.0–46.7) | 31.4 (25.0–38.3) | 47.5 (39.9–55.2) |
| New sexual partner over past 12 mo | ||||
| No | 28.1 (23.6 – 33.0) | 27.7 (23.8–31.8) | 21.9 (18.6–25.4) | 33.9 (29.0–39.0) |
| Yes | 34.9 (27.4 – 43.0) | 34.9 (28.5–41.7) | 26.8 (20.8–33.6) | 43.0 (36.0–50.2) |
| Age of sexual debut, yc | ||||
| ≤16 | 42.1 (36.9–47.4) | 43.4 (38.7–48.2) | 35.5 (31.6–39.6) | 49.9 (44.1–55.7) |
| 17–18 | 24.8 (17.6–33.3) | 26.5 (21.7–31.8) | 19.0 (14.2–24.6) | 32.4 (25.3–40.1) |
| ≥19 | 13.6 (9.1–19.2) | 10.2 (6.3–15.3) | 7.3 (4.7–10.6) | 16.5 (12.0–22.0) |
All data are weighted using pooled Medical Examination Center (MEC) survey weights provided by the National Center for Health Statistics.
Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobin G; MBA, multiplex bead format assay; US, United States.
aDefinitions of positivity: ELISA, positive on the anti-pgp3 ELISA; MBA, positive on the anti-pgp3 MBA; both, positive on both an anti-pgp3 MBA and ELISA; either, positive on either the anti-pgp3 MBA and ELISA.
bNot asked of participants ages 18 to 19 years.
cOnly among those sexually experienced.
By MBA, seroprevalence was highest among non-Hispanic Black females (68.0% [95% CI, 61.1-74.4]). Seroprevalence was also higher in Mexican Americans (30.9% [95% CI, 26.2-36.0]), other Hispanics (35.0% [95% CI, 28.1-42.4]), and people of other race/multiracial (35.9% [95% CI, 22.5-51.2]) compared with non-Hispanic Whites (21.4% [95% CI, 17.5-25.7]). ELISA underestimated the seroprevalence in non-Hispanic Black females in comparison to MBA.
There was an inverse relationship between seroprevalence and both education and poverty status. Those with educational attainment of less than a high school degree (MBA, 41.6% [95% CI, 34.7-48.7]) or who were under the federal poverty level (MBA, 42.5% [95% CI, 35.8-49.2]) had the highest seroprevalence, while those with a college degree or higher (MBA, 25.4% [95% CI, 22.0-29.0]) or who were at or above the poverty level (MBA, 25.0% [95% CI, 21.0-29.3]) had the lowest seroprevalence.
Seroprevalence by Sexual Behaviors
There were differences in seroprevalence by both reported lifetime and recent sexual behaviors. Seroprevalence increased with both an increase of lifetime sexual partners and increase of past years sexual partners. Among sexually experienced persons, seroprevalence was higher among those whose sexual debut was at 16 years or younger (MBA, 43.4% [95% CI, 38.7-48.2]) compared with those who initiated sex at 19 years or older (MBA, 10.2% [95% CI, 6.3-15.3]).
Associations with C. trachomatis Seroprevalence
Associations with anti-C. trachomatis Pgp3 IgG are shown in Table 2. Characteristics associated with being positive by ELISA were similar to characteristics associated with being positive by MBA. In multivariable analyses, racial/ethnic minorities were significantly associated with being seropositive. Compared with non-Hispanic White females, non-Hispanic Black females had 2.7 (95% CI, 2.3-3.3, MBA) times the anti-C. trachomatis Pgp3 IgG prevalence. Having more lifetime sexual partners was also associated with being seropositive, as well as having a younger age at sexual debut. Although household poverty status and being widowed/divorced/separated (vs being never married) were significant predictors of anti-C. trachomatis Pgp3 IgG seropositivity in a univariable analysis, this was not observed in multivariable analysis.
Table 2.
Factors Associated With Positivity for Anti-C. trachomatis Pgp3 IgG
| ELISA | MBA | |||
|---|---|---|---|---|
| Characteristics | PR (95% CI) | adjPRa (95% CI) | PR (95% CI) | adjPRa (95% CI) |
| Age | ||||
| 18–24 | 1 | 1 | 1 | 1 |
| 25–29 | 1.1 (0.8–1.4) | 1.0 (0.8–1.2) | 1.0 (0.8–1.3) | 0.8 (0.7–1.0) |
| 30–34 | 1.1 (1.0–1.4) | 1.0 (0.9–1.2) | 1.1 (0.8–1.4) | 0.9 (0.7–1.2) |
| 35–39 | 1.2 (1.0–1.5) | 1.1 (0.9–1.4) | 1.2 (0.9–1.5) | 1.0 (0.8–1.2) |
| Race/ethnicity | ||||
| Non-Hispanic White | 1 | 1 | 1 | 1 |
| Non-Hispanic Black | 2.6 (2.1–3.3) | 2.4 (1.8–3.1) | 3.2 (2.7–3.8) | 2.7 (2.3–3.3) |
| Mexican American | 1.4 (1.1–1.8) | 1.5 (1.2–2.1) | 1.4 (1.1–1.8) | 1.5 (1.2–1.9) |
| Other Hispanic | 1.5 (1.2–2.0) | 1.8 (1.3–2.5) | 1.6 (1.3–2.1) | 1.9 (1.4–2.5) |
| Non-Hispanic Asian | 0.7 (0.5–1.1) | 1.0 (0.6–1.5) | 0.7 (0.5–1.1) | 1.1 (0.7–1.7) |
| Other race, including multiracial | 1.9 (1.3–2.8) | 1.6 (1.0–2.6) | 1.7 (1.1–2.6) | 1.3 (0.9–2.0) |
| Born in the 50 US states | ||||
| No | 1 | 1 | 1 | 1 |
| Yes | 0.9 (0.8–1.2) | 0.9 (0.7–1.2) | 0.9 (0.7–1.1) | 1.0 (0.8–1.2) |
| Poverty | ||||
| Below the poverty level | 1 | 1 | 1 | 1 |
| At or above poverty level | 0.8 (0.7–0.9) | 0.9 (0.8–1.0) | 0.7 (0.6–0.8) | 0.9 (0.8–1.0) |
| Education | ||||
| Less than high school | 1 | 1 | 1 | 1 |
| GED/high school degree/some college | 0.8 (0.7–0.9) | 0.8 (0.7–1.0) | 0.7 (0.6–0.8) | 0.7 (0.6–0.9) |
| College degree of higher | 0.9 (0.8–1.1) | 1.0 (0.8–1.2) | 1.0 (0.8–1.1) | 1.0 (0.8–1.2) |
| Marital status | ||||
| Never married | 1 | 1 | 1 | 1 |
| Married/living with partner | 0.7 (0.6–0.9) | 0.9 (0.7–1.1) | 0.8 (0.6–0.9) | 1.1 (0.9–1.3) |
| Widowed/divorced/separated | 1.4 (1.1–1.8) | 1.1 (0.9–1.5) | 1.3 (1.1–1.7) | 1.1 (0.8–1.5) |
| Not askedb | 0.7 (0.5–0.9) | 0.9 (0.7–1.3) | 0.6 (0.4–0.8) | 0.9 (0.6–1.2) |
| Lifetime sexual partners | ||||
| 0–1 | 1 | 1 | 1 | 1 |
| 2–3 | 2.1 (1.3–3.4) | 1.9 (1.2–3.1) | 2.5 (1.6–3.9) | 2.2 (1.5–3.3) |
| 4–7 | 3.3 (2.3–4.8) | 2.8 (1.9–4.1) | 5.5 (3.8–7.8) | 5.0 (3.5–7.1) |
| 8–15 | 3.3 (2.3–4.7) | 3.0 (2.1–4.5) | 5.2 (3.5–7.6) | 5.4 (3.7–7.9) |
| >15 | 3.8 (2.5–6.0) | 3.6 (2.2–6.0) | 6.3 (4.3–9.5) | 6.9 (4.7–10.3) |
| Sexual partner over past 12 mo | ||||
| 0 | 1 | - | 1 | - |
| 1 | 1.5 (0.9–2.4) | - | 1.9 (1.3–2.9) | - |
| 2 | 2.1 (1.4–3.2) | - | 2.8 (1.8–4.3) | - |
| New sexual partner over past 12 mo | ||||
| No | 1 | 1 | 1 | 1 |
| Yes | 1.2 (1.0–1.5) | 0.9 (0.7–1.1) | 1.3 (1.0–1.5) | 0.9 (0.7–1.1) |
| Age of sexual debut, yc | ||||
| ≤16 | 1 | 1 | 1 | 1 |
| 17–18 | 0.6 (0.5–0.8) | 0.7 (0.5–0.9) | 0.6 (0.5–0.7) | 0.8 (0.7–0.9) |
| ≥19 | 0.3 (0.2–0.4) | 0.5 (0.4–0.7) | 0.2 (0.2–0.3) | 0.4 (0.2–0.6) |
All data are weighted using pooled Medical Examination Center (MEC) survey weights provided by the National Center for Health Statistics. Prevalence ratios were estimated by Poisson regression. Boldface indicate significant association, where the 95% confidence interval does not cross the null value of 1.0.
Abbreviations: adjPR, adjusted prevalence ratio; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobin G; MBA, multiplex bead format assay; Pgp3, plasmid gene product 3; PR, prevalence ratio; US, United States.
aMultivariable model includes age, race, place of birth, poverty status, education, marital status, lifetime sexual partners, and a new sexual partner over the past 12 months. Multivariable model for age of sexual debut was a separate model that included an additional covariate for age of sexual debut.
bNot asked of participants ages 18 to 19 years.
cOnly among those sexually experienced.
Age- and Race-Specific Seroprevalence of C. trachomatis and HSV-2
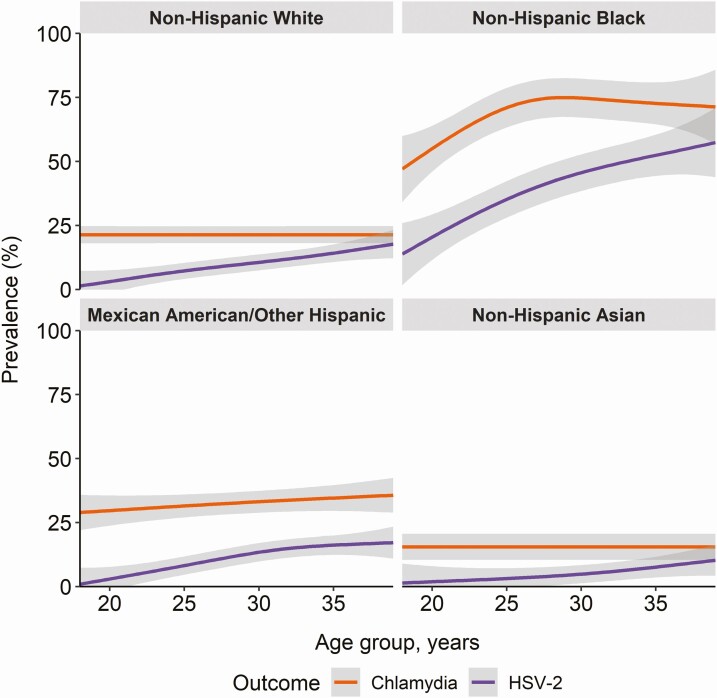
Figure 2 compares the seroprevalence of C. trachomatis measured by the anti-Pgp3 MBA assay with HSV-2 seroprevalence by race/ethnicity and age. In general, although the HSV-2 seroprevalence is lower than C. trachomatis seroprevalence, there appeared to be similar distributions by race/age categories that have higher prevalence of HSV-2 also have higher prevalence of C. trachomatis, with highest prevalence in non-Hispanic Black females.While HSV-2 seroprevalence increased with older age across race/ethnicity groups, C. trachomatis seroprevalence remained relatively stable for all race/ethnicity groups except non-Hispanic black females. Among non-Hispanic black females, C. trachomatis seroprevalence rapidly increased with older age until age 25 but then plateaued.
Figure 2.
Seroprevalence of Chlamydia trachomatis (C. trachomatis) and herpes simplex virus 2 by age and race among US females age 18 to 39 years. Note: C. trachomatis prevalence as determined by a multiplex bead array (MBA). Abbreviations: HSV-2, herpes simplex virus 2; US, United States.
Sensitivity Analyses
Factors associated with being seropositive on both the ELISA and MBA, or by at least 1 of the assays were similar to those observed with the individual assays (Supplemental Table 4). Results from the multiple imputation are shown in Supplemental Tables 5 and 6. Seroprevalence estimates and measures of association were insensitive to imputation of missing data.
DISCUSSION
This study found a high prevalence of anti-C. trachomatis Pgp3 IgG (~30%) among young adult females in the US household population. In addition to providing the first national estimates, this study demonstrates that seroprevalence is dramatically higher among racial/ethnic minorities. This study also shows that, although the ELISA and MBA for Pgp3 IgG perform well, they do not provide consistent results and the MBA assay is likely superior.
In this study, seroprevalence of C. trachomatis was disproportionately higher among racial/ethnic minorities. Non-Hispanic Black females had the highest seroprevalence, with approximately two-thirds having prior exposure to C. trachomatis infection. Seroprevalence of C. trachomatis was also elevated among Mexican American, other Hispanic, and multiracial/other race females compared with non-Hispanic Whites. These are among the first nationally representative serological data demonstrating such striking racial/ethnic disparities in C. trachomatis infection in the United States. These data are consistent with findings of national surveillance data for reported current infections in minorities in the United States, as well as serosurveys of C. trachomatis Pgp3 conducted in other high-income settings including England [6, 30]. Some of the factors that may contribute to the observed racial disparities in C. trachomatis infection include differences in individual-level sexual behaviors, differences in sexual networks, differential access to sexually transmitted infection prevention and treatment services, and structural racism more broadly [6, 31, 32].
Seroprevalence was also elevated with an increase of lifetime sexual partners and high-risk sexual behaviors, indicating that these data are a good measure of the tremendous total burden of previous and current infection, which is significantly higher than current infections. Antibodies to HSV-2 have been thought of as a biological marker for sexual behavior and a way to indicate risk of other sexually transmitted infections [33–36]. As demonstrated in this study, HSV-2 seroprevalence is lower than C. trachomatis seroprevalence; however, they largely follow similar distributions by age and race with a large increase over younger ages in non-Hispanic Black females. Previous studies have demonstrated that C. trachomatis antibody detection decreases over time, but remain longer for women who have been infected multiple times [37, 38]. This suggests that the lifetime seroprevalence is generally underestimated using Pgp3-antibody assays and the racial disparities in C. trachomatis exposure may potentially be greater than that observed here. Sensitivity of Pgp3 ELISAs have also shown to decrease over time since infection [37].
A strength of this study is the comparison between the ELISA and MBA for Pgp3 IgG. Although both assays generally yielded similar associations and prevalence estimates, the lack of concordance between assays is concerning. The MBA assay is likely superior than the ELISA, as demonstrated by the slightly higher percentage agreement with currently detected C. trachomatis infection by urine nucleic acid amplification test (NAAT), females diagnosed with C. trachomatis during the past year, and also with females reporting being previously treated for PID. The higher concordance of the MBA with NAAT positivity compared with the Pgp3 ELISA has also been observed previously in women with PID [22]. The MBA assay has also been shown to be a superior method for detection of antibodies to C. trachomatis in trachoma [39].
There are several limitations to this study. The study population only included females between 18 and 39 years of age. It would have been ideal to include adolescent females as well as males, although Pgp3 testing is less sensitive in males [9, 12]. There is no widely accepted gold standard assay for chlamydia IgG, and this study used an indirect ELISA, which has been shown to be less sensitive than a double antigen ELISA [12]. Another weakness is that Pgp3 antibody may decrease over time, which may lead to underestimated seroprevalence estimates. In addition, urine testing in women has a lower sensitivity than vaginal swabs for C. trachomatis detection [40]. There is also possible selection bias because participants had to attend the MEC and agree to provide both urine and serum samples. Additionally, because high-risk persons including incarcerated individuals and homeless persons were not in the sampling frame, these data may not be generalizable to the entire US population. Nonetheless, NHANES is designed to be a nationally representative survey, and these data provide among the first population-based serological data for C. trachomatis.
This population-based study demonstrates that antibodies to C. trachomatis Pgp3 correlate with current detection of C. trachomatis infection, recent history of chlamydia diagnosis, and increased sexual partners, validating their use for serosurveillance, particularly using the MBA assay. As such, C. trachomatis serosurveillance data will be invaluable for performance and evaluation of future vaccine trials [3, 8], as well as other prevention measures including screening. Notably, these data also highlight stark racial/ethnic disparities in exposure to C. trachomatis. Increased public health interventions are needed to improve racial inequities in sexual health in the United States.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are grateful to the NHANES study staff and participants, without whom this study would not have been possible.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Financial support. This work was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID) and extramural support from NIAID (R01AI120938 and R01AI128779 to A.A.R.T. and T32AI102623 to E.U.P.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Torrone E, Papp J, Weinstock H; Centers for Disease Control and Prevention (CDC) . Prevalence of Chlamydia trachomatis genital infection among persons aged 14-39 years–United States, 2007-2012. MMWR Morb Mortal Wkly Rep 2014; 63:834–8. [PMC free article] [PubMed] [Google Scholar]
- 2. CDC. Sexually transmitted disease surveillance 2018. Atlanta, GA: US Department of Health and Human Services, CDC. 2019. Available at: https://www.cdc.gov/std/stats18/STDSurveillance2018-full-report.pdf. Accessed 1 August 2020. [Google Scholar]
- 3. Woodhall SC, Gorwitz RJ, Migchelsen SJ, et al. . Advancing the public health applications of Chlamydia trachomatis serology. Lancet Infect Dis 2018; 18:e399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geisler WM, Uniyal A, Lee JY, et al. . Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med 2015; 373:2512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patton DL, Sweeney YT, Stamm WE. Significant reduction in inflammatory response in the macaque model of chlamydial pelvic inflammatory disease with azithromycin treatment. J Infect Dis 2005; 192:129–35. [DOI] [PubMed] [Google Scholar]
- 6. Fine D, Thomas KK, Nakatsukasa-Ono W, Marrazzo J. Chlamydia positivity in women screened in family planning clinics: racial/ethnic differences and trends in the northwest U.S., 1997-2006. Public Health Rep 2012; 127:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Der Pol B. Sexually transmitted infections in women. Scand J Clin Lab Invest Suppl 2014; 244:68–74; discussion 73. [DOI] [PubMed] [Google Scholar]
- 8. Horner P. Can Chlamydia serology be used to help inform a potential future chlamydia vaccination strategy? Sex Transm Dis 2017; 44:722–4. [DOI] [PubMed] [Google Scholar]
- 9. Wills GS, Horner PJ, Reynolds R, et al. . Pgp3 antibody enzyme-linked immunosorbent assay, a sensitive and specific assay for seroepidemiological analysis of Chlamydia trachomatis infection. Clin Vaccine Immunol 2009; 16:835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaur H, Dize L, Munoz B, Gaydos C, West SK. Evaluation of the reproducibility of a serological test for antibodies to Chlamydia trachomatis pgp3: a potential surveillance tool for trachoma programs. J Microbiol Methods 2018; 147:56–8. [DOI] [PubMed] [Google Scholar]
- 11. Gwyn S, Cooley G, Goodhew B, et al. . Comparison of platforms for testing antibody responses against the Chlamydia trachomatis antigen Pgp3. Am J Trop Med Hyg 2017; 97:1662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horner PJ, Wills GS, Righarts A, et al. . Chlamydia trachomatis Pgp3 antibody persists and correlates with self-reported infection and behavioural risks in a blinded cohort study. PLoS One 2016; 11:e0151497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berry A, Hall JV. The complexity of interactions between female sex hormones and Chlamydia trachomatis infections. Curr Clin Microbiol Rep 2019; 6:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: sample design, 2011–2014. Vital Health Stat 2014; 2:1–33. [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS). Interviewer Procedures Manual. Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2015–2016/manuals/2016_Interviewer_Procedures_Manual.pdf. Accessed 1 August 2020.
- 16. Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS). NHANES response rates and population totals. Available at: https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx. Accessed 1 August 2020.
- 17. Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS). Laboratory procedures manual. Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2015–2016/manuals/2016_MEC_Laboratory_Procedures_Manual.pdf. Accessed 1 August 2020.
- 18. National Center for Health and Vital Statistics. National Health and Nutrition Examination Survey. 2015–2016 data documentation, codebook, and frequencies. Data File: CHLMDA_I.xpt Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2015–2016/CHLMDA_I.htm. Accessed 1 September 2020.
- 19. Van Der Pol B, Taylor SN, Lebar W, et al. . Clinical evaluation of the BD ProbeTec™ Chlamydia trachomatis Qx amplified DNA assay on the BD Viper™ system with XTR™ technology. Sex Transm Dis 2011; 38:603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Biospecimen Program. Available at: https://www.cdc.gov/nchs/nhanes/biospecimens/biospecimens.htm. Accessed 1 August 2020.
- 21. Goodhew EB, Priest JW, Moss DM, et al. . CT694 and pgp3 as serological tools for monitoring trachoma programs. PLoS Negl Trop Dis 2012; 6:e1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dize L, Martin D, Gwyn S, Perin J, Gaydos C, Trent M. Comparison of three serological assays to measure antibody response to Chlamydia antigen Pgp3 in adolescent and young adults with pelvic inflammatory disease. Int J STD AIDS 2018; 29:1324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Center for Health and Vital Statistics. National Health and Nutrition Examination Survey. 2015–2016 data documentation, codebook, and frequencies. Data File: SSCT_I.xpt. Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2015–2016/SSCT_I.htm. Accessed 5 December 2020.
- 24. Lee FK, Coleman RM, Pereira L, Bailey PD, Tatsuno M, Nahmias AJ. Detection of herpes simplex virus type 2-specific antibody with glycoprotein G. J Clin Microbiol 1985; 22:641–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu F, Sternberg MR, Kottiri BJ, et al. . Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006; 296:964–73. [DOI] [PubMed] [Google Scholar]
- 26. National Center for Health and Vital Statistics. National Health and Nutrition Examination Survey. 2015–2016 data documentation, codebook, and frequencies. Data File: HSV_I.xpt. Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2015–2016/HSV_I.htm.
- 27. Korn EL, Graubard BIJSM. Confidence intervals for proportions with small expected number of positive counts estimated from survey data. Surv Methodol 1998; 24:193–201. [Google Scholar]
- 28. Lumley T. survey: analysis of complex survey samples.2020. R package version 4.0.
- 29. van Buuren S, Groothuis-Oudshoorn K. Mice: Multivariate Imputation by Chained Equations in R. J Stat Softw 2011; 45:1–67. Available at: https://www.jstatsoft.org/v45/i03/. [Google Scholar]
- 30. Woodhall SC, Wills GS, Horner PJ, et al. . Chlamydia trachomatis Pgp3 antibody population seroprevalence before and during an era of widespread opportunistic chlamydia screening in England (1994-2012). PLoS One 2017; 12:e0152810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Geisler WM, Chyu L, Kusunoki Y, Upchurch DM, Hook EW 3rd. Health insurance coverage, health care-seeking behaviors, and genital chlamydial infection prevalence in sexually active young adults. Sex Transm Dis 2006; 33:389–96. [DOI] [PubMed] [Google Scholar]
- 32. Kreisel K, Torrone E, Bernstein K, Hong J, Gorwitz R. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age—United States, 2013-2014. MMWR Morb Mortal Wkly Rep 2017; 66:80–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abu-Raddad LJ, Schiffer JT, Ashley R, et al. . HSV-2 serology can be predictive of HIV epidemic potential and hidden sexual risk behavior in the Middle East and North Africa. Epidemics 2010; 2:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dukers NH, Bruisten SM, van den Hoek JA, de Wit JB, van Doornum GJ, Coutinho RA. Strong decline in herpes simplex virus antibodies over time among young homosexual men is associated with changing sexual behavior. Am J Epidemiol 2000; 152:666–73. [DOI] [PubMed] [Google Scholar]
- 35. Cowan FM, Johnson AM, Ashley R, Corey L, Mindel A. Antibody to herpes simplex virus type 2 as serological marker of sexual lifestyle in populations. BMJ 1994; 309:1325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patel EU, Laeyendecker O, Hsieh YH, Rothman RE, Kelen GD, Quinn TC. Parallel declines in HIV and hepatitis C virus prevalence, but not in herpes simplex virus type 2 infection: a 10-year, serial cross-sectional study in an inner-city emergency department. J Clin Virol 2016; 80:93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Horner PJ, Wills GS, Reynolds R, et al. . Effect of time since exposure to Chlamydia trachomatis on chlamydia antibody detection in women: a cross-sectional study. Sex Transm Infect 2013; 89:398–403. [DOI] [PubMed] [Google Scholar]
- 38. Blomquist PB, Mighelsen SJ, Wills G, et al. . Sera selected from national STI surveillance system shows Chlamydia trachomatis PgP3 antibody correlates with time since infection and number of previous infections. PLoS One 2018; 13:e0208652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martin DL, Wiegand R, Goodhew B, et al. . Serological measures of trachoma transmission intensity. Sci Rep 2015; 5:18532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fang J, Husman C, DeSilva L, Chang R, Peralta L. Evaluation of self-collected vaginal swab, first void urine, and endocervical swab specimens for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in adolescent females. J Pediatr Adolesc Gynecol 2008; 21:355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.