Abstract
Background
Noninvasive markers of liver fibrosis such as aspartate aminotransferase-to-platelet ratio (APRI) and transient elastography (TE) have largely replaced liver biopsy for staging hepatitis C virus (HCV). As there is little longitudinal data, we compared changes in these markers before and after sustained virologic response (SVR) in human immunodeficiency virus (HIV)-HCV coinfected patients.
Methods
Participants from the Canadian Coinfection Cohort study who achieved SVR after a first treatment with either interferon/ribavirin or direct acting antivirals (DAAs), with at least 1 pre- and posttreatment fibrosis measure were selected. Changes in APRI or TE (DAA era only) were modeled using a generalized additive mixed model, assuming a gamma distribution and adjusting for sex, age at HCV acquisition, duration of HCV infection, and time-dependent body mass index, binge drinking, and detectable HIV RNA.
Results
Of 1981 patients, 151 achieved SVR with interferon and 553 with DAAs; 94 and 382 met inclusion criteria, respectively. In the DAA era, APRI increased (0.03 units/year; 95% credible interval (CrI): −.05, .12) before, declined dramatically during, and then changed minimally (−0.03 units/year; 95% CrI: −.06, .01) after treatment. TE values, however, increased (0.74 kPa/year; 95% CrI: .36, 1.14) before treatment, changed little by the end of treatment, and then declined (−0.55 kPa/year; 95% CrI: −.80, −.31) after SVR.
Conclusions
TE should be the preferred noninvasive tool for monitoring fibrosis regression following cure. Future studies should assess the risk of liver-related outcomes such as hepatocellular carcinoma according to trajectories of fibrosis regression measured using TE to determine if and when it will become safe to discontinue screening.
Keywords: HIV-HCV coinfection, APRI, transient elastography, sustained virologic response, fibrosis regression
Among human immunodeficiency virus-hepatitis C virus coinfected patients, transient elastography (TE) better reflects changes in fibrosis than aminotransferase-to-platelet ratio A pretreatment TE should be considered for all patients with advanced fibrosis and then periodically after treatment to monitor evolution of fibrosis after cure.
(See the Editorial Commentary by Rockstroh on pages 478–9.)
Hepatitis C virus (HCV) is the leading cause of cirrhosis, hepatocellular carcinoma (HCC), and transplantation, and in many countries including Canada, causes more years of life lost than any other infectious disease [1–4] especially for those also living with human immunodeficiency virus (HIV) [5–7]. Direct acting antiviral (DAA) therapy, with cure rates that exceed 95% in real-world settings [8, 9], provide an unprecedented opportunity to reverse these negative health impacts.
Fibrosis staging is important for risk-stratification and in predicting individual-level adverse outcomes, treatment response, and the need for posttreatment HCC surveillance. Determining the degree of liver fibrosis prior to treatment is now commonly established using noninvasive markers such as the aspartate aminotransferase-to-platelet ratio index (APRI), the Fibrosis-4 (FIB-4) index, or transient elastography (TE). However, use of these measures in monitoring fibrosis regression after HCV cure is the subject of ongoing study [10, 11]. Although several studies have shown that sustained virologic response (SVR) following interferon- or DAA-based therapy is associated with a rapid decline in these noninvasive markers, few studies have followed these markers—TE in particular—far beyond SVR [12–20]. Establishing a good longitudinal marker that does not primarily reflect changes in hepatic inflammation but actual liver fibrosis regression is clinically and epidemiologically important. To inform liver fibrosis monitoring after cure, the primary objective of this study was to compare changes in noninvasive markers before and after SVR, in both the interferon and DAA eras, among HIV-HCV coinfected patients, while accounting for factors that are known to affect fibrosis progression.
METHODS
Study Population and Data Collection
We used data from the Canadian Coinfection Cohort Study [21], an open prospective multicenter study recruiting patients 16 years of age and older with documented HIV infection and with chronic HCV infection or evidence of previous HCV exposure (eg, HCV seropositive by enzyme-linked immunosorbent assay [ELISA] or if serologically false-negative, HCV RNA-positive). From April 2003 to June 2019, 1962 patients were enrolled from 18 sites across 6 Canadian provinces. Patients underwent an initial evaluation followed by study visits approximately every 6 months. At each visit, laboratory analyses were performed. Starting in 2014, a substudy was instituted at sites with access to TE (n = 8 sites) where evaluations were performed every 6 months (before and after treatment). The study was approved by the community advisory committee of the Canadian Institutes of Health Research (CIHR)-Canadian HIV Trials Network and by all institutional ethics boards of participating centers.
All patients with at least 1 measure of a fibrosis marker (APRI in the interferon era, APRI, FIB-4, or TE in the DAA era) before successful treatment and 1 measure of that same marker after SVR were included. In our analysis in the interferon era, we included patients successfully treated with a pegylated-interferon regimen with or without ribavirin. We excluded any patient successfully retreated with such a regimen after an earlier treatment failure or coinfected with hepatitis B virus. In our analyses in the DAA era, we included patients successfully treated with an all oral second-generation DAA regimen (the first of which was simeprevir/sofosbuvir, approved in Canada in November 2013). We also excluded any patient retreated after an earlier unsuccessful DAA treatment in the same era, starting a DAA within 6 months of an unsuccessful treatment with interferon, reinfected after a successful treatment with interferon or coinfected with hepatitis B virus.
Statistical Analysis
We analyzed measurement sequences from each patient with a generalized additive mixed model [22]. This model was fit in R (version 3.5.2) using the mgcv package (version 1.8–28). For this model, we assumed measurements were distributed gamma, with the mean estimated from model parameters through a natural log link function; we included fixed effects for prespecified covariates and 2 smoothing terms. The first smoothing term was a random intercept to accommodate repeated measurements from the same patient; the second smoothing term was a spline function representing the change in the mean measurement over time, both before, during, and after treatment. The date treatment began was designated time zero, and measurement sequences were left censored until at least 6 months after any earlier unsuccessful treatment and right censored after any reinfection.
The prespecified covariates were selected based on their reported association with liver fibrosis and included: age at HCV acquisition (per 10 years), duration of infection when starting treatment (per 10 years), sex and time dependent binge drinking, detectable HIV viral load and body mass index (BMI) (per 5 kg/m2). Age at HCV acquisition and BMI were represented in the model by linear splines [23], with a single knot (at age 40 and at a BMI of 25, respectively). Binge drinking was self-reported and defined as at least 6 or more drinks on at least 1 occasion each month in the last 6 months.
We then used the fitted model to calculate the rate of change during one year periods of relatively constant slope before and after treatment. We estimated a slope between 1.5 and 0.5 years before treatment started; in the DAA era, we estimated a slope between 0.5 and 1.5 years after treatment ended, but in the interferon era—because of the slower response to interferon treatment—we estimated a slope between 1.0 and 2.0 years after treatment ended. By sampling model parameters from their posterior distribution [24], we repeatedly estimated measurement slopes before and after treatment for the following reference patient: a man infected with HCV at age 25 and initiating treatment 25 years after infection (to reflect the median age when starting treatment; Table 1), with a BMI of 25 (median BMI; Table 1), an undetectable HIV viral load and not reporting binge drinking. We then calculated an approximate 95% credible interval (95% CrI) for the slope from the 2.5 and 97.5 percentiles of a distribution of 10 000 slope estimates.
Table 1.
Patient Characteristics When Starting Treatment for Those Successfully Treated in the Interferon (IFN) and Direct Acting Antiviral (DAA) Eras
| Era: | IFN | DAA | DAA | DAA |
|---|---|---|---|---|
| Marker: | APRI | APRI | FIB-4 | TE |
| Median or Percent | n = 94 | n = 382 | n = 381 | n = 149 |
| Demographic characteristics | ||||
| Age, years | 46 | 52 | 52 | 51 |
| Female, % | 15 | 31 | 31 | 30 |
| Body mass index, kg/m2 | 25 | 25 | 25 | 24 |
| Risk factors | ||||
| Men who have sex with men, % | 36 | 30 | 30 | 28 |
| History of injection drug use, % | 72 | 77 | 77 | 78 |
| Current injection drug use, % | 16 | 23 | 23 | 26 |
| History of binge drinking, % | 24 | 32 | 32 | 24 |
| Current binge drinking, % | 15 | 14 | 13 | 13 |
| Disease characteristics | ||||
| Time since HIV diagnosis, years | 12 | 18 | 18 | 17 |
| Duration of HCV infection, years | 16 | 24 | 24 | 22 |
| Prior AIDS diagnosis, % | 28 | 27 | 27 | 24 |
| Nadir CD4 + cell count, cells/µl | 200 | 170 | 170 | 190 |
| CD4 + cell count, cells/µl | 420 | 460 | 450 | 530 |
| HIV RNA < 50 copies/ml, % | 70 | 74 | 74 | 77 |
| Prior diagnosis of end-stage liver disease, % | 17 | 18 | 17 | 17 |
| HCV genotype 1 or 4, % | 55 | 77 | 77 | 77 |
| Medications | ||||
| On antiretroviral therapy, % | 85 | 98 | 98 | 97 |
| HCV treatment naive, % | 90 | 68 | 68 | 67 |
| Duration of HCV treatment, weeks | 45 | 12 | 12 | 12 |
| Markers of fibrosis | ||||
| APRI | 0.92 | 0.72 | ||
| APRI > 1.5, % | 32 | 25 | ||
| FIB-4 | 1.9 | |||
| FIB-4 > 3.25, % | 23 | |||
| TE, kPa | 7.3 | |||
| TE > 7.2 kPa, % | 50 | |||
| Measurement frequency | ||||
| Measures before treatment, number | 2 | 5 | 5 | 3 |
| Measures after treatment, number | 7 | 3 | 3 | 3 |
| Most recent measure before treatment, weeks | 17 | 12 | 12 | 13 |
| Most recent measure after treatment, weeks | 16 | 16 | 16 | 20 |
| Follow-up before treatment, weeks | 55 | 153 | 172 | 78 |
| Follow-up after treatment, weeks | 252 | 86 | 84 | 96 |
| Time between measures, weeks | 28 | 27 | 27 | 28 |
Abbreviations: APRI, aspartate aminotransferase to platelet ratio index; FIB-4, fibrosis-4 index; HCV, hepatitis C virus; HIV, human immunodeficiency virus; TE, transient elastography.
Sensitivity Analysis
In planned sensitivity analyses, we added additional covariates to our generalized linear mixed model and made a number of covariate substitutions. We considered the effect of these changes only on the analysis of APRI in the DAA era, reasoning that any material differences ought to be apparent in this analysis as it contained the most information among our main analyses. We assessed whether these changes had a material effect by comparing slope estimates. We also refit our model for APRI in the DAA era to 2 patient subsets: those with (APRI ≥ 1.5) and without (APRI < 1.5) fibrosis when starting treatment.
In an unplanned sensitivity analysis, we refit our model for TE in the DAA era to these same 2 patient subsets. We did not a priori expect to have sufficient TE data to successfully model the response in patients with fibrosis, but our modeling of all TE data suggested a simple response over time that would require less data to estimate than anticipated. In a second unplanned sensitivity analysis, we added additional data to the analysis of APRI in the DAA era. These data were collected during treatment monitoring, rather than during the 6-monthly follow-up visits. In a third unplanned sensitivity analysis, we refit our models for TE in the DAA era to FIB-4 in the DAA era overall, and to 2 patient subsets: those with (FIB-4 ≥ 3.25) and without (FIB-4 < 3.25) fibrosis when starting treatment, in response to a reviewer’s request.
RESULTS
Patient Characteristics
A total of 1981 HIV-HCV coinfected patients were enrolled by the end of the study period (Supplementary material, Figure S1). A total of 151 and 553 coinfected patients achieved SVR with interferon and second-generation DAAs, respectively. Of these, 94 and 382 patients had pre- and post-SVR APRI data for the interferon and DAA eras, respectively; 149 patients had pre- and post-SVR TE data in the DAA era and were included in the study. Patient sociodemographic and clinical characteristics are presented in Table 1. In the DAA era, patients contributing APRI data were more likely to be on combination antiretroviral therapy (cART) and have an HIV RNA below 50 copies/mL and less likely to be HCV treatment naive and have advanced fibrosis (APRI > 1.5) compared to patients contributing APRI data in the interferon era. Among the 149 participants with available TE data in the DAA era, 50% had a fibrosis stage of ≥ F2 when starting HCV treatment.
Fibrosis Regression Before, During, and After Treatment
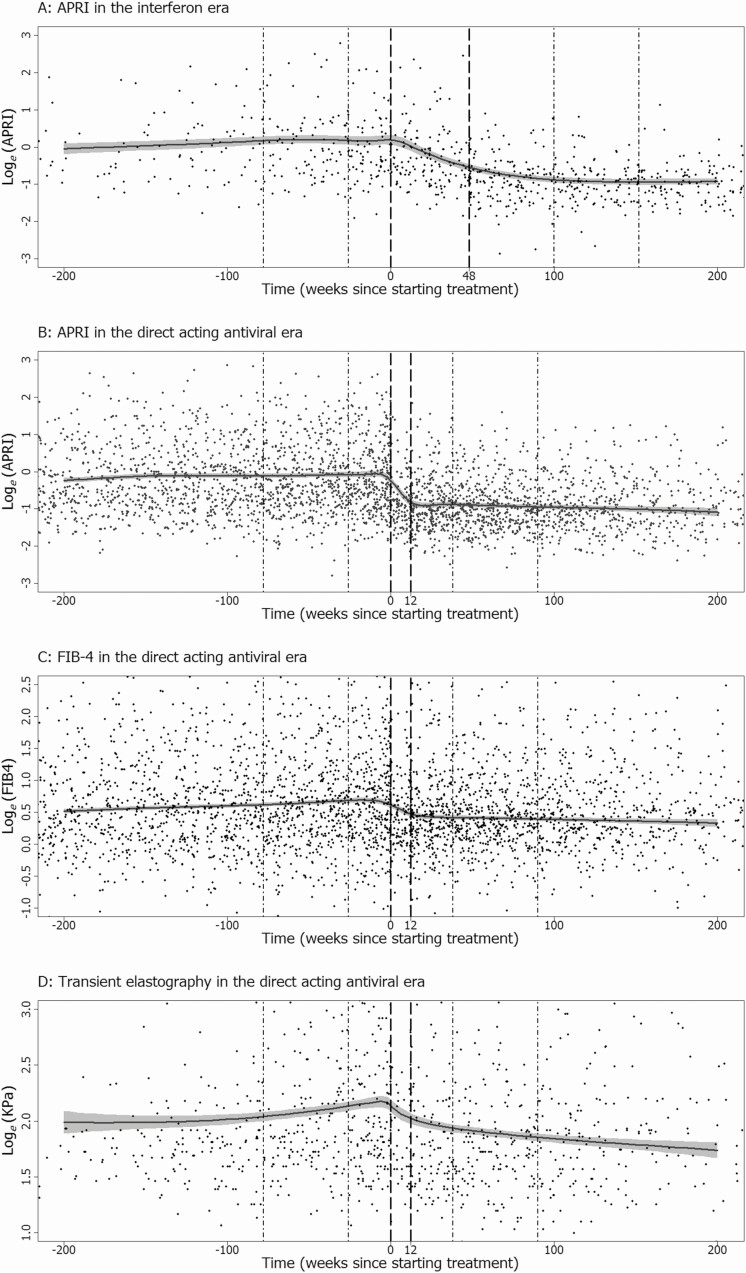
In the interferon era, the response curve suggests a gradual rise in APRI before treatment, a period of decline during treatment that continues after treatment completion (duration of interferon therapy: median 45 weeks; interquartile range [IQR], 25–49 weeks), but little evidence of decline in the longer term (Figure 1A). In contrast, in the DAA era, the decline in APRI during treatment appears more rapid and confined to the treatment period (duration of DAA therapy: median 12 weeks; IQR 12–13 weeks), with little decline after treatment completion (Figure 1B). This same pattern is seen in FIB-4 in the DAA era (Figure 1C). Conversely, the response curve suggests that changes in TE are more gradual: a period of increase prior to treatment, minimal decline during the treatment period, followed by further decline after treatment completion (Figure 1D). When fitting generalized additive models, subjective decisions need to be made about how to construct a smooth response curve from the underlying data. In Supplementary Material, we describe the process we followed and show alternative response curves for all data (Supplementary material, Figures S2 to S5) and report estimated associations between covariates and markers (Supplementary material, Table S1).
Figure 1.
Response curves from generalized additive models for APRI in the interferon era (A); and for APRI (B), FIB-4 (C), and transient elastography (D) in the direct acting antiviral era. Reference lines show the usual treatment period (– – –) and the 1 year periods during which slopes were estimated both before and after treatment (• – •). Abbreviations: APRI, aspartate aminotransferase to platelet ratio index; FIB-4, fibrosis-4 index.
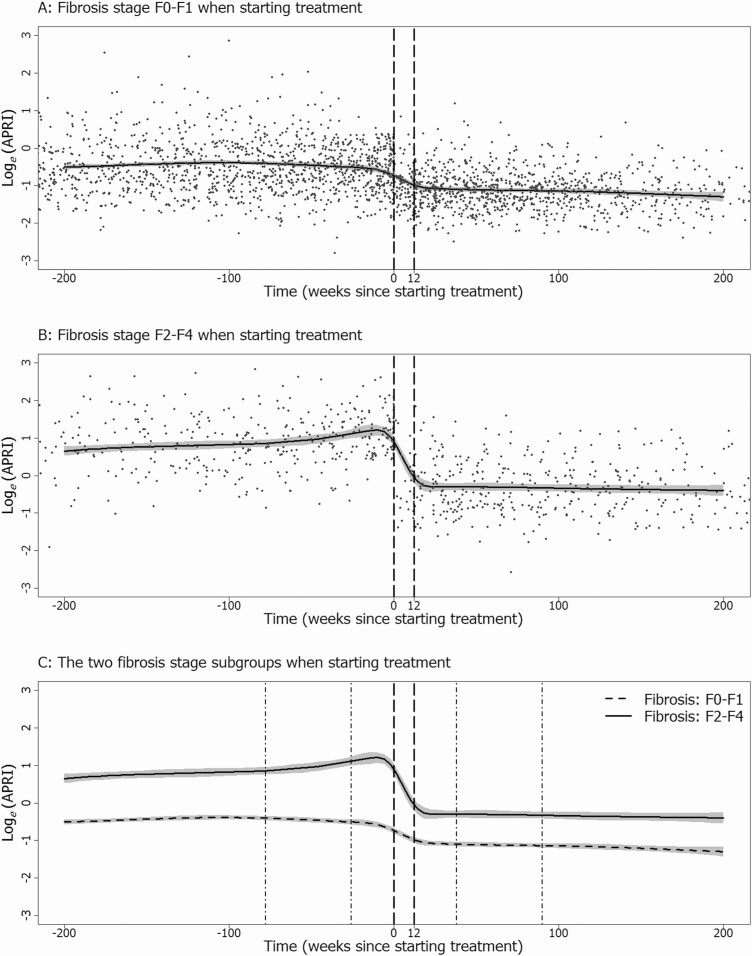
In a sensitivity analysis, we fitted identical models for APRI measurements in the DAA era to 2 patient subgroups (Figure 2). There was little change in APRI over time among patients without fibrosis when starting treatment (Figure 2A), whereas there was a rapid decline in APRI during treatment among patients with fibrosis when starting treatment (Figure 2B). APRI changed little after treatment in either subgroup. Subgroup response curves were similar for both APRI and FIB-4 (Supplementary material, Figures S6 and S7).
Figure 2.
Response curves from generalized additive models for aspartate APRI in the direct acting antiviral era. Curves are shown for patients without fibrosis (APRI < 1.5) when starting treatment (A), for patients with fibrosis (APRI ≥ 1.5) when starting treatment (B), and for both subgroups together (C). Reference lines show the usual treatment period (– – –) and 1 year periods during which slopes were estimated both before and after treatment (• – •). Abbreviation: APRI, aminotransferase to platelet ratio index.
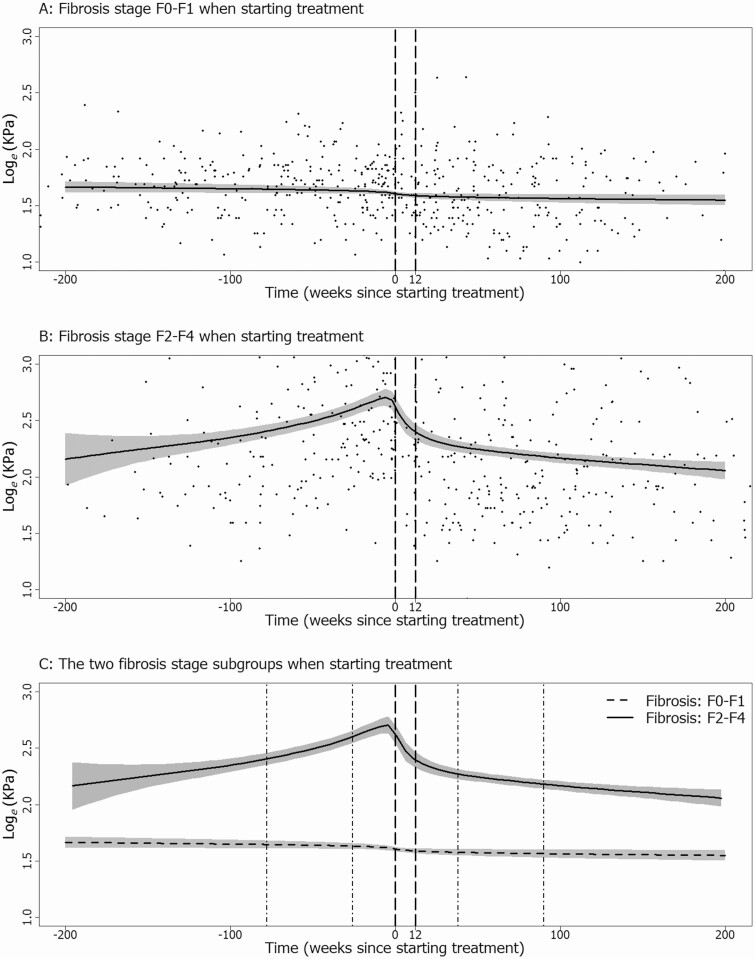
We fitted identical models for TE measurements in the DAA era to 2 patient subgroups (Figure 3; Supplementary material, Figure S8). There was little change in TE over time among patients without fibrosis (Figure 3A). In patients with fibrosis, TE measurements increased before treatment, declined after treatment initiation, and continued to decline after treatment completion (Figure 3B).
Figure 3.
Response curves from generalized additive models for TE in the direct acting antiviral era. Curves are shown for patients without fibrosis (TE < 7.2) when starting treatment (A), for patients with fibrosis (TE ≥ 7.2) when starting treatment (B), and for both subgroups together (C). Reference lines show the usual treatment period (– – –) and 1 year periods during which slopes were estimated both before and after treatment (• – •). Abbreviation: TE, transient elastography.
Adding additional data collected during treatment monitoring to the analysis of APRI in the DAA era had no real influence on the response curve outside that period (Supplementary material, Figure S9). The response curve with these additional data was essentially equivalent to the curve found from 6 monthly follow-up data alone (Figure 1B).
Estimates of Change Before and After Treatment
Given these response curves, we estimated changes in each marker over 1 year periods of relatively constant slope. These estimates show little change in APRI or FIB-4 both before and after treatment (Table 2). The exception was an increase in both APRI and FIB-4 before treatment among patients with fibrosis when starting treatment. In contrast, overall there was evidence of an increase in TE prior to treatment initiation and a decrease following treatment completion. Changes in TE were essentially limited to those with fibrosis when starting treatment; in those without fibrosis, there was a slight but constant decrease over time.
Table 2.
Slope Estimates for Fibrosis Markers: Change Before and After Treatment for Patients Successfully Treated in the Interferon and Direct Acting Antiviral Eras
| Marker | Era | Slope Before (95% CrI)a | Slope After (95% CrI)b |
|---|---|---|---|
| APRI | Interferon | .01 (−.16, .18) | −.02 (−.06, .01) |
| Direct acting antiviral | .03 (−.05, .12) | −.03 (−.06, .01) | |
| With monitoring data | .02 (−.08, .12) | −.03 (−.07, .01) | |
| F0–F1 when starting | −.04 (−.10, .01) | −.02 (−.04, .01) | |
| F2–F4 when starting | 1.84 (.48, 3.53) | .01 (−.32, .34) | |
| FIB-4 | Direct acting antiviral | .13 (.04, .22) | −.03 (−.10, .03) |
| F0–F1 when starting | .01 (−.05, .06) | −.02 (−.06, .02) | |
| F2–F4 when starting | .91 (.46, 1.42) | −.01 (−.22, .20) | |
| TE | Direct acting antiviral | .74 (.36, 1.14) | −.55 (−.80, −.31) |
| F0–F1 when starting | −.07 (−.14, −.02) | −.06 (−.10, −.01) | |
| F2–F4 when starting | 4.2 (2.4, 6.4) | −1.5 (−2.6, −.5) |
Abbreviations: APRI, aspartate aminotransferase to platelet ratio index; CrI; credible interval; FIB-4, fibrosis-4 index; TE. transient elastography.
aSlope between 1.5 and 0.5 years before starting treatment.
bSlope between 1 and 2 years after treatment ends in the interferon era, and between 0.5 and 1.5 years after treatment ends in the direct acting antiviral era.
Other Sensitivity Analyses
In separate analyses, we added additional covariates for HCV genotype 3, a CD4 cell count of < 200 cells/mm3 when starting treatment, type 2 diabetes, and a protease inhibitor as part of the antiretroviral regimen. We also replaced binge drinking with hazardous drinking as defined by the AUDIT-C score [25], and age at HCV acquisition and duration of infection when starting treatment with age when starting treatment. None of these changes made an appreciable difference to slope estimates (Supplementary Material, Table S2).
DISCUSSION
Our study prospectively examined changes in APRI and TE before and after SVR, and in both the interferon and DAA eras, in a large cohort of HIV-HCV coinfected patients to determine the most promising noninvasive tool for monitoring fibrosis regression over time. In both eras, we observed minimal change in APRI prior to treatment, a rapid and steep decline in APRI confined primarily to the treatment periods, and essentially no change in APRI following SVR. These findings suggest that changes observed in APRI over time are related to changes in hepatic inflammation rather than fibrosis. The slower decline in APRI with interferon therapy compared to DAAs appears to parallel differences in the rate of HCV RNA suppression on these regimens, further supporting that the changes in APRI observed are related to a reduction in inflammation associated with HCV replication [12]. Although we had limited data, particularly in the interferon era, among those with available HCV RNA data during treatment, 54% versus 84% suppressed HCV RNA below the limit of quantification by week 4, rising to 90% and 99% at week 12, in the interferon and DAA eras, respectively. APRI does not also appear to be useful for monitoring fibrosis regression among those with significant fibrosis at treatment initiation. Although APRI increased prior to treatment in this group, no decline was seen in the posttreatment period. Not surprisingly, FIB-4 behaved similarly to APRI, both overall and in those with advanced fibrosis when starting treatment. Studies have suggested that posttreatment APRI and FIB-4 correlate well with paired liver biopsy scores [26]. It is possible that concurrent inflammatory processes not affected by HCV treatment, such as steatohepatitis and/or alcoholic liver disease continue to drive fibrosis among a subset of patients after HCV eradication—in such patients, APRI or FIB-4 may remain associated with the degree of fibrosis.
In contrast, the picture with TE is quite different—TE measures increased prior to DAA treatment, declined minimally during treatment and, more importantly, continued to decline following cure. These findings imply that the changes in TE following SVR likely reflect true reversal of fibrosis. Changes in TE were essentially restricted to those having advanced fibrosis prior to treatment; an average regression of −1.5 kPA (95% CrI: −2.6, −.5) between 6 months and 1.5 years after treatment completion was observed in F2-F4 reference patients. This suggests that SVR may lead to meaningful fibrosis regression over a relatively short period in the absence of other risk factors for fibrosis. Taken together, TE is more useful than APRI in monitoring fibrosis regression after cure. As with APRI, it has been suggested that TE can be influenced by necroinflammatory hepatic activity [16, 27] and changes in TE may overestimate regression post-SVR [28]. Indeed, we did observe some decline in TE over the treatment period. Thus, estimation of fibrosis regression should only begin after this inflammatory period has resolved—this postinflammatory TE measure is likely a truer estimate of the level of fibrosis than the pretreatment TE measure. Changes thereafter reflect regression of fibrosis in the absence of HCV-related inflammation.
As the use of noninvasive markers for liver fibrosis assessment has become widespread, understanding their clinical utility throughout the treatment trajectory, and particularly post-SVR, is important. Other groups have studied changes in noninvasive markers but have only compared these measures at a single time point before, and 1 or 2 time points at or shortly after SVR [13, 29]. This approach will misattribute changes that occur during the treatment period to fibrosis resolution following cure and potentially overestimate the degree of regression. Furthermore, very few studies have examined fibrosis regression among HIV-HCV coinfected persons [29–33], and the median follow-up using TE has been short, ranging from 12 to 52 weeks [29, 32]. Thus, a major strength of our study was the availability of multiple routinely performed measures over a long follow-up period, allowing us to longitudinally follow the course of noninvasive markers to 200 weeks after treatment.
Currently, the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases recommend lifelong monitoring for HCC in patients with advanced fibrosis or cirrhosis who achieve SVR [34, 35]. Some studies have shown that a post-SVR APRI or FIB-4 can be used to predict the risk of HCC, including among coinfected patients [36–39]. It would be expected that a single posttreatment measure might retain some predictive value for fibrosis and clinical outcomes as these measures are directly related to the pretreatment value. However, their predictive value would likely diminish further out from SVR given they essentially do not change over time. What is of greater interest, however, is whether HCC risk differs by the degree of posttreatment fibrosis resolution given fibrosis regression is variable after SVR, and progression of fibrosis has been observed in some cases [26]. Our methods provide a means of estimating fibrosis regression longitudinally, and our results suggest that TE should be the preferred noninvasive marker when investigating how various fibrosis trajectories predict HCC and other clinical outcomes. Our findings also underscore that TE should be performed routinely following SVR among individuals with advanced fibrosis/cirrhosis before treatment (ie, among those who require indefinite screening for HCC post-SVR). Although APRI and FIB-4 are now frequently used instead of TE to stage liver disease before treatment to facilitate HCV treatment uptake both in high- and low-income settings [35], our results present a strong case for not abandoning TE and for expanding its access in resource-limited countries.
The Canadian Coinfection Cohort comprises a diverse patient population followed at various primary and tertiary care clinics in urban and semi-urban areas and is thus representative of the HIV-HCV coinfected Canadian population in care [21]. We used statistical methods that allowed us to model irregularly repeated measurements that are not normally distributed, before, during, and after treatment, without artificially grouping (eg, binning) data, which potentially masks changes over time. Our study has limitations. First, our results may not be generalizable to HCV-monoinfected individuals, although a recent study suggested similar degrees of regression in TE 12 weeks posttreatment among HCV-monoinfected and coinfected patients on cART [32]. Second, when fitting generalized additive models, subjective decisions need to be made regarding how a smooth response curve is constructed from the underlying data. Although we explain our rationale in Supplementary material, our choices may hide or exaggerate elements of a response curve. Third, the credible intervals of our slope estimates will be too narrow as they do not allow for uncertainly in the smoothing parameter that controls the tradeoff between fit to the data and smoothness. However, simulation suggests the underestimate will be trivial for the relatively simple models in Figure 2 [40].
In conclusion, although APRI and FIB-4 may be useful to stage patients for fibrosis before treatment, TE should be the preferred noninvasive tool for monitoring fibrosis regression following cure. Such monitoring should begin during the postinflammatory period– that is, after SVR, and continue periodically thereafter. As meaningful regression of fibrosis occurs in those with advanced liver disease, future studies should assess the risk of HCC and other liver-related outcomes according to trajectories of fibrosis regression measured using TE to determine if and when it will become safe to discontinue HCC screening.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The Canadian Coinfection Cohort investigators (CTN222) are: Drs Lisa Barrett, QEII Health Science Center for Clinical Research, Halifax, NS; Jeff Cohen, Windsor Regional Hospital Metropolitan Campus, Windsor, ON; Brian Conway, Vancouver Infectious Diseases Research and Care Centre, Vancouver, BC; Curtis Cooper, Ottawa Hospital Research Institute, Ottawa, ON; Pierre Côté, Clinique du Quartier Latin, Montréal, QC; Joseph Cox, MUHC IDTC-Montréal General Hospital, Montréal, QC; John Gill, Southern Alberta HIV Clinic, Calgary, AB; Shariq Haider, McMaster University, Hamilton, ON; Mark Hull, BC Centre for Excellence in HIV/AIDS, Vancouver, BC; Marina Klein, McGill University Health Centre, Division of Infectious Diseases and Chronic Viral Illness Service, Montreal, QC; Julio Montaner, St. Paul’s Hospital, Vancouver, BC; Erica Moodie, McGill University, Montreal, QC; Neora Pick, Oak Tree Clinic, Children’s and Women’s Health Centre of British Columbia, University of British Columbia, Vancouver, BC; Anita Rachlis, Sunnybrook & Women’s College Health Sciences Centre, Toronto, ON; Danielle Rouleau, Centre Hospitalier de l’Université de Montréal, Montréal, QC; Roger Sandre, HAVEN Program, Sudbury, ON; Mark Tyndall, Department of Medicine, Infectious Diseases Division, University of Ottawa, Ottawa, ON; Steve Sanche, SHARE University of Saskatchewan, Saskatoon, SK; Marie-Louise Vachon, Centre Hospitalier Universitaire de Québec, Québec, QC; Sharon Walmsley, University Health Network, Toronto, ON; Alex Wong, Regina Qu’Appelle Health Region, Regina General Hospital, Regina, SK.; and David Wong, University Health Network, Toronto, ON. The authors thank all study coordinators and nurses for their assistance with study coordination, participant recruitment, and care.
Financial support. This work was supported by Fonds de recherche du Québec—Santé (FRQ-S); Réseau SIDA/maladies infectieuses, the Canadian Institute for Health Research (CIHR) (FDN-143270) and the CIHR Canadian HIV Trials Network (CTN222). N. K. and V. M. L. are supported by career awards from the Fonds de Recherche Québec—Santé (FRQ-S; Junior 1). M. B. K. is supported by a Tier I Canada Research Chair.
Potential conflicts of interest. The authors: No reported conflicts of interest. N. K. received grants and consulting fees from ViiV Healthcare and Gilead, as well as consulting fees from Merck. J. Y. received consulting fees from ViiV Healthcare. S. W. received grants, consulting fees, lecture fees, nonfinancial support, and fees for the development of educational presentations from Merck, ViiV Healthcare, GlaxoSmithKline, Pfizer, Gilead, Abbvie, Bristol-Myers Squibb, and Janssen. M. H. has served as a consultant for Merck, Vertex Pharmaceuticals, Pfizer, Viiv Healthcare, and Ortho-Jansen. M. H. has also received grants from the National Institute on Drug Abuse, as well as payment for lectures from Merck and Ortho-Janssen. J. C. received grants and consulting fees from ViiV Healthcare, Merck, and Gilead and personal fees from Bristol-Myers Squibb. C. C. has received personal fees for being a member of the national advisory boards of Gilead, Merck, Janssen, and Bristol-Myers Squibb. M. B. K. reports grants from ViiV Healthcare, Abbvie, Merck, and Gilead; and honoraria for lectures from Janssen, ViiV Healthcare, and Merck. A. W. reports grants from Merck, ViiV Healthcare, Janssen, Gilead, and Schering-Plough, consulting fees from ViiV Healthcare and AbbVie, and lecture fees from ViiV Healthcare and Gilead. V. M.-L. reports grants and personal fees from Merck, Gilead, and AbbVie. A. W. reports consulting fees and honoraria from Merck, Gilead, ViiV Heathcare, and AbbVie. N. P. reports honoraria from Gilead and ViiV Healthcare. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Canadian Coinfection Cohort Study Investigators:
Lisa Barrett, Jeff Cohen, Brian Conway, Curtis Cooper, Pierre Côté, Joseph Cox, John Gill, Shariq Haider, Mark Hull, Marina Klein, Julio Montaner, Erica Moodie, Neora Pick, Anita Rachlis, Danielle Rouleau, Roger Sandre, Mark Tyndall, Steve Sanche, Marie-Louise Vachon, Sharon Walmsley, Alex Wong, and David Wong
References
- 1. Myers RP, Liu M, Shaheen AA. The burden of hepatitis C virus infection is growing: a Canadian population-based study of hospitalizations from 1994 to 2004. Can J Gastroenterol 2008; 22:381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Myers RP, Krajden M, Bilodeau M, et al. Burden of disease and cost of chronic hepatitis C infection in Canada. Can J Gastroenterol Hepatol 2014; 28:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schanzer DL, Paquette D, Lix LM. Historical trends and projected hospital admissions for chronic hepatitis C infection in Canada: a birth cohort analysis. CMAJ Open 2014; 2:E139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwong JC, Ratnasingham S, Campitelli MA, et al. The impact of infection on population health: results of the Ontario Burden of Infectious Diseases Study. PLoS One 2012; 7:e44103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kovari H, Ledergerber B, Cavassini M, et al. ; Swiss HIV Cohort Study . High hepatic and extrahepatic mortality and low treatment uptake in HCV-coinfected persons in the Swiss HIV cohort study between 2001 and 2013. J Hepatol 2015; 63:573–80. [DOI] [PubMed] [Google Scholar]
- 6. Klein MB, Rollet-Kurhajec KC, Moodie EE, et al. ; Canadian Co-infection Cohort Investigators . Mortality in HIV-hepatitis C co-infected patients in Canada compared to the general Canadian population (2003–2013). AIDS 2014; 28: 1957–65. [DOI] [PubMed] [Google Scholar]
- 7. Berenguer J, Alejos B, Hernando V, et al. ; CoRIS (AIDS Research Network Cohort) . Trends in mortality according to hepatitis C virus serostatus in the era of combination antiretroviral therapy. AIDS 2012; 26:2241–6. [DOI] [PubMed] [Google Scholar]
- 8. Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology 2016; 64:405–14. [DOI] [PubMed] [Google Scholar]
- 9. Alimohammadi A, Holeksa J, Thiam A, Truong D, Conway B. Real-world efficacy of direct-acting antiviral therapy for HCV infection affecting people who inject drugs delivered in a multidisciplinary setting. Open Forum Infect Dis 2018; 5:ofy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trivedi HD, Lin SC, T Y Lau D. Noninvasive assessment of fibrosis regression in hepatitis C virus sustained virologic responders. Gastroenterol Hepatol (N Y) 2017; 13:587–95. [PMC free article] [PubMed] [Google Scholar]
- 11. Pinzani M. Liver fibrosis in the post-HCV era. Semin Liver Dis 2015; 35:157–65. [DOI] [PubMed] [Google Scholar]
- 12. Singh S, Facciorusso A, Loomba R, Falck-Ytter YT. Magnitude and kinetics of decrease in liver stiffness after antiviral therapy in patients with chronic hepatitis C: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 16:27–38.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lledó GM, Carrasco I, Benítez-Gutiérrez LM, et al. Regression of liver fibrosis after curing chronic hepatitis C with oral antivirals in patients with and without HIV coinfection. AIDS 2018; 32:2347–52. [DOI] [PubMed] [Google Scholar]
- 14. Bachofner JA, Valli PV, Kröger A, et al. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int 2017; 37:369–76. [DOI] [PubMed] [Google Scholar]
- 15. Tada T, Kumada T, Toyoda H, et al. Improvement of liver stiffness in patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. J Gastroenterol Hepatol 2017; 32:1982–8. [DOI] [PubMed] [Google Scholar]
- 16. Chan J, Gogela N, Zheng H, et al. Direct-acting antiviral therapy for chronic HCV infection results in liver stiffness regression over 12 months post-treatment. Dig Dis Sci 2018; 63:486–92. [DOI] [PubMed] [Google Scholar]
- 17. Rout G, Nayak B, Patel AH, et al. Therapy with oral directly acting agents in hepatitis C infection is associated with reduction in fibrosis and increase in hepatic steatosis on transient elastography. J Clin Exp Hepatol 2019; 9:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hsu WF, Lai HC, Su WP, et al. Rapid decline of noninvasive fibrosis index values in patients with hepatitis C receiving treatment with direct-acting antiviral agents. BMC Gastroenterol 2019; 19:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Facciorusso A, Del Prete V, Turco A, Buccino RV, Nacchiero MC, Muscatiello N. Long-term liver stiffness assessment in hepatitis C virus patients undergoing antiviral therapy: results from a 5-year cohort study. J Gastroenterol Hepatol 2018; 33:942–9. [DOI] [PubMed] [Google Scholar]
- 20. Rial-Crestelo D, Sepúlveda MA, González-Gasca FJ, et al. Does fibrosis really regress in HIV/HCV co-infected patients after treatment with direct-antiviral agents? AIDS 2020; 34:427–32. . Volume published ahead of print. [DOI] [PubMed] [Google Scholar]
- 21. Klein MB, Saeed S, Yang H, et al. Cohort profile: the Canadian HIV-hepatitis C co-infection cohort study. Int J Epidemiol 2010; 39:1162–9. [DOI] [PubMed] [Google Scholar]
- 22. Wood SM. Mixed methods and GAMMs (Chapter 6). Generalized additive models: an introduction with R. Boca Raton, FL: Chapman & Hall, 2006:277–325. [Google Scholar]
- 23. Greenland A. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology 1995; 6:356–65. [DOI] [PubMed] [Google Scholar]
- 24. Wood SM. Bayesian confidence intervals for non-linear functions of parameters (Chapter 4.8.4). Generalized additive models: an introduction with R. Boca Raton, FL: Chapman & Hall, 2006:194. [Google Scholar]
- 25. Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG.. AUDIT: the alcohol use disorders identification test. Guidelines for use in primary care. 2nd ed. Geneva: World Health Organization, 2001. [Google Scholar]
- 26. Tachi Y, Hirai T, Toyoda H, et al. Predictive ability of laboratory indices for liver fibrosis in patients with chronic hepatitis C after the eradication of hepatitis C virus. PLoS One 2015; 10:e0133515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tapper EB, Cohen EB, Patel K, et al. Levels of alanine aminotransferase confound use of transient elastography to diagnose fibrosis in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol 2012; 10:932–937.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pan JJ, Bao F, Du E, et al. Morphometry confirms fibrosis regression from sustained virologic response to direct-acting antivirals for hepatitis C. Hepatol Commun 2018; 2:1320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fabbri G, Mastrorosa I, Vergori A, et al. Liver stiffness reduction and serum fibrosis score improvement in HIV/hepatitis C virus-coinfected patients treated with direct-acting antivirals. HIV Med 2018; 19:578–84. [DOI] [PubMed] [Google Scholar]
- 30. Casado JL, Quereda C, Moreno A, Pérez-Elías MJ, Martí-Belda P, Moreno S. Regression of liver fibrosis is progressive after sustained virological response to HCV therapy in patients with hepatitis C and HIV coinfection. J Viral Hepat 2013; 20:829–37. [DOI] [PubMed] [Google Scholar]
- 31. Labarga P, Fernandez-Montero JV, Barreiro P, et al. Changes in liver fibrosis in HIV/HCV-coinfected patients following different outcomes with peginterferon plus ribavirin therapy. J Viral Hepat 2014; 21:475–9. [DOI] [PubMed] [Google Scholar]
- 32. Malin JJ, Boesecke C, Schwarze-Zander C, et al. Liver stiffness regression after successful hepatitis C treatment is independent of HIV coinfection. HIV Med 2019; 20:230–6. [DOI] [PubMed] [Google Scholar]
- 33. ANRS CO13 HEPAVIH Cohort. Regression of liver stiffness after sustained hepatitis C virus (HCV) virological responses among HIV/HCV-coinfected patients. AIDS 2015; 29:1821–30. [DOI] [PubMed] [Google Scholar]
- 34. AASLD/IDSA. HCV guidance: recommendations for testing, managing, and treating hepatitis C: monitoring patients who are starting HCV treatment, are on treatment, or have completed therapy. 2014. [updated 24 May 2018; cited 2019 Aug 30]. Available at: https://www.hcvguidelines.org/sites/default/files/full-guidance-pdf/HCVGuidance_May_24_2018b.pdf. Accessed 25 October 2020.
- 35. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018; 69:461–511. [DOI] [PubMed] [Google Scholar]
- 36. Toyoda H, Tada T, Yasuda S, Mizuno K, Ito T, Kumada T. Dynamic evaluation of liver fibrosis to assess the risk of hepatocellular carcinoma in patients with chronic hepatitis C who achieved sustained virologic response. Clin Infect Dis 2020; 70:1208–14. [DOI] [PubMed] [Google Scholar]
- 37. Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology 2020; 71:44–55. [DOI] [PubMed] [Google Scholar]
- 38. Corma-Gomez A, Macias J, Téllez F, et al. Liver stiffness at the time of sustained virologicalresponse predicts the clinical outcome in people living with human immunodeficiency virus and hepatitis C virus with advanced fibrosis treated with direct-acting antivirals. CID 2020; 71:2354–62. [DOI] [PubMed] [Google Scholar]
- 39. Ioannou GN, Beste LA, Green PK, et al. Increased risk for hepatocellular carcinoma persists up to 10 years after HCV eradication in patients with baseline cirrhosis or high FIB-4 scores. Gastroenterology 2019; 157: 1264–1278.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wood SM. Confidence interval performance (Chapter 4.9). Generalized additive models: an introduction with R. Boca Raton, FL: Chapman & Hall, 2006: 196–204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.