Abstract
Background
Limited evidence suggests that the nonhormonal contraceptive copper intrauterine device (Cu-IUD) may increase bacterial vaginosis (BV) risk, possibly due to increased volume and duration of menses, a common side effect of Cu-IUD use. Although increases in bleeding typically resolve within 6–12 months following initiation, evaluations of the association between Cu-IUD and BV have not included more than 6 months of follow-up.
Methods
This secondary analysis of a human immunodeficiency virus type 1 prevention trial included 2585 African women ages 18–45 followed for up to 33 months. Women reported contraceptive use each month. BV was evaluated by Nugent score in 6-monthly intervals and, if clinically indicated, by Amsel criteria. Andersen-Gill proportional hazards models were used to (1) evaluate BV risk among Cu-IUD users relative to women using no/another nonhormonal contraceptive and (2) test changes in BV frequency before, while using, and following Cu-IUD discontinuation.
Results
BV frequency was highest among Cu-IUD users at 153.6 episodes per 100 person-years (95% confidence interval [CI]: 145.2, 162.4). In adjusted models, Cu-IUD users experienced 1.28-fold (95% CI: 1.12, 1.46) higher BV risk relative to women using no/another nonhormonal contraception. Compared to the 6 months prior to initiation, BV risk was 1.52-fold (95% CI: 1.16, 2.00) higher in the first 6 months of Cu-IUD use and remained elevated over 18 months of use (P < .05). Among women who discontinued Cu-IUD, BV frequency was similar to pre-initiation rates within 1 year.
Conclusions
Cu-IUD users experienced elevated BV risk that persisted throughout use. Women and their providers may wish to consider BV risk when discussing contraceptive options.
Keywords: bacterial vaginosis, copper intrauterine device, long-acting reversible contraception
In this prospective cohort analysis, women using copper intrauterine device (Cu-IUD) contraception experienced 1.28-fold (P < .001) elevated risk of bacterial vaginosis (BV). This elevated risk persisted through 18 months of use (P < .05) and declined to pre-initiation risk levels following Cu-IUD discontinuation.
Bacterial vaginosis (BV) is the most common vaginal infection among women of reproductive age [1] and is associated with multiple adverse outcomes, including pelvic inflammatory disease [2], preterm birth [3], and increased risk of acquisition and transmission of human immunodeficiency virus type 1 (HIV-1) [4, 5] and other sexually transmitted infections [6]. Among this same population of reproductive-aged women, use of long-acting reversible contraception (LARC) is increasing, particularly in sub-Saharan Africa, where use of progestin-only implants and copper intrauterine devices (Cu-IUD) has increased 4-fold over the previous decade [7].
Previous research has established a reduced risk of BV among women using either combined hormonal contraception containing both estrogen and progestins or progestin-only contraception [8, 9]. However, there are limited data on the effect of the nonhormonal Cu-IUD on BV risk. Cross-sectional studies of the association between Cu-IUD use and BV prevalence have reported mixed results [10, 11], as have 2 prior longitudinal studies. One prior longitudinal study did not find a statistically significant association between BV incidence and use of either Cu-IUD or a levonorgestrel intrauterine system, although an underpowered exploratory analysis suggested an increased risk of BV among women who reported irregular bleeding while using either method [12]. An additional study specifically designed to evaluate changes in prevalence of BV across contraceptive methods identified a nearly 2-fold increase in prevalence of BV at 6 months following initiation of Cu-IUD (P = .005) [13].
Two mechanisms of action have been proposed through which Cu-IUD may increase risk of BV. First, the presence of a foreign body in the uterus and vagina may facilitate the overgrowth of the mixed facultative and anaerobic bacteria associated with BV [12]. Second, the relative abundance of Gardnerella vaginalis and Lactobacillus species morphotypes fluctuates throughout the normal menstrual cycle, with an increase in Gardnerella vaginalis and decrease in Lactobacillus species during menses [14]. Cu-IUD initiation is typically accompanied by an increased volume and duration of menses [15, 16], potentially allowing preferential loss of Lactobacillus species and heme-stimulated growth of Gardnerella vaginalis during this time [14] to persist to the point of dysbiosis.
Prior longitudinal investigations of the association between Cu-IUD use and BV risk have included up to 6 months of follow-up time [12, 13], whereas bleeding patterns following Cu-IUD insertion show that initial increases in volume and duration of menses decline over 6 months to 1 year after Cu-IUD initiation [15, 16]. Should changes in bleeding patterns form a part of the biological mechanism through which Cu-IUD use increases BV risk, longer periods of follow-up time are needed to evaluate a potential time-varying effect of Cu-IUD on BV risk.
We therefore conducted a secondary analysis of data collected as part of a phase III biomedical HIV-1 prevention trial. Within the trial, a Contraceptive Action Team (CAT) was formed to scale up access to LARC methods [17]. These efforts to expand the contraceptive method mix provided a unique opportunity to (1) evaluate the association between current Cu-IUD use and BV frequency relative to women using no contraception or a nonhormonal method other than Cu-IUD, and (2) among new Cu-IUD users, assess changes in BV frequency prior to Cu-IUD initiation, during up to 18 months of use, and following discontinuation.
METHODS
We conducted a secondary analysis of data from the MTN-020/ASPIRE trial, a placebo-controlled, double-blind, randomized clinical trial of the HIV-1 prevention efficacy of a vaginal ring; detailed trial methods have been published previously [18]. Briefly, 2629 HIV-1-negative, healthy, sexually active women aged 18–45 were enrolled from 2012 to 2015 from Malawi, South Africa, Uganda, and Zimbabwe. Institutional review boards at each study site approved the study protocol, and all participants provided written informed consent. Analyses here include 2585 (98.3%) women confirmed to be HIV-1-negative at enrollment and with complete baseline data.
At study enrollment, participants were required to use an effective contraceptive method, including LARC methods (progestin-only implants, Cu-IUD), short-acting reversible contraceptives (SARC), including oral contraceptive pills (OCP) and the injectables intramuscular depot medroxyprogesterone acetate (DMPA-IM) and norethisterone enanthate (NET-EN), or tubal ligation. Throughout follow-up, women could choose a new contraceptive method or discontinue contraception at any time. Contraceptive method use was determined by either onsite provision of contraception, review of participant’s family planning records, and/or ascertained per participant self-report at monthly study visits.
Vaginal swabs for BV evaluation by Nugent score [19] were collected at enrollment and every 6 months thereafter. Additionally, clinical evaluation of BV by Amsel criteria [20] was performed at any visit if clinically indicated, with metronidazole treatment provided for clinical BV per the local standard of care [21–23]. At enrollment, demographic data were collected, and women were evaluated for Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and syphilis. Women reported intravaginal practices (insertion of soap or water; paper, cloth, tissue, rags, or cotton wool; products to make the vagina dry or tight) in the prior 3 months by audio computer-assisted self-interview at enrollment and the 3-month follow-up visit. Circumcision status of primary partners and sexual behavior data over the prior 3 months were collected in standardized surveys at enrollment and quarterly thereafter. Urine pregnancy tests and HIV-1 serologic tests were performed monthly. Participants reported unexpected heavy menstrual bleeding and/or intermenstrual bleeding as part of clinical trial adverse event reporting requirements.
We compared participant characteristics at enrollment across contraceptive methods with Cochran-Maentel-Haenzel χ 2 tests stratified by site for categorical variables and from analysis of variance adjusted for site for continuous variables. We used Andersen-Gill proportional hazards models with robust standard errors to evaluate the association between Cu-IUD use and BV frequency (including recurrent episodes), relative to women using no contraceptive or a nonhormonal contraceptive method other than Cu-IUD. Andersen-Gill models assume each BV episode is independent, with robust standard errors accounting for intra-individual correlation in events over time. Women were censored at HIV-1 seroconversion or first pregnancy. In our primary analysis, a time-varying, single contraceptive method exposure is defined as the method most recently initiated, and BV end points include both regularly scheduled BV evaluations as well as interim BV evaluations diagnosed by either Nugent score or Amsel criteria. We additionally report results for a model including only regularly scheduled BV evaluations. We conducted 2 sensitivity analyses to account for simultaneous exposure to additional exogenous hormones. First, we included an interaction term between current contraceptive method and recent exposure to an alternative hormonal contraceptive to account for overlap in contraceptive exposures following a method switch. Recent exposure was defined as within the prior 17 weeks for DMPA-IM and the prior 10 weeks for NET-EN. Second, some women reporting heavy and intermenstrual bleeding were prescribed additional estrogen and/or progestins to reduce excess bleeding. The second sensitivity analysis includes an interaction term between current contraceptive method and concomitant use or use within the prior 30 days of additional exogenous hormones. Finally, there may be residual confounding when using a reference group of women using no contraception or a nonhormonal contraceptive method other than Cu-IUD. We therefore conducted additional analyses using hormonal implant, OCP, and DMPA-IM users as referents. We used Andersen-Gill proportional hazards models with robust standard errors to evaluate changes in BV incidence prior to Cu-IUD initiation, while using, and following discontinuation. For all Andersen-Gill models, we evaluated the appropriateness of the proportional hazards assumption with tests of independence between time and Schoenfeld residuals. All models are stratified by study site and adjusted for variables hypothesized a priori to be confounders or expected to increase the precision of our analysis, including a new primary sex partner, number of sex partners, condom use at last vaginal sex, circumcision status of the primary partner, and any intravaginal practices reported at baseline and/or month 3. Quarterly values of retrospective data were carried backward. All analyses were performed in R software 3.5.0 [24].
RESULTS
At study enrollment, injectable contraceptive methods were used by over half of women (1412, 54.6%), followed by hormonal implants (492, 19.0%), Cu-IUD (323, 12.5%), OCP (280, 10.8%), and tubal ligation (78, 3.0%). Compared to women using a SARC, women using a LARC or with tubal ligation were somewhat older, more likely to be married, less likely to have completed secondary education, and less likely to test positive for C. trachomatis at enrollment (Table 1). Enrollment contraceptive use additionally varied by country, with relatively low prevalence of Cu-IUD (5.5%) and hormonal implant use (1.4%) at enrollment in South Africa, reflecting low background use of these methods and delayed access to LARC methods in South Africa within the trial.
Table 1.
Baseline Participant Characteristics by Contraceptive Method Used at Study Enrollment
| Cu-IUD n = 323 (12.4%) | Implant n = 492 (19.0%) | OCP n = 280 (10.8%) | DMPA-IM n = 1,044 (40.4%) | NET-EN n = 368 (14.2%) | Tubal Ligation n = 78 (3.0%) | P a | |
|---|---|---|---|---|---|---|---|
| Age, median (IQR) | 28(24, 33) | 28(24, 32) | 25(21, 31) | 26(22, 30) | 24(21, 28) | 36(33, 39) | <.001 |
| Currently married, n (%) | 184(57.0) | 396(80.5) | 52(18.6) | 368(35.2) | 19(5.2) | 47(60.3) | <.001 |
| Secondary school education or higher, n (%) | 261(80.8) | 376(76.4) | 259(92.5) | 881(84.4) | 359(97.6) | 49(62.8) | .028 |
| Current smoking, n (%) | 7(2.2) | 5(1.0) | 16(5.7) | 58(5.6) | 25(6.8) | 4(5.1) | .531 |
| Number of prior pregnancies, median (IQR) | 2(1, 3) | 3(2, 3) | 1(1, 2) | 2(1, 3) | 1(1, 2) | 4(3, 4) | <.001 |
| Number of live births, median (IQR) | 2(1, 3) | 2(2, 3) | 1(0, 2) | 2(1, 2) | 1(0, 2) | 4(3, 4) | <.001 |
| Sexually transmitted infection, n (%) | |||||||
| Chlamydia trachomatis b | 25(7.7) | 42(8.5) | 34(12.1) | 140(13.4) | 62(16.8) | 3(3.8) | .037 |
| Neisseria gonorrhoeae b | 13(4.0) | 26(5.3) | 12(4.3) | 41(3.9) | 12(3.3) | 5(6.4) | .614 |
| Trichomonas vaginalis c | 26(8.0) | 41(8.3) | 23(8.2) | 57(5.5) | 25(6.8) | 6(7.7) | .336 |
| Syphilisd | 4(1.2) | 13(2.6) | 5(1.8) | 17(1.6) | 0(0.0) | 0(0.0) | .164 |
| Bacterial vaginosise | 144(44.6) | 218(44.4) | 120(42.9) | 391(37.5) | 154(41.8) | 43(55.1) | <.001 |
| Two or more sexual partners, n (%) | 71(22.0) | 66(13.4) | 57(20.4) | 170(16.3) | 55(14.9) | 12(15.4) | .332 |
| Condom used at last sex, n (%) | 177(54.8) | 231(47.0) | 193(68.9) | 589(56.4) | 247(67.1) | 35(44.9) | .060 |
| Primary sex partner circumcised, n (%) | 123(38.1) | 126(25.6) | 125(44.6) | 459(44.0) | 215(58.4) | 32(41.0) | .029 |
Abbreviations: Cu-IUD, copper intrauterine device; DMPA-IM, intramuscular depot medroxyprogesterone acetate; IQR, interquartile range; NET-EN, norethisterone enanthate; OCP, oral contraceptive pill.
a P-values are obtained from Cochran-Maentel-Haenzel χ 2 tests stratified by site for categorical variables and from analysis of variance adjusted for site for continuous variables.
bEvaluated by nucleic acid amplification test.
cEvaluated by rapid test.
dEvaluated by serology.
eAt baseline, median time since method initiation was 16 days (IQR: 8, 112) across all methods, excluding women with a tubal ligation, as date of tubal ligation was unknown.
Contraceptive method switching was common, with 1003 (38.8%) women switching from their enrollment method at some point during trial participation. Among women who switched from their baseline method, 298 (29.7%) switched to Cu-IUD (Supplementary Figure 1). Continuation rates for Cu-IUD were high, with only 22% of women using Cu-IUD at baseline switching to a different method (Table 2). Throughout study follow-up, 619 (23.9%) women ever used a Cu-IUD, 803 (31.1%) ever used a hormonal implant, 520 (20.1%) ever used an OCP, 1185 (45.8%) ever used DMPA-IM, 477 (18.5%) ever used NET-EN, and 262 (10.1%) ever used another nonhormonal method (including tubal ligation, male and female condoms, and spermicide) or no contraception.
Table 2.
Contraceptive Method Discontinuation and Duration of Use During Trial Participation
| Cu-IUD n = 323 (12.5%) | Implant n = 492 (19.0%) | OCP n = 280 (10.8%) | DMPA-IM n = 1,044 (40.4%) | NET-EN n = 368 (14.2%) | None/Other Nonhormonala n = 78 (3.0%) | |
|---|---|---|---|---|---|---|
| Switched from baseline method, n (%) | 71(22.0) | 97(19.7) | 169(60.4) | 441(42.2) | 225(61.1) | 0(0.0) |
| Time to method switch (months), median (IQR)b | 9.2(5.2, 14.8) | 8.2(5.5, 12.9) | 4.0(2.0, 7.2) | 7.4(3.6, 14.7) | 7.8(3.9, 13.7) | … |
| Duration of method use (months), median (IQR)c | 16.4(9.9, 22.0) | 11.0(7.4, 18.2) | 5.6(2.8, 10.4) | 13.6(6.5, 21.2) | 11.4(5.2, 19.2) | 5.5(1.3, 16.6) |
Abbreviations: Cu-IUD, copper intrauterine device; DMPA-IM, intramuscular depot medroxyprogesterone acetate; IQR, interquartile range; NET-EN, norethisterone enanthate; OCP, oral contraceptive pill.
aAt baseline, includes only women with tubal ligation. At follow-up, includes all women using no contraceptive method or another nonhormonal contraceptive method.
bEstimated from among women who switched their baseline contraceptive method.
cEstimated from among women who used the method at any time during ASPIRE participation.
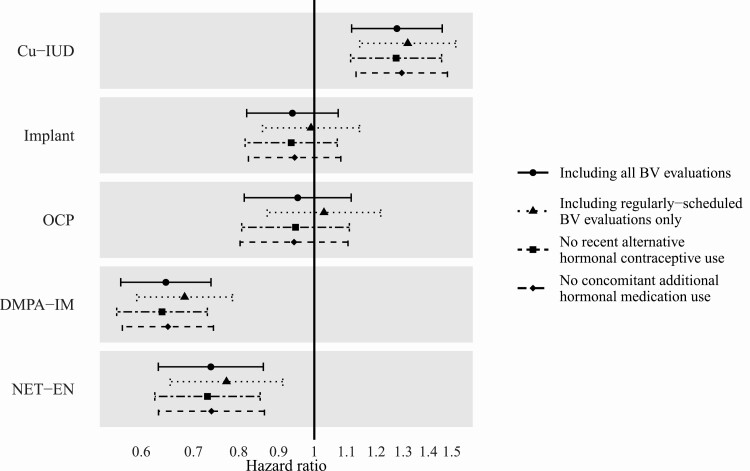
Across a median of 10.5 months of follow-up (interquartile range: 5.4, 16.8), women contributed 9552 BV evaluations, of which 93.9% were regularly scheduled evaluations and 3.9% were interim evaluations prompted by reported BV symptoms and conducted by Amsel criteria (the remainder were interim Nugent score evaluations). BV was common among trial participants at 101.6 episodes (95% confidence interval [CI]: 98.5, 104.7) per 100 person-years, ranging from 69.1 (95% CI: 64.9, 73.6) per 100-person years among DMPA-IM users to 153.6 (95% CI: 145.2, 162.4) per 100 person-years among Cu-IUD users (Supplementary Table 1). In adjusted models, BV incidence among Cu-IUD users was 1.28-fold (95% CI: 1.12, 1.46) higher than among women using no contraception or another nonhormonal contraceptive method (Figure 1). In contrast, DMPA-IM and NET-EN users had lower risk of BV (hazard ratio [HR]: 0.65, 95% CI: .56, .74; HR: 0.74, 95% CI: .63, .86, respectively), whereas hormonal implant and OCP users did not have significantly different BV risk compared to women using no contraception or another nonhormonal contraceptive method (HR: 0.94, 95% CI: .82, 1.07; HR: 0.95, 95% CI: .81, 1.12, respectively) (Figure 1). Results were similar in sensitivity analyses in which models included only regularly scheduled BV evaluations, accounted for recent exposure to an alternative hormonal contraceptive, or accounted for concomitant use of additional exogenous hormones to treat excess bleeding (Figure 1). The pattern of hazard ratios was also consistent across analyses with reference groups of women using implant, OCP, and DMPA-IM (Supplementary Table 2).
Figure 1.
Hazard ratio of BV by contraceptive method, relative to women using no contraception or a nonhormonal contraceptive method other than Cu-IUD. Analyses were conducted including (1) all BV evaluations, (2) only regularly scheduled BV evaluations, (3) estimated hazard ratio for those without recent exposure to an alternative hormonal contraceptive, and (4) estimated hazard ratio for those without current or recent concomitant additional hormonal medication use to treat heavy and/or intermenstrual bleeding. The proportional hazards assumption was appropriate for all models. Abbreviations: BV, bacterial vaginosis; Cu-IUD, copper intrauterine device; DMPA-IM, intramuscular depot medroxyprogesterone acetate; NET-EN, norethisterone enanthate; OCP, oral contraceptive.
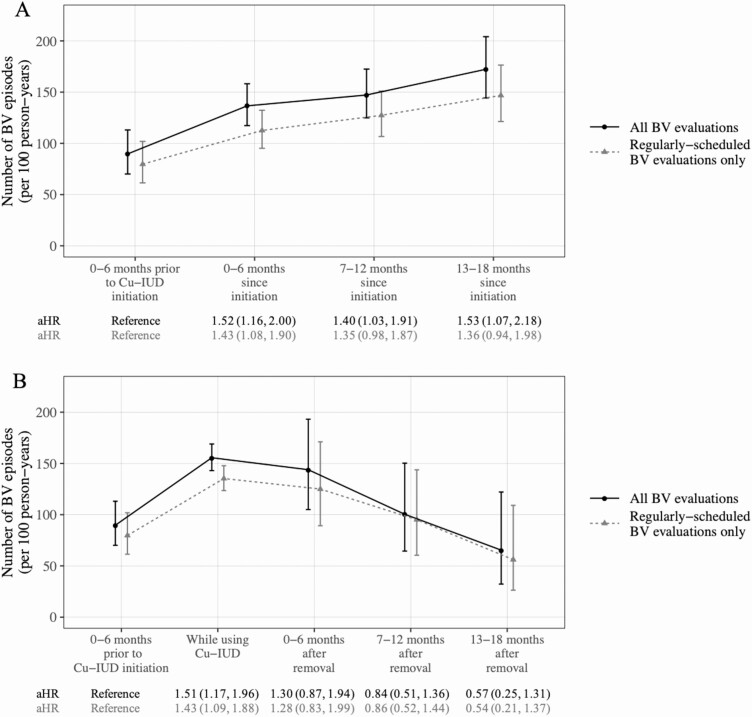
Among 298 women who initiated Cu-IUD during ASPIRE, use of DMPA-IM was most common in the 6 months prior to Cu-IUD initiation (147, 49.3%), followed by NET-EN (69, 23.2%), OCP (63, 21.1%), progestin-only implant (35, 11.7%), or no/another nonhormonal contraceptive method (5, 1.7%). BV frequency in the 6 months prior to Cu-IUD initiation was 89.7 episodes (95% CI: 70.1, 113.1) per 100 person-years, and increased to 136.6 episodes (95% CI: 117.3, 158.2) per 100 person-years in the first 6 months following Cu-IUD initiation and remained elevated for up to 18 months of use (Figure 2A). In adjusted models, the hazard of BV was significantly higher following Cu-IUD initiation over 18 months of use (all P < .05; Figure 2A). When including only regularly scheduled BV evaluations, the number of BV episodes at all time points was somewhat lower, and hazard ratios of BV following initiation relative to the 6 months prior to Cu-IUD initiation were attenuated (Figure 2A).
Figure 2.
Frequency of BV in (A) time period prior to Cu-IUD initiation and while using Cu-IUD and (B) in time period prior to Cu-IUD initiation, while using Cu-IUD, and following Cu-IUD discontinuation. Adjusted hazard ratios and 95% confidence intervals from Andersen-Gill proportional hazards models are shown below each figure. Abbreviations: BV, bacterial vaginosis; Cu-IUD, copper intrauterine device.
Seventy-four new Cu-IUD users subsequently discontinued Cu-IUD during ASPIRE participation. In the 6 months following discontinuation, women used OCP (28, 37.8%), implant (26, 35.1%), DMPA-IM (18, 24.3%), NET-EN (16, 21.6%) or no/another nonhormonal method (15, 20.3%). Among 70 women who reported a reason for Cu-IUD discontinuation, the primary side effect-related reasons were excessive bleeding (25, 35.7%) and increased menstrual pain (12, 17.1%), while only 1 woman (1.4%) reported recurrent genital symptoms as a reason for Cu-IUD discontinuation. Following discontinuation, BV frequency declined from 155.6 episodes (95% CI: 143.0, 169.0) per 100 person-years during use to 143.9 episodes (95% CI: 104.0, 193.2) per 100 person-years in the 6 months following discontinuation and 100.4 episodes (95% CI: 64.5, 150.3) per 100 person-years in the 7–12 months following discontinuation. In adjusted models, frequency of BV following Cu-IUD discontinuation was not significantly different from frequency in the 6 months prior to initiation (Figure 2B). Results were similar in a model including only regularly scheduled BV evaluations (Figure 2B).
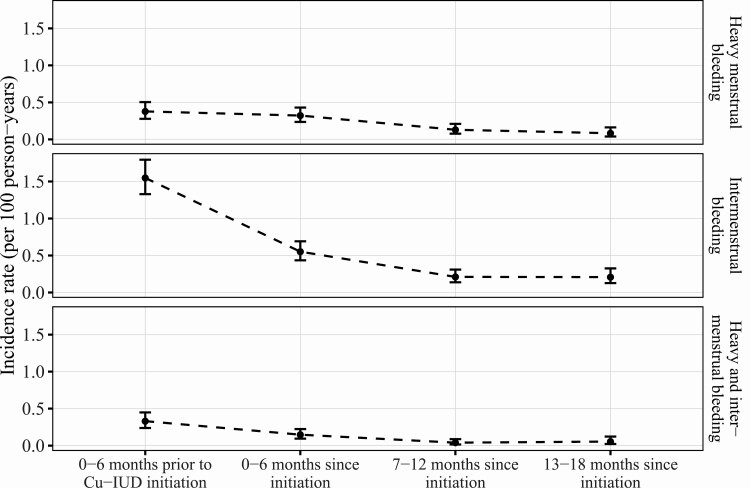
In adverse event reporting, incidence of unexpected heavy menstrual bleeding was similar in the 6 months prior to Cu-IUD initiation as in the 6 months following Cu-IUD initiation, with declining incidence over the subsequent year (Figure 3). Incidence of unexpected intermenstrual bleeding declined from the 6 months prior to initiation relative to the first 6 months following initiation and continued to decline over the following 12 months (Figure 3).
Figure 3.
Incidence rate of reported heavy menstrual bleeding, intermenstrual bleeding, and both heavy and intermenstrual bleeding in the 6 months prior to Cu-IUD initiation and in the 18 months following initiation. Abbreviation: Cu-IUD, copper intrauterine device.
DISCUSSION
In this large prospective analysis, Cu-IUD users experienced a 28% increased risk of bacterial vaginosis, relative to women using either no contraception or an alternative nonhormonal method. This finding was robust to multiple sensitivity analyses and adds to a growing body of literature demonstrating an elevated risk of BV among Cu-IUD users [13, 25]. These analyses additionally suggest that BV risk remains elevated through up to 18 months of Cu-IUD use and demonstrate that BV risk returns to pre-initiation levels following Cu-IUD discontinuation. Given this elevated risk, women and their contraceptive providers may wish to consider BV risk when discussing contraception options.
The Contraceptive Action Team within ASPIRE scaled up access to long-acting reversible contraceptives for study participants, with high initiation and continuation rates of these methods [26]. Similarly, there is a need to increase the contraceptive method mix to meet the diverse needs and preferences of women in sub-Saharan Africa, with provision of Cu-IUD included as part of this expansion. However, BV is associated with multiple adverse outcomes, regardless of symptomatology [1–6]. In particular, in regions of high HIV-1 prevalence, where BV may account for 15% of new HIV-1 infections [27], BV risk may be an especially important consideration when selecting contraceptives. The levonorgestrel intrauterine system (LNG-IUS) may be a preferable alternative for some women, as several longitudinal studies have shown either no significant changes in the composition of the vaginal microbiota among LNG-IUS users [28, 29] or a short-term increase in BV risk immediately following initiation, with a return to pre-initiation risk within 1 year [30]. However, as the LNG-IUS remains largely unavailable in sub-Saharan Africa, there were no users of the LNG-IUS in the present study, and so we were unable to evaluate the association between LNG-IUS and BV in this population.
Women using Cu-IUD experienced consistently elevated BV risk over 18 months of use, relative to the 6 months prior to Cu-IUD initiation. Over the same time period following Cu-IUD initiation, adverse event reports of unexpected heavy and intermenstrual bleeding declined, consistent with prior literature showing declining incidence of bleeding side effects in the 6–12 months following Cu-IUD insertion [15, 16]. Taken together, this suggests that heavy and intermenstrual bleeding secondary to Cu-IUD use may not mediate the association between Cu-IUD and increased BV incidence. However, adverse events of heavy and intermenstrual bleeding in ASPIRE were reported only when the change in bleeding was unexpected; systematic evaluation of bleeding patterns across methods can provide the data needed to formally evaluate a potentially mediating role of bleeding-related side effects in the relationship between Cu-IUD and BV incidence. Furthermore, although prior literature has established adhesion of Candida species to Cu-IUD [31], research is limited demonstrating increasing diversity and quantity of adhered microbes to Cu-IUD in situ with increasing duration of use [32]. Additional research is needed to investigate the biological mechanism that underlies the association between Cu-IUD use and BV risk.
Our estimates of the effect of DMPA-IM and NET-EN are consistent with prior literature [8, 9], and our analyses additionally replicate findings [13] showing no association between the progestin-only implant and BV frequency, consistent with the relatively small quantity of hormone released by this method [33, 34]. In contrast to prior literature [8, 9], we observed no association between OCP use and BV risk in this cohort. This null association may have been the result of lower exogenous hormonal exposure in our group of OCP users than in previous studies, as indicated by pregnancy incidence rates among women reporting OCP use in this cohort that were more than 3-fold higher than typical use failure rates [35, 36].
There are several limitations to the analyses presented here. Our comparison of BV frequency prior to initiation and while using Cu-IUD is limited by the presence of exogenous hormones prior to initiation, as women typically used a hormonal contraceptive method prior to Cu-IUD use. Estimates of the hazard of BV while using Cu-IUD relative to pre-initiation are likely higher than would be observed in the absence of additional exogenous hormones. Furthermore, the contraceptive method mix following Cu-IUD discontinuation was more likely to include methods not associated with BV frequency in this cohort, although the method mix in the 6 months prior to initiation was predominantly made up of methods associated with lower BV frequency. As a result, our estimate of the hazard of BV following Cu-IUD discontinuation relative to the 6 months prior to initiation may be higher than would be observed with a more comparable contraceptive method mix in both pre-initiation and following discontinuation. Finally, BV was evaluated infrequently, precluding a test-of-cure to define an endpoint of incident BV within this analysis. However, 60% of asymptomatic BV cases in a comparable cohort of African women resolved within 6 months [37], suggesting that the majority of recurrent episodes are incident cases. More frequent evaluation of BV would allow for more precise description of the pathogenesis of BV in relation to Cu-IUD use.
In conclusion, Cu-IUD users experienced a 1.28-fold increase in the risk of BV, relative to women using no contraception or another nonhormonal method. The elevated risk of BV persisted through up to 18 months of Cu-IUD use, with BV frequency declining to pre-initiation rates within 1 year of discontinuation. Women and their providers may wish to consider BV risk when discussing contraceptive options.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
MTN-020/ASPIRE Study team leadership . Jared Baeten, University of Washington (Protocol Chair); Thesla Palanee-Phillips, Wits Reproductive Health and HIV Institute (Protocol Cochair); Elizabeth Brown, Fred Hutchinson Cancer Research Center (Protocol Statistician); Lydia Soto-Torres, US National Institute of Allergy and Infectious Diseases (Medical Officer); Katie Schwartz, FHI 360 (Clinical Research Manager).
MTN-020/ASPIRE Study sites and site investigators of record. Malawi, Blantyre site (Johns Hopkins University, Queen Elizabeth Hospital): Bonus Makanani; Malawi, Lilongwe site (University of North Carolina, Chapel Hill): Francis Martinson; South Africa, Cape Town site (University of Cape Town): Linda-Gail Bekker; South Africa, Durban–Botha’s Hill, Chatsworth, Isipingo, Tongaat, Umkomaas, Verulam sites (South African Medical Research Council): Vaneshree Govender, Samantha Siva, Zakir Gaffoor, Logashvari Naidoo, Arendevi Pather, and Nitesha Jeenarain; South Africa, Durban, eThekwini site (Center for the AIDS Programme for Research in South Africa); Gonasagrie Nair; South Africa, Johannesburg site (Wits RHI): Thesla Palanee-Phillips; Uganda, Kampala site (John Hopkins University, Makerere University): Flavia Matovu; Zimbabwe, Chitungwiza, Seke South and Zengeza sites (University of Zimbabwe, University of California San Francisco): Nyaradzo Mgodi; Zimbabwe, Harare, Spilhaus site (University of Zimbabwe, University of California San Francisco): Felix Mhlanga. Data management was provided by The Statistical Center for HIV/AIDS Research & Prevention (Fred Hutchinson Cancer Research Center, Seattle, WA) and site laboratory oversight was provided by the Microbicide Trials Network Laboratory Center (Pittsburgh, PA).
Acknowledgments. The authors thank the MTN-020/ASPIRE study team for their tremendous efforts to conduct a rigorous trial and collect the data used in these analyses. In particular, we appreciate the data management provided by Daniel Szydlo and expert statistical consultation from Anu Mishra. They are also grateful to the Contraceptive Action Team for their dedication to increasing contraceptive access for women in the ASPIRE trial. The International Partnership for Microbicides developed the dapivirine ring and supplied rings for this trial. Finally, they sincerely thank the women who contributed their time and efforts to participate in ASPIRE, without whom this work could not be completed.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial support. This work was supported by The Microbicide Trials Network (MTN), which is funded by National Institute of Allergy and Infectious Disease (UM1AI068633, UM1AI068615, and UM1AI106707), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute of Mental Health, all components of the US National Institutes of Health (NIH).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
MTN-020/ASPIRE study team:
Jared Baeten, Thesla Palanee-Phillips, Elizabeth Brown, Lydia Soto-Torres, Katie Schwartz, Bonus Makanani, Francis Martinson, Linda-Gail Bekker, Vaneshree Govender, Samantha Siva, Zakir Gaffoor, Logashvari Naidoo, Arendevi Pather, Nitesha Jeenarain, Gonasagrie Nair, Thesla Palanee-Phillips, Flavia Matovu, Nyaradzo Mgodi, and Felix Mhlanga
References
- 1. Bradshaw CS, Brotman RM. Making inroads into improving treatment of bacterial vaginosis: striving for long-term cure. BMC Infect Dis 2015; 15:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis 2013; 40:117–22. [DOI] [PubMed] [Google Scholar]
- 3. Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 1995; 333:1737–42. [DOI] [PubMed] [Google Scholar]
- 4. Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med 2012; 9:e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Low N, Chersich MF, Schmidlin K, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med 2011; 8:e1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bautista CT, Wurapa E, Sateren WB, Morris S, Hollingsworth B, Sanchez JL. Bacterial vaginosis: a synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Mil Med Res 2016; 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacobstein R. Liftoff: the blossoming of contraceptive implant use in Africa. Glob Health Sci Pract 2018; 6:17–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riggs M, Klebanoff M, Nansel T, Zhang J, Schwebke J, Andrews W. Longitudinal association between hormonal contraceptives and bacterial vaginosis in women of reproductive age. Sex Transm Dis 2007; 34:954–9. [PubMed] [Google Scholar]
- 9. Vodstrcil LA, Hocking JS, Law M, et al. Hormonal contraception is associated with a reduced risk of bacterial vaginosis: a systematic review and meta-analysis. PLoS One 2013; 8:e73055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donders G, Bellen G, Janssens D, Van Bulck B, Hinoul P, Verguts J. Influence of contraceptive choice on vaginal bacterial and fungal microflora. Eur J Clin Microbiol Infect Dis 2017; 36:43–8. [DOI] [PubMed] [Google Scholar]
- 11. Kancheva Landolt N, Chaithongwongwatthana S, Nilgate S, et al. Use of copper intrauterine device is not associated with higher bacterial vaginosis prevalence in Thai HIV-positive women. AIDS Care 2018; 30: 1–5. [DOI] [PubMed] [Google Scholar]
- 12. Madden T, Grentzer JM, Secura GM, Allsworth JE, Peipert JF. Risk of bacterial vaginosis in users of the intrauterine device: a longitudinal study. Sex Transm Dis 2012; 39:217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Achilles SL, Austin MN, Meyn LA, Mhlanga F, Chirenje ZM, Hillier SL. Impact of contraceptive initiation on vaginal microbiota. Am J Obstet Gynecol 2018; 218:622.e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One 2010; 5:e10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hubacher D, Chen PL, Park S. Side effects from the copper IUD: do they decrease over time? Contraception 2009; 79:356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanders JN, Adkins DE, Kaur S, Storck K, Gawron LM, Turok DK. Bleeding, cramping, and satisfaction among new copper IUD users: a prospective study. PLoS One 2018; 13:e0199724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bunge K, Baeten J, Chappell C, et al. Expanding the mix of contraceptive methods in an HIV prevention trial. In: IAS Conference. Durban, South Africa, 18–22 July 2016.
- 18. Baeten JM, Palanee-Phillips T, Brown ER, et al. ; MTN-020–ASPIRE Study Team . Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 2016; 375:2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991; 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983; 74:14–22. [DOI] [PubMed] [Google Scholar]
- 21. World Health Organization. Guidelines for the management of sexually transmitted infections. Geneva, Switzerland: World Health Organization, 2003. [Google Scholar]
- 22. Ministry of Health, Malawi. Management of sexually transmitted infections using syndromic management approach. Lilongwe, Malawi: Ministry of Health, 2007. [Google Scholar]
- 23. South Africa National Department of Health. Sexually transmitted infections management guidelines 2015. Pretoria, South Africa: National Department of Health, 2015. [Google Scholar]
- 24. R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2008. [Google Scholar]
- 25. Jaspan H, Bunjun R, Brown B, et al. Contraceptive-induced changes in genital tract HIV-1 cellular targets and microbiota among women enrolled in the ECHO trial. In: 10th IAS Conference on HIV Science. Mexico City, Mexico, 21–24 July 2019.
- 26. Chappell CA, Harkoo I, Szydlo DW, et al. ; CAT and MTN-020/ASPIRE Study Team . Contraceptive method switching among women living in sub-Saharan Africa participating in an HIV-1 prevention trial: a prospective cohort study. Contraception 2019; 100:214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masese L, Baeten JM, Richardson BA, et al. Changes in the contribution of genital tract infections to HIV acquisition among Kenyan high-risk women from 1993 to 2012. AIDS (London, England) 2015; 29:1077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jacobson JC, Turok DK, Dermish AI, Nygaard IE, Settles ML. Vaginal microbiome changes with levonorgestrel intrauterine system placement. Contraception 2014; 90:130–5. [DOI] [PubMed] [Google Scholar]
- 29. Brooks JP, Edwards DJ, Blithe DL, et al. Effects of combined oral contraceptives, depot medroxyprogesterone acetate and the levonorgestrel-releasing intrauterine system on the vaginal microbiome. Contraception 2017; 95:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donders GGG, Bellen G, Ruban K, Van Bulck B. Short- and long-term influence of the levonorgestrel-releasing intrauterine system (Mirena®) on vaginal microbiota and Candida. J Med Microbiol 2018; 67:308–13. [DOI] [PubMed] [Google Scholar]
- 31. Chassot F, Negri MF, Svidzinski AE, et al. Can intrauterine contraceptive devices be a Candida albicans reservoir? Contraception 2008; 77:355–9. [DOI] [PubMed] [Google Scholar]
- 32. Pál Z, Urbán E, Dósa E, Pál A, Nagy E. Biofilm formation on intrauterine devices in relation to duration of use. J Med Microbiol 2005; 54:1199–203. [DOI] [PubMed] [Google Scholar]
- 33. Bayer AG. Jadelle: highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020544s010lbl.pdf. Accessed 13 August 2019.
- 34. Merck. Nexplanon: highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021529s011lbl.pdf. Accessed 13 August 2019.
- 35. Balkus JE, Palanee-Phillips T, Reddy K, et al. Brief report: dapivirine vaginal ring use does not diminish the effectiveness of hormonal contraception. J Acquir Immune Defic Syndr 2017; 76:e47–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodriguez J, Abutouk M, Roque K, Sridhar A. Personalized contraceptive counseling: helping women make the right choice. Open Access J Contracept 2016; 7:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pyra M, Abdala A, Mwinyikai K, et al. Natural History of Asymptomatic Bacterial Vaginosis among Kenyan Women at High Risk for HIV Infection. In: Annual Meeting of the Infectious Diseases Society for Obstetrics and Gynecology. Big Sky, Montana, 7–10 August 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.