Abstract
Background
Demonstration of influenza vaccine effectiveness (VE) against hospitalized illness in addition to milder outpatient illness may strengthen vaccination messaging. Our objective was to compare patient characteristics and VE between United States (US) inpatient and outpatient VE networks.
Methods
We tested adults with acute respiratory illness (ARI) for influenza within 1 outpatient-based and 1 hospital-based VE network from 2015 through 2018. We compared age, sex, and high-risk conditions. The test-negative design was used to compare vaccination odds in influenza-positive cases vs influenza-negative controls. We estimated VE using logistic regression adjusting for site, age, sex, race/ethnicity, peak influenza activity, time to testing from, season (overall VE), and underlying conditions. VE differences (ΔVE) were assessed with 95% confidence intervals (CIs) determined through bootstrapping with significance defined as excluding the null.
Results
The networks enrolled 14 573 (4144 influenza-positive) outpatients and 6769 (1452 influenza-positive) inpatients. Inpatients were older (median, 62 years vs 49 years) and had more high-risk conditions (median, 4 vs 1). Overall VE across seasons was 31% (95% CI, 26%–37%) among outpatients and 36% (95% CI, 27%–44%) among inpatients. Strain-specific VE (95% CI) among outpatients vs inpatients was 37% (25%–47%) vs 53% (37%–64%) against H1N1pdm09; 19% (9%–27%) vs 23% (8%–35%) against H3N2; and 46% (38%–53%) vs 46% (31%–58%) against B viruses. ΔVE was not significant for any comparison across all sites.
Conclusions
Inpatients and outpatients with ARI represent distinct populations. Despite comparatively poor health among inpatients, influenza vaccination was effective in preventing influenza-associated hospitalizations.
Keywords: influenza, vaccine effectiveness, severity, attenuation, hospitalization
We compared patient characteristics and influenza vaccine effectiveness (VE) between US hospital and outpatient studies, 2015–2018. Despite hospitalized patients being older and having a higher burden of comorbidities, VE estimates were similar, supporting vaccine protection against hospitalizations associated with influenza.
Seasonal influenza is a major cause of morbidity and mortality in the United States (US), resulting in an estimated 4.3–21 million outpatient visits and 140 000–810 000 hospitalizations annually over the previous decade [1]. Vaccination is the most effective means of preventing influenza-associated illnesses [2]. In the US, the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) recommends that all people ≥ 6 months of age should receive annual influenza vaccination [3]. However, vaccination rates in the US are < 50% in most age groups [4]. Suboptimal vaccine effectiveness (VE), with estimates in outpatient settings ranging from 33% to 67% depending on virus type [5], as well as the perception that influenza is not a severe illness, contributes to decisions not to get vaccinated [6]. Establishing VE against hospitalized influenza is important for policymakers [7, 8] and may influence people’s decisions on whether to get vaccinated.
A key outstanding question is whether influenza vaccination provides greater protection against severe disease compared with milder manifestations of illness, with influenza-associated hospitalization used as a proxy for severe influenza [9]. A comparison of published VE estimates found similar VE between outpatient and hospital studies within the same season and country [10]. However, even within the same geographic area and healthcare systems, patients who are hospitalized with acute respiratory illness (ARI) may differ from outpatients with respect to medical conditions that affect vaccine response and influenza severity [11–13]. This may therefore make direct comparisons of VE between hospital and outpatient settings problematic. Some studies provide support that vaccination reduces disease severity among influenza cases [14–16]. If influenza vaccination attenuates disease severity, we might expect higher VE in inpatient settings compared with outpatient settings, under the hypothesis that patients are generally comparable in most regards other than their disease severity.
The United States Influenza Vaccine Effectiveness Network (US Flu VE Network) has estimated outpatient-exclusive VE annually since the 2011–2012 seasons. A second US VE network with enrollment of adults in inpatient settings, the Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN), started in 2015–2016. Three study sites participate in both VE networks. This provided the opportunity to compare inpatient and outpatient populations used in VE studies and VE estimates across networks, as well as within the same source populations.
The objectives of this study were (1) to compare characteristics of adults enrolled in 2 outpatient and hospital-based networks during 3 seasons to inform the interpretation of VE comparison between populations; and (2) to compare estimated VE by season and influenza type/subtype between outpatient and hospital-based networks, across all network sites and shared network sites within the same geographic areas and health systems.
METHODS
Population and Settings
We included adults enrolled in the CDC-funded US Flu VE Network and HAIVEN during the 2015–2016, 2016–2017, and 2017–2018 influenza seasons, which have been described in detail previously [2, 17–19]. Details on vaccine strains are shown in Supplementary Table 1. Inclusion criteria differed for inpatient vs outpatient settings. In brief, the US Flu VE Network enrolled children and adults with ARI with cough of ≤ 7 days’ duration at approximately 52 participating clinics or emergency departments in 5 states (Michigan, Pennsylvania, Texas, Washington, and Wisconsin). HAIVEN enrolled hospitalized adults (≥ 18 years) with ARI with qualifying symptoms/syndromes of ≤ 10 days’ duration at 10 participating community and tertiary care hospitals in 4 states (Michigan, Pennsylvania, Texas, and Tennessee). Three sites—Michigan, Pennsylvania, and Texas—participate in both the outpatient and hospital networks. Prior to or at enrollment, combined nasal and throat swabs were systematically collected and tested by reverse-transcription polymerase chain reaction for influenza viruses, including by subtype and lineage.
Trained study staff collected demographic and clinical characteristics through structured patient interviews and/or medical records review. Information on high-risk medical conditions in the past year, as defined by the ACIP, was abstracted or extracted from electronic medical records using International Classification of Diseases (ICD) codes (Ninth Revision [2015–2016 season] and Tenth Revision [2016–2017 and 2017–2018 seasons]) [20, 21]. We grouped ICD codes into 14 categories: asthma, blood disorders, heart disease, cerebrovascular disease, diabetes mellitus (DM), endocrine disorders, immunosuppressive disorders, liver disease, malignancy in the previous year, non-DM metabolic diseases, morbid obesity (body mass index ≥ 40 kg/m2), and neurological/musculoskeletal, pulmonary, and renal conditions (Supplementary Table 2). We excluded ICD codes if they were not captured in both networks. Scores on the Charlson Comorbidity Index (CCI), a validated measure of chronic comorbidities predictive of long-term survival that has been used in both inpatient and outpatient settings [22, 23], were also derived using ICD codes [24]. This measure provides a prognostic survival risk score based on presence of select underlying medical conditions.
For this analysis, we excluded the following patients: < 18 years of age (as HAIVEN only enrolls adults); with time from symptom onset to enrollment > 7 days (the US Flu VE Network restricts ARI onset to ≤ 7 days); enrolled before the first laboratory-confirmed influenza case or after the last case specific to season and site; with missing or inconclusive vaccination status; or who received influenza vaccination < 14 days prior to enrollment. The VE network studies were approved by institutional review boards at the CDC and each participating site.
Current-season Vaccination History
Current-season influenza vaccination status was obtained through state immunization registries, electronic immunization or health insurance claim records, and employee health records. In the absence of documentation from these sources, for both networks vaccine receipt was also obtained through plausible self-report in which a participant identified the date and location of vaccination [25].
Characteristic of Network Participants
Baseline demographic characteristics including age, sex, race/ethnicity, self-reported health before illness (excellent/very good, good, or fair/poor), and presence and number of ICD codes by category and CCI scores were summarized for participants enrolled in the outpatient and hospital network by median and interquartile range (IQR) or number and percentage.
VE Estimates by Network
Using the test-negative design [26], we estimated VE by comparing the odds of seasonal influenza vaccination in influenza-positive cases to influenza-negative (test-negative) controls. We estimated VE using logistic regression models, for each network separately, where VE equals 100 × (1 – odds ratio). For this analysis, all models were adjusted a priori for site, age, sex, timing of illness onset defined relative to season- and site-specific peak influenza activity (defined within date tertiles for influenza cases), race/ethnicity, and days from symptoms onset to enrollment [18, 19]. Because the 2 networks consisted of distinct patient populations with differing baseline characteristics, we then controlled for confounding by considering network-specific variables. In the US Flu VE Network models, we adjusted for presence of ≥ 1 high-risk condition and self-reported health status as previously performed [27]. In HAIVEN, a priori we adjusted for patient/proxy-reported number of hospitalizations in the year before enrollment as a general indicator for baseline health status. Presence or sum of categories of high-risk conditions did not change adjusted VE estimates by ≥ 5%, the prespecified threshold for inclusion, so these variables were not added to the final adjusted model for HAIVEN. VE estimates were stratified by season, influenza type/subtype [A(H1N1)pdm09, A(H3N2), or B viruses], and age (18–49, 50–64, and ≥ 65 years). We also estimated overall and strain-specific VE across all seasons by adjusting for the above variables as well as season. VE was not estimated when the number of influenza cases in a stratum was < 10.
Secondary VE Analyses Among Restricted Populations
To estimate VE within more similar source populations, we estimated VE according to 2 prespecified subgroups: (1) restricted to populations at shared VE network sites in the same geographic areas and health systems (in Michigan, Pennsylvania, and Texas) to capture a similar source population; and (2) restricted to younger adults (18–64 years) in the same health systems and excluding patients with high-risk immunosuppressive conditions. This second prespecified analysis was selected because age distribution and presence of immunosuppressive conditions differ between hospital and outpatient networks, age is a potential effect modifier for VE [5, 28], and immunosuppressive conditions are associated with reduced vaccine immunogenicity [12].
VE Comparisons Between Outpatient and Hospital Networks
We further compared VE estimates between hospital and outpatient networks by absolute difference in VE estimates, defined as ΔVE = VEhospital – VEoutpatient, within each season and by influenza type/subtype. We derived 95% confidence intervals (CIs) for ΔVE estimates by a bootstrapped method in which we defined VE as 1 – exp(log odds ratio) × 100 and assumed the log odds ratio was normally distributed with mean and standard deviation equal to the regression coefficient and standard error estimated from the respective inpatient and outpatient VE models. We drew a random sample with 10 000 iterations from each distribution and calculated ΔVE and repeated this random sample selection 100 times. We assumed the mean of the 2.5th and 97.5th percentiles from the resulting distributions of ΔVE as the 95% CI of ΔVE [10], and considered ΔVE estimates with this 95% CI excluding the null value (ie, 0) as statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina) and RStudio Version 1.2 (Boston, Massachussetts).
RESULTS
Baseline Patient Characteristics for Outpatient and Hospital Networks
From 2015–2016 through 2017–2018, the outpatient network enrolled 14 573 (including 28% influenza-positive) outpatients and the hospital VE network enrolled 6769 (including 21% influenza-positive) hospitalized adults (13% [894/6763] were admitted to the intensive care unit [ICU], 6 missing ICU status). Hospitalized patients were older than adults enrolled in the outpatient study (median age, 62 vs 49 years), more likely to be non-Hispanic black (28% vs 6%), and more likely to have received current-season influenza vaccination (68% vs 53%) (Table 1). Among vaccinated study participants, hospitalized patients were more likely to have received high-dose rather than standard-dose vaccination compared to vaccinated outpatients (30% vs 17%) (Supplementary Table 3). At enrollment, 50% (2734) of hospitalized patients reported their general health as fair to poor, compared with 9% (1281) from the outpatient network. Hospitalized patients were more likely to have ≥ 1 high-risk medical condition (96% vs 57%), with a higher median number of categories with ICD diagnoses (4 vs 1) and higher median CCI scores (3 vs 0). The most common conditions among hospitalized patients were heart disease (62%), pulmonary conditions (chronic obstructive pulmonary disease or other, excluding asthma) (62%), and non-DM metabolic disorders (55%). In the outpatient network, the most common conditions included non-DM metabolic disorders (23%), asthma (16%), and heart disease (15%). Vaccinated patients were older and more likely to have ≥ 1 high-risk medical condition compared to unvaccinated patients (Supplementary Table 3).
Table 1.
Characteristics of Adults in the United States Influenza Vaccine Effectiveness Network (Outpatient) and Hospitalized Adult Influenza Vaccine Effectiveness Network (Hospital) Across All Sites
| Variable | US Flu VE Network (n = 14 573) | HAIVEN (n = 6769) |
|---|---|---|
| Year | ||
| 2015–2016 | 4495 (31) | 1309 (19) |
| 2016–2017 | 4553 (31) | 2345 (35) |
| 2017–2018 | 5525 (38) | 3115 (46) |
| Age, y, median (IQR) | 49 (34–62) | 62 (51–72) |
| Sex, malea | 5220 (36) | 2967 (44) |
| Race/ethnicitya | ||
| White, non-Hispanic | 11 602 (80) | 4377 (65) |
| Black, non-Hispanic | 879 (6) | 1881 (28) |
| Other, non-Hispanic | 1144 (8) | 213 (3) |
| Hispanic | 897 (6) | 291 (4) |
| Received current-season vaccination | 7756 (53) | 4619 (68) |
| Influenza-positive | 4144 (28) | 1452 (21) |
| Self-described general health status, no./No. (%)a | ||
| Excellent/very good | 8964/14 560 (62) | 986/5421 (18) |
| Good | 4315/14 560 (30) | 1701/5421 (31) |
| Fair/poor | 1281/14 560 (9) | 2734/5421 (50) |
| Charlson Comorbidity Index, median (IQR) | 0 (0–1) | 3 (1–6) |
| High-risk conditionsb | ||
| Heart disease | 2232 (15) | 4211 (62) |
| Pulmonary disease | 965 (7) | 4167 (62) |
| Metabolic diseases (not diabetes) | 3366 (23) | 3744 (55) |
| Chronic renal disease | 894 (6) | 2880 (43) |
| Diabetes mellitus | 1724 (12) | 2600 (38) |
| Neurological/musculoskeletal | 1420 (10) | 2339 (35) |
| Immunosuppressive disorders | 784 (5) | 2172 (32) |
| Asthma | 2283 (16) | 2059 (30) |
| Malignancy | 1272 (9) | 1765 (26) |
| Endocrine disorders | 1571 (11) | 1697 (25) |
| Morbid obesity | 1647 (11) | 1258 (19) |
| Liver diseases | 470 (3) | 908 (13) |
| Blood disorders | 192 (1) | 852 (13) |
| Cerebrovascular disease | 177 (1) | 572 (8) |
| Any high-risk category | 8319 (57) | 6486 (96) |
| Total high-risk categories, median (IQR) | 1 (0–2) | 4 (3–6) |
Data are presented as no. (%) unless otherwise indicated; no./No indicates number/total population with value recorded.
Abbreviations: HAIVEN, Hospitalized Adult Influenza Vaccine Effectiveness Network; IQR, interquartile range; US Flu VE Network, United States Influenza Vaccine Effectiveness Network.
aVariables with missing values: For HAIVEN, race/ethnicity missing for 7 (< 1%), self-reported health status for 1348 (20%); for the US Flu VE Network, sex missing for 2 (< 1%), race/ethnicity for 51 (< 1%), self-reported health for 13 (< 1%).
b International Classification of Diseases, Ninth or Tenth Revision conditions shared between outpatient and hospital network; see Supplementary Table 2 for list of codes.
In analysis restricted to those 18–64 years of age (Supplementary Table 4), most hospitalized patients had multiple medical comorbidities: 95% had ≥ 1 high-risk medical condition (median, 4 [IQR, 2–6]) and a median CCI score of 3 (IQR, 1–5), similar to all hospitalized patients including older adults. Patient characteristics restricted to shared network sites were similar compared to all network sites (Supplementary Table 5).
VE Across All Network Sites
Overall adjusted VE during the 3 seasons ranged from 28% to 39% in the outpatient network and 29% to 53% in the hospital network (Table 2), averaging 31% (95% CI, 26%–37%) among outpatients and 36% (95% CI, 27%–44%) among hospitalized patients. Strain-specific VE among outpatients vs hospitalized adults averaged 37% (95% CI, 25%–47%) vs 53% (95% CI, 37%–64%) against A(H1N1)pdm09; 19% (95% CI, 9%–27%) vs 23% (95% CI, 8%–35%) against A(H3N2); and 46% (95% CI, 38%–53%) vs 46% (95% CI, 31%–58%) against influenza B viruses. VE point estimates against H3N2 were consistently lower than against H1N1 and B across seasons, and no consistent patterns in differences in VE by subtype were observed between age strata in both networks (Supplementary Tables 6 and 7; Supplementary Figures 1 and 2). In a post hoc analysis, adjusted VE against hospitalized influenza was similar when we excluded patients admitted to the ICU and overall and type-specific VE similar for hospital and outpatient networks when we classified vaccination status by self-report alone (data not shown).
Table 2.
Adjusted Influenza Vaccine Effectiveness Against All Influenza Viruses and Stratified by Subtype Among Adults in the United States Influenza Vaccine Effectiveness Network (Outpatient) and Hospitalized Adult Influenza Vaccine Effectiveness Network (Hospital) Across All Sites
| Influenza Type | 2015–2016 | 2016–2017 | 2017–2018 | |||
|---|---|---|---|---|---|---|
| US Flu VE Network | HAIVEN | US Flu VE Network | HAIVEN | US Flu VE Network | HAIVEN | |
| All | 39 (28–48) | 53 (33–66) | 28 (17–38) | 29 (9–45) | 28 (19–37) | 34 (21–45) |
| A(H1N1)pdm09 | 35 (21–47) | 50 (26–66) | 39 (–67 to 78) | 46 (–115 to 86) | 41 (17–58) | 55 (30–71) |
| A(H3N2) | 27 (−29 to 59) | … | 23 (9–35) | 19 (−9 to 40) | 12 (−2 to 24) | 25 (6–40) |
| B | 49 (32–62) | 57 (9–80) | 43 (27–56) | 53 (28–70) | 48 (36–57) | 40 (17–56) |
Vaccine effectiveness (VE) models were adjusted for site, age, sex, race/ethnicity, season- and site-specific peak influenza activity, time to testing from symptoms onset, and underlying conditions (as presence of ≥ 1 high-risk conditions and self-reported health status in US Flu VE Network and number of hospitalizations in the previous year in HAIVEN). Measure includes adjusted VE and 95% confidence interval; VE not measured when the number of influenza cases was < 10.
Abbreviations: HAIVEN, Hospitalized Adult Influenza Vaccine Effectiveness Network; US Flu VE Network, United States Influenza Vaccine Effectiveness Network.
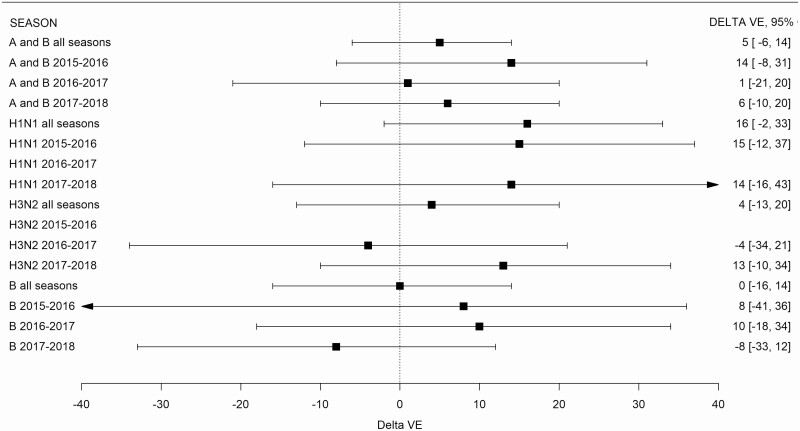
Differences in vaccine effectiveness (ΔVE) did not meet significance thresholds (ie, confidence limits included 0) for any season: 14% (95% CI, −8% to 31%) in 2015–2016; 1% (95% CI, −21% to 20%) in 2016–2017; and 6% (95% CI, −10% to 20%) in 2017–2018 (Table 3 and Figure 1).
Table 3.
Difference in Adjusted Vaccine Effectiveness Between Hospital and Outpatient Networks by Season and Influenza Type/Subtype
| Season | Type | All Sites, ΔVE (95% CI)a | Shared Sites, ΔVE (95% CI) | Shared Sites, < 65 y, No Immunosuppressive Condition, ΔVE (95% CI) |
|---|---|---|---|---|
| 2015–2016 | All | 14 (−8 to 31) | 31 (7–53) | 40 (12–64) |
| A(H1N1)pdm09 | 15 (−12 to 37) | 27 (1–52) | 30 (−7 to 59) | |
| A(H3N2) | … | … | … | |
| B viruses | 8 (−41 to 36) | 36 (−19 to 78) | 67 (43–104) | |
| 2016–2017 | All | 1 (−21 to 20) | 12 (−12 to 35) | −2 (−44 to 30) |
| A(H1N1)pdm09 | … | … | … | |
| A(H3N2) | −4 (−34 to 21) | 19 (−14 to 51) | −1 (−67 to 45) | |
| B viruses | 10 (−18 to 34) | 5 (−26 to 30) | −3 (−61 to 29) | |
| 2017–2018 | All | 6 (−10 to 20) | 10 (−8 to 27) | 17 (−7 to 37) |
| A(H1N1)pdm09 | 14 (−16 to 43) | 11 (−24 to 45) | −6 (−81 to 37) | |
| A(H3N2) | 13 (−10 to 34) | 17 (−7 to 40) | 21 (−13 to 50) | |
| B viruses | −8 (−33 to 12) | −5 (−32 to 18) | 18 (−14 to 43) |
Bold values indicate significant differences (defined as not crossing 0) in estimated VE between populations not crossing the null; ΔVE not shown for A(H3N2) in 2015–2016 and for A(H1N1)pdm09 in 2016–2017 because of small number of cases for comparisons.
Abbreviations: CI, confidence interval, VE, vaccine effectiveness.
a ΔVE = VEhospital – VEoutpatient.
Figure 1.
Difference in vaccine effectiveness (VE) between hospital and outpatient networks at all sites by season and influenza type. Delta VE (ΔVE) = VEhospital – VEoutpatient. ΔVE is not shown for A(H3N2) in 2015–2016 and for A(H1N1)pdm09 in 2016–2017 because of small number of cases for comparisons. Abbreviation: CI, confidence interval.
VE Restricted to Shared Network Sites and Network Comparisons
Restricted to shared sites, the overall pooled estimated VE was 29% (95% CI, 20%–36%) among outpatients and 43% (95% CI, 34%–51%) among hospitalized patients. Strain-specific estimated VE among outpatients vs hospitalized adults was 37% (95% CI, 21%–49%) vs 60% (95% CI, 44%–71%) against H1N1pdm09; 12% (95% CI, −2% to 24%) vs 31% (95% CI, 17%–43%) against H3N2; and 47% (95% CI, 36%–56%) vs 51% (95% CI, 36%–63%) against influenza B viruses (Supplementary Tables 8 and 9). Within these shared sites, VE in hospital settings was significantly higher compared to outpatient settings for the 2015–2016 season (ΔVE = 31% [95% CI, 7%–53%]), but without a significant difference in VE between settings for the 2016–2017 (ΔVE = 12% [95% CI, −12% to 35%]) or 2017–2018 seasons (ΔVE = 10% [95% CI, −8% to 27%]) (Table 3).
Further restricting to patients < 65 years of age at shared sites (Michigan, Pennsylvania, and Texas) without high-risk immunosuppressive conditions, overall adjusted VE against influenza averaged 31% (95% CI, 22%–39%) among outpatients and 49% (95% CI, 35%–60%) among hospitalized patients. VE in hospital settings was again higher compared to outpatient settings in 2015–2016 (ΔVE = 40% [95% CI, 12%–64%]), with no significant difference observed for the 2016–2017 and 2017–2018 seasons (Table 3). Notably, the 2015–2016 season had a predominance of H1N1pdm09 and B viruses, whereas later seasons had greater circulation of H3N2 viruses.
DISCUSSION
Our study highlights marked differences in characteristics of patients with ARI seeking medical care in outpatient and hospital settings within the same geographic areas and healthcare systems. Half of the hospitalized patients described their health before their illness as fair or poor vs < 10% of the outpatients. This was also reflected in a greater number of high-risk medical conditions and higher CCI scores among hospitalized patients. The differences in baseline health of the 2 patient populations persisted when restricting the analysis to adults aged 18–64 years. These findings suggest that patients enrolled in our 2 VE networks from outpatient and hospital settings represent distinct populations, with significant implications for interpreting estimates of influenza VE from outpatient and hospital settings.
Importantly, we documented that vaccination prevented influenza disease in both hospital and outpatient settings. Vaccine provided protection not only among outpatients, but also among hospitalized patients with multiple medical comorbidities, a population that has a high risk of ICU admissions for respiratory support, exacerbation of underlying health conditions, and other influenza-related complications [29, 30]. A large majority of the published data report vaccine protection against medically attended influenza in the outpatient setting [5]. Prevention of influenza disease among hospitalized patients is also an important benchmark to use in communicating the overall benefits of vaccination to policy makers and the general public. Preventing influenza-associated hospitalizations in high-risk patients also reduces healthcare expenditures and complications related to the hospitalization itself such as hospital-acquired infections, ventilator-associated morbidity, medication errors, and worsening frailty [31–33].
Vaccine effectiveness against hospitalized illness was not consistently higher than against outpatient illness when we restricted our analysis to outpatient and hospitalized patients in the same healthcare systems. However, without attenuation, we would have expected lower VE among hospitalized patients compared to outpatients because hospitalized patients had multiple high-risk comorbidities such as immunosuppressive disorders, conditions that have been historically associated with reduced vaccine protection [12]. When we restricted analysis to adults aged < 65 years without immunosuppressive conditions, we did identify significantly higher VE among hospitalized patients during 2015–2016 (ΔVE = 40%) but not during the 2017–2018 and 2018–2019 seasons. Virus circulation was variable with H1N1pdm09 and B-lineage viruses circulating during 2015–2016 compared with H3N2 viruses during the latter seasons. Differences in attenuation by virus type and vaccine match could partly explain these findings because VE has generally been lower against H3N2 viruses during the past decade [5]. However, this also could be a chance finding. A previous meta-analysis also identified no significant difference in VE between hospital and outpatient settings, matched by season and country [10].
Evidence of disease attenuation exists for several viral vaccines that provide imperfect protection against infection but reduce severity and postinfectious manifestations of disease, including rotavirus, measles, and varicella vaccines [34–36]. A limited number of studies provide support for attenuation of influenza disease severity with vaccination by evaluating outcomes such as reduced ICU admissions, length of stay, and death among those hospitalized with influenza [14–16]. Within VE networks, an observation of higher VE among hospitalized patients vs a comparable cohort of outpatients would further support a role of vaccination in attenuating disease severity. Our findings, however, illustrate that direct VE comparisons between settings should be interpreted with caution given a lack of comparability between populations. Longitudinal cohort studies might better assess severity attenuation compared to test-negative design studies, but are significantly more resource-intensive.
Our study had several limitations. First, we may have had unmeasured or residual confounding leading to biased estimates, and inpatient and outpatient studies may have been biased in different ways. We adjusted both VE models based on established confounders, such as age and time, as well as general measures of baseline health status relevant to the patients enrolled within each setting. Second, additional factors such as vaccine type (eg, greater uptake of adjuvanted or high-dose vaccines among high-risk inpatients or differential receipt of prior-season vaccination which may impact VE) may also influence VE estimates and were not included in our final models [37, 38]. Namely, high-dose vaccines were more frequently used in the hospitalized compared to outpatient population. Third, there could be information bias if data were collected differently between networks or sites. We standardized definitions to minimize the risk of information bias, for example by defining vaccination status the same between networks. Fourth, although we included a large number of patients from 3 influenza seasons in multisite studies, our study was likely underpowered to detect differences in VE within subgroups such as specific age strata.
In conclusion, patients within outpatient and hospital-based VE networks in our study represented unique populations challenging direct influenza VE comparisons between settings. Despite a high prevalence of comorbid high-risk conditions that may modify vaccine response among hospitalized patients, we documented that vaccination prevented influenza-associated hospitalization, which is associated with poor patient outcomes and high cost and additional resource burdens on the US healthcare system.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Dr Elaine Shuo Feng for sharing the R code used to calculate delta vaccine effectiveness estimates. Baylor Scott and White Health acknowledges the US Flu Vaccine Effectiveness Network investigators Michael Reis, Lydia Clipper, Michael Smith, Chandni Raiyani, Wencong Chen, Anne Robertson, Teresa O’Quinn, Ashley Kossie, Vanessa Hoelscher, Kimberley Walker, Marcus Volz, Arundhati Rao, Robert Fader, Yolanda Munoz-Maldonado; Baylor College of Medicine, Houston: Pedro Piedra, W. Paul Glezen, Vasanthi Avadhanula; Hospitalized Adult Influenza Vaccine Effectiveness Network investigators Tresa McNeal, Shekhar Ghamande, Kevin Chang, Heath White, Alejandro Arroliga, Laurel Kilpatrick, Meredith Wimberly, Victor Escobedo, JoAnn Nichols, Lydia Clipper, Chandni Raiyani, Wencong Chen, Anne Robertson, Arundhati Rao, Robert Fader, Kimberly Walker, Marcus Volz; and also acknowledges Juhee Song, Jessica Pruszynski, Archana Nangrani, Joann Nichols, Crystal Hodges, Ineshia Jackson, Deborah Furze, Gabriela Gonzales, Martha Zayed, Melissa Zdroik, Kevin Dunlap, Todd Crumbaker, Iosefo, Chooihoong Choo, Mary Kylberg, Lea Mallett, Hania Wehbe- Janek, Madhava Beeram, Natalie Settele, Jennifer Thomas, Jaime Walkowiak, Adelfa Alcozer, Evangeline Knight, Jeremy Ray, Renee Day, Deborah Price, Jennifer Fox, and Robert Probe.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Financial support. This study was supported by the CDC (cooperative agreements U01IP001034-U01IP001039, IP15-002, U01IP000969, 5U01 IP000473-05, 5U01 IP001039-01-02, and 5U01IP000972-01-03). At the University of Pittsburgh, the project was also supported by the National Institutes of Health (grant number UL1TR001857).
Potential conflicts of interest. E. A. B. reports grants from the CDC during the conduct of the study. J. M. F. reports nonfinancial support from the Institute for Influenza Epidemiology (funded in part by Sanofi Pasteur) outside the submitted work. M. G. reports grants from CDC during the conduct of the study and grants from MedImmune/AstraZeneca and CDC-Abt Associates outside the submitted work. M. L. J. reports grants from the CDC during the conduct of the study and grants from Sanofi Pasteur outside the submitted work. L. A. J. reports grants from the CDC during the conduct of the study and an organization research contract with Pfizer to fund clinical trials and studies outside the submitted work. E. T. M. reports personal fees from Pfizer and grants from Merck outside the submitted work. H. Q. M. reports grants from the CDC during the conduct of the study and grants from Seqirus outside the submitted work. A. S. M. reports personal fees from Sanofi Pasteur and Seqirus outside the submitted work. F. P. S. reports grants from the CDC during the conduct of the study; grants from Shire, Whiscon, and Ansun outside the submitted work; and nonfinancial support from Gilead outside the submitted work. H. K. T. has served on a data and safety monitoring board for Seqirus outside the submitted work. R. K. Z. reports grants from the CDC during the conduct of the study and grants from Merck & Co, Pfizer, and Sanofi Pasteur outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Disease burden of influenza. Available at: https://www.cdc.gov/flu/about/burden/index.html. Accessed 27 December 2019.
- 2. Rolfes MA, Flannery B, Chung J, et al. Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis 2019; 69:1845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019-20 influenza season. MMWR Recomm Rep 2019; 68:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2018–19 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed 27 December 2019.
- 5. Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16:942–51. [DOI] [PubMed] [Google Scholar]
- 6. Schmid P, Rauber D, Betsch C, Lidolt G, Denker ML. Barriers of influenza vaccination intention and behavior—a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS One 2017; 12:e0170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fell DB, Azziz-Baumgartner E, Baker MG, et al. WHO Taskforce to Evaluate Influenza Data to Inform Vaccine Impact and Economic Modelling . Influenza epidemiology and immunization during pregnancy: final report of a World Health Organization working group. Vaccine 2017; 35:5738–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ortiz JR, Neuzil KM. Influenza immunization of pregnant women in resource-constrained countries: an update for funding and implementation decisions. Curr Opin Infect Dis 2017; 30:455–62. [DOI] [PubMed] [Google Scholar]
- 9. Chow EJ, Doyle JD, Uyeki TM. Influenza virus-related critical illness: prevention, diagnosis, treatment. Crit Care 2019; 23:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng S, Cowling BJ, Sullivan SG. Influenza vaccine effectiveness by test-negative design—comparison of inpatient and outpatient settings. Vaccine 2016; 34:1672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andrew MK, Shinde V, Ye L, et al. Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN) . The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beck CR, McKenzie BC, Hashim AB, Harris RC, Nguyen-Van-Tam JS; University of Nottingham Influenza and the ImmunoCompromised (UNIIC) Study Group . Influenza vaccination for immunocompromised patients: systematic review and meta-analysis by etiology. J Infect Dis 2012; 206:1250–9. [DOI] [PubMed] [Google Scholar]
- 13. Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ 2013; 347:f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arriola C, Garg S, Anderson EJ, et al. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin Infect Dis 2017; 65:1289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arriola CS, Anderson EJ, Baumbach J, et al. Does influenza vaccination modify influenza severity? Data on older adults hospitalized with influenza during the 2012–2013 season in the United States. J Infect Dis 2015; 212:1200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thompson MG, Pierse N, Sue Huang Q, et al. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015. Vaccine 2018; 36:5916–25. [DOI] [PubMed] [Google Scholar]
- 17. Jackson ML, Chung JR, Jackson LA, et al. Influenza vaccine effectiveness in the United States during the 2015–2016 season. N Engl J Med 2017; 377:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferdinands JM, Gaglani M, Martin ET, et al. Prevention of influenza hospitalization among adults in the United States, 2015–2016: results from the US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). J Infect Dis 2019; 220:1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flannery B, Chung JR, Monto AS, et al. Influenza vaccine effectiveness in the United States during the 2016–2017 season. Clin Infect Dis 2019; 68:1798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fiore AE, Shay DK, Broder K, et al. Centers for Disease Control and Prevention . Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58:1–52. [PubMed] [Google Scholar]
- 21. O’Halloran AC, Lu PJ, Williams WW, Bridges CB, Singleton JA. Influenza vaccination coverage among people with high-risk conditions in the U.S. Am J Prev Med 2016; 50:e15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47:1245–51. [DOI] [PubMed] [Google Scholar]
- 23. Tang J, Wan JY, Bailey JE. Performance of comorbidity measures to predict stroke and death in a community-dwelling, hypertensive Medicaid population. Stroke 2008; 39:1938–44. [DOI] [PubMed] [Google Scholar]
- 24. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 25. Irving SA, Donahue JG, Shay DK, Ellis-Coyle TL, Belongia EA. Evaluation of self-reported and registry-based influenza vaccination status in a Wisconsin cohort. Vaccine 2009; 27:6546–9. [DOI] [PubMed] [Google Scholar]
- 26. Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol 2016; 184:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flannery B, Kondor RJG, Chung JR, et al. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis 2020; 221:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rondy M, El Omeiri N, Thompson MG, Levêque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75:381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reed C, Chaves SS, Perez A, et al. Complications among adults hospitalized with influenza: a comparison of seasonal influenza and the 2009 H1N1 pandemic. Clin Infect Dis 2014; 59:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 2018; 378:345–53. [DOI] [PubMed] [Google Scholar]
- 31. Putri WCWS, Muscatello DJ, Stockwell MS, Newall AT. Economic burden of seasonal influenza in the United States. Vaccine 2018; 36:3960–6. [DOI] [PubMed] [Google Scholar]
- 32. Segaloff HE, Petrie JG, Malosh RE, et al. Severe morbidity among hospitalised adults with acute influenza and other respiratory infections: 2014–2015 and 2015–2016. Epidemiol Infect 2018; 146: 1350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walker TA, Waite B, Thompson MG, et al. Risk of severe influenza among adults with chronic medical conditions. J Infect Dis 2020; 221:183–90. [DOI] [PubMed] [Google Scholar]
- 34. Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Human Rotavirus Vaccine Study Group . Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11–22. [DOI] [PubMed] [Google Scholar]
- 35. Cherry JD, Zahn M. Clinical characteristics of measles in previously vaccinated and unvaccinated patients in California. Clin Infect Dis 2018; 67:1315–9. [DOI] [PubMed] [Google Scholar]
- 36. Vázquez M, LaRussa PS, Gershon AA, Steinberg SP, Freudigman K, Shapiro ED. The effectiveness of the varicella vaccine in clinical practice. N Engl J Med 2001; 344:955–60. [DOI] [PubMed] [Google Scholar]
- 37. Van Buynder PG, Konrad S, Van Buynder JL, et al. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine 2013; 31:6122–8. [DOI] [PubMed] [Google Scholar]
- 38. van Aalst R, Gravenstein S, Mor V, et al. Comparative effectiveness of high dose versus adjuvanted influenza vaccine: a retrospective cohort study. Vaccine 2020; 38:372–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.